Abstract
Patients with refractory chronic migraine have substantial disability and have failed many acute and preventive medications. When aggressive intravenous therapy is indicated, both lidocaine and (R,S)-ketamine infusions have been used successfully to provide relief. Retrospective studies have shown that both agents may be associated with short-term analgesia. In this prospective, observational pilot study of 6 patients we compared the effects of lidocaine and (R,S)-ketamine infusions and performed metabolite analyses of (R,S)-ketamine to determine its metabolic profile in this population. One of (R,S)-ketamine’s metabolites, (2R,6R)-hydroxynorketamine, has been shown in animal studies to reduce pain but human studies in patients undergoing continuous (R,S)-ketamine infusions for migraine are lacking. All 6 patients tolerated both infusions well with mild adverse effects. The baseline mean pain rating (0–10 numeric rating scale) decreased from 7.5 ± 2.2 to 4.7 ± 2.8 by end of lidocaine treatment (p≤0.05) but increased to 7.0 ± 1.4 by the post-discharge visit at 4 weeks (p>0.05 versus baseline). The baseline mean pain rating prior to ketamine treatment was 7.4 ± 1.4, which decreased to 3.7 ± 2.3 by the end of the hospitalization (p≤0.05), but increased to 7.2 ± 1.7 by the post-discharge visit at 6 weeks (p>0.05 versus baseline). For the primary outcome the change in pain from baseline to end of treatment was greater for ketamine than lidocaine (−3.7 versus −2.8, p≤0.05) but this has minimal clinical significance. Ketamine metabolite analysis revealed that (2R,6R)-hydroxynorketamine was the predominant metabolite during most of the infusion, consistent with previous studies.
Introduction
Patients with refractory chronic migraine often have continuous pain and non-painful symptoms, substantial disability, and have failed multiple medications.1 The degree of intractability in refractory chronic migraine has been characterized based on response to preventive treatments, progressing from mild (class 1) to very severe (class 4).2 Patients in class 4 have often failed typical inpatient or outpatient infusion treatment and are left with few if any options. One medication that has shown promise in retrospective studies is (R,S)-ketamine.3 A 5-day continuous infusion of (R,S)-ketamine was associated with short-term improvement with manageable adverse effects in more than 75% of patients in one study.4 Of those who were short-term responders, 30–40% reported sustained response at 1 and 3 months.
(R,S)-ketamine is rapidly and extensively metabolized by cytochrome P450 enzymes (CYPs) into multiple metabolites, including a key metabolite (2R,6R;2S,6S)-hydroxynorketamine (HNK).5 Both (2R,6R)- and (2S,6S)-HNK enantiomers have been shown to produce anti-depressant responses in mouse models of depression.6 Recent studies in murine models of neuropathic pain demonstrated that (2R,6R)-HNK possesses intrinsic analgesic properties,7 appears to be more potent than (R,S)-ketamine.5 (2R,6R;2S,6S)-HNK was also the major circulating metabolite in a plasma sample from a patient receiving a continuous 5-day infusion of (R,S)-ketamine for the treatment of complex regional pain syndrome.8 These observations and our clinical experience suggest that (2R,6R;2S,6S)-HNK or one of its enantiomers may have a therapeutic role in the treatment of refractory chronic migraine patients with a 5-day infusion of (R,S)-ketamine. However, the distribution of (R,S)-ketamine metabolites in these patients has not yet been studied.
Retrospective evidence suggests that a 5-day infusion of (R,S)-ketamine may be beneficial in the treatment of refractory chronic migraine, but prospective evidence is lacking. Data on (R,S)-ketamine’s metabolism and distribution in such patients and how they may relate to individual response to treatment are not known. We therefore performed a prospective, observational pilot study comparing the analgesic benefit of a 5-day (R,S)-ketamine infusion to retrospectively collected standard treatment data with lidocaine infusion determined in the same patients during two separate treatment admissions. Since the enantiomeric composition of (2R,6R;2S,6S)-HNK during a 5-day continuous infusion of (R,S)-ketamine had not been previously determined, the secondary endpoint was to characterize the metabolic profile of (2R,6R)-HNK and (2S,6S)-HNK in patients with refractory chronic migraine and determine if an association exists between clinical response and metabolite levels.
Materials and Methods
General Methods and Data Collection
This study was approved by the Institutional Review Board of Thomas Jefferson University on March 20, 2019 and was carried out in accordance with the Declaration of Helsinski. All patients provided written informed consent. Patients were identified by headache physicians or the research coordinator at the time of their office visit after the initial hospitalization for lidocaine from December 2018 to October 2019 at Methodist Hospital in Philadelphia, PA. Once the patient was scheduled for a second inpatient hospitalization by the headache physician, which typically meant the patient had reported an improvement in average daily pain of either 0 or 1 on the 0–10 numeric rating scale since the initial hospitalization, the patient was approached for enrollment in the prospective, observational study. Hospitalizations for ketamine occurred from June 2019 to December 2019 at Thomas Jefferson University Hospital in Philadelphia, PA. All patient follow-up phone calls took place between 8 and 18 days of discharge from the hospital. The study was registered on clinicaltrials.gov prior to patient enrollment (NCT03896256) and was conducted according to recommendations from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.9 Patients age 18 years and older with a diagnosis of chronic migraine10 who met refractory criteria for class IV2 were eligible. Patients with a contraindication to ketamine, including schizophrenia, active psychosis, pregnancy, poorly controlled cardiovascular disease, cirrhosis,3 or previous treatment with intravenous (IV) ketamine, were excluded. Neither patients nor assessors were blinded as this was an open-label, prospective, observational study.
Historical data from the first hospitalization of patients receiving lidocaine and other IV agents were collected for all patients from manual queries of the electronic medical record. In addition, data during and after hospitalization for ketamine were prospectively collected by a research nurse. Data collected included: demographics, pain levels (baseline, throughout both hospitalizations, and at first post-discharge office visit), medications, and times of blood samples. Primary outcome data (pain ratings) were obtained from the electronic medical record (Epic) for lidocaine hospitalizations and from prospective patient assessment for ketamine hospitalizations. Pain ratings were recorded using a 0–10 verbal numerical rating scale where 0=no pain and 10=worst pain imaginable.
Lidocaine Treatment Protocol and Admission
Patients with refractory chronic migraine were admitted for 5 days to Methodist Hospital for lidocaine infusion. Methodist is a Jefferson-affiliated community hospital approximately 5 miles away from the main hospital and is the location of the Jefferson Headache Center Inpatient Unit. A baseline pain rating was obtained and a lidocaine infusion at 1 mg/min was started on day 1 or 2. The lidocaine infusion was titrated daily based on reported pain levels, the presence of adverse effects, and daily lidocaine plasma levels. Patients were questioned daily about the presence of adverse effects. The target plasma level was not to exceed 5 mcg/mL and the lidocaine infusion rate was not to exceed 4 mg/min. Preventive migraine medications taken at home were continued during the hospitalization. Additional IV medications were used as needed.
Ketamine Treatment Protocol and Admission
Patients were admitted for 5 days to Thomas Jefferson University Hospital for management of refractory chronic migraine with a continuous ketamine infusion. Baseline liver function tests were obtained as well as baseline metabolite levels prior to starting the infusion. (R,S)-ketamine (Ketalar) was started at 10 mg/h and was increased every 4–6 hours in increments of 5–10 mg/h up to the point of intolerable adverse effects or 1 mg/kg/h, whichever came first. Patients were questioned about the presence of adverse effects (hallucinations, nightmares, nausea, and vomiting) at the times of pain assessments and blood draws. Ketamine metabolite blood draws occurred at baseline, 24, 72, and approximately 120 hours from beginning of infusion. Preventive migraine medications taken at home were continued during the hospitalization.
Blood Sample Collection and Analysis
Ketamine metabolites have been studied previously in a patient with complex regional pain syndrome receiving a 5-day continuous ketamine infusion. Steady state plasma metabolites were analyzed on day 3 while the patient was reporting maximal pain relief and hydroxynorketamine was found to be the predominant circulating metabolite.8 We therefore designed this study to measure plasma samples at baseline, 24 hours, 72 hours, and at the end of infusion, which was approximately 120 hours. Samples were analyzed for the following compounds: (R,S)-ketamine, (R)- and (S)-norketamine, (R,S)-dehydronorketamine (DHNK), and (2R,6R)-HNK and (2S,6S)-HNK. Pain ratings were assessed at the times of sample collection and the rate of the ketamine infusion was also recorded.
Venous blood was collected in 5-mL ethylenediamine tetraacetic acid tubes by a research team member and immediately centrifuged and separated into plasma and blood components. They were then transported to a monitored freezer and stored at -70°C until they were shipped for analysis. Samples were shipped in temperature-controlled containers to an external laboratory for analysis. A total of 4 samples per patient were collected, stored, and shipped. Samples were shipped according to established protocols to ensure maintenance of sample storage temperature.
Plasma concentrations of ketamine and its metabolites were determined using a previously described achiral method on a Shimadzu Prominence high-performance liquid chromatography system and a triple quadrupole mass spectrometer, model API 4000 system from Applied Biosystems/MDS Sciex equipped with Turbo Ion Spray® (TIS).8, 11 Resolution of (2R,6R)-HNK, (2S,6S)-HNK, (R)-norketamine, and (S)-norketamine was achieved via a previously described method with slight modifications.12 Briefly, separation of the metabolites was accomplished on a Lux® Amylose-2, LC Column (150 × 4.6 mm, 3um, Phenomenex, Torrance, CA) at 40oC, with mobile phase A consisting of ammonium acetate (5mM, pH 9) and mobile phase B consisting of isopropanol and acetonitrile (4:1). Data were acquired using a Nexera XR HPLC (Shimadzu, Kyoto, Japan) coupled with a QTRAP 5500 (SCIEX) and analyzed with Analyst 1.6 (SCIEX).
Sample Size Calculation and Statistical Analysis
Prior work by our group has shown that a 5-day ketamine infusion is associated with a reduction in short-term pain of approximately 55%.4 Conservatively assuming a reduction in pain by 50%, the sample size was calculated using a paired t-test assuming a mean difference of 4 points with standard deviation (SD) of 2.5 in the 0–11 numerical rating scale for pain from pre- to post-treatment. Using those assumptions with alpha set at 0.05 and 87.5% power, a sample size of 6 pairs would detect a significant difference in pain between ketamine and standard treatment with lidocaine.
Comparisons of pain ratings between hospitalizations were performed using the Kruskal Wallis test. Comparisons of changes in pain over time between and within each hospitalization were performed using one and two-way analysis of variance with repeated measures with a Bonferroni correction for multiple comparisons. Plasma concentrations of ketamine, norketamine, HNK, and DHNK and their enantiomers were analyzed using one-way analysis of variance with repeated measures with Bonferroni correction for multiple comparisons. Correlations between the mean daily pain ratings and ketamine metabolite concentrations were analyzed using the Pearson correlation coefficient. Continuous data are reported as means ± SD. All statistical analyses were performed using Systat, version 13 (San Jose, CA). A p-value of 0.05 was considered statistically significant. All 6 patients had complete pain data for the ketamine and lidocaine hospitalizations.
Results
Demographics and Pain Outcomes
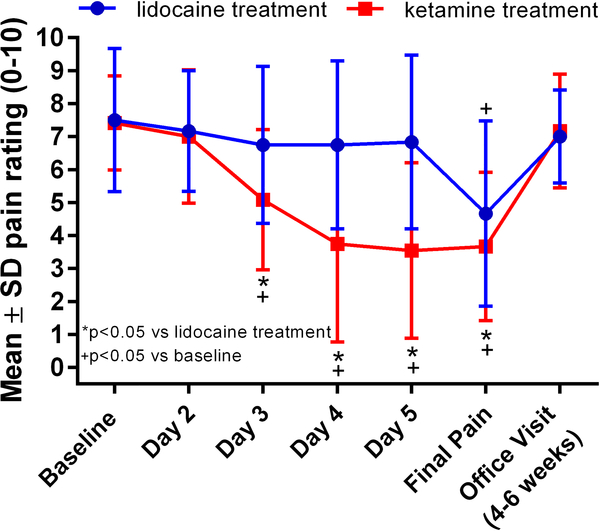
A total of 7 patients who had completed a treatment course of lidocaine during a previous hospitalization were screened for eligibility over a 6-month period. All 7 patients signed written informed consent and were enrolled in the study and underwent all study procedures and Supplemental Figure S1 shows the patient flow diagram. One of the patients was later determined to have a diagnosis other than chronic migraine and was excluded from analysis leaving 6 patients for analysis. The patients ranged in age from 20–55 years and half were female. Two patients had a diagnosis of depression. Five out of the 6 patients received dihydroergotamine during the initial hospitalization in addition to the lidocaine infusion, which is the typical protocol. All 6 patients had complete data for all pain outcomes, including baseline, end of treatment, and post-discharge pain ratings. Patients reported a mean baseline pain rating (0–10 numeric rating scale) of 7.5 ± 2.2 prior to initial admission for lidocaine, which decreased to 4.7 ± 2.8 by the end of the hospitalization (p≤0.05); when comparing baseline pain to pain at the first post-discharge office visit, there was no difference (7.5 ± 2.2 vs. 7.0 ± 1.4, p>0.05). This visit occurred at a mean time of 28 ± 8 days after treatment. The mean lidocaine infusion rate on day 1 was 1.6 ± 0.8 mg/min and 2.5 ± 0.7 mg/min on day 4.
At the time of the hospitalization for ketamine, no patients were taking opioids and all 6 patients had Migraine Disability Assessment (MIDAS) scores of grade 4, indicating severe disability.13 All 6 patients had complete pain data, including baseline, end of treatment, and post-discharge pain ratings. Prior to ketamine treatment the mean baseline pain rating was 7.4 ± 1.4, which decreased to 3.7 ± 2.3 by the end of the hospitalization (p≤0.05). There was no statistical difference between baseline pain and pain at the first post-discharge office visit (7.4 ± 1.4 vs. 7.2 ± 1.7, p>0.05), which occurred at a mean time of 41 ± 7 days after treatment. Ketamine infusions were started at 10 mg/h for all 6 patients and the mean infusion rate by the end of day 2 was 46.7 ± 9.8 mg/h. The mean maximum infusion rate achieved during admission was 72.5 ± 10.4 mg/h for all patients and occurred on day 5.
When comparing the primary outcome between the two treatments, there was a statistically significant difference. Patients experienced a statistically greater decrease in pain rating (0–10 numeric rating scale) after ketamine than lidocaine: −3.7 with ketamine versus −2.8 with lidocaine (p≤0.05). The pain trajectory for patients during both treatment admissions is shown in Figure 1.
Figure 1.
Pain over time during the two treatment admissions for lidocaine (blue line) and (R,S)-ketamine (red line). The office visits were 4–6 weeks after discharge.
Adverse Effects and Additional Medications
Four of the 6 patients experienced some adverse effects attributed to lidocaine. One patient experienced four episodes of bradycardia/junctional heart rhythm and nausea; the second experienced hallucinations and blurry vision; the third experienced nausea and blurry vision; and the fourth experienced insomnia. All were transient in nature.
All 6 patients experienced some AEs during treatment with ketamine. These included hallucinations, nightmares, vivid dreams, blurry vision, and nausea/vomiting (see Table 1). None of the AEs required complete discontinuation of ketamine and all patients completed the full 5-day treatment course. None of the patients required discontinuation of either lidocaine or ketamine infusion for hemodynamic reasons.
Table 1.
Adverse effects experienced by 6 patients with refractory chronic migraine who underwent 5-day continuous ketamine infusion.
| Patient | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|---|
| A: 20 yo female, 64 kg | Maximum ketamine rate (mg/h) | 30 (0.5 mg/kg/h) | 60 (0.9 mg/kg/h) | 68 (1 mg/kg/h) | 60 (0.9 mg/kg/h) | 64 (1 mg/kg/h) |
| Adverse Effects | None | Nausea/vomiting | Blurry vision; nausea/vomiting | Blurry vision; hallucinations; nausea/vomiting | Blurry vision; nausea/vomiting | |
| B: 54 yo male, 84 kg | Maximum ketamine rate (mg/h) | 30 (0.4 mg/kg/h) | 60 (0.7 mg/kg/h) | 60 (0.7 mg/kg/h) | 85 (1 mg/kg/h) | 60 (0.7 mg/kg/h) |
| Adverse Effects | None | Blurry vision; hallucinations | Blurry vision; hallucinations | Blurry vision | Blurry vision | |
| C: 48 yo female, 83 kg | Maximum ketamine rate (mg/h) | 30 (0.4 mg/kg/h) | 60 (0.7 mg/kg/h) | 65 (0.8 mg/kg/h) | 70 (0.8 mg/kg/h) | 75 (0.9 mg/kg/h) |
| Adverse Effects | None | Blurry vision; nausea/vomiting | Blurry vision; nausea/vomiting | Blurry vision | Blurry vision; nausea/vomiting | |
| D: 28 yo male, 82 kg | Maximum ketamine rate (mg/h) | 30 (0.4 mg/kg/h) | 40 (0.5 mg/kg/h) | 50 (0.6 mg/kg/h) | 55 (0.7 mg/kg/h) | 50 (0.6 mg/kg/h) |
| Adverse Effects | None | Blurry vision; vivid dreams | Blurry vision; vivid dreams | Blurry vision; hallucinations; nightmares | Blurry vision | |
| E: 55 yo female, 69 kg | Maximum ketamine rate (mg/h) | 10 (0.1 mg/kg/h) | 40 (0.6 mg/kg/h) | 60 (0.9 mg/kg/h) | 72 (1 mg/kg/h) | 65 (0.9 mg/kg/h) |
| Adverse Effects | None | Nausea/vomiting | None | None | Blurry vision; nausea/vomiting | |
| F: 36 yo male, 94 kg | Maximum ketamine rate (mg/h) | 30 (0.3 mg/kg/h) | 50 (0.5 mg/kg/h) | 70 (0.7 mg/mg/h) | 80 (0.9 mg/kg/h) | 80 (0.9 mg/kg/h) |
| Adverse Effects | None | Blurry vision; nausea/vomiting | Blurry vision; nausea/vomiting | Blurry vision; nausea/vomiting | Nausea/vomiting |
Additional medications given to patients during the second treatment admission for ketamine are shown in Supplemental Table T1. A literature review was conducted to determine if metabolic interactions between ketamine and the co-administered drugs had been reported. No such reports were identified.
Metabolite Analyses
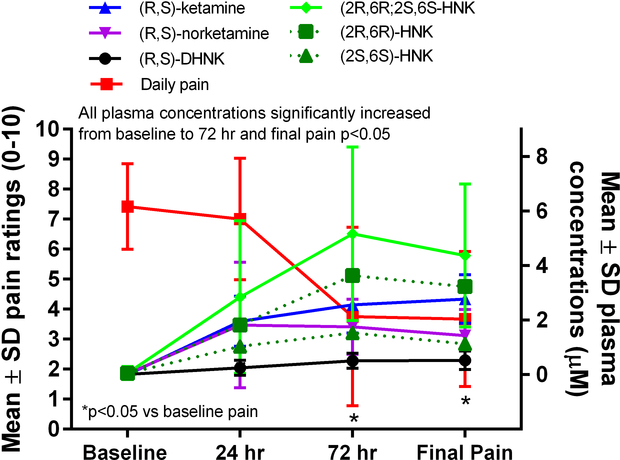
All 6 patients had complete ketamine metabolite and concurrent medication data for analysis. The circulating plasma concentrations of (R,S)-ketamine increased throughout the 5-day treatment and were greatest at the end of the infusion on day 5. Concentrations of (R,S)-norketamine and (R,S)-DHNK remained fairly stable from 24 h until the end of infusion, while hydroxynorketamine levels peaked at 72 h (Table 2, Figure 2). Pearson correlations between mean daily pain ratings and mean plasma concentrations of (R,S)-ketamine and its metabolites were as follows: (R,S)-ketamine [R= −0.82, p>0.05]; (R,S)-norketamine [R= −0.53, p>0.05]; (2R,6R,2S,6S)-HNK [R= −0.89, p>0.05)]; (R)-norketamine [R= −0.55, p>0.05]; (S)-norketamine [R= −0.48, p>0.05]; (2R,6R)-HNK [R= −0.93, p>0.05]; (2S,6S)-HNK [R= −0.79, p>0.05]; and (R,S)-DHNK [R= −0.95, p>0.05].
Table 2.
Mean circulating concentrations (μM) of ketamine and its metabolites in 6 patients with refractory chronic migraine who underwent 5-day continuous infusion.
| Racemic Molecule | Mean ± SD concentration (μM)* | Enantiomers | Mean ± SD concentration (μM) |
|---|---|---|---|
| (R,S)-ketamine | |||
| Baseline | 0.0 ± 0.0 | ||
| 24 hours | 2.0 ± 0.4 | -- | -- |
| 72 hours | 2.6 ± 0.4 | ||
| End of infusion | 2.8 ± 0.4 | ||
|
| |||
| (R)-norketamine | |||
| Baseline | 0.0 ± 0.0 | ||
| 24 hours | 1.1 ± 0.5 | ||
| (R,S)-norketamine | 72 hours | 1.0 ± 0.3 | |
| Baseline | 0.0 ± 0.0 | End of infusion | 0.9 ± 0.2 |
| 24 hours | 1.8 ± 0.9 | ||
| 72 hours | 1.8 ± 0.4 | (S)-norketamine | |
| End of infusion | 1.4 ± 0.4 | Baseline | 0.0 ± 0.0 |
| 24 hours | 0.8 ± 0.4 | ||
| 72 hours | 0.7 ± 0.2 | ||
| End of infusion | 0.6 ± 0.2 | ||
|
| |||
| (2R,6R)-HNK | |||
| Baseline | 0.0 ± 0.0 | ||
| 24 hours | 1.8 ± 0.7 | ||
| (RR,SS)-HNK | 72 hours | 3.6 ± 0.9 | |
| Baseline | 0.0 ± 0.0 | End of infusion | 3.2 ± 0.8 |
| 24 hours | 2.8 ± 1.1 | ||
| 72 hours | 5.2 ± 1.3 | (2S,6S)-HNK | |
| End of infusion | 4.4 ± 1.1 | Baseline | 0.0 ± 0.0 |
| 24 hours | 1.0 ± 0.5 | ||
| 72 hours | 1.5 ± 0.5 | ||
| End of infusion | 1.1 ± 0.3 | ||
|
| |||
| (R,S)-DHNK | |||
| Baseline | 0.0 ± 0.0 | ||
| 24 hours | 0.3 ± 0.1 | -- | -- |
| 72 hours | 0.5 ± 0.1 | ||
| End of infusion | 0.5 ± 0.1 | ||
Racemic concentrations may differ slightly from the sum of the enantiomer concentrations because of rounding.
SD=standard deviation; HNK=hydroxynorketamine; DHNK=dehydronorketamine
Figure 2.
Plasma concentrations of (R,S)-ketamine and its metabolites and pain ratings over time. DHNK=dehydronorketamine, HNK=hydroxynorketamine.
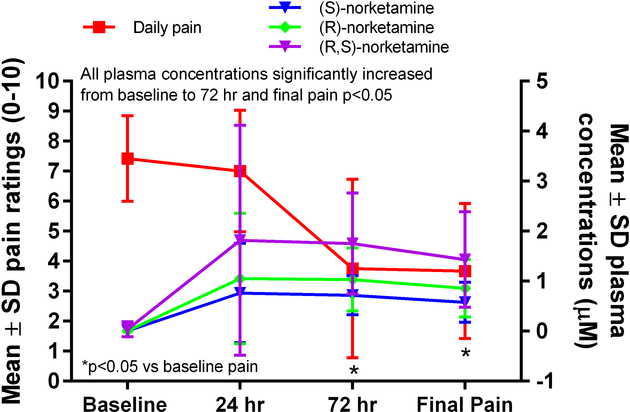
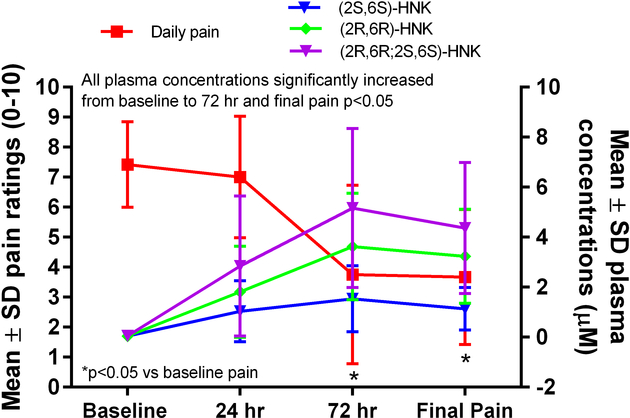
The mean concentrations of (R,S)-ketamine, (R)-norketamine, (S)-norketamine, (2R,6R)-HNK, (2S,6S)-HNK, and (R,S)-DHNK all increased from baseline to 24 h (Table 2, Figure 2). The mean concentrations of (R)-norketamine were greater than those of (S)-norketamine at 24 h, 72 h, and the end of infusion (Table 2, Figure 3; p≤0.05). Similarly, the mean concentrations of (2R,6R)-HNK were greater than those of (2S,6S)-HNK at 72 h and the end of infusion (Table 2, Figure 4; p≤0.05). The ratio of (2R,6R)-HNK to (2S,6S)-HNK increased from 24 h to the end of infusion (Table 3; p≤0.05) while the ratio of (R)-norketamine to (S)-norketamine did not, indicating stereoselective formation of the (2R,6R) enantiomer preferentially over the (2S,6S) enantiomer or perhaps stereoselective renal clearance of (2S,6S)-HNK.
Figure 3.
Plasma concentrations of (R,S)-norketamine and its enantiomers and pain ratings over time.
Figure 4.
Plasma concentrations of (2R,6R;2S,6S)-hydroxynorketamine and its enantiomers and pain ratings over time. HNK=hydroxynorketamine.
Table 3.
Ratios of ketamine metabolite enantiomers over time
| Metabolite Ratios | Ratio Value Mean ± SD | P value* |
|---|---|---|
| (R)-norketamine:(S)-norketamine | ||
| 24 hours | 1.4 ± 0.1 | - |
| 72 hours | 1.4 ± 0.1 | p>0.05 vs. 24 h |
| End of infusion | 1.5 ± 0.2 | p>0.05 vs. 24 h |
|
| ||
| (2R,6R)-HNK:(2S,6S)-HNK | ||
| 24 hours | 1.9 ± 0.7 | - |
| 72 hours | 2.5 ± 0.7 | p≤0.05 vs. 24 h** |
| End of infusion | 2.9 ± 0.5 | p≤0.05 vs. 24 h** |
Analyzed using analysis of variance
Statistically significant
SD=standard deviation; HNK=hydroxynorketamine
Discussion
In this pilot study we have shown that both (R,S)-ketamine and lidocaine infusions have the potential to reduce short-term pain in patients with refractory chronic migraine. The reduction in pain associated with (R,S)-ketamine was statistically greater than that with lidocaine, but this difference has unclear clinical significance given that the minimum clinically significant change in numeric rating scale pain has been reported as 2 on the 0–10 scale.14 In addition, the time course of pain relief differed between the infusions in that pain ratings did not decrease until the end of the lidocaine treatment, while pain relief began to occur on day 3 during ketamine treatment and remained greater than that obtained from lidocaine until the end of hospitalization. By the time patients were seen 6 weeks after both treatments their pain level had returned to baseline. This reflects the clinical challenge of this refractory population. While long-term relief of pain and disability is the goal of both patients and physicians, short-term improvement is not insignificant for patients with nearly constant symptoms. While both infusions were associated with AEs, both were tolerated by all patients and no serious AEs occurred. The clinical improvement demonstrated in this study was consistent with previous retrospective studies of refractory chronic migraine patients who received lidocaine15 and (R,S)-ketamine,4 although the patients in this study reported lower pain ratings for a greater period of time with ketamine than lidocaine.
Patients with refractory chronic migraine often have disabling pain and those in classes 3 or 4 have failed a minimum of 3 classes of medication2 but often have failed dozens or more in our clinical experience. The patients in this pilot study all met the criteria for MIDAS grade 4, indicating a severe level of disability, and 5 of the 6 patients had scores of at least 140, indicating nearly constant pain. While clinical trials of patients with episodic migraine or even chronic migraine typically have clear inclusion and exclusion criteria, the inconsistency in how intractable or refractory headache is defined makes the inclusion of such patients in clinical trials difficult.2 Non-traditional treatments that have not been well studied, such as the IV infusions used in this study, are sometimes the only available options for these patients. Generating the necessary evidence to support such treatments can be complicated by inconsistent definitions and endpoints created for less refractory populations. Nevertheless, we have shown that both infusions can potentially improve short-term pain in the most challenging headache population and further prospective study is warranted.
(R,S)-ketamine is a chiral phencyclidine derivative that was initially developed as an anesthetic agent.16 Initial studies demonstrated that (R,S)-ketamine was extensively and stereoselectively metabolized, that only the (R,S)-norketamine metabolite produced anesthesia in the rat, and that (2R,6R;2S,6S)-HNK was inactive in this model.17 Thus, pharmacokinetic and pharmacodynamic studies following acute or sub-chronic (R,S)-ketamine administration concentrated on (R,S)-ketamine and (R,S)-norketamine or the separate enantiomers. The initial study of the enantioselective metabolism and distribution of (R,S)-ketamine during a 5-day infusion was conducted in patients with CRPS but was limited to the examination of (R)-ketamine, (S)-ketamine, (R)-norketamine and (S)-norketamine.18 The results of the study demonstrated that while the plasma concentrations of (R)-ketamine were slightly greater than (S)-ketamine, the (R)-norketamine concentrations were significantly higher than (S)-norketamine concentrations.18 While we did not determine the separate concentrations of (R)-ketamine and (S)-ketamine in the current study, the relative concentrations of (R)-norketamine and (S)-norketamine were consistent with the earlier study.
In the CRPS study, 10 of 16 patients reported a significant reduction (≥30%) in individual pain ratings relative to baseline ratings.19 Similar to the data in the current study, the mean pain ratings decreased over the length of the infusion. A pharmacodynamic analysis comparing plasma concentrations of the target compounds with the probability of achieving pain relief indicated that the antinociceptive activity produced by the continuous administration of (R,S)-ketamine could not be explained by the plasma concentrations of (R)-ketamine, (S)-ketamine, (R)-norketamine, or (S)-norketamine. The results suggested that other metabolites may play a role in the observed therapeutic effects. This led to the reanalysis of a plasma sample from a complex regional pain syndrome patient classified as a responder using a method able to quantify all of the major (R,S)-ketamine metabolites.8 The data indicated that (2R,6R;2S,6S)-hydroxynorketamine was a major circulating metabolite and that the plasma concentration of this metabolite was far greater than (R,S)-norketamine.
The current study is the first to re-examine the effect of a continuous 5-day infusion on the metabolism and distribution of (R,S)-ketamine. In this study, (2R,6R;2S,6S)-HNK was the predominant circulating compound followed by (R,S)-ketamine, (R,S)-norketamine, and (R,S)-DHNK. (R,S)-DHNK is rapidly cleared by renal excretion8,10 and its in vivo pharmacological activity has not been established. Since urine samples were not collected in the current study, our analysis was concentrated on the enantiomers of (2R,6R;2S,6S)-HNK.
In the current study, the average plasma concentration of (2R,6R;2S,6S)-HNK peaked at 72 h and it significantly exceeded the concentrations of both (R,S)-ketamine (p<0.05) and (R,S)-norketamine (p<0.05). The plasma concentrations of (R,S)-ketamine slowly increased throughout the infusion in 5 of the 6 patients while (R,S)-norketamine plasma concentrations peaked at 24 or 48 hours followed by a rapid decline. No statistically significant correlation was found between (R,S)-ketamine, (R,S)-norketamine, or (2R,6R;2S,6S)-HNK plasma concentrations and mean daily pain ratings, which is not surprising considering our limited sample size. However, there were clear non-significant correlations noted between (R,S)-ketamine concentrations and pain, R = −0.822 (p>0.05) and (2R,6R;2S,6S)-HNK concentrations and pain, R = −0.887 (p>0.05). Since previous studies had established that (2R,6R)-HNK has analgesic properties,7 the plasma samples were reanalyzed using an enantioselective chromatographic method to establish the relative concentrations of (2R,6R)-HNK and (2S,6S)-HNK as well as their immediate precursors (R)-norketamine and (S)-norketamine. The results indicate that the average plasma concentration of (2R,6R)-HNK exceeded (2S,6S)-HNK at 24 h and that this difference reached significance at the 72 h (p<0.05) and Day 5 (p<0.05) sampling times. Indeed, (2R,6R)-HNK plasma concentrations surpassed (R,S)-ketamine concentrations at 72 h in 5 of the 6 patients and in 4 of the 6 patients on Day 5.
The data indicate that the ratio of (2R,6R)-HNK:(2S,6S)-HNK increased over the course of the infusion from 1.9 ± 0.7 at 24 h to 2.9 ± 0.5 on Day 5. Our data identifying a ~2-fold difference in the circulating concentrations of (2R,6R)-HNK relative to (2S,6S)-HNK 24-h post-infusion are consistent with previous findings. In a recent study, 230 min after the administration of a single infusion of (R,S)-ketamine, (2R,6R)-HNK was present in greater concentrations than (2S,6S)-HNK11 and, in a separate single-subject study, (2R,6R)-HNK had a larger systemic exposure (~1.5 times) than (2S,6S)-HNK.20
The significant increases in these ratios observed at 72 h and at the end of the infusion suggest that there was a stereoselective induction of the metabolic transformation from (R,S)-norketamine to the corresponding HNK metabolites, which favors the production of (2R,6R)-HNK, or could indicate stereoselective renal clearance of (2S,6S)-HNK. Previous studies have identified CYP2A6 as the primary enzyme involved in the transformation of (R,S)-norketamine to (2R,6R;2S,6S)-HNK and determined that this process is stereoselective favoring the conversion of (R)-norketamine to (2R,6R)-HNK,5 and cell-based studies have demonstrated that (R,S)-ketamine, (2R,6R)-HNK, and (2S,6S)-HNK induce CYP2A6 expression and function.22 The latter study also demonstrated that the same compounds induce CYP2B6 expression and function.22 Since CYP2B6 mediates the conversion of (R,S)-norketamine to (R,S)-DHNK, one would expect to see this reflected in a change in the (R)-norketamine:(S)-norketamine ratio over time. However, this was not observed, suggesting that CYP2B6 induction did not occur, or that any changes were obscured by the rapid renal clearance of DHNK. Additional experiments, including the analysis of urine samples, are required in larger sample cohort to determine whether induction, clearance, or both are responsible for the significant increase of (2R,6R)-HNK over (2S,6S)-hHNK during a continuous infusion of (R,S)-ketamine.
The results of this study demonstrate that (2R,6R)-HNK was the major circulating compound at 72 h and at the end of the infusion and that these time points coincide with the optimum pain relief. While no correlation was found between overall improvement in pain and (2R,6R)-HNK, these preliminary findings should be further explored in a larger cohort to determine whether (2R,6R)-HNK could be a viable therapeutic agent for chronic pain conditions such as migraine or complex regional pain syndrome. Current evidence of the benefits have been from preclinical studies in animals using pain models7 and in refractory depression.6, 21 While the data are inconclusive, the indication that there has been an induction of CYP2A6 suggest a potential mechanism for the compound’s antinociceptive activity observed in animal pain models. Recent cell-based studies have demonstrated that (R,S)-ketamine, (2R,6R)-HNK, and (2S,6S)-HNK bind to the estrogen receptor α (ERα) and in coordination with estrogens enhance the ERα-induced transcription of CYP2A6 and CYP2B6 as well as the increased expression of ERα.22 Thus, the continuous infusion of (R,S)-ketamine and the buildup of (2R,6R)-HNK may invoke an ERα-related mechanism not observed after a single rapid infusion of (R,S)-ketamine, (2R,6R)-HNK, or (2S,6S)-HNK. This possibility has not been demonstrated in vivo and will be explored in future clinical studies.
Our results are generalizable to other clinical settings where 5-day ketamine infusions are used. However, the clinical significance of (2R,6R;2S,6S)-HNK being the predominant metabolite throughout much of the infusion is not yet known and our results could be used as the basis to study the role of these metabolites in pain, particularly during prolonged infusions.
Our study has several limitations. In addition to the small sample size, retrospectively collected pain ratings from the lidocaine hospitalizations were not always assessed at consistent intervals and could be subject to influence based on the time of day and medications given just prior to assessment. Follow-up pain ratings were in some cases obtained via telephone call several months after treatment and could be subject to recall bias. Our results may not be generalizable to other practices where lower (R,S)-ketamine or lidocaine doses are used or different adjunctive medications are used. Our results also may not apply to less refractory patients. Lastly, our study was open label and we were unable to control for the additional medications that patients were given during admission, such as dihydroergotamine and ketorolac. It is unknown how these or other medications might have influenced pain. Future studies including pre-treatment and post-treatment metabolic phenotyping for CYP2B6 and CYP2A6 activity and urinary excretion are necessary to help elucidate whether those enzymes play a role in the pharmacological mechanisms responsible for the observed effects.
Conclusions
Patients with refractory chronic migraine have continuous pain and substantial disability. We have shown that both lidocaine and (R,S)-ketamine infusions hold potential to reduce short-term pain and “break the cycle” of constant symptoms, with a more pronounced reduction in pain score with (R,S)-ketamine treatment. In this study, no statistically significant correlation was found between pain scores and circulating levels of (R,S)-ketamine or its metabolites. Circulating concentrations of (2R,6R)-HNK, however, were at their highest between Days 3 and 5, the period of time when pain was at its lowest, suggesting the possibility that this molecule could hold promise as an analgesic that lacks (R,S)-ketamine’s psychomimetic adverse effects, although this should be interpreted with caution given the small sample size. Our results suggest that future studies should be undertaken to explore this further. Preliminary data from this study suggest that (R,S)-ketamine may induce its metabolism throughout the treatment period in a stereoselective fashion that favored formation of the (2R,6R)-HNK isomer.
Supplementary Material
Acknowledgments
Funding: this work was partially funded by an internal provost grant awarded to Dr. Schwenk; this work was also supported in part by the Intramural Research Program at the National Institute on Aging, National Institutes of Health (Ruin Moaddel and Jacqueline Lovett).
Footnotes
Disclosures:
Eric Schwenk, Jacqueline Lovett, Daniel Katz, William Denk, and Clinton Lauritsen have nothing to disclose.
Ruin Moaddel, Marc Torjman, and Irving Wainer are listed as co-inventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro- and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; Ruin Moaddel and Irving Wainer are co-inventors on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. Ruin Moaddel and Irving Wainer assigned their patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Marc Torjman assigned his patent rights to the Cooper Health System.
Stephen Silberstein receives, or has received, honoraria from AbbVie.; Amgen; Aeon BioPharma; Axsome Therapeutics; Biohaven Pharmaceuticals; Cefaly; Curelator, Inc.; Epalex; GlaxoSmithKline Consumer Health Holdings, LLC.;; electroCore Medical, LLC; Impel NeuroPharma, Inc.; Lilly USA, LLC; Medscape, LLC; Lundbeck; Nocera; Novartis, Inc.; Revance: Salvia, Bioelectronics; Satsuma Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Teva Pharmaceuticals; Theranica; Thermaquil; and Trillen MedicalInc.
References
- 1.Schulman E Refractory migraine - a review. Headache. 2013;53(4): 599–613. [DOI] [PubMed] [Google Scholar]
- 2.Silberstein SD, Dodick DW, Pearlman S. Defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(9): 1499–1506. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SP, Bhatia A, Buvanendran A, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5): 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwenk ES, Dayan AC, Rangavajjula A, et al. Ketamine for Refractory Headache: A Retrospective Analysis. Reg Anesth Pain Med. 2018;43(8): 875–879. [DOI] [PubMed] [Google Scholar]
- 5.Desta Z, Moaddel R, Ogburn ET, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42(11): 1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroin JS, Das V, Moric M, Buvanendran A. Efficacy of the ketamine metabolite (2R,6R)-hydroxynorketamine in mice models of pain. Reg Anesth Pain Med. 2019;44(1): 111–117. [DOI] [PubMed] [Google Scholar]
- 8.Moaddel R, Venkata SL, Tanga MJ, et al. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82(5): 1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12): 1495–1499. [DOI] [PubMed] [Google Scholar]
- 10.Martelletti P, Katsarava Z, Lampl C, et al. Refractory chronic migraine: a consensus statement on clinical definition from the European Headache Federation. J Headache Pain. 2014;15: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmer CA, Gilbert JR, Moaddel R, et al. Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacology. 2020;45(8): 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasan M, Hofstetter R, Fassauer GM, Link A, Siegmund W, Oswald S. Quantitative chiral and achiral determination of ketamine and its metabolites by LC–MS/MS in human serum, urine and fecal samples. Journal of Pharmaceutical and Biomedical Analysis. 2017;139: 87–97. [DOI] [PubMed] [Google Scholar]
- 13.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1): S20–28. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2): 105–121. [DOI] [PubMed] [Google Scholar]
- 15.Rosen N, Marmura M, Abbas M, Silberstein S. Intravenous lidocaine in the treatment of refractory headache: a retrospective case series. Headache. 2009;49(2): 286–291. [DOI] [PubMed] [Google Scholar]
- 16.Domino EF, Chodoff P, Corssen G. Pharmacologic Effects of Ci-581, a New Dissociative Anesthetic, in Man. Clin Pharmacol Ther. 1965;6: 279–291. [DOI] [PubMed] [Google Scholar]
- 17.Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem. 1986;29(11): 2396–2399. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer IW. Pharmacodynamic profiles of ketamine (R)- and (S)- with 5-day inpatient infusion for the treatment of complex regional pain syndrome. Pain Physician. 2010;13(4): 379–387. [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg ME, Schwartzman RJ, Torjman MC, Wainer IW. Ketamine infusion successful in some patients. Pain Physician. 2010;13(6): E371–372; author reply E372–373. [PubMed] [Google Scholar]
- 20.Hasan M, Hofstetter R, Fassauer GM, Link A, Siegmund W, Oswald S. Quantitative chiral and achiral determination of ketamine and its metabolites by LC-MS/MS in human serum, urine and fecal samples. J Pharm Biomed Anal. 2017;139: 87–97. [DOI] [PubMed] [Google Scholar]
- 21.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4): 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho MF, Correia C, Ingle JN, et al. Ketamine and ketamine metabolites as novel estrogen receptor ligands: Induction of cytochrome P450 and AMPA glutamate receptor gene expression. Biochem Pharmacol. 2018;152: 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.