Abstract
Objective
This study aimed to assess the effect of endometrioma surgery on ovarian reserve by measuring anti-Müllerian hormone (AMH) levels.
Methods
This systematic review and meta-analysis included observational studies and randomized clinical trials published in English referenced in MEDLINE, SCOPUS and Cochrane (1982-2019). We included studies that reported AMH levels in the pre and post-operative period of patients undergoing laparoscopic surgery for endometrioma. Preoperative AMH was defined as the baseline AMH; short term AMH was measured no later than a month after surgery; medium term AMH was measured between one and six months after surgery; and long-term AMH was measured six or more months after surgery.
Results
Thirty-six studies met the inclusion criteria. A significant decrease was observed in short, medium and long-term post-operative AMH levels when compared with baseline AMH. However, there were no differences between short and long-term post-operative AMH levels, suggesting a non-significant recovery after one year of follow-up. A significant decrease in post-operative AMH was observed in bilateral endometriomas compared with unilateral cases. In addition, patients with endometriomas presented a significant decline in post-operative AMH compared with patients with other benign ovarian conditions. The decrease in post-operative AMH was significantly greater in bilateral cystectomy when compared with vaporization with bipolar energy or laser. We also observed a greater decrease in post-operative AMH with bipolar energy hemostasis compared with suture and hemostatic agents. These results should be taken with caution due to the high heterogeneity of the studies analyzed.
Conclusions
Endometrioma surgery has a deleterious effect on short, medium, and long-term post-operative AMH levels. Bilateral endometriomas and endometriomas greater than 7 cm have been associated with greater decreases in AMH. The mechanical resection of healthy tissue and the inflammatory damage on the ovarian cortex might explain the diminishing of ovarian reserve.
Keywords: anti-Müllerian hormone, endometriosis, endometrioma, laparoscopic surgery, ovarian reserve
INTRODUCTION
Endometriosis is a benign disease characterized by the presence of endometrial tissue, both stroma and glands, outside the uterine cavity. The disease has a high prevalence, as approximately 10% of women of childbearing age might suffer from it (Hirsch et al., 2018). The most frequent location is the ovary, followed by the pelvic peritoneum, uterosacral ligaments, and tubes. Ovarian endometriomas are found in up to 20% of patients with endometriosis (Zondervan et al., 2018). Clinical presentation varies, with 20-30% of the patients remaining asymptomatic, while others may develop hypogastric pain, dysmenorrhea, dyspareunia, chronic pelvic pain, menstrual disorders and infertility (Zondervan et al., 2018).
Surgical techniques for endometriosis include the excision of endometriomas larger than 4 cm and adhesiolysis (Duffy et al., 2014). Different treatments focus specifically on endometriomas. In a Cochrane review, Hart et al. (2008) concluded that laparoscopic cystectomy was the most favorable technique to avoid recurrences in the treatment of endometriomas compared with drainage and ablation, since the procedure also improved pregnancy rates compared with other techniques. The most common technique used for ovarian cystectomy is decapsulation. The consequent bleeding from the remaining ovarian stroma is usually controlled with bipolar coagulation and/or suturing.
In recent years, many studies have discussed the effect of ovarian surgery on ovarian reserve and the possible risk of early ovarian failure (Duffy et al., 2014; Saito et al., 2018; Choi et al., 2018). Ovarian reserve is defined as the reproductive potential at a certain moment; it is determined by the quantitative analysis of the follicular count by ultrasound or biochemical tests such as measurement of serum levels of FSH, estradiol, inhibin B, and anti-Müllerian hormone (AMH) (Kline et al., 2005; La Marca et al., 2010; Committee on Gynecologic Practice, 2015). The decline of the ovarian reserve is a continuous and irreversible physiological process, with great variability at an ethnic, family or individual level (Farquhar et al., 2019).
Decreases in ovarian reserve after laparoscopic cystectomy have been reported, and studies have attributed it to the irreversible damage caused by bipolar electrocoagulation to achieve proper hemostasis. This damage might be secondary to the thermal effects on the ovarian stroma and vascularization, in addition to accidental and involuntary damage to healthy follicles during cyst excision (Asgari et al., 2016).
AMH might be the most practical ovarian reserve marker for several reasons. AMH expressed by the granulosa cells of the active developing pre-antral follicles plays an important role in the physiology of the ovary since its initial recruitment (Durlinger et al., 2002). This phase is still independent of gonadotropins, making it a stable hormone between and during cycles. The decrease in the number of follicles that occurs over time leads to a gradual and physiological decrease in the levels of this hormone, starting at the age of 21 (Farquhar et al., 2019).
To avoid follicular damage and thus preserve ovarian function, new methods are being included to control bleeding, such as suturing, plasma-jet vaporization, and coagulation with other hemostatic agents (Asgari et al., 2016; Kang et al., 2015). However, there is controversy regarding the effectiveness of such methods (Song et al., 2014; Sönmezer et al., 2013).
Recent studies have reported that endometriosis surgery has a negative impact on ovarian reserve, as shown in post-operative follow-up of AMH and antral follicles. However, there is no consensus as to whether this is due to endometriosis itself, its severity, or to factors specific to surgery, such as surgical approach and hemostatic technique (Chang et al., 2010; Sugita et al., 2013; Alborzi et al., 2014; Iwase et al., 2016; Ozaki et al., 2016; Goodman et al., 2016).
In accordance with the Patients, Intervention, Comparison and Outcomes (PICO) statements, this study aimed to assess the effects of endometrioma surgery on ovarian reserve based on AMH levels at different times before and after surgery. Additionally, we evaluated the effect of laparoscopic cystectomy on ovarian reserve according to laterality and size of the lesions, the effect of laparoscopic surgery on ovarian reserve for endometrioma vs. surgery for benign ovarian conditions, and the effect of hemostatic technique during laparoscopic surgery for endometrioma on ovarian reserve.
MATERIAL AND METHODS
This systematic review of the literature and meta-analysis did not involve interventions in humans. Therefore, approval was not required from an Ethics Review Committee. We used the "Preferred Reporting Items for Systematic Reviews and Meta-analysis" (PRISMA) statement to report results (Shamseer et al., 2015).
Search Strategy
This systematic review of the literature and meta-analysis included observational studies evaluating the effect of endometrioma surgery on ovarian reserve measured through AMH levels. Searches were performed in the following databases: MEDLINE, SCOPUS and Cochrane from 1982 to January 2019. In addition, we searched for references cited in relevant studies. The search combined terms and descriptions associated with the variants of these interventions and the study population. They included ovarian endometriosis, gynecological laparoscopy, endometriosis surgery, and AMH. The search strategy was modified to make it compatible with the syntax of each database consulted.
Data collection and analysis
Two authors (CR and JM) independently reviewed the titles and abstracts of the studies that the search yielded. The ones that met the predefined criteria were selected for inclusion in the review. Full texts of all citations that might meet the predefined selection criteria were obtained. Disagreements and doubts were resolved by consensus of both authors. Both authors critically analyzed the results and used the GRADE System to rate the quality of the evidence and grade the strength of the recommendation for each result (Schünemann et al., 2013).
Selection criteria
Inclusion criteria: The review included retrospective or prospective observational studies and randomized clinical trials that reported AMH levels in the pre and post-operative period of patients undergoing laparoscopic surgery for endometrioma. The studies included patients with benign ovarian disease and patients treated without surgery. The selection criteria are described in Table 1.
Table 1.
Description of the studies included in the meta-analysis.
| Study | Location / Years | Study design | Participants | Outcomes | Study quality (NOS) |
|---|---|---|---|---|---|
| Uncu et al., 2013 | South Korea 2008 - 2009 | Prospective cohort | 27 | Post-operative AMH short term vs. pre-operative AMH Post-operative AMH medium term vs. pre-operative AMH |
6/9 |
| Uncu et al., 2013 | Turkey 2008 - 2011 | Prospective cohort | 60 | Post-operative AMH short term vs . pre-operative AMH Post-operative AMH long term vs. pre-operative AMH Post-operative AMH short vs. long term |
7/9 |
| Alborzi et al., 2014 | Iran 2010 - 2012 | Prospective cohort | 193 | Post-operative AMH short term vs . pre-operative AMH Post-operative AMH medium term vs. pre-operative AMH Post-operative AMH long term vs. pre-operative AMH Post-operative AMH short vs. long term Post-operative AMH EO unilateral vs. bilateral short term Post-operative AMH EO unilateral vs. bilateral medium term Post-operative AMH EO unilateral vs. bilateral long term |
7/9 |
| Tanprasertkul et al., 2014 | Thailand 2012 - 2013 | Prospective cohort | 77 | Post-operative AMH short term vs . pre-operative AMH Post-operative AMH medium term vs. pre-operative AMH Post-operative AMH long term vs. pre-operative AMH Post-operative AMH short vs. long term Post-operative AMH EO unilateral vs. bilateral short term Post-operative AMH EO unilateral vs. bilateral medium term Post-operative AMH EO unilateral vs. bilateral long term Post-operative AMH sealing agents vs. bipolar coagulation |
6/9 |
| Chen et al., 2014 | China 2013 - 2014 | Prospective cohort | 98 | Post-operative AMH short term vs. pre-operative AMH Post-operative AMH EO vs. No EO short term |
8/9 |
| Vignali et al., 2015 | Italy 2009 - 2010 | Prospective cohort | 22 | Post-operative AMH short term vs. pre-operative AMH Post-operative AMH medium term vs. pre-operative AMH Post-operative AMH long term vs. pre-operative AMH Post-operative AMH short vs. long term |
9/9 |
| Saito et al., 2018 | Japan 2011 - 2013 | Prospective cohort | 62 | Post-operative AMH short term vs. pre-operative AMH Post-operative AMH long term vs. pre-operative AMH Post-operative AMH short vs. long term Post-operative AMH EO unilateral vs. bilateral short term Post-operative AMH laser ablation vs. unilateral cystectomy Post-operative AMH laser ablation vs. bilateral cystectomy |
8/9 |
| Muzii et al., 2019 | Italy 2015 - 2016 | Prospective cohort | 52 | Post-operative AMH short term vs. pre-operative AMH Post-operative AMH long term vs. pre-operative AMH Post-operative AMH short vs. long term Post-operative AMH EO vs. No EO medium term |
8/9 |
| Biacchiardi et al., 2011 | Italy 2007 - 2009 | Prospective cohort | 43 | Post-operative AMH long term vs. pre-operative AMH | 7/10 |
| Ercan et al., 2011 | Turkey 2008 - 2010 | Prospective cohort | 36 | Post-operative AMH medium term vs. pre-operative AMH | 9/9 |
| Hwu et al., 2011 | Taiwan 2007 - 2010 | Prospective cohort | 1642 | Post-operative AMH medium term vs. pre-operative AMH Post-operative AMH EO unilateral vs. bilateral short term |
7/9 |
| Kitajima et al., 2011 | Japan 2007 - 2009 | Prospective cohort | 32 | Post-operative AMH medium term vs. pre-operative AMH | 7/9 |
| Celik et al., 2012 | Turkey 2009 - 2010 | Prospective cohort | 65 | Post-operative AMH medium term vs. pre-operative AMH Post-operative AMH long term vs. pre-operative AMH |
8/9 |
| Kwon et al., 2014 | South Korea 2011 - 2013 | Prospective cohort | 100 | Post-operative AMH medium term vs. pre-operative AMH Post-operative AMH EO vs. No EO medium term |
9/9 |
| Salihoğlu et al., 2016 | Turkey 2013 - 2014 | Prospective case control | 67 | Post-operative AMH medium term vs. pre-operative AMH Post-operative AMH EO vs. No EO short term |
7/9 |
| Kim et al., 2017 | South Korea 2011 - 2012 | Prospective cohort | 75 | Post-operative AMH medium term vs. pre-operative AMH Post-operative AMH EO vs. No EO medium term Post-operative AMH EO unilateral vs. bilateral medium term |
9/9 |
| Tsolakidis et al., 2010 | Greece 2005 - 2007 | Randomized clinical trial | 20 | Post-operative AMH long term vs. pre-operative AMH Post-operative AMH laser ablation vs. cystectomy |
9/9 |
| Iwase et al., 2016 | Japan 2008 - 2009 | Randomized clinical trial | 20 | Post-operative AMH long term vs. pre-operative AMH Post-operative AMH laser ablation vs. cystectomy |
9/9 |
| Chun et al., 2016 | Taiwan 2010 - 2013 | Prospective cohort | 65 | Post-operative AMH EO vs. No EO short term | 8/9 |
| Ergun et al., 2015 | Turkey 2011 - 2013 | Prospective cohort | 50 | Post-operative AMH EO vs. No EO medium term Post-operative AMH EO unilateral vs. bilateral medium term Post-operative AMH EO > 7 cm vs. < 7 cm |
7/9 |
| Hirokawa et al., 2011 | Japan 2008 - 2010 | Prospective cohort | 38 | Post-operative AMH EO unilateral vs. bilateral short term | 5/9 |
| Saito et al., 2014 | Japan 2011 - 2013 | Prospective cohort | 99 | Post-operative AMH EO unilateral vs . bilateral short term Post-operative AMH laser ablation vs. unilateral cystectomy Post-operative AMH laser ablation vs. bilateral cystectomy |
9/9 |
| Shao et al., 2016 | China 2012 - 2013 | Prospective cohort | 80 | Post-operative AMH EO unilateral vs. bilateral long term | 8/9 |
| Mehdizadeh Kashi et al., 2017 | Iran 2012 - 2013 | Prospective cohort | 70 | Post-operative AMH EO unilateral vs. bilateral long term | 8/8 |
| Kovačević et al., 2018 | Serbia 2013 - 2016 | Prospective cohort | 54 | Post-operative AMH EO unilateral vs. bilateral long term | 7/9 |
| Ercan et al., 2010 | Turkey 2007 - 2008 | Prospective cohort | 47 | Post-operative AMH EO unilateral vs. bilateral short term | 9/9 |
| Wang et al., 2019 | China 2014 - 2017 | Prospective cohort | 171 | Post-operative AMH EO > 7 cm vs. < 7 cm Post-operative AMH suture vs. bipolar coagulation |
5/8 |
| Giampaolino et al., 2015 | Italy 2012 - 2014 | Randomized clinical trial | 76 | Post-operative AMH laser ablation vs. unilateral cystectomy | 7/9 |
| Candiani et al., 2018 | Italy 2017 - 2018 | Randomized clinical trial | 60 | Post-operative AMH laser ablation vs. cystectomy | 6/9 |
| Ferrero et al., 2012 | Italy 2007 - 2010 | Randomized clinical trial | 100 | Post-operative AMH suture vs. bipolar coagulation | 8/9 |
| Li et al., 2013 | China 2008 - 2010 | Prospective cohort | 162 | Post-operative AMH suture vs. bipolar coagulation Post-operative AMH ultrasound vs. bipolar coagulation |
7/9 |
| Takashima et al., 2013 | Japan 2008 - 2010 | Prospective cohort | 44 | Post-operative AMH suture vs. bipolar coagulation | 8/9 |
| Song et al., 2015 | South Korea 2011 - 2014 | Prospective cohort | 125 | Post-operative AMH suture vs. bipolar coagulation | 8/9 |
| Zhang et al., 2016 | China 2013 | Randomized clinical trial | 207 | Post-operative AMH suture vs. bipolar coagulation Post-operative AMH ultrasound vs. bipolar coagulation |
9/9 |
| Sönmezer et al., 2013 | Turkey 2010 | Randomized clinical trial | 30 | Post-operative AMH sealing agents vs. bipolar coagulation | 9/9 |
| Song et al., 2014 | South Korea 2012 - 2013 | Randomized clinical trial | 100 | Post-operative AMH sealing agents vs. bipolar coagulation | 9/9 |
| Choi et al., 2018 | South Korea 2014 - 2016 | Randomized clinical trial | 80 | Post-operative AMH sealing agents vs. bipolar coagulation | 9/9 |
Exclusion criteria: The analysis was limited to evaluating the AMH and other studies that only considered other variables related to ovarian reserve. Reproductive results such as spontaneous pregnancy rates or assisted reproduction were excluded. Furthermore, patients undergoing laparoscopic surgery who presented other forms of endometriosis (superficial or deep) were not included.
Outcome Measures
Outcomes were measured in terms of post-operative AMH levels in different settings. The unit of measurement of AMH level was nanograms per milliliter and SD.
Preoperative AMH was defined as the baseline AMH; short term AMH was measured no later than a month after surgery; medium term AMH was measured between one and six months after surgery; and long-term AMH was measured six or more months after surgery.
Quality and risk of bias of included studies
Only cohort studies and randomized clinical trials were included in this systematic review and meta-analysis. Quality assessment was performed using the internationally accepted Newcastle-Ottawa scale (Stang, 2010). This scale is useful for assessing potential biases in the selection, comparability, exposure, and results stages. Table 2 shows the assessment of quality and risk of bias of the included studies.
Table 2.
Summary of the results.
| Outcome | Mean difference (95% CI) | Participants | Number of studies | Quality of evidence (GRADE) |
|---|---|---|---|---|
| Post-operative versus pre-operative AMH | ||||
| Short term | -1.62 [-2.21, -1.02] | 810 | 8 | ⊕⊕⊖⊖ low |
| Medium term | -1.31 [-1.68, -0.93] | 1376 | 13 | ⊕⊕⊖⊖ low |
| Long term | -1.54 [-1.89, -1.19] | 932 | 9 | ⊕⊕⊖⊖ low |
| Short term vs. long term | 0.11 [-0.08, 0.30] | 700 | 6 | ⊕⊕⊖⊖ low |
| Post-operative AMH Endometrioma vs. other benign ovarian pathology | ||||
| Short term | -0.96 [-1.34, -0.58] | 229 | 4 | ⊕⊕⊖⊖ low |
| Medium term | -1.40 [-2.09, -0.71] | 287 | 4 | ⊕⊕⊖⊖ low |
| Post-operative AMH Bilateral vs. Unilateral Endometrioma | ||||
| Short term | -0.86 [-1.24, -0.49] | 759 | 7 | ⊕⊕⊖⊖ low |
| Medium term | -0.86 [-1.44, -0.29] | 534 | 4 | ⊕⊕⊖⊖ low |
| Long term | -0.75 [-1.18, -0.33] | 617 | 5 | ⊕⊕⊖⊖ low |
| Post-operative AMH according to size | ||||
| Endometrioma> 7 cm vs. <7 cm | -0.50 [-0.65, -0.35] | 197 | 2 | ⊕⊕⊕⊖ moderate |
| Post-operative AMH according to surgical technique | ||||
| Bipolar ablation vs. unilateral cystectomy | 0.41 [-0.23, 1.04] | 114 | 3 | ⊕⊕⊕⊖ moderate |
| Bipolar ablation vs. bilateral cystectomy | 0.72 [0.19, 1.25] | 69 | 2 | ⊕⊕⊕⊕ high |
| Laser ablation vs. cystectomy | 0.86 [0.55, 1.16] | 80 | 2 | ⊕⊕⊕⊖ moderate |
| Post-operative AMH according to hemostatic technique | ||||
| Suture vs. bipolar energy | 0.50 [0.19, 0.81] | 686 | 6 | ⊕⊕⊕⊖ moderate |
| Sealing agents vs. bipolar energy | 0.53 [0.25, 0.81] | 258 | 4 | ⊕⊕⊕⊕ high |
| Ultrasound vs. bipolar energy | 0.00 [-0.24, 0.24] | 246 | 2 | ⊕⊕⊕⊕ high |
Statistical analysis
A meta-analysis was performed using REVMAN 5.3. To determine the combined effect of each variable, we used a Mantel-Haenszel model and applied the fixed effects model. Mean difference (MD) was calculated to measure the absolute difference between the mean values in two different groups, accompanied by 95% confidence intervals (CI). Statistical significance was established at values of p<0.05. The degree of variation between studies attributable to heterogeneity was assessed with the I2 statistical test. When heterogeneity was greater than 50% (I2> 50%), the random effects model was applied (Higgins et al., 2003).
RESULTS
Search results and characteristics of included studies
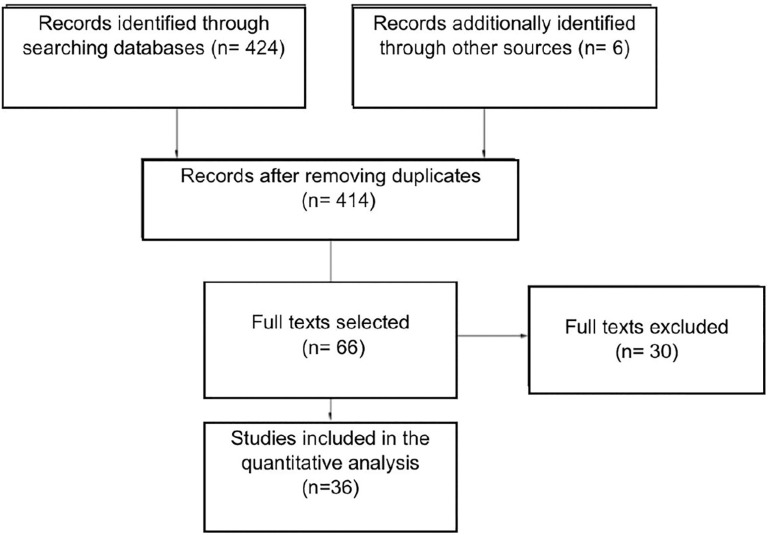
The search yielded 424 articles; however, 352 were excluded in the title/abstract selection. Six articles were added from the references of the most relevant publications. One or the two reviewers considered the remaining 66 studies eligible, but exclusions were made, as explained in Figure 1. In the end, 36 studies met the inclusion criteria and were included in the study.
Figure 1.
Flowchart detailing the selection of studies for inclusion in the meta-analysis. PRISMA.
Description of included studies
Of the 36 studies that met the inclusion criteria, studies with NOS scores > 7 were considered of high quality. The characteristics of the included studies are provided in Table 1.
Summary of results
Table 2 shows a summary of the study results.
I. Effect of laparoscopic cystectomy of an ovarian endometrioma on ovarian reserve: post-operative AMH versus baseline AMH
a. Post-operative short-term AMH versus baseline AMH
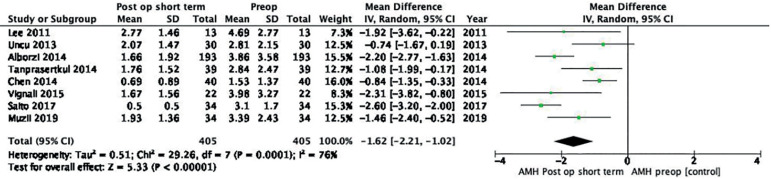
Eight studies were included in the analysis, with a total of 405 patients enrolled in each group.
Random effects analysis showed a mean difference (MD) of -1.62 (95% CI -2.21, -1-02; I2 = 76%) when short-term post-operative AMH and baseline AMH levels were compared (Figure 2). The quality of the evidence was low according to GRADE.
Figure 2.
Forest plot short-term post-operative AMH versus baseline AMH.
b. Post-operative medium-term AMH versus baseline AMH
Thirteen studies with a total of 688 patients in each group were compared. Random effects analysis showed an MD of -1.31 (95% CI -1.68, -0.93; I2 = 94%) when post-operative medium-term AMH and baseline AMH levels were compared (Figure 3). The quality of the evidence was low according to GRADE.
Figure 3.
Forest plot medium-term post-operative AMH versus baseline AMH.
c. Long-term post-operative AMH versus baseline AMH
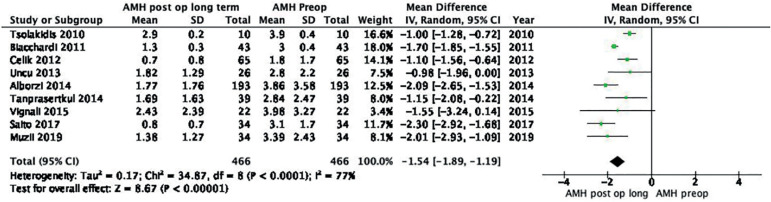
Random effects analysis of nine studies including 466 patients in each group showed an MD of -1.54 (95% CI -1.89, -1.19; I2 = 77%) when long-term post-operative AMH and baseline AMH were compared (Figure 4). The quality of the evidence was low according to GRADE.
Figure 4.
Forest plot long-term post-operative AMH versus baseline AMH.
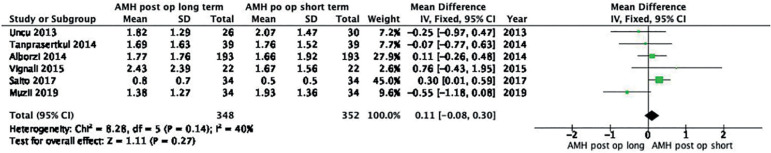
d. Long-term post-operative AMH versus short-term post-operative AMH
The analysis of six studies with 348 patients in the long-term post-operative AMH group and 352 in the short-term AMH group demonstrated an MD of 0.11 (95% CI -0.08, 0.30; I2 = 40%) when long-term post-operative AMH and short-term post-operative AMH were compared via fixed effects analysis (Figure 5). The quality of the evidence was low according to GRADE.
Figure 5.
Forest plot long-term post-operative AMH versus short-term post-operative AMH.
II. Effect of laparoscopic cystectomy on ovarian reserve according to laterality of the lesions
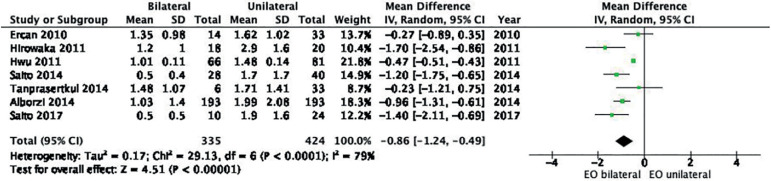
a. Short-term AMH in unilateral versus bilateral endometriomas
Seven studies were included in the analysis, with a total of 355 patients included in the group with bilateral endometriomas and 424 in the group with unilateral endometriomas. Random effects analysis showed lower post-operative AMH levels in patients with bilateral endometriomas compared with subjects with unilateral endometriomas in the short-term (MD -0.86, 95% CI -1.24, -0.49; I2 = 79%, Figure 6). The quality of the evidence was low according to GRADE.
Figure 6.
Forest plot AMH short term in unilateral versus bilateral endometriomas.
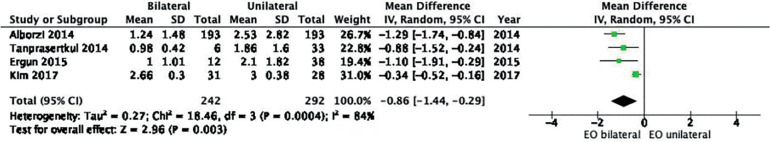
b. Medium-term AMH in unilateral versus bilateral endometriomas
The analysis of four studies with a total of 242 patients with bilateral endometriomas and 292 with unilateral endometriomas revealed lower post-operative AMH levels in patients with bilateral endometriomas compared with patients with unilateral endometriomas in the medium-term (MD of -0.86, 95% CI -1.44, -0.29; I2 = 84%, Figure 7). The quality of the evidence was low according to GRADE.
Figure 7.
Forest plot AMH in the medium term in unilateral versus bilateral endometriomas.
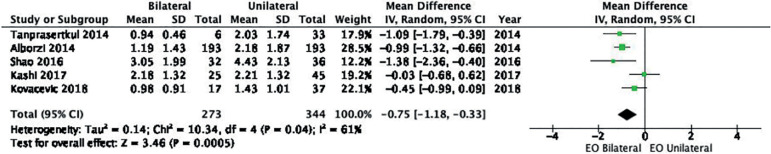
c. Long-term AMH in unilateral versus bilateral endometriomas
Five studies were included in the analysis, with a total of 273 patients with bilateral endometriomas and 344 with unilateral endometriomas. Random effects analysis showed lower post-operative AMH levels in patients with bilateral endometriomas compared with subjects with unilateral endometriomas in the long-term (MD of -0.75, 95% CI -1.18, -0.33; I2 = 61%, Figure 8). The quality of the evidence was low according to GRADE.
Figure 8.
Forest plot long-term AMH in unilateral versus bilateral endometriomas.
III. Effect of laparoscopic surgery for endometrioma on ovarian reserve compared with benign ovarian conditions
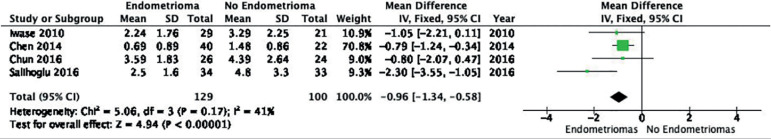
a. Post-operative short-term AMH in subjects with endometrioma versus benign ovarian disease
The four studies included in the analysis reported lower post-operative AMH levels in patients with endometriomas versus subjects without endometriomas in the short-term (MD of -0.96, 95% CI -1.34, -0.58; I2 = 41%, Figure 9). Fixed effect analysis included 129 patients in the endometrioma group and 100 in the non-endometrioma group. The quality of the evidence was low according to GRADE.
Figure 9.
Forest plot short-term post-operative AMH in endometriomas versus other benign ovarian pathology.
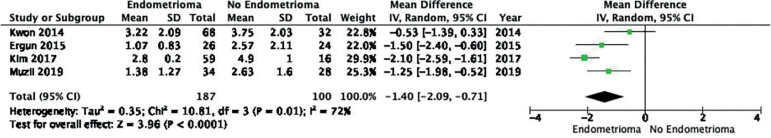
b. Post-operative medium-term AMH in subjects with endometrioma versus benign ovarian disease
Random effects analysis comparing four studies with a total of 187 patients in the endometrioma group and 100 in the non-endometrioma group showed lower post-operative AMH levels in patients with endometriomas in the medium-term (MD of -1.40, 95% CI -2.09, -0.71; I2 = 72%, Figure 10). The quality of the evidence was low according to GRADE.
Figure 10.
Forest plot AMH post-operative medium term in endometriomas versus benign ovarian pathology.
IV. Effect of laparoscopic cystectomy on ovarian reserve according to lesion size
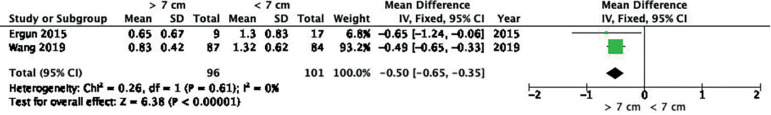
a. Post-operative AMH in endometriomas greater than 7 cm versus smaller than 7 cm
The comparison of two studies with a total of 96 patients included in the group of endometriomas greater than 7 cm and 101 in the group of endometriomas measuring less than 7 cm presented evidence of moderate quality according to GRADE. Fixed effects analysis showed lower post-operative AMH levels in patients with endometriomas greater than 7 cm in the short term (DM of -0.50, 95% CI -0.65, -0.35; I2 = 0%, Figure 11).
Figure 11.
Forest plot AMH post-operative in endometriomas greater than 7 cm versus less than 7 cm.
V. Effect of laparoscopic surgical technique on ovarian reserve in patients with endometriomas
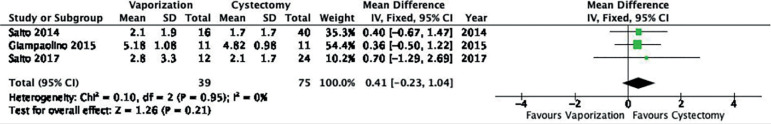
a. Post-operative AMH after vaporization with bipolar energy versus unilateral cystectomy
Three studies were included in the analysis, with a total of 39 patients included in the group treated with bipolar energy vaporization and 75 in the unilateral cystectomy group. Fixed effects analysis showed no significant differences between means when comparing AMH levels post-vaporization with bipolar energy versus unilateral cystectomy (MD of 0.41, 95% CI -0.23, 1.04; I2 = 0%, Figure 12). The quality of the evidence was moderate according to GRADE.
Figure 12.
Forest plot AMH post-operative post vaporization with bipolar energy versus unilateral cystectomy.
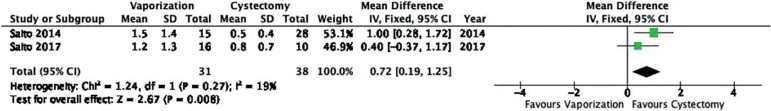
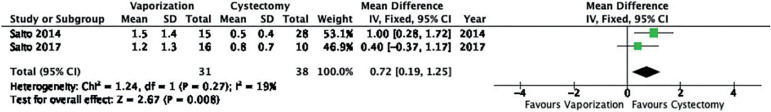
b. Post-operative AMH after vaporization with bipolar energy versus bilateral cystectomy
The analysis of two studies with only 31 patients in the group treated with bipolar energy vaporization and 38 in the group treated with bilateral cystectomy showed higher post-operative AMH levels after post-vaporization with bipolar energy (DM of 0.72, 95% CI 0.19, 1.25; I2 = 19%, Figure 13). The quality of the evidence was high according to GRADE.
Figure 13.
Forest plot AMH post-operative post vaporization with bipolar energy versus bilateral cystectomy.
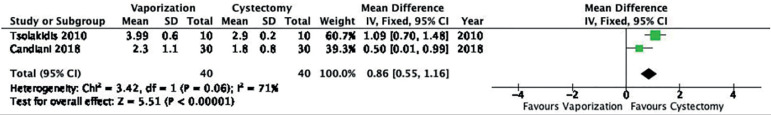
c. Post-operative AMH after laser vaporization versus cystectomy
The analysis of 40 patients included in each group from two studies revealed higher post-operative AMH levels after laser vaporization versus cystectomy (MD of 0.86, 95% CI 0.55, 1.16; I2 = 71%, Figure 14). The quality of the evidence was moderate according to GRADE.
Figure 14.
Forest plot AMH post-operative post laser vaporization versus cystectomy.
VI. Effect of hemostatic technique during laparoscopic endometrioma surgery on ovarian reserve
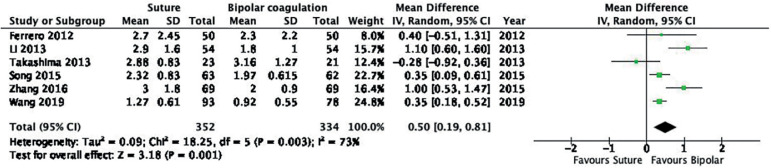
a. Post-operative AMH based on sutured hemostasis versus bipolar energy hemostasis
Six studies were included in the analysis, with a total of 352 patients treated with sutured hemostasis and 344 with bipolar energy hemostasis. Random effects analysis showed higher post-operative AMH levels after sutured hemostasis (MD of 0.50, 95% CI 0.19, 1.81; I2 = 73%, Figure 15). The quality of the evidence was moderate according to GRADE.
Figure 15.
Forest plot AMH post-operative according to hemostasis with suture versus hemostasis with bipolar energy.
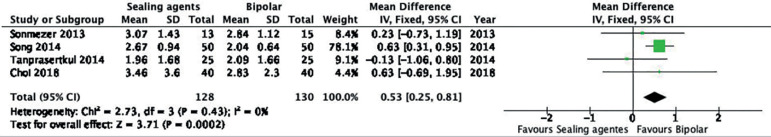
b. Post-operative AMH according to hemostasis with hemostatic agents versus hemostasis with bipolar energy
Four studies including a total of 128 patients treated with hemostatic agents and 130 with bipolar energy hemostasis revealed higher post-operative AMH levels after use of hemostatic agents according to fixed effects analysis (MD of 0.53, 95% CI 0.25, 0.81; I2 = 0%, Figure 16). The quality of the evidence was high according to GRADE.
Figure 16.
Forest plot AMH post-operative according to hemostasis treatment with hemostatic agentes versus hemostasis with bipolar energy.
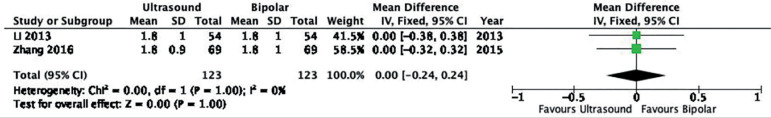
c. Post-operative AMH according to hemostasis with ultrasound versus hemostasis with bipolar energy
Two studies were included in the analysis, with a total of 123 patients in each group. Fixed effects analysis showed no significant differences between means when comparing post-operative AMH levels of patients offered hemostasis with ultrasound versus hemostasis with bipolar energy (MD of 0.00 (95% CI -0.24, 0.24; I2 = 0%, Figure 17). The quality of the evidence was high according to GRADE.
Figure 17.
Forest plot AMH post-operative according to hemostasis with ultrasound versus hemostasis with bipolar energy.
DISCUSSION
Main results
In this study, we systematically reviewed the effect of laparoscopic surgical treatment of endometriomas on ovarian reserve reported in 36 studies, including a total of 4374 patients. The results showed a significant decrease in short, medium and long-term post-operative AMH when compared with baseline AMH. However, there were no differences between short and long-term post-operative AMH levels, suggesting a non-significant recovery of AMH levels after one year of follow-up. Moreover, when the effect of laparoscopic cystectomy on ovarian reserve according to the laterality of the lesions was studied, a significant decrease in post-operative AMH was observed in the short, medium and long-term in bilateral endometriomas compared with unilateral endometriomas.
When evaluating the post-operative ovarian reserve of patients with endometriomas compared with other benign ovarian conditions, the decrease in AMH was significantly more pronounced in endometriomas, both in the short and medium term. In turn, the decrease in AMH was significantly greater when the size of the endometriomas exceeded 7 cm.
In relation to the effect of laparoscopic surgery on the ovarian reserve of patients with endometriomas, post-operative AMH levels were significantly lower after bilateral cystectomy when compared with vaporization with bipolar energy. Lower post-operative AMH levels were also observed after laser vaporization when compared with cystectomy. No difference was observed when comparing post-operative AMH of patients submitted to unilateral cystectomy versus bipolar energy vaporization.
Post-operative AMH also decreased significantly with bipolar energy hemostasis compared with suture and hemostatic agents. On the other hand, this difference was not significant when compared with hemostasis with ultrasound.
Strengths
Our study analyzed a large number of patients compared with previous reviews (Somigliana et al., 2012; Younis et al., 2019) because we included the results from several recent studies. Furthermore, a large proportion of the included studies were randomized clinical trials with good methodological quality. The included studies also stand out for having adjusted for important confounding variables such as surgical indication, fertility status, operating time, days of hospitalization, etc. These factors significantly increased the validity of our findings.
Limitations
Several studies were excluded due to language barriers and presentation of partial results or findings that did not match our inclusion criteria. However, it was not possible to contact all authors in order to gain access to their databases. Several retrospective observational studies were included, and it was not possible to know what the AMH measurement method was.
Serum levels are not altered with prior use of hormonal contraceptives or other drugs, although decreases have been observed during the administration of anovulatory drugs or GnRH analogues, an effect that must be considered, but which seems to disappear after withdrawal of these treatments.
A disadvantage of AMH is that there are several assay types for detection, making it difficult to compare levels obtained in different studies with different methods. In practice, the serum AMH level of a patient should be interpreted evaluating the method used and its cut-off levels (La Marca et al., 2010).
In addition, the meta-analysis showed some highly heterogeneous values through I2 for several of the primary outcomes, suggesting that the precision of the meta-analysis is of moderate quality for these results.
Comparison with other studies
Evolution of post-operative AMH
The decrease in ovarian reserve after endometrioma cystectomy has been described in numerous studies. However, these studies are highly heterogeneous and include different surgical techniques, follow-up periods, and measures to quantify the ovarian reserve.
In recent years, several authors have reported decreases in ovarian reserve sided by decreases in AMH after ovarian cystectomy (Chang et al., 2010; Ozaki et al., 2016). Nevertheless, some reports have observed a partial recovery of AMH in some women after three to 12 months of endometrioma removal (Sugita et al., 2013; Alborzi et al., 2014).
A prospective study followed up post-operative AMH levels at six and 12 months, observing a recovery of 36.4% and 72.2%, respectively, compared with baseline AMH (Vignali et al., 2015). Consistent with these studies, a recent systematic review reported a partial reversal of decreased post-operative AMH in the medium term compared with the short term, regardless of bilateralism (Younis et al., 2019). Our study, however, found no difference between post-operative AMH levels comparing the short with the long-term, after a follow-up of up to six months.
Two authors with similar findings found no differences, regardless of the surgical technique used (Chang et al., 2010; Ferrero et al., 2012). Our results should be evaluated with caution, in part due to the high heterogeneity observed during the analysis, as we wait for new studies reporting longer-term results.
In addition, some studies present limitations due to the use of combined contraceptives, since recent data indicate that they might decrease AMH levels temporarily (Kallio et al., 2013). However, it has been shown that a small portion of the patients included in each study group was on oral contraceptives patients, and that no difference was found when these patients were excluded from the analysis.
Evolution of post-operative AMH according to laterality
Our findings also indicated that greater decreases in post-operative AMH levels are observed after the resection of bilateral endometriomas compared with unilateral ones, as described in some studies (Hirokawa et al., 2011; Kim et al., 2017; Mehdizadeh Kashi et al., 2017) and refuted in others (Alborzi et al., 2014; Tanprasertkul et al., 2014; Ding et al., 2015a). However, these results are questionable due to the small number of patients studied, the loss of patients during follow-up, and the fact that surgical techniques were not evaluated.
A recent systematic review reported maximum decreases in post-operative AMH of 39.5% and 57.0% in individuals with unilateral and bilateral endometriomas, respectively (Younis et al., 2019).
Evolution of post-operative AMH according to the nature of the injury
Ovarian endometriomas per se might damage the ovarian reserve and cystectomy in patients with endometriomas cause extra damage to the ovarian reserve compared with other benign cysts (Muzii et al., 2019). This study observed that the decreases in post-operative AMH levels were greater in patients with endometriomas compared with benign ovarian cysts. However, a recent meta-analysis reported a similar magnitude of reduction of post-operative AMH for both groups of around 38% (Mohamed et al., 2016).
Evolution of post-operative AMH according to the size of the endometrioma
In agreement with our results, some studies have shown differences in post-operative AMH according to the size of endometriomas, with a more pronounced decrease observed in endometriomas measuring more than 5 cm in diameter (Wang et al., 2019). Conversely, other studies found no relationship between the diameter of the endometrioma and the percentage decrease in post-operative AMH. Many such studies enrolled small populations and took the laterality of the lesions into account in their analyses (Hirokawa et al., 2011; Celik et al., 2012; Uncu et al., 2013).
Evolution of post-operative AMH according to the surgical technique used
To date, there is no consensus on the effect of endometrioma decapsulation per se on ovarian reserve. A randomized clinical trial revealed that ablation of the cyst wall by vaporization with bipolar energy might be associated with better preservation of the ovarian reserve than cystectomy (Var et al., 2011). Another study revealed that ablation with plasma energy, in spite of producing similar decreases in post-operative AMH levels in the short term compared with cystectomy, yields a more significant long-term recovery (Roman et al., 2011).
In line with the above results, we agree that ablation by vaporization with both bipolar and laser energy is the technique of choice over cystectomy in patients wishing to preserve ovarian reserve for reproductive purposes.
Evolution of post-operative AMH according to the hemostatic technique used
The most used hemostatic techniques in endometrioma surgery are based on laser energy, bipolar energy or suture. However, a randomized clinical trial reported that post-operative AMH decreases significantly regardless of hemostatic method (Ferrero et al., 2012).
This has prompted other researchers to develop new methods to prevent ovarian damage. In a recent prospective study, bipolar coagulation was compared with suture, with the latter leading to better results in terms of preservation of the ovarian reserve (Song et al., 2015). The advantage of hemostatic suture includes lower cost and less biochemical complications compared with hemostatic sealants. Nonetheless, it might cause ischemic damage and adhesions.
Another study did not find a significant decrease in post-operative AMH when using hemostatic suture techniques, suggesting that a good surgical technique, without the use of bipolar energy, does not necessarily imply a reduction in ovarian reserve (Litta et al., 2013).
However, other studies found no significant differences in terms of changes in the post-operative ovarian reserve with either of the two hemostatic methods (Tanprasertkul et al., 2014; Takashima et al., 2013). Variations in surgical technique, surgeon experience or the different methods to measure AMH levels might explain the differences in the results described in the literature.
Another study compared the effect of hemostatic sealants and bipolar coagulation on post-operative ovarian reserve, concluding that short-term damage was more severe with bipolar coagulation. It was also reported that there were no significant differences in AMH levels between the two groups. Moreover, the levels of AMH were equal to the levels seen three months after surgery (Sönmezer et al., 2013). However, the sample size of this study was very small, limiting the conclusions derived from its results. A subsequent study was the first to show statistically significant differences in terms of less post-operative decrease in AMH at three months using hemostatic sealants compared with bipolar energy (Choi et al., 2018).
According to the results obtained in our study, three systematic reviews with meta-analysis have suggested that the use of bipolar energy is associated with greater decreases in AMH compared with non-thermal hemostatic techniques, including suturing and sealing agents (Ding et al., 2015b; Ata et al., 2015; Deckers et al., 2018). In any case, our study has a larger sample size, which increases the precision of the results. Long-term follow-up studies are needed, as most only assess post-operative AMH in the medium term.
Interpretation of the Results
Many hypotheses have been formulated to explain the relationship between endometrioma excision and AMH reduction. Several authors demonstrated that the removal of ovarian endometriomas is usually more difficult due to the absence of a clear cleavage plane between the cyst capsule and the healthy ovarian parenchyma. This might cause accidental mechanical damage to the ovarian cortex causing loss of follicles and decreased ovarian reserve. On the other hand, the electrical damage of the hemostatic method used might cause microvascular and inflammatory damage to the residual ovarian tissue leading to acute damage, which might be recovered in the long-term due to revascularization and the reduction of inflammation. The relative recovery of AMH levels expressed in some articles (Sugita et al., 2013; Goodman et al., 2016) might also be attributed to a phenomenon of restructuring and growth of more primordial follicles mediated by angiogenic and inflammatory factors, especially when surgery is performed meticulously, avoiding the removal of healthy ovarian tissue. This data cannot be confirmed with our results, but long-term monitoring of the ovarian reserve of the patients might be helpful.
Regarding cyst laterality, the differences in AMH decrease after unilateral versus bilateral cystectomy might be attributed to the fact that a larger surgical procedure, such as the one associated with bilateral endometriomas, might imply, on the one hand, a greater use of suture and hemostasis, and on the other, an eventual removal of a greater proportion of healthy ovarian tissue (Alammari et al., 2017).
Cystectomy of endometriomas generate an apparently greater impact on ovarian reserve compared with cystectomy of other benign ovarian conditions, although it is difficult to determine whether the differences in the magnitude of the decrease in AMH levels are inherent to endometriosis or to the influence of surgical techniques (Alammari et al., 2017). One study postulated that endometrioma surgery led to greater damage to residual ovarian tissue compared with other types of cystectomies (Kitajima et al., 2011). Furthermore, theoretically the risk of accidental excision of healthy ovarian tissue is greater in endometrioma due to its inflammatory nature and the adhesions that the disease causes. A study found ovarian stroma in 80.3% of endometrioma samples, compare to only 17.2% of dermoid cyst samples (Muzii et al., 2019). Another study concluded that the ovarian volume was significantly lower 36 months after surgery for endometrioma than after dermoid cyst surgery (Exacoustos et al., 2004).
When the impact of the size of endometriomas was compared, it has been found that the ovarian cortex of endometriomas larger than 4 cm might have fibrosis and stromal loss, leading to significantly lower follicular density, compared with the contralateral ovary. In addition, the larger the cyst to be removed, the greater the amount of healthy ovarian tissue removed, and the greater the loss of ovarian reserve (Goodman et al., 2016; Wang et al., 2019). This indicates that the larger the diameter of the cyst, the greater the damage to the ovarian reserve, although this must be confirmed with further research.
Surgical technique and hemostasis
There are two possible mechanisms by which ovarian damage might occur during endometrioma surgery: mechanical damage and electrical damage to growing follicles. On the one hand, our results indicate that cystectomy is associated with a greater impact on ovarian reserve compared with ablation by different means. The mechanical damage and the resection of healthy tissue might explain the consequent loss of follicles (Wang et al., 2019).
However, it has been proposed that different hemostatic techniques used during cyst removal might compromise the vascularization of the remaining ovarian tissue and cause inflammation (Goodman et al., 2016). This might explain the recovery of AMH levels described in different articles (Vignali et al., 2015; Younis et al., 2019), since tissue revascularization and decreased inflammation might cause the improvement of the ovarian reserve. Although this finding could not be demonstrated in our study, possibly longer monitoring of AMH levels in operated patients might modify the results.
Implications of the study and future directions
Endometriomas are frequent findings in clinical practice and often require surgical treatment. Our results indicate that laparoscopic surgery negatively affects the ovarian reserve, and that bilateralism and size are compounding adverse factors. However, other factors that were not evaluated, such as surgeon experience and the influence of different methods to measure AMH, might also affect the decrease in ovarian reserve after surgery.
Furthermore, it is important to determine what constitutes optimal endometrioma management based on patient subtypes, and assess measures such as conservative management (ablation). Although it has been associated with less involvement of the ovarian reserve compared with cystectomy, it is ineffective in terms of gestation rates and exposes the patient to a higher risk of recurrence.
Based on our results, we conclude that patients should be advised and informed of all aspects related to different surgical techniques and both the potential risks of decreased ovarian reserve and possible recurrence tied to each different surgical technique. Special consideration must be given to infertile patients before undergoing IVF treatment. Importantly, not all studies showed that low AMH is associated with low pregnancy rates in patients undergoing IVF.
Therefore, surgical planning must be individualized according to patient symptoms and reproductive objectives; preference should be given to conservative methods if the patient wishes to preserve her fertility.
Taking into account that the size of endometrioma is associated with significant post-operative decreases in AMH levels and that endometriosis is a progressive disease, early detection and conservative management might prevent future reproductive problems. Patients with severe endometriosis and older patients wishing to get pregnant should be informed that surgery might cause irreversible damage to the ovaries (Lessey et al., 2018).
Future directions
In order to lessen the impact of surgery on AMH, new surgical techniques are under development. One of them is sclerotherapy guided by transvaginal ultrasound. In this technique, different substances are injected into the endometrioma in order to shrink it and make it lose its pathogenic potential (Gordts & Campo, 2019). Tetracycline, methotrexate and ethanol are among the sclerosing agents being studied, with promising results (Cohen et al., 2017). Local alternatives are also in development of the hormonal type in order to reduce the risks of surgery (Benagiano et al., 2016).
New techniques such as orthotopic transplantation of ovarian tissue or cryopreservation of ovarian tissue should be considered in patients with worse reproductive prognoses and individuals requiring surgery to manage their symptoms (Donnez et al., 2004; 2005). Researchers in this area should guide their efforts to develop randomized clinical trials with good methodological quality in order to obtain better quality of evidence. New clinical trials with long-term monitoring of ovarian reserve are required to define the impact of different endometrioma management strategies on AMH levels.
CONCLUSIONS
Laparoscopic resection of endometriomas produces deleterious effects on short, medium, and long-term post-operative AMH levels.
There are no differences in the decrease of post-operative AMH in the short and long-term; however, these results should be taken with caution due to the high heterogeneity of the studies included in the analysis.
Bilateral endometriomas and endometriomas greater than 7 cm are associated with a greater decrease in AMH levels compared with unilateral endometriomas and endometriomas measuring less than 7 cm.
Laparoscopic resection of endometriomas produces greater deleterious effects on post-operative AMH levels compared with other benign ovarian conditions.
Ovarian cystectomy is associated with greater post-operative AMH involvement than bipolar energy ablation in bilateral endometriomas and laser energy ablation in all endometriomas. When hemostatic techniques are compared, bipolar energy produces a greater decrease in post-operative AMH levels than suture and sealing agents.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Alammari R, Lightfoot M, Hur HC. Impact of Cystectomy on Ovarian Reserve: Review of the Literature. J Minim Invasive Gynecol. 2017;24:247–257. doi: 10.1016/j.jmig.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Alborzi S, Keramati P, Younesi M, Samsami A, Dadras N. The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometriomas. Fertil Steril. 2014;101:427–434. doi: 10.1016/j.fertnstert.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Asgari Z, Rouholamin S, Hosseini R, Sepidarkish M, Hafizi L, Javaheri A. Comparing ovarian reserve after laparoscopic excision of endometriotic cysts and hemostasis achieved either by bipolar coagulation or suturing: a randomized clinical trial. Arch Gynecol Obstet. 2016;293:1015–1022. doi: 10.1007/s00404-015-3918-4. [DOI] [PubMed] [Google Scholar]
- Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar dessication. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2015;22:363–372. doi: 10.1016/j.jmig.2014.12.168. [DOI] [PubMed] [Google Scholar]
- Benagiano G, Petraglia F, Gordts S, Brosens I. A new approach to the management of ovarian endometrioma to prevent tissue damage and recurrence. Reprod Biomed Online. 2016;32:556–562. doi: 10.1016/j.rbmo.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Biacchiardi CP, Piane LD, Camanni M, Deltetto F, Delpiano EM, Marchino GL, Gennarelli G, Revelli A. Laparoscopic stripping of endometriomas negatively affects ovarian follicular reserve even if performed by experienced surgeons. Reprod Biomed Online. 2011;23:740–746. doi: 10.1016/j.rbmo.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Candiani M, Ottolina J, Posadzka E, Ferrari S, Castellano LM, Tandoi I, Pagliardini L, Nocun A, Jach R. Assessment of ovarian reserve after cystectomy versus 'one-step' laser vaporization in the treatment of ovarian endometrioma: a small randomized clinical trial. Hum Reprod. 2018;33:2205–2211. doi: 10.1093/humrep/dey305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik HG, Dogan E, Okyay E, Ulukus C, Saatli B, Uysal S, Koyuncuoglu M. Effect of laparoscopic excision of endometriomas on ovarian reserve: serial changes in the serum antimüllerian hormone levels. Fertil Steril. 2012;97:1472–1478. doi: 10.1016/j.fertnstert.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Chang HJ, Han SH, Lee JR, Jee BC, Lee BI, Suh CS, Kim SH. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94:343–349. doi: 10.1016/j.fertnstert.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pei H, Chang Y, Chen M, Wang H, Xie H, Yao S. The impact of endometrioma and laparoscopic cystectomy on ovarian reserve and the exploration of related factors assessed by serum anti-Mullerian hormone: a prospective cohort study. J Ovarian Res. 2014;7:108–108. doi: 10.1186/s13048-014-0108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Kim WY, Lee DH, Lee SH. Usefulness of hemostatic sealants for minimizing ovarian damage during laparoscopic cystectomy for endometriosis. J Obstet Gynaecol Res. 2018;44:532–539. doi: 10.1111/jog.13542. [DOI] [PubMed] [Google Scholar]
- Chun S, Cho HJ, Ji YI. Comparison of early postoperative decline of serum antiMüllerian hormone levels after unilateral laparoscopic ovarian cystectomy between patients categorized according to histologic diagnosis. Taiwan J Obstet Gynecol. 2016;55:641–645. doi: 10.1016/j.tjog.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Cohen A, Almog B, Tulandi T. Sclerotherapy in the management of ovarian endometrioma: systematic review and meta-analysis. Fertil Steril. 2017;108:117–24.e5. doi: 10.1016/j.fertnstert.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Committee opinion no. 618: Ovarian reserve testing. Obstet Gynecol. 2015;125:268–273. doi: 10.1097/01.AOG.0000459864.68372.ec. [DOI] [PubMed] [Google Scholar]
- Deckers P, Ribeiro SC, Simões RDS, Miyahara CBDF, Baracat EC. Systematic review and meta-analysis of the effect of bipolar electrocoagulation during laparoscopic ovarian endometrioma stripping on ovarian reserve. Int J Gynaecol Obstet. 2018;140:11–17. doi: 10.1002/ijgo.12338. [DOI] [PubMed] [Google Scholar]
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive Assessment of the Impact of Laparoscopic Ovarian Cystectomy on Ovarian Reserve. J Minim Invasive Gynecol. 2015a;22:1252–1259. doi: 10.1016/j.jmig.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Ding W, Li M, Teng Y. The impact on ovarian reserve of haemostasis by bipolar coagulation versus suture following surgical stripping of ovarian endometrioma: a meta-analysis. Reprod Biomed Online. 2015b;30:635–642. doi: 10.1016/j.rbmo.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Dolmans MM, Martinez-Madrid B, Jadoul P, Van Langendonckt A. Orthotopic transplantation of fresh ovarian cortex: a report of two cases. Fertil Steril. 2005;84:1018–1018. doi: 10.1016/j.fertnstert.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Duffy JM, Arambage K, Correa FJ, Olive D, Farquhar C, Garry R, Barlow DH, Jacobson TZ. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014:CD011031–CD011031. doi: 10.1002/14651858.CD011031.pub2. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- Ercan CM, Sakinci M, Duru NK, Alanbay I, Karasahin KE, Baser I. Antimullerian hormone levels after laparoscopic endometrioma stripping surgery. Gynecol Endocrinol. 2010;26:468–472. doi: 10.3109/09513591003632134. [DOI] [PubMed] [Google Scholar]
- Ercan CM, Duru NK, Karasahin KE, Coksuer H, Dede M, Baser I. Ultrasonographic evaluation and anti-mullerian hormone levels after laparoscopic stripping of unilateral endometriomas. Eur J Obstet Gynecol Reprod Biol. 2011;158:280–284. doi: 10.1016/j.ejogrb.2011.04.043. [DOI] [PubMed] [Google Scholar]
- Ergun B, Ozsurmeli M, Dundar O, Comba C, Kuru O, Bodur S. Changes in Markers of Ovarian Reserve After Laparoscopic Ovarian Cystectomy. J Minim Invasive Gynecol. 2015;22:997–1003. doi: 10.1016/j.jmig.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Exacoustos C, Zupi E, Amadio A, Szabolcs B, De Vivo B, Marconi D, Elisabetta Romanini M, Arduini D. Laparoscopic removal of endometriomas: sonographic evaluation of residual functioning ovarian tissue. Am J Obstet Gynecol. 2004;191:68–72. doi: 10.1016/j.ajog.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, Boivin J. Female subfertility. Nat Rev Dis Primers. 2019;5:7–7. doi: 10.1038/s41572-018-0058-8. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Venturini PL, Gillott DJ, Remorgida V, Leone Roberti Maggiore U. Hemostasis by bipolar coagulation versus suture after surgical stripping of bilateral ovarian endometriomas: a randomized controlled trial. J Minim Invasive Gynecol. 2012;19:722–730. doi: 10.1016/j.jmig.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93. doi: 10.1016/j.ejogrb.2015.09.046. [DOI] [PubMed] [Google Scholar]
- Goodman LR, Goldberg JM, Flyckt RL, Gupta M, Harwalker J, Falcone T. Effect of surgery on ovarian reserve in women with endometriomas, endometriosis and controls. Am J Obstet Gynecol. 2016;215:589.e1–589.e6. doi: 10.1016/j.ajog.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Gordts S, Campo R. Modern approaches to surgical management of endometrioma. Best Pract Res Clin Obstet Gynaecol. 2019;59:48–55. doi: 10.1016/j.bpobgyn.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008:CD004992–CD004992. doi: 10.1002/14651858.CD004992.pub3. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa W, Iwase A, Goto M, Takikawa S, Nagatomo Y, Nakahara T, Bayasula B, Nakamura T, Manabe S, Kikkawa F. The post-operative decline in serum anti-Mullerian hormone correlates with the bilaterality and severity of endometriosis. Hum Reprod. 2011;26:904–910. doi: 10.1093/humrep/der006. [DOI] [PubMed] [Google Scholar]
- Hirsch M, Begum MR, Paniz É, Barker C, Davis CJ, Duffy J. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG. 2018;125:556–564. doi: 10.1111/1471-0528.14838. [DOI] [PubMed] [Google Scholar]
- Hwu YM, Wu FS, Li SH, Sun FJ, Lin MH, Lee RK. The impact of endometrioma and laparoscopic cystectomy on serum anti-Müllerian hormone levels. Reprod Biol Endocrinol. 2011;9:80–80. doi: 10.1186/1477-7827-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Nakamura T, Kato N, Goto M, Takikawa S, Kondo M, Osuka S, Mori M, Kikkawa F. Anti-Müllerian hormone levels after laparoscopic cystectomy for endometriomas as a possible predictor for pregnancy in infertility treatments. Gynecol Endocrinol. 2016;32:293–297. doi: 10.3109/09513590.2015.1114078. [DOI] [PubMed] [Google Scholar]
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99:1305–1310. doi: 10.1016/j.fertnstert.2012.11.034. [DOI] [PubMed] [Google Scholar]
- Kang JH, Kim YS, Lee SH, Kim WY. Comparison of hemostatic sealants on ovarian reserve during laparoscopic ovarian cystectomy. Eur J Obstet Gynecol Reprod Biol. 2015;194:64–67. doi: 10.1016/j.ejogrb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Cha SW, Kim HO. Serum anti-Müllerian hormone levels decrease after endometriosis surgery. J Obstet Gynaecol. 2017;37:342–346. doi: 10.1080/01443615.2016.1239071. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Khan KN, Hiraki K, Inoue T, Fujishita A, Masuzaki H. Changes in serum anti-Müllerian hormone levels may predict damage to residual normal ovarian tissue after laparoscopic surgery for women with ovarian endometrioma. Fertil Steril. 2011;95:2589–91.e1. doi: 10.1016/j.fertnstert.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Kline J, Kinney A, Kelly A, Reuss ML, Levin B. Predictors of antral follicle count during the reproductive years. Hum Reprod. 2005;20:2179–2189. doi: 10.1093/humrep/dei048. [DOI] [PubMed] [Google Scholar]
- Kovačević VM, Anđelić LM, Mitrović Jovanović A. Changes in serum antimüllerian hormone levels in patients 6 and 12 months after endometrioma stripping surgery. Fertil Steril. 2018;110:1173–1180. doi: 10.1016/j.fertnstert.2018.07.019. [DOI] [PubMed] [Google Scholar]
- Kwon SK, Kim SH, Yun SC, Kim DY, Chae HD, Kim CH, Kang BM. Decline of serum antimüllerian hormone levels after laparoscopic ovarian cystectomy in endometrioma and other benign cysts: a prospective cohort study. Fertil Steril. 2014;101:435–441. doi: 10.1016/j.fertnstert.2013.10.043. [DOI] [PubMed] [Google Scholar]
- La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- Lee DY, Young Kim N, Jae Kim M, Yoon BK, Choi D. Effects of laparoscopic surgery on serum anti-Müllerian hormone levels in reproductive-aged women with endometrioma. Gynecol Endocrinol. 2011;27:733–736. doi: 10.3109/09513590.2010.538098. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Gordts S, Donnez O, Somigliana E, Chapron C, Garcia-Velasco JA, Donnez J. Ovarian endometriosis and infertility: in vitro fertilization (IVF) or surgery as the first approach? Fertil Steril. 2018;110:1218–1226. doi: 10.1016/j.fertnstert.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Li CZ, Wei DY, Wang F, Wang HQ, Yang CR. Impact on ovarian reserve function by different homostasis methods during laparoscopic cystectomy in treatment of ovarian endometrioma. Zhonghua Fu Chan Ke Za Zhi. 2013;48:11–15. [PubMed] [Google Scholar]
- Litta P, D'Agostino G, Conte L, Saccardi C, Cela V, Angioni S, Plebani M. Anti-Müllerian hormone trend after laparoscopic surgery in women with ovarian endometrioma. Gynecol Endocrinol. 2013;29:452–454. doi: 10.3109/09513590.2012.758704. [DOI] [PubMed] [Google Scholar]
- Mehdizadeh Kashi A, Chaichian S, Ariana S, Fazaeli M, Moradi Y, Rashidi M, Najmi Z. The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometrioma. Int J Gynaecol Obstet. 2017;136:200–204. doi: 10.1002/ijgo.12046. [DOI] [PubMed] [Google Scholar]
- Mohamed AA, Al-Hussaini TK, Fathalla MM, El Shamy TT, Abdelaal II, Amer SA. The impact of excision of benign nonendometriotic ovarian cysts on ovarian reserve: a systematic review. Am J Obstet Gynecol. 2016;215:169–176. doi: 10.1016/j.ajog.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Muzii L, Di Tucci C, Di Feliciantonio M, Galati G, Pecorella I, Radicioni A, Anzuini A, Piccioni MG, Patacchiola F, Benedetti Panici P. Ovarian Reserve Reduction With Surgery Is Not Correlated With the Amount of Ovarian Tissue Inadvertently Excised at Laparoscopic Surgery for Endometriomas. Reprod Sci. 2019;26:1493–1498. doi: 10.1177/1933719119828055. [DOI] [PubMed] [Google Scholar]
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9:37–37. doi: 10.1186/s13048-016-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H, Auber M, Mokdad C, Martin C, Diguet A, Marpeau L, Bourdel N. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96:1396–1400. doi: 10.1016/j.fertnstert.2011.09.045. [DOI] [PubMed] [Google Scholar]
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21:804–810. doi: 10.1016/j.jmig.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Saito N, Yamashita Y, Okuda K, Kokunai K, Terai Y, Ohmichi M. Comparison of the impact of laparoscopic endometriotic cystectomy and vaporization on postoperative serum anti-Mullerian hormone levels. Asian J Endosc Surg. 2018;11:23–29. doi: 10.1111/ases.12412. [DOI] [PubMed] [Google Scholar]
- Salihoğlu KN, Dilbaz B, Cırık DA, Ozelci R, Ozkaya E, Mollamahmutoğlu L. Short-Term Impact of Laparoscopic Cystectomy on Ovarian Reserve Tests in Bilateral and Unilateral Endometriotic and Nonendometriotic Cysts. J Minim Invasive Gynecol. 2016;23:719–725. doi: 10.1016/j.jmig.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Handbook. 2013. https://gdt.gradepro.org/app/handbook/handbook.html
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647–g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- Shao MJ, Hu M, He YQ, Xu XJ. AMH trend after laparoscopic cystectomy and ovarian suturing in patients with endometriomas. Arch Gynecol Obstet. 2016;293:1049–1052. doi: 10.1007/s00404-015-3926-4. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Berlanda N, Benaglia L, Viganò P, Vercellini P, Fedele L. Surgical excision of endometriomas and ovarian reserve: a systematic review on serum antimüllerian hormone level modifications. Fertil Steril. 2012;98:1531–1538. doi: 10.1016/j.fertnstert.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Song T, Lee SH, Kim WY. Additional benefit of hemostatic sealant in preservation of ovarian reserve during laparoscopic ovarian cystectomy: a multi-center, randomized controlled trial. Hum Reprod. 2014;29:1659–1665. doi: 10.1093/humrep/deu125. [DOI] [PubMed] [Google Scholar]
- Song T, Kim WY, Lee KW, Kim KH. Effect on ovarian reserve of hemostasis by bipolar coagulation versus suture during laparoendoscopic single-site cystectomy for ovarian endometriomas. J Minim Invasive Gynecol. 2015;22:415–420. doi: 10.1016/j.jmig.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Sönmezer M, Taşkın S, Gemici A, Kahraman K, Özmen B, Berker B, Atabekoğlu C. Can ovarian damage be reduced using hemostatic matrix during laparoscopic endometrioma surgery? A prospective, randomized study. Arch Gynecol Obstet. 2013;287:1251–1257. doi: 10.1007/s00404-012-2704-9. [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Sugita A, Iwase A, Goto M, Nakahara T, Nakamura T, Kondo M, Osuka S, Mori M, Saito A, Kikkawa F. One-year follow-up of serum antimüllerian hormone levels in patients with cystectomy: are different sequential changes due to different mechanisms causing damage to the ovarian reserve? Fertil Steril. 2013;100:516–22.e3. doi: 10.1016/j.fertnstert.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Takashima A, Takeshita N, Otaka K, Kinoshita T. Effects of bipolar electrocoagulation versus suture after laparoscopic excision of ovarian endometrioma on the ovarian reserve and outcome of in vitro fertilization. J Obstet Gynaecol Res. 2013;39:1246–1252. doi: 10.1111/jog.12056. [DOI] [PubMed] [Google Scholar]
- Tanprasertkul C, Ekarattanawong S, Sreshthaputra O, Vutyavanich T. Impact of hemostasis methods, electrocoagulation versus suture, in laparoscopic endometriotic cystectomy on the ovarian reserve: a randomized controlled trial. J Med Assoc Thai. 2014;97:95–101. [PubMed] [Google Scholar]
- Tsolakidis D, Pados G, Vavilis D, Athanatos D, Tsalikis T, Giannakou A, Tarlatzis BC. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94:71–77. doi: 10.1016/j.fertnstert.2009.01.138. [DOI] [PubMed] [Google Scholar]
- Uncu G, Kasapoglu I, Ozerkan K, Seyhan A, Oral Yilmaztepe A, Ata B. Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Hum Reprod. 2013;28:2140–2145. doi: 10.1093/humrep/det123. [DOI] [PubMed] [Google Scholar]
- Var T, Batioglu S, Tonguc E, Kahyaoglu I. The effect of laparoscopic ovarian cystectomy versus coagulation in bilateral endometriomas on ovarian reserve as determined by antral follicle count and ovarian volume: a prospective randomized study. Fertil Steril. 2011;95:2247–2250. doi: 10.1016/j.fertnstert.2011.03.078. [DOI] [PubMed] [Google Scholar]
- Vignali M, Mabrouk M, Ciocca E, Alabiso G, Barbasetti di Prun A, Gentilini D, Busacca M. Surgical excision of ovarian endometriomas: Does it truly impair ovarian reserve? Long term anti-Müllerian hormone (AMH) changes after surgery. J Obstet Gynaecol Res. 2015;41:1773–1778. doi: 10.1111/jog.12830. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ruan X, Lu D, Sheng J, Mueck AO. Effect of laparoscopic endometrioma cystectomy on anti-Müllerian hormone (AMH) levels. Gynecol Endocrinol. 2019;35:494–497. doi: 10.1080/09513590.2018.1549220. [DOI] [PubMed] [Google Scholar]
- Younis JS, Shapso N, Fleming R, Ben-Shlomo I, Izhaki I. Impact of unilateral versus bilateral ovarian endometriotic cystectomy on ovarian reserve: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:375–391. doi: 10.1093/humupd/dmy049. [DOI] [PubMed] [Google Scholar]
- Zhang CH, Wu L, Li PQ. Clinical study of the impact on ovarian reserve by different hemostasis methods in laparoscopic cystectomy for ovarian endometrioma. Taiwan J Obstet Gynecol. 2016;55:507–511. doi: 10.1016/j.tjog.2015.08.026. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9–9. doi: 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]