Abstract
Glioblastoma multiforme (GBM) is an aggressive cancer type, with fewer than 3–5% of patients surviving for more than 3 years. We describe a 48-year-old right-handed man who presented with generalized seizure attacks. Magnetic resonance imaging (MRI) revealed a heterogeneous gadolinium-enhancing lesion in the left inferior parietal lobule. The patient underwent awake surgery, and tumor resection included abnormalities on T2-weighted MRI, with subcortical mapping used to identify the deep functional boundaries. After supratotal resection, the tumor was diagnosed as GBM without isocitrate dehydrogenase (IDH) 1 and 2 mutations. At a follow-up evaluation, 9 years and 2 months after the surgery, the patient appeared healthy, and no relapse or recurrence was observed. We present the case of a long-term survivor of IDH-wildtype GBM. This case suggests that supratotal resection with intraoperative awake brain mapping can improve survival without impairing the patient’s neurological functions.
Keywords: IDH-wildtype glioblastoma, long-term survivor, awake brain mapping, supratotal resection
Introduction
Glioblastoma multiforme (GBM) is one of the most frequent and aggressive glial neoplasms in the primary central nervous system in adults, with a median survival time of 20 months from diagnosis even with aggressive treatment, including surgery, radiotherapy, and chemotherapy.1,2) Surgical tumor resection plays a critical role in the multimodal treatment of GBM. Large observational studies and literature reviews based on objective evaluation of the extent of resection (EOR) for GBM have revealed that maximal tumor resection is significantly associated with better outcomes for GBM.3,4) Even after aggressive surgery for GBM, tumor recurrence is inevitable, and most patients uniformly die.
Recently, some research groups reported that extended tumor resection beyond contrast-enhancing lesions in a magnetic resonance imaging (MRI)-verified area, also known as supratotal resection, improves the outcome of patients with GBM.5–10) These studies on gliomas found that tumor cells spread beyond visible abnormalities and that conventional MRI underestimates the actual spatial extent of gliomas. It has also been suggested that tumor cells might have the potential to invade areas 10–20 mm away from the MRI-defined tumor boundaries.11) Therefore, we attempted to achieve supratotal resection for gliomas whenever possible, beyond the tumor margins visible on MRI, with the aid of awake functional mapping.
Here, we present a case of a long-term survivor (still alive 9 years and 2 months after the surgery) of isocitrate dehydrogenase (IDH)-wildtype GBM, in which supratotal resection was achieved using awake brain mapping.
Case Presentation
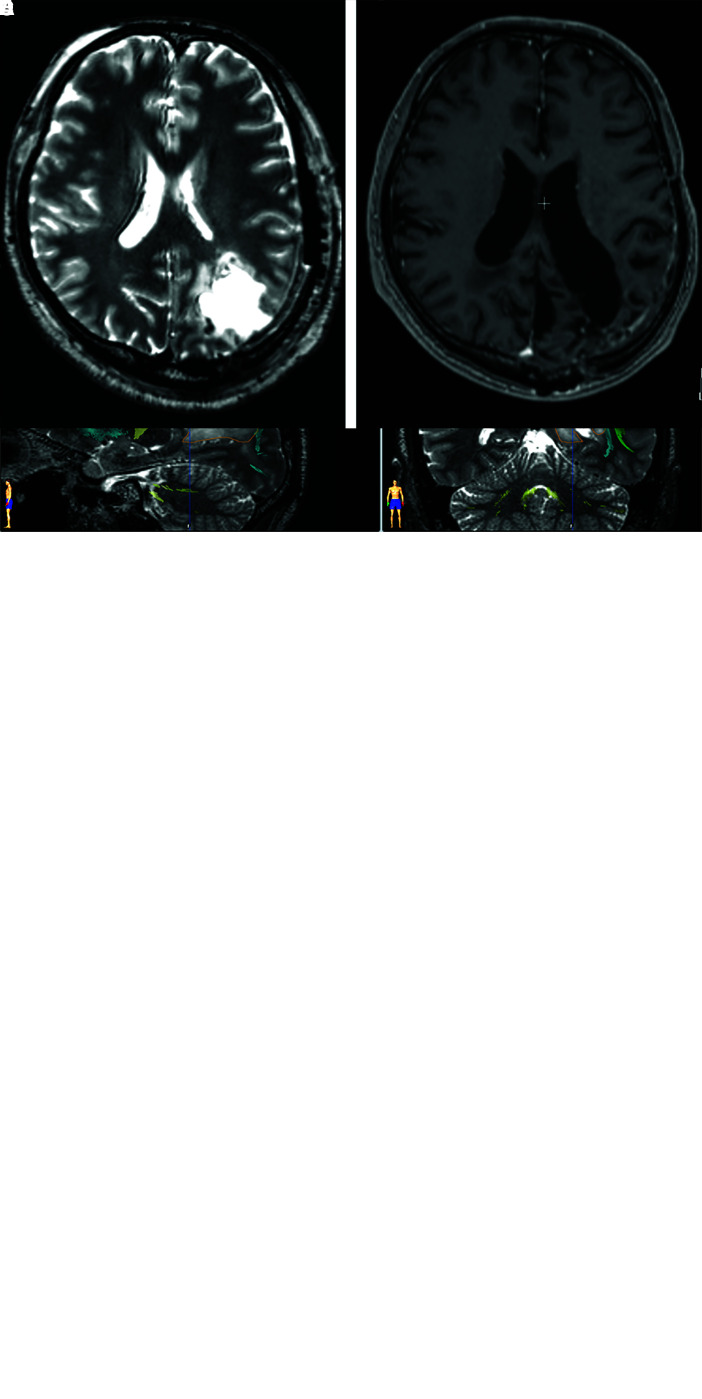
In 2013, a 48-year-old right-handed man presented with generalized seizure attacks and was transferred to the emergency room of a nearby hospital. Brain MRI revealed a heterogeneous gadolinium-enhancing lesion with a high-intensity area on T2-weighted images in the left inferior parietal lobule, with a maximum diameter of approximately 20 mm (Fig. 1A and 1B). The patient was transferred to our hospital for surgery. The white matter tracts, such as the pyramidal tract, superior longitudinal fasciculus (SLF), and inferior fronto-occipital fasciculus (IFOF), were computed from the preoperative diffusion tensor imaging (DTI) data set using BrainLAB iPlan Cranial version 3.0 (German HealthCare Export Group, Bonn, Germany) (Fig. 1C).12)
Fig. 1. Preoperative axial T2-weighted (A) and axial T1-weighted MRI with gadolinium enhancement (B), showing a high-intensity area in the left inferior parietal lobule and enhancing parts at the superficial lesion. Generation of a 3D object representing the fiber bundles of white matter tracts (C). After segmentation of the fiber tract bundle, a 3D object (yellow) was generated, representing the left pyramidal tract. The green-colored area shows the SLF, the blue-colored area shows the IFOF, and the tumor area is highlighted in orange. Postoperative axial T2-weighted MRI (D) showing no tumors due to supratotal resection. Axial T1-weighted MRI with gadolinium enhancement (E) performed 9 years and 2 months after the surgery, showing no recurrent tumors. 3D: three-dimensional, IFOF: inferior fronto-occipital fasciculus, MRI: magnetic resonance imaging, SLF: superior longitudinal fasciculus.
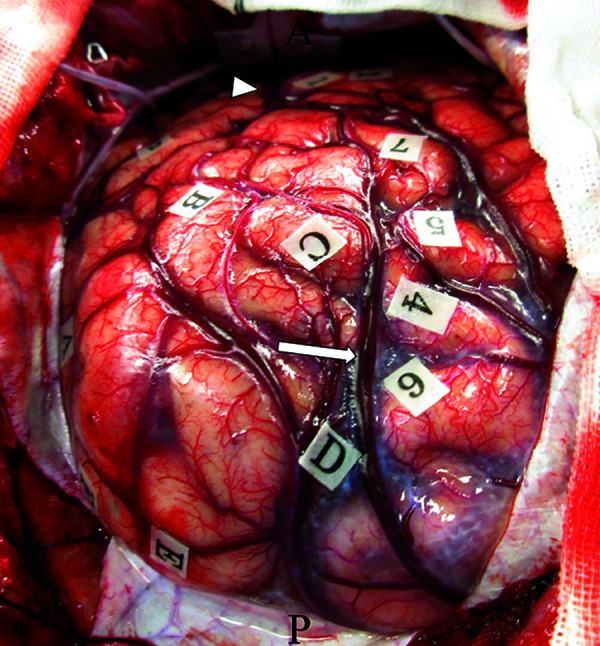
To achieve supratotal resection, which means a broad resection of the regions on T2-weighted MRI that extends beyond the contrast-enhancing portion of the tumor, the patient underwent awake surgery, assisted by both cortical and subcortical functional mapping. The tumor margins were letter-tagged (A–F) using the neuro-navigation system before the brain shift occurred (Fig. 2). Cortical mapping was performed with a double task combining picture-naming and right-arm movements. During cortical mapping using a biphasic current (pulse frequency, 60 Hz; single pulse phase duration, 0.5 ms) under a 4 mA stimulus with picture-naming tasks, a convulsion around the mouth was induced with postcentral gyrus stimulation (tags 1 and 2; Fig. 2). Next, the patient was asked to perform oral reading and writing tasks. Cortical stimulation did not induce any neurological disturbances during these tasks. After cortical mapping was completed, cortical resection was initiated at the anterior border of the tumor (letter tags; B–C) with negative mapping, and tumor resection was performed at the level of the white matter under the angular gyrus. At the end of the tumor resection, semantic paraphasia was induced by stimulating the deep side of the tumor cavity. The patient mistakenly stated “notebook” when the picture of “pencil” was presented on a monitor. Upon stimulation of this site, the patient was not able to perform the oral reading and writing tasks. Tumor resection included abnormalities on T2-weighted MRI, with subcortical mapping used to identify the deep functional boundaries (Fig. 1D). Supratotal resection was thus achieved with the aid of awake functional mapping. The extent of supratotal resection (EOsR) was calculated as follows: postoperative tumor cavity/preoperative tumor volume in T1 contrast-enhancing tumors. The final EOsR was 399.6%.
Fig. 2. Intraoperative photograph obtained before tumor resection, showing letter tags that indicate tumor boundaries (A–E). Postcentral gyrus stimulation induced convulsion around the mouth (tag: 1, 2). Posterior portion of the superior temporal gyrus stimulation induced phonemic paraphasia (tag: 3). Left superior parietal lobule stimulation induced a cessation in right upper limb movement (tag: 4–7). Oral reading and writing tasks did not induce any disturbances in the cortical mapping. Arrowhead: intraparietal sulcus, arrow: Sylvian fissure.
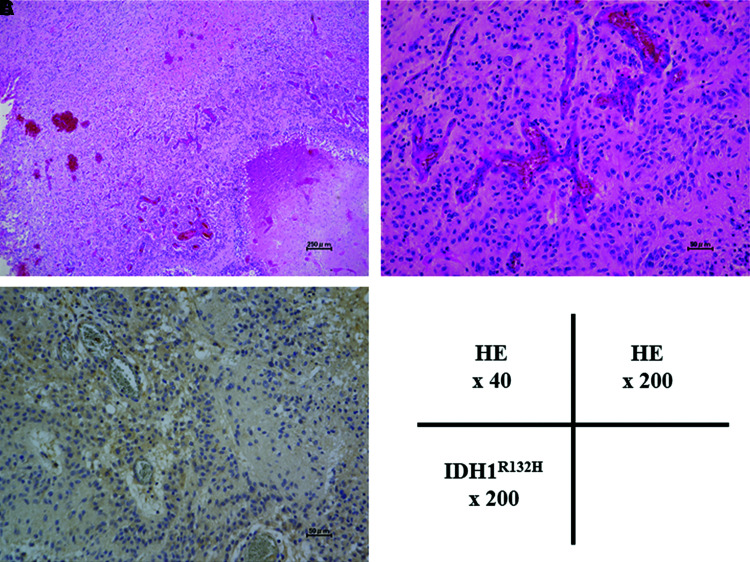
Postoperatively, the patient showed no remarkable neurological deteriorations in language, oral reading and writing, or movement of the upper and lower extremities. Histological examination of the tumor yielded a diagnosis of GBM, and Ki-67 expression was observed in 10% of the tumor cells (Fig. 3). Sanger sequencing revealed that this tumor harbored no IDH1, IDH2, H3F3A, HIST1H3B K27M, B-Raf proto-oncogene, serine/threonine kinase (BRAF) V600E, or telomerase reverse transcriptase (TERT) promoter mutations.13) By contrast, methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter was detected using quantitative pyrosequencing technology. The proneural subtype of GBMs with better prognosis, as described in several studies,14,15) was characterized by PDGFRA amplification, as well as TP53 and IDH1 mutations. No copy number changes in PDGFRA or TP53, nor IDH1 mutations, were detected by MLPA in this tumor. Furthermore, EGFR amplification and homozygous deletion of CDKN2A, CDKN2B, and PTEN loss, which is a frequent event in the other GBM subclass, were also not observed.
Fig. 3. Photomicrographs (magnification ×40 or ×200) of the current case. HE staining showed proliferation of atypical glial cells and necrosis. Abnormal proliferation of vessels was observed around necrosis (A). Microvascular proliferation was also observed, which strongly suggested glioblastoma pathology (B). Immunohistochemical staining of IDH1R132H yielded negative results (C). The absence of IDH1 and IDH2 mutations was also confirmed by Sanger sequencing. HE: hematoxylin–eosin, IDH: isocitrate dehydrogenase.
Following this surgery, the patient was treated with 60 Gy in 30 fractions of radiotherapy in combination with concurrent temozolomide (TMZ) chemotherapy. Moreover, the patient was still on a maintenance regimen of TMZ 200 mg/m2 on days 1–5 every 4 weeks at the time of the check-up.
At a follow-up evaluation 9 years and 2 months after the surgery, the patient appeared healthy and showed no signs of relapse or recurrence (Fig. 1E). Moreover, he continued to live a normal life and did not show any disturbances in neurocognitive status or motor, language, reading, and writing functions.
Discussion
Long-term survivors living at least 2 years from diagnosis comprise 13% of all patients with GBM.16) Due to the nature of the disease and the high recurrence, only 3%–5% of patients with GBM live for longer than 3 years after diagnosis.17) According to a systematic review by Tykocki et al.,18) fewer than 1% of all patients with GBM survive longer than 10 years. The prognosis of GBM depends on the age of the patient, with younger patients having more favorable outcomes. This may be the reason for the different molecular and genetic alterations observed in younger compared to older patients. However, the biological and molecular characteristics of short- and long-term GBM survivors are also remarkably different.19)
Regarding the relationship between molecular alterations and survival after GBM, some studies have revealed that patients with GBM and IDH1 or IDH2 mutations and MGMT promoter methylation have a better prognosis.20,21) The overall survival (OS) of patients with GBM with MGMT promoter methylation is significantly higher (21.7 months) than that of patients with an unmethylated promoter (15.3 months).22) IDH1 and IDH2 mutations occur in up to 10% of primary GBM cases and are associated with a substantially longer OS. The median OS was 3.8 years for patients with IDH1 mutations and 1.1 years for patients with wildtype IDH1.23) Other molecular biomarkers, such as TERT promoter mutations, have also been observed in GBM more frequently than in other types of gliomas. TERT promoter mutations in MGMT promoter unmethylated GBM have been associated with poorer OS.24) Although our patient showed MGMT promoter methylation and no IDH1, IDH2, or TERT promoter mutations, our case presents a favorable disease course, with no signs of tumor recurrence at more than 9 years after surgery.
Supratotal resection for gliomas, including both GBM and lower-grade gliomas, is a newly emerging concept in the field of neuro-oncology that can improve the survival of patients with glioma. In this aggressive surgery, awake functional mapping enables safe tumor resection of language-dominant and non-dominant GBMs while protecting cortical and subcortical neurological functions.
A comprehensive summary and analysis of several studies on supratotal resection for GBM that have been published, comparing supratotal resection and gross total resection for GBM is presented in detail in Table 1. In a retrospective study with 282 newly diagnosed GBM cases, the median PFS for supratotal resection was 24.5 ± 2.4 months and the median OS was 28.6 ± 5.2 months, compared with a gross total resection PFS of 11.9 ± 0.6 months and OS of 16.2 ± 1.2 months. Moreover, fewer patients showed worsening of deficits or developed new neurological deficits after supratotal resection (4.8%) than after gross total resection (13.3%) or subtotal resection (11.2%). This study suggests supratotal resection, that is, surgical resection beyond contrast-enhancing boundaries, as a promising strategy to improve clinical outcomes in patients with GBM.8) Eyüpoglo et al. reported on supratotal resection surgery using a dual intraoperative visualization approach for GBM.9) In this study, gross total resection (n = 75) with intraoperative MRI alone (control group) was compared with supratotal resection using a surgical strategy consisting of 5-aminolevulinic acid (5-ALA) administration and intraoperative MRI (n = 30). The median survival time in the control group treated according to current gold standards in surgical neuro-oncology was 14 months, whereas tumor resection with both 5-ALA administration and intraoperative MRI resulted in a significantly longer median survival time of 18.5 months. The authors emphasize that supratotal resection for glioma leads to a significant prolongation of the OS time in GBM patients. Li et al.5) investigated the role of supratotal resection in 876 GBM patients who achieved complete resection of T1 contrast-enhancing tumor, with the purpose to verify whether supratotal resection can improve the survival of GBM. They demonstrated that the resection of ≥53.21% of the FLAIR abnormality beyond contrast-enhancing lesions was related with a significant prolonged survival compared with that less extensive tumor resections (median survival: 20.7 and 15.5 months, respectively). They indicated that supratotal resection, along with the removal of a significant amount of the FLAIR abnormality region, can result in longer survival of GBM without significant increases in postoperative morbidity.
Table 1. Supratotal resection in glioblastoma.
| Number of cases | Definition of supratotal resection | Number of supratotal resection case (%) | OS | PFS | Adjuvant therapy | IDH status | MGMT promoter methylation | |
|---|---|---|---|---|---|---|---|---|
| Eyüpoglu et al., 20169) | n = 105 | Beyond obvious contrast enhancement using 5-ALA and iMRI | 30 (29%) | 18.5 vs 14 months (vs GTR) p = 0.0004 | NA | RT + TMZ | NA | +* |
| Li et al., 20165) | n = 643 | Resection over 53.21% of FLAIR | 159 (25%) | 20.7 vs 15.5 months (vs <53.21% of FLAIR ) p <0.001 | NA | NA | NA | NA |
| Esquenazi et al., 20177) | n = 86 | Beyond the zone of enhancement using subpial technique | 25 (29%) | 54 vs 16.5 months (vs GTR) p <0.01 | NA | RT + TMZ ± BCNU wafer | NA | NA |
| Grossmann et al., 20176) | n = 103 | ≤46% of remnant FLAIR (3 months post-operation) | NA | 26.6 ± 3.7 vs 13.5 ± 0.5 (vs GTR) p <0.001 | NA | Stupp protocol | +* | NA |
| Pessina et al., 20178) | n = 282 | Resection 100% of FLAIR | 21 (7%) | 28.6 ± 5.2 vs 16.2 ± 1.2 months (vs GTR) p <0.001 | 24.5 ± 2.4 vs 11.9 ± 0.6 months (vs GTR) p <0.001 | TMZ + RT, 6-8 cycles TMZ | NA | +* |
| Glenn et al., 201810) | n = 32 | Removal of at least 1 cm of brain tissue surrounding the enhancement | 7 (21.9%) | 24 vs 11 months (vs GTR) p = 0.002 | 15 vs 7 months (vs GTR) p = 0.003 | NA | +* | +** |
5-ALA: aminolevulinic acid, BCNU: carmustine, DC: dendritic cell, FLAIR: fluid-attenuated inversion recovery, GTR: gross total resection, iMRI: intraoperative magnetic resonance imaging, MGMT: O6-methylguanine-DNA methyltransferase, NA: not available, OS: overall survival, PFS: progression-free survival, RT: radiotherapy, STR: subtotal resection, TMZ: temozolomide.
+*, Relation between supratotal resection and IDH status or MGMT promoter status was not described; +**, multivariate analysis of PFS and OS with MGMT promoter status and supratotal resection was performed. Supratotal resection was an independent predictor of OS and PFS.
In the present case, despite IDH-wildtype GBM, maximal supratotal resection of GBM was possible with no permanent postoperative deficit and enabled him to live a normal life without tumor recurrence for 9 years and 2 months after the diagnosis. Considering the absence of molecular prognostic biomarkers, including IDH1 and IDH2 mutations in our case, therefore, our patient’s favorable outcome can be likely associated with high EOR of tumor by supratotal resection using awake functional mapping.
Conclusion
We present a case of a long-term survivor of IDH-wildtype GBM. This case suggests that supratotal resection with intraoperative awake brain mapping, when performed on the language-dominant side, can improve survival without impairing the patient’s neurological functions.
Acknowledgments
The authors thank Mr. Hiroyasu Yamamoto, Mr. Kyohei Koyama, Mr. Daisuke Hara, and Mr. Yasuyuki Matsui (Department of Rehabilitation, Nagoya University Hospital, Nagoya, Japan) for their excellent technical assistance.
Footnotes
Ethics Approval
The Ethics Committee at Nagoya University Hospital approved this retrospective data evaluation and the experimental design of the study (approval number: 2017-0459).
Funding
This work was supported by a Grant-in-Aid for Scientific Research (C) awarded to K.M. (No. 17K10862) from the Japan Society for the Promotion of Science (JSPS).
Conflicts of Interest Disclosure
The authors declare that they have no conflicts of interest.
References
- 1). Omuro A, DeAngelis LM: Glioblastoma and other malignant gliomas: a clinical review. JAMA 310: 1842– 1850, 2013 [DOI] [PubMed] [Google Scholar]
- 2). Bryukhovetskii IS, Bryukhovetskii AS, Khotimchenko YS: New biomolecular approaches to the treatment of glioblastoma multiforme. Bull Exp Biol Med 158: 794– 799, 2015 [DOI] [PubMed] [Google Scholar]
- 3). Sanai N, Berger MS: Glioma extent of resection and its impact on patient outcome. Neurosurgery 62: 753– 764; discussion 264-266, 2008 [DOI] [PubMed] [Google Scholar]
- 4). Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS: An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115: 3– 8, 2011 [DOI] [PubMed] [Google Scholar]
- 5). Li YM, Suki D, Hess K, Sawaya R: The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 124: 977– 988, 2016 [DOI] [PubMed] [Google Scholar]
- 6). Grossman R, Shimony N, Shir D, et al. : Dynamics of FLAIR volume changes in glioblastoma and prediction of survival. Ann Surg Oncol 24: 794– 800, 2017 [DOI] [PubMed] [Google Scholar]
- 7). Esquenazi Y, Friedman E, Liu Z, Zhu JJ, Hsu S, Tandon N: The survival advantage of “supratotal” resection of glioblastoma using selective cortical mapping and the subpial technique. Neurosurgery 81: 275– 288, 2017 [DOI] [PubMed] [Google Scholar]
- 8). Pessina F, Navarria P, Cozzi L, et al. : Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol 135: 129– 139, 2017 [DOI] [PubMed] [Google Scholar]
- 9). Eyüpoglu IY, Hore N, Merkel A, Buslei R, Buchfelder M, Savaskan N: Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget 7: 25755– 25768, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Glenn CA, Baker CM, Conner AK, et al. : An examination of the role of supramaximal resection of temporal lobe glioblastoma multiforme. World Neurosurg 114: e747– e755, 2018 [DOI] [PubMed] [Google Scholar]
- 11). Pallud J, Varlet P, Devaux B, et al. : Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology 74: 1724– 1731, 2010 [DOI] [PubMed] [Google Scholar]
- 12). Nimsky C, Ganslandt O, Fahlbusch R: Implementation of fiber tract navigation. Neurosurgery 58: ONS– 292–303;, discussion ONS–303–304, 2006 [DOI] [PubMed] [Google Scholar]
- 13). Motomura K, Natsume A, Kishida Y, et al. : Benefits of interferon-β and temozolomide combination therapy for newly diagnosed primary glioblastoma with the unmethylated MGMT promoter: a multicenter study. Cancer 117: 1721– 1730, 2011 [DOI] [PubMed] [Google Scholar]
- 14). Verhaak RG, Hoadley KA, Purdom E, et al. : Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17: 98– 110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Phillips HS, Kharbanda S, Chen R, et al. : Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9: 157– 173, 2006 [DOI] [PubMed] [Google Scholar]
- 16). Gately L, McLachlan SA, Philip J, Ruben J, Dowling A: Long-term survivors of glioblastoma: a closer look. J Neurooncol 136: 155– 162, 2018 [DOI] [PubMed] [Google Scholar]
- 17). Kitambi SS, Toledo EM, Usoskin D, et al. : Vulnerability of glioblastoma cells to catastrophic vacuolization and death induced by a small molecule. Cell 157: 313– 328, 2014 [DOI] [PubMed] [Google Scholar]
- 18). Tykocki T, Eltayeb M: Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci 54: 7– 13, 2018 [DOI] [PubMed] [Google Scholar]
- 19). Yuan GQ, Wei NL, Mu LY, et al. : A 4-miRNAs signature predicts survival in glioblastoma multiforme patients. Cancer Biomark 20: 443– 452, 2017 [DOI] [PubMed] [Google Scholar]
- 20). Gerber NK, Goenka A, Turcan S, et al. : Transcriptional diversity of long-term glioblastoma survivors. Neuro Oncol 16: 1186– 1195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Michaelsen SR, Urup T, Olsen LR, Broholm H, Lassen U, Poulsen HS: Molecular profiling of short-term and long-term surviving patients identifies CD34 mRNA level as prognostic for glioblastoma survival. J Neurooncol 137: 533– 542, 2018 [DOI] [PubMed] [Google Scholar]
- 22). Czapski B, Baluszek S, Herold-Mende C, Kaminska B: Clinical and immunological correlates of long term survival in glioblastoma. Contemp Oncol (Pozn) 22: 81– 85, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Parsons DW, Jones S, Zhang X, et al. : An integrated genomic analysis of human glioblastoma multiforme. Science 321: 1807– 1812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Arita H, Yamasaki K, Matsushita Y, et al. : A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun 4: 79, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]