Abstract
Background
Anaesthetic drugs during general anaesthesia are titrated according to sympathetic or somatic responses to surgical stimuli. It is now possible to measure depth of anaesthesia using electroencephalography (EEG). Entropy, an EEG‐based monitor can be used to assess the depth of anaesthesia using a strip of electrodes applied to the forehead, and this can guide intraoperative anaesthetic drug administration.
Objectives
The primary objective of this review was to assess the effectiveness of entropy monitoring in facilitating faster recovery from general anaesthesia. We also wanted to assess mortality at 24 hours, 30 days, and one year following general anaesthesia with entropy monitoring.
The secondary objectives were to assess the effectiveness of the entropy monitor in: preventing postoperative recall of intraoperative events (awareness) following general anaesthesia; reducing the amount of anaesthetic drugs used; reducing cost of the anaesthetic as well as in reducing time to readiness to leave the postanaesthesia care unit (PACU).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 10), MEDLINE via Ovid SP (1990 to September 2014) and EMBASE via Ovid SP (1990 to September 2014). We reran the search in CENTRAL, MEDLINE via Ovid SP and EMBASE via Ovid SP in January 2016. We added one potential new study of interest to the list of ‘Studies awaiting Classification' and we will incorporate this study into the formal review findings during the review update.
Selection criteria
We included randomized controlled trials (RCTs) conducted in adults and children (aged greater than two years of age), where in one arm entropy monitoring was used for titrating anaesthesia, and in the other standard practice (increase in heart rate, mean arterial pressure, lacrimation, movement in response to noxious surgical stimuli) was used for titrating anaesthetic drug administration. We also included trials with an additional third arm, wherein another EEG monitor, the Bispectral index (BIS) monitor was used to assess anaesthetic depth.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently extracted details of trial methodology and outcome data from trials considered eligible for inclusion. All analyses were made on an intention‐to‐treat basis. We used a random‐effect model where there was heterogeneity. For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs, we downgraded evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect or potential publication bias).
Main results
We included 11 RCTs (962 participants). Eight RCTs (762 participants) were carried out on adults (18 to 80 years of age), two (128 participants) involved children (two to 16 years) and one RCT (72 participants) included patients aged 60 to 75 years. Of the 11 included studies, we judged three to be at low risk of bias, and the remaining eight RCTs at unclear or high risk of bias.
Six RCTs (383 participants) estimated the primary outcome, time to awakening after stopping general anaesthesia, which was reduced in the entropy as compared to the standard practice group (mean difference (MD) ‐5.42 minutes, 95% confidence interval (CI) ‐8.77 to ‐2.08; moderate quality of evidence). We noted heterogeneity for this outcome; on performing subgroup analysis this was found to be due to studies that included participants undergoing major, long duration surgeries (off‐pump coronary artery bypass grafting, major urological surgery). The MD for time to awakening with four studies on ambulatory procedures was ‐3.20 minutes (95% CI ‐3.94 to ‐2.45). No trial reported the second primary outcome, mortality at 24 hours, 30 days, and one year with the use of entropy monitoring.
Eight trials (797 participants) compared the secondary outcome, postoperative recall of intraoperative events (awareness) in the entropy and standard practice groups. Awareness was reported by only one patient in the standard practice group, making meaningful estimation of benefit of entropy monitoring difficult; moderate quality of evidence.
All 11 RCTs compared the amount of anaesthetic agent used between the entropy and standard practice groups. Six RCTs compared the amount of propofol, four compared the amount of sevoflurane and one the amount of isoflurane used between the groups. Analysis of three studies (166 participants) revealed that the MD of propofol consumption between the entropy group and control group was ‐11.56 mcg/kg/min (95% CI ‐24.05 to 0.92); low quality of evidence. Analysis of another two studies (156 participants) showed that the MD in sevoflurane consumption in the entropy group compared to the control group was ‐3.42 mL (95% CI ‐6.49 to ‐0.35); moderate quality of evidence.
No trial reported on the secondary outcome of the cost of general anaesthesia.
Three trials (170 participants) estimated MD in time to readiness to leave the PACU of the entropy group as compared to the control group (MD ‐5.94 minutes, 95% CI ‐16.08 to 4.20; low quality of evidence). Heterogeneity was noted, which was due to the difference in anaesthetic technique (propofol‐based general anaesthesia) in one study. The remaining two studies had used volatile‐based general anaesthesia. The MD in time to readiness to leave the PACU was ‐4.17 minutes (95% CI ‐6.84 to ‐1.51) with these two studies.
Authors' conclusions
The evidence as regards time to awakening, recall of intraoperative awareness and reduction in inhalational anaesthetic agent use was of moderate quality. The quality of evidence of as regards reduction in intravenous anaesthetic agent (propofol) use, as well as time to readiness to leave the PACU was found to be of low quality. As the data are limited, further studies consisting of more participants will be required for ascertaining benefits of entropy monitoring.
Further studies are needed to assess the effect of entropy monitoring on focal issues such as short‐term and long‐term mortality, as well as cost of general anaesthesia.
Keywords: Adult; Child; Humans; Anesthesia, General; Anesthesia, General/mortality; Entropy; Anesthesia Recovery Period; Anesthetics; Anesthetics/administration & dosage; Intraoperative Awareness; Intraoperative Awareness/prevention & control; Isoflurane; Isoflurane/administration & dosage; Memory, Short‐Term; Methyl Ethers; Methyl Ethers/administration & dosage; Propofol; Propofol/administration & dosage; Randomized Controlled Trials as Topic; Sevoflurane; Time Factors
Plain language summary
Entropy or EEG‐based depth of anaesthesia monitoring for adults and children undergoing general anaesthesia
Review question
We wanted to assess if giving anaesthetic medicines according to the values shown in the entropy monitor would help in avoiding overdosing or underdosing of patients with these drugs.
Background
General anaesthesia is a reversible state of unconsciousness produced by administering anaesthetic medicines that enable patients to undergo surgery without pain or recollection of intraoperative events. Electroencephalography (EEG) is a method whereby sensors attached on the scalp are used to pick up and record electrical activity of the brain. The entropy monitor measures the irregularity of the processed EEG signals and displays it as a numerical value, denoting level of anaesthesia.
Too little anaesthesia can cause the patient to awaken during surgery, feel pain, hear conversations and realize that they are paralysed. Recollection of these experiences after awakening can lead to severe mental distress, anxiety and inability to function normally. Excessive anaesthesia can lead to delayed awakening and increased anaesthetic costs, as well as contribute to an increase in incidence of death within 24 hours, or up to a year after surgery.
An entropy monitor, by displaying values indicating adequate level of anaesthesia, can guide anaesthetic medicine administration, without increasing chances of awakening during surgery. Further, it can facilitate faster awakening at the end of surgery, reduce costs and decrease chances of death.
Study characteristics
We included studies that compared entropy monitoring to the standard practice of administering anaesthetic drugs according to changes in heart rate, blood pressure, tearing, sweating or movement in response to surgery. The evidence is current to September 2014. We included adults and children aged two to 16 years. The participants underwent all types of surgery, except brain surgery, under general anaesthesia. We reran the search in January 2016. We identified one potential new study of interest; we will incorporate it into the formal review findings during the review update.
Key results
We found 11 studies, with a total of 962 participants.
Six studies (383 participants) found minimally shorter time to awakening in the entropy group. No study reported on death occurring in the first 24 hours after surgery or within 30 days to a year after surgery.
Eight studies (797 participants) evaluated recollection of intraoperative events (awareness). Adverse events were rare and no benefit was evident.
All 11 studies compared anaesthetic medicine use: six compared propofol (given in the vein) and five evaluated anaesthetic gas (sevoflurane or isoflurane). Limited studies were analysed because of differences in methodology and units of measurement. Analysis of three studies (166 participants) found reduced propofol use, and two studies (156 participants) found lower sevoflurane use in the entropy group.
No study reported on cost of general anaesthesia. Three studies found shorter length of stay in the postanaesthesia care unit (PACU) in the entropy group.
Quality of evidence
The evidence for assessing reduction in time to awakening, recall of intraoperative events and amount of inhalation of anaesthetic agents used is of moderate quality. The quality of evidence as regards intravenous anaesthetic agent used and length of stay in the PACU is of low quality.
Summary of findings
Summary of findings for the main comparison. Entropy versus Standard for adults and children undergoing general anaesthesia.
| Entropy versus Standard for adults and children undergoing general anaesthesia | ||||||
| Patient or population: adults and children undergoing general anaesthesia Settings: People undergoing various surgical procedures under general anaesthesia in hospitals in Europe (France, Germany, Bulgaria, Finland, Norway, Sweden) and Asia (South Korea, China, Saudi Arabia, India, Taiwan). Intervention: Entropy versus Standard | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Entropy versus Standard | |||||
| Time to awakening minutes1 | The mean time to awakening in the intervention groups was 5.42 lower (8.77 to 2.08 lower) | 383 (6 studies) | ⊕⊕⊕⊝ moderate2 | |||
| Decrease in mortality with the use of the entropy monitor at 24 hours, 30 days and one year Assessng mortality at 24 hours, 30 days and one year | Study population | Not estimable | 0 (03) | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Postoperative recall of intraoperative events Validated questionnaire/ not validated interview4 | 2 per 1000 | 0 per 1000 (0 to 0) | Not estimable5 | 797 (8 studies) | ⊕⊕⊕⊝ moderate6 | |
| Anaesthetic agent (Intravenous used) mcg/kg/min7 | The mean reduction in anaesthetic agent (intravenous) used was in mcg/kg/min | The mean anaesthetic agent (intravenous requirement) in the intervention groups was 11.56 lower (24.05 lower to 0.92 higher) | 166 (3 studies) | ⊕⊕⊝⊝ low8,9 | ||
| Anaesthetic agent (Inhalational used) ml10 | The mean reduction in anaesthetic agent (inhalation) used was in ml | The mean anaesthetic agent (inhalational requirement) in the intervention groups was 3.42 lower (6.49 to 0.35 lower) | 156 (2 studies) | ⊕⊕⊕⊝ moderate11 | ||
| Cost of general anaesthesia GBP or USD | Study population | Not estimable | 0 (012) | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Time to readiness to leave PACU minutes13 | The mean time to readiness to leave PACU in the intervention groups was 5.94 lower (16.08 lower to 4.20 higher) | 170 (3 studies) | ⊕⊕⊝⊝ low14,15 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Defined as either time to extubation in intubated patients, or eye opening in response to verbal commands or spontaneous movements in non‐intubated patients) after stopping the anaesthetic agent 2 We downgraded the quality of evidence by one level because of the 6 included studies, only 2 RCTs had low risk of bias ( Choi 2010;Talawar 2010). Three RCTs had unclear risk of bias in 3, 2 and 1 domains respectively (Aimé 2006; Jiahai 2012; Marinova 2012). In one study GE Healthcare Monitoring Solutions Helsinki, loaned the authors a S5 monitor and probes (other bias) however authors did not find decreased time to awakening in the entropy group (Aimé 2006) . Only one RCT had high risk of bias because the outcome assessor was not blinded (Ellerkmann 2010). 3 No RCT has studied this outcome, therefore no data available. 4 Five studies (Aimé 2006; Ellerkmann 2010; Jiahai 2012; Marinova 2012; Vakkuri 2005) used validated questionnaires [Brice (Brice 1970), Nordstrom (Nordström 1997), modified Brice (Enlund 2002) , Dowd (Dowd 1998)] to elicit recall of intraoperative events whereas three studies (Choi 2010; Gruenewald 2007; Riad 2007) used non‐validated interview for eliciting incidence of awareness. 5 As postoperative recall of intraoperative events (awareness) is an extremely rare event and was observed in only one participant out of the 797 participants studied, any estimation of the relative risk for the event on comparison of the groups would be misleading 6 We downgraded the quality of evidence by one level because of the 8 RCTs included,5 RCTs had unclear risk of bias in one or more domains (Aimé 2006; Gruenewald 2007; Jiahai 2012; Marinova 2012; Riad 2007) . Two RCTs had high risk of bias in one domain each (Ellerkmann 2010; Vakkuri 2005). Only one RCT had low risk of bias (Choi 2010). 7 Three studies (Ellerkmann 2010; Gruenewald 2007; Marinova 2012) calculated propofol requirement in mcg/kg/min 8 We downgraded quality of evidence by one level because of risk of bias. Two studies had unclear risk of bias in one or more domains (Gruenewald 2007; Marinova 2012). One study (Ellerkmann 2010) had high risk of bias in one domain. 9 We downgraded the quality of evidence by one level because of the wide confidence intervals observed ‐11.56 [‐24.05, 0.92] mcg/kg/min in propofol consumption. The upper boundary of the confidence interval denoted no effect but the lower boundary denotes appreciable benefit. This was due to the heterogeneity introduced by one study (Marinova 2012) that had a small sample size. 10 Two studies assessed total sevoflurane requirement for the procedure, in one study (Aimé 2006) this was assessed in mg and in the other (Wu 2008) in ml. The values obtained from the first study were converted into ml. 11 We downgraded the quality of evidence by one level because of the unclear risk of bias in more than one domains in both the studies (Aimé 2006; Wu 2008). 12 No RCT studied this outcome, therefore no data is available. 13 Time to readiness to leave PACU was evaluated from analysis of three studies (Choi 2010; El Hor 2013; Marinova 2012) in minutes. In the study by Choi 2010, authors were contacted and time to complete recovery i.e. an Aldrete score of 9 (Aldrete 1970) or more was confirmed to be equivalent to time to readiness to leave PACU. Another study (Marinova 2012) provided us time to readiness to discharge from the PACU after retrospectively calculating it. 14 We downgraded quality of evidence by one level because in one RCT (Marinova 2012) had an unclear risk of bias in one domain. The other two RCTs had low risk of bias (Choi 2010; El Hor 2013). 15 We downgraded the quality of evidence by one level because of the wide confidence intervals observed ‐5.94 [‐16.08, 4.20] min. The upper boundary of the confidence interval denoted no effect but the lower boundary denotes appreciable benefit. This was due to a single study (Marinova 2012) which had unclear risk of bias due to allocation concealment.
Background
Anaesthetic drug administration has classically been titrated according to clinical signs, such as increases in heart rate or mean arterial pressure, lacrimation or movement to noxious stimuli (ASA Task Force 2006). The development of microprocessor technology and Fourier transformation has made it possible to use electroencephalographic (EEG)‐based depth of anaesthesia monitors in the operating room (Bruhn 2006; Punjasawadwong 2014). 'Entropy' is such a monitor, which can be used to assess the depth of anaesthesia in patients undergoing general anaesthesia (Vakkuri 2004), thereby providing the clinician with an alternative tool to titrate anaesthetic agents.
Description of the condition
Anaesthesia is a drug‐induced reversible loss of consciousness which is produced for the purpose of facilitating a surgical procedure. The drugs used in anaesthesia are very potent and produce a global loss of consciousness. Despite this, there is a 0.1% to 0.2% incidence of awareness or recall of intraoperative events in patients undergoing surgery under general anaesthesia (Myles 2004; Spitellie 2002). The incidence of awareness is higher in patients undergoing caesarean sections, cardiac surgery or trauma surgery than other surgeries (Myles 2004; Spitellie 2002). As many as 70% of participants with awareness during anaesthesia can develop unpleasant sequale such as sleep disturbances, nightmares, distressing flashbacks, anxiety attacks, inability to concentrate and, in severe cases, prolonged or chronic post‐traumatic stress disorder (Leslie 2010a; Messina 2008: Spitellie 2002). Therefore the underdosage of anaesthetic drugs, which may result in awareness, is to be avoided.
On the other hand, literature has revealed that anaesthetic drugs can lead to neuronal cell apoptosis, especially in the elderly and in children (Lu 2010; Xie 2006). In addition, there are reports of increased mortality at 30 days, six months, as well as many years after surgery in participants found to have increased depth of anaesthesia by another EEG monitor, the Bispectral index (BIS) monitor (Kertai 2010; Leslie 2010b; Monk 2005; Monk 2011). The relationship may be an epiphenomenon rather than directly related to anaesthetic drug overdose (Kertai 2010; Leslie 2010b; Monk 2005; Monk 2011). However, the ability to titrate the anaesthetic drugs and avoid underdosage or overdosage of these drugs may benefit patients.
Description of the intervention
Entropy is an EEG‐based monitor that measures the randomness or irregularity of a signal. In the awake state, the EEG signal has greater irregularity or randomness and therefore a higher value. With increasing depth of anaesthesia, the EEG signal becomes regular and the entropy value decreases (Vakkuri 2004). The entropy monitor, a proprietary software of GE Datex‐Ohmeda measures two indices, state entropy (SE) and response entropy (RE). SE is computed from signals of 0.8 to 32 hertz (Hz) frequencies and reflects cortical EEG activity. RE measures higher frequencies, from 0.8 to 47 Hz, and in addition, also assesses electromyogram (EMG) signals originating from contraction of the frontalis muscle (Vakkuri 2004; Viertiö‐Oja 2004). It was hoped that measuring RE and SE would enhance the ability of the entropy monitor to assess the depth of anaesthesia as compared to other EEG‐based monitors, such as Bispectral index (BIS). SE and RE values in the range of 40 to 65 indicate adequate depth of anaesthesia (Vakkuri 2004; Viertiö‐Oja 2004); the maximum awake value of SE is 91 and of RE is 100 (Vakkuri 2004; Viertiö‐Oja 2004). An increase in the difference of RE and SE values of 10 or more is thought to reflect pain or recovery from muscle relaxants (Vakkuri 2004; Viertiö‐Oja 2004).
Many studies (adults and children) found the entropy values decrease with induction of anaesthesia, recovering to preinduction awake values after anaesthetic drugs have been stopped (Davidson 2005; Klockars 2006; Klockars 2012; Moller 2008; Weil 2008). In other studies, entropy values (40 to 65) have been found to correlate with similar BIS values denoting adequate depth of anaesthesia (Baulig 2010; Bonhomme 2006; Meybohm 2010; Vanluchene 2004; White 2006). The entropy values also correlate with verbal rating scores, denoting adequate depth of sedation (Iannuzzi 2005; Moller 2008; Vanluchene 2004). Some studies have questioned participants in the postoperative period about postoperative recollection of intraoperative events (awareness) (Baulig 2010; Moller 2008). Another method of assessing intraoperative awareness with the entropy monitor would be by the use of the isolated forearm technique in anaesthetized patients. However, such information is lacking at present. Most studies use either validated (Baulig 2010), or non‐validated questionnaires (Moller 2008), to ascertain postoperative recollection of intraoperative events.
How the intervention might work
Monitoring anaesthetic depth with the entropy monitor could facilitate reduction in amount of anaesthetic drug used without the risk of recollection of intraoperative events. Reduced anaesthetic agent administration could facilitate faster awakening after the surgery (Aimé 2006; Choi 2010; Vakkuri 2005). The ability of the RE index to sense impulses originating from frontalis muscle contractions in response to nociceptive stimuli, further improves the efficiency of the monitor (Vakkuri 2004; Viertiö‐Oja 2004).
Why it is important to do this review
Entropy is used to ensure adequate depth of anaesthesia. This is ascertained by observing a decrease in the SE (from a maximum awake value of 91) and RE (from a maximum awake value of 100) indices to SE and RE values between 40 to 65, following induction of anaesthesia. In addition, an increase in the RE‐SE difference of ≥ 10 indicates either a need for analgesia or recovery from muscle relaxants in paralysed patients. This review aimed to ascertain whether titrating anaesthetic depth with entropy (RE and SE values maintained between 40 to 65) resulted in faster awakening of patients at the end of surgery, together with decreased incidence of awareness and reduced anaesthetic use. This was assessed in adult and paediatric patients undergoing elective and emergency surgery (other than intracranial surgery) under volatile anaesthetic‐based general anaesthesia or propofol‐based general anaesthesia.
The entropy monitor module is only compatible with a particular workstation, and its use involves the standing cost of the module as well as the cost of the disposable sensors. The costs have been estimated as GBP 5352 per operating theatre for the machine module and GBP 8.68 per sensor, assuming use of a single sensor per person (NICE 2011). One study found evidence that monitoring depth of anaesthesia using the BIS monitor, decreased anaesthetic agent consumption by 19%, and reduced the time spent in the recovery room by four minutes, however this did not lead to a reduction in costs due to the cost of the disposable BIS sensors (Liu 2004).
We carried out this review to assess the benefits obtained from using an entropy monitor in terms of faster awakening from anaesthesia, mortality at 24 hours, 30 days and one year, decreased incidence of awareness, reduction in amount of anaesthetic agents used, reduction in cost of the anaesthetic agents and reduction in time to readiness to leave the postanaesthesia care unit (PACU).
Objectives
The primary objective of this review was to assess the effectiveness of entropy monitoring in facilitating faster recovery from general anaesthesia. We also wanted to assess mortality at 24 hours, 30 days, and one year following general anaesthesia with entropy monitoring.
The secondary objectives were to assess the effectiveness of the entropy monitor in: preventing postoperative recall of intraoperative events (awareness) following general anaesthesia; reducing the amount of anaesthetic drugs used; reducing cost of the anaesthetic as well as in reducing time to readiness to leave the postanaesthesia care unit (PACU).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) in adults and children (aged two to 16 years of age) where in one arm entropy was used for titrating anaesthesia and in the other arm standard practice (monitoring mean arterial pressure or heart rate or clinical signs such as lacrimation or movement in response to noxious stimuli) was used for titrating anaesthetic drug administration. We also included studies that had a third arm in which another EEG‐based depth of anaesthesia monitor, such as Bispectral index (BIS), was used.
We included studies where entropy monitoring was used with volatile anaesthetic‐based general anaesthesia or propofol‐based general anaesthesia, and where patients breathed spontaneously or were mechanically ventilated after muscle paralysis.
Types of participants
We included a heterogenous population with the following characteristics.
Adults (> 16 years) and children (aged two to 16 years) of both genders.
Participants undergoing any elective or emergency surgery, except intracranial surgery, under general anaesthesia.
We excluded:
infants and children aged less than two years of age; and
patients undergoing intracranial surgery under general anaesthesia.
Types of interventions
Intervention: entropy monitoring during general anaesthesia. The entropy monitoring could have been started preinduction or postinduction (children), and continued up to postoperative awakening of the patients (defined as either time to extubation in intubated patients, or eye opening in response to verbal commands, or spontaneous movements in non‐intubated patients following stopping of the intravenous or inhalational agent).
The control or standard practice group included patients monitored by conventional haemodynamic variables (mean arterial pressure or heart rate) or clinical signs such as lacrimation, sweating or movement in response to noxious stimuli to titrate anaesthetic agent administration.
Types of outcome measures
Primary outcomes
Time to awakening (defined as either time to extubation in intubated patients, or eye opening in response to verbal commands, or spontaneous movements in non‐intubated patients) after stopping the anaesthetic agent.
Mortality with the use of the entropy monitor at 24 hours, 30 days, and one year.
Secondary outcomes
Postoperative recall of intraoperative events (awareness) in the immediate postoperative period, at 24 hours, one week, and one month.
Anaesthetic agent (intravenous or inhalational) used.
Cost of general anaesthesia.
Time to readiness to leave the postanaesthesia care unit (PACU).
Search methods for identification of studies
Electronic searches
We searched for eligible trials in the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 10 see Appendix 1), MEDLINE via Ovid SP (1990 to September 2014, see Appendix 2), EMBASE via Ovid SP (1990 to September 2014, see Appendix 3). We applied the highly sensitive filter for randomized controlled trials (RCTs) in MEDLINE and EMBASE.
We adapted our MEDLINE search strategy for searching all other databases.
We did not impose any language restrictions.
We reran the search in CENTRAL, MEDLINE via Ovid SP and EMBASE via Ovid SP in January 2016. We added one potential new study of interest to the list of ‘Studies awaiting Classification' and we will incorporate this new study into the formal review findings during the review update.
Searching other resources
We searched for relevant ongoing trials (1990 to February 2015) on specific web sites such as:
Data collection and analysis
We recorded the data obtained from the above publications on a predesigned data collection form (Appendix 4).
Selection of studies
Using the results of the above searches, we screened all titles and abstracts for eligibility. Two authors (AC and SP) independently performed this screening. We obtained and assessed the full articles of all potentially eligible RCTs for relevance based on the preplanned checklist. We avoided duplication of studies. Each author independently documented the reason for excluding a trial. We resolved any disagreement by discussion with a third review author (HP), who decided on the inclusion or exclusion of the study. We compiled a list of all eligible trials.
If further information was required to make a decision about trial inclusion, AC contacted the first/corresponding author of the relevant trial. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and 'Characteristics of excluded studies' table. We did not impose any language restrictions
Data extraction and management
We used a data collection form for study characteristics and outcome (Appendix 4). Two review authors [AC, SP] extracted study characteristics from included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (RS, AS) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third review author (HP). One review author (AC) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (HP) spot‐checked study characteristics for accuracy against the trial report.’
Assessment of risk of bias in included studies
Two review authors (AC, AS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (HP).
We assessed the risk of bias according to the following domains.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We completed a 'Risk of bias' table for each eligible study and outcome using the categories of low, high or unclear risk of bias. For each outcome, we presented the summary 'Risk of bias' assessments within domains in the 'Risk of bias' graphs or figures, and across domains in the 'Summary of findings' table.
Measures of treatment effect
For binary outcomes, such as whether entropy (state entropy (SE) values of 40 to 65) was associated with postoperative recall of intraoperative events (awareness) in patients receiving general anaesthesia, we planned to estimate the risk ratio (RR) with its 95% confidence interval (CI) from a random‐effects model analysis. However, the event was rare and observed in only one patient, therefore, we did not estimate the risk ratio.
For continuous data, such as time to awakening, reduction in amount of anaesthetic agent used, and time to readiness to leave the PACU, we calculated the mean difference (MD).
In studies where propofol‐based general anaesthesia had been used, we calculated the dose of propofol used in mcg/kg/min. In studies where volatile anaesthetic‐based general anaesthesia was used we calculated the volume of volatile anaesthetic used. In cases where particular information regarding an outcome was not available, we contacted the authors. If the authors could not or did not provide required data, it was not entered in analysis of that particular outcome.
Unit of analysis issues
We included RCTs with a parallel group design in the review.
Dealing with missing data
We performed quantitative analysis on an intention‐to‐treat (ITT) basis and contacted the authors in order to obtain any missing data.
Assessment of heterogeneity
We planned not to perform meta‐analysis if on examining the included trials we suspected important clinical heterogeneity. We used the Q statistic to test the statistical heterogeneity between trials and considered P ≤ 0.05 as indicating significant heterogeneity (Higgins 2002; Higgins 2003). We used the I2 statistic to assess the magnitude of heterogeneity (Higgins 2002). When I2 > 50% we performed a subgroup analysis based on preplanned criteria. We also performed a sensitivity analysis to check robustness of results. We planned to used a random‐effects model analysis if the I2 statistic was between 30% and 50% (Higgins 2011).
Assessment of reporting biases
We planned to test for funnel plot asymmetry if more than 10 studies (for each outcome) were included in the meta‐analysis.
Data synthesis
We quantitatively reviewed the included data and combined the data by intervention, outcome and population using Cochrane statistical software, Review Manager 5 (RevMan 2014). We synthesized the data in the absence of important clinical or statistical heterogeneity. We pooled estimates of the MD for continuous variables and risk ratio (RR) for proportions, as described above.
Subgroup analysis and investigation of heterogeneity
Where appropriate, based on obvious clinical or statistical (I2 > 50%) heterogeneity, we considered subgroup analysis. We observed heterogeneity for the outcome, time to awakening (I2 = 95%). On performing preplanned subgroup analysis we found that studies in which patients underwent major surgeries (off‐pump coronary artery bypass graft (Jiahai 2012), and major urological surgery (Marinova 2012) were heterogenous. Four studies comprising patients undergoing short, ambulatory procedures showed no heterogeneity (I2 = 0%) (Aimé 2006; Choi 2010; Ellerkmann 2010; Talawar 2010). The difference in time to awakening between the entropy and control group was MD ‐3.20 minutes (95% CI ‐3.94 to ‐2.45).
We observed heterogeneity for intravenous anaesthetic agent use (I2 = 49%). This could not be explained on preplanned subgroup analysis.
Similarly, we observed heterogeneity in time to readiness to leave the PACU (I2 = 78%). We performed a preplanned subgroup analysis and found that this was due to the difference in anaesthetic technique (propofol‐based general anaesthesia in the study by Marinova 2012). The remaining two studies that used volatile‐based general anaesthesia were homogenous (Choi 2010; El Hor 2013).
We did not carry out subgroup analysis based on effect of age on risk of postoperative recall of intraoperative events (awareness), long‐acting opioids compared to ultrashort‐acting opioids, or regional anaesthesia alone, due to paucity of data.
Sensitivity analysis
We had planned to perform sensitivity analyses to explore the consistency of effect size measures in trials with low risk of bias versus high risk of bias, and to investigate the impact of any missing data using the imputation method described above. We observed heterogeneity for intravenous anaesthetic agent use (I2 = 49%) however, we could not perform a sensitivity analysis because of the three included studies, two had unclear risk of bias in one or more domain (Gruenewald 2007; Marinova 2012) and one had high risk of bias in one domain (Ellerkmann 2010).
We noted heterogeneity in time to readiness to leave the PACU (I2 = 78%) (Choi 2010; El Hor 2013; Marinova 2012). On performing sensitivity analysis this was found to be due to a single study (Marinova 2012) that had unclear risk of bias due to allocation concealment.
'Summary of findings' table and GRADE
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (Guyatt 2008). We constructed Summary of findings table 1 using GRADE software (GRADEproGDT 2015). We included the following outcomes in Summary of findings table 1: time to awakening, mortality with the use of the entropy monitor at 24 hours, 30 days, and one year, postoperative recall of intraoperative events, intravenous or inhalational anaesthetic agent use, cost of general anaesthesia, and time to readiness to leave the PACU. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The assessment of the quality of a body of evidence considers within study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
Results of the search
We identified a total of 876 trials through electronic database searching. After we had removed duplicate trials (trials that were identical), 510 trials remained. We did not obtain trials from other sources. Twelve trials fulfilled the criteria of our review and we obtained the full‐text of these trials. We excluded one trial because the patient population was undergoing intracranial procedures. Therefore, we included 11 studies in this review (Figure 1).
1.
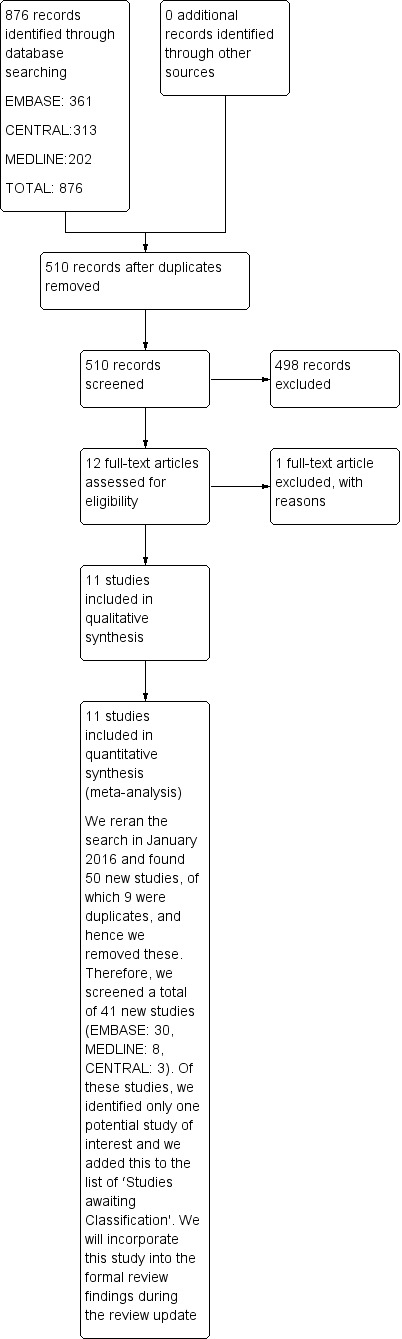
Study flow diagram.
We reran the search in CENTRAL, MEDLINE via Ovid SP, EMBASE via Ovid SP in January 2016. One potential new study of interest was added to the list of ‘Studies awaiting Classification' and will be incorporated into the formal review findings during the review update.
We contacted the lead authors of all the included studies by email to gather missing data. The corresponding authors of four studies either did not respond to our request or did not provide further information (Aimé 2006; Gruenewald 2007; Vakkuri 2005; Wu 2008). The authors of the remaining seven studies confirmed that studying mortality and the cost of the anaesthetic was not a part of their studies (Choi 2010; El Hor 2013; Ellerkmann 2010; Jiahai 2012; Marinova 2012; Riad 2007; Talawar 2010).
See: Characteristics of included studies; Characteristics of excluded studies.
Included studies
We included 11 studies with 962 participants in our review (Aimé 2006; Choi 2010; El Hor 2013; Ellerkmann 2010; Gruenewald 2007; Jiahai 2012; Marinova 2012; Riad 2007; Talawar 2010; Vakkuri 2005; Wu 2008). All studies were of a parallel design and included patients undergoing surgical procedures conducted under general anaesthesia, wherein in one arm entropy was used for titrating anaesthesia, and in the other arm standard practice (monitoring mean arterial pressure or heart rate or clinical signs such as lacrimation or movement in response to noxious stimuli) was used for titrating anaesthetic drug administration. Two of these studies had an additional third arm, wherein Bispectral index (BIS) was also used for titrating depth of anaesthesia (Aimé 2006; Ellerkmann 2010). Eight studies were carried out on adults (> 18 years to 80 years) (Aimé 2006; El Hor 2013; Ellerkmann 2010; Gruenewald 2007; Jiahai 2012; Marinova 2012; Vakkuri 2005; Wu 2008), two studies exclusively involved children (two years to 16 years) (Choi 2010; Talawar 2010), and one study involved participants from 60 to 75 years of age (Riad 2007). Only one study was in the Bulgarian language (Marinova 2012); all other studies were in English.
Excluded studies
We excluded Reviron 2008 for the reasons detailed in Characteristics of excluded studies. This study involved entropy monitoring during cerebral artery embolism, an intracranial procedure that may interfere with entropy monitoring (exclusion criteria in our protocol).
Studies awaiting classification
We reran the search in CENTRAL, MEDLINE via Ovid SP, and EMBASE via Ovid SP in January 2016. We added one potential new study of interest to the list of ‘Characteristics of studies awaiting classification' and we will incorporate this study into the formal review findings during the review update.
Ongoing studies
There are no ongoing studies.
Risk of bias in included studies
We assessed the risk of bias of included studies using the 'Risk of bias' tool developed by Cochrane (Higgins 2011). The risk of bias tool invites judgement on five items for each trial (selection bias, performance bias, detection bias, attrition bias, reporting bias). All authors independently assessed risk of bias for each study. We resolved disagreements by discussion. The characteristics of the included studies for assessment of the risk of bias are shown in Figure 2 and Figure 3. We found three studies to be of high methodological quality (Choi 2010; El Hor 2013; Talawar 2010). The majority of remaining studies had unclear bias in one or more domains (Aimé 2006; Gruenewald 2007; Jiahai 2012; Marinova 2012; Riad 2007; Wu 2008). However two studies had at least one domain with high risk of bias (Ellerkmann 2010; Vakkuri 2005). See Characteristics of included studies.
2.
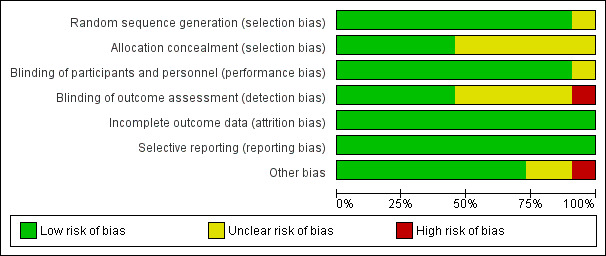
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
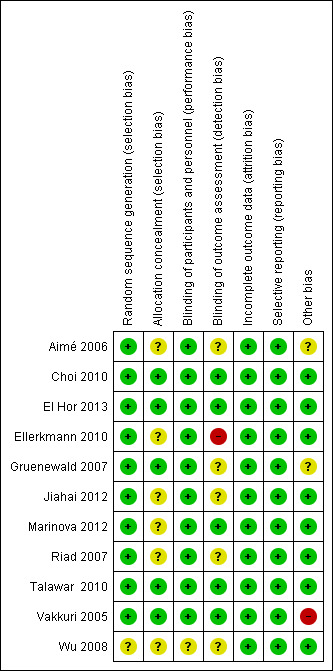
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 11 studies included, only five reported allocation concealment (Choi 2010; El Hor 2013; Gruenewald 2007; Talawar 2010; Vakkuri 2005). In the remaining six studies, either allocation concealment was not done or not described. However, the review authors feel that this domain does not affect the outcomes of the review.
Blinding
Of the 11 included studies, blinding of participants and personnel was unclear in one study (Wu 2008).
Blinding of outcome assessor was not done in one study (Ellerkmann 2010) and unclear in five studies (Aimé 2006; Gruenewald 2007; Jiahai 2012; Riad 2007; Wu 2008).
Incomplete outcome data
Attrition bias was not observed in any of the 11 included studies. The studies wherein participants were excluded due to missing data, or technical problems, had less than 15% of participants excluded from the analysis (Aimé 2006; Choi 2010; El Hor 2013; Ellerkmann 2010; Marinova 2012; Vakkuri 2005; Wu 2008)
Selective reporting
We found that all planned outcomes were reported in the studies. The authors reported all the outcomes which they mentioned in their methodology.
Other potential sources of bias
Three studies had potential risk of bias. GE Healthcare Monitoring Solutions in Helsinki, loaned a S5 monitor and probes to the authors in one study (Aimé 2006).
Gruenewald 2007 acknowledged GE Healthcare for supplying the M‐Entropy module and electrodes.
Technical assistance, financial support, and equipment for data collection and analysis were provided for another study by Datex‐Ohmeda, Helsinki, Finland and two authors of this study Drs. Vakkuri and Yli‐Hankala were medical advisors for GE healthcare Finland, Helsinki, Finland (Vakkuri 2005).
Effects of interventions
See: Table 1
Primary outcomes
1. Time to awakening (defined as either time to extubation in intubated participants, or eye opening in response to verbal commands, or spontaneous movements in non‐intubated participants) after stopping the anaesthetic agent
Six studies consisting of 383 participants (39.8% of total included participants in the meta analysis) estimated the primary outcome, time to awakening after general anaesthesia (Aimé 2006 (91 participants); Choi 2010 (78 participants); Ellerkmann 2010 (52 participants); Jiahai 2012 (70 participants); Marinova 2012 (42 participants); Talawar 2010 (50 participants). Time to awakening decreased in the entropy as compared to the standard practice group (mean difference (MD) ‐5.42 minutes; 95% CI ‐8.77 to ‐2.08, P < 0.00001; Analysis 1.1). We noted heterogeneity for this outcome (I2 = 95%). On performing subgroup analysis of major surgeries and ambulatory procedures, we found that two studies that included participants undergoing major surgeries of long duration, off‐pump coronary artery bypass graft (Jiahai 2012), and major urological surgery (Marinova 2012), contributed to the heterogeneity. The MD for time to awakening after ambulatory surgeries for the entropy as compared to the control group was found to be MD ‐3.20 minutes (95% CI ‐3.94 to ‐2.45) (Aimé 2006; Choi 2010; Ellerkmann 2010; Talawar 2010). These studies were homogenous.
1.1. Analysis.
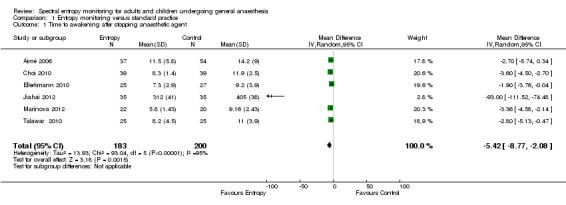
Comparison 1 Entropy monitoring versus standard practice, Outcome 1 Time to awakening after stopping anaesthetic agent.
Of the six included studies, only two studies had a low risk of bias across all domains (Choi 2010; Talawar 2010; Figure 3). Three studies had unclear risk of bias in one or more domains (Aimé 2006; Jiahai 2012; Marinova 2012). One study had high risk of bias in one domain (blinding of outcome assessor or detection bias) (Ellerkmann 2010).
For this outcome, we downgraded the evidence from high to moderate quality because of risk of bias (selection and detection bias mainly, other source of bias in one study because equipment was loaned by GE Healthcare Monitoring Solutions to the study authors).
2. Mortality with the use of the entropy monitor at 24 hours, 30 days, and one year
No study reported this outcome.
Secondary outcomes
1. Postoperative recall of intraoperative events (awareness) in the immediate postoperative period, at 24 hours, one week, and one month
Eight trials with 797 participants (82.8% of total included participants) compared the secondary outcome, postoperative recall of intraoperative events in the entropy and standard practice groups (Aimé 2006 (91 participants); Choi 2010 (78 participants); Ellerkmann 2010 (52 participants); Gruenewald 2007 (72 participants); Jiahai 2012 (70 participants); Marinova 2012 (42 participants); Riad 2007 (72 participants); Vakkuri 2005 (320 participants).
Of the 797 participants included in the eight studies (391 participants in the entropy group; 406 in the standard practice group), only one participant in the standard practice group reported explicit recall of intraoperative events (Gruenewald 2007). As postoperative recall of intraoperative events (awareness) is an extremely rare event and was observed in only one participant out of the 797 participants studied, any estimation of the risk ratio for the event in the standard practice group as compared to the entropy group would be inaccurate. Five studies used a validated score to assess postoperative recall of intraoperative events (Aimé 2006; Ellerkmann 2010; Jiahai 2012; Marinova 2012; Vakkuri 2005).
Seven of the eight studies evaluated this outcome (postoperative recall of intraoperative events) on the first postoperative day (Aimé 2006; Choi 2010; Ellerkmann 2010; Gruenewald 2007; Jiahai 2012; Marinova 2012; Vakkuri 2005). One study evaluated incidence of postoperative recall of intraoperative events on the second postoperative day (Riad 2007). Three studies repeated evaluation on the third postoperative day (Aimé 2006; Ellerkmann 2010; Jiahai 2012). Only one study evaluated awareness at one week postsurgery (Marinova 2012). No study evaluated awareness at one month postsurgery. See Characteristics of included studies.
Of the eight included studies, only one study had a low risk of bias across all domains (Choi 2010; Figure 3). The majority of remaining studies had unclear bias in one or more domains (Aimé 2006; ; Gruenewald 2007; Jiahai 2012; Marinova 2012; Riad 2007). However two studies had at least one domain with high risk of bias (Ellerkmann 2010; Vakkuri 2005). We downgraded the evidence from high to moderate quality because of risk of bias (selection, performance, detection bias, as well as other potential sources of bias).
2. Anaesthetic agent (intravenous or inhalational) used
All 11 RCTs compared anaesthetic agent consumption between the entropy and standard practice groups. Six studies compared propofol consumption (Ellerkmann 2010; Gruenewald 2007; Jiahai 2012; Marinova 2012; Riad 2007; Vakkuri 2005). Four studies evaluated sevoflurane consumption (Aimé 2006; Choi 2010; El Hor 2013; Wu 2008), and one study (Talawar 2010), compared isoflurane consumption between the groups. However, the parameters and the units of measurement used were different in the different studies.
Analysis of three studies consisting of 17.2% of total included participants (Ellerkmann 2010 (52 participants); Gruenewald 2007 (72 participants); Marinova 2012 (42 participants)), revealed that the MD of propofol use between the entropy group and control group was ‐11.56 mcg/kg/min (95% CI ‐24.05 to 0.92, P = 0.07; Analysis 1.2). We observed heterogeneity for intravenous anaesthetic agent consumption (I2 = 49%). This could not be explained on preplanned subgroup analysis. We could not perform sensitivity analyses because two of these studies had unclear risk of bias in one or more domain (Gruenewald 2007; Marinova 2012) and the third had a high risk of bias in one domain (Ellerkmann 2010).
1.2. Analysis.
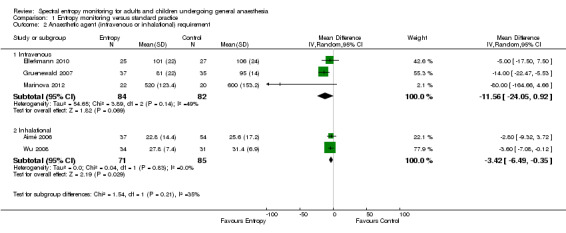
Comparison 1 Entropy monitoring versus standard practice, Outcome 2 Anaesthetic agent (intravenous or inhalational) requirement.
For this outcome, we downgraded the evidence by two levels from high to low quality because of risk of bias (selection, performance and detection bias, other potential sources of bias), and also because the results were imprecise (wide CIs observed: MD ‐11.56 mcg/kg/min, 95% CI ‐24.05 to 0.92 in propofol consumption).
Analysis of another two studies (Aimé 2006 (91 participants); Wu 2008 (65 participants)), consisting of 16.2% of total included participants, showed that the MD in sevoflurane consumption in the entropy group compared to the control group was ‐3.42 mL (95% CI ‐6.49 to ‐0.35, P = 0.03; Analysis 1.2).
Both studies had unclear risk of bias in more than one domain (Figure 3).
For this outcome, we downgraded the evidence from high to moderate quality because of unclear risk of bias (selection, performance and detection bias, other potential sources of bias).
3. Cost of general anaesthesia
No trial reported this outcome. The authors of one study commented on cost of anaesthesia, taking into account the cost of the entropy electrodes (Aimé 2006). The authors stated that: "the saving in sevoflurane realized in the monitor groups, 1 euro per hour, accounts for 8% of the price of the electrode for each hour of anaesthesia." Therefore, the savings in cost because of decreased anaesthetic agent consumption were overshadowed by the cost of the entropy electrodes (see Characteristics of included studies).
4. Time to readiness to leave the postanaesthesia care unit (PACU)
Time to readiness to leave the PACU was assessed by one study with 50 participants (El Hor 2013). Time to achieve an Aldrete score of nine or more (Aldrete 1970), was considered to be equivalent to time to readiness to leave the PACU (after corresponding with the authors of another study (Choi 2010, 78 participants)). Authors of yet another study provided us with time to readiness to leave the PACU after retrospectively calculating it (Marinova 2012, 42 participants). We evaluated time to readiness to leave the PACU from analysis of three studies consisting 17.6% of total included participants (Choi 2010; El Hor 2013; Marinova 2012). It was found that time to readiness to leave the PACU was shorter in the entropy when compared to the standard practice group (MD ‐5.94 minutes, 95% CI ‐16.08 to 4.20, P = 0.001). We performed a preplanned subgroup analysis and found that this was due to the difference in anaesthetic technique (propofol‐based general anaesthesia in the study by Marinova 2012). The remaining two studies that used volatile‐based general anaesthesia were homogenous (I2 = 0%): MD ‐4.17 minutes, 95% CI ‐6.84 to ‐1.51 (Choi 2010; El Hor 2013; Analysis 1.3).
1.3. Analysis.
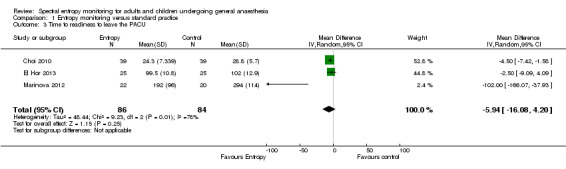
Comparison 1 Entropy monitoring versus standard practice, Outcome 3 Time to readiness to leave the PACU.
Of the three studies, two had a low risk of bias (Choi 2010; El Hor 2013; Figure 3). The third study had unclear risk of bias in one domain (selection bias) (Marinova 2012). For this outcome, we downgraded the quality of evidence by two levels from high to low because of the unclear risk of bias and because the results were imprecise (wide CIs observed ‐5.94 minutes, ‐16.08 to 4.20). The upper boundary of the CI denoted no effect, but the lower boundary denoted appreciable benefit. We noted heterogeneity in time to readiness to leave the PACU (I2 = 78%) on performing sensitivity analysis, this was due to the study (Marinova 2012) which had unclear risk of bias due to allocation concealment.
Discussion
The review was carried out to ascertain if the use of entropy monitoring with general anaesthesia would result in faster awakening, reduced anaesthetic drug consumption and cost, and shorter stay in the postanaesthesia care unit (PACU) without increasing risk of intraoperative awareness. Most importantly the review addressed the question of whether entropy monitoring and titrated use of anaesthetic agents would lead to reduction in mortality at 24 hours, one month, and one year after surgery.
Summary of main results
Six studies consisting of 383 participants estimated the primary outcome, time to awakening after general anaesthesia, which was reduced in the entropy as compared to the standard practice group (Aimé 2006; Choi 2010; Ellerkmann 2010; Jiahai 2012; Marinova 2012; Talawar 2010). We noted heterogeneity for this outcome (I2 = 95%). On performing subgroup analysis of major surgeries and ambulatory procedures, we found that two studies that included participants undergoing major surgeries of long duration such as off‐pump coronary artery bypass graft (Jiahai 2012), and major urological surgery (Marinova 2012), contributed to the heterogeneity.
None of the studies evaluated the second primary objective i.e. postoperative mortality at 24 hours, 30 days, and one year in the entropy and standard practice groups.
Eight trials with 797 participants compared the secondary outcome, postoperative recall of intraoperative events in the entropy and standard practice groups (Aimé 2006; Choi 2010; Ellerkmann 2010; Gruenewald 2007; Jiahai 2012; Marinova 2012; Riad 2007; Vakkuri 2005). Postoperative recall of intraoperative events or awareness is an extremely rare event and only one participant in the standard practice group had awareness. In view of the scarcity of available data, no benefit of entropy monitoring on awareness was evident.
All 11 studies estimated anaesthetic agent consumption. Six studies compared propofol consumption (Ellerkmann 2010; Gruenewald 2007; Jiahai 2012; Marinova 2012; Riad 2007; Vakkuri 2005). Four studies evaluated sevoflurane consumption (Aimé 2006; Choi 2010; El Hor 2013; Wu 2008), and one study (Talawar 2010) compared isoflurane consumption between the groups.
Analysis of three studies consisting of 166 participants, demonstrated that the entropy group participants consumed a lower dose of propofol compared to the standard practice group participants (Ellerkmann 2010; Gruenewald 2007; Marinova 2012). Heterogenity was observed for this outcome (I2 = 49%). This could not be explained on subgroup analysis. We could not perform sensitivity analysis because two of these studies had unclear risk of bias in one or more domain (Gruenewald 2007; Marinova 2012) and the third had a high risk of bias in one domain (Ellerkmann 2010). Two other studies found that sevoflurane consumption was lower in the entropy compared to the standard practice group (Aimé 2006; Wu 2008).
Only one study commented on cost of anaesthesia, taking into account the cost of the entropy electrodes (Aimé 2006). The authors stated that the saving in sevoflurane in the entropy group was less than the cost of the disposable, single‐use electrodes.
Evaluation of data from three studies consisting of 170 participants showed that time to readiness to leave the PACU was shorter in the entropy when compared to the standard practice group (Choi 2010; El Hor 2013; Marinova 2012). We noted heterogeneity for this outcome (I2 = 78%). This was found on subgroup analysis to be due to the anaesthetic technique (propofol‐based general anaesthesia in the study by Marinova 2012).
Overall completeness and applicability of evidence
The methodological quality of only three studies was good (Choi 2010; El Hor 2013; Talawar 2010).
We observed heterogeneity in studies on time to awakening after the end of the anaesthesia, intravenous anaesthetic requirement, and time to readiness to discharge from the PACU.
No data were available on the important clinical and primary outcome of mortality, or the secondary outcome, cost of anaesthesia in the entropy and standard practice groups.
We found that there was no evidence of benefit of entropy monitoring on postoperative recall of intraoperative events or awareness.
Due to methodological differences, differences in anaesthetic agents used, and differences in units of measurement, we used data from limited studies for evaluating time to awakening, anaesthetic agent consumption, and time to readiness to leave the PACU. The reduction in time to awakening, intravenous (propofol) and inhalational (sevoflurane) anaesthetic agent consumption, and time to readiness to leave the PACU, although statistically significant, may not have much clinical significance.
The above findings should be interpreted with caution, keeping in mind that data were collected from a limited number of studies.
Quality of the evidence
We selected RCTs in participants undergoing anaesthesia for different type of surgeries, under entropy monitoring or under standard monitoring. The majority of studies (10 RCTs, 90.9% of included studies) reported on the method of randomization, five RCTs (45.4% of included studies) also carried out allocation concealment. Blinding of participants and personnel was carried out in 10 of the 11 included RCTs (90.9% of the studies). However, the outcome assessors were blinded in only five RCTs (45.4% of included studies).
We estimated the quality of evidence for all outcomes studied using Summary of findings table 1. We found the quality of evidence for the outcome of time to awakening and reduction in amount of inhalational agent used to be of moderate quality, primarily due to risk of bias (selection, detection, performance and other bias). The quality of evidence as regards awareness, intravenous agent (propofol) use as well as time to readiness to leave the PACU, was of low quality because of risk of bias and imprecise results. Therefore, we are uncertain about the estimates.
Potential biases in the review process
In an attempt to minimize bias, we followed the guidelines recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The eligibility for inclusion and exclusion and assessment of risk of bias of the studies was carried out by two authors independently.
We reran the search in CENTRAL, MEDLINE via Ovid SP, and EMBASE via Ovid SP in January 2016. We added one potential new study of interest to the list of ‘Studies awaiting Classification' and we will incorporate this study into the formal review findings during the review update.
Agreements and disagreements with other studies or reviews
There is a systematic review on the clinical effect and cost‐effectiveness of all depth of anaesthesia monitors (E‐Entropy, Bispectral Index and Narcotrend) in participants undergoing various types of surgery under general anaesthesia (Shepherd 2013).
In agreement with our review, Shepherd et al concluded that there is evidence that monitoring depth of anaesthesia resulted in faster recovery times. Evaluation of reduction in mortality with entropy monitoring was not done in this review (Shepherd 2013). The authors opined that the impact of all depth of anaesthesia monitors, including entropy monitoring, on reducing the likelihood of intraoperative awareness is limited, as is the effect on reducing general anaesthetic drug consumption. The authors opined that the cost‐effectiveness of depth of anaesthesia monitors appears to be dependent on a number of factors, including the probability of awareness. The base‐case cost per quality‐adjusted life‐year (QALY) for entropy compared with standard clinical monitoring ranged from GBP 14,421 to GBP 31,430.
Authors' conclusions
Implications for practice.
The difference observed in time to awakening, reduction in amount of intravenous and inhalational anaesthetic agents used, as well as time to readiness to leave the PACU between the entropy and standard practice group is more of statistical than clinical significance. We do not have sufficient evidence to support entropy monitoring for detecting awareness. In view of the limited data available, there is insufficient evidence to support the use of entropy monitoring for monitoring depth of anaesthesia in routine practice. However larger, multicentric studies of uniform design are needed before this can be conclusively ascertained.
Implications for research.
Due to methodological differences and different units of measurement used, we could only include limited numbers of studies in the analysis for determining time to awakening after stopping the anaesthetic agent, reduction in amount of anaesthetic agents used, and time to readiness to leave the PACU.
There is a need to conduct multicentric RCTs with uniform methodology, adequately powered, to assess the above outcomes, as well as mortality and cost of anaesthetic for participants undergoing surgery under general anaesthesia, with and without entropy monitoring. This would also be useful for assessing incidence of postoperative recall of intraoperative events (awareness), which is extremely rare.
Acknowledgements
We would like to thank Javier Eslava‐Schmalbach (content editor), Vibeke E Horstmann (statistical editor), Kate Leslie, Jamie Sleigh, Yodying Punjasawadwong (peer reviewers), Shunjie Chua (consumer referee), Jane Cracknell (Managing Editor, Cochrane Anaesthesia, Critical and Emergency Care (ACE) Group) for their help and editorial advice during the preparation of this systematic review. We would also like to thank Karen Hovhannisyan, former Trials Search Co‐ordinator, ACE, for helping us develop a search strategy.
We would like to thank Jane Cracknell for helping us with every step of the project from title registration to protocol development; and Karen Hovhannisyan for helping us develop a search strategy and also Mathew Zacharias (content editor), Marialena Trivella (statistical editor), Ian Russell, Yodying Punjasawadwong, Jamie Sleigh and Andrew Davidson (peer reviewers) for their help and editorial advice during the preparation of the protocol for this systematic review (Chhabra 2012).
Appendices
Appendix 1. CENTRAL search strategy
#1 (spectra* or entropy) or ((entropy near monitor*) or (spectral near (entropy or monitor*))) #2 MeSH descriptor: [Anesthesia, General] explode all trees #3 an?esth* #4 #2 or #3 #5 #1 and #4
Appendix 2. MEDLINE (Ovid SP) search strategy
1. (spectra* or entropy).ti,ab. or ((entropy adj3 monitor*) or (spectral adj3 (entropy or monitor*))).af. 2. exp Anesthesia, General/ or an?esth*.ti,ab. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. (spectra* or entropy).ti,ab. or ((entropy adj3 monitor*) or (spectral adj3 (entropy or monitor*))).af.
2. general anesthesia/ or an?esth*.ti,ab.
3. 1 and 2
4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh.
5. 2 and 3
Appendix 4. Data Collection Form
CARG
Data collection form
Intervention review – RCTs only
| Review title or ID |
|
Spectral entropy monitoring for adults and children undergoing general anaesthesia |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
Notes: |
1. General Information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/ abstract/ report that data are extracted from) |
|
|
Report ID (ID for this paper/ abstract/ report) |
|
|
Reference details |
|
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
|
Notes: | |
2. Study Eligibility
| Study Characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomized Controlled Trials | |||||
| Participants | Children > 2years up to 16years and adults > 16 to 65 years, participants > 65 years undergoing surgery under general anaesthesia or sedation | |||||
| Types of intervention | RCTS with 2 arms ‐ where in the control/standard practice group anaesthetic agent administration is monitored according to conventional parameters (HR, MAP, movement, lacrimation, sweating etc) and in the entropy group anaesthetic agent administration is going to be titrated according to the entropy values |
|||||
| Types of outcome measures | Primary outcomes 1. Postoperative recall of intraoperative events within 24 hours, 1 week, and 1 month postoperatively 2. Anaesthetic agents ‐ Propofol/Remifentanil/Fentanyl/Sevoflurane/Isoflurane/Desflurane requirement intraoperatively 3. Cost of the anaesthesia Secondary outcomes 1. Time to awakening (defined as either time to extubation in intubated participants or eye opening in response to verbal commands/spontaneous movements in non‐intubated participants) after stopping the anaesthetic agent 2. Time to readiness for discharge from the postanaesthesia care unit (PACU) 3. Mortality at 24 hours, 1 week, and 1 year postoperatively |
|||||
| INCLUDE participants of the above age group undergoing elective or emergency surgery under general anaesthesia or sedation |
EXCLUDE Children < 2 years of age Participants undergoing intracranial surgery |
|||||
| Reason for exclusion | ||||||
|
Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg & ¶/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
|
Informed consent obtained |
Yes No Unclear |
||
| Notes: | |||
4. Methods
|
Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Aim of study |
|||
| Design(e.g. parallel, crossover, cluster) | |||
|
Unit of allocation (by individuals, cluster/groups or body parts) |
|||
|
Start date |
|
||
|
End date |
|
||
|
Total study duration |
|||
| Ethical approval needed/obtained for study | Yes No Unclear |
||
| Notes: | |||
5. 'Risk of bias' assessment
See Chapter 8 of the Cochrane Handbook
| Domain |
Risk of bias |
Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: All/ |
||||
| (if required) |
Outcome group: |
||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: All/ |
||||
| (if required) |
Outcome group: |
||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
|
Other bias |
|||||
| Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomized (or total pop. at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| ASA grade | ||
|
Comorbidities |
||
| Treatment received for comorbidities | ||
| Premedication given | ||
| Surgery performed | ||
|
Subgroups measured |
||
|
Subgroups reported |
||
| Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention Group (entropy group)
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
||
|
No. randomized to group (specify whether no. people or clusters) |
||
|
Theoretical basis(include key references) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of anaesthesia | ||
| Timing of entropy monitoring(prior to induction or after induction of anaesthesia) | ||
| Delivery of anaesthetic agents(whether propofol(TIVA), inhalational agents or opioids titrated) | ||
| Anaesthetic agent titrated by blinded observer or not | ||
| Cointerventions Need for muscle relaxants/analgesics assessed | ||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
| Notes: | ||
Control group (Standard Practice group)
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
||
|
No. randomized to group (specify whether no. people or clusters) |
||
|
Theoretical basis(include key references) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of anaesthesia | ||
| Timing of entropy monitoring(prior to induction or after induction of anaesthesia) | ||
| Delivery of anaesthetic agents(whether propofol(TIVA), inhalational agents or opioids titrated) | ||
| Anaesthetic agent titrated by blinded observer or not | ||
| Cointerventions Need for muscle relaxants/analgesics assessed | ||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
| Notes: | ||
8. Outcomes
Copy and paste table for each outcome.
Outcome 1
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name Any report of postoperative recall of intraoperative events in the entropy and standard practice group. |
|||
|
Time points measured Immediately postoperatively, within 24 hours up to 1 week postoperatively, 1 month postoperatively |
|||
| Time points reported | |||
|
Outcome definition – YES/NO |
|||
| Person measuring/reporting: Anaesthesiologist not involved in the study, other experts | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits entropy & BIS levels: 40‐65 indicate adequate depth of anaesthesia | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
| Power | |||
| Notes: | |||
Outcome 2
| Outcome name: Mortality at 24 hours, 1 week, and 1 year postoperatively | |||
| Time points measured: at 24 hours, 1 week, and 1 year postoperatively | |||
| Time points reported‐ | |||
|
Outcome definition – YES/NO |
|||
| Person measuring/reporting Blinded as to the technique used for titrating anaesthetic agent (entropy/standard practice group) | |||
|
Unit of measurement: (if relevant) |
|||
| Scales: upper and lower limits, entropy levels: 40‐65 indicate adequate depth of anaesthesia | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
| Power | |||
| Notes: | |||
Outcome 3
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name: Time to awakening (defined as either time to extubation in intubated participants or eye opening in response to verbal commands/spontaneous movements in non‐intubated participants) after stopping the anaesthetic agent | |||
| Time points measured : at the end of the procedure | |||
| Time points reported‐ the above endpoint | |||
| Outcome definition – Difference in awakening in minutes in the entropy group and standard practice group | |||
| Unit of measurement: minutes | |||
| Is outcome/tool validated? | Yes No Unclear | ||
|
Person measuring/reporting: Blinded as to the technique used for titrating anaesthetic agent ( entropy/standard practice group) |
|||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
| Power | |||
| Notes: | |||
Outcome 4
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name Anaesthetic agent requirement ‐ Propofol/ Remifentanil/Fentanyl/Sevoflurane/Isoflurane/ Desflurane requirement intraoperatively in entropy and standard practice group |
|||
|
Total anaesthetic agent used for the procedure: Inhalation agents: MAC‐hour Propofol/fentanyl/remifentanil = mL |
|||
|
Time points measured: after the end of surgery |
|||
| Time points reported: | |||
| Outcome definition: Mean difference in drug used in entropy and standard practice group | |||
| Person measuring/reporting: Anaesthesiologist administering anaesthetic | |||
|
Inhalational agent=MAC equivalents |
|||
| Scales: upper and lower limits entropy 40‐65 indicate adequate depth of anaesthesia | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
| Power | |||
| Notes: | |||
Outcome 5
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name Cost of anaesthetic |
|||
| Time points measured: after the end of surgery | |||
| Time points reported All the above endpoints | |||
| Outcome definition: Mean difference in cost of anaesthetic in USD/GBP between entropy and standard practice group | |||
| Person measuring/reporting Anaesthesiologist administering anaesthetic | |||
| Quantity of inhalation or intravenous agent in millilitres | |||
| Scales: upper and lower limits, entropy 40‐65 indicate adequate depth of anaesthesia | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
| Power | |||
| Notes: | |||
Outcome 6
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name: Difference in the time to readiness for discharge from the postanaesthesia care unit (PACU) in the entropy and standard practice group. | |||
| Time points measured: In the PACU when patient meets discharge criteria according to study protocol | |||
| Time points reported‐ | |||
| Outcome definition: Patient meets criteria for discharge from the PACU according to study protocol | |||
| Unit of measurement: minutes | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
| Power | |||
| Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcome
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Timepoint (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
|
Unit of analysis(by individuals, cluster/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
| Notes: | ||||||
Continuous outcome
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Timepoint (specify whether from start or end of intervention) | ||||||||||
| Postintervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, cluster/ groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Other outcome
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Timepoint (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
| Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations, and possible differences in the intervention effect) | Yes No Unclear |
|
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear |
|
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
|
| Notes: | ||
11. Other information
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
Appendix 5. Formula for calculating MAC‐hour or MAC equivalents for ascertaining inhalational agent cost
For inhalation agents we will calculate MAC‐hour and calculate the cost of the inhalation agent used by the following formula: Cost per MAC hour ($) = [(Concentration)(FGF)(duration)(MW)(cost/mL)]/[(2412)(D)], where concentration is the (%) of gas delivered (i.e. the delivered vaporizer setting), fresh gas flow rate (FGF in L/min), duration of inhaled anaesthetic delivery (min), cost per mL ($), molecular weight (MW in gm), density (D in gm/mL), and a factor to account for the molar volume of a gas at 21°C (2412) (Dion 1992a).
The MAC of isoflurane, sevoflurane and desflurane for adults will be 1.15, 1.8, 6.0 respectively. For participants > 65 years the values would be 1.0, 1.45 and 5.17 respectively (Mayer 2007). The MAC of isoflurane, sevoflurane for children 1 to12 years would be 1.6, 2.5 (Cameron 1984;Lerman 1994). For desflurane the MAC would be as follows in children 1 to 3 yr 8.72 ± 0.59%, in children 3 to 5 yr 8.62 ± 0.45%, and in children 5 to 12 yr 7.98 ± 0.43% (Taylor 1991).
Data and analyses
Comparison 1. Entropy monitoring versus standard practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to awakening after stopping anaesthetic agent | 6 | 383 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐8.77, ‐2.08] |
| 2 Anaesthetic agent (intravenous or inhalational) requirement | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Intravenous | 3 | 166 | Mean Difference (IV, Random, 95% CI) | ‐11.56 [‐24.05, 0.92] |
| 2.2 Inhalational | 2 | 156 | Mean Difference (IV, Random, 95% CI) | ‐3.42 [‐6.49, ‐0.35] |
| 3 Time to readiness to leave the PACU | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐5.94 [‐16.08, 4.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aimé 2006.
| Methods | RCT, parallel design, single centre, Department of Anaesthesiology, Hospital Foch, Suresnes, France. Duration of study period not mentioned | |
| Participants | Total of 140 adult participants of both genders were randomly allocated to one of 3 groups: standard practice (numbers = 60), entropy (numbers = 40) and Bispectral index (numbers = 40) M/F: entropy: 23/33; standard practice: 23/14 Inclusion criteria: ASA I‐III participants of 18‐80 years scheduled for elective abdominal, gynaecological, urological, or orthopaedic surgery expected to last at least 1 hour Exclusion criteria: history of any disabling central nervous or cerebrovascular disease, hypersensitivity to opioids or substance abuse, treatment with opioids or any psychoactive medication, or a body weight < 70% or more than 130% of ideal body weight |
|
| Interventions | 1. Standard practice group: (n = 60) (sevoflurane titrated using routine clinical signs) 2. Entropy group: (n = 40) (sevoflurane adjusted to keep SE, and RE values, in the range of 40–60) 3. BIS group (n = 40) sevoflurane adjusted to keep values in the range of 40–60) |
|
| Outcomes | 1. Awareness assessed (in the PACU, on the first and third postoperative days) 2. Assessed sevoflurane requirement intraoperatively 3. Emergence from anaesthesia |
|
| Notes | Intraoperative recall assessed using the Brice protocol On contacting corresponding author, no information provided regarding time to readiness for discharge from PACU, mortality |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization list performed with computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Authors contacted Quote: "too old study, sorry" Comment: Probably not done. However, the review authors feel that this domain does not affect the outcomes of the review |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 1. All participants had BIS & entropy electrodes applied to the forehead preinduction, therefore they were blinded 2. In the standard practice group, the screen monitor was customized to make BIS and entropy values invisible to the attending anaesthesiologist. In the BIS and in the spectral entropy‐guided groups, only the guiding parameter was displayed to the users. Therefore, the observers were also blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 15% excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Unclear risk | GE Healthcare Monitoring Solutions Helsinki, loaned the authors a S5 monitor and probes. However authors did not find decreased time to awakening in the entropy group |
Choi 2010.
| Methods | RCT, parallel design, single centre, Department of Anaesthesiology & Pain Medicine, Dong‐A University Hospital, Busan South Korea Duration of study period: 'late 2008 to early 2009, study duration: 3 months', information obtained after contacting authors |
|
| Participants | A total of 80 children M/F: entropy: 27/12; standard practice: 25/14 Inclusion criteria: ASA physical status I & II children of both genders aged 3‐12 years scheduled for tonsillectomy/adenoidectomy Exclusion criteria: participants with neurological disease and participants on anticonvulsants |
|
| Interventions | 1. Standard practice group: (n = 40) (sevoflurane adjusted to maintain heart rate and systolic blood pressure within 20% of baseline value) 2. Entropy group: (n = 40) (sevoflurane titrated to keep state entropy values maintained between 40‐50) |
|
| Outcomes | 1. Intraoperative recall in the PACU & the first postoperative day 2. Emergence from anaesthesia 3. Time to fitness for discharge from the PACU |
|
| Notes | Authors were contacted to ascertain whether time to complete recovery could be considered equivalent to time for fitness for discharge from the PACU in their hospital, and they replied in the affirmative. Cost of anaesthetic not studied. Research funds from Dong‐A University | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors contacted. Quote: "The method of randomization have been described in the my paper"/ in text stated that "participants were randomly assigned to the standard practice (Standard) group or to the spectral entropy‐guided (entropy) group by parents opening a sealed envelope" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing randomization number opened by parents |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 1. Entropy electrodes were applied for children of both groups 2. Anaesthetist in standard practice group could not see entropy values which were noted by a study nurse |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All data recorded by independent study nurse |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one patient per group has been excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Low risk | Nothing suggestive |
El Hor 2013.
| Methods | RCT, parallel design, single centre, University Hospital Center of Brugmann, Brussels, Belgium Duration of study period: June 2006 to October 2009 |
|
| Participants | Total of 50 participants, of both genders M/F: entropy: 17/8; standard practice: 17/8 Inclusion criteria: participants older than 18 years scheduled for elective laparoscopic recto sigmoidectomy lasting more than 2 hours Exclusion criteria: participants with impaired liver (plasma transaminases > 2 times normal); renal (creatinine > 2 mg/dL); History of drug abuse |
|
| Interventions | Entropy group (n = 25) (SE value maintained between 40‐60 by titration by increasing or decreasing sevoflurane) In the control group (n = 25) (sevoflurane titrated on the basis of the haemodynamic and clinical assessment) |
|
| Outcomes | 1. Sevoflurane uptake between the groups 2. Extubation time 3. PACU length of stay |
|
| Notes | Values of extubation time are available in median and 25‐75 interquartile range. Authors contacted, but they could not provide the values in mean (standard deviation) as data did not have a normal distribution. Authors provided values of time to readiness for discharge from the PACU using mean (standard deviation). Institutional and departmental sources used for the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of 10 envelopes, 5 entropy and 5 control were prepared and sealed in envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were chosen from a block and opened by a responsible anaesthetist who then assigned the patient to the control or the entropy group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants had entropy electrodes applied on forehead prior to induction, irrespective of group; therefore blinded In the control group, the anaesthesiologist was blinded to the SE values, and in the study or entropy group, the anaesthetists were blinded to the actual inspiratory and expiratory fractions of sevoflurane by concealing values using a swab on the screen |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants were managed by the same anaesthetic team who were not involved in data collection or analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 15% excluded from analysis (5 excluded after enrolment of 55 participants) |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Low risk | Nothing suggestive |
Ellerkmann 2010.
| Methods | RCT, parallel design, single centre, University of Bonn, Bonn, Germany Duration of study period: not mentioned; awaiting response from authors |
|
| Participants | Total of 90 participants of both genders randomized M/F: entropy: 10/15; standard practice: 12/15 Inclusion criteria: ASA physical status I‐ III participants, aged 18‐80 years undergoing minor orthopaedic surgery of upper limb or lower limb lasting at least one hour under general anaesthesia with regional anaesthesia to provide intra and postoperative pain control/analgesia Exclusion criteria: history of any disabling central nervous or cerebrovascular a diseases, hyper‐ sensitivity to opioids, substance abuse, or a treatment with opioids or any psychoactive medication |
|
| Interventions | 1. Standard practice group: (n = 30) (titration of propofol using routine clinical signs) 2. Entropy group: (n = 30) (propofol infusion adjusted to keep SE, target values of 50) 3. BIS group (n = 30) propofol infusion adjusted to keep BIS target values of 50, not relevant to the present meta‐analysis |
|
| Outcomes | 1. Awareness assessed in the PACU & on the 1st and 3rd postoperative day 2. Propofol consumption in the entropy as compared to standard practice group assessed 3. Time to recovery from anaesthesia 4. Time taken to achieve modified Aldrete score of 10 considered to be equivalent to time to readiness for discharge from the PACU after contacting authors |
|
| Notes | Duration of study period was not informed, regarding cost of anaesthetic authors commented in the published study "Since we did not find a significant effect in propofol saving etc. I would conclude that Entropy‐Monitoring is more expensive (Costs of one electrode about 10‐12 Euro at that time)" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing lots from a box |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned.However, the review authors feel that this domain does not affect the outcomes of the review |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants had BIS and entropy electrodes applied on forehead prior to induction, irrespective of group; therefore blinded Entropy and BIS monitors hidden behind curtain in standard practice group and either monitor visible in the respective group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The person collecting recovery times was not blinded to the groups; this was ascertained after contacting the authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 15% excluded from analysis. In all, 13.3% excluded from analysis: 3 excluded in standard group (insufficient EEG data = 1; insufficient regional anaesthesia = 2); 5 participants excluded in entropy group (insufficient EEG data = 2; insufficient regional anaesthesia = 3) |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Low risk | Not suggestive |
Gruenewald 2007.
| Methods | RCT, parallel design, single centre, University Hospital Schleswig‐Holstein, Kiel, Germany Duration of study period: not known; authors contacted, information not provided |
|
| Participants | Total: 72 participants (only females) enrolled in the study Inclusion criteria: ASA physical status I‐ II participants undergoing laparoscopic gynaecological surgery lasting at least an hour Exclusion criteria: participants were excluded if they were pregnant, or there was any neuromuscular or neurological disease, or there was use of CNS‐active medication or abuse of alcohol or illicit drugs |
|
| Interventions | 1. Standard practice group:(n = 35) (titration of general anaesthesia using routine clinical signs) 2. Entropy group: (n = 37) (propofol infusion adjusted to keep SE, values of between 40‐60) |
|
| Outcomes | 1. Awareness assessed on 1st postoperative day 2. Assessing propofol consumption between the two groups 3. Time to awakening assessed |
|
| Notes | Authors contacted for duration of study, but despite reply that author will "respond as soon as possible" no information sent despite repeated reminders | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by opening sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened, but not specified if envelopes opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants had BIS & entropy electrodes applied to forehead prior to induction, irrespective of group; therefore blinded Entropy and BIS monitors screen obscured in standard practice group and entropy monitor visible in the entropy group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned, and unlikely it was done in view of the statement in the Discussion section where authors have written that "As entropy and standard practice guidance could not be performed in a blinded fashion, bias cannot be totally excluded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in final analysis (37 assigned to entropy group and 35 to the standard practice group) |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Unclear risk | Authors acknowledged GE Healthcare for supplying the M‐Entropy module and electrodes, but did not find decreased time to awakening in entropy group |
Jiahai 2012.
| Methods | RCT, parallel design, single centre, Yantai Yuhuangding Hospital, Medical College, Qingdao University, Yantai, China Duration of study: January 2010‐Oct 2011, information obtained after contacting authors |
|
| Participants | Participants of both genders undergoing first‐time off‐pump coronary artery bypass surgery. Total participants enrolled: 70 M/F: entropy: 19/6; standard practice: 20/5 Inclusion criteria: were participants with good or only slightly reduced left ventricular function (ejection fraction > 40%, left ventricular end‐diastolic pressure <15 mmHg) and participants below 70 years of age Exclusion criteria: participants requiring CPB either electively or during the course of surgery; participants with renal insufficiency (creatinine > 1.5 mg/dL) or hepatic impairment (alanine aminotransferase or aspartate aminotransferase > 40 U/mL); and participants who misused alcohol or drugs |
|
| Interventions | 1.Entropy group: (n = 35) (propofol infusion rate adjusted to maintain SE range between 45‐55) 2.Control group: (n = 35) (propofol and sufentanil adjusted based on haemodynamic parameters and clinical signs of inadequate or too deep anaesthesia) |
|
| Outcomes | 1. Awareness assessed immediately after tracheal extubation and 3 days later 2. Consumption of anaesthetics (propofol and sufentanil) assessed in cumulative dose of mg/kg 3. Time to awakening assessed |
|
| Notes | Authors contacted for converting cumulative mg/kg dose to microgram/kg/min, but authors did not provide these values As regards cost of anaesthetic, authors commented that "The cost of anaesthesia in the entropy group is about 150 Yuan (Renminbi) less than standard practice group in my study" Study supported by the Science and Technology Programme Foundation of Yantai, Yantai, China (grant number 2010148‐03) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment done, information obtained after contacting authors.However, the review authors feel that this domain does not affect the outcomes of the review |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 1. All participants had entropy electrodes applied on forehead prior to induction irrespective of group; therefore were blinded 2. Entropy values were apparent only to the entropy group anaesthetists, in control group entropy data collected in laptop every 5 seconds but obscured from anaesthetist |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No, apparently all participants included in final analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Low risk | Not suggestive |
Marinova 2012.
| Methods | Prospective, RCT, single‐blind study, parallel design, single centre in Bulgaria; duration: October 2010‐September 2011 | |
| Participants | 42 patients, ASA II‐III of both genders. All patients undergoing elective urological surgery of 45‐150 mins duration under general anaesthesia M/F: entropy: 12/10; standard practice: 11/9 Inclusion criteria: 19‐80 year old, both genders, elective urological surgery of 45‐150 mins duration under general anaesthesia Exclusion criteria: history of psychiatric or neurological disease; previous head trauma with loss of consciousness; addictions (alcohol, drugs); on treatment with drugs with CNS activity; abnormalities of the scalp or skull; hypertension (baseline systolic blood pressure > 180 mmHg or baseline diastolic blood pressure > 105 mmHg); baseline systolic blood pressure < 90 mmHg heart rate < 55 beats/minute; insulin dependent diabetes mellitus; kidney or liver disease; body mass index > 33 kg/m2; any serious illness/condition which would affect the cardiovascular response; use of epidural anaesthesia/analgesia; emergency operations |
|
| Interventions | 1.Entropy group: (n = 22) (propofol infusion rate adjusted to maintain SE range between 40‐60) 2.Control group: (n = 20) (propofol adjusted based on haemodynamic parameters and clinical signs) |
|
| Outcomes | 1.Time to awakening 2. Awareness assessed, the second hour after awakening of anaesthesia and on the first postoperative day 3. Intraoperative total dose of anaesthetic agents (propofol) 4. Time to readiness to leave the PACU |
|
| Notes | Authors provided time to readiness to discharge from the PACU | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors were contacted to ascertain method of randomization and they said they used a random number table |
| Allocation concealment (selection bias) | Unclear risk | Authors contacted, reply: "there was no concealment of allocation". However, the review authors feel that this domain does not affect the outcomes of the review |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors contacted, reply: "the patients and the personnel were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors contacted, reply: "the outcomes were measured from a blinded observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors contacted, reply: "After randomization we excluded 1 patient from the entropy group and 3 patients from the control group" |
| Selective reporting (reporting bias) | Low risk | All the outcomes mentioned in methodology have been reported |
| Other bias | Low risk | Nothing suggestive |
Riad 2007.
| Methods | RCT, parallel design, single centre, King Khaled Eye Specialist Hospital, Riyadh, Kingdom of Saudi Arabia. Duration of study: 2005‐2007, obtained after contacting authors | |
| Participants | Total of 72 participants of both genders studied M/F: entropy: 27/9; standard practice: 24/12 Inclusion criteria: were elderly participants aged between 60 and 75 yrs of age, ASA physical status I–II, scheduled for elective ophthalmic surgery under general anaesthesia Exclusion criteria: with a history of cardiac, pulmonary, liver or renal disease; significant obesity (body mass index > 30); diagnosed Alzheimer disease, dementia, brain atrophy, previous cerebrovascular accident and other neurological disorders; those on long‐term use of drugs affecting the central nervous system, benzodiazepines and/or opioids were also excluded because of the possible effect on their preanaesthesia EEG |
|
| Interventions | 1.Entropy group: (n = 36) (propofol boluses adjusted until RE values dropped to 50 and the RE–SE difference was less than 10) 2.Control group: (n = 36) (propofol adjusted based on haemodynamic parameters and clinical signs to ensure adequate hypnosis. If the patient is still responding verbally, additional increments of 30 mg each were given) |
|
| Outcomes | 1. Awareness assessed 2 days postoperatively 2. Propofol requirement during induction of anaesthesia in elderly participants |
|
| Notes | Authors contacted, a validated interview tool for assessing postoperative recall of intraoperative events not used Propofol dose in mg/kg used during induction of anaesthesia The authors confirmed that entropy monitoring was continued until after extubation. Authors did not receive any external funding for the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No concealment of allocation done; this was elicited after contacting the authors.However, the review authors feel that this domain does not affect the outcomes of the review |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 1. Brain function monitoring using the entropy monitor used for both group participants; therefore they were blinded 2. During induction of anaesthesia in the control group, the anaesthesiologist was not guided by a fall in EEG entropy reading, whereas in the entropy group, propofol was given in successive 30 mg doses every 2 min until RE values dropped to 50 and the RE–SE difference was less than 10 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from all participants included in study were analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Low risk | Nothing suggestive |
Talawar 2010.
| Methods | RCT, parallel design, single centre, All India Institute of Medical Sciences, Ansari Nagar, New Delhi.Duration of study: Sept 2006‐Nov 2008 | |
| Participants | Total participants: 50. M/F: entropy: 25/0; standard practice: 22/3 Inclusion criteria: ASA grade I‐II children, aged 2–12 years, scheduled for lower abdominal or urological surgeries Exclusion criteria: Children whose parents refused consent; those with known neurological disorders; with history of major head injury; those on antiepileptic drugs; those having any contraindications to laryngeal mask airway insertion |
|
| Interventions | 1.Entropy group: (n = 25) (isoflurane adjusted to maintain SE, RE range between 40‐60) 2.Control group: (n = 25) (isoflurane adjusted based on haemodynamic parameters and clinical signs) |
|
| Outcomes | 1. Comparison of the intraoperative end tidal isoflurane concentrations, between the entropy and control group 2. Time to awakening evaluated |
|
| Notes | Dr Chhabra did not decide on the inclusion of this trial in the meta‐analysis; two other co‐authors (SP, HP) decided that the trial met the inclusion criteria. No funding taken from external sources, departmental equipment used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 1. Entropy electrodes were applied on all children postinduction; therefore they were blinded 2. An anaesthesiologist not involved in the anaesthetic management of the patient opened the envelope, and either obscured, or kept the entropy values visible on the monitor; therefore personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Time to awakening was assessed by resident anaesthesiologist who was blinded to the groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data of all participants analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Low risk | Nothing suggestive |
Vakkuri 2005.
| Methods | RCT, parallel design, multiple centre, 3 hospitals from Finland, 2 in Sweden, and 1 in Norway. Duration not known; authors contacted but no response | |
| Participants | Data of 368 participants (48 historical controls, 160 controls, and 160 entropy participants) were analysed M/F: entropy: 44/116; standard practice: 39/121 Inclusion criteria were patients of either sex, aged between 18 and 80 years, ASA physical status I–III with the ability to read and understand the consent form and scheduled for elective surgery procedures estimated to last from 45 to 150 min Exclusion criteria: 1. Those with known psychiatric or neurologic disorders; history of major head injury; substance abuse; medication affecting the central nervous system; acquired scalp or skull abnormalities 2. Uncontrolled hypertension (baseline systolic blood pressure > 160 mmHg or baseline diastolic blood pressure > 105 mmHg); baseline systolic pressure below 90 mmHg; baseline heart rate below 55 beats/min 3. Insulin‐dependent diabetes mellitus; renal or hepatic disease 4. Pregnancy; body mass index over 33.0 kg/m2 5. Any serious medical condition that would interfere with cardiovascular response assessment; cardiac, vascular, or cranial neurosurgery; intraoperatively activated epidural analgesia; and emergency or other non‐elective surgery |
|
| Interventions | 1.Standard practice group: (n = 160) ( titration of general anaesthesia using routine clinical signs) 2. Entropy group: (n = 160) (propofol infusion adjusted to keep SE, RE values between 45‐65) |
|
| Outcomes | 1.All participants were interviewed twice regarding possible intraoperative memories with a modified Brice interview, first in the PACU and during the first postoperative day 2. The study tested the hypothesis that intraoperative entropy monitoring would decrease propofol consumption during propofol‐nitrous oxide‐alfentanil anaesthesia 3. Time of stopping the infusions to recovery of spontaneous breathing and extubation, time to eye opening, time to squeezing of the anaesthesiologist’s hand on command, and orientation to time and place were recorded 4. The time of discharge from the PACU was recorded |
|
| Notes | The propofol consumption in mg/kg/ hour and recovery times i.e. times to spontaneous breathing, extubation, eye opening, complete orientation to time place and person are all recorded in median (range); authors contacted but no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Each study site provided with a sufficient number of "closed" randomization envelopes. They were in sequential coding and subjects were treated in blocks of 10 (5 in each group) Envelopes were opened in, or immediately prior to, induction of anaesthesia |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 1. All participants had entropy electrodes applied preinduction 2. The enrolled participants were randomized to receive propofol–nitrous oxide–alfentanil anaesthesia with either entropy values shown (entropy group) or with entropy values not shown (control group) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Entropy indices were collected with 5‐s intervals on a laptop computer but were not displayed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 17 participants out of 385 were excluded: 48 historical controls, 160 control and 160 entropy group participants included in final analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | High risk | Technical assistance, financial support, and equipment for data collection and analysis for this study were provided by Datex‐Ohmeda, Helsinki, Finland. The authors Drs. Vakkuri and Yli‐Hankala are medical advisors for GE Healthcare Finland, Helsinki, Finland |
Wu 2008.
| Methods | RCT, parallel design, single centre, Chang Gung Memorial Hospital, Kaohsiung 833, Taiwan, ROC | |
| Participants | Total participants = 68, data of 3 participants excluded because of missing data M/F: entropy: 28/6; standard practice: 25/6 Inclusion criteria: ASA physical status I or II participants of either gender, scheduled for total knee replacement, all participants received general anaesthesia without regional analgesia reinforcement Exclusion criteria: history of cerebrovascular disease, treatment with psychoactive medication, existing cardiac dysrhythmia, weight less than 70% or more than 130% of ideal body weight |
|
| Interventions | 1.Standard practice group: (n = 31) (sevoflurane titrated using routine clinical signs) 2. Entropy group: (n = 34) (sevoflurane titrated to keep SE, RE values between 35‐45) |
|
| Outcomes | 1.All participants were interviewed in the PACU in the 72 hour postoperative period about explicit recollection of the procedure they had undergone 2.The consumption of sevoflurane, the sole inhalational agent used, in the entropy‐assisted group and in the conventional group was also assessed |
|
| Notes | Authors contacted for information on duration of study and other queries, but no response from authors. This work was supported in part by the National Science Council (NMRPG 850311), the Statistical Analysis Laboratory, the Department of Medical Research of Kaohsiung Medical University Hospital and the University itself for its help | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors have stated patients were randomized, but method of randomization not described; authors contacted, no response |
| Allocation concealment (selection bias) | Unclear risk | Not specified; authors contacted, no response.However, the review authors feel that this domain does not affect the outcomes of the review |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | 1. All participants had entropy sensors attached before operation and anaesthesia induction; therefore were blinded 2. Entropy values were used to titrate sevoflurane in entropy group and haemodynamic changes and clinical signs in the conventional group, but there is no mention if the personnel/anaesthetists were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned; authors contacted, no response |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No, data of 65 out of 68 participants analysed. Only 3 participants of control group excluded because of missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology have been reported |
| Other bias | Low risk | Nothing suggestive |
ASA ‐ American Society of Anesthesiologists BIS: Bispectral Index CNS: central nervous system
CPB: cardiopulmonary bypass
EEG: electroencephalography F: female ICU: intensive care unit M: male PACU: postanaesthesia care unit RCT: randomized controlled trial RE: response entropy SE: state entropy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Reviron 2008 | This study was excluded because it involved entropy monitoring during cerebral artery embolism, an intracranial procedure that can interfere with entropy monitoring, and was an exclusion criteria in our protocol |
Characteristics of studies awaiting assessment [ordered by study ID]
Rao 2015.
| Methods | Randomized controlled trial |
| Participants | 72 American Society of Anesthesiologists’ physical status I and II patients undergoing general and orthopaedic surgeries |
| Interventions | Entropy monitoring |
| Outcomes | To assess propofol dose required for induction of general anaesthesia in patients with entropy monitoring (state entropy < 50 and state and response entropy difference < 10 ) as compared to the dose titrated to loss of response to verbal commands |
| Notes |
We reran the search in CENTRAL, MEDLINE via Ovid SP, and EMBASE via Ovid SP in January 2016. We added one potential new study of interest to the list of ‘Studies awaiting Classification' and we will incorporate this study into the formal review findings during the review update.
Differences between protocol and review
We included a new author Paranjape S in the Review after the protocol had been published.
We changed the wording of secondary outcome number two, from Anaesthetic agent (intravenous or inhalational) 'requirement' to Anaesthetic agent (intravenous or inhalational) 'used'.
We had intended, in the protocol (Chhabra 2012), that where the dose of propofol was given in mg/kg/min, we would calculate the total dose used by multiplying the mg/kg/minute dose by the duration of the procedure. This was not possible because we could not get individual patient data from the authors and therefore we could not calculate the mean and standard deviation values of the total dose in mg/kg.
For inhalation agents we had proposed to calculate minimum alveolar concentration over one hour (MAC‐hour) and the cost of the inhalation agent used by the formula given in Appendix 5 (Dion 1992a). This would have been possible if the end tidal values of the inhalational agents were available, together with data of individual patients showing duration, so that we could calculate mean duration with standard deviation. This was not possible, therefore we could not calculate the MAC equivalents of the inhalation agents based on the MAC for that age multiplied by the end tidal concentration (Punjasawadwong 2014). The cost of the inhalation agent also could not be calculated by replacing concentration and fresh gas flow (FGF) in the above equation with the MAC equivalent and calculating the MAC‐hour costs.
The first objective mentioned in the protocol was to assess the effectiveness of entropy monitoring in preventing postoperative recall of intraoperative events (awareness) following general anaesthesia. The second and third objectives were to assess the effectiveness of entropy monitoring in decreasing the cost of the anaesthetic as well as in facilitating faster recovery from general anaesthesia. These objectives were changed in the review, in order to avoid overlap with another review (Messina 2008). The objectives of the review now are to assess the effectiveness of entropy monitoring in facilitating faster recovery from general anaesthesia as well as assessing the mortality at 24 hours, 30 days, and one year with the use of the entropy monitor. The other objectives are to assess the effectiveness of entropy monitoring in preventing postoperative recall of intraoperative events (awareness) following general anaesthesia; in decreasing the cost of the anaesthetic as well as reduction in time to readiness for discharge from the PACU.
The primary outcome (number one) 'postoperative recall of intraoperative events (awareness) in the immediate postoperative period, at 24 hours, one week, and one month' was made secondary outcome (number one). and secondary outcome (number one) 'time to awakening (defined as either time to extubation in intubated patients, or eye opening in response to verbal commands or spontaneous movements in non‐intubated patients) after stopping the anaesthetic agent' was made primary outcome number one to avoid overlap with another review (Messina 2008).
'Summary of findings' section in the protocol stated that we would include the outcome 'awareness'; this has been changed in the text of the review to 'postoperative recall of intraoperative events'. Further the outcome 'length of time in the PACU' in the same section has been changed in the text of the review to 'time to readiness to leave the PACU'.
We did not do a manual search of the reference lists of journals and texts, and abstracts from proceedings of international conferences.
We did not carry out subgroup analysis based on effect of age on risk of postoperative recall of intraoperative events (awareness), long‐acting opioids compared to ultrashort‐acting opioids or regional anaesthesia alone, and total intravenous and inhalational (including balanced) anaesthesia for assessing time to awakening because of paucity of data.
Total intravenous anaesthesia (TIVA) has been replaced by propofol‐based general anaesthesia and inhalational (including balanced) anaesthesia replaced by volatile anaesthetic‐based general anaesthesia.
We replaced the term 'awareness recall' by 'awareness'.
In the 'Assessment of heterogeneity' section we replaced, 'We considered an I2 statistic > 50% to indicate that a meta‐analysis was not appropriate' with 'When I2 > 50% we performed a subgroup analysis based on preplanned criteria.' We also performed a sensitivity analysis to check robustness of results.
Contributions of authors
Conceiving the review: Anjolie Chhabra (AC)
Co‐ordinating the review: AC
Undertaking manual searches: AC, Saloni Paranjape (SP)
Screening search results: AC, SP
Organizing retrieval of papers: AC, Rajeshwari Subramaniam (RS)
Screening retrieved papers against inclusion criteria: AC, SP
Appraising quality of papers: Anurag Srivastava (AS), AC
Abstracting data from papers: AC, SP, Hemanshu Prabhakar (HP)
Writing to authors of papers for additional information: AC
Providing additional data about papers: AC, SP
Obtaining and screening data on unpublished studies: RS, AC
Data management for the review: AC, HP
Entering data into Review Manager (RevMan 2014): AC, HP
RevMan statistical data: Mani Kalaivani (MK), AC
Other statistical analysis not using RevMan: MK, AS
Interpretation of data: AC, HP
Statistical inferences: MK, AC
Writing the review: AC, HP
Securing funding for the review: none
Performing previous work that was the foundation of the present study: AC
Guarantor for the review (one author): AC
Person responsible for reading and checking review before submission: AC, HP, AS
Sources of support
Internal sources
-
None, Other.
No financial support taken
External sources
-
None, Other.
No financial support taken
Declarations of interest
Anjolie Chhabra published a RCT (Talawar 2010). Dr Chhabra did not decide on the inclusion of this trial in the meta‐analysis; two other co‐authors (SP, HP) decided that the trial met the inclusion criteria. No financial aid was received for the study, only departmental equipment was used.
Rajeshwari Subramaniam: none known.
Anurag Srivastava: none known.
Hemanshu Prabhakar: none known.
Mani Kalaivani: none known.
Saloni Paranjape: none known.
New
References
References to studies included in this review
Aimé 2006 {published data only}
- Aimé I, Verroust N, Masson‐Lefoll C, Taylor G, Laloë PA, Liu N, et al. Does monitoring bispectral index or spectral entropy reduce sevoflurane use?. Anesthesia and Analgesia 2006;103(6):1469‐77. [PUBMED: 17122226] [DOI] [PubMed] [Google Scholar]
Choi 2010 {published data only}
- Choi SR, Lim YH, Lee SC, Lee JH, Chung CJ. Spectral entropy monitoring allowed lower sevoflurane concentration and faster recovery in children. Acta Anaesthesiologica Scandinavica 2010;54(7):859‐62. [DOI: 10.1111/j.1399-6576.2010.02212.x] [DOI] [PubMed] [Google Scholar]
El Hor 2013 {published data only}
- Hor T, Linden P, Hert S, Mélot C, Bidgoli J. Impact of entropy monitoring on volatile anesthetic uptake. Anesthesiology 2013;118(4):868‐73. [DOI: 10.1097/ALN.0b013e3182850c36.; PUBMED: 23337606 ] [DOI] [PubMed] [Google Scholar]
Ellerkmann 2010 {published data only}
- Ellerkmann RK, Soehle M, Riese G, Zinserling J, Wirz S, Hoeft A, et al. The Entropy Module and Bispectral Index as guidance for propofol‐remifentanil anaesthesia in combination with regional anaesthesia compared with a standard clinical practice group. Anaesthesia and Intensive Care 2010;38(1):159‐66. [PUBMED: PMID: 20191792] [DOI] [PubMed] [Google Scholar]
Gruenewald 2007 {published data only}
- Gruenewald M, Zhou J, Schloemerkemper N, Meybohm P, Weiler N, Tonner PH, et al. M‐Entropy guidance vs standard practice during propofol‐remifentanil anaesthesia: a randomised controlled trial. Anaesthesia 2007;62:1224–9. [DOI: 10.1111/j.1365-2044.2007.05252.x; PUBMED: 17991257] [DOI] [PubMed] [Google Scholar]
Jiahai 2012 {published data only}
- Jiahai M, Xueyan W, Yonggang X, Jianhong Y, Qunhui H, Zhi L, et al. Spectral entropy monitoring reduces anesthetic dosage for patients undergoing off‐pump coronary artery bypass graft surgery. Journal of Cardiothoracic and Vascular Anesthesia 2012;26(5):818‐21. [DOI: 10.1053/j.jvca.2012.01.028; PUBMED: 22502772] [DOI] [PubMed] [Google Scholar]
Marinova 2012 {published data only}
- Marinova R, Temelkov AT, Lazarov M. Spectral entropy monitoring, propofol consumption and emergence from anesthesia [Мониторинг на спектрална ентропия, консумация на Propofol и събуждане след анестезия]. Anaesthesiology and Intensive Care 2012;41(3):16‐20. [Google Scholar]
Riad 2007 {published data only}
- Riad W, Schreiber M, Saeed AB. Monitoring with EEG entropy decreases propofol requirement and maintains cardiovascular stability during induction of anaesthesia in elderly patients. European Journal of Anaesthesiology 2007;24:684–8. [DOI: 10.1017/S026502150700018X; PUBMED: 17425814] [DOI] [PubMed] [Google Scholar]
Talawar 2010 {published data only}
- Talawar P, Chhabra A, Trikha A, Arora MK, Chandralekha. Entropy monitoring decreases isoflurane concentration and recovery time in pediatric day care surgery ‐ a randomized controlled trial. Paediatric Anaesthesia 2010;20(12):1105‐10. [PUBMED: 21260942] [DOI] [PubMed] [Google Scholar]
Vakkuri 2005 {published data only}
- Vakkuri A, Yli‐Hankala A, Sandin R, Mustola S, Høymork S, Nyblom S, et al. Spectral entropy monitoring is associated with reduced propofol use and faster emergence in propofol‐nitrous oxide‐alfentanil anesthesia. Anesthesiology 2005;103(2):274‐9. [PUBMED: 16052109] [DOI] [PubMed] [Google Scholar]
Wu 2008 {published data only}
- Wu SC, Wang PC, Liao WT, Shih TH, Chang KA, Lin KC, et al. Use of spectral entropy monitoring in reducing the quantity of sevoflurane as sole inhalational anesthetic and in decreasing the need for antihypertensive drugs in total knee replacement surgery. Acta Anaesthesiologica Taiwanica 2008;46(3):106‐11. [DOI: 10.1016/S1875-4597(08)60003-X; PUBMED: 18809520] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Reviron 2008 {published data only}
- Reviron P, Lenfant F, Seltzer S, Binnert M, Freysz M. Interest of entropy monitoring during low grade cerebral aneurysm’s embolisation [Inte ´reˆt du monitorage de la profondeur d’anesthe ´sie par entropie pour l’embolisation d’ane ´vrismes intracraˆniens de bas grade]. Annales Franc ¸aises d’Anesthe ´sie et de Re ´animation 2008;27:106–7. [DOI: 10.1016/j.annfar.2007.11.003] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Rao 2015 {published data only}
- Rao AK, Gurajala I, Gopinath R. Comparison of electroencephalogram entropy versus loss of verbal response to determine the requirement of propofol for induction of general anaesthesia. Indian Journal of Anaesthesia 2015;59(6):348‐52. [DOI: 10.4103/0019-5049.158738; PUBMED: PMC4481753] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aldrete 1970
- Aldrete JA, Kroulik D. A post‐anaesthesia recovery score. Anesthesia and Analgesia 1970;49:924–34. [PubMed] [Google Scholar]
ASA Task Force 2006
- American Society of Anesthesiology Task Force on Intraoperative Awareness. Practice advisory for intraoperative awareness and brain function monitoring: a report by the American Society of Anesthesiologists Task Force on intraoperative awareness. Anesthesiology 2006;104(4):847–64. [PUBMED: 16571982] [DOI] [PubMed] [Google Scholar]
Baulig 2010
- Baulig W, Seifert B, Schmid E, Schwarz U. Comparison of spectral entropy and bispectral index electroencephalography in coronary artery bypass graft surgery. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(4):544‐9. [DOI: 10.1053/j.jvca.2009.09.013] [DOI] [PubMed] [Google Scholar]
Bonhomme 2006
- Bonhomme V, Deflandre E, Hans P. Correlation and agreement between bispectral index and state entropy of the electroencephalogram during propofol anaesthesia. British Journal of Anaesthesia 2006;97(3):340‐6. [DOI: 10.1093/bja/ael171] [DOI] [PubMed] [Google Scholar]
Brice 1970
- Brice DD, Hetherington RR, Utting JE. A simple study of awareness and dreaming during anaesthesia. British Journal of Anaesthesia 1970;6:535‐42. [PUBMED: PMID: 5423844 ] [DOI] [PubMed] [Google Scholar]
Bruhn 2006
- Bruhn J, Myles PS, Sneyd R, Struys MMRF. Depth of anaesthesia monitoring: what's available, what's validated and what's next?. British Journal of Anaesthesia 2006;97(1):85‐94. [DOI: 10.1093/bja/ael120] [DOI] [PubMed] [Google Scholar]
Cameron 1984
- Cameron CB, Robinson S, Gregory GA. The minimum anesthetic concentration of isoflurane in children. Anesthesia and Analgesia 1984;4:418‐20. [PUBMED: 6703367] [PubMed] [Google Scholar]
Davidson 2005
- Davidson AJ, Huang GH, Rebmann CS, Ellery C. Performance of entropy and Bispectral Index as measures of anaesthesia effect in children of different ages. British Journal of Anaesthesia 2005;95(5):674‐9. [DOI: 10.1093/bja/aei247] [DOI] [PubMed] [Google Scholar]
Dion 1992a
- Dion P. The cost of anaesthetic vapours. Canadian Journal of Anaesthesia 1992;39(6):633. [PUBMED: PMID: 1643690] [DOI] [PubMed] [Google Scholar]
Dowd 1998
- Dowd NP, Cheng DC, Karski JM, Wong DT, Munro JA, Sandler AN. Intraoperative awareness in fast‐track cardiac anesthesia. Anesthesiology Nov 1998;89(5):1068‐73. [PUBMED: PMID: 9821994] [DOI] [PubMed] [Google Scholar]
Enlund 2002
- Enlund M, Hassan HG. Intraoperative awareness: detected by the structured Brice interview?. Acta anaesthesiologica Scandinavica Apr 2002;46(4):345‐9. [PUBMED: 11952430] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Jaeschke R, Helfand M, Liberati A, et al. Incorporating considerations of resources use into grading recommendations. BMJ 2008;336(7654):1170‐3. [PUBMED: 18497416] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539–58. [DOI: 10.1002/sim.1186] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, ThompsonSG, Deeks JJ. Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Iannuzzi 2005
- Iannuzzi M, Iannuzzi E, Rossi F, Berrino L, Chiefari M. Relationship between bispectral index, electroencephalographic state entropy and effect‐site EC50 for propofol at different clinical endpoints. British Journal of Anaesthesia 2005;94(5):613‐6. [PUBMED: PMID: 15734785] [DOI] [PubMed] [Google Scholar]
Kertai 2010
- Kertai MD, Pal N, Palanca BJA, Lin N, Searleman SA, Zhang L. Association of perioperative risk factors and cumulative duration of low bispectral index with intermediate‐term mortality after cardiac surgery in the B‐Unaware Trial. Anesthesiology 2010;112(5):1116–27. [DOI] [PubMed] [Google Scholar]
Klockars 2006
- Klockars JGM, Hiller A, Ranta S, Talja P, Gils MJ, Taivainen T. Spectral entropy as a measure of hypnosis in children. Anesthesiology 2006;104(4):708‐17. [PUBMED: 16571966 ] [DOI] [PubMed] [Google Scholar]
Klockars 2012
- Klockars JGM, Hiller A, Münte S, Gils MJ, Taivainen T. Spectral entropy as a measure of hypnosis and hypnotic drug effect of total intravenous anesthesia in children during slow induction and maintenance. Anesthesiology 2012;116(2):340‐51. [DOI: 10.1097/ALN.0b013e3182410b5e] [DOI] [PubMed] [Google Scholar]
Lerman 1994
- Lerman J, Sikich N, Kleinman S, Yentis S. The pharmacology of sevoflurane in infants and children. Anesthesiology 1994;80(4):814‐24. [PUBMED: 8024136] [DOI] [PubMed] [Google Scholar]
Leslie 2010a
- Leslie K, Chan MTV, Myles PS, Forbes A, McCulloch TJ. Posttraumatic stress disorder in aware patients from the B‐aware trial. Anesthesia and Analgesia 2010;110(3):823‐8. [DOI: 10.1213/ANE.0b013e3181b8b6ca] [DOI] [PubMed] [Google Scholar]
Leslie 2010b
- Leslie K, Myles PS, Forbes A, Chan MTV. The effect of bispectral index monitoring on long‐term survival in the B‐aware trial. Anesthesia and Analgesia 2010;110(3):816‐22. [DOI: 10.1213/ANE.0b013e3181c3bfb2] [DOI] [PubMed] [Google Scholar]
Liu 2004
- Liu SS. Effects of Bispectral Index monitoring on ambulatory anesthesia: a meta‐analysis of randomized controlled trials and a cost analysis. Anesthesiology 2004;101(2):311‐5. [ISSN0003‐3022] [DOI] [PubMed] [Google Scholar]
Lu 2010
- Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 2010;112(6):1404‐16. [PUBMED: 20460993] [DOI] [PubMed] [Google Scholar]
Messina 2008
- Messina AG, Wang M, Ward MJ, Wilker CC, Eggers M, Miller DB, et al. The effectiveness of anaesthetic interventions for prevention of wakefulness and awareness during and after surgery. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007272] [DOI] [Google Scholar]
Meybohm 2010
- Meybohm P, Gruenewald M, Höcker J, Renner J, Graesner J‐T, Ilies C, et al. Correlation and agreement between the bispectral index vs. state entropy during hypothermic cardio‐pulmonary bypass. Acta Anaesthesiologica Scandinavica 2010;54(2):169‐75. [DOI: 10.1111/j.1399-6576.2009.02138.x] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher 2009: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Moller 2008
- Moller DH, Rampil IJ. Spectral entropy predicts auditory recall in volunteers. Anesthesia and Analgesia 2008;106(3):873‐9. [DOI: 10.1213/ane.0b013e318163201d] [DOI] [PubMed] [Google Scholar]
Monk 2005
- Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one‐year mortality after noncardiac surgery. Anesthesia and Analgesia 2005;100:4–10. [DOI: 10.1213/01.ANE.0000147519.82841.5E] [DOI] [PubMed] [Google Scholar]
Monk 2011
- Monk TG, Weldon BC. Does depth of anesthesia monitoring improve postoperative outcomes?. Current Opinion in Anaesthesiology Dec 2011;24(6):665‐9. [doi: 10.1097/ACO.0b013e32834c7acf] [DOI] [PubMed] [Google Scholar]
Myles 2004
- Myles PS, Leslie K, McNeil J, Forbes A, Chan MTV. Bispectral index monitoring to prevent awareness during anaesthesia: the B‐Aware randomised controlled trial. Lancet 2004;363(9423):1757‐63. [DOI: 10.1016/S0140-6736(04)16300-9] [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence Diagnostics Assessment Programme. Depth of anaesthesia monitors (E‐Entropy, BIS and Narcotrend): Final scope, November 2011. http://www.nice.org.uk/guidance/dg6/documents/depth‐of‐anaesthesia‐monitors‐eentropy‐bis‐and‐narcotrend‐scope2 accessed 12 April 2012.
Nordström 1997
- Nordström O, Engström AM, Persson S, Sandin R. Incidence of awareness in total i.v. anaesthesia based on propofol, alfentanil and neuromuscular blockade. Acta Anaesthesiologica Scandinavica 1997;8:978‐84. [PUBMED: 9311394] [DOI] [PubMed] [Google Scholar]
Punjasawadwong 2014
- Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD003843.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shepherd 2013
- Shepherd J, Jones J, Frampton G, Bryant J, Baxter L, Cooper K. Clinical effectiveness and cost‐effectiveness of depth of anaesthesia monitoring (E‐Entropy, Bispectral Index and Narcotrend): a systematic review and economic evaluation. Health Technology Assessment 2013;17(34):1‐264. [DOI: 10.3310/hta17340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitellie 2002
- Spitellie PH, Holmes MA, Domino KB. Awareness during anesthesia. Anesthesiology Clinics of North America 2002;20(3):555‐70. [DOI] [PubMed] [Google Scholar]
Taylor 1991
- Taylor RH, Lerman J. Minimum alveolar concentration of desflurane and hemodynamic responses in neonates, infants, and children. Anesthesiology 1991;75(6):975‐9. [PUBMED: 1741518] [DOI] [PubMed] [Google Scholar]
Vakkuri 2004
- Vakkuri A, Yli‐Hankala A, Talja P, Mustola S, Tolvanen‐Laakso H, Sampson T, et al. Time‐frequency balanced spectral entropy as a measure of anesthetic drug effect in central nervous system during sevoflurane, propofol, and thiopental anesthesia. Acta Anaesthesiologica Scandinavica 2004;48(2):145‐53. [PUBMED: 14995935] [DOI] [PubMed] [Google Scholar]
Vanluchene 2004
- Vanluchene ALG, Struys MMRF, Heyse BEK, Mortier EP. Spectral entropy measurement of patient responsiveness during propofol and remifentanil. A comparison with the bispectral index. British Journal of Anaesthesia 2004;93(5):645‐54. [DOI: 10.1093/bja/aeh251] [DOI] [PubMed] [Google Scholar]
Viertiö‐Oja 2004
- Viertiö‐Oja H, Maja V, Särkelä M, Talja P, Tenkanen N, Tolvanen‐Laakso H, et al. Description of the Entropy algorithm as applied in the Datex‐Ohmeda S/5 Entropy Module. Acta Anaesthesiologica Scandinavica 2004;48(2):154‐61. [PUBMED: 14995936] [DOI] [PubMed] [Google Scholar]
Weil 2008
- Weil G, Passot S, Servin F, Billard V. Does spectral entropy reflect the response to intubation or incision during propofol‐remifentanil anesthesia?. Anesthesia and Analgesia 2008;106(1):152‐9. [DOI: 10.1213/01.ane.0000296454.00236.fc] [DOI] [PubMed] [Google Scholar]
White 2006
- White PF, Tang J, Romero GF, Wender RH, Naruse R, Sloninsky A, et al. A comparison of state and response entropy versus bispectral index values during the perioperative period. Anesthesia and Analgesia 2006;102(1):160‐7. [DOI: 10.1213/01.ane.0000183668.53139.fc] [DOI] [PubMed] [Google Scholar]
Xie 2006
- Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 2006;104(5):988‐94. [PUBMED: 16645451] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chhabra 2012
- Chhabra A, Subramaniam R, Srivastava A, Prabhakar H, Kalaivani M. Spectral entropy monitoring for adults and children undergoing general anaesthesia. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010135] [DOI] [PMC free article] [PubMed] [Google Scholar]


