Abstract
Cerebral hyperperfusion syndrome (CHS) after surgical clipping for cerebral aneurysm is a rare entity. The authors present a 76-year-old woman with a large left internal carotid-posterior communicating artery aneurysm. After successful clipping with temporary occlusion of the internal carotid artery, the patient exhibited motor aphasia. 15O-gas positron emission tomography (PET) showed extreme elevation of the regional cerebral blood flow (rCBF) along with a mildly decreased regional cerebral metabolic rate for oxygen (rCMRO2) and a remarkable decrease in the oxygen extraction fraction (OEF) in the territory of the ipsilateral superior trunk of the middle cerebral artery. These data indicated local hyperperfusion. She had fully recovered from the aphasia by postoperative day (POD) 18. PET showed normalization of CBF on POD 27. To our knowledge, this is the first case report to show hyperperfusion syndrome, clearly detected by 15O-gas PET, after aneurysmal neck clipping.
Keywords: clipping, unruptured aneurysm, hyperperfusion
Introduction
Cerebral hyperperfusion syndrome (CHS) after clipping for an unruptured aneurysm is extremely rare, and few cases have been reported to date.1–4) In these cases, regional cerebral blood flow (rCBF) was measured using perfusion computed tomography (CT) or single photon emission computed tomography (SPECT). Herein, we describe a patient developing CHS after clipping of a large unruptured internal carotid-posterior communicating artery (IC-PC) aneurysm. To our knowledge, this is the first report of CHS diagnosed by 15O-gas positron emission tomography (PET). Furthermore, 15O-gas PET findings over time were evaluated in relation to changes in symptoms.
Case Report
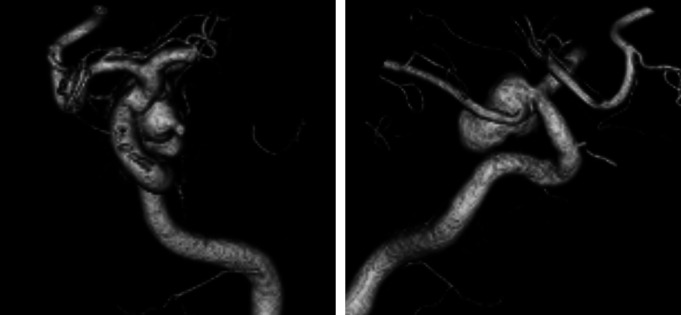
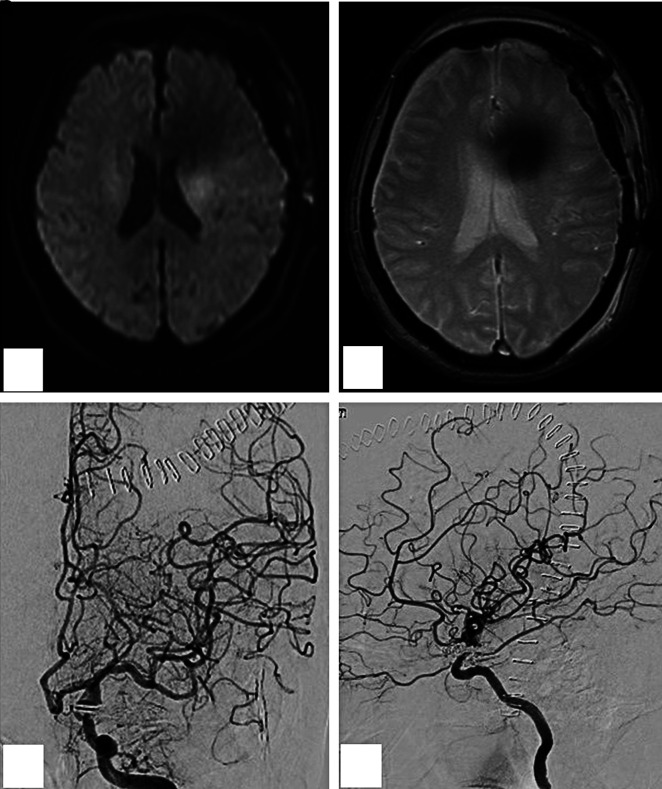
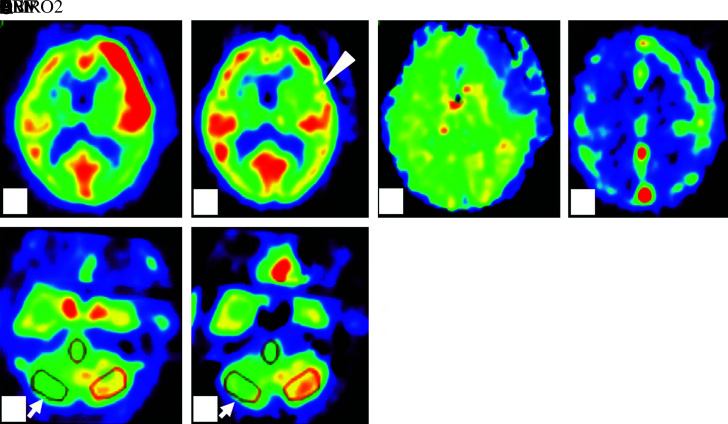
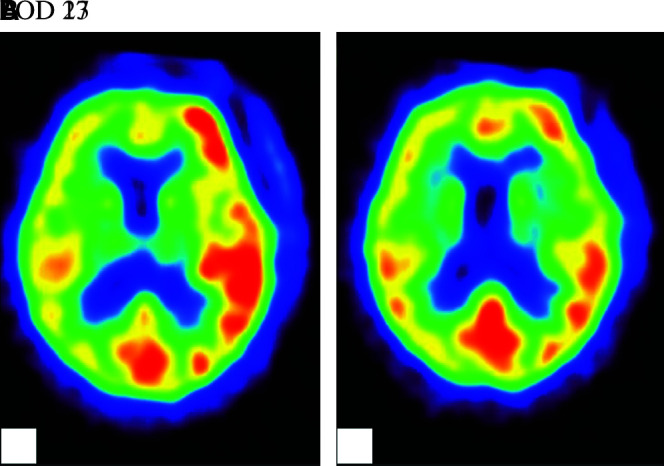
A 76-year-old woman presented with a 3-month history of double vision and photophobia affecting her left eye. She had no history of stroke or head trauma. CT of the brain on admission showed no hemorrhage. Neurological findings revealed mild left oculomotor nerve paralysis. Digital subtraction angiography (DSA) showed a large left IC-PC aneurysm with a maximum diameter of 15.8 mm (Fig. 1). Intracranial blood flow distal to the aneurysm did not delay. Because this aneurysm was estimated to be at risk for imminent rupture, we performed surgical neck clipping on the day of admission. During surgery, the internal carotid artery was temporarily clipped four times for 23 minutes in total (each clipping time ≤10 minutes), due to premature rupture of the aneurysm. The aneurysm was completely clipped using four titanium clips. CT just after surgery showed neither hemorrhage nor brain edema. She was extubated 18 hours after surgery and showed no clear symptoms at that time, though slight motor aphasia was suspected. Thirty hours later (48 hours after surgery), motor aphasia became evident. Magnetic resonance (MR) images showed neither infarction nor hemorrhage (Figs. 2A and 2B) and DSA showed no spasm (Figs. 2C and 2D) on postoperative day (POD) 2. Her symptoms gradually worsened, and by POD 4 had deteriorated to total aphasia. Electroencephalography showed no abnormalities. 15O-gas PET performed on POD 5 showed remarkably increased rCBF in the territory of the superior trunk of the middle cerebral artery (MCA) (Fig. 3A), and the regional cerebral metabolic rate for oxygen (rCMRO2) was slightly decreased in this territory (Fig. 3B). Consequently, the regional oxygen extraction fraction (rOEF) was extremely decreased (Fig. 3C). Regional cerebral blood volume (rCBV) was slightly elevated (Fig. 3D). Moreover, crossed cerebellar diaschisis was recognized, along with decreased blood flow and metabolism, in the contralateral cerebellar hemisphere (Figs. 3E and 3F). These PET findings and the focal neurological deficits strongly suggested CHS. She was maintained in a normotensive state to prevent intracerebral hemorrhage and medicated with levetiracetam (1000 mg/day) to prevent secondary epilepsy. Her symptoms showed amelioration starting on POD 9 and the follow-up 15O-gas PET on POD 13 revealed moderate improvement of hyperperfusion in the MCA superior trunk area, whereas the MCA inferior trunk area had newly become hyperemic (Fig. 4A). Her symptoms showed full resolution by POD 18, and rCBF normalization was confirmed on POD 27 (Fig. 4B).
Fig. 1. Preoperative 3D-DSA showing a lt. IC-Pcom aneurysm with a maximum diameter of 15.8 mm. 3D-DSA: three-dimensional digital subtraction angiography, IC-Pcom: internal carotid-posterior communicating artery.
Fig. 2. Post-surgical MRI showing no new infarction on DWI (A) or hemorrhage on T2* (B). And post-surgical DSA showed no spasm (C, D). DSA: digital subtraction angiography, DWI: diffusion-weighed imaging.
Fig. 3. 15O-gas PET performed on POD 5 shows elevated CBF (A) and CBV (D) in the area supplied by the lt. superior trunk of the MCA. CMRO2 (B) was slightly decreased (arrowhead) while OEF (C) was decreased in the same area. CBF (E) and CMRO2 (F) were decreased in the contralateral cerebellar hemisphere (arrow). CBF: cerebral blood flow, CBV: cerebral blood volume, CMRO2: cerebral metabolic rate for oxygen, MCA: middle cerebral artery, OEF: oxygen extraction fraction, PET: positron emission tomography, POD: postoperative day.
Fig. 4. CBF on 15O-gas PET revealed the CHS involvement to extend to the area of the lt. inferior trunk of the MCA on POD 13 (A) and to have normalized by POD 27 (B). CBF: cerebral blood flow, POD: postoperative day, PET: positron emission tomography.
Discussion
We experienced a rare case of CHS after clipping for an unruptured cerebral aneurysm, and according to our literature search, this is the first report to show CHS clearly detected by 15O-gas PET. To date, only five cases developing CHS after clipping for an unruptured cerebral aneurysm have been reported.1–4) In these cases, only rCBF was measured, using either perfusion CT or SPECT. However, diagnosing cerebral hyperperfusion based only on increased CBF is unreliable because such elevation can also occur during epileptic seizures.5–7) Ictal PET revealed hypermetabolism both at the focus and in the contralateral cerebellum.8) In our case, CBF increased remarkably with no elevation of CMRO2 in the operated cerebral hemisphere. Moreover, CBF and CMRO2 decreased in the contralateral cerebellar hemisphere (crossed cerebellar diaschisis). These findings strongly suggested CHS.
Autoregulatory failure caused by any form of preoperative or intraoperative ischemia is a possible mechanism of hyperperfusion after clipping of cerebral aneurysms. Large aneurysms are reportedly among the reasons for hyperperfusion. In the area distal to a large aneurysm, chronic hypoperfusion may exist preoperatively because the aneurysm acts as a reservoir.9–11) This phenomenon, reported as “Damping effect” or “Windkessel effect” causes drop in cerebrovascular reactivity (CVR).11) In addition, the maximum flow rate increased after clipping of an aneurysm lead to hyperperfusion.9) As well as neck clipping, flow-diverting stents for large aneurysms can also reduce blood flow into the aneurysmal sac by directing flow to the distal vessel, leading to hyperperfusion.9) Temporary clipping can also be associated with hyperperfusion. It has been reported that reperfusion after dysautoregulation of CBF caused by temporary clipping can lead to cerebral hyperperfusion.1,2,12–14) Among cases with ruptured aneurysms, intraoperative total temporary clipping time and the maximum single temporary clipping time were significantly longer in those with than in those without cerebral hyperperfusion (31.9 vs 13.9 minutes, p = 0.0157) (18.4 vs 8.6, p = 0.0313).12) In three reported cases with unruptured aneurysms, cerebral hyperperfusion developed after 7–10 minutes, in total, of temporary clipping.1–3) The threshold of the temporary clipping time as regards hyperperfusion remains unknown, because it depends on the degree of collateral flow which varies among individuals. In our case, presurgical CVR might have been normal because DSA did not show damping effect. During surgery, the maximum temporary clipping time was 10 minutes and the total temporary clipping time was 23 minutes. Long time blockage of blood flow caused autoregulatory failure. Compression of brain tissue with a spatula and intraoperative injury to the superficial sylvian vein might also cause hyperperfusion, according to one reported.4)
In most unruptured aneurysm cases including ours, CHS developed within 24 hours and disappeared within 30 days after clipping surgery.1–4) In our case, the symptoms clearly manifested at 48 hours after surgery and was revealed improvement tendency on POD 9. However, the area of rCBF increasing was spreading posterior to the MCA on POD 13 (Fig. 4A). The reason of this phenomenon was unclear but may have been related to heterogeneity of the cerebral vascular response to chronic or transient ischemic insult, or some electrophysiological factors could be involved. The symptom had disappeared by POD 18 and rCBF showed normalization on POD 27 (Fig. 4B). The resolution of her symptoms was followed by improved imaging findings.
Conclusion
In the present case, CHS could be clearly demonstrated by measuring both rCBF and rCMRO2, using 15O-gas PET, after clipping of a large unruptured aneurysm. 15O-gas PET can easily distinguish CHS form post-surgical epilepsy. Although CHS after clipping is extremely rare, this clinical entity must be considered when a large aneurysm is treated by clipping with the aid of prolonged temporary occlusion of the parent artery.
Footnotes
Ethics Approval
This case report was approved by the Ethics Committee of the National Cerebral and Cardiovascular Center (#M30-013). Informed consent was obtained from our patient.
Conflicts of Interest Disclosure
The authors have no financial interests in the materials and devices described in this article.
References
- 1). Kayahara T, Takeda R, Kikkawa Y, Take Y, Kurita H: Hyperperfusion syndrome after aneurysm surgery: a case report. Acta Neurochir (Wien) 157: 1855– 1857, 2015 [DOI] [PubMed] [Google Scholar]
- 2). Kuroki K, Taguchi H, Yukawa O: Hyperperfusion syndrome after clipping of an unruptured aneurysm. Case report. Neurol Med Chir (Tokyo) 46: 248– 250, 2006 [DOI] [PubMed] [Google Scholar]
- 3). Murakami H, Inaba M, Nakamura A, Ushioda T: Ipsilateral hyperperfusion after neck clipping of a giant internal carotid artery aneurysm. Case report. J Neurosurg 97: 1233– 1236, 2002 [DOI] [PubMed] [Google Scholar]
- 4). Sugino T, Ohtaki M, Wanibuchi M, Kin S, Houkin K: Hyperperfusion syndrome after clipping an unruptured cerebral aneurysm: two case reports. Neurol Med Chir (Tokyo) 50: 306– 309, 2010 [DOI] [PubMed] [Google Scholar]
- 5). Kuge Y, Hasegawa Y, Yokota C, et al. : Effects of single and repetitive spreading depression on cerebral blood flow and glucose metabolism in cats: a PET study. J Neurol Sci 176: 114– 123, 2000 [DOI] [PubMed] [Google Scholar]
- 6). Kurauchi Y, Mokudai K, Mori A: l-Citrulline ameliorates cerebral blood flow during cortical spreading depression in rats: Involvement of nitric oxide- and prostanoids-mediated pathway. J Pharmacol Sci 133: 146– 155, 2017 [DOI] [PubMed] [Google Scholar]
- 7). Park HR, Seong MJ, Shon YM, Joo EY, Seo DW, Hong SB: SPECT perfusion changes during ictal automatisms with preserved responsiveness in patients with right temporal lobe epilepsy. Epilepsy Behav 80: 11– 14, 2018 [DOI] [PubMed] [Google Scholar]
- 8). Kawai N, Kawanishi M, Tamiya T, Nagao S: Crossed cerebellar glucose hypermetabolism demonstrated using PET in symptomatic epilepsy–case report. Ann Nucl Med 19: 231– 234, 2005 [DOI] [PubMed] [Google Scholar]
- 9). Chiu AH, Wenderoth J: Cerebral hyperperfusion after flow diversion of large intracranial aneurysms. J Neurointerv Surg 5: e48, 2013 [DOI] [PubMed] [Google Scholar]
- 10). Heros RC, Scott RM, Kistler JP, Ackerman RH, Conner ES: Temporary neurological deterioration after extracranial-intracranial bypass. Neurosurgery 15: 178– 185, 1984 [DOI] [PubMed] [Google Scholar]
- 11). Hussein AE, Esfahani DR, Linninger A, et al. : Aneurysm size and the Windkessel effect: an analysis of contrast intensity in digital subtraction angiography. Interv Neuroradiol 23: 357– 361, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Araki Y, Andoh H, Yamada M, Nakatani K, Andoh T, Sakai N: Permissible arterial occlusion time in aneurysm surgery: postoperative hyperperfusion caused by temporary clipping. Neurol Med Chir (Tokyo) 39: 901– 906; discussion 906-907, 1999 [DOI] [PubMed] [Google Scholar]
- 13). Matsumoto M, Yamagata S, Minamikawa J, Hashimoto K, Kikuchi H, Kodama N: Delayed cerebral dysautoregulation after recirculation of cerebral ischemia. No Shinkei Geka 21: 503– 508, 1993. (Japanese) [PubMed] [Google Scholar]
- 14). Nagashima H, Miwa T, Horiguchi T, Tomio R, Nakagawa Y, Yoshida K: Hyperperfusion after clipping of aneurysm: a rare entity. J Stroke Cerebrovasc Dis 27: 1425– 1430, 2018 [DOI] [PubMed] [Google Scholar]