Abstract
Background:
In-hospital mortality in patients with coronavirus disease 2019 (COVID-19) is high. Simple prognostic indices are needed to identify patients at high-risk of COVID-19 health outcomes. We aimed to determine the usefulness of the CONtrolling NUTritional status (CONUT) index as a potential prognostic indicator of mortality in COVID-19 patients upon hospital admission.
Methods:
Our study design is of a retrospective observational study in a large cohort of COVID-19 patients. In addition to descriptive statistics, a Kaplan–Meier mortality analysis and a Cox regression were performed, as well as receiver operating curve (ROC).
Results:
From February 5, 2020 to January 21, 2021, there was a total of 2969 admissions for COVID-19 at our hospital, corresponding to 2844 patients. Overall, baseline (within 4 days of admission) CONUT index could be scored for 1627 (57.2%) patients. Patients’ age was 67.3 ± 16.5 years and 44.9% were women. The CONUT severity distribution was: 194 (11.9%) normal (0–1); 769 (47.2%) light (2–4); 585 (35.9%) moderate (5–8); and 79 (4.9%) severe (9–12). Mortality of 30 days after admission was 3.1% in patients with normal risk CONUT, 9.0% light, 22.7% moderate, and 40.5% in those with severe CONUT (P < 0.05). An increased risk of death associated with a greater baseline CONUT stage was sustained in a multivariable Cox regression model (P < 0.05). An increasing baseline CONUT stage was associated with a longer duration of admission, a greater requirement for the use of non-invasive and invasive mechanical ventilation, and other clinical outcomes (all P < 0.05). The ROC of CONUT for mortality had an area under the curve (AUC) and 95% confidence interval of 0.711 (0.676–0746).
Conclusion:
The CONUT index upon admission is potentially a reliable and independent prognostic indicator of mortality and length of hospitalization in COVID-19 patients.
Keywords: Admission, Clinical risk, CONUT, COVID-19, Prognosis, Score
Introduction
The global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) producing the current coronavirus disease 2019 (COVID-19) pandemic has taken health systems around the world to the brink of collapse.[1,2] There is still a need for evidence about its pathogeny and clinical course, and their determining factors, among others. The clinical expression of COVID-19 is highly heterogenous and with severity ranging from mild to critical, it produces a dearth of systemic effects and damage in diverse organs, including the respiratory, circulatory, and neurological systems.
It is of the utmost importance to identify, among admitted COVID-19 patients, those who are at the highest risk of complications during hospitalization, not only to achieve better quality of clinical care but also to optimize the use of health care resources. Therefore, it is vital to identify and implement a valid prognostic index upon admission, due to the limited value of classical semiology in this disease, where anamnesis, physical exploration, and complementary tests do not provide sufficient evidence to forecast individual outcomes. An index of clinical risk with enough sensitivity and predictive ability can help to identify promptly those COVID-19 patients that will develop severe disease. It can also have great utility in monitoring the disease during the follow-up of COVID-19.
Since the beginning of the pandemic, diverse investigations have tried to identify a parameter/index with prognostic utility,[3–5] although with uneven results. This is because of the poor and premature reporting, complexity, high risk of bias, and several other limitations. Hence, the need to explore new prognostic indices to evaluate the risk of COVID-19 is considered as a research and clinical priority.
Our aim was to test the usefulness and validity of the CONtrolling NUTritional status (CONUT), an already available and valid score for early detection and continuous control of undernutrition in hospitalized patients,[6] as a prognostic tool to evaluate the risk of worse progression and increased mortality in COVID-19 admitted patients. CONUT is based on serum thresholds of albumin, cholesterol, and total lymphocytes, with a range from 0 to 12. It is an easy score obtainable from parameters available in routine blood tests, calculated either mentally or automatically by an algorithm implemented in the laboratory informatic system, either in primary or specialized medical care. Therefore, the CONUT predictive power to help in individual medical decision-making, as well as aiding in the monitoring of high-risk patients, was tested.
Methods
Ethical approval
Research protocol was approved by the Ethics Committee for Medical and Drug Research of Hospital Universitario de La Princesa on May 20, 2021, acta CEIm 10/21 with No. 4468.
Study design and settings
This is a retrospective observational study in a cohort of hospitalized patients from February 5th, 2020 to January 21st, 2021 at the Hospital Universitario de La Princesa, Madrid, Spain.
Participants
This study included information from clinical records of all adult patients (age ≥ 18 years) with a positive COVID-19 clinical diagnosis upon hospital admission, confirmed either by positive antigen or polymerase tests.
Variables and outcome
The primary outcome of interest was in-hospital mortality up to 30 days from admission, which was obtained from the electronic medical records, with right truncation after discharge. There was no further follow-up over the phone or by other methods. The biometric, laboratory, and comorbidity variables were obtained and analyzed. Among routine laboratory variables, albumin, cholesterol, and total count of lymphocytes were used to calculate the CONUT score. Considering blood test results up to the fourth day was a pragmatic decision, to take into account biochemistry (of cholesterol, lymphocytes, and albumin) not assessed upon admission, mainly in the emergency room, due to collapse of laboratories during the peak of the first pandemic wave. Patients were classified in four stages according to the score obtained in the CONUT index (normal risk 0–1, light risk 2–4, moderate risk 5–8, and severe risk 9–12), depending on the blood stages thresholds of albumin, total cholesterol, and total lymphocytes [Table 1].
Table 1.
Thresholds and calculation of the CONUT index.
| Undernutrition Degree | ||||
| Parameter | Normal | Light | Moderate | Severe |
| Serum albumin (g/dL) | 3.5–4.5 | 3.0–3.5 | 2.5–2.9 | < 2.5 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocytes (count /mL) | > 1600 | 1200–1599 | 800–1199 | < 800 |
| Score | 0 | 1 | 2 | 3 |
| Cholesterol (mg/dL) | > 180 | 140–180 | 100–139 | < 100 |
| Score | 0 | 1 | 2 | 3 |
| Screening total score | 0–1 | 2–4 | 5–8 | 9–12 |
CONUT: CONtrolling NUTritional status.
Statistical methods
A first descriptive analysis of the patients’ characteristics was performed by calculating central tendency and dispersion measures of quantitative variables. For qualitative variables, comparison of proportions was tested by using the χ2 test or the Fisher exact test, whenever necessary. We performed Kolmogorov–Smirnov and Shapiro–Wilk tests in all continuous variables and confirmed their normal distribution. In addition to descriptive statistics, a Kaplan–Meier analysis of in-hospital mortality up to 30 days from admission was performed. To obtain the hazards ratios, a Cox proportional regression (univariable and multivariable analysis) was fitted over the statistically significant variables obtained from the univariable analysis (age, sex, smoker, and CONUT were used as categorical variables; and height, weight and body mass index [BMI] as continuous variables). A receiver operating curve (ROC) for CONUT on mortality and its area under the curve (AUC) with 95% confidence interval were also estimated. Data management, statistical calculations, and graphical plots were conducted using the R statistical software (https://www.R-project.org/); the particular packages used were survival, survminer, cmprsk, and ggplot.
Results
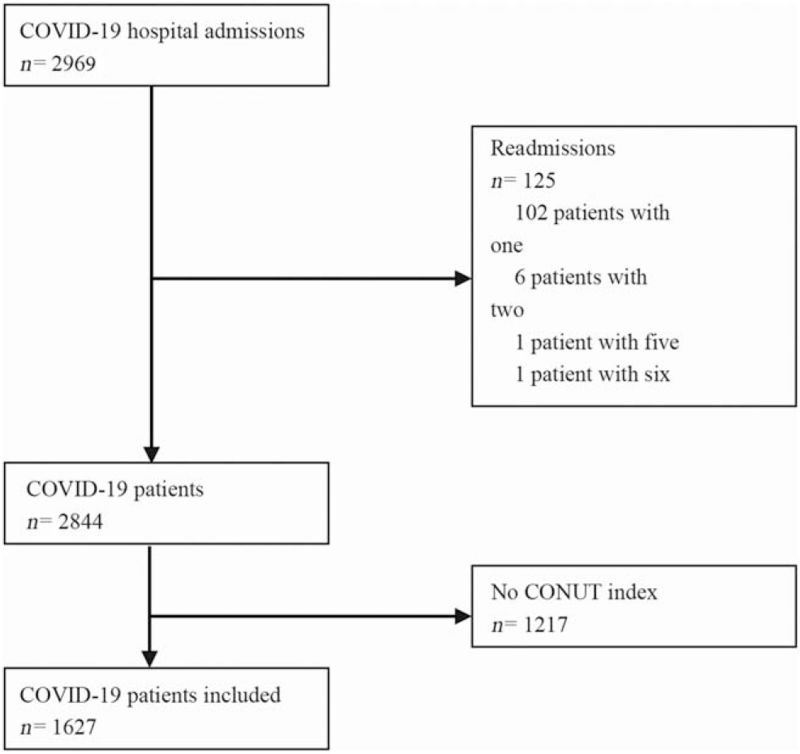
Overall, during the study period there were 2969 adult admissions with positive COVID-19 clinical diagnosis. After excluding the episodes corresponding to re-admissions (n = 125; 102 patients with one re-admission, six patients with two readmissions, one patient with five re-admissions, and one patient with six re-admissions), these admissions corresponded to 2844 single COVID-19 patients. Out of them, any of the three CONUT variables were unavailable in 1217 patients. Accordingly, clinical data from 1627 patients, from which a CONUT index could be calculated from the first blood test results obtained up to the fourth day of admission, were finally analyzed [Figure 1]. The survival rate of the whole cohort can be found in [Supplementary Figure 1].
Figure 1.
Flowchart of participation in the study. No information on albumin, cholesterol and lymphocytes to calculate the CONUT index upon admission or within 4 days after. CONUT: CONtrolling NUTritional status; COVID-19: Coronavirus disease 2019.
The distribution of patients according to the CONUT stages was as follows: 11.9% (n = 194) of the subjects were classified as normal risk, 47.2% (n = 769) as light, 35.9% (n = 585) as moderate and 4.9% (n = 79) as severe.
A direct, clinically, and statistically significant association of baseline CONUT with length of hospitalization was observed. The higher the CONUT stage, the longer the duration of hospitalization, starting at 7.9 ± 9.2 days on low-risk stage, up to 22.1 ± 25.2 days in severe risk CONUT stage (P < 0.001). Having a higher CONUT stage (moderate or high risk) was also related (P < 0.001) to increased use of resources, including non-invasive mechanic ventilation (NIMV), invasive mechanic ventilation (IMV), and management in intermediate care respiratory units (ICRU) and intensive care units (ICU). Further, a high score in the CONUT index was also related (P < 0.001) to higher risk of 30-day mortality, starting on 3.1% on the normal risk CONUT stage, increasing up to a 40.5% in the severe CONUT stage [Table 2].
Table 2.
Sociodemographic and clinical characteristics of the COVID-19 La Princesa cohort, according to baseline CONUT stage.
| Items | Low (0–1), n = 194 | Light (2–4), n = 769 | Moderate (5–8), n = 585 | Severe (9–12), n = 79 | P value |
| Age, years | 59.7 ± 16.1 | 63.8 ± 17.2 | 72.9 ± 13.4 | 79.1 ± 11.0 | <0.001 |
| Age (categorical, years) | |||||
| 18–50 | 52 (20.1) | 176 (67.7) | 31 (11.9) | 1 (0.3) | <0.001 |
| 51–60 | 56 (19.3) | 156 (53.8) | 74 (25.5) | 4 (1.4) | <0.001 |
| 61–70 | 40 (12.2) | 142 (43.5) | 132 (40.5) | 12 (3.7) | <0.001 |
| 71–80 | 21 (6.1) | 144 (42.2) | 152 (44.6) | 24 (7) | <0.001 |
| 81–90 | 18 (5.9) | 111 (36.3) | 152 (49.8) | 24 (7.9) | <0.001 |
| >90 | 7 (6.7) | 40 (38.1) | 44 (41.9) | 14 (13.3) | <0.001 |
| Female | 119 (61.3) | 352 (45.7) | 236 (40.3) | 23 (29.1) | <0.001 |
| Height in cm | 162.6 ± 10.0 (22) | 163.5 ± 19.7 (92) | 168.1 ± 10.5 (85) | 167.6 ± 9.1 (14) | 0.076 |
| Weight in kg | 78.8 ± 19.9 (27) | 76.3 ± 19.1 (120) | 76.6 ± 17.8 (108) | 65.6 ± 12.6 (16) | 0.137 |
| BMI in kg/m | 30.5 ± 8.0 (19) | 27.4 ± 5.5 (74) | 27.4 ± 5.0 (71) | 22.8 ± 3.3 (12) | 0.006 |
| Smokers | |||||
| Former | 24 (12.3) | 141 (18.3) | 123 (21.0) | 22 (27.8) | 0.010 |
| Current | 11 (5.6) | 26 (3.3) | 29 (4.9) | 5 (6.3) | 0.284 |
| Comorbidities | |||||
| COPD | 30 (15.5) | 85 (11.1) | 88 (15.0) | 13 (16.5) | 0.094 |
| Cancer | 8 (4.1) | 33 (4.3) | 32 (5.5) | 9 (11.4) | 0.043 |
| PTE | 4 (2.1) | 35 (4.6) | 24 (4.1) | 8 (10.1) | 0.030 |
| Duration of admission, days | 7.9 ± 9.2 | 10.9 ± 12.2 | 14.4 ± 16.7 | 22.1 ± 25.2 | <0.001 |
| Laboratory test | |||||
| Albumin in g/dL | 3.9 ± 0.3 | 3.8 ± 0.3 | 3.3 ± 0.3 | 2.6 ± 0.3 | <0.001 |
| Cholestrol in mg/dL | 182.2 ± 28.6 | 151.5 ± 32.0 | 129.7 ± 36.2 | 108.7 ± 23.9 | <0.001 |
| Lymphocyte in count/mL | 1.9 ± 0.5 | 1.2 ± 0.6 | 0.9 ± 0.5 | 0.7 ± 0.6 | <0.001 |
| CONUT score | 0.7 ± 0.5 | 3.0 ± 0.8 | 6.1 ± 1.0 | 9.7 ± 0.9 | <0.001 |
| Use of health services, % | |||||
| NIMV | 2.6 (5) | 3.9 (30) | 7.0 (41) | 10.1 (8) | <0.001 |
| IMV | 1.0 (2) | 5.6 (43) | 8.5 (50) | 19.0 (15) | <0.001 |
| IRCU | 0.5 (1) | 0.5 (16) | 2.0 (18) | 8.8 (7) | <0.001 |
| ICU | 2.5 (5) | 7.1 (55) | 11.2 (66) | 20.2 (16) | <0.001 |
| Death | 6 (3.1) | 69 (9.0) | 133 (22.7) | 32 (40.5) | <0.001 |
Data are shown as n (%), mean ± standard deviation (SD) or mean ± SD (n). BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; CONUT: CONtrolling NUTritional status; COVID-19: Coronavirus disease 2019; IRCU: intermediate respiratory care unit; ICU: Intensive care unit; IMV: Invasive mechanical ventilation; NIMV: Non-invasive mechanic ventilation; n: Number of patients; PTE: Pulmonary thromboembolism.
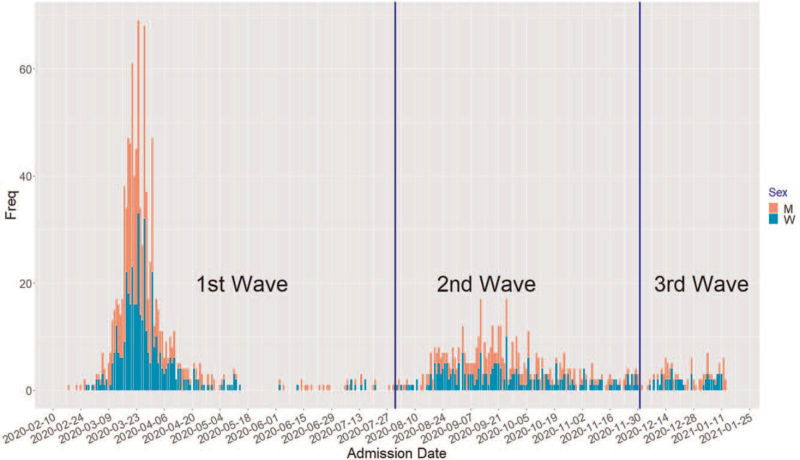
Overall, men with COVID-19 had a higher CONUT stage than COVID-19 women (P < 0.001), and men had a higher proportion of hospital admissions for COVID-19 than women [Figure 2]. In addition, higher scores of CONUT index were also related with lower BMI (P = 0.003) and increased age (P < 0.001). The categorization of BMI (i.e., underweight, normal, overweight, and obesity) was tested but did not present the statistically significant results (data not shown).
Figure 2.
Histogram of frequency of daily admissions for COVID-19 in the Hospital de La Princesa cohort (stacked by sex). “M” for men (red) vs. “W” for women (green). COVID-19: Coronavirus disease 2019.
These results are consistent with those expressed in [Table 3], corresponding to an analysis of the risk of mortality according to the CONUT score, where it can be appreciated that in the crude analysis as well as in a multivariable Cox regression analysis adjusted by age and sex, a higher CONUT stage was associated with a higher risk of mortality: for light CONUT, hazard ratio (HR) = 1.72, 95% CI: 0.75 to 3.98; for moderate CONUT, HR = 2.61, 95% CI:1.14 to 5.95; and severe CONUT, HR = 2.77, 95% CI:1.14 to 6.73. Specifically, there was a statistically significant difference from the normal CONUT vs. moderate (P < 0.023) and vs. severe-risk stages (P < 0.024), although there was no statistical significance between the normal and light stages (P = 0.202).
Table 3.
Hazard ratio (with 95% CI) of mortality by baseline CONUT stage.
| Univariable | Multivariable Cox regression | ||||
| Variable | Category | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Age (years) | 18–50 | Reference | Reference | ||
| 51–60 | 2.27 (0.73–7.03) | 0.156 | 2.03 (0.65–6.32) | 0.221 | |
| 61–70 | 2.76 (0.96–7.94) | 0.059 | 2.28 (0.79–6.60) | 0.129 | |
| 71–80 | 6.84 (2.49–18.84) | <0.001 | 5.58 (2.01–15.46) | <0.001 | |
| 81–90 | 15.21 (5.59–41.37) | <0.001 | 12.10 (4.41–33.24) | <0.001 | |
| >90 | 16.80 (5.98–47.19) | <0.001 | 13.50 (4.76–38.30) | <0.001 | |
| Sex | Female | Reference | Reference | ||
| Male | 0.90 (0.69–1.16) | 0.433 | 1.01 (0.77–1.315) | 0.949 | |
| Height | 0.99 (0.97–1.00) | 0.079 | Not included | ||
| Weight | 0.99 (0.98–1.01) | 0.717 | Not included | ||
| BMI | 0.96 (0.89–1.04) | 0.325 | Not included | ||
| Smoking status | Non-smoker | Reference | Not included | ||
| Former- | 1.188 (0.87–1.62) | 0.280 | |||
| Current- | 0.80 (0.42–1.51) | 0.491 | |||
| CONUT | Normal | Reference | |||
| Light | 1.97 (0.86–4.55) | 0.111 | 1.72 (0.75–3.98) | 0.202 | |
| Moderate | 3.77 (1.66–8.57) | <0.001 | 2.61 (1.14–5.95) | 0.023 | |
| Severe | 4.58 (1.90–11.01) | <0.001 | 2.77 (1.14–6.73) | 0.024 | |
Reference group: unit of each continuous measurement; female; never-smoker; normal risk CONUT stage. BMI: Body mass index; CI: Confidence interval; CONUT: CONtrolling NUTritional status.
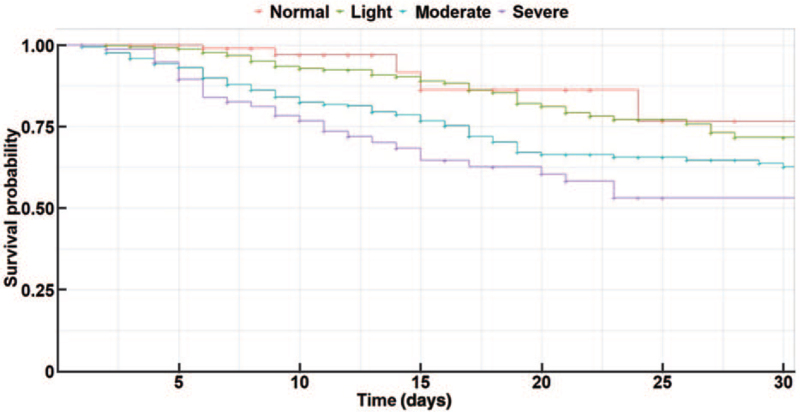
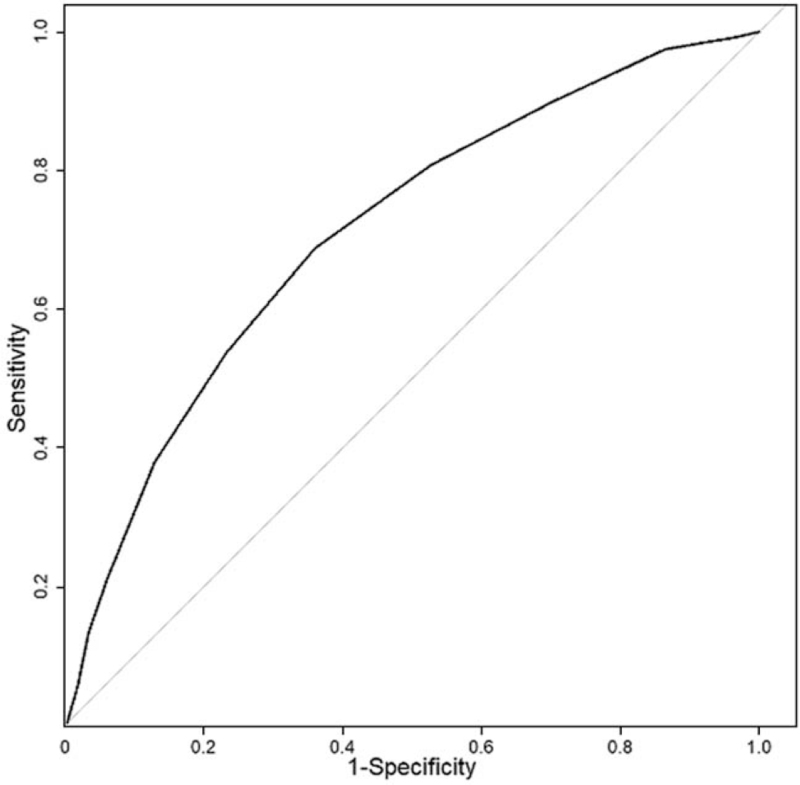
The Kaplan–Meier survival curves [Figure 3] identify a clear distinction in the survival probability of the four groups from admission, in particular between the CONUT stages light to severe from day three upon admission, with only some curve overlap in those with normal or light CONUT stages (log-rank test P < 0.001). Finally, the ROC of CONUT for mortality in 1627 admitted patients is in Figure 4, with an AUC and 95% CI of 0.711 (0.676–0746).
Figure 3.
Kaplan-Merier survival curve according to baseline CONUT stage. Log-rank test P < 0.001. CONUT: CONtrolling NUTritional status.
Figure 4.
ROC of CONUT for mortality in 1627 admitted patients. AUC and 95% CI of 0.711 (0.676–0746). AUC: Area under the curve; CI: Confidence interval; CONUT: CONtrolling NUTritional status; ROC: Receiver operating curve.
Discussion
The COVID-19 pandemic has had a great impact in health care systems all over the world, making health workers struggle in their duty due to the absence of appropriate tests to aid them in the correct and confident decision-making process on these new patients.[7] This has steered into a breach in patient care, not only because of the underlying lack of knowledge of a new disease with a rapid expansion and burden but also because of the uncertainty about the possible health outcomes from each particular patient, causing a negative influence on the functioning of health care systems and disease control.[8,9]
In consequence, prognostic systems and tools which could enable an early recognition of patients with higher risk for severe health outcomes, especially on the most vulnerable ones, remain a research gap.[10] The CONUT index is a potential candidate in this respect, because it has accumulated evidence in the past, has been applied to a wide range of severe conditions, and was tested during aggressive therapeutic procedures. They include a great variety of cancers in diverse locations and types, as well as being useful in many acute and chronic conditions, and predictive studies after medical, surgical, radio- and chemo-therapies.[11–14]
Recently, a first use of CONUT in COVID-19 was reported, although with a limited sample size. Wei et al[15] first concluded that a moderate or severe CONUT stage is an independent risk factor for greater mortality that can help in the recognition of high-risk patients. This was also described by Chen et al,[16] Wang et al,[17] Fong et al,[18] and Zhou et al.[19] CONUT has also been applied to the elaboration of recommendations in the nutritional treatment of oncologic patients before the advance of COVID-19.[20] It has also demonstrated its applicability in primary care, enabling a more efficient medical monitoring of COVID-19 patients, by adjusting patients’ needs without transferring them.[21]
Our study has confirmed that CONUT is an independent potential predictor of severe disease after infection by SARS-CoV-2, both in terms of mortality and hospitalization duration. In this manner, CONUT allows the identification of those patients which will most probably require management in intermediate and ICU admission, and will prospectively entail NIMV or IVM, warning physicians to apply a closer monitoring, and helping them to act as early as possible, limiting the risk of complications and permitting a more efficient allocation of resources.
The CONUT index has been comprehensively confirmed by independent researchers and in many settings as a fine indicator of both short- and long-term prognosis for cancer patients. Also, CONUT has been used for research in inflammatory conditions (infectious or not), degenerative diseases, as well as to explore the side effects of therapeutic procedures such as surgery, radio/chemotherapy, etc. This is due because CONUT captures and quantifies the impact of all these clinical conditions on the physiological balance and homeostasis of the cellular environment. The diversity of organs affected by COVID-19 is one of the main reasons that led us to study the usefulness of the method in this pathology, as there was equipoise about the usefulness of CONUT in COVID-19 before starting this research. Thus, CONUT being an adequate score, we envisage it could be tested in the three main phases of COVID-19. First, it might help physicians in taking decisions ensuring the minimum harm to patients and the most efficient allocation of resources upon admission. Further on, in the care period, it might enable professionals to limit the standby periods and guide them on whether to continue or modify therapies without the need of waiting for the emergence of symptoms, as the parameters on which the CONUT index is based, experiment changes before the appearance of symptoms. Finally, in the recovery phase, it can help to identify variations in the patient situation due to relapse/changes in their condition.
Strengths of our research include a large and unbiased cohort of COVID-19 patients, the simplicity of CONUT and the rapid clinical applicability of a tool that predicts clinically relevant outcomes by using standard routine blood tests. However, a number of limitations might be considered: First, a substantial proportion of patients had missing values of cholesterol, lymphocytes and/or albumin, which preclude the calculation of CONUT. As an alternative to imputation, we think the large number of patients in our clinical series gives robust estimates. Second, key variables like weight, height, and smoking are not systematically included in electronic health records, precluding some nutrition-based analysis, such as determining baseline BMI in all patients. However, a manual search of these variables is under way and will be reported in due course. The hidden effect of time, as our assessment encompasses three different COVID-19 waves in Spain, should be explored in other settings and populations. Finally, our analysis focused on baseline CONUT, yet we can anticipate high interest in exploring clusters of CONUT trajectories in subsets of COVID-19 patients of varied severity.
As of June 2021, the COVID-19 end-game is far from completion, but we endorse calls for elimination rather than mitigation strategies.[22,23] In future analysis, the usefulness of CONUT might be further evaluated, not only upon hospital admission, but also before and after admission, during the entire clinical course, enabling professionals to take early decisions on treatment effectiveness, and other relevant outcomes, including the need to manage patients in special units like IRCU or ICU. Other investigators and hospitals are encouraged to study CONUT in diverse populations, to expand the applicability of this system.
Conclusions
We conclude that CONUT can be helpful as a potential prognostic index of clinical risk in COVID-19 hospitalized patients, with utility in mortality and hospitalization duration. Although <5% of hospitalized patients had a severe baseline CONUT stage, even light and moderate CONUT stages were capable of identifying subgroups of COVID-19 patients with a higher risk in all clinical outcomes analyzed. CONUT holds potential for a tighter control of the clinical course during hospitalization, and calls for the deployment of health resources such as IMV and NIMV, and management in Intermediate Respiratory and ICUs, simply by scoring only three parameters that are easily obtained in blood tests either in primary or hospital care.
Availability of data and materials
Data and coding can be requested by contacting the author for correspondence and study team
Funding
The work is supported by a grant from the European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement (No 101016216).
Conflicts of interest
The authors declare there are no conflicts of interest to report related with this research.
Supplementary Material
Footnotes
How to cite this article: Bengelloun AK, Ortega GJ, Ancochea J, Sanz-Garcia A, Rodríguez-Serrano DA, Fernández-Jiménez G, Girón R, Ávalos E, Soriano JB, Ulíbarri JI. Usefulness of the CONUT index upon hospital admission as a potential prognostic indicator of COVID-19 health outcomes. Chin Med J 2022;135:187–193. doi: 10.1097/CM9.0000000000001798
Adrián K. Bengelloun and Guillermo J. Ortega contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Muller JE, Nathan DG. COVID-19, nuclear war, and global warming: lessons for our vulnerable world. Lancet 2020; 395:1967–1968. doi: 10.1016/S0140-6736(20)31379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng YL, He YK, Ma XQ, Gao ZC. Feasibility of coronavirus disease 2019 eradication. Chin Med J 2020; 133:1387–1389. doi: 10.1097/CM9.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ 2020; 369:m1328.doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin BG, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol 2021; 31:1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Dong Y, Wang L, Xie H, Li B, Chang C. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun 2020; 112:102473.doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Ulíbarri JI, González-Madroño A, de Villar NGP, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005; 20:38–45. [PubMed] [Google Scholar]
- 7.Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J 2020; 133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blake H, Bermingham F, Johnson G, Tabner A. Mitigating the psychological impact of COVID-19 on healthcare workers: a digital learning package. Int J Environ Res Public Health 2020; 17:2997.doi: 10.3390/ijerph17092997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Liang M, Li Y, Guo J, Fei D, Wang L, et al. Mental health care for medical staff in China during the COVID-19 outbreak. Lancet Psychiatry 2020; 7:e15–e16. doi: 10.1016/S2215-0366(20)30078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J 2020; 133:1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 2018; 21:204–212. doi: 10.1007/s10120-017-0744-3. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto S, Ureshino H, Kidoguchi K, Kusaba K, Kizuka-Sano H, Sano H, et al. Clinical impact of the CONUT score in patients with multiple myeloma. Ann Hematol 2020; 99:113–119. doi: 10.1007/s00277-019-03844-2. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz A, Tekin SB, Bilici M, Yilmaz H. The Significance of Controlling Nutritional Status (CONUT) score as a novel prognostic parameter in small cell lung cancer. Lung 2020; 198:695–704. doi: 10.1007/s00408-020-00361-2. [DOI] [PubMed] [Google Scholar]
- 14.Kato T, Yaku H, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, et al. Association with Controlling Nutritional Status (CONUT) score and in-hospital mortality and infection in acute heart failure. Sci Rep 2020; 10:3320.doi: 10.1038/s41598-020-60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei C, Liu Y, Li Y, Zhang Y, Zhong M, Meng X. Evaluation of the nutritional status in patients with COVID-19. J Clin Biochem Nutr 2020; 67:116–121. doi: 10.3164/jcbn.20-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Zhao Y, Du X, Liu Y, Chen J, Peng L, et al. Comparison of the clinical implications among two different nutritional indices in hopitalized patients with COVID-19. medRxiv 2020; 04.28.20082644. doi: 10.1101/2020.04.28.20082644. [Google Scholar]
- 17.Wang R, He M, Yue J, Bai L, Liu D, Huang Z, et al. CONUT score is associated with mortality in patients with COVID-19: a retrospective study in Wuhan. PREPRINT (Version 1) available at Research Square 2020; [ 10.21203/rs.3.rs-32889/v1]. [DOI] [Google Scholar]
- 18.Song F, Ma H, Wang S, Qin T, Xu Q, Yuan H, et al. Nutritional screening based on objective indices at admission predicts in-hospital mortality in patients with COVID-19. PREPRINT (Version 1) available at Research Square 2020; [ 10.21203/rs.3.rs-108125/v1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Ma Y, Liu Y, Xiang Y, Tao C, Yu H, et al. A correlation analysis between the nutritional status and prognosis of COVID-19 patients. J Nutr Health Aging 2021; 25:84–93. doi: 10.1007/s12603-020-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez D, Guerrero M, Maldonado M, Veintimilla DR, Tapia MG, Moran SH, et al. Recommendations in the nutritional treatment for cancer patients before Covid-19. J Health Med Sci 2020; 6:303–314. [Google Scholar]
- 21.Piñera M, de Esteban C, Rodríguez A, Arrieta-Blanco F. Grupo de Trabajo de Nutricion de Somamfyc. Recomendaciones para la prevención de la desnutrición en pacientes COVID 19 en seguimiento por Atencion Primaria: role of CONUT. Aten Primaria 2021; 53:101948.doi: 10.1016/j.aprim.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ya-Li Z, Yu-Kun H, Xin-Qian M, Zhan-Cheng G. Feasibility of coronavirus disease 2019 eradication. Chin Med J 2020; 133:1387–1389. doi: 10.1097/CM9.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliu-Barton M, Pradelski BSR, Aghion P, Artus P, Kickbusch I, Lazarus JV, et al. SARS-CoV-2 elimination, not mitigation, creates best outcomes for health, the economy, and civil liberties. Lancet 2021; 397:2234–2236. doi: 10.1016/S0140-6736(21)00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and coding can be requested by contacting the author for correspondence and study team