Abstract
This study investigated temporal trends in the epidemiology of primary myopia and associations with key environmental risk factors in a UK population. Data were collected at recruitment (non-cycloplegic autorefraction, year of birth, sex, ethnicity, highest educational attainment, reason and age of first wearing glasses and history of eye disease) from 107,442 UK Biobank study participants aged 40 to 69 years, born between 1939 and 1970. Myopia was defined as mean spherical equivalent (MSE) ≤-1 dioptre (D). Temporal changes in myopia frequency by birth cohort (5-year bands using date of birth) and associations with environmental factors were analysed, distinguishing both type (childhood-onset, <18 years versus adult-onset) and severity (three categories: low -1.00 to -2.99D, moderate -3.00 to -5.99D or high ≥-6.00D). Overall myopia frequency increased from 20.0% in the oldest cohort (births 1939–1944) to 29.2% in the youngest (1965–1970), reflecting a relatively higher increase in frequency of adult-onset and low myopia. Childhood-onset myopia peaked in participants born in 1950–54, adult-onset myopia peaked in the cohort born a decade later. The distribution of MSE only shifted for childhood-onset myopia (median: -3.8 [IQR -2.4, -5.4] to -4.4 [IQR -3.0, -6.2]). The magnitude of the association between higher educational attainment (proxy for educational intensity) and myopia overall increased over time (adjusted Odds Ratio (OR) 2.7 [2.5, 2.9] in the oldest versus 4.2 [3.3, 5.2] in the youngest cohort), being substantially greater for childhood-onset myopia (OR 3.3 [2.8, 4.0] to 8.0 [4.2, 13]). Without delineating childhood-onset from adult-onset myopia, important temporal trends would have been obscured. The differential impact of educational experience/intensity on both childhood-onset and high myopia, amplified over time, suggests a cohort effect in gene-environment interaction with potential for increasing myopia frequency if increasing childhood educational intensity is unchecked. However, historical plateauing of myopia frequency does suggest some potential for effective intervention.
Introduction
Myopia is one form of refractive error, placed at the opposite end of the distribution of this quantitative trait to hypermetropia. As it arises as a consequence of ocular growth [1] that is unchecked by normal homeostatic control, it has long intrigued clinicians and scientists. However, it is now a pressing public health concern internationally, with an emerging ‘epidemic’ of myopia, characterised by increased prevalence accompanied by a whole population shift in distribution towards younger age at onset and greater severity [2, 3]. This places a growing population of people at risk of the potentially blinding sequelae associated with greater severity of myopia, as evidenced in Asia [4, 5] but less strikingly in Europe [6–9]. The economic impact of myopia per se and associated visual impairment is already considerable [10] and set to escalate [11].
Severity and timing of onset of myopia (childhood versus adult-onset) are related [12]: childhood-onset myopia generally has a clear familial/hereditary basis, is progressive into adulthood and often of greater severity [13]. Onset, conventionally defined by age at first wearing optical correction, is infrequently considered in myopia research, although it offers the opportunity to investigate the relative importance of genetic predisposition and the nature of environmental risk factors: for example, to advance the current focus in both aetiological research and in preventive public health interventions, on the role of educational experience and intensity in childhood. Although severity (as a quantitative trait) is always measured in research, it is usual for this to be the intermediate rather than final measure, as the latter which can only be assigned in middle/late adulthood, and this risks non-random/systematic misclassification of severity. We hypothesised that if changing environmental factors, in particular educational experience, are accounting for increasing frequency of myopia in the UK, a cohort effect would be discernible in changing associations with myopia, with different profiles for childhood and adult-onset forms. We investigated this using the UK Biobank Study, a unique large contemporary adult population sample whose members, born over a period of more than three decades, have undergone a detailed ophthalmic examination. This affords the opportunity to analyse ‘historical’ cohorts covering a period of important socio-demographic, economic, and educational change in the UK from which current and emerging trends may be identified and examined. Drawing on our proof-of-concept study [14], we investigated whether there were differences between childhood-onset versus adult-onset myopia in temporal trends in both frequency and severity and in associations with key environmental factors.
Methods
Study population
Between 2006 and 2010, more than half a million UK adults aged between 40 and 69 years and registered with the UK National Health Service [15, 16] consented to participate in the UK Biobank study (UKBB). Since that time they have reported through serial questionnaires and in repeated examinations on their lifestyle, environment and medical history [16, 17]. Specifically, data have been collected on age at first use and reason for optical correction (glasses or contact lenses) and any eye illness or eye surgery. An enhanced ophthalmic assessment including non-cycloplegic autorefraction (Tomey RC 5000 auto-refkeratometer, Tomey Corp., Japan) as per the UK Biobank protocol (see participation flowchart; S1 Fig) was undertaken at the time of registration on a large subsample, as detailed elsewhere [18].
Available UK Biobank data
Participants reported diverse socio-demographic information including: sex, age at recruitment, ethnicity using the UK Office for National Statistics classification of White, mixed, Asian or Asian British (including Indian, Pakistani and Bangladeshi), Black or Black British), Chinese, Mixed and other group, and socio-economic status assessed using conventional individual level markers (versus less granular area-based proxy markers) of housing tenure (council rental, private rental, home-ownership with a mortgage and outright ownership) available for all participants. In the absence of specific data on educational experience or intensity over the participants lifetime, we used a priori their highest educational attainment i.e. ‘none’, ‘O’ levels (examinations at statutory school-leaving age in the UK), ‘A’ levels (examinations at age 18 years in the UK) or higher level of education (e.g. Degree) in two ways. We used ‘no educational qualification’ a sensitive proxy for lowest educational intensity and experience during school years and the gradient of qualifications achieved from ‘O’ levels to Degree/higher Level qualification as a proxy for intensity during school years. In addition, participants reported any eye conditions, eye surgery or other treatment received including optical correction and reason for prescription, as well as age of diagnosis or treatment e.g. surgery or first prescription for glasses.
Ethics statement
UK Biobank has received approval from the Northwest Multi-Centre Research Ethics committee, which covers the United Kingdom. UK Biobank also obtained approval in England and Wales from the National Information Governance Board for Health & Social Care, which allows access to information for inviting individuals to participate. In Scotland, UK Biobank received approval from the Community Health Index Advisory Group. Participants in the UK Biobank were voluntarily enrolled and gave their written informed consent. This research has been conducted using the UK Biobank Resource under Application Number 669.
Outcome measures
As detailed previously [18] the conventional metric of spherical equivalent (SE) (algebraic sum of sphere + 0.5 cylinder) in diopters (D) was used to categorise severity of refractive error in a clinically meaningful way, in keeping with international guidelines [19, 20]: emmetropia (SE -0.99D to +0.99D), low myopia (SE -1.0D to -2.99D), moderate myopia (SE -3.0D to -5.99D), high myopia (SE -6.0D or more extreme), low hypermetropia (SE +1.0D to +2.99D) and moderate/high hypermetropia (SE +3.0D or more extreme) [19]. Mean spherical equivalent (MSE) of the two eyes for each individual (or single eye measure if both measurements were not available) was used to report frequency. Importantly, in order to reliably analyse primary myopia, individuals who reported treatments that might alter refraction, including cataract surgery, refractive laser surgery, vitrectomy or retinal detachment, and those with high inter-ocular discordance (hypermetropia in one eye and myopia in the other) were excluded as detailed elsewhere [18].
Individuals were also characterised as having either childhood-onset (by the age of 17 years) or adult-onset myopia using self-reported age at first glasses/contact lenses wear and reason for first optical correction, based on prior work evaluating the utility of self-report in this population [21]. To enable a ‘deep dive’, we dichotomised those in the childhood-onset group into early (under 10 years) and late (10 to 17 years) childhood onset using the conventional threshold of 18 years for defining adulthood.
Participants were assigned into one of six cohorts (five-year age bands) based on their year of birth: the oldest cohort comprising those born between 1939 and 1944 (cohort 1) and the youngest (cohort 6), births between 1965 and 1970.
Statistical methods
Frequency and distribution of refractive error (myopia, emmetropia and hypermetropia) in the UK Biobank population, by demographic and environmental factors are reported. Multivariable logistic regression was used to model associations between myopia (all and by onset) and socio-demographic factors described earlier, including sex and ethnicity, with emmetropia as the reference category. Sandwich variance estimates allowed for correlation within test centres. Results are given as point estimates alongside interquartile range (IQR) or 95% confidence intervals. All analyses were carried out using Stata 15.0 (StataCorp, College Station, Texas).
Results
Participation and study sample
The final study sample of 107,442 (of 115,785 subjects eligible for ophthalmic examination, S1 Fig) was older and more affluent and with fewer males, but similar ethnic distribution as the general UK population, as detailed elsewhere [18].
Trends in frequency of myopia
The overall frequency of primary myopia in the UK Biobank population was 26.9% [26.6%, 27.1%], comprising 4.0% [3.9%, 4.1%] high myopia, 9.5% [9.3%, 9.7%] moderate and 13.3% [13.0%, 13.6%] low myopia. Altogether 45.6% [45.3%, 45.9%] of subjects were emmetropic and 27.6% [27.3%, 27.8%] hypermetropic.
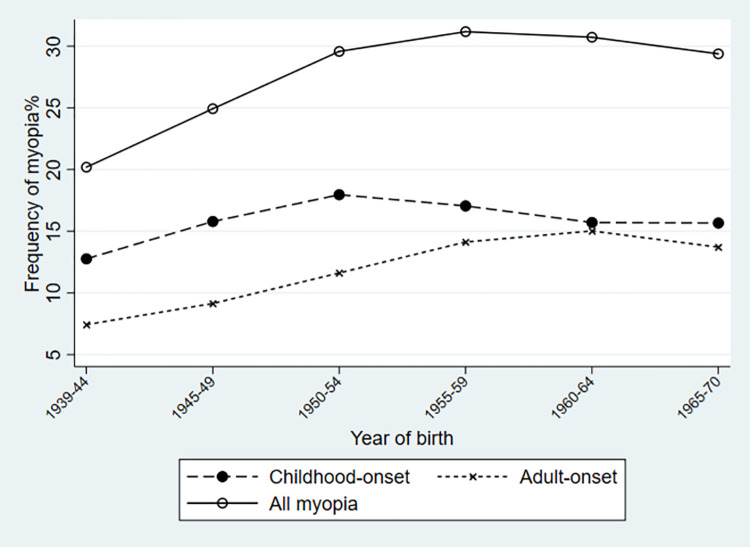
Fig 1 and Table 1 show that from a baseline overall myopia frequency of 20.0% (19.5% to 20.6%) amongst those born in cohort 1 (1939–44), there was a steady increase in subsequent birth cohorts, peaking in cohort 4 (1955–54) and then plateauing. However, the frequency of childhood-onset myopia peaked at 17.8% in cohort 3, two decades earlier than the peak at 15.0% of adult-onset myopia in cohort 5 (1960–1964).
Fig 1. Frequency of myopia (all, childhood-onset and adult-onset).
By year of birth as 5-year bands.
Table 1. Frequency of myopia (all, childhood-onset and adult-onset) and emmetropia in the UK Biobank population: Distribution of socio-demographic and environmental factors.
| Overall | Myopia | Emmetropia | Hypermetropia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Childhood-onset | Adult-onset | All myopia | ||||||||
| N | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| Population | 107,442 | 16,786 | 15.6 (15.4,15.8) | 12,066 | 11.2 (11.0,11.4) | 28,852 | 26.9 (26.6,27.1) | 48,960 | 45.6 (45.3,45.9) | 29,630 | 27.6 (27.3,27.8) |
| Year of birth | |||||||||||
| 1939–44 | 20,042 | 2,529 | 12.6 (12.2,13.1) | 1,482 | 7.4 (7.0,7.8) | 4,011 | 20.0 (19.5,20.6) | 6,884 | 34.4 (33.7,35.0) | 9,147 | 45.6 (45.0,46.3) |
| 1945–49 | 26,963 | 4,219 | 15.6 (15.2,16.1) | 2,473 | 9.2 (8.8,9.5) | 6,692 | 24.8 (24.3,25.3) | 10,353 | 38.4 (37.8,39.0) | 9,918 | 36.8 (36.2,37.4) |
| 1950–54 | 18,998 | 3,378 | 17.8 (17.2,18.3) | 2,207 | 11.6 (11.2,12.1) | 5,585 | 29.4 (28.8,30.0) | 8,418 | 44.3 (43.6,45.0) | 4,995 | 26.3 (25.7,26.9) |
| 1955–59 | 16,139 | 2,722 | 16.9 (16.3,17.5) | 2,263 | 14.0 (13.5,14.6) | 4,985 | 30.9 (30.2,31.6) | 8,081 | 50.1 (49.3,50.8) | 3,073 | 19.0 (18.4,19.7) |
| 1960–64 | 14,010 | 2,182 | 15.6 (15.0,16.2) | 2,104 | 15.0 (14.4,15.6) | 4,286 | 30.6 (29.8,31.4) | 8,071 | 57.6 (56.8,58.4) | 1,653 | 11.8 (11.3,12.3) |
| 1965–70 | 11,290 | 1,756 | 15.6 (14.9,16.2) | 1,537 | 13.6 (13.0,14.3) | 3,293 | 29.2 (28.3,30 0) | 7,153 | 63.4 (62.5,64.2) | 844 | 7.5 (7.0,8.0) |
| Sex | |||||||||||
| Female | 58,445 | 9,689 | 16.6 (16.3,16.9) | 6,048 | 10.3 (10.1,10.6) | 15,737 | 26.9 (26.6,27.3) | 25,823 | 44.2 (43.8,44.6) | 16,885 | 28.9 (28.5,29.3) |
| Male | 48,997 | 7,097 | 14.5 (14.2,14.8) | 6,018 | 12.3 (12.0,12.6) | 13,115 | 26.8 (26.4,27.2) | 23,137 | 47.2 (46.8,47.7) | 12,745 | 26.0 (25.6,26.4) |
| Highest educational qualification¥ | |||||||||||
| None | 15,776 | 1,009 | 6.4 (6.0,6.8) | 1,054 | 6.7 (6.3,7.1) | 2,063 | 13.1 (12.6,13.6) | 6,951 | 44.1 (43.3,44.8) | 6762 | 42.9 (42.1,43.6) |
| O-level | 28,272 | 3,500 | 12.4 (12.0,12.8) | 3,344 | 11.8 (11.5,12 2) | 6,844 | 24.2 (23.7,24.7) | 13,849 | 49.0 (48.4,49.6) | 7,579 | 26.8 (26.3,27.3) |
| A-level | 19,161 | 2,854 | 14.9 (14.4,15.4) | 2,248 | 11.7 (11.3,12.2) | 5,102 | 26.6 (26.0,27.3) | 8,974 | 46.8 (46.1,47.5) | 5,085 | 26.5 (25.9,27.2) |
| Higher-level | 42,767 | 9,329 | 21.8 (21.4,22.2) | 5,292 | 12.4 (12.1,12.7) | 14,621 | 34.2 (33.7,34.6) | 18,446 | 43.1 (42.7,43.6) | 9,700 | 22.7 (22.3,23.1) |
| Missing | 1,466 | 94 | - | 128 | - | 222 | - | 740 | - | 504 | - |
| Accommodation tenure | |||||||||||
| Rent from council | 7,299 | 782 | 10.7 (10.0,11.4) | 642 | 8.8 (8.2,9.5) | 1,424 | 19.5 (18.6,20.4) | 3,720 | 51.0 (49.8,52.1) | 2,155 | 29.5 (28.5,30.6) |
| Rent from private | 4,351 | 571 | 13.1 (12.1,14.2) | 455 | 10.5 (9.6,11.4) | 1,026 | 23.6 (22.3,24.9) | 2,328 | 53.5 (52.0,55.0) | 997 | 22.9 (21.7,24.2) |
| Own with mortgage | 37,853 | 5,937 | 15.7 (15.3,16.1) | 5,044 | 13.3 (13.0,13.7) | 10,981 | 29.0 (28.6,29.5) | 19,441 | 51.4 (50.9,51.9) | 7,431 | 19.6 (19.2,20.0) |
| Own outright | 55,837 | 9,251 | 16.6 (16.3,16.9) | 5,684 | 10.2 (9.9,10.4) | 14,935 | 26.4 (26.4,27.1) | 22,458 | 40.2 (39.8,40.6) | 18,444 | 33.0 (32.6,33.4) |
| Missing | 2,102 | 245 | - | 241 | - | 486 | - | 1,013 | - | 603 | - |
| Ethnicity | |||||||||||
| White | 95,783 | 15,257 | 15.9 (15.7,16.2) | 10,659 | 11.1 (10.9,11.3) | 25,916 | 27.6 (26.8,27.3) | 42,625 | 44.5 (44.2,44.8) | 27,242 | 28.4 (28.2,28.7) |
| Mixed ethnicity | 974 | 168 | 17.2 (15.0,19.8) | 122 | 12.5 (10.6,14.8) | 290 | 29.8 (27.0,32.7) | 516 | 53.0 (49.8,56.1) | 168 | 17.2 (15.0,19.8) |
| Asian or Asian British§ | 4,032 | 528 | 13.1 (12.1,14.2) | 472 | 11.7 (10.7,12.7) | 1,000 | 24.8 (23.5,26.2) | 2,185 | 54.2 (52.6,55.7) | 847 | 21.0 (18.5,21.0) |
| Black or Black British | 3,758 | 396 | 10.5 (9.6,11.6) | 479 | 12.8 (11.7,13.9) | 875 | 23.3 (22.0,24.7) | 2,143 | 57.0 (55.4,58.6) | 740 | 19.7 (18.5,21.0) |
| Chinese | 490 | 169 | 34.5 (30.3,38.8) | 63 | 12.7 (10.1,16.1) | 232 | 47.4 (42.9,51.8) | 205 | 41.8 (37.5,46.3) | 53 | 10.8 (8.3,13.9) |
| Other | 1,627 | 166 | 10.2 (8.8,11.8) | 189 | 11.6 (10.2,13.3) | 355 | 21.8 (19.9,23.9) | 908 | 55.8 (53.4,58.2) | 364 | 22.4 (20.4,24.5) |
| Missing | 778 | 102 | - | 82 | - | 184 | - | 378 | - | 216 | - |
¥O level: State examination at age 16 years; A level: State examination at age 18 years.
§Asian category includes Indian, Pakistani and Bangladeshi.
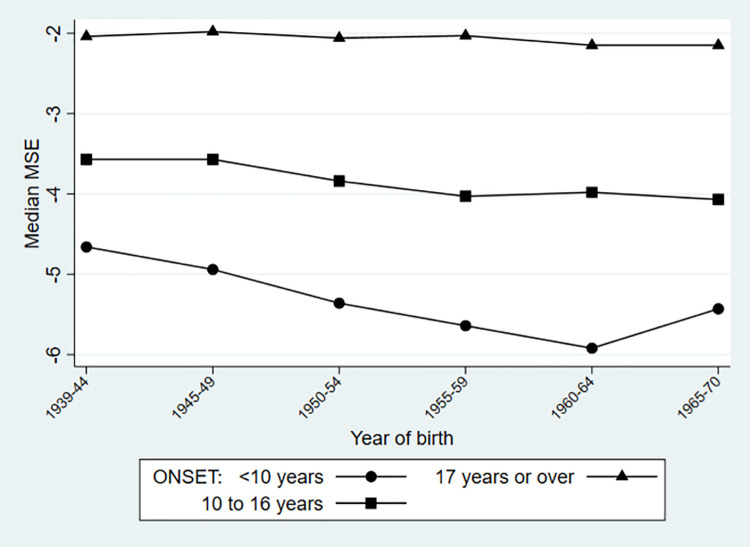
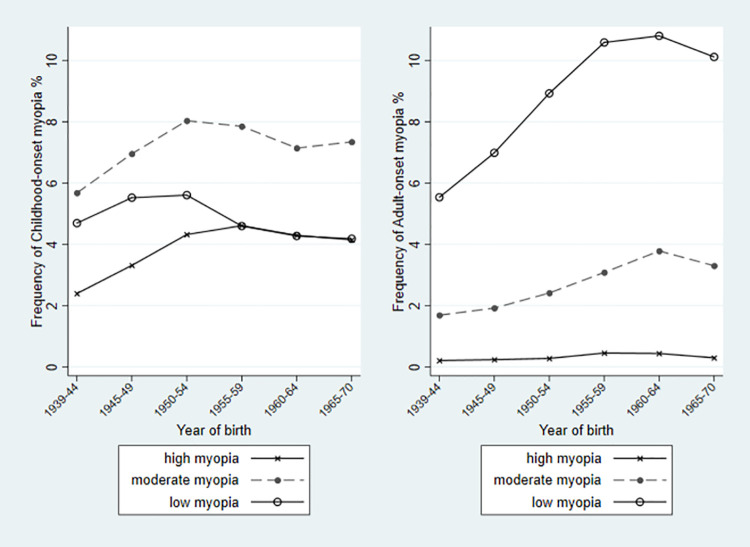
The overall median MSE in childhood-onset myopia decreased from -3.8D [IQR -2.4D, -5.4D] by a clinically meaningful amount of -0.6D, to -4.4D [IQR -3.0D, -6.2D] (S1 Table). Although moderate myopia accounted for more than half of all childhood-onset myopia in all cohorts, the largest relative increase over time (doubling of frequency) in childhood-onset was of high myopia, driving this change in MSE. The subgroup analysis showed that amongst those with childhood onset before the age of 10 years, the median MSE changed by -1.3D over time (cohort 1 versus cohort 5) whilst amongst those with onset between 11 and 17 years, the median MSE changed by only -0.5D (cohort 1 versus cohort 6, Fig 2; S1 Table). By contrast, the median MSE in adult-onset myopia was stable at around -2.1D. Low myopia accounted for at least three-quarters of adult-onset myopia in all birth cohorts and also had the greatest relative increase (doubling) in frequency, without any change in the small contribution of high myopia (Fig 3).
Fig 2. Median MSE (mean spherical equivalent): Childhood-onset myopia (subdivided: Before age 10 and aged 10 to 16 years) and adult-onset myopia, by year of birth as 5-year bands.
Fig 3. Frequency of childhood-onset and adult-onset myopia by severity (high, moderate and low), by year of birth as 5-year bands.
Distribution of socio-demographic and environmental risk factors
The distribution of sociodemographic factors overall and by type of myopia is shown in Table 1. There were important and anticipated variations overall with respect to sex (higher proportion of women had childhood-onset myopia, S2 Fig), highest educational attainment (the overall proportion with no educational qualifications declined over time from 30% to 5%, S3 Fig), socio-economic status (accommodation tenure) and ethnicity. Notably 47.4% of those of Chinese ethnicity had myopia (almost twice the frequency of any other ethnic group), mainly childhood-onset.
Associations of myopia with socio-demographic and environmental risk factors
Fully adjusted analysis, to model associations between myopia (all and by onset) and socio-demographic factors, showed later year of birth (younger cohort) was associated with reduced odds of myopia overall, but this was driven by a progressive decline (independent protective effect) in odds of childhood-onset myopia across all cohorts, resulting in an approximately 30% reduction in risk of childhood-onset myopia to those born from the 1960s onwards compared to the baseline cohort 1 (born between 1939 and 1944). By comparison, the odds of adult-onset myopia increased in cohorts 2 to 5 (Table 2). Females were at increased independent risk of myopia overall, but this comprised 24% increased risk of childhood-onset and a 12% decreased risk of adult-onset myopia (Table 2). Chinese ethnicity was associated with a 90% increased risk of myopia overall but specifically a 19% increased risk of adult-onset and 240% increased risk of childhood-onset myopia compared to White ethnicity, with all other ethnic groups being ‘protective’. Highest educational attainment was strongly associated with increased risk of childhood-onset myopia, with a clear ‘dose response’ relationship. There was a similar gradient, with smaller effect sizes, for adult-onset myopia. Thus, the largest effect sizes for associations with educational attainment after the age of 18 years in both types, with a greater effect size for males (S2 and S3 Tables). This is compatible with capturing the highest educational intensity/experience during school as either a ‘trajectory’ or ‘threshold’ effect with respect to myopia risk. Higher current socio-economic status (using the conventional index of housing tenure) was associated with increased risk of adult-onset myopia with a gradient across categories whereas only highest category of socio-economic status was associated with childhood-onset myopia.
Table 2. Association of myopia (all, childhood-onset and adult-onset), with socio-demographic and environmental factors in 75,297 participants.
| Factors | Childhood-onset myopia | Adult-onset myopia | All myopia | Emmetropia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Crude OR* | Adjusted OR ** | n | Crude OR* | Adjusted OR ** | n | Crude OR* | Adjusted OR ** | n | |
| 16,389 | (95% CI) | 11,685 | (95% CI) | 28,074 | (95% CI) | 47,223 | ||||
| Year of birth | ||||||||||
| 1939–1944 | 2,468 | 1 | 1 | 1,436 | 1 | 1 | 3,904 | 1 | 1 | 6,639 |
| 1945–1949 | 4,132 | 1.11 | 1.03 (0.98, 1.08) | 2,392 | 1.10 | 1.07 (1.03, 1.12) | 6,524 | 1.11 | 1.04 (1.00, 1.09) | 10,034 |
| 1950–1954 | 3,313 | 1.09 | 0.98 (0.87, 1.10) | 2,143 | 1.21 | 1.16 (1.08, 1.26) | 5,456 | 1.14 | 1.04 (0.96, 1.13) | 8,164 |
| 1955–1959 | 2,672 | 0.92 | 0.85 (0.76, 0.96) | 2,213 | 1.31 | 1.26 (1.22, 1.30) | 4,885 | 1.06 | 1.00 (0.94, 1.07) | 7,823 |
| 1960–1964 | 2,114 | 0.73 | 0.70 (0.62, 0.79) | 2,022 | 1.21 | 1.17 (1.09, 1.25) | 4,136 | 0.91 | 0.87 (0.80, 0.94) | 7,753 |
| 1965–1970 | 1,690 | 0.67 | 0.64 (0.55, 0.75) | 1,479 | 1.00 | 0.97 (0.93, 1.01) | 3,169 | 0.79 | 0.76 (0.70, 0.82) | 6,810 |
| Sex | ||||||||||
| Male | 6,929 | 1 | 1 | 5,821 | 1 | 1 | 12,750 | 1 | 1 | 22,246 |
| Female | 9,460 | 1.22 | 1.24 (1.13, 1.35) | 5,864 | 0.90 | 0.88 (0.84, 0.93) | 15,324 | 1.07 | 1.08 (1.01, 1.15) | 24,977 |
| Highest educational qualification ¥ | ||||||||||
| None | 984 | 1 | 1 | 1,025 | 1 | 1 | 2,009 | 1 | 1 | 6,738 |
| O-level | 3,426 | 1.73 | 1.91 (1.75, 2.09) | 3,269 | 1.58 | 1.52 (1.38, 1.66) | 6,695 | 1.65 | 1.72 (1.58, 1.88) | 13,590 |
| A-level | 2,793 | 2.18 | 2.39 (2.17, 2.64) | 2,201 | 1.65 | 1.57 (1.43, 1.72) | 4,994 | 1.91 | 1.97 (1.85, 2.10) | 8,783 |
| Higher-level | 9,186 | 3.47 | 3.82 (3.63, 4.02) | 5,190 | 1.88 | 1.80 (1.66, 1.95) | 14,376 | 2.66 | 2.76 (2.62, 2.90) | 18,112 |
| Accommodation tenure | ||||||||||
| Council rental | 759 | 1 | 1 | 615 | 1 | 1 | 1,374 | 1 | 1 | 3,584 |
| Private rental | 564 | 1.18 | 0.99 (0.89, 1.10) | 442 | 1.14 | 1.04 (0.92, 1.19) | 1,006 | 1.17 | 1.01 (0.90, 1.13) | 2,252 |
| Own with mortgage | 5,888 | 1.45 | 1.09 (0.90, 1.33) | 5,002 | 1.52 | 1.33 (1.13, 1.56) | 10,890 | 1.48 | 1.19 (1.00, 1.41) | 19,207 |
| Own | 9,178 | 1.95 | 1.28 (1.04, 1.57) | 5,626 | 1.48 | 1.36 (1.14, 1.63) | 14,804 | 1.74 | 1.31 (1.09, 1.58) | 22,180 |
| Ethnicity | ||||||||||
| White | 15,024 | 1 | 1 | 10,455 | 1 | 1 | 25,479 | 1 | 1 | 41,731 |
| Mixed ethnicity | 163 | 0.94 | 1.04 (0.83, 1.31) | 119 | 0.99 | 1.02 (0.89, 1.17) | 282 | 0.96 | 1.03 (0.89, 1.19) | 480 |
| Asian or Asian British§ | 499 | 0.70 | 0.78 (0.71, 0.86) | 433 | 0.87 | 0.87 (0.80, 0.98) | 932 | 0.77 | 0.82 (0.75, 0.90) | 1,983 |
| Black or Black British | 380 | 0.53 | 0.62 (0.53, 0.73) | 447 | 0.89 | 0.95 (0.92, 0.99) | 827 | 0.67 | 0.76 (0.70, 0.84) | 2,008 |
| Chinese | 166 | 2.40 | 2.33 (1.72, 3.16) | 57 | 1.18 | 1.20 (1.04, 1.38) | 223 | 1.90 | 1.83 (1.43, 2.36) | 192 |
| Other | 157 | 0.53 | 0.56 (0.47, 0.66) | 174 | 0.84 | 0.90 (0.82, 0.99) | 331 | 0.65 | 0.70 (0.62, 0.79) | 829 |
*OR = Odds Ratio. 95% CI = 95% confidence interval.
** Adjusted by year of birth, sex, educational qualification, accommodation tenure, ethnicity, variance adjustment for test centre. ¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi. Bold fonts indicate significant associations at the 5% level. For this analysis we have excluded 2,515 with missing data in the selected factors (as detailed in Table 1).
Effect modification (interaction) by sociodemographic and environmental risk factors
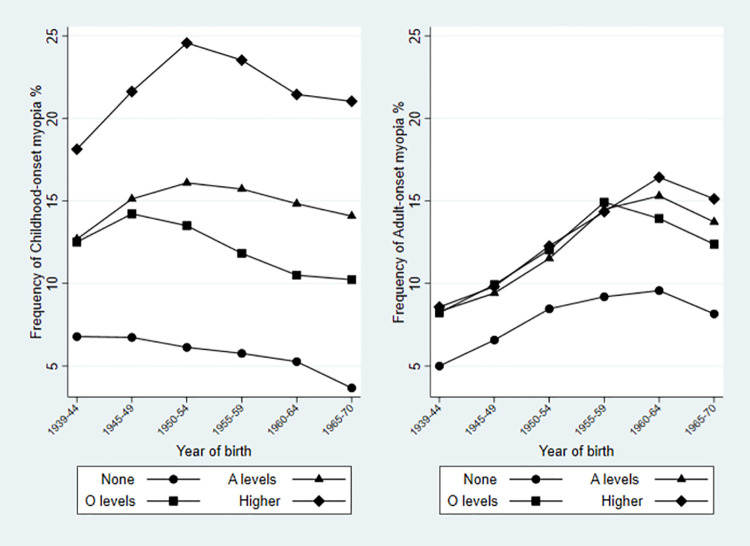
Effect modification was examined by adding interaction terms to the fully adjusted model reported in Table 2. Evidence of effect modification with highest educational attainment between sex (p<0.001), year of birth (p<0.001) and ethnicity (p<0.001) was observed. Table 3 and Fig 4 show a clear temporal trend of increasing magnitude of adjusted association of higher educational attainment (evident for each category of educational attainment) for childhood-onset myopia, notably in the association with the top category of post-school higher education, OR 3.3 [2.8, 4.0] in cohort 6 (youngest) compared to OR 8.0 [4.2, 13] in cohort 1 (oldest). By contrast the size of the associations for adult-onset myopia remained largely unchanged (with overlapping 95% confidence intervals) over time. Interestingly, an increasingly stronger protective effect of having no educational qualifications (plausibly capturing reduced educational experience or intensity during childhood) over time, was attributable to the relationship with childhood-onset myopia, with this protective effect increasing over time—from 15% risk reduction in cohort 1 compared to 77% risk reduction in the cohort 6. Taken together these changes over time in the associations with education are compatible with a cohort effect in gene-environment interaction in myopia causation.
Table 3. Interactions in associations with myopia (all, childhood-onset and adult-onset) between year of birth and no educational qualifications.
| Educational attainment adjusted Odds Ratios (95% confidence interval) | |||||
|---|---|---|---|---|---|
| No qualification | O level | A level | Higher level | ||
| All myopia, n = 28,074 | |||||
| Year of birth | |||||
| 1939–44 | 1 | 1.91 (1.73, 2.10) | 1.91 (1.65, 2.20) | 2.70 (2.51, 2.90) | |
| 1945–49 | vs 1939–44 | 0.97 (0.90, 1.05) | 0.99 (0.86, 1.15) | 1.08 (0.99, 1.18) | 1.10 (0.98, 1.23) |
| vs No qualification | 1 | 1.95 (1.72, 2.21) | 2.12 (1.86, 2.41) | 3.06 (2.72, 3.43) | |
| 1950–54 | vs 1939–44 | 0.92 (0.75, 1.13) | 0.94 (0.68, 1.30) | 1.02 (0.77, 1.35) | 1.04 (0.83, 1.31) |
| vs No qualification | 1 | 1.87 (1.45, 2.41) | 2.20 (1.73, 2.80) | 3.47 (2.77, 4.35) | |
| 1955–59 | vs 1939–44 | 0.86 (0.69, 1.07) | 0.88 (0.65, 1.18) | 0.96 (0.76, 1.20) | 0.98 (0.84, 1.14) |
| vs No qualification | 1 | 1.94 (1.61, 2.35) | 2.33 (1.95, 2.80) | 3.50 (2.81, 4.36) | |
| 1960–64 | vs 1939–44 | 0.77 (0.58, 1.03) | 0.79 (0.62, 1.19) | 0.86 (0.62, 1.19) | 0.88 (0.69, 1.11) |
| vs No qualification | 1 | 1.71 (1.34, 2.17) | 2.37 (1.77, 3.17) | 3.52 (2.59, 4.78) | |
| 1965–70 | vs 1939–44 | 0.58 (0.49, 0.68) | 0.59 (0.44, 0.80) | 0.64 (0.48, 0.86) | 0.65 (0.51, 0.84) |
| vs No qualification | 1 | 1.96 (1.59, 2.41) | 2.69 (2.23, 3.25) | 4.17 (3.32, 5.22) | |
| Adult-onset myopia, n = 11,685 | |||||
| Year of birth | |||||
| 1939–44 | 1 | 1.81 (1.44, 2.29) | 1.77 (1.44, 2.19) | 2.05 (1.81, 2.32) | |
| 1945–49 | vs 1939–44 | 1.14 (0.98, 1.32) | 1.03 (0.87, 1.22) | 1.06 (0.96, 1.17) | 1.08 (0.96, 1.20) |
| vs No qualification | 1 | 1.64 (1.47, 1.83) | 1.65 (1.51, 1.80) | 1.94 (1.76, 2.14) | |
| 1950–54 | vs 1939–44 | 1.24 (0.96, 1.62) | 1.12 (0.82, 1.55) | 1.16 (0.83, 1.62) | 1.18 (0.88, 1.57) |
| vs No qualification | 1 | 1.54 (1.17, 2.03) | 1.61 (1.22, 2.12) | 2.03 (1.50, 2.75) | |
| 1955–59 | vs 1939–44 | 1.22 (1.02, 1.45) | 1.10 (0.74, 1.64) | 1.13 (0.90, 1.43) | 1.15 (0.96, 1.38) |
| vs No qualification | 1 | 1.77 (1.36, 2.31) | 1.86 (1.44, 2.40) | 2.19 (1.74, 2.76) | |
| 1960–64 | vs 1939–44 | 1.13 (0.83, 1.53) | 1.02 (0.69, 1.50) | 1.05 (0.79, 1.39) | 1.07 (0.87, 1.31) |
| vs No qualification | 1 | 1.52 (1.15, 2.00) | 1.92 (1.39, 2.63) | 2.43 (1.71, 3.44) | |
| 1965–70 | vs 1939–44 | 0.88 (0.67, 1.14) | 0.80 (0.58, 1.10) | 0.82 (0.58, 1.15) | 0.83 (0.61, 1.13) |
| vs No qualification | 1 | 1.59 (1.24, 2.04) | 2.02 (1.55, 2.65) | 2.63 (2.03, 3.41) | |
| Childhood-onset myopia, n = 16,389 | |||||
| Year of birth | |||||
| 1939–44 | 1 | 1.99 (1.77, 2.36) | 2.06 (1.68, 2.52) | 3.33 (2.77, 3.99) | |
| 1945–49 | vs 1939–44 | 0.85 (0.72, 1.00) | 0.97 90.81, 1.16) | 1.09 (0.99, 1.20) | 1.12 (0.99, 1.25) |
| vs No qualification | 1 | 2.27 (1.90, 2.72) | 2.66 (2.25, 3.11) | 4.37 (3.92, 4.87) | |
| 1950–54 | vs 1939–44 | 0.67 (0.53, 0.85) | 0.76 (0.50, 1.17) | 0.86 (0.61, 1.20) | 0.88 (0.67, 1.15) |
| vs No qualification | 1 | 2.37 (1.91, 2.94) | 3.13 (2.49, 3.93) | 5.80 (4.59, 7.34) | |
| 1955–59 | vs 1939–44 | 0.58 (0.43, 0.78) | 0.66 (0.42, 1.06) | 0.74 (0.49, 1.14) | 0.76 (0.53, 1.09) |
| vs No qualification | 1 | 2.26 (1.71, 2.99) | 3.21 (2.66, 3.88) | 5.96 (4.27, 8.30) | |
| 1960–64 | vs 1939–44 | 0.49 (0.36, 0.68) | 0.56 (0.33, 0.95) | 0.63 (0.41, 0.97) | 0.65 (0.45, 0.92) |
| vs No qualification | 1 | 2.07 (1.55, 2.77) | 3.32 (2.40, 4.57) | 5.83 (4.05, 8.40) | |
| 1965–70 | vs 1939–44 | 0.33 (0.26, 0.41) | 0.37 (0.26, 0.53) | 0.42 (0.31, 0.57) | 0.43 (0.34, 0.55) |
| vs No qualification | 1 | 2.85 (1.74, 4.69) | 4.36 (2.65, 7.22) | 8.04 (4.19, 13.1) | |
*Adjusted by sex vs educational qualification interaction, accommodation tenure, ethnicity, variance adjustment by test centre. Bold fonts indicate significant within cohort associations at the 5% level.
Fig 4.
Frequency of (a) childhood-onset and (b) adult-onset myopia by highest educational attainment*, by year of birth as 5-year bands. *No qualifications, ‘O’ levels (examinations at statutory school-leaving age in the UK), ‘A’ levels (examinations at age 18 years in the UK) or higher level of education.
The association between higher educational attainment and myopia risk was evident for all ethnic groups in relation to childhood-onset myopia, and in a gradient with the category of higher qualifications after the age of 18 years having the strongest effect size except amongst Black/Black British participants (Table 4). By contrast the association between higher educational attainment and adult-onset myopia was only evident for White and Asian ethnicities, despite similar sample sizes between the latter and participants of African ancestry. No educational qualifications appeared to have a greater protective effect amongst those from ethnic minority populations versus White participants, although the smaller sample sizes resulted in wide confidence intervals for the effect estimates. Finally, despite the limited sample size, there was a striking interaction between Chinese ethnicity and education which could reflect the relationship between genetic predisposition and environment-environment interactions: Chinese participants without any educational qualifications were not at increased risk of either childhood-onset or adult-onset myopia whilst those with higher highest educational qualifications had the highest risk of both. Together, these findings provide indirect evidence of gene-environment interaction, in particular for childhood-onset myopia.
Table 4. Interactions in associations with myopia (all, childhood-onset and adult -onset) between ethnicity and no educational qualifications.
| Educational attainment adjusted Odds Ratios (95% confidence interval) | |||||
|---|---|---|---|---|---|
| No qualification | O level | A level | Higher level | ||
| All myopia, n = 28,074 | |||||
| Ethnicity | |||||
| White | 1 | 1.67 (1.53, 1.82) | 1.96 (1.82, 2.11) | 2.74 (2.60, 2.89) | |
| Mixed | vs White | 1.16 (0.81, 1.67) | 1.06 (0.81, 1.39) | 0.92 (0.58, 1.46) | 1.05 (0.91, 1.21) |
| vs No qualification | 1 | 1.52 (0.97, 2.40) | 1.54 (0.75, 3.18) | 2.47 (1.56, 3.91) | |
| Asian/British Asian | vs White | 0.61 (0.47, 0.81) | 0.85 (0.76, 0.96) | 0.89 (0.75, 1.06) | 0.82 (0.72, 0.94) |
| vs No qualification | 1 | 2.34 (1.59, 3.43) | 2.85 (2.30, 3.54) | 3.70 (2.61, 5.25) | |
| Black/Black British | vs White | 0.76 (0.47, 1.24) | 1.24 (1.11, 1.38) | 0.67 (0.54, 0.83) | 0.60 (0.56, 0.66) |
| vs No qualification | 1 | 2.72 (1.73, 4.27) | 1.72 (0.95, 3.13) | 2.18 (1.43, 3.33) | |
| Chinese | vs White | 1.13 (0.57, 2.23) | 1.66 (0.90, 3.07) | 1.66 (1.46, 1.89) | 2.06 (1.67, 2.55) |
| vs No qualification | 1 | 2.46 (1.27, 4.74) | 2.89 (1.42, 5.88) | 5.00 (2.76, 9.05) | |
| Other | vs White | 0.61 (0.46, 0.81) | 0.72 (0.53, 0.99) | 0.59 (0.47, 0.74) | 0.72 (0.65, 0.81) |
| vs No qualification | 1 | 1.98 (1.28, 3.09) | 1.89 (1.21, 2.95) | 3.26 (2.29, 4.63) | |
| Adult-onset myopia, n = 11,685 | |||||
| Ethnicity** | |||||
| White | 1 | 1.49 (1.35, 1.64) | 1.58 (1.44, 1.74) | 1.77 (1.63, 1.92) | |
| Mixed | vs White | 1.32 (0.52, 3.34) | 1.04 (0.85, 1.28) | 0.68 (0.50, 0.93) | 1.12 (0.90, 1.39) |
| vs No qualification | 1 | 1.17 (0.50, 2.75) | 0.82 (0.25, 2.65) | 1.49 (0.51, 4.36) | |
| Asian/British Asian | vs White | 0.63 (0.37, 1.07) | 0.89 (0.82, 0.97) | 0.82 (0.69, 0.97) | 0.96 (0.79, 1.16) |
| vs No qualification | 1 | 2.11 (1.13, 3.91) | 2.06 (1.26, 3.35) | 2.69 (1.46, 4.96) | |
| Black/Black British | vs White | 0.90 (0.60, 1.37) | 1.21 (1.04, 1.41) | 0.70 (0.66, 0.74) | 0.92, (0.87, 0.98) |
| vs No qualification | 1 | 2.00 (1.26, 3.16) | 1.23 (0.80, 1.90) | 1.80 (1.24, 2.63) | |
| Chinese | vs White | 1.13 (0.40, 3.21) | 0.97 (0.47, 2.00) | 0.38 (0.23, 0.61) | 1.55 (1.41, 1.71) |
| vs No qualification | 1 | 1.27 (0.35, 4.59) | 0.53 (0.12, 2.28) | 2.42 (0.78, 7.51) | |
| Childhood-onset myopia, n = 16,389 | |||||
| Ethnicity** | |||||
| White | 1 | 1.82 (1.69, 1.97) | 2.33 (2.11, 2.58) | 3.78 (3.61, 3.96) | |
| Mixed | vs White | 0.93 (0.44, 2.92) | 1.05 (0.70, 1.58) | 1.11 (0.63, 1.95) | 1.01 (0.86, 1.19) |
| vs No qualification | 1 | 2.06 (0.75, 5.66) | 2.78 (1.70, 4.53) | 4.11 (2.11, 8.01) | |
| Asian/British Asian | vs White | 0.56 (1.07, 1.96) | 0.81 (0.65, 1.01) | 0.95 (0.75, 1.20) | 0.75 (0.68, 0.84) |
| vs No qualification | 1 | 2.64 (1.92, 3.64) | 3.93 (3.02, 5.12) | 5.09 (4.36, 5.94) | |
| Black/Black British | vs White | 0.57 (1.16, 4.16) | 1.25 (1.09, 1.42) | 0.63 (0.44, 0.91) | 0.42 (0.36, 0.49) |
| vs No qualification | 1 | 4.00 (2.26, 7.10) | 2.59 (1.11, 6.05) | 2.78 (1.58, 4.90) | |
| Chinese | vs White | 1.07 (1.28, 3.54) | 2.28 (1.24, 4.22) | 2.79 (2.24, 3.43) | 2.38 (1.78, 3.18) |
| vs No qualification | 1 | 3.88 (2.36, 6.38) | 6.05 (2.38, 15) | 8.37 (4.09, 17) | |
*Adjustment by year of birth, sex, accommodation tenure, variance adjustment for test centre.
**Estimates by Other ethnicity cannot be made due to low numbers. Bold fonts indicate significant within cohort associations at the 5% level.
Discussion
A moderate increase in myopia frequency over three decades in the UK took the overall baseline from 20.0% of older adults in cohort 1 to 29.2% of the youngest, in cohort 6. However temporal trends varied by type of myopia; with a substantially greater increase in adult-onset (doubling) and in low myopia, as well as plateauing of frequency a decade later (those born in 1960s) for adult-onset myopia. By comparison, a clinically important shift in distribution resulting in increase in severity was only observed in childhood-onset myopia. There were notable differences in patterns of associations with sex, ethnicity, socio-economic status and education by type of myopia along with complex interactions (effect modification) between these predictors over time. Notably the strong positive association across increasing levels of higher educational attainment (proxy for prior educational experience/intensity) and the negative (protective) effect of no educational qualifications was greater for childhood-onset myopia. Amplification of these associations over time (effect modification), more so in men, was substantially greater for childhood-onset than adult-onset myopia indicating greater cumulative experience in those with childhood-onset myopia. Importantly the observed associations with education did not account fully for the cohort effect in myopia frequency and observed interactions between ethnicity and education.
The design and scale of UK Biobank afforded the opportunity to carefully dissect temporal trends taking account of type of myopia, enabling a more meaningful analysis of associations with key risk factors than is achieved by combining all myopia. Standardised ophthalmic assessment and structured standardised collection of information on key environmental factors enabled high quality data to be collected. Using our systematic approach [21] to combining refraction data and age at first optical correction together with self-report on eye conditions enabled both exclusion of those with myopia as a secondary condition and categorisation of type of primary myopia in the remaining individuals. The potential for misclassification using non-cycloplegic autorefraction [22] is low in the age group in our study and its effects minimal given our use of the recently accepted consensus threshold for clinically meaningful myopia of -1.0D [19, 20, 23, 24]. Whilst significant efforts have been directed to measuring educational intensity and experience for example using diaries or ‘wearable’ devices, proxy measures of educational intensity/exposure such as years of education are conventionally used in large scale whole population studies when myopia research has not been the sole/primary purpose. We used educational attainment (lifetime highest educational qualifications), as a proxy for educational experience and intensity, as it directly captures a combination of years of schooling along with childhood time spent on educational activities outside of school (e.g. homework) and educational activities into adult life. We analysed childhood-onset and adult-onset myopia separately, partly to address the potential for confounding by parental history of myopia, which was not measured in Biobank. As UK Biobank comprises volunteers, population prevalence estimates cannot be estimated but the overall frequency of primary myopia in the UKBB is consistent with other European studies, taking into account differences in age range and threshold values for refractive errors between studies [25, 26]. Moreover, risk factor associations in UK Biobank are accepted to be generalisable [27] but as in any cross-sectional study, the possibility of residual confounding exists, so associations reported here do not imply causality.
Conventionally temporal trends and cohort effects are investigated using longitudinal data, but we exploited the scope of the UK Biobank to create historical cohorts of adults who had also fortuitously been assessed at an age when the final myopia phenotype, comprising both type and severity of myopia, could be reliably assigned. As such there are no studies with which we can directly compare our findings. The findings of the present study are consistent with the findings of our prior research in the population-based UK 1958 British birth cohort at age 44 years with respect to frequency (after accounting for small differences in myopia definition) [14, 19], the strong predominance of adult-onset myopia and strikingly similar associations with sex, social class and educational attainment and additionally cognitive performance during childhood. Furthermore, temporal trends observed in the present study are comparable to those reported in a meta-analysis of heterogeneous European studies in terms of both point prevalence and increase in prevalence of all myopia comparing those born by 1939 versus by 1970 [26] This supports the generalisability of our findings.
Myopia is a multifactorial trait and numerous environmental factors e.g. prenatal growth and maternal health during pregnancy, cumulative education/near work activities, socioeconomic status and time spent outdoors are all reported to involved [5, 13, 14, 28–33]. We report here evidence that supports a ‘complex’ of changing environmental factors and presumably changing gene-environment interactions, accounting for increasing overall myopia frequency and shift in distribution to increase in the UK. We show that differential patterns by type of myopia underlying these temporal trends. For example, by finding of interactions between cohort (year of birth) and sex which may explain the conflicting prior reports on all myopia and sex [6, 26, 34–36]. Equally our study shows that the shift in median MSE (increasing severity) over time occurred only for childhood onset (and in particular onset before 10 years), by contrast with adult-onset myopia which increased in frequency but the average MSE remained stable over time. Our findings regarding the different eras for peak/plateau of risk of childhood-onset versus adult-onset myopia could be analogous to different ‘waves’ in a long-duration epidemic. For example, the peak of childhood-onset representing the impact of universal schooling and raising of the school leaving and broader changes in maternal and child health, whilst the ongoing increase in risk of adult-onset myopia reflecting the broader changes in lifestyles with more time spent indoors, increased near viewing occupations or activities.
Interesting variations by ethnicity were evident in the relationship between year of birth and education but only for childhood-onset myopia in UK Biobank. Interpretation of findings relating to UK Biobank participants of Chinese ethnicity– 80.5% of whom were born outside the UK and immigrated aged 17 years or older–is difficult because information is lacking about relevant childhood exposures and also, because they frequently (58.5%) had a degree/higher qualification. The present myopia boom in Asian populations originated after the period studied in Biobank but it is noteworthy that most of the Chinese participants in our study had attained higher education qualifications prior to this epidemic. Ongoing investigations of other ethnic groups born in the UK have not yet demonstrated important gene-environment interaction in myopia frequency in the UK [37].
Since Kepler in the early 17th century, aetiological research has been especially directed to the role of retinal blur (defocus) affecting the peripheral retina during “near work” viewing, such as in an educational context [38, 39], with a more recent focus on the active protective effect of ‘distance’ viewing achieved during time spent outdoors [40] and data-driven approaches to distinguishing the two [41]. Since it would be unethical to undertake randomised clinical trials of different levels of education provision, recent studies have utilised Mendelian randomisation and findings support a causal relationship between a more time spent in education and subsequent myopia [42, 43]. Nevertheless, the association with education is not the sole cause of increasing myopia prevalence [6, 8]. For example, myopia in older populations in China is primarily early-onset ‘genetic’ myopia and independent of educational experience, whereas environmentally-induced myopia associated with education parameters is seen in more recent cohorts [44]. This aligns with our finding that adult-onset myopia accounted for the majority of increase in myopia frequency in the present study, was positively associated with more time in education (although nevertheless to a lesser degree than for childhood-onset) but the effect size of the association changed little over time. By contrast, the ‘protective’ effect of minimal educational experience (no lifetime qualifications) was only evident for childhood-onset myopia and increasingly so over time. Together these findings point to other environmental factors, related to complex societal and behavioural changes that occur with urbanisation, having a differential influence the development of adult-onset myopia which predominates in the UK today.
The childhoods captured in UK Biobank occurred during important changes in education, health and nutrition. Older study participants were children during World War II when many aspects of life including education and access to a varied diet were disrupted/limited. Prior to the formation of the NHS in 1948 and the introduction of vision screening and free prescription glasses affected children may not have been tested or worn glasses [45]. There has been a shift over time in the proportion of children opting to stay in higher and further education and, in parallel, changing methods of teaching, widespread use of TV and more recently the widespread use of electronic screen devices and extension of such activities into free time has altered the pattern of near work activity. In future, although the prevalence of myopia may increase the association between myopia and education parameters may not be as strong and educational attainment as a marker of socioeconomic status and lifestyle may not be a singularly useful marker. The changing lifestyles of children, from pre-school to school age, is raising concerns about health and well-being including about the potential impact of use of screen devices on sleep patterns and cognitive development [46]. In the UK, as part of a public health strategies for children [47–49] physical activity initiatives have been introduced which as a by-product of balancing indoor (near viewing) and outdoor (distance viewing) activity, may help curb the risk of myopia.
Our demonstration of complex temporal trends in the UK, points to a dynamic complex of environmental risk factors and cohort effects in gene-environment interactions, discernible only by distinguishing by between childhood and adult-onset myopia. The evidence of historical plateauing of frequency suggests that stabilisation or reversal of temporal trends is possible. Evidence of a differential impact of educational experience/intensity on childhood-onset and high myopia and its amplification over time predicts future increasing frequency of childhood-onset myopia without attention to educational intensity/experience continues to increase. But attention to childhood-onset myopia alone will not address the considerable public health impact of myopia. Whilst adult-onset myopia is generally less severe, it remains as/more common in the UK population and thus confers considerable personal and societal burden. A mixed economy of research is needed to improve our understanding of modifiable risk factors across the life course and how to tackle them.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
* Numbers do not include n = 120 individuals who did not report age of first wearing glasses and were assigned category based on other aggregate evidence.
(PDF)
* OR = Odds Ratio. 95% CI = 95% confidence interval. ** Adjusted by year of birth, sex, educational qualification, accommodation tenure, ethnicity, variance adjustment for test centre. ¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi. Bold fonts indicate significant associations at the 5% level.
(PDF)
*OR = Odds Ratio. 95% CI = 95% confidence interval. ** Adjusted by year of birth, sex, educational qualification, accommodation tenure, ethnicity, variance adjustment for test centre. ¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi. Bold fonts indicate significant associations at the 5% level.
(PDF)
¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi.
(PDF)
¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi.
(PDF)
Acknowledgments
This research has been conducted using the UK Biobank Resource under Application Number 669. The authors thank the study participants, the field investigators responsible for recruiting and the data management staff. The UK Biobank Eye and Vision consortium is supported by a grant from the Special Trustees of Moorfields Eye Hospital. The main contact for this consortium is Prof Paul Foster (p.foster@ucl.ac.uk). Membership of the UK Biobank Eye and Vision consortium is provided on their website: https://www.ukbiobankeyeconsortium.org.uk/people
Jugnoo Rahi is a National Institute for Health Research (NIHR) Senior Investigator, supported by the National Institute for Health Research (NIHR) Biomedical Research Centres at Moorfields Eye Hospital/UCL Institute of Ophthalmology, and at the UCL Institute of Child Health/Great Ormond Street Hospital. Phillippa Cumberland was supported by the Ulverscroft Foundation (https://www.ulverscroft-foundation.org.uk). Views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health, all of which had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data Availability
Dissemination of the findings to study participants will be through the UK Biobank website. Data cannot be shared publicly because of restrictions according to the material transfer agreement. Data are available from the UK Biobank for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from UK Biobank (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access).
Funding Statement
The authors received no specific funding for this specific work.
References
- 1.Flitcroft DI. Is myopia a failure of homeostasis? Experimental eye research. 2013;114:16–24. doi: 10.1016/j.exer.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 2.Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Annals of the Academy of Medicine, Singapore. 2004;33(1):27–33. [PubMed] [Google Scholar]
- 3.Lin Z, Gao T, Vasudevan B, Jhanji V, Ciuffreda KJ, Zhang P, et al. Generational difference of refractive error and risk factors in the Handan Offspring Myopia Study. Investigative ophthalmology & visual science. 2014. 2014;55(9):5711–7. doi: 10.1167/iovs.13-13693 [DOI] [PubMed] [Google Scholar]
- 4.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–42. doi: 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists). 2012;32(1):3–16. doi: 10.1111/j.1475-1313.2011.00884.x [DOI] [PubMed] [Google Scholar]
- 6.Bar Dayan Y, Levin A, Morad Y, Grotto I, Ben-David R, Goldberg A, et al. The changing prevalence of myopia in young adults: a 13-year series of population-based prevalence surveys. Investigative ophthalmology & visual science. 2005;46(8):2760–5. doi: 10.1167/iovs.04-0260 [DOI] [PubMed] [Google Scholar]
- 7.Vitale S, Sperduto RD, Ferris FL 3rd. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Archives of ophthalmology. 2009;127(12):1632–9. doi: 10.1001/archophthalmol.2009.303 [DOI] [PubMed] [Google Scholar]
- 8.Williams KM, Bertelsen G, Cumberland P, Wolfram C, Verhoeven VJ, Anastasopoulos E, et al. Increasing Prevalence of Myopia in Europe and the Impact of Education. Ophthalmology. 2015;122(7):1489–97. doi: 10.1016/j.ophtha.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parssinen O. The increased prevalence of myopia in Finland. Acta ophthalmologica. 2012;90(6):497–502. doi: 10.1111/j.1755-3768.2011.02210.x [DOI] [PubMed] [Google Scholar]
- 10.Chua SYL, Foster PJ. The Economic and Societal Impact of Myopia and High Myopia. In: Ang M, Wong TY, editors. Updates on Myopia: A Clinical Perspective. Singapore: Springer Singapore; 2020. p. 53–63. [Google Scholar]
- 11.Smith TS, Frick KD, Holden BA, Fricke TR, Naidoo KS. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bulletin of the World Health Organization. 2009;87(6):431–7. doi: 10.2471/blt.08.055673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua SY, Sabanayagam C, Cheung YB, Chia A, Valenzuela RK, Tan D, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists). 2016;36(4):388–94. doi: 10.1111/opo.12305 [DOI] [PubMed] [Google Scholar]
- 13.Morgan I, Rose K. How genetic is school myopia? Progress in retinal and eye research. 2005;24(1):1–38. doi: 10.1016/j.preteyeres.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 14.Rahi JS, Cumberland PM, Peckham CS. Myopia over the lifecourse: prevalence and early life influences in the 1958 British birth cohort. Ophthalmology. 2011;118(5):797–804. doi: 10.1016/j.ophtha.2010.09.025 [DOI] [PubMed] [Google Scholar]
- 15.Palmer LJ. UK Biobank: bank on it. The Lancet. 2007;369(9578):1980–2. [DOI] [PubMed] [Google Scholar]
- 16.Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, et al. UK Biobank: Current status and what it means for epidemiology. Health Policy and Technology. 2012;1(3):123–6. [Google Scholar]
- 17.UK Biobank. Enable Your Research [updated 2020, December 11th. Available from: https://www.ukbiobank.ac.uk/enable-your-research.
- 18.Cumberland PM, Bao Y, Hysi PG, Foster PJ, Hammond CJ, Rahi JS. Frequency and Distribution of Refractive Error in Adult Life: Methodology and Findings of the UK Biobank Study. PloS one. 2015;10(10):e0139780. doi: 10.1371/journal.pone.0139780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cumberland PM, Bountziouka V, Rahi JS. Impact of varying the definition of myopia on estimates of prevalence and associations with risk factors: time for an approach that serves research, practice and policy. The British journal of ophthalmology. 2018;102(10):1407–12. doi: 10.1136/bjophthalmol-2017-311557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flitcroft DI, He M, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, et al. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investigative ophthalmology & visual science. 2019;60(3):M20–m30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cumberland PM, Chianca A, Rahi JS. Accuracy and Utility of Self-report of Refractive Error. JAMA ophthalmology. 2016;134(7):794–801. doi: 10.1001/jamaophthalmol.2016.1275 [DOI] [PubMed] [Google Scholar]
- 22.Sanfilippo PG, Chu BS, Bigault O, Kearns LS, Boon MY, Young TL, et al. What is the appropriate age cut-off for cycloplegia in refraction? Acta ophthalmologica. 2014;92(6):e458–62. doi: 10.1111/aos.12388 [DOI] [PubMed] [Google Scholar]
- 23.Fotouhi A, Morgan IG, Iribarren R, Khabazkhoob M, Hashemi H. Validity of noncycloplegic refraction in the assessment of refractive errors: the Tehran Eye Study. Acta ophthalmologica. 2012;90(4):380–6. doi: 10.1111/j.1755-3768.2010.01983.x [DOI] [PubMed] [Google Scholar]
- 24.Krantz EM, Cruickshanks KJ, Klein BE, Klein R, Huang GH, Nieto FJ. Measuring refraction in adults in epidemiological studies. Archives of ophthalmology. 2010;128(1):88–92. doi: 10.1001/archophthalmol.2009.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfram C, Hohn R, Kottler U, Wild P, Blettner M, Buhren J, et al. Prevalence of refractive errors in the European adult population: the Gutenberg Health Study (GHS). The British journal of ophthalmology. 2014;98(7):857–61. doi: 10.1136/bjophthalmol-2013-304228 [DOI] [PubMed] [Google Scholar]
- 26.Williams KM, Verhoeven VJ, Cumberland P, Bertelsen G, Wolfram C, Buitendijk GH, et al. Prevalence of refractive error in Europe: the European Eye Epidemiology (E(3)) Consortium. European journal of epidemiology. 2015;30(4):305–15. doi: 10.1007/s10654-015-0010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batty G D, Gale C R, Kivimäki M, Deary I J, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis BMJ 2020;368:m131. doi: 10.1136/bmj.m131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherwin JC, Hewitt AW, Coroneo MT, Kearns LS, Griffiths LR, Mackey DA. The association between time spent outdoors and myopia using a novel biomarker of outdoor light exposure. Investigative ophthalmology & visual science. 2012;53(8):4363–70. doi: 10.1167/iovs.11-8677 [DOI] [PubMed] [Google Scholar]
- 29.Mirshahi A, Ponto KA, Hoehn R, Zwiener I, Zeller T, Lackner K, et al. Myopia and level of education: results from the Gutenberg Health Study. Ophthalmology. 2014;121(10):2047–52. doi: 10.1016/j.ophtha.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 30.Williams C, Miller LL, Gazzard G, Saw SM. A comparison of measures of reading and intelligence as risk factors for the development of myopia in a UK cohort of children. The British journal of ophthalmology. 2008;92(8):1117–21. doi: 10.1136/bjo.2007.128256 [DOI] [PubMed] [Google Scholar]
- 31.Ip JM, Saw SM, Rose KA, Morgan IG, Kifley A, Wang JJ, et al. Role of near work in myopia: findings in a sample of Australian school children. Investigative ophthalmology & visual science. 2008;49(7):2903–10. [DOI] [PubMed] [Google Scholar]
- 32.Dirani M, Shekar SN, Baird PN. The role of educational attainment in refraction: the Genes in Myopia (GEM) twin study. Investigative ophthalmology & visual science. 2008;49(2):534–8. doi: 10.1167/iovs.07-1123 [DOI] [PubMed] [Google Scholar]
- 33.Mackey DA. Myopia-The future progression of myopia: Seeing where we are going. Ophthalmic genetics. 2016;37(4):361–5. doi: 10.1080/13816810.2016.1232416 [DOI] [PubMed] [Google Scholar]
- 34.Lee KE, Klein BE, Klein R, Wong TY. Changes in refraction over 10 years in an adult population: the Beaver Dam Eye study. Investigative ophthalmology & visual science. 2002;43(8):2566–71. [PubMed] [Google Scholar]
- 35.Vitale S, Ellwein L, Cotch MF, Ferris FL 3rd, Sperduto R. Prevalence of refractive error in the United States, 1999–2004. Archives of ophthalmology. 2008;126(8):1111–9. doi: 10.1001/archopht.126.8.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology. 1999;106(6):1066–72. doi: 10.1016/S0161-6420(99)90251-8 [DOI] [PubMed] [Google Scholar]
- 37.Logan NS, Davies LN, Mallen EA, Gilmartin B. Ametropia and ocular biometry in a U.K. university student population. Optometry and vision science: official publication of the American Academy of Optometry. 2005;82(4):261–6. [DOI] [PubMed] [Google Scholar]
- 38.Curtin BJ. Myopia:a review of its etiology, pathogenesis and treatment. Survey of ophthalmology. 1970(15):1–17. [Google Scholar]
- 39.Weale RA. Epidemiology of refractive errors and presbyopia. Surv Ophthalmol. 2003;48(5):515–43. doi: 10.1016/s0039-6257(03)00086-9 [DOI] [PubMed] [Google Scholar]
- 40.Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: Aetiology and prevention. Progress in retinal and eye research. 2018;62:134–49. doi: 10.1016/j.preteyeres.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 41.Ye B, Liu K, Cao S, Sankaridurg P, Li W, Luan M, et al. Discrimination of indoor versus outdoor environmental state with machine learning algorithms in myopia observational studies. Journal of translational medicine. 2019;17(1):314. doi: 10.1186/s12967-019-2057-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mountjoy E, Davies NM, Plotnikov D, Smith GD, Rodriguez S, Williams CE, et al. Education and myopia: assessing the direction of causality by mendelian randomisation. BMJ (Clinical research ed). 2018;361:k2022. doi: 10.1136/bmj.k2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuellar-Partida G, Lu Y, Kho PF, Hewitt AW, Wichmann HE, Yazar S, et al. Assessing the Genetic Predisposition of Education on Myopia: A Mendelian Randomization Study. Genetic epidemiology. 2016;40(1):66–72. doi: 10.1002/gepi.21936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jonas JB, Xu L, Wang YX, Bi HS, Wu JF, Jiang WJ, et al. Education-Related Parameters in High Myopia: Adults versus School Children. PloS one. 2016;11(5):e0154554. doi: 10.1371/journal.pone.0154554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart-Brown SL, Haslum MN, Howlett B. Preschool vision screening: a service in need of rationalisation. Archives of disease in childhood. 1988;63(4):356–9. doi: 10.1136/adc.63.4.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheung CH, Bedford R, Saez De Urabain IR, Karmiloff-Smith A, Smith TJ. Daily touchscreen use in infants and toddlers is associated with reduced sleep and delayed sleep onset. Scientific reports. 2017;7:46104. doi: 10.1038/srep46104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan RA, Kuzel AH, Vaandering ME, Chen W. The Association of Physical Activity and Academic Behavior: A Systematic Review. The Journal of school health. 2017;87(5):388–98. doi: 10.1111/josh.12502 [DOI] [PubMed] [Google Scholar]
- 48.Sporting Future (Second annual report) 2018 [Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/677557/2018_Second_annual_report_to_parliament_on_Sporting_Future.pdf.
- 49.Childhood Obesity: a plan for action 2018 [Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/718903/childhood-obesity-a-plan-for-action-chapter-2.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
* Numbers do not include n = 120 individuals who did not report age of first wearing glasses and were assigned category based on other aggregate evidence.
(PDF)
* OR = Odds Ratio. 95% CI = 95% confidence interval. ** Adjusted by year of birth, sex, educational qualification, accommodation tenure, ethnicity, variance adjustment for test centre. ¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi. Bold fonts indicate significant associations at the 5% level.
(PDF)
*OR = Odds Ratio. 95% CI = 95% confidence interval. ** Adjusted by year of birth, sex, educational qualification, accommodation tenure, ethnicity, variance adjustment for test centre. ¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi. Bold fonts indicate significant associations at the 5% level.
(PDF)
¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi.
(PDF)
¥O level: State examination at age 16 years; A level: State examination at age 18 years. §Asian category includes Indian, Pakistani and Bangladeshi.
(PDF)
Data Availability Statement
Dissemination of the findings to study participants will be through the UK Biobank website. Data cannot be shared publicly because of restrictions according to the material transfer agreement. Data are available from the UK Biobank for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from UK Biobank (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access).