Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a newly discovered coronavirus and has rapidly spread to most of the world and resulted in a global pandemic. However, there is a paucity of information available to characterize the immunodeficient population in the COVID-19 pandemic, especially information that focuses on patients after renal transplantation as the typical representative of this population. Understanding the susceptibility, clinic characteristics, treatment strategy, and prognosis of this population is of substantial importance for the management of immunodeficient patients in the pandemic.
This is a retrospective, multicenter, observational cohort study, and conducted in all the transplant centers within Hubei province, China. All renal allograft recipients who lived in Hubei province and were performing their follow-up within these hospitals were enrolled. We obtained the medical records and compiled the data of recipients who were infected with COVID-19 between December 11, 2019 and May 1, 2020. The study was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-C20200166), written informed consent was obtained from the participants for the publication of their individual details in this manuscript.
Hubei province has a population of 59.17 million. As of May 1, 2020, 68,128 cases of COVID-19 had been diagnosed, in accompaniment with 4512 deaths. The overall incidence of infection is 0.12%, with a mortality of 6.60%.[1] Currently, there are 4468 renal allograft recipients located in Hubei province participating in follow-up at these hospitals. Until May 1, 2020, 31 recipients had been diagnosed with COVID-19 [Supplementary Table 1].
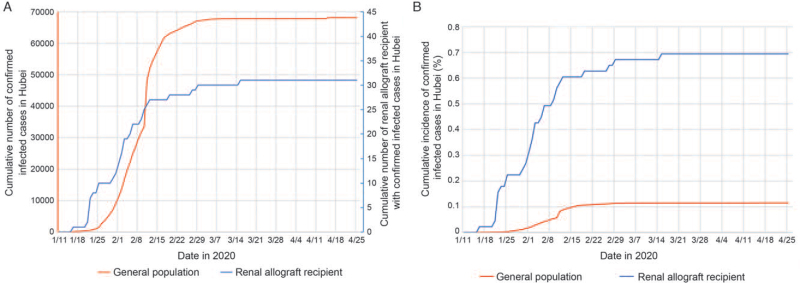
The diagnosis period was from January 17 to March 16, 2020. Both the cumulative number and incidence of confirmed infected cases of COVID-19 in the Hubei general population and renal allograft recipients are shown in Figure 1. The incidence of COVID-19 in renal allograft recipients in Hubei was 0.69%, which was nearly six times more than the incidence among the general population of Hubei (0.69% vs. 0.12%, P < 0.001) [Supplementary Figure 1A]. The patients were divided into two groups, not severe (n = 16) and severe (n = 15), based on their clinical manifestations. The basic characteristics of the recipients are shown in Supplementary Table 2.
Figure 1.
(A) Red line: the cumulative number of confirmed infected cases of COVID-19 in Hubei from January 11, 2020 to April 26, 2020. A total of 68,128 people were infected. As of April 26, a total of 63,616 patients were cured, 4512 patients died, and the number of existing infected patients is 0.[1] Blue line: 31 renal allograft recipients had been diagnosed as having COVID-19 from January 17 to March 16, three recipients died, and 29 recipients were discharged. (B) The cumulative incidence of confirmed infected cases of COVID-19. COVID-19: Coronavirus disease 2019.
All of the patients had abnormal findings upon chest computed tomography (CT) examination. In patients presenting with severe manifestations, the incidence of ground-glass opacity and interstitial abnormalities in the CT were higher than the not severe group (13/15 vs. 6/16, P = 0.005; 6/15 vs. 0, P = 0.005; respectively). The number of patients with lymphocyte counts <1000 per mm3 was statistically different (15/15 vs. 10/16, P = 0.008). The incidence of acute respiratory distress syndrome (ARDS) was also significantly higher in the severe than in the not severe group (6/15 vs. 0, P = 0.007). The overall incidence of acute kidney injury (AKI) was 9/31 (29%) among all recipients, and the incidence in the severe group was significantly higher than in the not severe group (7/15 vs. 2/16, P = 0.030) [Supplementary Table 3].
Once diagnosed, maintenance of immunosuppressant regimens were adjusted immediately for all but one patient. In 25/31 (81%) cases, withdrawal involved mycophenolic acid and calcineurin inhibitors (CNI), while the maintenance immunosuppressants were simply oral or bolus steroids; 12/31 (39%) patients received oral steroids only, 12/31 (39%) received low doses of 40 to 80 mg/day methylprednisolone, and one received a high dose of >250 mg/day methylprednisolone. In the severe group, 13/15 patients needed some means of mechanical ventilation. In the not severe group, none required mechanical ventilation (13/15 vs. 0, P < 0.001).
As to the outcomes, 28/31 (90%) patients recovered and were finally discharged from the hospital. In the severe group, 3/31 (10%) patients died, of whom one had chronic obstructive pulmonary disease as an underlying disease, one was accompanied with gastrointestinal hemorrhage, and the other case was accompanied with severe renal allograft dysfunction that required dialysis. This mortality was slightly higher than that of the general population of Hubei (4512 out of 68,128, 6.60%, P = 0.490) [Supplementary Table 4].
We compared these data with those of the general population published by Guan et al,[2] and found that COVID-19 in renal allograft recipients was significantly more severe (48% vs. 16%, P < 0.001), required more mechanical ventilation (42% vs. 6%, P < 0.001), needed more intensive care unit (ICU) admission (13% vs. 5%, P = 0.050), and had greater incidence of AKI (29% vs. 0.5%, P < 0.001). The mortality rate in the recipient group was higher than that in the general population, but there was no statistical significance (10% vs. 7%, P = 0.490) [Supplementary Figure 1B–F].
Our data indicated that an immunocompromised person does have higher susceptibility for COVID-19 infection. The incidence of COVID-19 disease in this cohort was 0.69%, which was nearly six times the overall incidence of 0.12% in Hubei. Currently, it is well accepted that the immunocompromised population has higher susceptibility to transmitted diseases.[3] Despite this, it is very difficult to assess the exact differences when comparing the immunocompromised with the general population. The lockdown of Hubei province not only helped to control the transmission of the virus, but also provided us an opportunity to know the epidemiology of a special herd. These data suggested that COVID-19, like most viruses, is more likely to be transmitted to the immunosuppressed population. Thus, in a high risk area, stricter self-protection strategies should be adopted to avoid infection.
The most common symptoms of COVID-19 include cough, fatigue, fever, dyspnea, muscle aches, and gastrointestinal symptoms.[4] Unlike the general population, renal allograft recipients had lower incidence of fever as an initial symptom. Of the recipients, six had never had a fever until discharge. This is consistent with a recent report from New York, in which only 30.7% patients had a fever as a presenting symptom. This diminishing of the fever is due to the use of antimetabolites as immunosuppressants, which can result in the absence of fever in up to 40% of infections.
Once infected, an immunocompromised person is at higher risk of developing a severe case.[5] In this cohort, almost half (48%) of cases included were severe, much higher than that in the general population (16%). When compared with the general population, more cases needed mechanical ventilation, developed ARDS, and were admitted into the ICU. It is obvious that, in the immunocompromised population, antivirus ability is impaired significantly. This finding is consistent with an influenza study, in which the authors found that patients receiving immunosuppressive therapy are at risk for more severe or complicated influenza-induced disease. Furthermore, lymphocyte count has been associated with increased disease severity in COVID-19. Patients who died from COVID-19 are reported to have had significantly lower lymphocyte counts than survivors. The high incidence of lymphopenia in this cohort might explain why they were more prone to develop the severe syndrome.
Our data also shows that hospitalized COVID-19 infection is correlated with a high incidence of AKI in renal allograft recipients. Although the COVID-19 virus nucleic acid can be detected in the urine, it is not certain that this virus can damage the kidneys directly. The incidence of AKI in the general population varies from 0 to 23% among hospitalized patients and 19% to 29% in critical care patients. In a large cohort reported by Guan et al, the incidence of AKI was only 0.5%. Our data reported an incidence of 29% among all the patients and 47% among severe patients, and these numbers are the highest among current publications. A single renal allograft within the body, facing immunological rejection and CNI renal toxicity every day, has a low capacity to endure the inflammatory environment and anti-infection medicines. Our opinion is that the high incidence of AKI is not caused by rejection, since renal allograft function could recover without anti-rejection therapy. Thus, treating COVID-19 infection among renal allograft recipients should involve the consideration of more strategies to protect renal allograft function.
Our data showed that, although they face more severe clinical manifestations, immunocompromised patients do not have to have a worse outcome. In this cohort, 28 (90%) patients recovered and were discharged from the hospital. Only three out of 31 (10%) patients died of COVID-19 pneumonia, and this is significantly lower than the report from New York, which indicated a figure of 28%[6] and only slightly higher than the mortality of the general population in Hubei (6.6%). Early admission to the hospital, lower dose of maintenance immunosuppressants following transplantation, and early reduction of immunosuppressants might account for the relatively lower incidence of death.
Moreover, although the use of corticosteroids in treating COVID-19 pneumonia remains controversial, a low dose of bolus corticosteroid (methylprednisolone 40–80 mg/day) was widely used in replacement of maintenance immunosuppressants in this cohort. According to experience from the management of cytomegalovirus infection, which is a common infectious complication after renal transplantation, a low dose of corticosteroid is a well-tolerable selection for the reduction of inflammation and cytokine release. The effect of a low-dose bolus steroid should not be excluded for achieving a relatively benign outcome.
We found during the COVID-19 breakout in Hubei province, China, the immunocompromised population, as represented by renal allograft recipients, had higher susceptibility for virus infection, more severe clinical manifestations, longer lengths of hospital stay, and more AKI complications. However, these patients did not have to have a worse outcome if disease management was carefully handled.
Funding
This work is funded by a Key Project of Health and Family Planning Commission of Hubei Province of China (No. WJ2019Z007), the National Key Research & Development Program of China (2018YFA0108804), the National Natural Science Foundation of China (Nos. 81970650 and 81770753), the Youth Program of National Natural Science Foundation of China (No. 81800661), the Fundamental Research Funds for the Central Universities (No. 20ykpy34), and the China Postdoctoral Science Foundation Funded Project (No. 2020M683083).
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Zhang W, Han F, Wu X, Wang Z, Wang Y, Guo X, Chen S, Qiu T, Li H, Tu Y, Zhong Z, He J, Liu B, Zhang H, Cai Z, Zhang L, Lu X, Zhu L, Chen D, Zhou J, Sun Q, Chen Z. COVID-19 in the immunocompromised population: data from renal allograft recipients throughout full cycle of the outbreak in Hubei province, China. Chin Med J 2022;135:228–230. doi: 10.1097/CM9.0000000000001538
Weijie Zhang and Fei Han contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.https://news.sina.cn/project/fy2020/yq_province.shtml?province=hubei. Accessed January 13, 2021. [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaltsas A, Sepkowitz K. Community acquired respiratory and gastrointestinal viral infections: challenges in the immunocompromised host. Curr Opin Infect Dis 2012; 25:423–430. doi: 10.1097/QCO.0b013e328355660b. [DOI] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. Covid-19 and kidney transplantation. N Engl J Med 2020; 382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.