Abstract
Objectives
To analyze the temporal trends in thrombolysis rates after implementation of a regional emergency network for acute ischemic stroke (AIS).
Methods
We conducted a retrospective study based on a prospective multicenter observational registry. The AIS benefited from reperfusion therapy included in 1 of the 5 primary stroke units or 1 comprehensive stroke center and 37 emergency departments were included using a standardized case report form. The population covers 3 million inhabitants.
Results
In total, 32,319 AIS was reported in the regional hospitalization database of which 2215 thrombolyzed AIS patients were included in the registry and enrolled in this study. The annual incidence rate of thrombolysis continuously and significantly increased from 2010 to 2018 (10.2% to 17.3%, P‐trend = 0.0013). The follow‐up of the onset‐to‐door and the door‐to‐needle delays over the study period showed stable rates, as did the all‐cause mortality rate at 3‐months (13.2%).
Conclusion
Although access to stroke thrombolysis has increased linearly since 2010, the 3‐month functional outcome has not evolved as favorably. Further efforts must focus on reducing hospital delays.
Keywords: acute ischemic stroke, evolution of professional practices, reperfusion therapy, temporal trend
1. INTRODUCTION
1.1. Background
Stroke represents the third cause of mortality and the first cause of disability in high‐income countries with 84% of acute ischemic stroke (AIS). 1
The latest recommendations from the American Heart Association/American Stroke Association still recommend that the benefit of intravenous reperfusion therapy among eligible patients was time dependent and that treatment should be initiated as quickly as possible, without first magnetic resonance imaging (MRI) . 2 Mechanical thrombectomy has been recommended since 2015 in case of proximal occlusion. 3 Intravenous thrombolysis with recombinant tissue‐type plasminogen activator (tPA) is currently the standard treatment within 3 to 4.5 hours from the symptom onset.
1.2. Importance
The implementation of these recommendations implies that the guidelines need to adapt to territorial constraints and population specificities particularly in regard to respecting prehospital management by emergency physicians. Also, the decision regarding thrombolysis is the joint responsibility of the emergency physician and the neurologist.
Covering 3 million inhabitants in the Rhône‐Alpes region in France, the regional emergency network was established in 2008 including 5 primary stroke units, 1 comprehensive stroke center, and 37 emergency medical services (EMS). With written standardized protocols for prehospital stroke care and emergency transport to the closest facility with a stroke center, the prospective observational registry collects real‐life professional practices concerning the prehospital and hospital management of adult AIS patients eligible for thrombolysis.
1.3. Goals of the investigation
The objective of this study was to assess 8 consecutive years of data collection concerning the access to reperfusion therapy. We specifically analyzed whether the rate of thrombolysis was changing over time by comparing the registry rates with the number of ischemic strokes and whether there was a trend toward shorter treatment times and better short‐term prognosis.
2. METHODS
2.1. Study design and setting
The registry is funded by the Regional Agency for Health (Agence Régionale de Santé Auvergne Rhône‐Alpes) to organize emergency care pathways and improve clinical practices throughout the territory. All patients included in the prospective observational registry were also included in this study (see STROBE Guidelines), received oral and written information about their participation in the registry, and did not oppose to their data collection. The registry received approval from the French Commission for Liberties and Data Protection (n°1528226).
2.2. Selection of participants
Consecutive AIS patients treated with intravenous tPA, irrespective of age and treatment delays for thrombolysis, were included between October 2010 and September 2018. We chose the study period from October to September to keep data on the first incomplete collection year. Patients treated with intraarterial thrombolysis were also included. Stroke was defined according to the World STROKE Organization's Roadmap Implementation Guide for stroke. 4 The diagnosis of stroke subtype was based on physical examination, radiology imaging, and complementary exams. The classification of stroke was as follows: atherothrombotic, cardiac embolism, dissection, lacunar, undetermined, other ischemic etiologies, stroke mimics, and missing information. All patients were managed by attending neurologists with expertise in neurocritical care. The main exclusion criteria were based on the European product (tPA) license, derived from the European Cooperative Acute Stroke Study (ECASS) protocol 5 patients older than 80 years with more than 3 hours from onset to treatment (OTT), enhanced risk of bleeding, particularly intracranial hemorrhage and oral anticoagulant treatment, history of both prior stroke and diabetes, initial stroke severity evaluated by the National Institutes of Health Stroke Scale (NIHSS) >25 or <5, and blood glucose <50 mg/dl or >400 mg/dl3. Age and stroke severity became less restrictive exclusion criteria in 2014 after a meta‐analysis reported favorable effects of tPA irrespective of these factors. Specialized stroke centers often apply less restrictive criteria to treat patients with 1 or more violations of the license. The findings showed similar outcomes for patients treated with thrombolysis outside the European license (off‐label use; age >80 years old, Vitamin K antagonists (VKA) administered or international normalized ratio (INR) > 1.7, patient on anticoagulant or arterial pressure >185‐110 mmHg), and patients treated within the license (on‐label use). 6
2.3. Measurements
Data from consecutive patients were collected by neurologists using a standardized case report form (CRF). Immediate data entry was required on admission to ensure high data quality. The following variables were used for analysis: age, sex, call to the emergency dispatch center, transportation by fire brigade or mobile ICU (MICU), direct admission to stroke unit, distance from home to stroke unit (km), prestroke disability (modified Rankin scale [mRS] >1), and baseline characteristics such as NIHSS, wake‐up stroke, systolic and diastolic blood pressure, blood glucose, oral anticoagulant, INR > 1.7, off label use of thrombolysis, brain computed tomography (CT) scan or MRI, delays (onset to first medical contact, onset to direct admission), and OTD (onset‐to‐door), DIT (door‐to‐imaging time), ITN (imaging‐to‐needle), DTN (door‐to‐needle), and OTT times. Outcomes were also recorded in the CRF: hemorrhagic complications, such as systemic and symptomatic intracerebral hemorrhage, according to the current classification, 7 24‐hour NIHSS, mRS, and mortality at 3 months. The mRS is a 7‐point scale ranging from 0 (recovery to an asymptomatic state) through 2 (symptoms causing loss of independence in a previously independent daily activity, with preserved mobility) and 5 (bedbound, with severe disability) to 6 (death). 8 The mRS was dichotomized as a favorable outcome (score of 0 to 1) or an unfavorable outcome (score to 2 to 6). Symptomatic intracranial hemorrhage was defined as evidence of intracranial hemorrhage on neuroimaging that was associated with a neurologic deterioration (increase of 4 points or more on the NIHSS) or that led to death. 5 Early neurologic recovery was defined by an NIHSS score < 5. 9
2.4. Regional hospitalization database
Abstracts of AIS patients admitted to participating hospitals in our territory were extracted between October 2010 and September 2018 over the 8‐year period from the regional hospitalization database (PMSI, Programme de Médicalisation des Systèmes d'Information). Patients assigned ischemic stroke codes (International Classification of Diseases Tenth Revision [ICD‐10] codes I63, I64, and G46) were used as the denominator to report annual incidence rates of thrombolysis. The exclusion criteria were ICD‐10 (codes for transient cerebral ischemic attack (G45), subarachnoid hemorrhage (I60), and intracerebral hemorrhage (I61 and I62).
The Bottom Line.
Stroke centers along with emergency care pathways have proliferated in an attempt to expand early treatment with intravenous thrombolytics. A French registry study (2010–2018) of 37 emergency departments along with 5 primary and 1 comprehensive stroke center showed an increase in cases of thrombolysis but no improvement in onset‐to‐door and door‐to‐needle times.
2.5. Statistical analysis
Baseline characteristics were described by the median and interquartile range (IQR) for continuous variables because of their abnormal distributions and by numbers and percentages for categorical variables. Temporal linear trends were assessed using the Pearson Χ2 test for categorical variables and a Mann‐Kendall trend test for numerical variables, noted P‐trend. The annual incidence rate of thrombolysis was calculated by dividing the annual number of tPA‐treated patients in the RESUVal registry by the midyear number of patients abstracted from the regional PMSI data. The percentages of patients with DIT ≤25 minutes, ITN time ≤35 minutes, OTT time ≤155 minutes, and DTN time ≤60 minutes were estimated according to national and international stroke guidelines. The significance of differences was set at a P‐value < 0.05. All analyses were performed with R statistical software (R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R‐project.org/).
3. RESULTS
3.1. Characteristics of study subjects
Between October 2010 and September 2018, data from 32,319 AIS were extracted from the regional hospitalization database. Data from 4272 patients treated with thrombolysis were collected in the prospective RESUVal registry. Eighteen patients had missing information for stroke etiology (0.8%). The study population included 4272 thrombolysis patients, with an overall incidence rate of 17.3%. Of these patients, 3839 (97.7%) received intravenous thrombolysis, 17 (0.43%) received intraarterial thrombolysis, 40 (1.02%) received intravenous plus intraarterial thrombolysis, and 31 (0.79%) had missing information for the route of thrombolysis administration. The annual incidence rate of thrombolysis increased modestly by approximately 1% from the first year (10.2%; 369/3617) to the second (11%; 401/3628) and third year (12.5%; 463/3701) and remained stable in the fourth (12.1%; 466/3848) and fifth (11.9%; 502/4192) years (Figure 1). The stroke subtypes were atherothrombotic (26%), cardiac embolism (43%), dissection (2%), lacunar (4%), other ischemic etiologies (23%), stroke mimics (1%) and missing information (1%).
FIGURE 1.
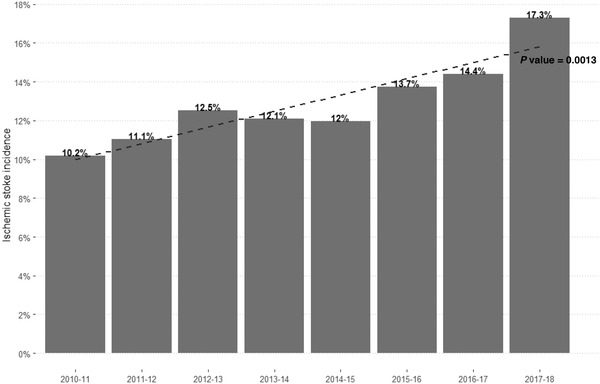
Annual incidence rate of AIS thrombolysis from October 2010 to September 2018. AIS, acute ischemic stroke
The median age was 74 years (IQR 63–82), and half of the patients were men (54%). The patients were similar in terms of most characteristics during the 8‐year period (Table 1, P‐value for trend ≥0.05): median age, sex ratio, median distance from home to stroke unit (18 km; IQR 7–34), median systolic (150; IQR 133–165 mmHg) and diastolic (80; IQR 70–90 mmHg) blood pressure values, prestroke use of oral anticoagulant therapy (approximately 5% of patients, among whom less than 1% had an INR above 1.7), and median initial blood glucose (6.5; IQR 5.6‐7.7 mmol/L). Preadmission calls to the medical dispatch center were high and stable at approximately 85%, in contrast with direct admission to the stroke unit, which remained low, at approximately 10% annually. Some trends were observed during the study period (P‐value for trend < 0.05). The proportion of patients older than 80 years significantly increased, representing roughly one quarter in the first year and one third in the last year. In this older population, there was a positive trend toward more female patients treated annually, whereas the trend remained stable for male patients. Transportation by fire brigade increased over time and almost doubled between the first and the last years, and transportation by EMS (MICU) and by ambulance decreased. Transportation by private vehicles and in‐hospital transportation remained low (nearly 5%) throughout the study period. Direct admission to brain imaging decreased from 57% in the first year to 50% in the last year. The median stroke severity at admission was stable over time (NIHSS 10; IQR = 5‐16), even though there was a small increase (6%) in patients with milder stroke (NIHSS 0–4). The percentage of patients with prestroke disability (mRS >1) doubled from 11.7% in the first year to 16.1% in the last year. A small percentage of patients reported wake‐up stroke, although this percentage doubled from 2% to 5% during the study period.
TABLE 1.
Temporal trend in baseline characteristics of patients treated for an acute ischemic stroke with intravenous or intraarterial tPA
| All | October 2010–September 2011 | October 2011–September 2012 | October 2012–September 2013 | October 2013–September 2014 | October 2014–September 2015 | October 2015–September 2016 | October 2016–September 2017 | October 2017–September 2018 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 4272 | Missing values | n = 369 | n = 403 | n = 468 | n = 469 | n = 509 | n = 604 | n = 649 | n = 801 | P‐trend | |
| Age, y (median [IQR]) | 74 [63;82] | 2 (0.05%) | 73 [63;80] | 74 [62;81] | 73 [62;81.25] | 75 [62;83] | 75 [64;82] | 73 [64;82] | 73 [62;82] | 74 [62;83] | X0.0643 |
| Age > 80 (%) | 1280 (29.96%) | 2 (0.05%) | 89 (24.12%) | 103 (25.56%) | 131 (27.99%) | 156 (33.26%) | 161 (31.63%) | 177 (29.30%) | 199 (30.66%) | 264 (32.96%) | 0.0013↑ |
| Male sex (%) | 2273 (53.21%) | 15 (0.35%) | 203 (55.01%) | 220 (54.59%) | 271 (57.91%) | 245 (52.24%) | 256 (50.29%) | 325 (53.81%) | 340 (52.39%) | 413 (51.56%) | 0.0823 |
| Male sex and age > 80 (%) | 498 (11.66%) | 4 (0.09%) | 35 (9.49%) | 47 (11.66%) | 52 (11.11%) | 56 (11.94%) | 63 (12.38%) | 75 (12.42%) | 78 (12.02%) | 92 (11.49%) | 0.3927 |
| Female sex and age > 80 (%) | 778 (18.21%) | 6 (0.14%) | 53 (14.36%) | 54 (13.40%) | 78 (16.67%) | 100 (21.32%) | 98 (19.25%) | 102 (16.89%) | 121 (18.64%) | 172 (21.47%) | 0.0009↑ |
| Call to dispatch center (%) | 3543 (82.94%) | 118 (2.76%) | 316 (85.64%) | 347 (86.10%) | 393 (83.97%) | 395 (84.22%) | 434 (85.27%) | 480 (79.47%) | 513 (79.04%) | 665 (83.02%) | 0.0036↓ |
| Transportation | |||||||||||
| ‐ Fire brigade (%) | 2511 (58.78%) | 337 (7.89%) | 136 (36.86%) | 230 (57.07%) | 269 (57.48%) | 300 (63.97%) | 345 (67.78%) | 385 (63.74%) | 403 (62.10%) | 443 (55.31%) | < 0.0001↑ |
| ‐ Ambulance (%) | 433 (10.14%) | 337 (7.89%) | 46 (12.47%) | 58 (14.39%) | 64 (13.68%) | 54 (11.51%) | 42 (8.25%) | 60 (9.93%) | 44 (6.78%) | 65 (8.11%) | < 0.0001↓ |
| ‐ Private vehicle (%) | 248 (5.81%) | 337 (7.89%) | 18 (4.88%) | 20 (4.96%) | 26 (5.56%) | 28 (5.97%) | 26 (5.11%) | 30 (4.97%) | 59 (9.09%) | 41 (5.12%) | 0.1825 |
| ‐ EMS (%) | 482 (11.28%) | 337 (7.89%) | 86 (23.31%) | 55 (13.65%) | 52 (11.11%) | 45 (9.59%) | 47 (9.23%) | 48 (7.95%) | 62 (9.55%) | 87 (10.86%) | < 0.0001↓ |
| In‐hospital transportation (%) | 261 (6.11%) | 337 (7.89%) | 17 (4.61%) | 17 (4.22%) | 24 (5.13%) | 17 (3.62%) | 29 (5.70%) | 44 (7.28%) | 47 (7.24%) | 66 (8.24%) | 0.0001↑ |
| Direct admission to stroke unit (%) | 461 (10.79%) | 172 (4.03%) | 54 (14.63%) | 58 (14.39%) | 63 (13.46%) | 63 (13.43%) | 57 (11.20%) | 61 (10.10%) | 55 (8.47%) | 50 (6.24%) | < 0.0001↓ |
| Direct admission to brain imaging (%) | 2008 (47.00%) | 172 (4.03%) | 211 (57.18%) | 206 (51.12%) | 210 (44.87%) | 199 (42.43%) | 248 (48.72%) | 258 (42.72%) | 274 (42.22%) | 402 (50.19%) | 0.0224↓ |
| Admission to ED (%) | 1546 (36.19%) | 172 (4.03%) | 69 (18.70%) | 129 (32.01%) | 167 (35.68%) | 194 (41.36%) | 188 (36.94%) | 254 (42.05%) | 265 (40.83%) | 280 (34.96%) | < 0.0001↑ |
| Admission to interventional neuroradiology | 67 (1.57%) | 172 (4.03%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 6 (0.99%) | 26 (4.01%) | 35 (4.37%) | < 0.0001↑ |
| Distance to stroke unit, km (median [IQR]) | 17 [7;32] | 160 (3.75%) | 17 [7;33.5] | 18 [8;34] | 20 [7;35] | 19 [8;32] | 17 [8;33] | 17 [6.625;30] | 18 [7;32] | 16 [5;31] | 0.0228X |
| Distance to stroke unit < 17 km | 1976 (46.25%) | 160 (3.75%) | 172 (46.61%) | 187 (46.40%) | 207 (44.23%) | 199 (42.43%) | 229 (44.99%) | 284 (47.02%) | 301 (46.38%) | 397 (49.56%) | 0.1204 |
| Prestroke disability mRS ≥ 1 (%) | 823 (19.26%) | 137 (3.21%) | 43 (11.65%) | 75 (18.61%) | 103 (22.01%) | 116 (24.73%) | 125 (24.56%) | 104 (17.22%) | 128 (19.72%) | 129 (16.10%) | 0.8196 |
| Prestroke disability mRS ≥ 2 (%) | 459 (10.74%) | 137 (3.21%) | 26 (7.05%) | 43 (10.67%) | 69 (14.74%) | 84 (17.91%) | 69 (13.56%) | 54 (8.94%) | 60 (9.24%) | 54 (6.74%) | 0.0021↓ |
| NIHSS score at admission, (median [IQR]) | 10 [5;17] | 128 (3%) | 11 [6;17] | 11 [6;16] | 10 [6;17] | 8 [5;15] | 10 [5;16] | 9 [5;17] | 9 [5;17] | 9 [4;16] | 0.0001X |
| NIHSS score < 5 (%) | 869 (20.34%) | 128 (3%) | 53 (14.36%) | 65 (16.13%) | 77 (16.45%) | 95 (20.26%) | 108 (21.22%) | 133 (22.02%) | 131 (20.18%) | 207 (25.84%) | < 0.0001↑ |
| NIHSS score > 25 (%) | 60 (1.40%) | 128 (3%) | 8 (2.17%) | 3 (0.74%) | 2 (0.43%) | 5 (1.07%) | 9 (1.77%) | 12 (1.99%) | 12 (1.85%) | 9 (1.12%) | X |
| Wake‐up stroke (%) | 296 (6.93%) | 2666 (62.41%) | 9 (2.44%) | 14 (3.47%) | 30 (6.41%) | 26 (5.54%) | 27 (5.30%) | 42 (6.95%) | 60 (9.24%) | 88 (10.99%) | < 0.0001↑ |
| Blood pressure, mmHg | |||||||||||
| ‐ Systolic (median [IQR]) | 150 [133;165] | 133 (3.11%) | 148.5 [132;164] | 147 [131;163] | 148 [130;162] | 151 [137;168] | 150 [134;165] | 150 [133;166] | 149 [133;164] | 150 [134;169] | 0.0526X |
| ‐ Systolic < 150 mmHg | 2017 (47.21%) | 133 (3.11%) | 187 (50.68%) | 211 (52.36%) | 235 (50.21%) | 201 (42.86%) | 240 (47.15%) | 280 (46.36%) | 318 (49.00%) | 345 (43.07%) | 0.0066↓ |
| ‐ Diastolic (median [IQR]) | 80 [70;90] | 164 (3.84%) | 80 [70;89] | 80 [70;90] | 80 [70;90] | 80 [70;90] | 80 [70;90] | 80 [70;90] | 80 [70;90] | 80 [70;90] | 0.1262X |
| ‐ Diastolic < 80 mmHg | 1854 (43.40%) | 164 (3.84%) | 166 (44.99%) | 191 (47.39%) | 210 (44.87%) | 197 (42.00%) | 224 (44.01%) | 252 (41.72%) | 286 (44.07%) | 328 (40.95%) | 0.0596 |
| ‐ Blood pressure > 180/110 mmHg (%) | 316 (7.40%) | 410 (9.6%) | 24 (6.50%) | 29 (7.20%) | 24 (5.13%) | 37 (7.89%) | 43 (8.45%) | 39 (6.46%) | 53 (8.17%) | 67 (8.36%) | 0.1119 |
| Initial blood glucose mmol/l | 6.5 [5.6;7.8] | 265 (6.2%) | 6.4 [5.55;7.64] | 6.6 [5.8;7.745] | 6.595 [5.5;7.7] | 6.6 [5.6;7.7] | 6.5 [5.61;7.8] | 6.43 [5.6;7.8] | 6.38 [5.5;7.8] | 6.57 [5.6;8.03] | 0.5963X |
| Initial blood glucose < 6.5 mmol/l | 1963 (45.95%) | 265 (6.2%) | 180 (48.78%) | 174 (43.18%) | 203 (43.38%) | 201 (42.86%) | 226 (44.40%) | 293 (48.51%) | 321 (49.46%) | 365 (45.57%) | 0.2690 |
| Prestroke use of oral anticoagulants (median [IQR] | 159 (3.72%) | 427 (10%) | 16 (4.34%) | 19 (4.71%) | 20 (4.27%) | 27 (5.76%) | 26 (5.11%) | 12 (1.99%) | 17 (2.62%) | 22 (2.75%) | 0.0029↓ |
| INR > 1.7 (%) | 21 (0.49%) | 4119 (96.42%) | 6 (1.63%) | 0 (0.00%) | 3 (0.64%) | 4 (0.85%) | 6 (1.18%) | 1 (0.17%) | 1 (0.15%) | 0 (0.00%) | X |
Note: Temporal linear trends were assessed using the Pearson Χ 2 for categorical variables and Mann‐Kendall trend test for continuous variables.
Abbreviations: ED, emergency department; EMS, emergency medical services; INR, international normalized ratio; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator.
3.2. Hospital management
Nearly three quarters of the patients (n = 3248; 76.03%) were evaluated by MRI diffusion, which was rather stable over the study period (Table 2), as was the use of MRI angiography. The use of CT angiography doubled (from 10.6% to 20.1%), whereas the use of MRI perfusion gradually decreased from 33% to 9%. Approximately 40% of the patients each year had proximal artery occlusion. The percentages of patients treated with thrombolysis within 3 hours (62%), between 3 and 4.5 hours (33%), and beyond 4.5 hours (4.7%) of stroke presentation remained stable during the study period. Patients became less likely to be treated with intraarterial thrombolysis (n = 17; 0.43%) or intraarterial plus intravenous thrombolysis (n = 40; 1.02%) over time. Approximately one third (39.5%) of the patients were treated with off‐label thrombolysis each year.
TABLE 2.
Temporal trends in timeliness of care delivery of patients treated for an acute ischemic stroke with intravenous or intraarterial tPA
| All | October 2010–September 2011 | October 2011–September 2012 | October 2012–September 2013 | October 2013–September 2014 | October 2014–September 2015 | October 2015–September 2016 | October 2016–September 2017 | October 2017–September 2018 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 4272 | Missing values | n = 369 | n = 403 | n = 468 | n = 469 | n = 509 | n = 604 | n = 649 | n = 801 | P‐trend | |
| CT scan | |||||||||||
| ‐ CT angiography (%) | 846 (19.80%) | 13 (0.3%) | 39 (10.57%) | 57 (14.14%) | 94 (20.09%) | 96 (20.47%) | 123 (24.17%) | 131 (21.69%) | 145 (22.34%) | 161 (20.10%) | < 0.0001↑ |
| ‐ CT perfusion (%) | 247 (5.78%) | 27 (0.63%) | 12 (3.25%) | 15 (3.72%) | 25 (5.34%) | 25 (5.33%) | 31 (6.09%) | 37 (6.13%) | 28 (4.31%) | 74 (9.24%) | 0.0001↑ |
| MRI | |||||||||||
| ‐ MRI‐angiography (%) | 3161 (73.99%) | 13 (0.3%) | 263 (71.27%) | 299 (74.19%) | 335 (71.58%) | 314 (66.95%) | 363 (71.32%) | 462 (76.49%) | 489 (75.35%) | 636 (79.40%) | 0.0001↑ |
| ‐ MRI‐diffusion (%) | 3248 (76.03%) | 12 (0.28%) | 266 (72.09%) | 308 (76.43%) | 346 (73.93%) | 345 (73.56%) | 370 (72.69%) | 469 (77.65%) | 493 (75.96%) | 651 (81.27%) | 0.0006↑ |
| ‐ MRI‐perfusion (%) | 752 (17.60%) | 40 (0.94%) | 122 (33.06%) | 72 (17.87%) | 39 (8.33%) | 28 (5.97%) | 47 (9.23%) | 86 (14.24%) | 110 (16.95%) | 248 (30.96%) | 0.0004↑ |
| Proximal artery occlusion (ICA, M1, vertebral, BA) (%) | 1784 (41.76%) | 41 (0.96%) | 157 (42.55%) | 159 (39.45%) | 195 (41.67%) | 170 (36.25%) | 198 (38.90%) | 285 (47.19%) | 278 (42.84%) | 342 (42.70%) | 0.1273 |
| Time to treatment initiation | |||||||||||
| ≤3 hours (%) | 2480 (58.05%) | 384 (8.99%) | 229 (62.06%) | 256 (63.52%) | 282 (60.26%) | 274 (58.42%) | 304 (59.72%) | 341 (56.46%) | 360 (55.47%) | 434 (54.18%) | 0.0001↓ |
| >3 to ≤4.5 hours (%) | 1141 (26.71%) | 384 (8.99%) | 122 (33.06%) | 126 (31.27%) | 140 (29.91%) | 154 (32.84%) | 143 (28.09%) | 149 (24.67%) | 143 (22.03%) | 164 (20.47%) | < 0.0001↓ |
| 4.5 hours (%) | 152 (3.56%) | 384 (8.99%) | 18 (4.88%) | 18 (4.47%) | 31 (6.62%) | 26 (5.54%) | 11 (2.16%) | 18 (2.98%) | 12 (1.85%) | 18 (2.25%) | < 0.0001↓ |
| On fluid attenuation inversion response | 115 (2.69%) | 384 (8.99%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.21%) | 13 (2.55%) | 40 (6.62%) | 28 (4.31%) | 33 (4.12%) | < 0.0001↑ |
| tPA administration | 3927 (91.92%) | 0 (0%) | 365 (98.92%) | 399 (99.01%) | 457 (97.65%) | 459 (97.87%) | 483 (94.89%) | 550 (91.06%) | 552 (85.05%) | 662 (82.65%) | < 0.0001↓ |
| ‐ Intravenous tPA (%) | 3839 (97.76%) | 31 (0.79%) | 337 (92.33%) | 394 (98.75%) | 435 (95.19%) | 451 (98.26%) | 475 (98.34%) | 545 (99.09%) | 546 (98.91%) | 656 (99.09%) | < 0.0001↑ |
| ‐ Intraarterial tPA (%) | 17 (0.43%) | 31 (0.79%) | 11 (3.01%) | 2 (0.50%) | 2 (0.44%) | 0 (0.00%) | 1 (0.21%) | 0 (0.00%) | 0 (0.00%) | 1 (0.15%) | < 0.0001↓ |
| ‐ Intravenous plus intra‐arterial tPA (%) | 40 (1.02%) | 31 (0.79%) | 13 (3.56%) | 1 (0.25%) | 18 (3.94%) | 1 (0.22%) | 5 (1.04%) | 0 (0.00%) | 2 (0.36%) | 0 (0.00%) | X |
| ‐ Unknown administration route | 31 (0.79%) | 0 (0%) | 4 (1.10%) | 2 (0.50%) | 2 (0.44%) | 7 (1.53%) | 2 (0.41%) | 5 (0.91%) | 4 (0.72%) | 5 (0.76%) | X |
| ‐ Off‐label tPA (%) | 1551 (39.50%) | 74 (1.88%) | 168 (46.03%) | 156 (39.10%) | 163 (35.67%) | 195 (42.48%) | 197 (40.79%) | 211 (38.36%) | 202 (36.59%) | 259 (39.12%) | 0.0996 |
| Primary thrombectomy | 308 (7.21%) | 2 (0.05%) | 4 (1.08%) | 4 (0.99%) | 8 (1.71%) | 8 (1.71%) | 22 (4.32%) | 50 (8.28%) | 91 (14.02%) | 121 (15.11%) | < 0.0001↑ |
| Delays (minutes) | |||||||||||
| Onset‐to‐first medical contact (median [IQR]) | 34.5 [15;73] | 1762 (41.25%) | 35 [15;72.25] | 30 [15;60] | 30 [12;60] | 35 [15;69.75] | 40 [15.5;80] | 40 [20;90] | 30 [15;80] | 30 [15;69.5] | 0.2585X |
| Onset‐to‐first medical contact < 35 minutes (%) | 1255 (29.38%) | 1762 (41.25%) | 132 (35.77%) | 151 (37.47%) | 159 (33.97%) | 138 (29.42%) | 132 (25.93%) | 160 (26.49%) | 176 (27.12%) | 207 (25.84%) | < 0.0001↓ |
| Onset‐to‐direct admission (median [IQR]) | 86 [64;116] | 855 (20.01%) | 90 [65.5;120] | 90 [65;115] | 85 [60;113] | 90 [64;121] | 85 [64;115] | 85 [65;120] | 85 [63;114] | 85 [65;120] | 0.4280X |
| Onset‐to‐direct admission < 88 minutes (%) | 1704 (39.89%) | 855 (20.01%) | 148 (40.11%) | 158 (39.21%) | 184 (39.32%) | 183 (39.02%) | 198 (38.90%) | 234 (38.74%) | 273 (42.06%) | 326 (40.70%) | 0.4497 |
| DIT (median [IQR]) | 24 [12;45] | 532 (12.45%) | 15 [9;37] | 20.5 [10;42.75] | 20 [10;40] | 26 [13;45.25] | 21 [10;42] | 28 [17;50] | 25 [13;48] | 25 [13;51] | < 0.0001X |
| DIT ≤ 25 minutes (%) | 1985 (46.47%) | 532 (12.45%) | 197 (53.39%) | 193 (47.89%) | 237 (50.64%) | 191 (40.72%) | 257 (50.49%) | 246 (40.73%) | 290 (44.68%) | 374 (46.69%) | 0.0135↓ |
| ITN (median [IQR]) | 35 [25;45] | 598 (14%) | 40 [30;50.25] | 37 [28;48] | 37 [29;48] | 32 [25;45] | 34 [25;45] | 32 [23;44] | 32 [23;46] | 33 [25;44] | < 0.0001X |
| ITN < 35 minutes (%) | 1832 (42.88%) | 598 (14%) | 108 (29.27%) | 168 (41.69%) | 174 (37.18%) | 242 (51.60%) | 237 (46.56%) | 286 (47.35%) | 281 (43.30%) | 336 (41.95%) | 0.0013↑ |
| OTT (median [IQR]) | 154 [123;195] | 792 (18.54%) | 160 [135;198] | 155 [127;196.25] | 154 [122;195] | 160 [123.5;195] | 150 [120;190] | 150 [125;195] | 151 [120;195] | 150 [120;195] | 0.0291X |
| OTT < 155 minutes (%) | 1749 (40.94%) | 792 (18.54%) | 150 (40.65%) | 167 (41.44%) | 194 (41.45%) | 188 (40.09%) | 220 (43.22%) | 248 (41.06%) | 258 (39.75%) | 324 (40.45%) | 0.6925 |
| DTN (median [IQR]) | 60 [45;85] | 594 (13.9%) | 60 [45;86] | 60 [47;89] | 60 [47;85] | 60 [44;82.25] | 56 [45;78] | 63 [45.25;91] | 60 [44;90] | 59 [45;83.25] | 0.2787X |
| DTN ≤ 60 minutes (%) | 1932 (45.22%) | 594 (13.9%) | 176 (47.70%) | 203 (50.37%) | 225 (48.08%) | 240 (51.17%) | 266 (52.26%) | 243 (40.23%) | 257 (39.60%) | 322 (40.20%) | < 0.0001↓ |
Note: Temporal linear trends were assessed using the Pearson Χ 2 for categorical variables and Mann‐Kendall trend test for continuous variables.
Abbreviations: BA, basilar artery; CT, computed tomographic; DIT, door‐to‐imaging time; DTN, door‐to‐needle; ICA, intracranial carotid artery; IQR, interquartile range; ITN, imaging‐to‐needle time; M1, anterior cerebral artery; MRI, magnetic resonance imaging; OTT, onset‐to‐treatment time; tPA, tissue plasminogen activator.
3.3. Timeliness of care delivery
Over the study period, there was no improvement in most prehospital and hospital times except for ITN time, which decreased from a median of 40 minutes in the first year to 34 minutes (Table 2). Less than half of the patients had an ITN time less than 35 minutes, although this proportion doubled over time. The onset‐to‐first medical contact delay had a relatively stable median of 35 minutes (IQR 15–69.3), and there was a median delay of 89 minutes for onset to direct admission (IQR 64–115). The overall median DIT was 21 minutes (IQR 10–41 minutes) and remained stable over the study period. Less than half of the patients had a recommended DIT within 25 minutes throughout the study period. DTN time remained unchanged, with a borderline recommended median of 60 minutes (IQR 45–83). Half of the patients had a recommended DTN time within 60 minutes during the study period. The OTT time was stable throughout the study period, with an overall median of 155 minutes, corresponding to less than 3 hours (2.6 hours) (Figure 2).
FIGURE 2.
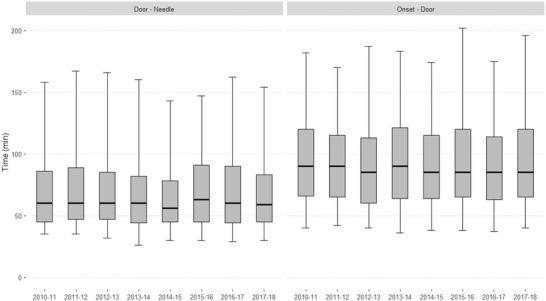
Comparison of the onset‐to‐door and the door‐to‐needle delays over the study period
3.4. Main results
The 3‐month all‐cause mortality rate remained stable at 13.2% throughout the study period. The rates of systemic hemorrhage and symptomatic intracerebral hemorrhage were nearly 3% over time (Table 3). The 24‐hour median NIHSS after thrombolysis decreased from 6 (IQR 2–14) in the first year to 4 (IQR 1–12) in the last year. The median Rankin scale score was 2 (IQR 0–4) and remained stable, as did the percentage of patients with a good recovery (mRS ≤1) (33.3%). Approximately 40% of the patients had a good early recovery after thrombolysis (Figure 3).
TABLE 3.
Temporal trends in 24‐hours and 90‐days outcomes of acute ischemic stroke in patients treated with intravenous or intraarterial tPA
| All | October 2010–September 2011 | October 2011–September 2012 | October 2012–September 2013 | October 2013–September 2014 | October 2014–September 2015 | October 2015–September 2016 | October 2016–September 2017 | October 2017–September 2018 | P‐trend | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 4224 | Missing values | n = 369 | n = 401 | n = 463 | n = 466 | n = 502 | n = 595 | n = 638 | n = 790 | ||
| Any ICH (%) | |||||||||||
| ‐ HI type 1 (%) | 252 (5.97%) | 63 (1.49%) | 24 (6.50%) | 21 (5.24%) | 25 (5.40%) | 23 (4.94%) | 28 (5.58%) | 32 (5.38%) | 45 (7.05%) | 54 (6.84%) | 0.2343 |
| ‐ HI type 2 (%) | 185 (4.38%) | 63 (1.49%) | 19 (5.15%) | 15 (3.74%) | 18 (3.89%) | 14 (3.00%) | 18 (3.59%) | 28 (4.71%) | 33 (5.17%) | 40 (5.06%) | 0.2423 |
| ‐ PH type 1 (%) | 122 (2.89%) | 63 (1.49%) | 11 (2.98%) | 14 (3.49%) | 15 (3.24%) | 10 (2.15%) | 10 (1.99%) | 19 (3.19%) | 22 (3.45%) | 21 (2.66%) | 0.8436 |
| ‐ PH type 2 (%) | 138 (3.27%) | 63 (1.49%) | 15 (4.07%) | 11 (2.74%) | 16 (3.46%) | 24 (5.15%) | 15 (2.99%) | 13 (2.18%) | 18 (2.82%) | 26 (3.29%) | 0.2969 |
| Symptomatic ICH (%) | 139 (3.29%) | 102 (2.41%) | 13 (3.52%) | 12 (2.99%) | 8 (1.73%) | 14 (3.00%) | 21 (4.18%) | 22 (3.70%) | 25 (3.92%) | 24 (3.04%) | 0.4414 |
| Systemic hemorrhage (%) | 144 (3.41%) | 82 (1.94%) | 6 (1.63%) | 20 (4.99%) | 11 (2.38%) | 16 (3.43%) | 24 (4.78%) | 15 (2.52%) | 22 (3.45%) | 30 (3.80%) | 0.4316 |
| 24 hours‐NIHSS (median [IQR]) | 5 [1;12] | 974 (23.06%) | 6 [2;14] | 6 [2;14] | 5 [2;13] | 5 [1;12] | 4 [1;11.75] | 5 [1;12] | 4 [1;11] | 3 [1;11] | < 0.0001X |
| 24 hours‐NIHSS < 5 | 1620 (38.35%) | 974 (23.06%) | 153 (41.46%) | 148 (36.91%) | 191 (41.25%) | 190 (40.77%) | 212 (42.23%) | 236 (39.66%) | 242 (37.93%) | 248 (31.39%) | 0.0015 |
| mRS at 3 months (median [IQR]) | 2 [0;4] | 1020 (24.15%) | 2 [1;4] | 2 [1;4] | 2 [0;4] | 2 [0;4] | 2 [0;4] | 1 [0;4] | 2 [0;4] | 2 [0;4] | 0.22931X |
| mRS ≤ 1 (%) | 1461 (34.59%) | 1020 (24.15%) | 137 (37.13%) | 119 (29.68%) | 155 (33.48%) | 147 (31.55%) | 176 (35.06%) | 235 (39.50%) | 225 (35.27%) | 267 (33.80%) | 0.3563 |
| mRS ≤ 2 (%) | 1874 (44.37%) | 1020 (24.15%) | 178 (48.24%) | 167 (41.65%) | 193 (41.68%) | 202 (43.35%) | 220 (43.82%) | 293 (49.24%) | 277 (43.42%) | 344 (43.54%) | 0.9721 |
| All‐cause mortality at 3 months (%) | 559 (13.23%) | 1780 (42.14%) | 54 (14.63%) | 42 (10.47%) | 59 (12.74%) | 67 (14.38%) | 57 (11.35%) | 76 (12.77%) | 84 (13.17%) | 120 (15.19%) | 0.2791 |
Note: Temporal linear trends were assessed using the Pearson Χ 2 for categorical variables and Mann‐Kendall trend test for continuous variables.
Abbreviations: HI, hemorrhagic infarction; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; PH, parenchymal hematoma.
FIGURE 3.
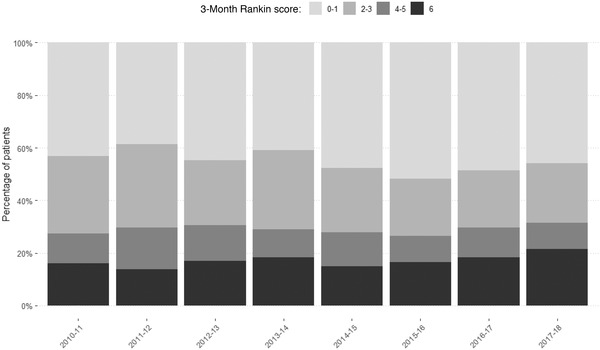
Functional outcome (3‐month Rankin score) over the study period
3.5. Limitations
Our work is a real‐life observational study, for which there are some limitations. First, we have no information regarding the etiology of the whole study period, as well as the evolution of the epidemiological profile of patients. The interventions and infrastructures put in place to improve access to health care are specific to the health zone and the registry (management recommendations specific to the unit). There is also a limitation concerning our territorial approach: our study was conducted in urban and peri‐urban areas with different accessibility constraints depending on the units and the availability of hospitals with no neurovascular units, which may extend delays in certain areas.
4. DISCUSSION
In accordance with the objective of the study, it was demonstrated that the annual rate of thrombolysis continuously increased throughout the study period (from 10.2% to 17.3%). In 2008, after the publication of the ECASS III study, 10 the American Heart Association/American Stroke Association extended the intravenous‐tPA time window from 3 to 4.5 hours. Time window excludes many patients from receiving thrombolysis. However, skeptical reports demonstrated that expanding the time window of thrombolysis up to 4.5 hours would only marginally increase the thrombolysis rate (0.5% to 1.4%) and that the time window within 3 hours was more effective. Because of the less restrictive use of thrombolysis, there was a gradual increase in the number of patients older than 80 years during the study period, which resulted in a trend toward more patients with a prestroke disability or a milder stroke. In the RESUVal registry, approximately one third of the patients were older than 80 years, so the median age shifted from 73 years in the first year to 75 years in the last 2 years. Kelly et al. 11 reported an increase in tPA‐treated patients older than 85 years, who accounted for 10.5% of patients from 2003 to 2005 and 16.4% of patients from 2010 to 2011. The proportion of tPA‐treated patients with milder stroke severity almost doubled over time.
For stroke care management, the guidelines recommend initial transportation by EMS (MICU) to prompt triage, prehospital care including resuscitation (when necessary), transport to the stroke unit available in the shortest time and prehospital notification. 2 Our findings showed in‐depth changes in the profile of transportation by fire brigade, whereas transportation by MICU and private ambulances decreased. Direct admission to stroke units decreased the OTD and OTT times of admitted patients and was associated with better functional outcomes. Bypassing the emergency department to directly admit patients to brain imaging was associated with a lower DTN time. 12
The goal of the RESUVal network to improve prehospital and in‐hospital delays to thrombolysis was partially achieved. Also, during the study period, quality changes projects have probably indirectly improved the outcomes such as a training of neurovascular referents in the network's neurovascular unit carried out on site by emergency physicians. The referents have trained their teams, annual meetings on the latest stroke recommendations were organized, the promotion of the transition from direct admission to MRI and also sharing of referentials and updates with European recommendations, allowing easy access to information.
A lack of improvement in prehospital and in‐hospital delays to thrombolysis were observed, such as symptom onset to first medical contact, symptom onset to first medical contact, symptom onset to direct and secondary admission, OTD, DIT, and DTN. However, the ITN time significantly decreased. Some positive points in line were observed with the stroke guidelines, such as gradual decrease in perfusion imaging for both CT and MRI over time in the RESUVal registry, which are long procedures. 2 Another positive result in the RESUVal registry was the improvement in the ITN time over time, even though more than half of tPA‐treated patients had an ITN time greater than 35 minutes. The ITN time is a complex component of the DTN time that is related to decision making and laboratory tests and is more difficult to improve than the DIT. 2 In a recent study, a Mobile Interventional Stroke Team (MIST) model was assessed as an alternative model to transferring patients and demonstrated that transferring a MIST to a Thrombectomy Capable Stroke Center to perform endovascular therapy was time efficient. 13 The PHANTOM study in Berlin demonstrated that the use of an ambulance‐based CT scanner followed by thrombolysis was safe and significantly increased the thrombolysis rate (32.6% vs 21% for standard care) and reduced the alarm‐to‐treatment time by a mean of 25 minutes. 14 The second solution could be to reduce the DTN time via a further decrease in the DIT, which was still longer than 25 minutes for more than half of the patients. Our results contrasted with those of interventional and observational studies, which reported a consistent decrease in the DIT. 15 Some measures could be implemented to improve DIT: prehospital notification calls to stroke physicians and EMS, the relocation of CT near the ED, routing patients directly to brain imaging, and prioritization of a non‐contrast CT scan over MRI unless there is diagnostic uncertainty that requires CT angiography and perfusion imaging. 12 , 16 , 17
A positive trend in access to reperfusion therapy was demonstrated with the increase in the rate of thrombolysis over the study period and patients with a good recovery represented nearly one third of the tPA‐treated patients. From 2010 to 2018, intraarterial treatment seems to be an area for improvement highlighted by the low rate of its use. Outcomes were improved by different intervention and infrastructures put in place, which paved the way toward shorter treatment times and better short‐term prognosis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
CEK, MB, KBL, SC, FP, PS, NN, AEV and LD conceived the study and designed the trial. CEK, LF, CC, MB and PS supervised the conduct of the trial and data collection. CEK, LF, CC, MB, KBL, SC, FP, PS, NN, AEV and LD undertook recruitment of participating centers and patients and managed the data, including quality control. CEK, LF, CC and LD provided statistical advice on study design and analyzed the data; CEK shared the data oversight committee. CEK, CBE, LF and LD drafted the manuscript, and all authors contributed substantially to its revision. CEK and LD take responsibility for the paper as a whole. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank the referents of stroke hospitals: Hôpital Louis Pradel of Bron (Laurent Derex, MD, PhD), Centre Hospitalier of Valence (Karine Blanc‐Lasserre, MD), Centre Hospitalier of Vienne (Anne‐Evelyne Vallet, MD), Centre Hospitalier of Bourg‐en‐Bresse (Frédéric Philippeau, MD), Centre Hospitalier of Villefranche‐sur‐Saône (Serkan Cakmak, MD), and Centre Hospitalier of Montélimar (Chérif Heroum, MD). The authors also thank Emeline Moderni, French medical translator specialized in cardiology, pharmacology and neurology within the RESCUe RESUVal networks, for medical writing assistance and English proofreading in accordance with the European Writers Association guidelines and Good Publication Practice.
Biography
Carlos El Khoury, MD, PhD, is an Emergency Physician and Head of the Clinical Research Division at Médipôle Hôpital Mutualiste in Villeurbanne, France.

El Khoury C, Aboa‐Eboule C, Fraticelli L, et al., Temporal trends in reperfusion therapy for patients with acute ischemic stroke. JACEP Open. 2022;3:e12654. 10.1002/emp2.12654
Supervising Editor: Christian Tomaszewski, MD, MS.
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
REFERENCES
- 1. Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. 2020;48(11):1654‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. 2019;50(12):e344‐e418. [DOI] [PubMed] [Google Scholar]
- 3. Paliniswami M, Yan B. Mechanical thrombectomy is now the gold standard for acute ischemic stroke: implications for routine clinical practice. Interv Neurol. 2015;4(1‐2):18‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindsay MP, Norrving B & Furie KL. Global Stroke Guidelines and Action Plan: A Road Map for Quality Stroke Care Roadmap Implementation Guide. 2016. https://www.world‐stroke.org/assets/downloads/Global_Stroke_Guidelines_and_Action_Plan_All_in_one_English.pdf
- 5. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317‐1329. [DOI] [PubMed] [Google Scholar]
- 6. Cappellari M, Moretto G, Micheletti N, et al. Off‐label thrombolysis versus full adherence to the current European alteplase license: impact on early clinical outcomes after acute ischemic stroke. J Thromb Thrombolysis. 2014;37(4):549‐556. [DOI] [PubMed] [Google Scholar]
- 7. Zhang J, Yang Y, Sun H, Xing Y. Hemorrhagic transformation after cerebral infarction: current concepts and challenges. Ann Transl Med. 2014;2(8):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sauser K, Levine DA, Nickles AV, Reeves MJ. Hospital variation in thrombolysis times among patients with acute ischemic stroke: the contributions of door‐to‐imaging time and imaging‐to‐needle time. JAMA Neurol. 2014;71(9):1155‐1161. [DOI] [PubMed] [Google Scholar]
- 9. Irvine HJ, Battey TWK, Ostwaldt AC, et al. Early neurological stability predicts adverse outcome after acute ischemic stroke. Int J Stroke Off J Int Stroke Soc. 2016;11(8):882‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de los Ríos la Rosa F, Khoury J, Kissela BM, et al. Eligibility for Intravenous Recombinant Tissue‐Type Plasminogen Activator within a population: the effect of the European Cooperative Acute Stroke Study (ECASS) III trial. Stroke J Cereb Circ. 2012;43(6):1591‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly AG, Hellkamp AS, Olson D, Smith EE, Schwamm LH. Predictors of rapid brain imaging in acute stroke: analysis of the Get With the Guidelines‐stroke program. Stroke. 2012;43(5):1279‐1284. [DOI] [PubMed] [Google Scholar]
- 12. Rubin MN, Barrett KM. What to do with wake‐up stroke. The Neurohospitalist. 2015;5(3):161‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morey JR, Oxley TJ, Wei D, et al. Mobile Interventional stroke team model improves early outcomes in large vessel occlusion stroke. Stroke. 2020;51(12):3495‐3503. [DOI] [PubMed] [Google Scholar]
- 14. Oluwole SA, Wang K, Dong C, et al. disparities and trends in door‐to‐needle time. Stroke. 2017;48(8):2192‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kruyt ND, Nederkoorn PJ, Dennis M, et al. Door‐to‐needle time and the proportion of patients receiving intravenous thrombolysis in acute ischemic stroke: uniform interpretation and reporting. Stroke. 2013;44(11):3249‐3253. [DOI] [PubMed] [Google Scholar]
- 16. Ebinger M, Winter B, Wendt M, et al. effect of the use of ambulance‐based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA. 2014;311(16):1622‐1631. [DOI] [PubMed] [Google Scholar]
- 17. Sung SF, Huang YC, Ong CT, Chen YW. A parallel thrombolysis protocol with nurse practitioners as coordinators minimized door‐to‐needle time for acute ischemic stroke. Stroke Res Treat. 2011;2011:198518. [DOI] [PMC free article] [PubMed] [Google Scholar]


