Abstract
The pathogenesis of infection induced by cytopathogenic isolates from the newly identified genetic cluster Id of bovine viral diarrhea virus (BVDV) type I was studied in two experimental infections of previously seronegative, immunocompetent calves. Experiment 1 focused on the evaluation of clinical patterns, viremia, and serological responses. All infected calves in this experiment developed respiratory symptoms and seroconverted to BVDV positivity. Contact calves also contracted a respiratory tract infection following exposure to infected animals. Viremia was demonstrated between postinfection days 2 and 17, and the virus was detected in organ specimens of all but one each of the infected and contact calves. In experiment 2, the distribution of BVDV in various tissues of calves euthanized at defined days postinfection was studied. In two of these calves recurrent shedding of BVDV in nasal secretions was shown. BVDV was detected in various tissues of all infected calves throughout the experiment and also following seroconversion and the clearance of BVDV from the circulatory system. Despite the widespread distribution of the virus in various organs, significant tissue damage was found mainly in respiratory tract and lymphoid tissues. These experiments revealed that viruses from cluster Id of BVDV are able to induce primary respiratory disease in previously seronegative, immunocompetent calves. Contact transmission and virus recurrence, contrary to observations from acute experimental infections with noncytopathogenic BVDV, are likely to reflect differences in biological features of these cytopathogenic isolates. Virus shedding and its presence in tissues following peripheral clearance and in the presence of antibodies may have implications in the diagnosis, pathogenesis, and epidemiology of BVDV-induced syndromes in cattle.
Bovine viral diarrhea virus (BVDV) is a member of the genus Pestivirus, which also includes classical swine fever virus and border disease virus, within the family Flaviviridae (34). The genome of BVDV is a positive-sense RNA of about 12.5 kb in length and encodes a single large polyprotein, which is co- and posttranslationally processed into mature viral proteins by host cell- and virus-encoded proteases (31).
Genetic typing has shown that BVDV strains can be segregated into two genotypes, BVDV type I and BVDV type II (Pestivirus type 1 and type 4, respectively, in the new proposed division of pestiviruses) (4, 23, 24, 27, 33). BVDV type I has been further subdivided genetically and serologically into subgroups Ia and Ib (22, 23, 24, 32). Further genome characterization studies have shown an extensive antigenic and genetic diversity among BVDV type I strains (3, 5, 22, 23). Strain heterogeneity and differences in pathogenicity may have a determinant role in the pathogenesis and clinical outcome of infections induced by BVDV.
On the basis of their ability to induce a cytopathic effect on cell cultures, BVDV strains are divided into a cytopathogenic (cp) biotype and a noncytopathogenic (ncp) biotype. The majority of acute infections are caused by the ncp biotype, while the cp biotype is commonly isolated, together with the ncp biotype, in animals suffering from mucosal disease (MD) (19). This fatal condition develops when animals persistently infected (PI) with an ncp strain are superinfected with a cp strain that is either of exogenous origin or arises from genetic changes in a resident ncp virus (reviewed in 18).
Acute infections of seronegative immunocompetent cattle with BVDV type I are often subclinical or result in mild disease. Clinical signs of acute infection include fever, leukopenia, nasal discharge, diarrhea, erosions in the oral mucosa, and immunosuppression (reviewed in 1). This immunosupression has been documented to enhance susceptibility to infection with secondary pathogens such as the ones causing respiratory disease (reviewed in 26). The production of neutralizing antibodies and clearance of the virus are the normal outcome of acute infections (1).
Most studies on the in vivo biological effects of cp BVDV have mainly focused on their role in combination with ncp BVDV in the induction of MD (11, 12, 13, 16, 20). We have previously identified two new genetic clusters within BVDV type I, distinct from subgroups Ia and Ib, and have preliminarily termed them clusters Ic and Id (3). Of these, cluster Id viruses were found to be predominantly associated with field cases of respiratory tract disease in local cattle from the southern part of Africa (3). To define in vivo biological features of these viruses under controlled conditions, we have characterized here two cp isolates representative of cluster Id but not associated with classical MD. The clinical, virological, and serological responses following infection of previously seronegative, immunocompetent calves were evaluated in the first experiment. In the second experiment, the distribution of virus in different tissues of experimentally infected calves was studied.
MATERIALS AND METHODS
Cells and viruses.
Secondary bovine turbinate cells were grown in Eagle's minimum essential medium supplemented with 10% fetal calf serum (FCS). The cells and serum were tested to ensure their freedom from adventitious contamination with BVDV, and the FCS was found to be free from antibodies against BVDV. The cp isolates Mo1 and Mo2, referred to as M1118-8CK/95 and M1096-5IN/95 in a previous work (3), were propagated on these cells maintained in Eagle's minimum essential medium with 2% FCS at 37°C and 5% CO2. Prior to infection of calves, the inocula were checked to ensure that they were free from bovine respiratory syncytial virus (BRSV), bovine herpesvirus type 1 (BHV-1), and bovine adenoviruses (BAV) by using our previously developed reverse transcription-PCR (RT-PCR) and PCR procedures (28; K. Öhman-Forslund, personal communication).
Calves.
The calves were of the Holstein-Friesian breed, 2 to 3 months old, and of either sex. They originated from a single BVDV-free farm and were kept in isolation for 2 weeks prior to infection. Nasal swabs, white blood cells (WBCs), and sera from all of the calves were tested by RT-PCR for BVDV at days −15, −5, and 0 to confirm the absence of BVDV infection. Nasal swabs taken from all of the calves at day −5 preinfection were tested for BRSV, BHV-1, and BAV, as indicated above.
Preinfection sera were tested for antibodies to BVDV in an indirect enzyme-linked immunosorbent assay (ELISA) (SVANOVA Biotech, Uppsala, Sweden) to confirm the seronegative status of the calves.
Experimental infections and sampling. (i) Experiment 1.
Twelve calves were allocated into three groups—I, II and III. Each group was kept in an isolated room, and the calves were individually tethered. Group I contained five calves, of which two (no. 30 and 31) were infected intranasally and two (no. 33 and 34) were infected intravenously, each with 2 ml of cell culture supernatant containing 106 50% tissue culture-infective doses (TCID50) of isolate Mo1. Group II also contained five calves, of which two (no. 35 and 36) were infected intranasally and two (no. 37 and 38) were infected intravenously, each with 2 ml of cell culture supernatant containing 105 TCID50 of isolate Mo2. The fifth calf in each group (no. 32 [group I] and 39 [group II]) remained uninfected but in contact with the infected calves. Group III contained two calves (no. 40 and 42) that were each mock infected with 2 ml of culture medium from noninfected bovine turbinate cells as controls. The calves were monitored daily for clinical signs, and rectal temperatures were determined throughout a 30-day observation period. Nasal swabs were collected from all calves at postinfection days 1, 2, 4, 7, 11, 15, 21, and 30. At days 1, 4, 11, 15, 21, and 30 postinfection, blood samples for sera and EDTA anticoagulated blood were collected for isolation of peripheral WBCs. At the end of the observation period, all of the calves were euthanized, and a set of specimens from nine different tissues was collected from each calf and frozen at −70°C until use.
(ii) Experiment 2.
Ten calves were each infected intranasally with 5 ml of cell culture supernatant containing 106 TCID50 of BVDV isolate Mo1. The calves were kept in isolation during the course of the experiment. A control calf was euthanized at day 0 to provide tissues from a noninfected animal. At days 2, 3, 5, 7, 10, 12, 14, 17, 19, 21 and 31 postinfection, nasal swabs and blood with and without EDTA were collected from all calves. One infected calf was euthanized each day on days 3, 5, 7, 10, 12, 14, 17, 19, 21, and 31 and examined for gross pathological changes, and a set of specimens from 26 different tissues was collected for each euthanized calf.
Detection of BVDV. (i) Isolation of BVDV.
Virus isolation was performed following standard procedures (9). Prior to cell infection, nasal swabs were soaked in 2 ml of phosphate-buffered saline containing antibiotics, and WBCs were purified from whole blood by centrifugation on a Ficoll-Paque cushion. The tissue samples were homogenized in phosphate-buffered saline without Ca2+ and Mg2+ to obtain 10% (wt/vol) suspensions. Secondary cultures of bovine turbinate cells were inoculated with nasal swabs, WBC lysates, and tissue homogenates and passaged three times at weekly intervals.
(ii) Detection of BVDV genomic RNA.
RNA was extracted from nasal swabs, WBCs, sera, and tissue homogenates using the TRIzol LS reagent (Gibco BRL) according to the manufacturer's instructions. Synthesis of cDNA was carried out by random priming and by using M-MLV RT (Gibco BRL). A nested PCR was used to amplify part of the 5′ noncoding region (5′NCR) of the viral genome essentially as previously described (10). Briefly, 50-μl reaction mixtures with primers OPES13A (nucleotides 102 through 123 in the genome of BVDV strain NADL) and OPES14A (nucleotides 400 through 376 in NADL) were subjected to a program consisting of initial denaturation at 95°C for 2 min and 5 amplification cycles of 94°C for 45 s, 53°C for 1 min, and 72°C for 1 min, followed by 30 cycles of 94°C for 45 s, 48°C for 1 min, and 72°C for 1 min. A final extension at 72°C for 7 min was also included. The second PCR was performed using primers OPES11 (nucleotides 179 through 206 in NADL) and OPES12A (nucleotides 351 through 329 in NADL), and the cycling program was 95°C for 2 min for initial denaturation and 5 amplification cycles of 94°C for 45 s, 57°C for 1 min, and 72°C for 1 min, followed by 30 cycles of 94°C for 45 s, 52°C for 1 min, and 72°C for 1 min, ending with a final extension at 72°C for 7 min. The 169-bp specific PCR products were visualized by ethidium bromide staining following electrophoresis on 2% agarose gels. Precautions to avoid contamination were followed throughout the RT-PCR, as previously described (6).
Detection of heterologous viruses.
To rule out concurrent infections with other respiratory tract viruses, the following samples were tested by using our routine PCR assays to detect BRSV, BHV-1, and BAV (as described above): (i) in experiment 1, samples included nasal swabs from days 0, 4, 7, 11, 15 and 21 postinfection and postmortem respiratory tissue samples from calves 30, 32, and 33 (group I) and calves 35, 37, 38, and 39 (group II); (ii) in experiment 2, samples included nasal swabs from days 0, 5, 7, 12, 17, 19, 21 and 31 postinfection and postmortem respiratory tissue samples from calves 136, 4, 222, 3, and 115, euthanized at days 5, 10, 14, 19, and 31, respectively.
Serology.
Sera from consecutive samplings were tested in the same plate in fivefold dilutions starting from 1:10, using an indirect ELISA for BVDV (SVANOVA Biotech), following the manufacturer's instructions. End points were determined as the highest dilutions giving a corrected mean optical density at 450 nm that was higher than 0.2.
For BRSV, bovine coronavirus (BCV), parainfluenza 3 virus (PI3), and BHV-1, pre- and postinfection sera were tested at a single dilution on commercial antibody ELISA (SVANOVA Biotech). Tests for BAV antibodies were done using the Bio-X adenovirus 3 ELISA kit (Bio-X, Marche-en-Famenne, Belgium), following the manufacturer's instructions.
Seroconversion was considered to have occurred if calves with negative preinfection sera showed an optical density at 450 nm of 0.2 or higher in postinfection sera.
Gross and histopathological examinations.
Gross pathological examination was done for experiment 2 only, following standard methodology. For histopathological examinations, tissue samples were placed into 8% buffered formaldehyde. Fixed tissues were embedded in paraffin, and 6-μm sections were cut and stained with hematoxylin-eosin.
RESULTS
Clinical observations.
In experiment 1, all infected calves had clinical symptoms. Figure 1A shows the clinical picture of representative animals, including one of the infected calves and the respective contact calf from each group. Isolate Mo1 (group I) induced mainly nasal discharge and fever (rectal temperatures above 39.8°C). One calf (no. 30) also showed ocular discharge, abnormal breathing, and coughing, and two calves (no. 30 and 33) had erosions in their oral mucosa. The clinical picture induced by isolate Mo2 (group II) was more pronounced and included ocular discharge, nasal discharge, fever, coughing, abnormal breathing, and lesions in the oral mucosa. Calves no. 36 and 38 also had transient diarrhea. The earliest symptom recorded was nasal discharge, while the remaining symptoms developed from day 7 on, in the majority of infected calves, regardless of the route of infection and the virus inoculated. The contact calves developed clinical symptoms from day 11 (no. 32 [group I]) and day 12 (no. 39 [group II]). These consisted of nasal discharge in the calf exposed to isolate Mo1, whereas the calf exposed to Mo2 showed symptoms as recorded for Mo2-infected calves. Both mock-infected control calves remained healthy.
FIG. 1.
Pattern of clinical symptoms, viremia, and serology. Data for representative animals from experiment 1 (A) and experiment 2 (B) are shown. (A) Seronegative, immunocompetent calves were experimentally infected with cp isolates of BVDV Mo1 (group 1, calf no. 31) and Mo2 (group II, calf no. 36) in experiment 1. Calves no. 32 and 39 were contact controls in the respective groups. (B) In experiment 2, seronegative, immunocompetent calves were experimentally infected with cp isolate BVDV Mo1 and euthanized at different days postinfection. Solid bars, duration of clinical symptoms; V, detection of virus; +, virus positive; −, virus negative (for details, see Table 1); S, seroconversion; †, time point after initial infection when the calf was euthanized. For both panels A and B, the top row of each table indicates day(s) postinfection and the left column of each table indicates type of diagnosis or event (see key in panel B).
In experiment 2, the main clinical signs recorded were nasal discharge, coughing, and fever. Similar to Mo1-induced symptoms in experiment 1, there was an increased incidence of coughing and extended duration of fever, as shown in Fig. 1B. Two calves, no. 118 and 134, also showed erosion in the oral mucosa.
Detection of BVDV.
In the two experiments, viremia was demonstrated on at least two sampling occasions, between days 2 and 11 by virus isolation and between days 2 and 17 by RT-PCR. Viremia was detected more frequently by analysis of WBCs than of nasal swabs (Table 1). In experiment 2, recurrent shedding of BVDV in nasal secretions was detected in two calves (no. 34 and 115) (Fig. 1B and Table 1).
TABLE 1.
Detection of viruses in nasal swabs and WBCs
| Experiment and day(s) postinfection | No. samples from nasal swabs that were positive for BVDV by
|
No. samples from WBCs that were positive for BVDV by
|
||||||
|---|---|---|---|---|---|---|---|---|
| VIa | RT-PCR | Total positive | Total tested | VI | RT-PCR | Total positive | Total tested | |
| Experiment 1 | ||||||||
| 1 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 |
| 2 | 2 | 2 | 2 | 10 | 1 | 2 | 2 | 10 |
| 4 | 2 | 4 | 4 | 10 | 2 | 5 | 6 | 10 |
| 7 | 3 | 5 | 5 | 10 | 4 | 8 | 8 | 10 |
| 11 | 1 | 2 | 2 | 10 | 2 | 7 | 7 | 10 |
| 15 | 0 | 0 | 0 | 10 | 1 | 2 | 2 | 10 |
| 21 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 |
| 30 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 |
| Experiment 2 | ||||||||
| 2 | 5 | 5 | 5 | 10 | 2 | 7 | 7 | 10 |
| 3 | 2 | 3 | 3 | 10 | 4 | 6 | 7 | 10 |
| 5 | 3 | 5 | 5 | 9 | 3 | 9 | 9 | 9 |
| 7 | 3 | 5 | 6 | 8 | 8 | 8 | 8 | 8 |
| 10 | 1 | 1 | 1 | 7 | 3 | 7 | 7 | 7 |
| 12 | 0 | 0 | 0 | 6 | 3 | 5 | 5 | 6 |
| 14 | 0 | 1 | 1 | 5 | 1 | 3 | 3 | 5 |
| 17 | 0 | 0 | 0 | 4 | 1 | 3 | 3 | 4 |
| 19 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 |
| 21 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 2 |
| 31 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
VI, virus isolation.
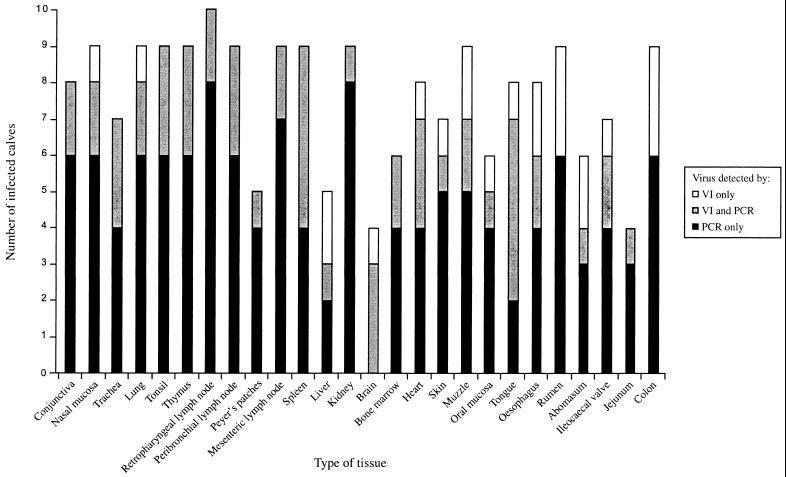
When organ specimens were analyzed at the end of experiment 1, BVDV was detected in tissues from seven of the eight infected calves and from the two contact control calves by virus isolation and RT-PCR. The virus-positive tissues were bone marrow (in calves no. 30, 31, 32, 33, 35, and 37), nasal mucosa (in calves no. 31, 35, 36, and 39), lung (in calves no. 32, 35, and 39), spleen (calf no. 38), and the peribronchial lymph node (calf no. 39). Virus was not detected in tissues from the mock-infected calves. In experiment 2, BVDV was detected in organ specimens from all of the infected calves, regardless of the time point after infection, when the calves were euthanized. All types of tissues analyzed were found to contain virus by either virus isolation, RT-PCR, or both methods (Fig. 2). More specimens were tested positive by RT-PCR alone than by virus isolation alone. BVDV was most frequently detected in (i) all lymphoid tissues, (ii) the conjunctiva and oronasal cavity (nasal mucosa, tongue, muzzle, and oral mucosa), (iii) respiratory tract (lungs and trachea), (iv) gastrointestinal tract (colon, ileocecal valve, rumen, and esophagus), and (v) urinary tract (kidney). Tissues like heart muscle, skin, bone marrow, liver, and brain were also found to harbor virus. In some cases, mostly from the gastroenteric tissue, BVDV was detected only after passages in cell cultures. Virus was still present in different tissues of calves euthanized at the end of the experiment, 31 days postinfection, when virus was no longer detected in blood cell fractions.
FIG. 2.
Distribution of BVDV in tissues in experiment 2. Specimens from 26 tissues were collected from each calf at different days following experimental infection with cp isolate BVDV Mo1. The specimens were analyzed by virus isolation (VI) and reverse transcription-PCR (PCR).
Detection of heterologous viruses.
Nucleic acids of respiratory pathogens BRSV, BHV-1, and BAV were detected neither in the nasal swabs collected pre- and postinfection nor in the postmortem respiratory specimens analyzed.
Serology.
All of the infected calves seroconverted to BVDV positivity during the course of the experiments, with serum antibody titers between 1:50 and 1:250. In experiment 1, antibodies to BVDV were detected in the sera of three of the infected calves from day 15 on (no. 30, 35, and 38) and later in the remaining infected animals. The two contact calves (no. 32 and 39) did not show detectable antibody levels to BVDV by the time they were sacrificed. The uninfected calves remained seronegative.
In experiment 2, BVDV antibody titers between 1:50 and 1:250 were detected in three of the four animals euthanized from day 17 on (no. 134, 34, and 115).
Testing of preinfection and postinfection sera for BRSV, PI3, BCV, BAV, and BHV-1 gave no indication of seroconversion to those pathogens during the course of the two experiments, except for one calf in experiment 1 (no. 37) which seroconverted to BCV positivity.
Gross and histopathological examinations.
In experiment 2, the following main changes were observed at gross pathological examination. In the nasal cavity a serous exudate was seen. Petechial hemorrhages were observed in the tracheal mucosa. In the lungs focal catarrhal bronchopneumonia and focal atelectasis were detected. Examination of the oral cavity showed erosions on the tongue and cheeks. In the gastroenteric tract, erosions occurred in the abomasum, and mucus was present in the small intestine. The tonsils and the retropharyngeal, peribronchial, and mesenteric lymph nodes were enlarged in calves euthanized within the first days.
The changes found at histopathological examination are listed in Table 2. The most relevant findings were the following:
TABLE 2.
Histopathological lesions in tissue specimens of calves infected with cp isolate BVDV Mo1 in experiment 2 and euthanized at different days postinfection
| Tissue analyzed | Lesion type | Presence of lesion after euthanasia on postinfection daya
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 5 | 7 | 10 | 12 | 14 | 17 | 19 | 21 | 31 | ||
| Oral cavity | Intraepithelial vesicles | X | X | X | |||||||
| Erosion with reparation | X | X | X | ||||||||
| Perivascular lymphocytic inflammation in submucosa, focal degeneration in the epithelium, inclusions in the cytoplasm of epithelial cells | X | ||||||||||
| Esophagus | Vacuolization in the stratum spinosum and stratum splanocellulare | X | |||||||||
| Rumen | Localized chronic purulent inflammation- | X | X | ||||||||
| Focal epithelial regeneration | X | ||||||||||
| Abomasum | Subacute inflammation in the mucosa | X | X | ||||||||
| Epithelial regeneration | X | ||||||||||
| Small and large intestines | Eosinophil infiltration | X | X | X | X | X | X | X | X | X | X |
| Peyer's patches and ileocecal valve | Eosinophil granulocyte infiltration | X | X | X | X | ||||||
| Lymphoid depletion | X | X | X | ||||||||
| Lymphoid depletion and mitosis | X | X | X | ||||||||
| Mesenteric lymph node | Hyperplasia of germinal centers and eosinophil granulocyte infiltration | X | X | X | X | ||||||
| Hyperplasia of germinal centers and necrosis | X | ||||||||||
| Lymphoid depletion and mitosis | X | X | X | X | X | ||||||
| Thymus | Mild lymphoid depletion in the cortex | X | X | X | X | X | X | ||||
| Tonsils | Follicular hyperplasia | X | X | X | X | ||||||
| Hyperplasia of germinal centers and necrosis | X | X | |||||||||
| Lymphoid degeneration, mild lymphoid depletion | X | ||||||||||
| Lymphoid depletion and mitosis in the germinal centers | X | X | X | ||||||||
| Retropharyngeal and peribronchial lymph node | Hyperplasia of germinal centers | X | X | X | X | X | X | ||||
| Hyperplasia of germinal centers and necrosis | X | ||||||||||
| Lymphoid depletion and mitosis in the germinal centers | X | X | X | ||||||||
| Nasal cavity and trachea | Acute catarrhal inflammation | X | X | X | X | ||||||
| Subacute inflammation | X | X | X | X | X | X | |||||
| Lung | Peribronchial lymphoid hyperplasia | X | X | X | |||||||
| Focal intralobular interstitial pneumonia | X | X | X | X | X | X | X | ||||
| Bronchiolitis and focal atelectasis | X | ||||||||||
| Hyperplasia of the media of small arteries | X | X | |||||||||
| Heart muscle | Subacute focal myocardial necrosis | X | |||||||||
| Lymphocytic perivasculitis, lymphocytic myocarditis | X | ||||||||||
| Brain | Perivascular lymphohistiocytic inflammation in the brain stem | X | X | ||||||||
| Panencephalitis | X | ||||||||||
| Spleen | Infiltration with neutrophil granulocytes in the red pulp | X | X | ||||||||
| Hyperplasia of Malpighian bodies | X | X | X | ||||||||
| Hyperplasia of Malpighian bodies and necrosis in the lymphocytes | X | ||||||||||
| Lymphoid depletion in the Malpighian bodies | X | X | |||||||||
| Lymphoid depletion in the Malpighian bodies and mitosis | X | X | X | ||||||||
| Liver | Focal subacute inflammation | X | X | X | X | ||||||
| Kidney | Mild focal interstitial inflammation | X | X | X | X | ||||||
| Proliferation of mesangium cells in the glomeruli | X | X | |||||||||
X, lesion present.
(i) All lymphoid organs showed hyperplasia of germinal centers within the first 10 days postinfection, followed by lymphoid depletion and mitosis. In two calves (no. 34 and 115), lesions in the tonsils, retropharyngeal and peribronchial lymph nodes, and spleen were similar to those present in calves euthanized at early stages (days 3 and 5) of infection.
(ii) Apart from an eosinophilic infiltration in the intestinal submucosa, which was present in all infected calves, lesions in the gastroenteric tract were present at mid to late stage (from day 14) of infection. The eosinophilic infiltration was also present in Peyer's patches and ileocecal valves of calves euthanized up to day 10.
(iii) In the nasal cavity and trachea, acute catarrhal inflammation was seen up to day 10 and thereafter was a subacute inflammation.
(iv) The lungs of calves euthanized up to day 7 showed peribronchial lymphoid hyperplasia, whereas focal intralobular interstitial pneumonia was the predominant lesion thereafter.
DISCUSSION
The ability of BVDV to induce primary respiratory disease has for decades been a controversial issue. The immunosuppressive effect of BVDV has been considered determinant in facilitating secondary infections with potential respiratory tract pathogens such as PI3, BHV-1, or Pasteurella hemolytica (25, 26). Mounting evidence from field cases has indicated a primary role of BVDV in production of respiratory tract disease (2, 30); however, experimental data supporting those observations have been limited. We have investigated the in vivo biological effects of two cp isolates of BVDV belonging to genetic cluster Id in immunocompetent calves, and we have shown that these viruses were able to induce a primary respiratory disease. The symptoms produced in infected calves were typical of a respiratory syndrome. As respiratory pathogens such as BRSV, PI3, BHV-1, and BAV were not found on examination of nasal swabs and of postmortem respiratory tissues, there is a strong indication that the respiratory symptoms developed by the infected calves were induced by the inoculated BVDV isolates. Absence of seroconversion to BRSV, PI3, BCV, BHV-1, and BAV further ruled out a concurrent infection with other respiratory viruses. When present, these pathogens may aggravate the picture, resulting in more severe symptoms and disease (25, 26).
When the clinical symptoms induced by the two isolates were compared, infection with Mo2 resulted in a wider range of clinical manifestations, suggesting a difference in pathogenicity between the two isolates. The clinical reactions in the contact calves support this conclusion, since the calf contact exposed to Mo2 showed more pronounced symptoms of nasal discharge, coughing, abnormal breathing, and oral lesions. In contrast, in the contact calf exposed to Mo1, the symptoms were limited to a brief period of nasal discharge. The differences in clinical manifestation do not appear to correlate with the infectious doses since isolate Mo2 at lower doses induced more pronounced clinical signs. Increasing the dose of Mo1 in experiment 2 (5 × 106 TCID50) resulted in extended duration of fever and increased incidence of coughing. Nevertheless, we judge the overall clinical picture to be closer to the results obtained in experiment 1 using the same virus than to those observed using isolate Mo2 in experiment 1.
The development of clinical signs and virus detection in the contact calves indicate that the two viruses were readily transmitted by contact, suggesting horizontal virus spread from an acute infection. Acutely infected animals are regarded as poor transmitters of virus, and experiments done with ncp BVDV strains have not shown this ability of contact transmission (21). It is therefore speculated that the cp biotype replicates in the nasal mucosa to a higher titer than the ncp biotype, resulting in efficient spread to susceptible animals.
Viremia was more frequently detected based on analysis of WBCs than of nasal swabs, suggesting that WBC fractions are more suitable than nasal swabs to assess the presence of virus during acute infection. Virus appeared to be cleared earlier in nasal secretions, because the majority of infected calves shed virus between days 2 and 7.
The distribution of virus in tissues of calves following infection was studied in a second experiment. BVDV and BVDV RNAs were detected in all types of postmortem specimens analyzed, indicating a systemic distribution following primary replication. Virus associated with migratory WBCs and particularly with lymphocytes and lymphoblasts is considered to have been responsible for virus dissemination to different organs or for the signals detected from those tissues, particularly with a highly sensitive technique like RT-PCR. A complementary study is being carried out by means of immunohistochemistry and in situ hybridization to investigate which resident cell types become subsequently infected.
Despite the presence of virus in all types of tissues analyzed, the association of BVDV infection with significant gross and histopathological changes was seen mostly in the organs of the respiratory tract and lymphoid tissue. Lesions such as acute catarrhal inflammation of the nasal mucosa and of the trachea and interstitial pneumonia were consistent with the clinical symptoms observed in the infected calves. All lymphoid tissues, regardless of the drainage site in the body, showed hyperplasia of germinal centers, followed by lymphoid depletion and mitosis, signs of infection, and destruction of lymphocytes followed by regeneration. It is interesting that the two calves that shed virus in the nasal mucosa at days 21 and 31 (calves no. 34 and 115) showed lesions associated with an early infection in lymphoid organs, in parallel with “old” lesions in other tissues. This further supports the conclusion that those calves were undergoing a recurrent infection, as indicated by virus isolation. The generalized infection of the lymphoid tissues is consistent with the lymphotropic nature of BVDV. We did not observe the restriction of cp BVDV to gut-associated lymphoid tissues as described by others (7, 29). Thus we consider this feature to be associated with mucosal disease or with primarily enteric forms of BVDV infection.
The lack of clinical signs such as diarrhea in the infected calves in the present study (except for two calves with transient diarrhea in experiment 1) indicates that infection of gastrointestinal tissue did not result in significant impairment of gastrointestinal functions. This is consistent with the fact that gastrointestinal lesions were mild or absent in these experiments. In some cases, detection of BVDV in gastroenteric tissues (such as the rumen, abomasum, and colon) was achieved only after cultivation, indicating rather low amounts of virus in the tissues or presence of substances affecting the quality and/or yield of RNA. The presence of an eosinophilic infiltration in the intestinal submucosa (all infected calves) and in the Peyer's patches and ileocecal valve (up to day 10) seems remarkable. Similar scattered eosinophilic infiltration has been reportedly associated with lymphocytic infiltrates in ovaries of cattle subsequent to experimental acute infection with BVDV and immunization with a modified live BVDV vaccine (14, 15). The significance of this phenomenon is, however, not known.
Reports on the ability of BVDV to infect postnatal brain tissue have been contradictory. Although some experiments have shown evidence of brain infection in calves (17, 29), virus could not be detected in other studies of similar tissue (8). We found BVDV in the brain tissue of 4 out of the 10 infected calves in experiment 2. As this was not a generalized feature, it appears that infection of brain tissue must be conditioned by the state of development of the blood brain barrier, which varies from calf to calf. When infection of brain tissue becomes established, it seems extremely effective, as shown by ready isolation of BVDV from these specimens.
Since BVDV is detected for a longer period in other tissues than in peripheral WBC fractions or nasal secretions, determination of infection status based solely on analysis of these specimens may lead to false-negative results in an infected animal. The time of virus elimination in different tissues was not evaluated in this study. Shedding of virus in nasal secretions of two calves after clearance from the circulatory system suggests virus recurrence. Fray et al. (11) have reported intermittent shedding of a cp BVDV in nasal secretions of an experimentally superinfected PI calf in the absence of viremia but in the presence of serum antibodies. Among others, immunoprivileged tissues such as the brain and bone marrow, which in the present study were found to harbor virus even late in infection, might represent potential sites of virus sequestering. The overall implications of these findings are that sequestered cp BVDV may constitute a source of virus for recurrent infections and for recombination events with resident ncp BVDV upon infection of PI cattle.
All infected calves had seroconverted to BVDV positivity by day 21, which is in accordance with the 2 to 4-week time period reported for seroconversion to this virus (1). The reasons for lack of a detectable antibody response in the contact calves, despite the development of clinical symptoms and viremia, are not clear. Probably a longer time is required for detection of measurable antibody following contact-mediated exposure.
In conclusion, these experiments revealed that viruses from cluster Id of BVDV are able to induce primary respiratory disease in previously seronegative, immunocompetent calves. Contact transmission and virus recurrence, contrary to observations from acute experimental infections with ncp BVDV, are likely to reflect differences in biological features of these cp isolates. Virus shedding and the presence of BVDV in tissues following peripheral clearance and in the presence of antibodies may have implications in the diagnosis, pathogenesis, and epidemiology of BVDV-induced syndromes in cattle.
ACKNOWLEDGMENTS
This work was supported by a grant from the Department for Research Cooperation SAREC/SIDA, and by internal funds from the National Veterinary Institute, Uppsala, Sweden; from the State Control Institute for Veterinary Biologicals, Drugs and Feeds, Budapest, Hungary; and from the National Veterinary Research Institute (INIVE), Maputo, Mozambique.
We are also grateful to Stefan Alenius for fruitful discussions during preparation of the manuscript.
REFERENCES
- 1.Baker J C. The clinical manifestations of bovine viral diarrhea infection. Vet Clin North Am Food Anim Pract. 1995;11:425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- 2.Baszler T V, Evermann J F, Kaylor P S, Byington T C, Dilbeck P M. Diagnosis of naturally occurring bovine viral diarrhea virus infections in ruminants using monoclonal antibody-based immunohistochemistry. Vet Pathol. 1995;32:609–618. doi: 10.1177/030098589503200601. [DOI] [PubMed] [Google Scholar]
- 3.Baule C, van Vuuren M, Lowings J P, Belák S. Genetic heterogeneity of bovine viral diarrhoea viruses isolated in Southern Africa. Virus Res. 1997;52:205–220. doi: 10.1016/s0168-1702(97)00119-6. [DOI] [PubMed] [Google Scholar]
- 4.Becher P, König M, Paton D J, Thiel H-J. Further characterisation of border disease virus isolates: evidence for the presence of more than three species within the genus Pestivirus. Virology. 1995;209:200–206. doi: 10.1006/viro.1995.1243. [DOI] [PubMed] [Google Scholar]
- 5.Becher P, Orlich M, Shannon A D, Horner G, König M, Thiel H-J. Phylogenetic analysis of pestiviruses from domestic and wild ruminants. J Gen Virol. 1997;78:1357–1366. doi: 10.1099/0022-1317-78-6-1357. [DOI] [PubMed] [Google Scholar]
- 6.Belák S, Ballagi-Pordány A. Experiences on the application of the polymerase chain reaction in a diagnostic laboratory. Mol Cell Probes. 1993;7:241–248. doi: 10.1006/mcpr.1993.1035. [DOI] [PubMed] [Google Scholar]
- 7.Bielefeldt Ohmann H. BVD virus antigens in tissues of persistently viraemic, clinically normal cattle: implications for the pathogenesis of clinically fatal disease. Acta Vet Scand. 1988;29:77–84. doi: 10.1186/BF03548395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruschke C J M, Weerdmeester K, Van Oirschot J T, Van Rijn P A. Distribution of bovine virus diarrhoea virus in tissues and white blood cells of cattle during acute infection. Vet Microbiol. 1998;64:23–32. doi: 10.1016/s0378-1135(98)00249-1. [DOI] [PubMed] [Google Scholar]
- 9.Dinter Z. Diagnostic procedures at the NVI. Diagnostic virology: special part. In: Moreno-Lopez J, editor. Diagnostic virology: a review of methods at the National Veterinary Institute. Uppsala, Sweden: Swedish University of Agricultural Sciences/National Veterinary Institute/Swedish International Developing Authority; 1989. p. 63. [Google Scholar]
- 10.Elvander M, Baule C, Persson M, Egyed L, Ballagi-Pordány A, Belák S, Alenius S. An experimental study of concurrent primary infection with bovine respiratory syncytial virus (BRSV) and bovine viral diarrhoea virus (BVDV) in calves. Acta Vet Scand. 1998;39:251–264. doi: 10.1186/BF03547797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fray M D, Clarke M C, Thomas L H, MaCauley J W, Charleston B. Prolonged nasal shedding and viraemia of cytopathogenic bovine virus diarrhoea virus in experimental late-onset mucosal disease. Vet Rec. 1998;143:608–611. doi: 10.1136/vr.143.22.608. [DOI] [PubMed] [Google Scholar]
- 12.Fritzemeier J, Greiser-Wilke I, Haas L, Pitoco E, Moennig V, Liess B. Experimentally induced “late-onset” mucosal disease—characterization of the cytopathogenic viruses isolates. Vet Microbiol. 1995;46:285–294. doi: 10.1016/0378-1135(95)00093-p. [DOI] [PubMed] [Google Scholar]
- 13.Greiser-Wilke I, Liebler E, Haas L, Pohlenz J, Moennig V. Distribution of cytopathogenic and non-cytopathogenic bovine virus diarrhea virus in tissues from a calf with experimentally induced mucosal disease using antigenic and genetic markers. Arch Virol Suppl. 1993;7:295–301. doi: 10.1007/978-3-7091-9300-6_22. [DOI] [PubMed] [Google Scholar]
- 14.Grooms D L, Brock K V, Ward L A. Detection of bovine viral diarrhea virus in the ovaries of cattle acutely infected with bovine viral diarrhea virus. J Vet Diagn Invest. 1998;10:125–129. doi: 10.1177/104063879801000201. [DOI] [PubMed] [Google Scholar]
- 15.Grooms D L, Brock K V, Ward L A. Detection of cytopathic bovine viral diarrhea virus in the ovaries of cattle following immunization with a modified live bovine viral diarrhea virus vaccine. J Vet Diagn Invest. 1998;10:130–134. doi: 10.1177/104063879801000202. [DOI] [PubMed] [Google Scholar]
- 16.Liebler-Tenorio E M, Greiser-Wilke I, Pohlenz J F. Organ and tissue distribution of the antigen of the cytopathogenic bovine virus diarrhea virus in the early and advanced phase of experimental mucosal disease. Arch Virol. 1997;142:1613–1634. doi: 10.1007/s007050050184. [DOI] [PubMed] [Google Scholar]
- 17.Marshall D J, Moxley R A, Kelling C L. Distribution of virus and viral antigen in specific pathogen-free calves following inoculation with noncytopathic bovine viral diarrhoea virus. Vet Pathol. 1996;33:311–318. doi: 10.1177/030098589603300308. [DOI] [PubMed] [Google Scholar]
- 18.Meyers G, Thiel H J. Molecular characterization of pestiviruses. Adv Virus Res. 1996;47:53–119. doi: 10.1016/s0065-3527(08)60734-4. [DOI] [PubMed] [Google Scholar]
- 19.Moennig V, Frey H-R, Liebler E, Pohlenz J, Liess B. Reproduction of mucosal disease with cytopathogenic bovine viral diarrhoea virus selected in vitro. Vet Rec. 1990;127:200–203. [PubMed] [Google Scholar]
- 20.Moennig V, Greiser-Wilke I, Frey H-R, Haas L, Liebler E, Pohlenz J, Liess B. Prolonged persistence of cytopathogenic bovine viral diarrhea virus (BVDV) in a persistently viremic cattle. J Vet Med B. 1993;40:371–377. doi: 10.1111/j.1439-0450.1993.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 21.Niskanen R, Lindberg A, Larsson B, Alenius S. Lack of transmission from bovine viral diarrhoea virus infected calves to susceptible peers. Acta Vet Scand. 2000;41:93–99. doi: 10.1186/BF03549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton D J. Pestivirus diversity. J Comp Pathol. 1995;112:215–236. doi: 10.1016/s0021-9975(05)80076-3. [DOI] [PubMed] [Google Scholar]
- 23.Paton D J, Sands J J, Lowings J P, Smith J E, Ibata G, Edwards S. A proposed division of the Pestivirus genus using monoclonal antibodies, supported by cross-neutralisation assays and genetic sequencing. Vet Res. 1995;26:92–109. [PubMed] [Google Scholar]
- 24.Pellerin C, van den Hurk J, Lecomte J, Tijssen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology. 1994;203:260–267. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- 25.Potgieter L N D, McCracken M D, Hopkins F M, Walker R D, Guy J S. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am J Vet Res. 1984;45:1582–1585. [PubMed] [Google Scholar]
- 26.Potgieter L N D. Immunology of bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract. 1995;11:501–520. doi: 10.1016/s0749-0720(15)30464-3. [DOI] [PubMed] [Google Scholar]
- 27.Ridpath J F, Bolin S R, Dubovi E J. Segregation of bovine viral diarrhea virus into genotypes. Virology. 1994;205:66–74. doi: 10.1006/viro.1994.1620. [DOI] [PubMed] [Google Scholar]
- 28.Ros C, Riquelme M E, Öhman-Forslund K, Belák S. Improved detection of five closely related ruminant alphaherspesviruses by specific amplification of viral genomic sequences. J Virol Methods. 1999;83:55–65. doi: 10.1016/s0166-0934(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 29.Spagnuolo-Weaver M, Allan G M, Kennedy S, Foster J C, Adair B M. Distribution of cytopathic and noncytopathic bovine viral diarrhea virus antigens in tissues of calves following acute experimental infection. J Vet Diagn Invest. 1997;9:287–297. doi: 10.1177/104063879700900310. [DOI] [PubMed] [Google Scholar]
- 30.Taylor L F, Janzen E D, Ellis J A, van den Hurk J V, Ward P. Performance, survival, necropsy, and virological findings from calves persistently infected with the bovine viral diarrhea virus originating from a single Saskatchewan beef herd. Can Vet J. 1997;38:29–37. [PMC free article] [PubMed] [Google Scholar]
- 31.Thiel H-J, Plagemann P G W, Moennig V. Pestiviruses. In: Fields B N, Knipe D N, Howley P M, editors. Fields virology. Philadelphia, Pa: Raven Press; 1996. pp. 1059–1074. [Google Scholar]
- 32.Van Rijn P A, Van Gennip H G P, Leendertse C H, Bruschke C J M, Paton D J, Moormann R J M, Van Oirschot J T. Subdivision of the Pestivirus genus based on envelope glycoprotein E2. Virology. 1997;237:337–348. doi: 10.1006/viro.1997.8792. [DOI] [PubMed] [Google Scholar]
- 33.Vilcek S, Nettleton P F, Paton D J, Belák S. Molecular characterisation of ovine pestiviruses. J Gen Virol. 1996;78:725–735. doi: 10.1099/0022-1317-78-4-725. [DOI] [PubMed] [Google Scholar]
- 34.Wengler G D, Bradley D W, Collett M S, Heinz F X, Schlesinger R W, Strauss J H. Flaviviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy. Sixth report of the International Committee on Taxonomy of Viruses. Vienna, Austria, and New York, N.Y: Springer-Verlag; 1995. pp. 415–427. [Google Scholar]