PURPOSE
Next-generation sequencing is increasingly used in gynecologic and breast cancers. Multidisciplinary Molecular Tumor Board (MTB) may guide matched therapy; however, outcome data are limited. We evaluate the effect of the degree of matching of tumors to treatment as well as compliance to MTB recommendations on outcomes.
METHODS
Overall, 164 patients with consecutive gynecologic and breast cancers presented at MTB were assessed for clinicopathologic data, next-generation sequencing results, MTB recommendations, therapy received, and outcomes. Matching score (MS), defined as percentage of alterations targeted by treatment over total pathogenic alterations, and compliance to MTB recommendations were analyzed in context of oncologic outcomes.
RESULTS
Altogether, 113 women were evaluable for treatment after MTB; 54% received matched therapy. Patients with MS ≥ 40% had higher overall response rate (30.8% v 7.1%; P = .001), progression-free survival (PFS; hazard ratio [HR] 0.51; 95% CI, 0.31 to 0.85; P = .002), and a trend toward improved overall survival (HR 0.64; 95% CI, 0.34 to 1.25; P = .082) in univariate analysis. The PFS advantage remained significant in multivariate analysis (HR 0.5; 95% CI, 0.3 to 0.8; P = .006). Higher MTB recommendation compliance was significantly associated with improved median PFS (9.0 months for complete; 6.0 months for partial; 4.0 months for no compliance; P = .004) and overall survival (17.1 months complete; 17.8 months partial; 10.8 months none; P = .046). Completely MTB-compliant patients had higher MS (P < .001). In multivariate analysis comparing all versus none MTB compliance, overall response (HR 9.5; 95% CI, 2.6 to 35.0; P = .001) and clinical benefit (HR 8.8; 95% CI, 2.4 to 33.2; P = .001) rates were significantly improved with higher compliance.
CONCLUSION
Compliance to MTB recommendations resulted in higher degrees of matched therapy and correlates with improved outcomes in patients with gynecologic and breast cancers.
INTRODUCTION
Next-generation sequencing (NGS) is being integrated into routine cancer care, providing patients and their oncologists with molecular characterization of their tumors and a promise for personalized therapy. Molecular analysis reports include genomic alterations in signaling pathways, DNA repair pathways, and biomarkers for response to immunotherapy. Several large meta-analyses, examining a total of over 85,000 patients in clinical trials, found significantly improved response rates in patients receiving biomarker-directed therapy compared with unselected therapies.1-3 The goal of precision oncology is to customize targeted regimens, often navigating patients to specific clinical trials or combining several agents repurposed from US Food and Drug Administration approvals for other tumors to an individual's unique tumor profile.4-7
CONTEXT
Key Objective
Does molecular matching of therapy with genomic alterations correlate with improved oncologic outcomes in patients with gynecologic and breast cancers?
Knowledge Generated
Patients with higher degrees of matched therapy had higher overall response rates, progression-free survival, and a trend toward improved overall survival. Higher compliance to Molecular Tumor Board recommendations was associated with higher degrees of matched therapy and improved outcomes.
Relevance
Molecular Tumor Boards are a valuable resource for gynecologic and breast cancer treatment; we advocate for increased access in this patient population to assist with treatment decisions as they may improve oncologic outcomes.
Precision medicine has a long history in breast oncology, with early incorporation of hormone status and HER2 overexpression guiding treatment.5,8,9 Gynecologic cancers have more recently seen the benefits of biomarker-directed therapy. Most notably, BRCA alterations and homologous recombination deficiency predict improved response rates to poly (ADP-ribose) polymerase inhibitors as single agent10-14 or combined with bevacizumab15 in maintenance therapy in epithelial ovarian cancer. Higher tumor mutation burden (TMB) and microsatellite instability high-molecular phenotypes predict response to checkpoint blockade in solid cancers, especially uterine, cervical, and vulvar malignancies.16-19 Extrapolating from data in breast cancer, trastuzumab has been used for patients with HER2-positive uterine serous cancer with exciting results,20 although combination of everolimus and letrozole has not proven to be reliably biomarker-dependent in uterine cancer.21
Targeting single pathways may be insufficient given tumor heterogeneity, escape pathways, and low response rates.22 Evidence is emerging that patients may benefit most from an N-of-one approach, examining all available NGS to determine a customized, often multiagent, treatment-to-target driver alterations, taking into context biomarkers of resistance, drug interactions, and clinical history.6,7,23-26 Evidence using multiple agents to target various alterations in an individual's gynecologic or breast cancer is limited,5,27 likely due at least in part to the complexity of managing combination therapy dosing and treatment-related adverse events.
This complex strategy requires a multidisciplinary approach, best addressed by a Molecular Tumor Board (MTB).23-26 The UC San Diego Moores Cancer Center MTB is a meeting led by medical oncologists with expertise in precision medicine and therapeutics, which incorporates the patient's clinical history and treatment course, relevant pathology, imaging, and all molecular profiling, as well as medical, surgical, radiation, and gynecologic oncologists; bioinformaticians; basic and translational scientists; geneticists; clinical trial coordinators; and medication acquisition specialists. After discussion, a targeted regimen is recommended to the patient's primary oncologist. Here, we provide outcome data on 164 patients with gynecologic or breast cancers who underwent NGS and were presented at our MTB. We show that high degrees of molecular matching of therapy with genomic alterations correlates with following MTB suggestions, which in turn associates favorably with outcome.
METHODS
Molecular Tumor Board
A midlevel and a senior medical oncologist with expertise in genomics, clinical trials, and immunotherapy moderated the face-to-face MTB meetings three times per month. Treating physicians submitted cases, including patient age, malignancy, prior treatment, biopsy site(s) and date(s), and molecular profile results. The treating physician or a proxy presented the clinical case; a pathologist reviewed pertinent histologic slides; and a radiologist presented key images and chronological comparisons if applicable. All molecular tests were performed at the College of American Pathologist–accredited and clinical laboratory improvement amendments–licensed clinical laboratories. Clinical trial coordinators and medical acquisition specialists screened patients for eligibility in clinical trials and medication acquisition. The MTB selected therapies to maximally target individual molecular alterations thought to be driving an individual's cancer, often combining drugs to address as many targetable molecular alterations while considering toxicity limitations. Combination therapies involved unique combinations, established combinations, or clinical trial experimental drugs. If multiple drugs were administered, they were administered at the same time and not sequentially. MTB recommendations were provided, but the treating physician made the final therapeutic decision. MTB complied with Health Insurance Portability and Accountability Act privacy standards.
Patients
Patients with consecutive gynecologic or breast cancers presented at the face-to-face MTB from December 2012 to September 2018 were assessed for eligibility, as described previously.26 All investigations followed the UC San Diego Internal Review Board–approved Profile-Related Evidence Determining Individualized Cancer Therapy (PREDICT) study (ClinicalTrials.gov identifier: NCT02478931) guidelines for data acquisition28 and for any investigational therapies or procedures for which the patients consented.
NGS of Tissue and Blood-Derived Cell-Free Circulating Tumor DNA
The treating physician determined which NGS tests to order. Options included tissue-based sequencing, liquid biopsy, and/or immunotherapy biomarkers. Tissue-based sequencing panels included 47-397 genes while circulating tumor DNA panels included 54-73 genes depending on the test laboratory and date29,30 (Data Supplement). Some physicians presented NGS results at MTB even if their patient was tolerating current therapy without progressive disease. In these situations, they often did not change treatment within 6 months of MTB presentation. Other physicians ordered the tests or presented older NGS tests at disease progression or intolerance of current treatment to change the treatment regimen. Only patients who had a change in treatment within 6 months of MTB presentation were included in the outcome analyses.
Evaluating the Degree to Which Therapy Was Matched With Genomic Alterations (matching score)
Matching score (MS), reflecting the degree of matching of patients to treatment, was calculated by evaluating the total number of alterations targeted by administered drugs divided by the total number of alterations. For example, if a tumor had four alterations and the patient received two therapies that targeted two of these alterations, the score would be 50% (two of four alterations targeted). A receiver operating characteristic curve (Data Supplement) identified 40% as the optimal cut point for MS in our cohort. MSs were calculated by investigators blinded to patient outcomes. Further details regarding MS calculation have been reported previously.7
Compliance to MTB Suggestions
Compliance to MTB recommendations was determined by examining the recorded recommendations of the MTB and the patient's first line of therapy after MTB presentation. If the patient received all recommended treatments, it was considered completely compliant. If they received part of the recommendation, it was partial. Physicians could choose therapy after MTB discussion; for this publication, physicians who provided their choice of therapy without adhering to any MTB suggestions were considered noncompliant.
Statistical Methods
Descriptive statistics were used to summarize patient and molecular characteristics. Progression-free survival (PFS) was defined as the start date of treatment after MTB presentation to the date of progression or death, as determined by radiographic, serologic, or clinical findings. Overall survival (OS) was defined as the start date of therapy after MTB presentation until the date of death. Patients who had not progressed or were still alive at the last follow-up for PFS and OS, respectively, were censored on the date of last follow-up. RECIST v1.1 was used to evaluate response rates. Overall response rate (ORR) was defined as complete response (CR) or partial response (PR) while clinical benefit rate also included stable disease (SD) for ≥ 6 months. Patients (n = 2) who had SD ongoing at less than 6 months were excluded from the clinical benefit rate analysis (SD ≥ 6 months, PR or CR) as they were not considered assessable for SD ≥ 6 months (although they were included in the PFS and OS analysis). Kaplan-Meier curves were constructed, and the Wilcoxon test compared survival outcomes by MS and compliance to MTB recommendations. Univariate and multivariate Cox regressions were used to compare survival and response rates by MS and compliance to MTB; covariates with P < .2 were included in multivariate analyses. Because patients who received completely compliant treatment had higher MSs (P < .001), separate multivariate analyses were conducted for MTB compliance and MS. Statistical analyses were performed with SPSS, version 25.
RESULTS
Patient and Tumor Molecular Characteristics
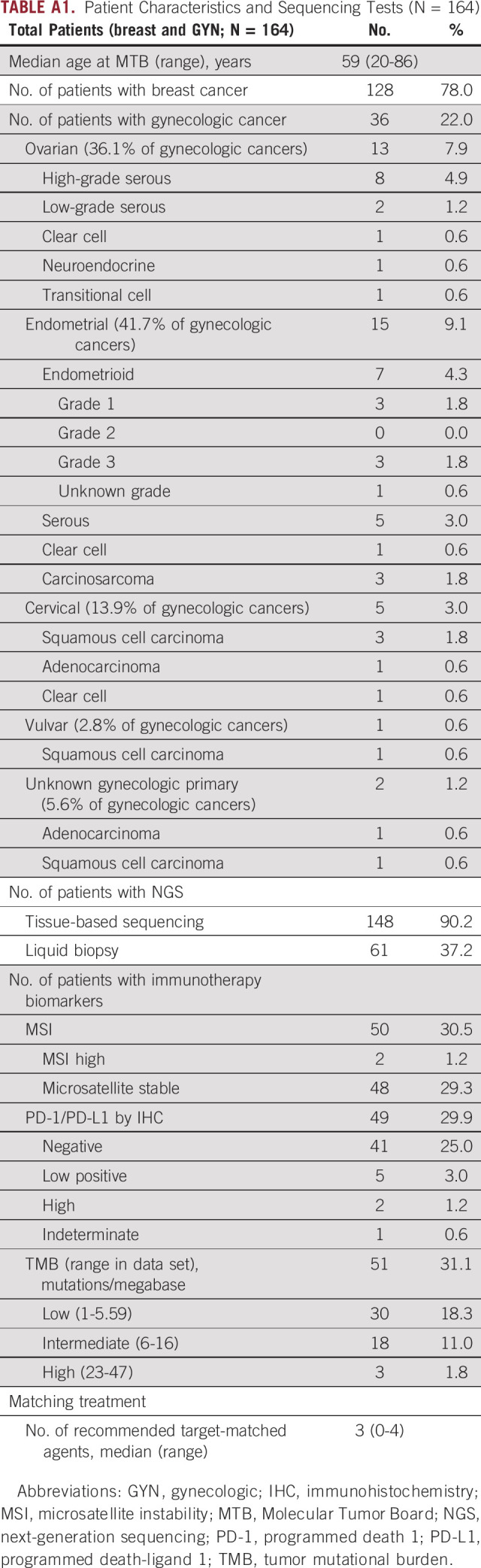
A total of 164 female patients with breast (n = 128, 78.0%) and gynecologic (n = 36, 22.0%) cancers were presented at MTB. One male patient with breast cancer was excluded from this study as all other study participants were female. Of the patients with gynecologic cancer, 36.1% had ovarian cancer, 41.7% had endometrial cancer, 13.9% had cervical cancer, 2.8% had vulvar cancer, and 5.6% had presumed gynecologic cancer of undetermined primary site (Appendix Table A1).
The majority (n = 148, 90.2%) had tissue-based NGS while 37.2% of patients had a liquid biopsy (9.8% had liquid biopsy alone while 27.4% had both tissue and liquid biopsies). The majority of submitted tumor samples were microsatellite stable (n = 48 out of 50, 96%), programmed death-ligand 1 (PD-L1)–negative (n = 41 out of 49, 83.7%), and TMB low (n = 30 out of 51, 58.8%; Appendix Table A1). Of the 135 patients with Foundation Medicine31 tissue NGS, the most common alterations were in the following genes: TP53 (54.1% of patients), PIK3CA (27.4% of patients), MYC (20.7% of patients), CCND1 (16.3% of patients), and PTEN (14.8% of patients; Data Supplement). The 30 most common alterations in patients with Guardant circulating tumor DNA32 NGS are detailed in the Data Supplement (n = 58), with the top four in the following genes: TP53 (50.0% of patients), PIK3CA (34.5% of patients), BRAF (20.7% of patients), and MYC (20.7% of patients).
Overall, 113 women were evaluable for treatment after MTB presentation (Appendix Fig A1). Of the evaluable patients, the median age was 50 (range, 20-80) years. Eighty-one (71.7%) patients were White, 15 (13.3%) were Hispanic, nine (8.0%) were Asian, four (3.5%) were Black, two (1.8%) were mixed, and two (1.8%) declined to answer. There were no differences in outcomes by race; White patients had a median PFS of 10.1 months, and non-White patients had a median PFS of 9.6 months (P = .894). Patients received a median of three prior lines of therapy (range, 0-14). Two patients (1.8%) had primary disease while the remainder (98.2%) had recurrent disease. Information about gynecologic (n = 28) and breast (n = 85) cancer cohorts is included in the Data Supplement.
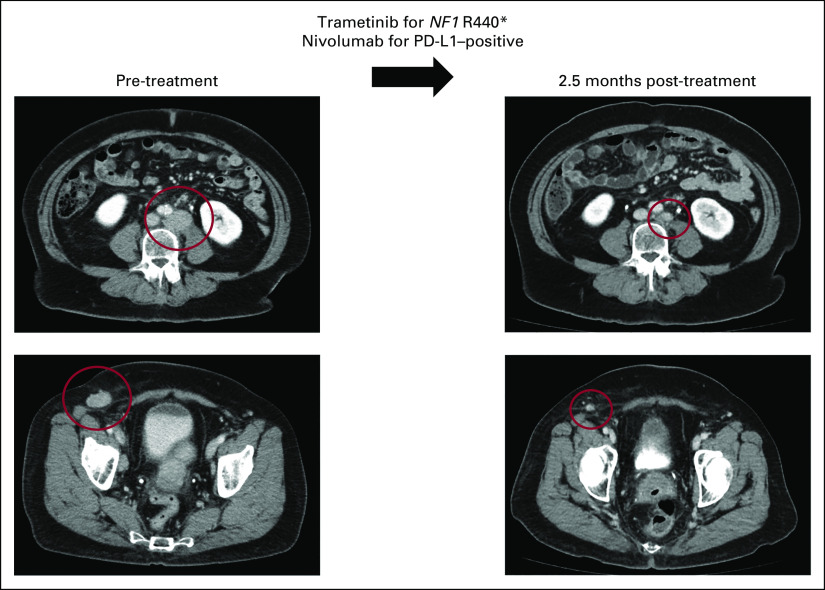
All 165 patients were on the PREDICT trial (ClinicalTrials.gov identifier: NCT02478931). Two patients were also consented to the IPREDICT trial (ClinicalTrials.gov identifier: NCT02534675).7 In addition, four patients (3.5%) were navigated to secondary trials for which they consented. Thirty-two of 113 patients (28%) received advised therapies that were standard of care for their disease at the time of the MTB. Details including NGS results, treatments, demographics, and secondary clinical trial enrollment of the 113 patients who received a change in treatment after MTB are included in the Data Supplement. A representative case of a patient with metastatic high-grade adenocarcinoma who responded to matched therapy is included in Figure 1.
FIG 1.
Representative case of metastatic high-grade serous adenocarcinoma of likely gynecologic (favor tubo-ovarian) primary managed with matched targeted combination therapy approach. This is a 68-year-old woman who initially noticed enlargement of a right groin node (study ID: 4275). Biopsy was consistent with metastatic high-grade serous adenocarcinoma. CT showed multiple enlarged abdominal lymph nodes (left), with no obvious primary tumor on positron emission tomography. She was counseled on options for standard-of-care therapy with paclitaxel and carboplatin or enrollment in clinical trial. Tissue molecular profiling through Foundation Medicine showed alterations in NF1 R440*, CCND2 amplification, CCNE1 amplification, KDM5A amplification, and TP53 T231fs*9. Tumor mutation burden was low (3 mutations per megabase), microsatellite stable, and PD-L1 by immunohistochemistry showed low positivity (5%, Ventana SP142). The case was discussed at the Molecular Tumor Board with suggestion of trametinib (MEK inhibitor for NF1 R440*) and nivolumab (anti–programmed death 1 inhibitor for PD-L1 positivity). Patient also gave informed consent for an open-label navigational I-PREDICT study (ClinicalTrials.gov identifier: NCT02534675).7 The patient was started on trametinib 1 mg by mouth daily and nivolumab 240 mg intravenous every 2 weeks. CT 2.5 months after therapy was initiated showed partial response (left to right, best response 53% reduction by RECIST 1.1). Along with the reduction of tumor, tumor markers also normalized (CA153 before the therapy: 68.5 U/mL, nadir: 25 U/mL [normal range: 0-25 U/mL], CA125 before the therapy: 80 U/mL, nadir: 12 U/mL [normal range: 0-34 U/mL]). The patient tolerated the therapy well without major drug-related adverse events (experienced grade 1 rash). Treatment is ongoing for 19 months at the time of last follow-up. CA, cancer antigen; CT, computed tomography; I-PREDCIT, Investigation of molecular Profile-Related Evidence Determining Individualized Cancer Therapy for patients with aggressive malignancies; PD-L1, programmed death-ligand 1.
Matching Drugs to Patients With Gynecologic and Breast Cancers After MTB Discussion Was Feasible
Overall, 61 patients (54%) received matched therapy. The median MS for the entire cohort was 9.1% (range, 0%-100%). For the 61 patients who received matched therapy, the median MS was 33.3% (range, 6.7%-100%); eight patients (13.1%) received completely matched therapy.
Patients With MS ≥ 40% Had Significantly Higher Response Rates and PFS With a Trend Toward Improved OS
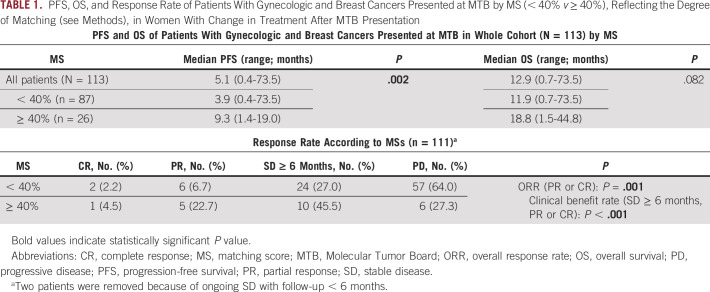
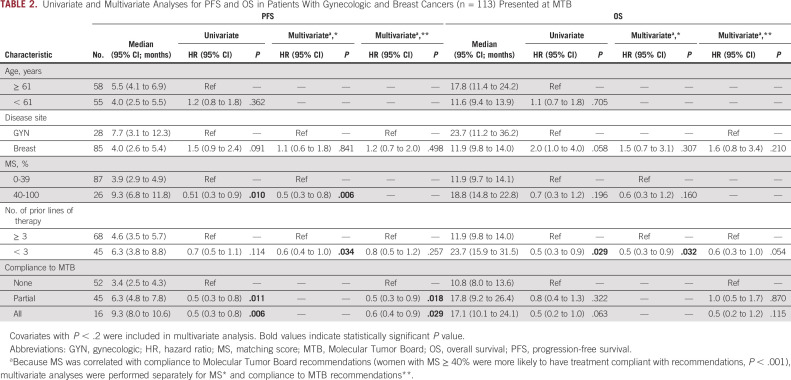
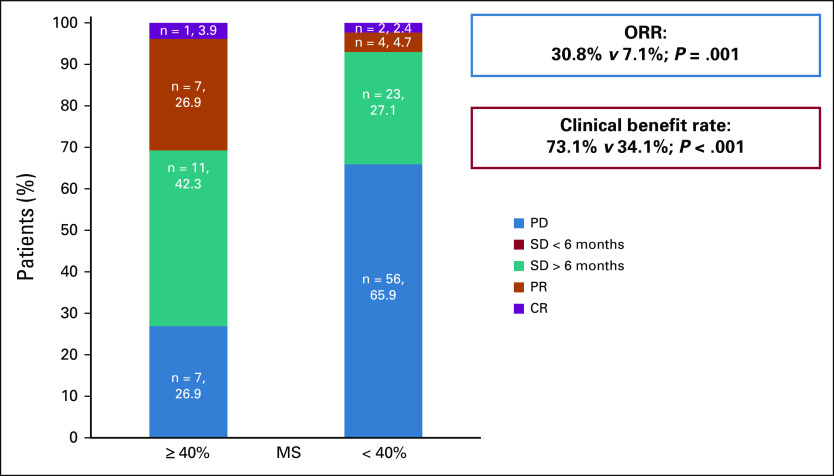
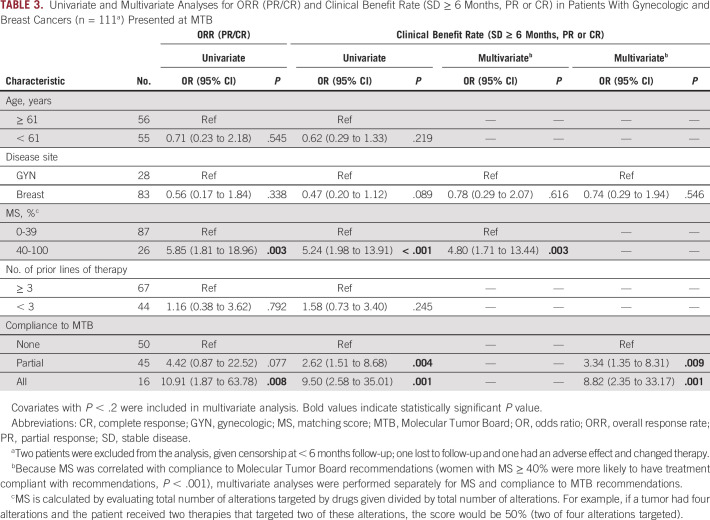
In all evaluable patients, the median PFS was 5.1 months (range, 0.4-73.5) and OS was 12.9 months (range, 0.7-73.5). Patients with MS < 40% (n = 87) had a median PFS of 3.9 months (range, 0.4-73.5) while patients with MS ≥ 40% (n = 26) had a median PFS of 9.3 months (range, 1.4-19.0; hazard ratio [HR] 0.51; 95% CI, 0.31 to 0.85; P = .002; Table 1, Fig 2A). This remained significant in multivariate analysis, adjusting for disease site and number of prior lines of therapy (adjusted HR 0.3; 95% CI, 0.3 to 0.8; P = .006; Table 2). Similarly, patients with MS < 40% had a median OS of 11.9 months (range, 0.7-73.5) while patients with MS ≥ 40% had a median OS of 18.8 months (range, 1.5-44.8; HR 0.65; 95% CI, 0.34 to 1.25; P = .082; Table 1, Fig 2B). Only number of prior lines of therapy was significantly associated with OS (Table 2). Patients with higher MSs had higher CRs (4.5% v 2.2%), PRs (22.7% v 6.7%), and SD ≥ 6 months (45.5% v 27.0%) compared with patients with lower scores. Patients with higher MSs had significantly improved ORRs (30.8% v 7.1%; P = .001) and clinical benefits rates (SD ≥ 6 months, PR or CR; 73.1% v 34.1%; P < .001; Table 1, Fig 3). This remained significant in multivariate analysis for clinical benefit rate (SD ≥ 6 months, PR or CR; adjusted HR 4.80; 95% CI, 1.71 to 13.44; P = .003). Multivariate analysis for ORR was not performed as no other covariate (age, disease site, or number of lines of prior therapy) had P < .2 in univariate analysis (Table 3).
TABLE 1.
PFS, OS, and Response Rate of Patients With Gynecologic and Breast Cancers Presented at MTB by MS (< 40% v ≥ 40%), Reflecting the Degree of Matching (see Methods), in Women With Change in Treatment After MTB Presentation
FIG 2.
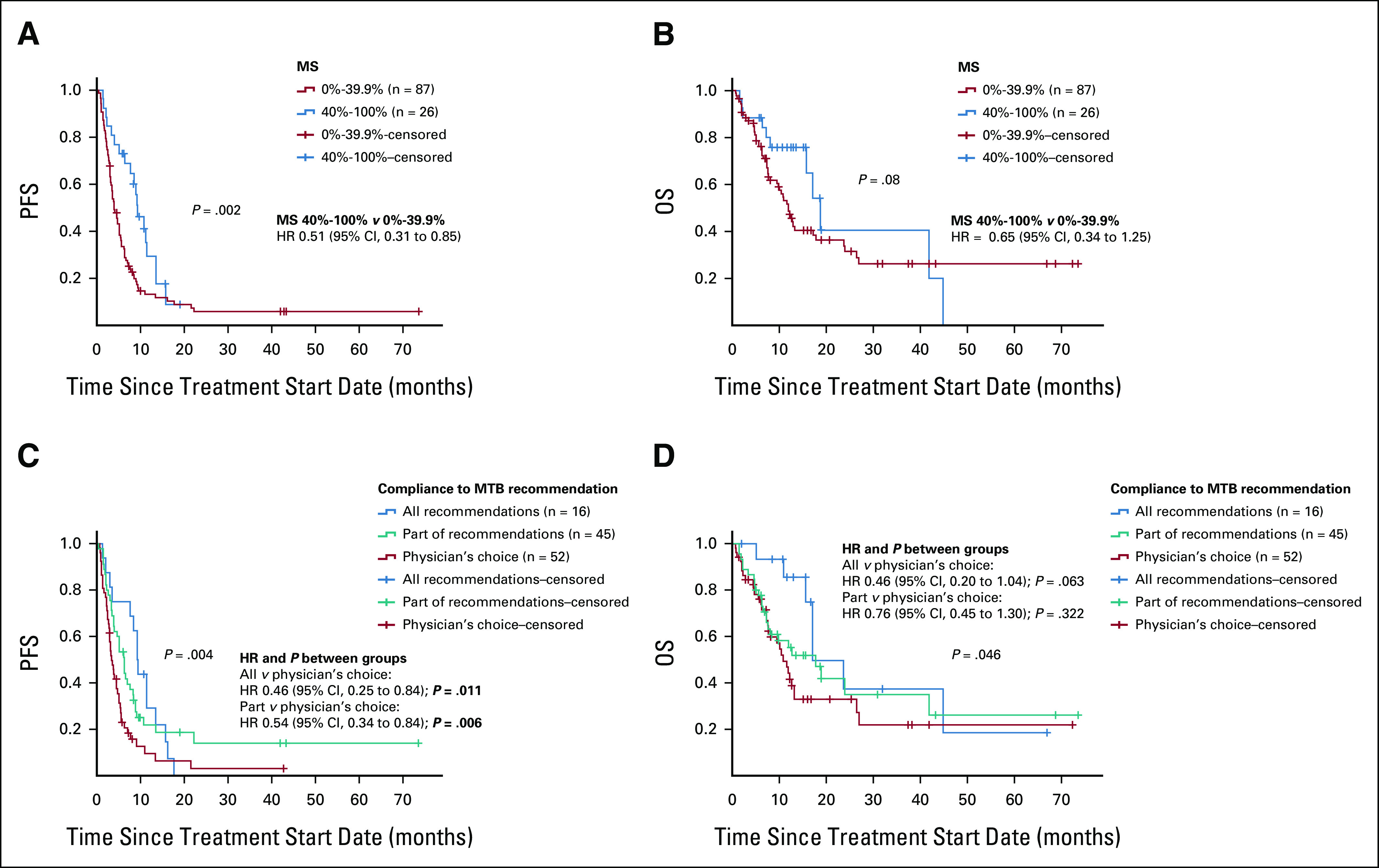
Survival outcomes by MS and compliance to MTB recommendations. (A) PFS by MS reflecting degree of matching (see Methods; n = 113 patients). The median PFS for the whole cohort was 5.1 months (95% CI, 3.8 to 6.4) and by MS category: 0%-39% 3.9 months (95% CI, 2.9 to 4.8) and 40%-100% 9.3 months (95% CI, 6.8 to 11.8). Higher MS was statistically significantly related to longer PFS (Wilcoxon P = .002). HR was calculated by Cox regression. (B) OS by MS reflecting degree of matching (see Methods; n = 113). The median OS for the whole cohort was 12.9 months (95% CI, 9.0 to 16.8) and by MS category: 0%-39% 11.9 months (95% CI, 9.7 to 14.1) and 40%-100% 18.8 months (95% CI, 14.8 to 22.8; Wilcoxon P = .08). (C) PFS according to the compliance to recommendation of MTB. Median PFS by compliance with MTB recommendation: all recommendations followed 9.0 months (95% CI, 7.7 to 10.3), part of recommendations followed 6.0 months (95% CI, 4.0 to 8.0), and physician's choice (did not follow any recommendations) 4.0 months (95% CI, 2.9 to 5.1; Wilcoxon P = .004). HR was calculated by Cox regression. (D) OS according to the compliance to recommendation of MTB. Median OS by compliance with MTB recommendation: all recommendations followed 17.1 months (95% CI, 10.1 to 24.1), part of recommendations followed 17.8 months (95% CI, 9.2 to 26.4), and physician's choice (did not follow any recommendations) 10.8 months (95% CI, 8.0 to 13.6; Wilcoxon P = .046). HR was calculated by Cox regression. HR, hazard ratio; MS, matching score; MTB, Molecular Tumor Board; OS, overall survival; PFS, progression-free survival.
TABLE 2.
Univariate and Multivariate Analyses for PFS and OS in Patients With Gynecologic and Breast Cancers (n = 113) Presented at MTB
FIG 3.
Response rates in patients whose therapy changed after Molecular Tumor Board stratified by MS reflecting degree of matching (see Methods; n = 111 patients [two patients were excluded from the analysis, given SD ongoing at < 6 months of follow-up]) Patients who had MSs ≥ 40% (n = 26) were more likely to have favorable clinical response than patients who had MSs < 40% (n = 85): ORR is defined as CR or PR (P = .001). Clinical benefit rate includes SD for ≥ 6 months, PR, and CR (P < .001). See also Table 3. Because of rounding, numbers do not add to 100% in cohort with MS < 40%. CR, complete response; MS, matching score; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease. Molecular Tumor Board–guided matched therapy correlated with improved breast and gynecologic cancer outcomes
TABLE 3.
Univariate and Multivariate Analyses for ORR (PR/CR) and Clinical Benefit Rate (SD ≥ 6 Months, PR or CR) in Patients With Gynecologic and Breast Cancers (n = 111a) Presented at MTB
Higher Compliance to MTB Recommendations Was Associated With Improved PFS, OS, and Response Rates
Women who received treatment that followed all MTB recommendations (PFS 9.3 months; 95% CI, 8.0 to 10.6; HR 0.46; 95% CI, 0.25 to 0.84; P = .011) or part of MTB recommendations (PFS 6.3 months; 95% CI, 4.8 to 7.8; HR 0.54; 95% CI, 0.34 to 0.84) had significantly longer PFS compared with women who received physician's choice treatment that did not follow MTB recommendations (PFS 3.4 months; 95% CI, 2.5 to 4.3 months; Table 2, Fig 2C). After adjusting for disease site and number of prior lines of therapy, this effect remained significant (adjusted HR partial recommendations versus physician's choice 0.5; 95% CI, 0.3 to 0.9; P = .018 and adjusted HR all recommendations versus physician's choice HR 0.6; 95% CI, 0.4 to 0.9; P = .029; Table 2). Women who received treatment that followed all (OS 17.1 months; 95% CI, 10.1 to 24.1; HR 0.46; 95% CI, 0.20 to 1.04; P = .063) or part (OS 17.8 months; 95% CI, 9.2 to 26.4; HR 0.76; 95% CI, 0.45 to 1.30; P = .322) of MTB recommendations also had longer OS compared with women who received physician's choice treatment (OS 10.8 months; 95% CI, 8.0 to 26.4); this effect was significant on Kaplan-Meier Wilcoxon overall comparison (chi-square 6.27, P = .046; Fig 2D). By Cox regression, MTB compliance showed a trend toward improved OS, which was not statistically significant (Table 2).
Compliance to MTB was significantly associated with improved objective response rates; women who received treatment that followed all MTB recommendations had 10.9 (95% CI, 1.9 to 63.8) times higher odds of objective response than women who did not receive any MTB-recommended treatments (P = .008; Table 3). Additionally, women who received part (odds ratio 2.6; 95% CI, 1.5 to 8.7; P = .004) or all (odds ratio 9.5; 95% CI, 2.6 to 35.0; P = .001) of recommended treatments had significantly higher odds of clinical benefit compared with women who received physician's choice regimen; this effect remained significant in multivariate analysis adjusting for disease site (Table 3).
DISCUSSION
The utilization of NGS to help inform treatment options for patients with gynecologic and breast cancers is increasing in frequency. Importantly, however, the findings can be difficult to interpret and implement in clinical practice. Understanding and prioritizing driver aberrations, considering alterations that may signify resistance, combining and dosing multiple targeted therapies, and directing patients to applicable clinical trials is a complex process. Multiple institutions have initiated MTBs to assist clinicians, with suggestion of improved outcomes.6,7,24,26,33-35
We identified 164 gynecologic and breast cancer patients with NGS presented at our MTB. The genomic landscape in our study was similar to that reported in The Cancer Genome Atlas (TCGA) publication of over 2,500 patients with breast and gynecologic cancers.36 Both studies showed high rates of alterations in TP53 (44% of patients in TCGA v 54% in this study), PIKC3CA (32% v 27%), PTEN (20% v 15%), and ARID1A (14% v 15%) in breast and gynecologic cancers (Data Supplement). Of the 113 women evaluable for treatment, 54% received matched therapy, which is similar to other studies.6,33,37
Clinical benefit rate (SD ≥ 6 months, PR or CR) was also similar to prior studies. In one study of 198 patients, including 5% of patients with breast cancer and < 1% with gynecologic cancer, 58% who received MTB-recommended therapy showed SD or PR.33 Another study of 67 patients with gynecologic cancer showed a 64% clinical benefit rate.37 Patients in our study who received at least part of MTB-recommended therapy had a 59% clinical benefit rate. We also stratified clinical benefit rates by MS and showed a significant association between higher MS and clinical benefit rate, with 73% of women with MS ≥ 40% having SD > 6 months, PR or CR. Patients with MS < 40% had a significantly lower rate of SD ≥ 6 months, PR or CR of 34% (P < .001). MS ≥ 40% was also associated with improved objective response rate (CR or PR; 31% v 7%, P = .001).
Previous studies have used 50% or 25% as the MS cut point6,7,26; however, a high MS in this cohort was better described as ≥ 40%, probably because of the limited number of patients with MS > 50% (n = 22). It is important to accumulate more data to determine whether there are thresholds of degrees of matching that predict benefit from matched combination therapy or there is a linear relationship between degree of matching and outcome. It is also conceivable that matching thresholds may be different by disease site, a subject that should be evaluated in future studies. Regardless, higher percentage of alterations targeted by a treatment regimen seems to be correlated with improved response rates and survival in the pan-cancer setting.7
Higher compliance to MTB recommendations was also associated with improved outcomes in univariate and multivariate analyses. The pooled experience and expertise, especially with combination therapy and navigating patients to clinical trials, may be important in improving patient results. The logistics of establishing MTBs at individual institutions may be limiting. One study evaluated the use of a virtual tumor board38 to improve access. Remote access may be important in expanding availability to expert opinion, especially in patients with complicated NGS results and treatment histories.
Limitations of our study include that it was a heterogeneous cohort, with breast and various gynecologic cancers. TCGA analysis has shown that breast and gynecologic cancers had significant molecular commonalities but also differences.39 Our MTB also tends to evaluate patients with a more tumor agnostic approach, prioritizing molecular characteristics. Additionally, primary disease site was not found to be associated with any specific oncologic outcome on multivariate analyses. This study is also limited by lack of random assignment to control for differences in treatment decisions or standardization for molecular analysis time points, tests, and follow-up, reflecting the real-world nature of this study.
On the basis of the current observations, we advocate for expanding MTBs to assist with treatment decisions for patients with breast and gynecologic cancers since compliance with our MTB was associated with greater degrees of matching patients with therapy, which in turn was correlated with improvements in response rates and survival outcomes.
APPENDIX
FIG A1.
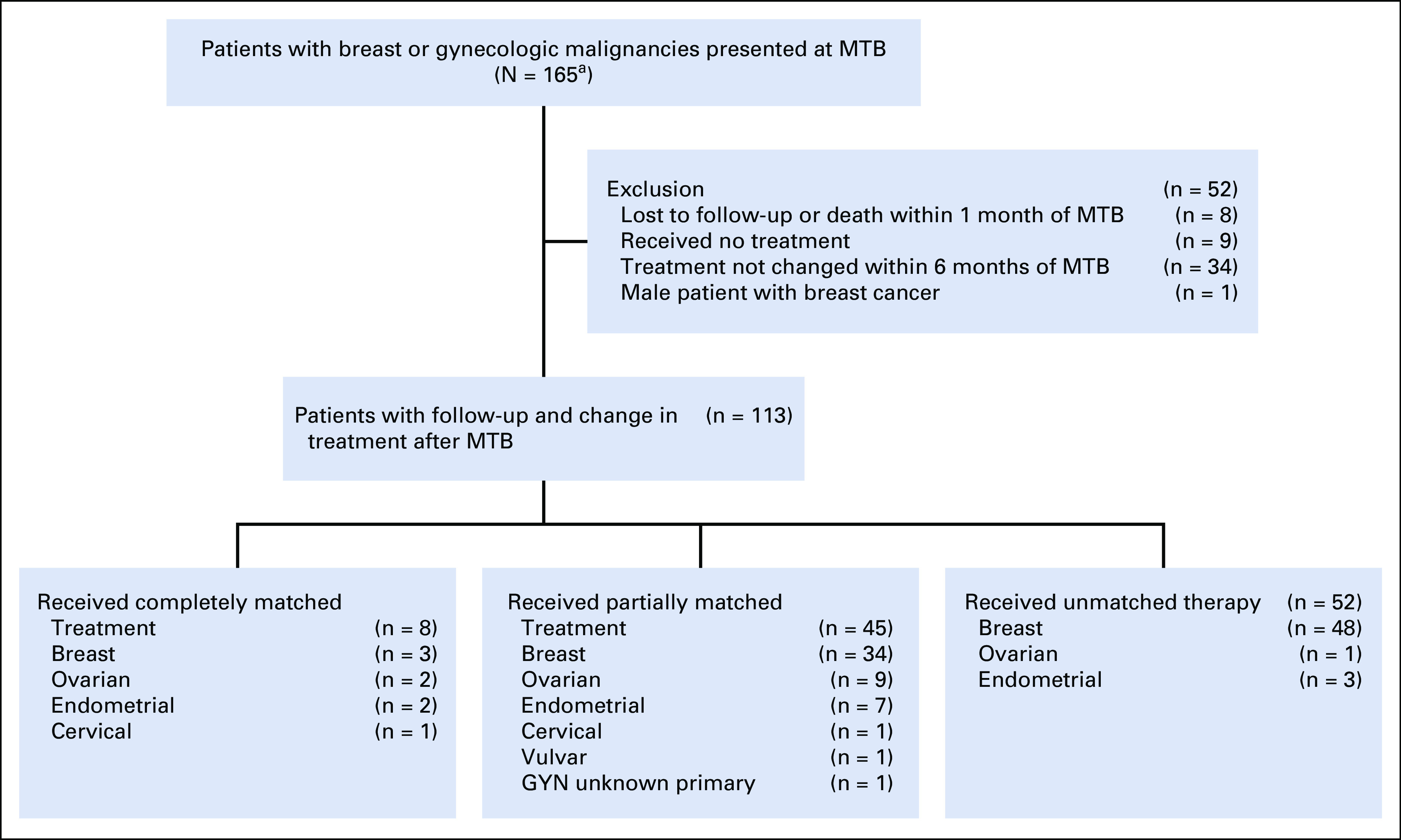
CONSORT diagram of patients with breast (n = 129) and GYN (n = 36) cancers presented at MTB. aTwo patients (1.2% of 165) were also part of the published IPREDICT study7; the majority of patients were different because the IPREDICT study included a face-to-face MTB as well as an electronic MTB, although this study includes only patients presented in a face-to-face MTB. GYN, gynecologic; MTB, Molecular Tumor Board.
TABLE A1.
Patient Characteristics and Sequencing Tests (N = 164)
Ramez N. Eskander
Consulting or Advisory Role: Pfizer, Clovis Oncology, AstraZeneca/MedImmune, Tesaro, Merck, Eisai, Agenus, Myriad Genetics, Daiichi Sankyo/Lilly
Speakers' Bureau: AstraZeneca/MedImmune, Myriad Genetics
Travel, Accommodations, Expense: AstraZeneca/MedImmune, Merck, Eisai
Jason Sicklick
Stock and Other Ownership Interests: Personalis
Consulting or Advisory Role: Deciphera
Speakers' Bureau: QED Therapeutics, Foundation Medicine, Roche, Deciphera, MJH Life Sciences
Research Funding: Foundation Medicine, Amgen
Richard Schwab
Leadership: Procend Inc
Stock and Other Ownership Interests: Samumed (I)
Patents, Royalties, Other Intellectual Property: The patent covers sialylated glycans and antibodies that specifically bind to them for early detection and diagnosis of cancer (Inst)
Rebecca Shatsky
Honoraria: SOTERIA Precision Medicine, Horizon CME, OncoSec, Relevate Health Group, The Dedham Group
Consulting or Advisory Role: SOTERIA Precision Medicine, OncoSec, The Dedham Group
Speakers' Bureau: Horizon CME
Research Funding: Oncternal Therapeutics (Inst), Phoenix Molecular Designs (Inst), Genentech (Inst), OncoSec (Inst), CytomX Therapeutics (Inst), Merck (Inst)
Steven Plaxe
Stock and Other Ownership Interests: Pfizer, Merck, Zimmer BioMet, GlaxoSmithKline, AstraZeneca, Bristol Myers Squibb/Pfizer, Johnson & Johnson/Janssen
Research Funding: Endocyte (Inst), Incyte (Inst), MedImmune (Inst), Novartis (Inst), Pfizer (Inst), Janssen Oncology (Inst), BIND Therapeutics (Inst), PharmaMar (Inst), AstraZeneca (Inst), Kevelt (Inst), Millennium (Inst), Tesaro (Inst)
Shumei Kato
Honoraria: Roche
Consulting or Advisory Role: Foundation Medicine, Pfizer/EMD Serono
Research Funding: ACT Genomics, Sysmex, Konica Minolta, OmniSeq
Razelle Kurzrock
Leadership: CureMatch, CureMetrix Inc
Stock and Other Ownership Interests: CureMatch, IDbyDNA
Honoraria: Roche, EUSA Pharma, NeoGenomics Laboratories, Biocom, NeoMed Therapeutics, Advanced Therapeutics, LEK, AACR, Chugai Pharma USA, Wiley, Merck, Pfizer, Meyer Consulting, Foundation Medicine, Turning Point Therapeutics, Bicara
Consulting or Advisory Role: Actuate Therapeutics, Loxo, XBiotech, Neo-Med, Roche, Gaido Soluventis, Pfizer, Merck, Turning Point Therapeutics, TD2/Volastra, Bicara Therapeutics Inc
Speakers' Bureau: Roche
Research Funding: Guardant Health (Inst), Sequenom (Inst), Merck Serono (Inst), Genentech (Inst), Pfizer (Inst), Foundation Medicine (Inst), Incyte (Inst), Konica Minolta (Inst), Grifols (Inst), OmniSeq (Inst), Debiopharm Group (Inst), Boerhinger Ingelheim (Inst), Top Alliance BioScience (Inst), Takeda (Inst), MedImmune (Inst)
Travel, Accommodations, Expenses: Roche, EUSA Pharma, NeoGenomics Laboratories, Biocom, NeoMed Therapeutics, Advanced Therapeutics, LEK, AACR, Chugai Pharma USA, Wiley
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by the Joan and Irwin Jacobs Fund and National Cancer Institute at the National Institutes of Health (Grant No. NIH P30 CA023100 [R.K.]).
AUTHOR CONTRIBUTIONS
Conception and design: Lindsey M. Charo, Ramez N. Eskander, Ki Hwan Kim, Hyo Jeong Lim, Shumei Kato
Administrative support: Suzanna Lee
Provision of study materials or patients: Richard Schwab
Collection and assembly of data: Lindsey M. Charo, Ramez N. Eskander, Ki Hwan Kim, Hyo Jeong Lim, Ryosuke Okamura, Suzanna Lee, Rupa Subramanian, Richard Schwab, Rebecca Shatsky, Shumei Kato
Data analysis and interpretation: Lindsey M. Charo, Ramez N. Eskander, Jason Sicklick, Ki Hwan Kim, Hyo Jeong Lim, Richard Schwab, Steven Plaxe, Razelle Kurzrock
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ramez N. Eskander
Consulting or Advisory Role: Pfizer, Clovis Oncology, AstraZeneca/MedImmune, Tesaro, Merck, Eisai, Agenus, Myriad Genetics, Daiichi Sankyo/Lilly
Speakers' Bureau: AstraZeneca/MedImmune, Myriad Genetics
Travel, Accommodations, Expense: AstraZeneca/MedImmune, Merck, Eisai
Jason Sicklick
Stock and Other Ownership Interests: Personalis
Consulting or Advisory Role: Deciphera
Speakers' Bureau: QED Therapeutics, Foundation Medicine, Roche, Deciphera, MJH Life Sciences
Research Funding: Foundation Medicine, Amgen
Richard Schwab
Leadership: Procend Inc
Stock and Other Ownership Interests: Samumed (I)
Patents, Royalties, Other Intellectual Property: The patent covers sialylated glycans and antibodies that specifically bind to them for early detection and diagnosis of cancer (Inst)
Rebecca Shatsky
Honoraria: SOTERIA Precision Medicine, Horizon CME, OncoSec, Relevate Health Group, The Dedham Group
Consulting or Advisory Role: SOTERIA Precision Medicine, OncoSec, The Dedham Group
Speakers' Bureau: Horizon CME
Research Funding: Oncternal Therapeutics (Inst), Phoenix Molecular Designs (Inst), Genentech (Inst), OncoSec (Inst), CytomX Therapeutics (Inst), Merck (Inst)
Steven Plaxe
Stock and Other Ownership Interests: Pfizer, Merck, Zimmer BioMet, GlaxoSmithKline, AstraZeneca, Bristol Myers Squibb/Pfizer, Johnson & Johnson/Janssen
Research Funding: Endocyte (Inst), Incyte (Inst), MedImmune (Inst), Novartis (Inst), Pfizer (Inst), Janssen Oncology (Inst), BIND Therapeutics (Inst), PharmaMar (Inst), AstraZeneca (Inst), Kevelt (Inst), Millennium (Inst), Tesaro (Inst)
Shumei Kato
Honoraria: Roche
Consulting or Advisory Role: Foundation Medicine, Pfizer/EMD Serono
Research Funding: ACT Genomics, Sysmex, Konica Minolta, OmniSeq
Razelle Kurzrock
Leadership: CureMatch, CureMetrix Inc
Stock and Other Ownership Interests: CureMatch, IDbyDNA
Honoraria: Roche, EUSA Pharma, NeoGenomics Laboratories, Biocom, NeoMed Therapeutics, Advanced Therapeutics, LEK, AACR, Chugai Pharma USA, Wiley, Merck, Pfizer, Meyer Consulting, Foundation Medicine, Turning Point Therapeutics, Bicara
Consulting or Advisory Role: Actuate Therapeutics, Loxo, XBiotech, Neo-Med, Roche, Gaido Soluventis, Pfizer, Merck, Turning Point Therapeutics, TD2/Volastra, Bicara Therapeutics Inc
Speakers' Bureau: Roche
Research Funding: Guardant Health (Inst), Sequenom (Inst), Merck Serono (Inst), Genentech (Inst), Pfizer (Inst), Foundation Medicine (Inst), Incyte (Inst), Konica Minolta (Inst), Grifols (Inst), OmniSeq (Inst), Debiopharm Group (Inst), Boerhinger Ingelheim (Inst), Top Alliance BioScience (Inst), Takeda (Inst), MedImmune (Inst)
Travel, Accommodations, Expenses: Roche, EUSA Pharma, NeoGenomics Laboratories, Biocom, NeoMed Therapeutics, Advanced Therapeutics, LEK, AACR, Chugai Pharma USA, Wiley
No other potential conflicts of interest were reported.
REFERENCES
- 1.Schwaederle M, Zhao M, Lee JJ, et al. : Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: A meta-analysis. JAMA Oncol 2:1452-1459, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Jardim DL, Fontes Jardim DL, Schwaederle M, et al. : Impact of a biomarker-based strategy on oncology drug development: A meta-analysis of clinical trials leading to FDA approval. J Natl Cancer Inst 107:djv253, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwaederle M, Zhao M, Lee JJ, et al. : Impact of precision medicine in diverse cancers: A meta-analysis of phase II clinical trials. J Clin Oncol 33:3817-3825, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwaederle M, Kurzrock R: Actionability and precision oncology. Oncoscience 2:779-780, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shatsky R, Parker BA, Bui NQ, et al. : Next-generation sequencing of tissue and circulating tumor DNA: The UC San Diego Moores Center for personalized cancer therapy experience with breast malignancies. Mol Cancer Ther 18:1001-1011, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Rodon J, Soria JC, Berger R, et al. : Genomic and transcriptomic profiling expands precision cancer medicine: The WINTHER trial. Nat Med 25:751-758, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sicklick JK, Kato S, Okamura R, et al. : Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat Med 25:744-750, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists' Collaborative Group : Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med 319:1681-1692, 1988 [DOI] [PubMed] [Google Scholar]
- 10.González-Martín A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Coleman RL, Oza AM, Lorusso D, et al. : Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:1949-1961, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirza MR, Monk BJ, Herrstedt J, et al. : Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154-2164, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Ledermann J, Harter P, Gourley C, et al. : Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366:1382-1392, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Ledermann JA, Harter P, Gourley C, et al. : Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol 17:1579-1589, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Ray-Coquard I, Pautier P, Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Le DT, Uram JN, Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le DT, Durham JN, Smith KN, et al. : Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409-413, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ott PA, Bang YJ, Berton-Rigaud D, et al. : Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study. J Clin Oncol 35:2535-2541, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Ott PA, Bang YJ, Piha-Paul SA, et al. : T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol 37:318-327, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Fader AN, Roque DM, Siegel E, et al. : Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol 36:2044-2051, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Slomovitz BM, Jiang Y, Yates MS, et al. : Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol 33:930-936, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Westin SN: Rational selection of biomarker driven therapies for gynecologic cancers: The more we know, the more we know we don't know. Gynecol Oncol 141:65-71, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker BA, Schwaederlé M, Scur MD, et al. : Breast cancer experience of the molecular tumor board at the University of California, San Diego Moores Cancer Center. J Oncol Pract 11:442-449, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Schwaederle M, Parker BA, Schwab RB, et al. : Molecular tumor board: The University of California-San Diego Moores Cancer Center experience. Oncologist 19:631-636, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel M, Kato SM, Kurzrock R: Molecular tumor boards: Realizing precision oncology therapy. Clin Pharmacol Ther 103:206-209, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato S, Kim KH, Lim HJ, et al. : Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat Commun 11:4965, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charo LM, Eskander RN, Okamura R, et al. : Clinical implications of plasma circulating tumor DNA in gynecologic cancer patients. Mol Oncol 15:67-79, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwaederle M, Parker BA, Schwab RB, et al. : Precision oncology: The UC San Diego Moores Cancer Center PREDICT experience. Mol Cancer Ther 15:743-752, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Frampton GM, Fichtenholtz A, Otto GA, et al. : Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 31:1023-1031, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas RK, Nickerson E, Simons JF, et al. : Sensitive mutation detection in heterogeneous cancer specimens by massively parallel picoliter reactor sequencing. Nat Med 12:852-855, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Foundation Medicine. https://www.foundationmedicine.com/
- 32.Guardant 360. http://www.guardant360.com/
- 33.Hoefflin R, Geißler AL, Fritsch R, et al. : Personalized clinical decision making through implementation of a molecular tumor board: A German single-center experience. JCO Precis Oncol 10.1200/PO.18.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore DA, Kushnir M, Mak G, et al. : Prospective analysis of 895 patients on a UK genomics review board. ESMO Open 4:e000469, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolfo C, Manca P, Salgado R, et al. : Multidisciplinary molecular tumour board: A tool to improve clinical practice and selection accrual for clinical trials in patients with cancer. ESMO Open 3:e000398, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger AC, Korkut A, Kanchi RS, et al. : A comprehensive pan-cancer molecular study of gynecologic and breast cancers. Cancer Cell 33:690-705.e9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Rodriguez L, Hirshfield KM, Rojas V, et al. : Use of comprehensive genomic profiling to direct point-of-care management of patients with gynecologic cancers. Gynecol Oncol 141:2-9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pishvaian MJ, Blais EM, Bender RJ, et al. : A virtual molecular tumor board to improve efficiency and scalability of delivering precision oncology to physicians and their patients. JAMIA Open 2:505-515, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoadley KA, Yau C, Hinoue T, et al. : Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173:291-304.e6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]