Abstract
In recent years, the gram-negative bacterium Stenotrophomonas maltophilia has become increasingly important in biotechnology and as a nosocomial pathogen, giving rise to a need for new information about its taxonomy and epidemiology. To determine intraspecies diversity and whether strains can be distinguished based on the sources of their isolation, 50 S. maltophilia isolates from clinical and environmental sources, including strains of biotechnological interest, were investigated. The isolates were characterized by in vitro antagonism against pathogenic fungi and the production of antifungal metabolites and enzymes. Phenotypically the strains showed variability that did not correlate significantly with their sources of isolation. Clinical strains displayed remarkable activity against the human pathogenic fungus Candida albicans. Antifungal activity against plant pathogens was more common and generally more severe from the environmental isolates, although not exclusive to them. All isolates, clinical and environmental, produced a range of antifungal metabolites including antibiotics, siderophores, and the enzymes proteases and chitinases. From 16S ribosomal DNA sequencing analysis, the isolates could be separated into three clusters, two of which consisted of isolates originating from the environment, especially rhizosphere isolates, and one of which consisted of clinical and aquatic strains. In contrast to the results of other recent investigations, these strains could be grouped based on their sources of isolation, with the exception of three rhizosphere isolates. Because there was evidence of nucleotide signature positions within the sequences that are suitable for distinguishing among the clusters, the clusters could be defined as different genomovars of S. maltophilia. Key sequences on the 16S ribosomal DNA could be used to develop a diagnostic method that differentiates these genomovars.
Stenotrophomonas maltophilia (29), previously referred to as Pseudomonas maltophilia (18) and Xanthomonas maltophilia (35), is ubiquitous in a wide variety of environments and geographical regions, and it occupies various ecological niches (11). This bacterium is often associated with plants and has been isolated from numerous diverse rhizospheres (4, 6, 22). Investigations have indicated a potential role for this species in biotechnology, e.g., as a biological control agent of fungal plant pathogens in agriculture (5, 20, 25) and in bioremediation (8). In the last decade, S. maltophilia has also become important as a nosocomial multidrug-resistant pathogen associated with significant case/fatality ratios in certain patient populations, particularly those who are severely debilitated or immunosuppressed (for a review, see reference 12). S. maltophilia is now the second most frequently isolated nosocomial bacterium after Pseudomonas aeruginosa (39).
Several physiological and molecular studies have revealed considerable heterogeneity among strains tentatively classified as S. maltophilia. Palleroni and Bradbury (29) highlighted the high intraspecies diversity in the type description of S. maltophilia; the physiological parameters also displayed a wide range of heterogeneity (35, 38) which was later confirmed by genotypic studies (9, 17). Nesme et al. (27) assumed that S. maltophilia species form a less coherent taxon as indicated by evidence of different restriction endonuclease sites within the 16S rRNA genes. Epidemiological studies have suggested that the majority of patients with S. maltophilia infections had unique types of S. maltophilia, indicating that most infections were independently acquired (12). Today, it is still unclear how S. maltophilia finds its way into the clinical environment, and it is also not possible to distinguish the sources of the strains (7, 12, 17).
Although there have been many reports in recent years on the antifungal activity of and production of antifungal compounds by environmental Stenotrophomonas isolates (6, 19, 25), there has been no report on the antifungal properties of clinical isolates. Therefore, the question remains whether strains present in hospitals possess antifungal properties similar to those of environmental isolates.
Berg et al. (7) investigated S. maltophilia strains of clinical and environmental origin by phenotypic profiling and by molecular methods. All strains were identified as S. maltophilia but with considerable variability in their properties. The question then arose as to how closely related the strains were.
We investigated 50 isolates of S. maltophilia with different clinical and environmental origins, including some strains of biotechnological interest and type strain DSMZ 50170 (18). Various phenotypic methods included analyses of in vitro activity against human and plant pathogenic fungi and the production of antifungal metabolites and enzymes. Genotypic methods included a comparison of 50 complete 16S rRNA gene sequences. With these methods, we developed a system that characterizes variability among S. maltophilia species as well as distinguishing between clinical and environmental (aquatic and plant-associated) isolates.
MATERIALS AND METHODS
Strains.
A total of 50 isolates was investigated. Most of these isolates (c1 through 20 and e1 through e20) had already been characterized pheno- and genotypically (7). Strains c1 through c20 were isolated in the Rigshospitalet, Copenhagen, Denmark, from various patient sites (tracheal aspirates, sputa, blood, throat, wounds, skin, ulcers, drainage fluids and aspirates, catheters, urine, etc. [14]). Strains c21 through c25, originally isolated from humans, were obtained from a reference laboratory in Leipzig, Germany. The environmental aquatic (e-a) strains originated from a brackish lagoon (Zingster Strom) in the southern Baltic region (e-a1 and e-a2), from a sewage treatment plant in Brunswick, Germany (e-a21 and e-a22), and from eye care solution (e-a23 and e-a24) (L. Bader, K. G. Riedel, G. Maydl, E. Ritter, C. Wirsing von König, A. Meroe, J. Billing, G. Hensel, and J. Heesemann. 1999. Augeninfekt. Herstell. kontam. intraokul. Spüllösung. abstr. 6P6, p. 241, 1999. Deutsche Gesellschaft für Hygiene und Microbiologie-Tagung, Regensburg, Germany.). The other environmental strains are plant associated (e-p) and were isolated from the rhizosphere of oilseed rape (e-p3 through e-p13), the rhizosphere of the potato (e-p14 through e-p16 and e-p20), and the geocaulosphere of the potato (e-p17 through e-p19) (7, 22). Strain e-p3 was used as a biocontrol agent against phytopathogenic fungi (6), and e-p8 has shown antifungal properties (6, 19). S. maltophilia DSMZ 50170 (ATCC 13637, type strain isolated from pleural fluid of a patient with oral carcinoma [18]) was used as a reference strain for comparison. All isolates were identified using the API system (BioMérieux, Mercy Etoile, France) and the BIOLOG identification system (Biolog Inc., Hayward, Calif.) (7).
Bioassay for antifungal activity in vitro.
Antifungal activity was determined by a dual-culture in vitro assay on Waksman agar (WA) containing 5 g of proteose-peptone (Merck, Darmstadt, Germany), 10 g of glucose (Merck), 3 g of meat extract (Chemex, München, Germany), 5 g of NaCl (Merck), 20 g of agar (Difco, Detroit, Mich.), and distilled water (to 1 liter) (pH 6.8). Zones of inhibition were measured after 5 days of incubation at 20°C by the method of Berg (4). All strains were tested in three independent replicates. Fungi used in this bioassay included Rhizoctonia solani, Verticillium dahliae, Sclerotinia sclerotiorum, and Candida albicans. The fungal strain R. solani DSMZ 63010 was obtained from the Deutsche Sammlung für Mikroorganismen and Zellkulturen GmbH, Braunschweig, Germany. The other pathogenic fungi were obtained from the strain collection of the Department of Microbiology, University of Rostock. These fungi were routinely grown on Sabouraud medium (Gibco, Paisley, United Kingdom) and stored in broth containing 15% glycerol at −70°C.
Production of antifungal secondary metabolites.
Antibiosis against V. dahliae by the bacterial strains was assayed on WA plates (15 ml) containing 5 ml of sterile culture filtrate (64-h culture, nutrient broth II [Sifin]). The pH was adjusted to between 7 and 8. A 5-mm plug from a V. dahliae agar plate was placed in the center of a WA plate. As a control, WA plates (20 ml) were similarly inoculated with mycelial plugs. Colony diameters were measured daily for 10 days, and the reduction in linear growth of the fungi was calculated. Siderophore production was assayed by the method of Schwyn and Neilands (33).
Production of lytic enzymes.
Colonies were screened by plating on chitin-agar plates containing 1.62 g of nutrient broth (Sifin), 0.5 g of NaCl, 6 g of M9 salts, 2 g of colloidal chitin, 0.1 mM CaCl2, 1 mM MgSO4, 3 nM Thiamin-HCl (all from Sigma, Deisenhofen, Germany), 15 g of Bacto Agar (Difco), and distilled water (to 1 liter). Clearance halos indicating chitin degradation or protease activity (nutrient agar [Sifin] containing 2% gelatine) were measured after 5 days of incubation at 30°C. β-1,3-Glucanase activity was determined by measuring the production of reducing sugars from laminarin (Fluka, Buchs, Switzerland) by the method of Daugrois et al. (10).
DNA preparation and amplification.
Bacterial DNA was prepared following the protocol of Andersen and McKay (3), modified for genomic DNA. 16S ribosomal DNA (rDNA) was amplified by PCR using the procaryote-specific forward primer 16F27 and reverse primer 16R1525 (numbering in Escherichia coli 16S rDNA sequence [21]) synthesized by MWG-Biotech, Ebersberg, Germany. A fifty-microliter reaction mixture contained at least 100 ng of genomic DNA (in 10 mM Tris-HCl [pH 8]), 0.2 μM each primer, and PCR SuperMix High Fidelity (Gibco, Eggenstein, Germany). PCR reactions were performed in a peltier thermal cycler PTC-200 (BIOzym, Oldendorf, Germany) using the following conditions: initial denaturation for 5 min at 95°C; 10 cycles of denaturation (30 s at 95°C), annealing (30 s at 52°C), and extension (1.5 min at 70°C); 20 cycles of the same program to prolong the extension by 10 s per cycle; and a final extension of 5 min at 70°C. The PCR products were separated on a 0.8% agarose gel in TAE (40 mM Tris-acetate, 1 mM EDTA). The amplified bands of 16S rDNA were eluted from the agarose and purified by GFX PCR DNA and the Gel Band purification kit (Amersham Pharmacia Biotech, Piscataway, N.J.) following the manufacturer's instructions. The elution efficiency was estimated by electrophoresis.
DNA cloning.
16S rDNA fragments were cloned into the pGEM-T vector (Promega, Madison, Wis.), a linear plasmid with T overhangs at both ends. The 10-μl ligation mixture contained 50 ng of vector, at least 100 ng of PCR product, 3 U of T4 DNA ligase per μl, and 5 μl of 2× buffer (Rapid Ligation kit; Promega). The mixture was incubated for 2 h at room temperature and overnight at 4°C. Preparation of competent E. coli cells and transformation were carried out using Hanahan's protocol (16) with slight modifications. The complete ligation mixture was transformed into 200 μl of competent cells by heat shock for 1.5 min at 42°C. Luria-Bertani broth (LB) medium (800 μl; Difco) was added, and the suspension was shaken at 37°C for 1 h. The efficiency of transformation was tested by blue-white screening on ampicillin agar plates (100 μg of ampicillin per ml, 1.2 mg of isopropyl-β-d-thiogalactopyranosid, and 1.0 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside in N,N-dimethylformamid per plate). Positive colonies were picked and boiled for 5 min and then used for PCR as described previously. Plasmid-specific primers USP 5′- GTA AAA CGA CGG CCA GT -3′ (universal sequencing primer, acting here as the forward primer) and RSP 5′- CAG GAA ACA GCT ATG ACC -3′ (reverse sequencing primer) were used.
DNA sequencing.
Plasmid isolation was carried out using the GFX Micro Plasmid Prep kit (Amersham Pharmacia Biotech). Cells of a single clone were incubated overnight at 37°C in LB medium. After cell lysis, the DNA was adsorbed onto the fiberglass column and washed according to the manufacturer's instructions. The DNA was eluted from the column in 100 μl of 10 mM Tris-HCl. The 16S rDNA fragment was sequenced with the SequiTherm EXCEL II Long Read DNA sequencing kit-LC (BIOzym) according to the method of Sanger et al. (31). The gel run was performed using a LI-COR automated DNA-sequencing machine (MWG-Biotech). In addition to the plasmid-specific primers USP and RSP, the procaryote-specific forward primers 16F530 and 16F926 and reverse primers 16R519 and 16R907 (21) were used as infrared dye-labeled dideoxyoligonucleotides. The sequence data were collected and analyzed with the MWG-Biotech software package BaseImagIR version 4.1.
16S rRNA gene sequence comparison and phylogenetic analysis.
The 16S rDNA sequence of each strain was aligned with 16S rRNA gene sequences from the GenBank EMBL, and DBJ sequence databases using the BLAST algorithm (1). Sequence similarities were calculated for the complete sequence using unambiguously determined nucleotide positions with the sequence alignment program ALIGN Plus version 2.0 (Scientific and Educational Software). Distance and bootstrap analyses were performed with Clustal X (36) using the neighbor-joining method (30). Dendrograms were constructed with the TreeView version 1.5 software program (28). The results were verified by ARB software using maximum parsimony and maximum likelihood methods (13).
Statistical data analysis.
The physiological-analysis data were converted to a binary code, and interisolate relationships were measured by the Euclidian metric unweighted pair-group average method using the program STATISTICA (StatSoft, Hamburg, Germany).
Nucleotide sequence accession numbers.
The nucleotide sequences of 14 16S rDNAs from the isolates investigated in this study (e-p14, e-p17, e-p10, e-p3, e-p19, e-p20, c5, c6, c20, e-a21, e-a1, e-a23, e-p13, and e-a22) have been deposited in the EMBL data library under accession numbers AJ293461 to AJ293474.
RESULTS
Antifungal activity and production of antifungal metabolites.
Table 1 shows the results of in vitro tests of activity against the human pathogenic fungus C. albicans and the plant pathogens V. dahliae, S. sclerotiorum (ascomycetes with a chitin-glucan-containing cell wall), and R. solani (a basidiomycete with a chitin-glucan-containing cell wall). Generally, the fungi grew as well as the Stenotrophomonas isolates on WA. Inhibition was clearly discerned by limited growth or the complete absence of fungal mycelium in the inhibition zone surrounding a bacterial colony. Although nearly half of the isolates were antifungal, they showed a high variability in their activity. Only one clinical strain (c10) was active against plant pathogenic fungi. However, 8 of the 25 strains (32%) were active against the human-associated pathogen C. albicans. In contrast, 62% of the environmental isolates demonstrated antifungal activity against the chosen plant pathogenic fungi, while only 21% were effective against C. albicans. Thirteen isolates showed activity against only one fungus in vitro. Four environmental isolates (e-p8, e-p12, e-p13, and e-p14) were active against all of the tested fungi. The majority of the isolates were active against V. dahliae and C. albicans (16 and 13 active strains, respectively).
TABLE 1.
In vitro activity against plant- and human-pathogenic fungi and production of antagonistic agents by S. maltophilia isolates
| Strain no. | Antagonism towarda
|
Production of
|
Group by antifungal activityg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antifungal metabolites
|
Enzymes
|
|||||||||
| C. albicans | R. solani | S. sclerotiorum | V. dahliae | Antibioticsb | Siderophoresc | Proteasesd | Glucanasese | Chitinasesf | ||
| c1 | − | − | − | − | ++ | + | + | − | + | 1 |
| c2 | − | − | − | − | +++ | + | + | − | + | 1 |
| c3 | − | − | − | − | ++ | + | + | − | + | 1 |
| c4 | − | − | − | − | +++ | + | + | − | + | 1 |
| c5 | + | − | − | − | +++ | + | + | − | + | 1 |
| c6 | − | − | − | − | +++ | + | + | − | + | 1 |
| c7 | − | − | − | − | +++ | + | + | − | + | 1 |
| c8 | ++ | − | − | − | +++ | + | + | − | + | 1 |
| c9 | − | − | − | − | +++ | + | + | − | + | 1 |
| c10 | − | +++ | − | +++ | +++ | + | + | − | + | 3 |
| c11 | + | − | − | − | +++ | + | + | − | + | 1 |
| c12 | ++ | − | − | − | +++ | + | + | − | + | 1 |
| c13 | − | − | − | − | +++ | + | + | − | + | 1 |
| c14 | + | − | − | − | +++ | + | + | − | + | 1 |
| c15 | − | − | − | − | + | + | + | − | + | 1 |
| c16 | + | − | − | − | ++ | + | + | − | + | 1 |
| c17 | − | − | − | − | ++ | + | + | − | + | 1 |
| c18 | + | − | − | − | +++ | + | + | − | + | 1 |
| c19 | − | − | − | − | +++ | + | + | − | + | 1 |
| c20 | + | − | − | − | +++ | + | + | − | + | 1 |
| c21 | − | − | − | − | +++ | + | + | + | + | 1 |
| c22 | − | − | − | − | +++ | + | + | ++ | + | 2 |
| c23 | − | − | − | − | +++ | + | + | − | + | 1 |
| c24 | − | − | − | − | +++ | + | + | ++ | + | 1 |
| c25 | − | − | − | − | +++ | + | + | ++ | + | 1 |
| e-a1 | − | − | − | − | +++ | + | + | − | + | 1 |
| e-a2 | − | − | − | − | +++ | + | + | − | + | 1 |
| e-p3 | +++ | +++ | +++ | +++ | + | + | + | + | 4 | |
| e-p4 | +++ | − | +++ | +++ | + | + | − | + | 3 | |
| e-p5 | ++ | − | − | + | +++ | + | + | − | + | 1 |
| e-p6 | − | − | − | + | +++ | + | + | + | + | 1 |
| e-p7 | − | − | − | + | +++ | + | + | − | + | 1 |
| e-p8 | +++ | +++ | +++ | +++ | +++ | + | + | − | + | 5 |
| e-p9 | − | − | − | + | +++ | + | + | − | + | 1 |
| e-p10 | − | ++ | ++ | ++ | +++ | + | + | + | + | 4 |
| e-p11 | − | ++ | ++ | ++ | + | + | + | − | + | 4 |
| e-p12 | +++ | +++ | +++ | +++ | + | + | + | − | + | 5 |
| e-p13 | +++ | +++ | +++ | +++ | + | + | + | − | + | 5 |
| e-p14 | ++ | +++ | +++ | ++ | ++ | + | + | + | + | 4 |
| e-p15 | − | − | − | + | ++ | + | + | − | + | 1 |
| e-p16 | − | − | − | + | + | + | + | − | + | 1 |
| e-p17 | − | − | − | − | + | + | + | − | + | 1 |
| e-p18 | − | − | − | − | +++ | + | + | − | + | 1 |
| e-p19 | − | − | − | − | +++ | + | + | − | + | 1 |
| e-p20 | − | + | − | + | +++ | + | + | + | − | 1 |
| e-a21 | − | − | − | − | +++ | + | + | + | + | 2 |
| e-a22 | − | − | − | − | +++ | + | + | + | + | 1 |
| e-a23 | − | − | − | − | +++ | + | + | + | + | 1 |
| e-a24 | − | − | − | − | +++ | + | + | + | − | 1 |
| DSMZ 50170 | − | − | − | − | +++ | + | + | − | + | 1 |
Dual-culture assay results: −, no antagonism; +, 0-5-mm-wide zone; ++, 5- to 10-mm-wide zone; +++, >10-mm-wide zone of inhibition.
Antibiosis (sterile filtrate test against V. dahliae) inhibition zones: +++, 100–60%, ++, 60–30%, +, 30–1%.
Siderophore activity: +++, 20-mm-wide orange zone; ++, 5 to 20-mm-wide orange zone; +, 5 to 3-mm orange zone.
Protease activity plate assay: +, hydrolysis; −, no hydrolysis.
β-1,3-Glucanase activity: ++, >10 U ml−1; ++, 10 to 1 U ml−1; +, 0.1 to 1 U ml−1; −, no activity.
Chitinase activity plate assay: +, hydrolysis; −, no hydrolysis.
Grouping on the basis of 75% similarity according to cluster analysis.
Mechanisms of fungal inhibition were elucidated by tracing secondary-metabolite production and fungal cell wall-degrading activity (Table 1). Antibiotic effects and siderophore production were shown for all strains. In addition, all strains were able to produce proteases in various amounts. Four out of 25 (16%) clinical and 9 out of 24 (38%) environmental strains showed fungal cell-wall-degrading enzyme β-1,3-glucanase activity. With the exception of strains e-p20 and e-a24, chitinolytic activity was detected for all Stenotrophomonas strains. In summary, the potential mode of action was strain dependent.
16S rDNA gene sequencing.
By using primers annealing at the 9- through 27-bp forward and 1,525- through 1,545-bp reverse positions of the 16S rDNA, nearly the complete gene could be sequenced as based on a comparison with the E. coli 16S rRNA gene sequence. Between 1,531 and 1,534 bases were determined, corresponding to about 99.4% of the gene. Each gene sequence was confirmed by determining contiguous overlapping sequences.
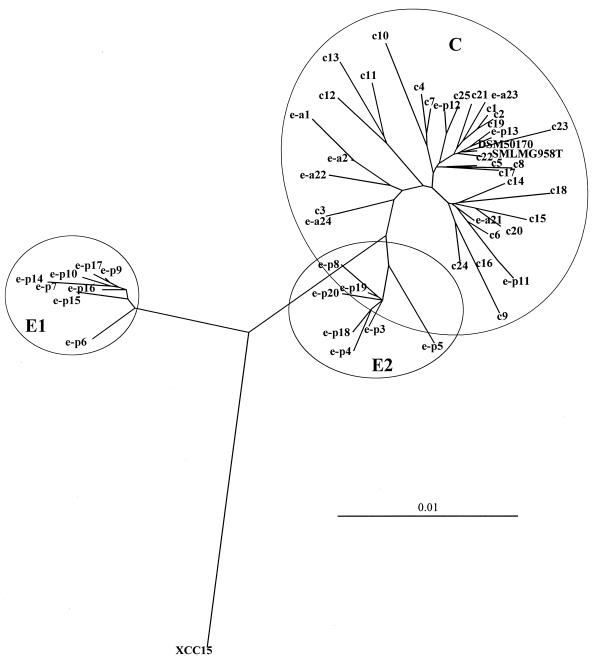
By the BLAST algorithm, all strains could be determined as S. maltophilia with 98 to 99% certainty. The most similar S. maltophilia strains were LMG 958T (EMBL accession number X95923) and LMG 957 (EMBL accession number AJ131114) for the clinical isolates and LMG 11087 (EMBL accession number X95924) for some environmental strains. Because of phylogenetic nearness to the genus Xanthomonas, Xanthomonas campestris strain XCC15 (GenBank accession number AF123092.2) was submitted to sequence investigation. It was found to be the closest to S. maltophilia according to analysis with BLAST. Figure 1 shows the inferred phylogenetic relationship between the investigated strains, S. maltophilia LMG 958T and X. campestris strain XCC15. At an identity level of about 99.5%, three clusters could be distinguished. The clinical isolates were grouped on their own in cluster C. Nine environmental strains (e-a1, e-a2, e-p11, e-p12, e-p13, e-a21, e-a22, e-a23, and e-a24) were included in cluster C, with e-a1, e-a2, and e-a22 forming a separate group within cluster C. The other environmental strains could be ordered into two clusters, E1 and E2, with about 2.0% and 0.5% differences from the clinical cluster, respectively.
FIG. 1.
Inferred phylogenetic relationships among the investigated S. maltophilia isolates. Evolutionary distances were calculated from pairwise sequence comparison using CLUSTAL X (neighbor-joining method). The dendrogram was constructed with TREEVIEW 1.5 software.
A pairwise comparison of the sequence data and the similarity data for S. maltophilia LMG 958T, X. campestris XCC15, and E. coli showed that clinical strains had average sequence similarities of 99.2% to each other and the reference strain DSM 50170. The lowest values were obtained with strain c18. The environmental strains showed an average similarity of 98.5% to each other, for which cluster E1 strains had the lowest values. Within the E1 and E2 clusters, the similarity was about 99.5%, with a 2.2% difference between the clusters. Differences with clinical cluster C were 1.1% in cluster E2 and 3.3% in E1. The average similarities to XCC15 and E. coli were 96.5 and 82.6%, respectively, for clinical cluster C, 96.8 and 82.9%, respectively, for E2, and 97.4 and 83.7%, respectively, for E1.
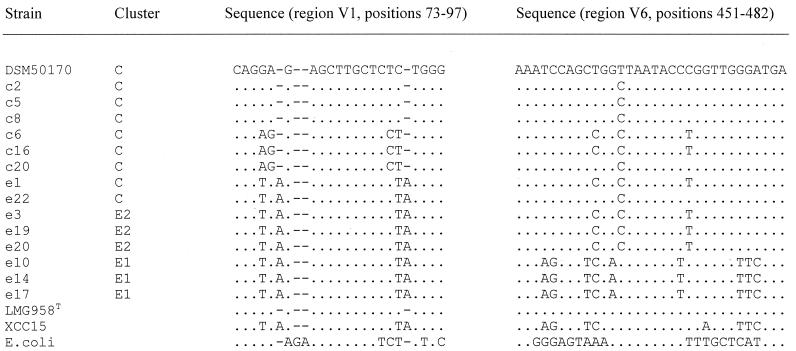
Key sequences of typical strains could be used to differentiate between the clinical and the environmental as well as between the two environmental clusters. These differences were most prominent in the variable regions: V1 with helix 6 (E. coli positions 69 through 100) had four variable positions; V2 (E. coli positions 143 through 222) had three to seven variable positions; V6 (E. coli positions 447 through 487) had one and three to nine variable positions; V7 (E. coli positions 589 through 650) had three to five variable positions; and V9 (E. coli positions 1,240 through 1,298) had four variable positions. As examples, the variable regions V1 and V6 are represented in Fig. 2. In these regions mismatches were found to be distributed throughout all sequences. In the V1 region, the clinical strains formed two different groups, whereas the aquatic and environmental isolates were more homologous and fitted in with the X. campestris strain XCC15. However, some exceptions existed; e-p5, e-p11, e-p12, e-p13, and e-a23 were found to be similar to one of the clinical groups (data not shown). In V6, the clinical and E2 cluster strains were not distinguishable. The highest variability was expressed by the strains of cluster E1. In variable regions V2 and V9, only the cluster E1 strains showed divergent base compositions. Additionally, these strains were different not only in isolated positions of semivariable regions but also in some universal regions such as positions 983 and 1,211.
FIG. 2.
Sequences for the hypervariable regions V1, including helix 6, and V6 of selected S. maltophilia strains from each cluster.
DISCUSSION
The investigated S. maltophilia strains showed high variability in their in vitro activity against pathogenic fungi and the production of antifungal metabolites and enzymes. Overall, more environmental isolates than clinical isolates showed antifungal activity. Clinical strains demonstrated remarkable activity against the human pathogenic fungus C. albicans. Antifungal activity against plant pathogens was more common and generally more severe in the environmental isolates, but it was not exclusive to this group. These investigations confirmed the strain specificity of the antifungal activity found for other species, e.g., Pseudomonas fluorescens (4, 26). In addition, the homogeneity of the investigated strains for the production of potential antifungal metabolites is of interest. The known mode of action for S. maltophilia is the production of antifungal agents, e.g., maltophilin, a novel macrocyclic lactam agent (19); xanthobaccins (25); siderophores with unknown structure (6); and the β-1, 3-glucanase, chitinase (6), and protease (12) lytic enzymes. Note that the antifungal mode of action of plant-associated bacteria is often strain specific (20, 25, 26). In conclusion, no significant differences in antifungal features were observed between clinical and environmental isolates, but the environmental population showed more diverse reactions than did the clinical group.
16S rDNA sequencing allowed the investigated clinical, aquatic, and plant-associated S. maltophilia strains to be distinguished. Two clusters with only environmental isolates (E1 and E2) and one with all of the clinical and a few of the environmental strains (C) were defined. While the isolates in clusters E1 and E2 were exclusively derived from rhizospheres, the few environmental strains in cluster C were isolated from a brackish lagoon (e-a1 and e-a2), from the rhizosphere of oilseed rape (e-p11, e-p12, and e-p13), from sewage (e-a21 and e-a22), and from eye care solution (e-a23 and e-a24). Because the eye care solution strains caused endophthalmitis after application in the clinic (Bader et al., Augeninfekt. Herstell. Kontam. intraokul. Spüllösung, abstr. 6P6), they could also be classified as clinical strains. The same is true for strains isolated from sewage containing a high input of communal and clinical waters and from the brackish lagoon, which acts as a prefilter basin in the Baltic Sea, with a high amount of sewage from households and hospitals (32). A comparison of these results with the investigated phenotypic features revealed that these six aquatic strains had the same characteristics as clinical isolates and could therefore be ordered together. A high degree of genetic similarity of the original environmental-rhizosphere isolates to clinical strains was shown by strains e-p11, e-p12, and e-p13. These three strains could strongly inhibit fungal growth, but they must have been placed in the clinical cluster according to 16S rDNA sequence analysis. Thus, strains e-p11, e-p12, and e-p13 were the only exceptions, which makes the exact differentiation between clinical-aquatic and rhizosphere isolates by 16S rDNA sequence analysis impossible.
Cluster E1 tended to be more closely related to the xantomonads. However, S. maltophilia was formerly classified as X. maltophilia (35), but because of significant physiological and genotypic differences with other Xanthomonas strains, a new genus was defined (29). Moore et al. (23) showed a 3% difference in 16S rDNA sequences between the two genera, which corresponded to 45 to 68 nucleotide bases. We report here that we obtained 48 different nucleotides out of 1,534 between the S. maltophilia type strain DSM 50170 and X. campestris XCC15, which exactly corresponds to 3%. Normally, sequence differences of about 3% suggest that strains belong to different species, not different genera. With 3% as the yardstick to differentiate between two genera, the isolates of genomovar E1 and eventually of E2 could in future be regarded as different species within the Stenotrophomonas complex. Recent investigations have raised the possibility of various different species existing in this group (17, 27). Stackebrandt and Goebel (34) considered the limitations of 16S rDNA sequence data for defining bacterial identities. Especially at sequence homologies over 99%, DNA-DNA hybridization of the whole genome is still the requirement most often proposed to describe new species (23).
The relatively high number of isolates in each cluster (eight in E1 and seven in E2) contradicts the hypothesis that nucleotide differences are random mutations independent of evolutionary pressure. In these clusters, the base changes can be considered as “signature positions” which are characteristic for the individual clusters. Hence, the clusters can be regarded as genomovars in the S. maltophilia complex with their own type strains (37). The sequence variable “hot spots” were regions V1, V2, V6, V7, and V9. The V6 region was the only one that allowed differentiation of cluster E1 within a narrow range of nucleotides. Most clinical strains had only one mismatch, but some had two additional changes, which corresponded to cluster E2. Cluster E1 strains differed in nine different positions. Because of the close proximity of the variable positions within the V1 and V6 regions, these areas could be ideal for probe design for diagnostic and epidemiological analysis of clinical and environmental S. maltophilia isolates by, for example, in situ hybridization (2). Additionally, a larger section of the V6 region with suitable primers can be used in denaturing gradient gel electrophoresis (24). So, we can suggest variable regions on the 16S rDNA as potential differentiation markers between clinical and environmental S. maltophilia strains; however, simple and rapid identification methods suitable for standard pathology laboratories should be developed.
S. maltophilia has an ambivalent character, first as a biocontrol and bioremediation agent and second as a multiresistant pathogen in nosocomial infections. The question is whether the environment can serve as a potential origin of clinical S. malthophilia infections. Recent investigations showed a high genetic variability within the species without any correlation to the source of isolation (7, 16, 27). The diversity of genomic patterns obtained by different molecular fingerprinting techniques suggests that a reservoir of S. maltophilia strains exists in the environment, from which certain strains adapt as opportunists. In this study, we could separate the environmental, especially the rhizosphere, isolates from the clinical strains by 16S rDNA analysis, with the exception of three. This result complicates the accurate determination of the environmental source(s) for clinical S. maltophilia infections. Additionally, it is not known whether the investigated environmental strains can cause infections in humans or whether clinical strains originate from environmental S. maltophilia.
ACKNOWLEDGMENTS
We thank Hella Goschke for valuable technical assistance and Lutz Bader (Munich), Britta Bruun (Copenhagen), Petra Marten and Jana Lottmann (Rostock), Kornelia Smalla (Braunschweig), and Matthias Scholz (Leipzig) for providing the Stenotrophomonas strains.
The study was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson D G, McKay L L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983;46:549–559. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg G. Rhizobacteria of oilseed rape antagonistic to Verticillium dahliae. J Plant Dis Protect. 1996;103:20–30. [Google Scholar]
- 5.Berg G, Knaape C, Ballin G, Seidel D. Biological control of Verticillium dahliae KLEB by naturally occurring rhizosphere bacteria. Arch Phytopathol Diss Prot. 1994;29:249–262. [Google Scholar]
- 6.Berg G, Marten P, Ballin G. Stenotrophomonas maltophilia in the rhizosphere of oilseed rape—occurrence, characterization and interaction with phytopathogenic fungi. Microbiol Res. 1996;151:19–27. [Google Scholar]
- 7.Berg G, Roskot N, Smalla K. Genotypic and phenotypic relationships between clinical and environmental isolates of Stenotrophomonas maltophilia. J Clin Microbiol. 1999;37:3594–3600. doi: 10.1128/jcm.37.11.3594-3600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binks P R, Nicklin S, Bruce N C. Degradation of RDX by Stenotrophomonas maltophilia PB1. Appl Environ Microbiol. 1995;61:1813–1822. doi: 10.1128/aem.61.4.1318-1322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatelut M, Dournes J L, Chabanon G, Marty N. Epidemiological typing of Stenotrophomonas (Xanthomonas) maltophilia by PCR. J Clin Microbiol. 1995;33:912–914. doi: 10.1128/jcm.33.4.912-914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugrois H J, Lafitte C, Barthe J P, Touze A. Induction of β-1.3-glucanase and chitinase activity in compatible and incompatible interactions between Colletotrichum lindemuthianum and bean cultivars. J Phytopathol. 1990;130:225–234. [Google Scholar]
- 11.Denton M, Kerr K G. Microbiological and clinical aspects of infections associated with Stenotrophomonas maltophilia. Clin Microbiol Rev. 1998;11:7–80. doi: 10.1128/cmr.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denton M, Todd N J, Kerr K G, Hawkey P M, Littlewood J M. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J Clin Microbiol. 1998;36:1953–1958. doi: 10.1128/jcm.36.7.1953-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsenstein J. Phylogensis from restriction sites: a maximum-likelihood approach. Evolution. 1992;46:159–173. doi: 10.1111/j.1558-5646.1992.tb01991.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerner-Smidt P, Bruun B, Arpi M, Schmidt J. Diversity of nosocomial Xanthomonas maltophilia (Stenotrophomonas maltophilia) as determined by ribotyping. Eur J Clin Microbiol Infect Dis. 1995;14:137–140. doi: 10.1007/BF02111874. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–562. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 16.Hauben L, Vauterin L, Swings J, Moore E R B. Comparison of 16S ribosomal DNA sequences of all Xanthomonas species. Int J Syst Bacteriol. 1997;47:328–335. doi: 10.1099/00207713-47-2-328. [DOI] [PubMed] [Google Scholar]
- 17.Hauben L, Vauterin L, Moore E R B, Hoste M, Swings J. Genomic diversity of the genus Stenotrophomonas. Int J Syst Bacteriol. 1999;49:1749–1760. doi: 10.1099/00207713-49-4-1749. [DOI] [PubMed] [Google Scholar]
- 18.Hugh R, Ryschenko E. Pseudomonas maltophilia, an Alcaligenes like species. J Gen Microbiol. 1961;26:123–132. doi: 10.1099/00221287-26-1-123. [DOI] [PubMed] [Google Scholar]
- 19.Jacobi M, Kaiser D, Berg G, Jung G, Winkelmann G, Bahl H. Maltophilin—a new antifungal compound produced by Stenotrophomonas maltophilia R3089. J Antibiot. 1996;49:1101–1104. doi: 10.7164/antibiotics.49.1101. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi D Y, Gugliemoni M, Clarke B B. Isolation of chitinolytic bacteria Xanthomonas maltophilia and Serratia marcescens as biological control agents for summer patch disease of turf grass. Soil Biol Biochem. 1995;27:1479–1487. [Google Scholar]
- 21.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester, United Kingdom: John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- 22.Lottmann J, Heuer H, Smalla K, Berg G. Influence of transgenic T4-lysozyme-producing plants on beneficial plant-associated bacteria. FEMS Microbiol Ecol. 1999;29:365–377. [Google Scholar]
- 23.Moore E, Krüger A, Hauben L, Seal S, De Baere R, De Wachter K, Timmis K, Swings J. 16S rRNA gene sequence analyses and inter- and intrageneric relationship of Xanthomonas species and Stenotrophomonas maltophilia. FEMS Microbiol Lett. 1997;151:145–153. doi: 10.1111/j.1574-6968.1997.tb12563.x. [DOI] [PubMed] [Google Scholar]
- 24.Muyzer G, de Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis by polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama T, Homma Y, Hashidoko Y, Mitzutani J, Tahara S. Possible role of xanthobaccins produced by Stenotrophomonas sp. strain SB-K88 in suppression of sugar beet damping-off disease. Appl Environ Microbiol. 1999;65:4334–4339. doi: 10.1128/aem.65.10.4334-4339.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neiendam-Nielson M, Sörensen J, Fels J, Pedersen H C. Secondary metabolite- and endochitinase-dependent antagonism toward plant-pathogenic microfungi of Pseudomonas fluorescens isolates from sugar beet rhizosphere. Appl Environ Microbiol. 1998;64:3563–3569. doi: 10.1128/aem.64.10.3563-3569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nesme X, Vaneechoutte M, Orso S, Hoste B, Swings J. Diversity and genetic relatedness within genera Xanthomonas and Stenotrophomonas using restriction endonuclease site differences of PCR-amplified 16S rRNA gene. Syst Appl Microbiol. 1995;18:127–135. [Google Scholar]
- 28.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 29.Palleroni N J, Bradbury J F. Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) Swings et al. 1983. Int J Syst Bacteriol. 1993;43:606–609. doi: 10.1099/00207713-43-3-606. [DOI] [PubMed] [Google Scholar]
- 30.Saitou N, Nei M. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlungbaum G, Baudler H, Nausch G. Die Darβ-Zingster Boddenkette—ein typisches Flachwasserästuar an ler südlichen Ostseeküste. Rostocker Meeresbiol Beitr. 1994;2:5–25. [Google Scholar]
- 33.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 34.Stackebrandt E, Goebel B M. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 35.Swings J, de Vos P, van den Mooter M, De Ley J. Transfer of Pseudomonas maltophilia Hugh 1981 to the genus Xanthomonas as Xanthomonas maltophilia (Hugh 1981) comb. nov. Int J Syst Bacteriol. 1983;33:409–413. [Google Scholar]
- 36.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ursing J B, Roselló-Mora R A, García-Valdés E, Lalucat J. Taxonomic note: a pragmatic approach to the nomenclature of phenotypically similar genomic groups. Int J Syst Bacteriol. 1995;45:604. [Google Scholar]
- 38.Van Den Mooter M, Swings J. Numerical analysis of 295 phenotypic features of 266 Xanthomonas strains and related strains and an improved taxonomy of the genus. Int J Syst Bacteriol. 1990;40:348–369. doi: 10.1099/00207713-40-4-348. [DOI] [PubMed] [Google Scholar]
- 39.Zuraleff J J, Yu V L. Infection caused by Pseudomonas maltophilia with emphasis on bacteraemia: reports and review of the literature. Rev Infect Dis. 1982;4:1242–1246. doi: 10.1093/clinids/4.6.1236. [DOI] [PubMed] [Google Scholar]