Abstract
Background
It is not clear whether previous thyroid diseases influence the course and outcomes of COVID-19.
Methods
The study is a part of a multicentric cohort of patients with confirmed COVID-19 diagnosis from 37 hospitals. Matching for age, sex, number of comorbidities, and hospital was performed for the paired analysis.
Results
Of 7,762 patients with COVID-19, 526 had previously diagnosed hypothyroidism and 526 were matched controls. The median age was 70 years, and 68.3% were females. The prevalence of comorbidities was similar, except for coronary and chronic kidney diseases that were higher in the hypothyroidism group (p=0.015 and p=0.001). D-dimer levels were lower in patients with hypothyroid (p=0.037). In-hospital management was similar, but hospital length-of-stay (p=0.029) and mechanical ventilation requirement (p=0.006) were lower for patients with hypothyroidism. There was a trend of lower in-hospital mortality in patients with hypothyroidism (22.1% vs 27.0%; p=0.062).
Conclusion
Patients with hypothyroidism had a lower requirement of mechanical ventilation and showed a trend of lower in-hospital mortality. Therefore, hypothyroidism does not seem to be associated with a worse prognosis.
Keywords: Hypothyroidism, COVID-19, Mortality, Prognosis, Epidemiology
List of Abbreviations
- COVID-19
Coronavirus disease 19
- NTIS
Non-thyroidal illness syndrome
- T3
Triiodothyronine
- T4
Thyroxine
- TSH
Thyroid-stimulating hormone
- ICU
Intensive Care Unit
- REDCap
Research Data Capture
- COPD
Chronic obstructive pulmonary disease
- SF ratio
Peripheral oxygen saturation/fraction of inspired oxygen ratio
- GCS
Glasgow coma score
- HR
Heart rate
- RR
Respiratory rate
- Sat O2
Peripheral capillary oxygen saturation
- aPTT
Activated partial thromboplastin time
- AST
Aspartate transaminase
- ALT
Alanine aminotransferase
- BNP
B-type natriuretic peptide
- CPK
Creatine phosphokinase
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- INR
International normalized ratio
- VTE
Venous thromboembolism
- ACE 2
Angiotensin-converting enzyme 2
Background
A global health crisis was established with the emergence of COVID-19 (Gelfand et al., 2021). It is well known that individuals with some underlying medical conditions, such as cardiovascular diseases, cancer, obesity, diabetes, and hypertension, are more likely to develop severe COVID-19, require hospitalization and intensive care, and have higher mortality rates (Marcolino et al., 2021, Sanyaolu et al., 2020, Centers for Disease Control and Prevention. People with Certain Medical Conditions. September 4th, 2021). Nevertheless, other conditions such as asthma and Chagas disease surprisingly have not been related to more severe cases of COVID-19 (Soeroto et al., 2021). However, many factors that interfere with COVID-19 clinical characteristics, symptoms, and prognosis are still unknown and require further investigation to improve disease prevention and patient management.
It is not clear whether a previous thyroid disease influences COVID-19 course (Duntas and Jonklaas, 2021). Previous studies suggested that patients’ thyroid status might have a direct impact on the course of COVID-19 owing to the effects of thyroid hormone on multiple organs systems, including the cardiovascular and respiratory systems (Duntas and Jonklaas, 2021). Some studies have indeed observed that hypothyroidism may be associated with an increased risk for COVID-19 or a poorer prognosis in patients with the disease (Bakshi and Kalidoss, 2021, Zhang et al., 2021). However, these studies are limited due to small sample sizes and lack of comparison with patients without hypothyroidism.
Other studies have found that previous diagnosis of hypothyroidism or other thyroid diseases were not a risk factor for COVID-19 infection and were not associated with poorer outcomes of the disease (Daraei et al., 2020, van Gerwen et al., 2020 Aug 18, Martins et al., 2021, Shabrawishi et al., 2020). It is well known that critically ill patients with no previous history of thyroid dysfunction usually present alterations in thyroid hormone levels, a situation known as nonthyroidal illness syndrome (NTIS) (Fliers et al., 2015). Patients with NTIS typically present with decreased concentrations of plasma triiodothyronine (T3), and low thyroxine (T4) levels, and usually slightly decreased concentrations of thyroid–stimulating hormone (TSH), which may be variable depending on the phase of the disease (Fliers et al., 2015). In patients at the intensive care unit, with conditions such as sepsis, major trauma, burn, or cardiac surgery, NTIS has been associated with disease severity and mortality, without cause-effect association. Rather, NTIS is more likely to be seen as an adaptive mechanism to critical illnesses (Fliers et al., 2015, Kim et al., 2021, Martins et al., 2021). As we could expect, NTIS has been frequently observed in patients with COVID-19 with the same pattern as in other critical conditions (Beltrão FEL, 2021, Gao et al., 2021, British Columbia Ministry of Health 2018).
Literature regarding the association between COVID-19 and previous hypothyroidism is still controversial, results may be biased by the inclusion of patients with NTIS, and more studies with larger sample sizes and comparison with patients without hypothyroidism are still needed. This study aims to compare the evolution of COVID-19 in patients with previous diagnosis of underlying hypothyroidism to patients without the disease to understand if hypothyroidism is an independent risk factor in the course of COVID-19.
Methods
Study design and subjects
This study is a part of the multicentric cohort study Brazilian COVID-19 Registry. Consecutive patients with confirmed COVID-19 by reverse transcriptase–polymerase chain reaction (RT-PCR) or serological tests (IgM) (Marcolino et al., 2021, World Health Organization 2020) who were admitted to the 37 participant hospitals from March 1, 2020, to September 31, 2020, were eligible (Marcolino et al., 2021). Patients were admitted from March 1, 2020, to September 31, 2020.
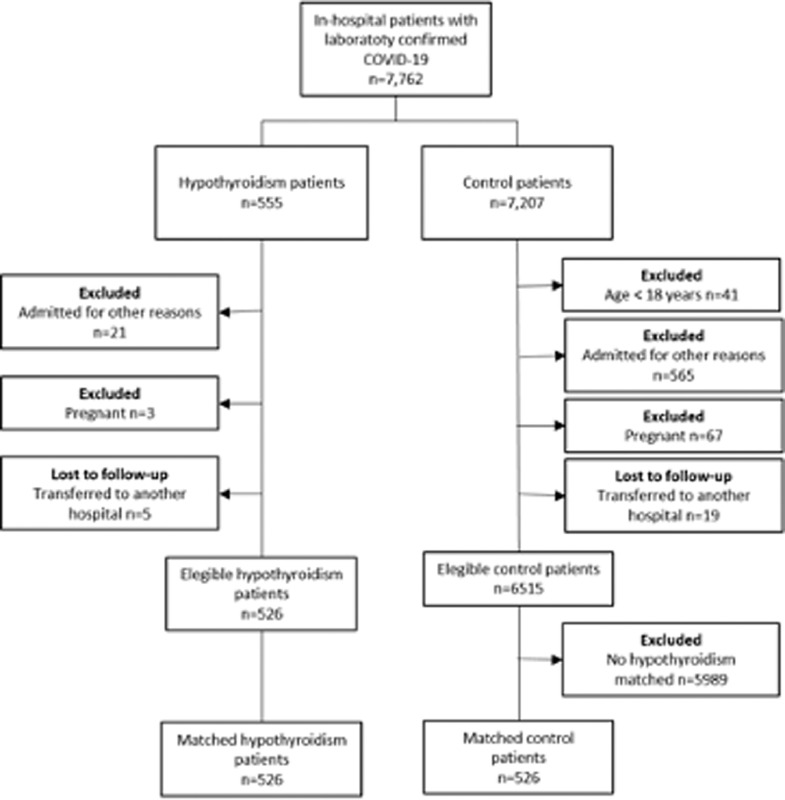
For the current analysis, “cases” were patients with a clinical history of previously diagnosed hypothyroidism, who were on levothyroxine replacement therapy. Of 7,762 patients with confirmed COVID–19 diagnosis, 555 (7.15%) had underlying hypothyroidism. Of those, 21 were excluded due to hospital admission for other reasons (not COVID-19), 3 were excluded due to pregnancy, and 5 were transferred to other hospitals, with 526 patients with hypothyroidism remaining eligible for the study (Figure 1 ). Those patients were admitted in 31 hospitals, which were located in 15 cities (Belo Horizonte, Betim, Curvelo, Ipatinga, Teófilo Otoni, Divinópolis, Porto Alegre, Bento Gonçalves, Santa Cruz do Sul, Canoas, Santa Maria, Lajeado, Chapecó, Florianópolis, and Botucatu) in 4 Brazilian states (Minas Gerais, Rio Grande do Sul, Santa Catarina, and São Paulo). Of those, 13 were public hospitals, 15 private hospitals, and 3 mixed hospitals. To compare patient outcomes and disease course, a paired analysis was made with matched controls with no previous history of hypothyroidism.
Fig. 1.
Flowchart of patients included in the study.
This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (STROBE) (von Elm et al., 2007), and it has been conducted by a protocol approved by the National Commission for Research Ethics (CAAE 30350820.5.1001.0008) (Barreto et al., 2012). Individual informed consent was waived due to the pandemic and to the fact that all data collected were unidentified and gathered through medical records.
Data collection
Data were collected through the analysis of medical records by trained health professionals or undergraduate students (from medical and nursing schools) using Research Data Capture (REDCap) tools (Harris et al., 2019). In the medical records, data collected concerned demographic and clinical characteristics, comorbidities, medication in use before admission, COVID-19 symptoms, clinical evaluation at admission, laboratory and radiological examinations, medication used during hospitalization, and outcomes. Study protocol and definitions were published elsewhere (Marcolino et al., 2021).
Statistical analysis
Statistical analysis was performed using R software (version 4.0.2). To adjust for potential confounding variables, patients who had underlying hypothyroidism were matched with patients who did not have underlying hypothyroidism (controls) on the basis of propensity score. Propensity score model was estimated by logistic regression and included sex, age, number of comorbidities (hypertension, diabetes mellitus, obesity, coronary artery disease, heart failure, atrial fibrillation or flutter, cirrhosis, chronic obstructive pulmonary disease, cancer, and previous stroke) (Marcolino et al., 2021), and hospital. Patients from the control group were searched to find those who had the closest propensity score from the hypothyroidism group (within 0.17 standard deviations of the logit of the propensity score on a scale from 0-1.00) using the MatchIt package in R software.
Categorical data were presented as absolute frequency and proportions, and continuous variables were expressed as medians and interquartile ranges. The chi-square and Fisher's exact tests were used to compare the distribution of categorical variables and the Wilcoxon–Mann–Whitney test for continuous variables. Results were considered statistically significant if the two-tailed P-value was < 0.05.
Results
The study sample comprehended 526 individuals with previous hypothyroidism history and 526 matched controls. The median age was 70 years (59-80), and 68.3% were female. Most patients (85.8%) had at least 1 comorbidity, with the most common being hypertension (72.0%), diabetes mellitus (38.8%), obesity (18.9%), and heart failure (11.2%). In comparison to the controls, patients with underlying hypothyroidism had similar prevalence of comorbidities and unhealthy habits, except for a higher prevalence of coronary disease (9.7% vs 5.7%, p=0.015) and chronic kidney disease (9.9% vs 4.8%, p= 0.001) among the hypothyroidism group (Table 1 ). [insert Table 1]
Table 1.
Demographics and clinical characteristics of the study cohort
| Hypothyroidism N = 5261 | Control patients N = 5261 | p-value2 | |
|---|---|---|---|
| Age (years) | 71.0 (60.0, 80.0) | 70.0 (59.0, 80.0) | 0.659 |
| Female sex | 356 (67.7%) | 363 (69.0%) | 0.643 |
| Cardiovascular diseases | |||
| Hypertension | 370 (70.3%) | 387 (73.6%) | 0.243 |
| Heart failure | 65 (12.4%) | 53 (10.1%) | 0.241 |
| Coronary artery disease | 51 (9.7%) | 30 (5.7%) | 0.015 |
| Atrial fibrillation/flutter | 36 (6.8%) | 37 (7.0%) | 0.903 |
| Stroke | 28 (5.3%) | 34 (6.5%) | 0.432 |
| Respiratory diseases | |||
| COPD | 42 (8.0%) | 44 (8.4%) | 0.822 |
| Asthma | 35 (6.7%) | 34 (6.5%) | 0.901 |
| Metabolic diseases | |||
| Diabetes mellitus | 215 (40.9%) | 193 (36.7%) | 0.164 |
| Obesity (BMI > 30 kg/m2) | 94 (17.9%) | 95 (18.1%) | 0.936 |
| Other conditions | |||
| Chronic kidney disease | 52 (9.9%) | 25 (4.8%) | 0.001 |
| Cancer | 29 (5.5%) | 38 (7.2%) | 0.256 |
| Dementia | 16 (3.0%) | 11 (2.1%) | 0.330 |
| Rheumatological disease | 16 (3.0%) | 9 (1.7%) | 0.157 |
| Cirrhosis | 4 (0.8%) | 7 (1.3%) | 0.363 |
| Transplant | 3 (0.6%) | 3 (0.6%) | >0.999 |
| HIV infection | 2 (0.4%) | 4 (0.8%) | 0.687 |
| Comorbidities (total number) | 0.549 | ||
| 0 | 71 (13.5%) | 78 (14.8%) | |
| 1 | 157 (29.8%) | 143 (27.2%) | |
| 2 | 163 (31.0%) | 173 (32.9%) | |
| 3 | 87 (16.5%) | 95 (18.1%) | |
| 4 | 36 (6.8%) | 31 (5.9%) | |
| >= 5 | 12 (2.3%) | 6 (1.2%) | |
| Toxic habits | |||
| Alcoholism | 10 (1.9%) | 10 (1.9%) | >0.999 |
| Smoking | 100 (19.0%) | 115 (21.9%) | 0.251 |
Median (IQR) or n (%)
Pearson's chi-square test; Wilcoxon rank sum test; Fisher's exact test.
COPD - Chronic obstructive pulmonary disease
The median duration of symptoms was 6 days (3-9 days) for both groups. The most predominant symptoms in disease presentation were dyspnea, fever, and dry cough, being similar in both groups. Upon hospital presentation, patients with hypothyroidism had a lower frequency of respiratory rate over 24 breaths per minute (36.1% vs 42.0%; p=0.050), as well as lower proportion of mechanical ventilation (4.0% vs 7.4%; p=0.016) and of inotropics (24.3% vs 29.8%; p=0.044) requirement. The median peripheral oxygen saturation/fraction of inspired oxygen ratio (SF ratio) was slightly higher in patients with hypothyroidism than in the control group (428.6 vs 423.8, p=0.034), as shown in Table 2 . [insert Table 2]
Table 2.
Clinical characteristics at hospital admission.
|
Hypothyroidism |
Control patients |
||||
|---|---|---|---|---|---|
| N = 5261 | Non missing cases | N = 5261 | Non missing cases | p-value2 | |
| Symptoms | |||||
| Duration of symptoms (days) | 6.0 (3.0, 9.0) | 524 | 6.0 (3.0, 9.0) | 524 | 0.635 |
| Adynamic | 138 (26.2%) | 526 | 143 (27.2%) | 526 | 0.728 |
| Ageusia | 25 (4.8%) | 526 | 34 (6.5%) | 526 | 0.228 |
| Anosmia | 44 (8.4%) | 526 | 48 (9.1%) | 526 | 0.662 |
| Headache | 95 (18.1%) | 526 | 94 (17.9%) | 526 | 0.936 |
| Rhinorrhea | 72 (13.7%) | 526 | 79 (15.0%) | 526 | 0.538 |
| Diarrhea | 84 (16.0%) | 526 | 77 (14.6%) | 526 | 0.549 |
| Dyspnea | 298 (56.7%) | 526 | 316 (60.1%) | 526 | 0.260 |
| Fever | 278 (52.9%) | 526 | 247 (47.0%) | 526 | 0.056 |
| Hyporexia | 71 (13.5%) | 526 | 66 (12.5%) | 526 | 0.647 |
| Neurological manifestations | 14 (2.7%) | 526 | 21 (4.0%) | 526 | 0.229 |
| Myalgia | 147 (27.9%) | 526 | 130 (24.7%) | 526 | 0.234 |
| Nausea/vomiting | 83 (15.8%) | 526 | 69 (13.1%) | 526 | 0.220 |
| Productive cough | 71 (13.5%) | 526 | 79 (15.0%) | 526 | 0.481 |
| Dry cough | 262 (49.8%) | 526 | 268 (51.0%) | 526 | 0.711 |
| Clinical assessment | |||||
| GCS < 15 | 84 (16.0%) | 526 | 83 (15.8%) | 526 | 0.933 |
| HR (bpm) | 85.0 (74.0, 96.0) | 505 | 87.0 (76.0, 98.0) | 507 | 0.169 |
| HR > 100 bpm | 116 (22.1%) | 526 | 131 (24.9%) | 526 | 0.275 |
| RR (bpm) | 20.0 (18.0, 23.0) | 443 | 20.0 (18.0, 24.0) | 430 | 0.216 |
| RR > 24 bpm | 190 (36.1%) | 526 | 221 (42.0%) | 526 | 0.050 |
| Sat O2 (%) | 94.0 (91.0, 96.0) | 513 | 94.0 (91.0, 97.0) | 513 | 0.845 |
| Sat O2 < 90% | 98 (18.6%) | 526 | 103 (19.6%) | 526 | 0.695 |
| Mechanical ventilation | 21 (4.0%) | 526 | 39 (7.4%) | 524 | 0.016 |
| Systolic blood pressure | 526 | 526 | 0.693 | ||
| SBP >= 90 mmHg | 482 (91.6%) | 464 (88.2%) | |||
| SBP < 90 mmHg | 25 (4.8%) | 28 (5.3%) | |||
| Inotropic use2 | 19 (3.6%) | 34 (6.5%) | |||
| SF ratio2 | 428.6 (339.3, 452.4) | 507 | 423.8 (304.7, 452.4) | 507 | 0.034 |
Median (IQR) or n (%)
Inotropic use at hospital presentation
SF ratio: peripheral capillary oxygen saturation/fraction of inspired oxygen (Spo2/Fio2 ratio)
GCS: Glasgow Coma Scale; HR: heart rate; RR: respiratory rate; Sat O2: peripheral capillary oxygen saturation
Laboratory examinations and biomarkers were similar between groups, except for D-dimer, which was lower in patients with hypothyroidism than in the controls (Table 3 ). [insert Table 3]
Table 3.
Findings of laboratory examinations.
|
Hypothyroidism |
Control patients |
||||
|---|---|---|---|---|---|
| N = 5261 | Non missing cases | N = 5261 | Non missing cases | p-value2 | |
| Hemogram | |||||
| Hemoglobin (g/L) | 12.6 (11.6, 13.9) | 515 | 12.9 (11.6, 13.8) | 509 | 0.301 |
| Leukocytes count (cels/mm3) | 6,785.0 (5,000.0, 9,302.5) | 512 | 6,940.5 (5,200.0, 9,132.5) | 510 | 0.317 |
| Neutrophils (cels/mm3) | 4,737.0 (3,310.5, 7,155.8) | 498 | 5,204.0 (3,481.8, 7,281.2) | 488 | 0.205 |
| Lymphocytes (cels/mm3) | 1,100.0 (760.0, 1,503.5) | 499 | 1,041.0 (710.0, 1,421.0) | 489 | 0.136 |
| Platelet count (109/L) | 195.0 (151.0, 244.8) | 508 | 196.3 (152.0, 261.0) | 505 | 0.499 |
| aPTT (secs/control) | 1.0 (0.9, 1.2) | 228 | 1.0 (0.9, 1.2) | 265 | 0.396 |
| Liver panel | |||||
| Albumin (g/dL) | 3.4 (3.0, 3.7) | 90 | 3.3 (3.0, 3.6) | 104 | 0.283 |
| Total bilirubin (mg/dL) | 0.4 (0.3, 0.6) | 253 | 0.4 (0.3, 0.6) | 249 | 0.150 |
| Direct bilirubin (mg/dL) | 0.2 (0.1, 0.3) | 251 | 0.2 (0.1, 0.3) | 249 | 0.013 |
| AST (U/L) | 37.4 (29.0, 55.0) | 326 | 38.0 (28.0, 52.0) | 334 | 0.750 |
| ALT (U/L) | 29.0 (20.0, 46.0) | 323 | 28.0 (19.0, 44.3) | 335 | 0.592 |
| Electrolytes | |||||
| Calcium (mmol/L) | 1.1 (1.1, 1.2) | 138 | 1.1 (1.1, 1.2) | 167 | 0.298 |
| Potassium (mmol/L) | 4.2 (3.8, 4.6) | 476 | 4.1 (3.7, 4.5) | 485 | 0.065 |
| Sodium (mmol/L) | 137.0 (134.0, 139.7) | 474 | 137.7 (135.0, 140.0) | 482 | 0.020 |
| Others | |||||
| BNP (pg/mL) | 78.0 (23.0, 230.5) | 47 | 110.0 (32.8, 312.0) | 48 | 0.218 |
| Creatinine (mg/dL) | 0.9 (0.8, 1.4) | 493 | 0.9 (0.7, 1.2) | 499 | 0.159 |
| CPK (U/L) | 81.5 (45.2, 145.8) | 150 | 74.5 (47.0, 189.9) | 149 | 0.668 |
| D-dimer* | 2.3 (1.2, 6.8) | 301 | 2.9 (1.2, 23.9) | 318 | 0.037 |
| Ferritin (ng/mL) | 80.1 (57.7, 142.0) | 19 | 66.0 (42.0, 175.3) | 17 | 0.447 |
| Fibrinogen (g/L) | 465.5 (334.5, 591.8) | 40 | 451.0 (343.0, 553.0) | 45 | 0.745 |
| Lactate dehydrogenase | 358.5 (255.2, 493.8) | 282 | 363.0 (270.0, 504.0) | 297 | 0.536 |
| NT-proBNP (pg/mL) | 310.0 (114.0, 785.7) | 55 | 302.0 (89.3, 1,197.5) | 70 | 0.895 |
| C-reactive protein (mg/L) | 75.3 (32.1, 134.3) | 466 | 74.8 (34.3, 143.3) | 441 | 0.933 |
| PTTa (secs/control) | 1.0 (0.9, 1.2) | 228 | 1.0 (0.9, 1.2) | 265 | 0.396 |
| INR | 1.1 (1.0, 1.2) | 291 | 1.1 (1.0, 1.2) | 317 | 0.229 |
| Troponin* | 0.3 (0.1, 0.5) | 171 | 0.3 (0.1, 0.5) | 184 | 0.261 |
| Lactate | 1.4 (1.0, 1.9) | 311 | 1.4 (1.1, 1.9) | 321 | 0.427 |
| Urea (mg/dL) | 39.1 (28.0, 58.0) | 492 | 38.0 (28.0, 55.8) | 484 | 0.539 |
Median (IQR) or n (%) 2Wilcoxon rank sum test; Fisher's exact test
aPTT: activated partial thromboplastin time; AST: aspartate transaminase; ALT: alanine aminotransferase; BNP B-type natriuretic peptide; CPK: creatine phosphokinase; NT-proBNP: N-terminal pro-brain natriuretic peptide; INR: international normalized ratio
* Times the reference value.
As for medications used during hospitalization, patients with hypothyroidism had a lower frequency of inotropes requirement than the control group (24.3% vs 29.8%, p=0.044). The other medications were similar between groups (Table S1).
Regarding patient outcomes, the median hospital length of stay (8 versus 9 days; p=0.029) and mechanical ventilation requirement (25.4% versus 33.1%; p=0.006) were lower in patients with hypothyroidism than in controls. A trend of lower in-hospital mortality was seen in patients with hypothyroidism (22.1% versus 27.0%; p=0.062). The other outcomes were similar between groups, as shown in Table 4 . [insert Table 4]
Table 4.
Patient outcomes
| HypothyroidismN = 5261 | Control patientsN = 5261 | p-value2 | |
| Hospital length of stay, days | 8.0 (4.0, 14.0) | 9.0 (5.0, 14.2) | 0.029 |
| ICU, days | 197 (37.5%) | 224 (42.6%) | 0.089 |
| Time of ICU admission | 1.0 (0.0, 3.0) | 1.0 (0.0, 2.0) | 0.089 |
| Days at ICU | 8.0 (3.0, 15.0) | 8.0 (4.0, 17.0) | 0.293 |
| Mechanical ventilation, number of patients | 133 (25.4%) | 172 (33.1%) | 0.006 |
| Dialysis | 57 (10.8%) | 63 (12.0%) | 0.553 |
| Sepsis | 72 (13.7%) | 90 (17.1%) | 0.124 |
| Disseminated intravascular coagulation | 0 (0.0%) | 3 (0.6%) | 0.249 |
| Decompensated chronic heart failure | 16 (3.0%) | 25 (4.8%) | 0.152 |
| Acute heart failure | 3 (0.6%) | 6 (1.1%) | 0.506 |
| Nosocomial infection | 48 (9.1%) | 48 (9.1%) | >0.999 |
| Miocardites | 0 (0.0%) | 3 (0.6%) | 0.249 |
| Hemorrhage | 10 (1.9%) | 9 (1.7%) | 0.817 |
| Vascular thrombosis | 18 (3.4%) | 22 (4.1%) | 0.519 |
| VTE | 16 (3.0%) | 21 (4.0%) | 0.403 |
| Arterial thrombosis | 2 (0.4%) | 1 (0.2%) | >0.999 |
| In hospital mortality | 116 (22.1%) | 142 (27.0%) | 0.062 |
Median (IQR) or n (%) 2Wilcoxon rank sum test; Pearson's chi-square test; Fisher's exact test
ICU: intensive care unit; VTE: venous thromboembolism
Discussion
In this multicenter cohort of in-hospital patients with COVID-19, those who had hypothyroidism had similar comorbidities, clinical manifestations, and laboratory parameters to the control group. Surprisingly, requirement for mechanical ventilation was remarkably lower in patients with underlying hypothyroidism, and there was a trend for lower hospital mortality than controls matched for age, sex, number of comorbidities, and hospital.
Underlying individual comorbidities were similar between groups, except for chronic kidney disease and coronary artery disease. There has been a great interest in the relationship between thyroid dysfunction and kidney function in recent years (Iglesias et al., 2017, Narasaki et al., 2021). Thyroid hormones have shown to directly affect the kidneys, influencing renal growth and development, glomerular filtration rate, renal transport systems, and sodium and water homeostasis. An indirect effect through modifications in cardiac and vascular function and disruptions in the renin-angiotensin system is also believed to play an important role in reducing glomerular filtration rate through impaired renal autoregulation. At the same time, patients with autoimmune thyroid disorders are also at risk for immune-mediated glomerular diseases (Narasaki et al., 2021).
As for the higher prevalence of coronary artery disease, the association between hypothyroidism and increased cardiovascular risk is controversial when TSH is normal (Decandia, 2018). Nonetheless, it was demonstrated that patients with underlying hypothyroidism with a poor disease control have a worse lipidic profile and a higher prevalence of atherosclerosis because thyroid hormones regulate lipid metabolism, especially TSH (Delitala et al., 2017, Kotwal et al., 2020, Tan et al., 2019 Oct 24). At the same time, it is well established that a key factor for adverse cardiovascular events prevention in patients with hypothyroidism is the achievement of symptom control and levels of TSH and T4 within the reference values with the use of hormonal replacement therapy (Sue and Leung, 2020). Another hypothesis to explain the higher frequency of coronary artery disease is the higher prevalence of chronic kidney disease, which is associated with increased cardiovascular risk.
There was no difference in frequency and severity of COVID-19 symptoms between groups, including anosmia and ageusia. Despite the previous evidence of an effect of hypothyroidism at gustatory and olfactory perceptual pathways, including receptors, central olfactory and gustatory areas, and high order cognitive systems, previous cohort studies did not observe a higher frequency of gustatory or olfactory COVID-19 symptoms in these patients. However, a case series has observed an independent association between hypothyroidism and a higher likelihood of persistent olfactory dysfunction among patients with COVID-19 (odds ratio [OR]: 21.1; 95% CI: 2.0–219.4) (Tsivgoulis et al., 2021). Further studies are needed to investigate whether hypothyroidism is indeed associated with a higher risk of persistent symptoms.
Overall, laboratorial examinations were similar between groups, except for D-dimer levels, which were slightly higher in the control group than in patients with hypothyroidism. However, the values were assessed as the number of times above the upper limit of normal, and the difference is not clinically relevant. These findings are different from a study from Wuhan, China, in the early phase of the pandemic, which observed that patients with thyroid dysfunction had persistently high levels of biomarkers for inflammatory response and cardiac injury. However, authors defined thyroid dysfunction by abnormal thyroid function test results within 3 days from admission and not previous underlying diseases (Zhang et al., 2021).
Current data show that COVID-19 is associated with a broad spectrum of thyroid dysfunction, ranging from thyrotoxicosis to NTIS and hypothyroidism, which may worsen disease course and affect prognosis (Baldelli et al., 2021, Malik et al., 2021, Ruggeri et al., 2021, Schwarz et al., 2021, Zou et al., 2020). This was observed even in mild to moderate COVID-19 cases, independently of SARS-CoV-2 viral load, age, and inflammation and tissue injury markers (Lui et al., 2021). Cytokine storm and dysregulated inflammation are believed to affect the thyroid gland through the proinflammatory cytokines, which resembles the immune activation that occurs in immune-mediated thyroid diseases, causing damage and compromising function (Ruggeri et al., 2021). It is also possible that thyroid abnormalities are caused by a direct effect of the virus, leading to subacute thyroiditis, a self-limiting destructive thyroiditis, or an indirect effect on angiotensin-converting enzyme 2 (ACE2) receptors of thyroid follicular cells, leading to a thyroid malfunction (Chakraborty et al., 2020 Dec 18, Ruggeri et al., 2021). Other contributing factors may be the oxidative stress due to augmented reactive oxygen species generation, which are associated with NTIS, and changes in the intracellular redox state that may disrupt deiodinase function by independent mechanisms, which might include depletion of the—as yet unidentified—endogenous thiol cofactor (Wajner and Maia, 2012). Nevertheless, there is no evidence that the administration of thyroid hormones improves prognosis in those patients. We might also wonder that in some studies that observed a correlation between thyroid dysfunction and more severe COVID-19 presentation, the alterations in thyroid hormone levels were actually due to NTIS, as opposed to an underlying previous clinical thyroid disease (Bakshi and Kalidoss, 2021). Therefore, these results have to be carefully analyzed.
Our findings suggest that underlying hypothyroidism does not lead to worse outcomes in patients with COVID-19. In fact, those patients presented indicators of better outcomes than patients without hypothyroidism. At hospital admission, respiratory rates over 24 breaths per minute were less frequent in patients with hypothyroidism than in the control group (36.1% vs 42.0%; p=0.050). It is known that tachypnea is one of the alarming COVID-19 symptoms as it may be an indicator of COVID-19 pneumonia (Izquierdo et al., 2020). Despite the fact that SF ratio, a strong predictor of COVID-19 prognosis and mortality (Marcolino et al., 2021, Mejía et al., 2020) , was slightly higher in patients with hypothyroidism, the difference was not clinically relevant (428.6 versus 423.8, p=0.034).
The lower requirement of mechanical ventilation had not been observed previously, and it was quite unexpected. It is known that patients with decompensated hypothyroidism may have abnormalities in pulmonary function, muscle myopathy, and neuropathy with respiratory muscle weakness, but these abnormalities improve with treatment (Laroche et al., 1988, Siafakas et al., 1992). In a previous propensity score matching analysis of patients from a New York health system, adjusted for age, sex, race, body mass index, smoking status, and number of comorbidities, hypothyroidism was not associated with an increased risk of mechanical ventilation (adjusted OR: 1.17 [95% CI: 0.81–1.69]) or death (adjusted OR: 1.07 [95% CI: 0.75–1.54]). However, this analysis was limited by the sample size of patients who were hospitalized, by limited number of events, and by fixed selection of 3 controls per case, which forces selection of controls (van Gerwen et al., 2020 Aug 18) The other previous cohort studies and case series were also limited by small sample sizes and lack of controls (Allam et al., 2021, Bakshi and Kalidoss, 2021, Daraei et al., 2020, van Gerwen et al., 2020 Aug 18, Lui et al., 2021, Tsivgoulis et al., 2021, Zhang et al., 2021). In this study, a caliper width of 0.17 was used for the matched analysis. It is considered conservative and restricts the selection of controls that are indeed similar to the cases, as recommended (Austin, 2011). Due to a successful matched analysis between samples, it is unlikely that age, sex, or other underlying diseases have interfered with the results, although patients with hypothyroidism showed a higher prevalence for coronary artery disease and chronic kidney disease. Medications used during hospitalization were also similar in both groups and therefore cannot explain the difference in prognosis as well.
This study has limitations to be addressed. It is a pragmatic study based on data from chart review. Thyroid disease status was based on patients’ information given at hospital admission, and therefore, it was not possible to know if the hypothyroidism was compensated. Nevertheless, to the best of our knowledge, this study has the largest sample of patients with COVID-19 with hypothyroidism published so far. TSH and thyroid hormone (T4 and T3) levels were not evaluated during the time of hospitalization and time in the ICU, as the health organizations recommend against testing for thyroid dysfunction during an acute illness, unless it is suspected the acute illness is due to thyroid dysfunction ((Van den Berghe, 2014, National Institute for Health and Care Excellence 2019), and it was not our aim to access the effects of NTIS. The assessment of thyroid hormone levels throughout the course of COVID-19 is still challenging owing to the possibility of overdiagnosis due to abnormal hormone levels during acute systemic disease. It is important to emphasize that these hormonal changes are adaptive processes and cannot be defined as a disease itself.
Conclusion
In this multicenter cohort of patients with COVID-19, those with underlying hypothyroidism had similar comorbidities, clinical presentation, and laboratory examinations as the control group. It was found, nonetheless, that a lower proportion of patients with hypothyroidism required mechanical ventilation and had a trend of lower in-hospital mortality. Therefore, hypothyroidism does not seem to be associated with a worse prognosis and should not be considered among the comorbidities that indicate a risk factor for COVID-19 severity.
Ethics approval
The study has been conducted by a protocol approved by the National Commission for Research Ethics(Barreto et al., 2012) (CAAE 30350820.5.1001.0008). Individual informed consent was waived due to the pandemic and to the fact that all data collected were unidentified and gathered through medical records.
Availability of data and materials
Data are available upon reasonable request.
Competing interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported in part by Minas Gerais State Agency for Research and Development (Fundação de Amparo à Pesquisa do Estado de Minas Gerais - FAPEMIG) [grant number APQ-00208-20], National Institute of Science and Technology for Health Technology Assessment (Instituto de Avaliação de Tecnologias em Saúde – IATS)/ National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq) [grant number 465518/2014-1 and 147122/2021-0].
DNP was funded by a research scholarship from IATS/CNPq [grant number 147122/2021-0].
Role of the funder/sponsor
The sponsors had no role in study design; data collection, management, analysis, and interpretation; writing the manuscript; and the decision to submit it for publication. DNP, LFGS, MMMG, MSM, and MCP had full access to all the data in the study and had responsibility for the decision to submit for publication.
Authors contributions
Substantial contributions for the conception or design of the manuscript: MSM, DNP, LFGS, and MMMG.
Substantial contributions for data acquisition, analysis, or interpretation: all authors.
Manuscript formulation: MSM, DNP, LFGS, MMMG, and MCP.
Critically revised the manuscript in relation to the important intellectual content: all authors.
Final version's approval: all authors agreed to be responsible for all aspects of the work, ensuring that issues related to precision of integrity in any of the work's parts will be properly investigated and solved: MSM and MCP.
Transparency declaration
The lead authors (DNP, MCP, and MSM) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the hospitals that are part of this collaboration for supporting this project: Hospitais da Rede Mater Dei; Hospital das Clínicas da UFMG; Hospital de Clínicas de Porto Alegre; Hospital Santo Antônio; Hospital Eduardo de Menezes; Hospital Tacchini; Hospital Márcio Cunha; Hospital Metropolitano Dr. Célio de Castro; Hospital Metropolitano Odilon Behrens; Hospital Risoleta Tolentino Neves; Hospital Santa Rosália; Hospital Santa Cruz; Hospital São João de Deus; Hospital Semper; Hospital Unimed-BH; Hospital Universitário Canoas; Hospital Universitário Santa Maria; Hospital Moinhos de Vento; Hospital Luxemburgo; Instituto Mario Penna; Hospital Nossa Senhora da Conceição; Hospital João XXIII; Hospital Júlia Kubitschek; Hospital Mãe de Deus; Hospital Regional do Oeste; Hospital das Clínicas da Faculdade de Medicina de Botucatu;Hospital Universitário Ciências Médicas; Hospital Bruno Born; Hospital SOS Cárdio; Hospital São Lucas da PUCRS.
We also thank all the clinical staff at those hospitals who cared for the patients and all undergraduate students who helped with data collection.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.01.016.
Appendix. Supplementary materials
References
- Allam MM, El-Zawawy HT, Ahmed SM, Aly Abdelhamid M. Thyroid disease and covid-19 infection: Case series. Clin Case Rep. 2021;9(6):e04225. doi: 10.1002/ccr3.4225. Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical Statistics. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi SS, Kalidoss VK. Is there an association between hypothyroidism and COVID 19?: A preliminary report. Wien Klin Wochenschr. 2021;133(7-8):414–415. doi: 10.1007/s00508-021-01813-2. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli R, Nicastri E, Petrosillo N, Marchioni L, Gubbiotti A, Sperduti I, et al. Thyroid dysfunction in COVID-19 patients. J Endocrinol Invest. 2021:1–5. doi: 10.1007/s40618-021-01599-0. Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto PA, Braga AS, Andrade M. Evaluation of completeness of dengue records: exploratory study of compulsory notices. Online braz. j. nurs. 2012;11(3) dec 21. [Google Scholar]
- Beltrão FEL, Beltrão DCA, Carvalhal G, Beltrão FEL, Brito AS, Capistrano KHR, et al. Thyroid Hormone Levels During Hospital Admission Inform Disease Severity and Mortality in COVID-19 Patients. Thyroid. ahead of print. 2021 doi: 10.1089/thy.2021.0225. [DOI] [PubMed] [Google Scholar]
- British Columbia Ministry of Health . Guidelines and Protocol Advisory Committee; 2018. Thyroid Function Testing in the Diagnosis and Monitoring of Thyroid Function Disorder.https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/thyroid-function-testing.pdf Oct 24. Available at: [Google Scholar]
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. September 4th, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- Chakraborty U, Ghosh S, Chandra A, Ray AK. Subacute thyroiditis as a presenting manifestation of COVID-19: a report of an exceedingly rare clinical entity. BMJ Case Rep. 2020 Dec 18;13(12) doi: 10.1136/bcr-2020-239953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraei M, Hasibi M, Abdollahi H, Mirabdolhagh Hazaveh M, Zebaradst J, Hajinoori M, et al. Possible role of hypothyroidism in the prognosis of COVID-19. Intern Med J. 2020;50(11):1410–1412. doi: 10.1111/imj.15000. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decandia F. Risk factors for cardiovascular disease in subclinical hypothyroidism. Ir J Med Sci. 2018;187(1):39–43. doi: 10.1007/s11845-017-1617-9. Feb. [DOI] [PubMed] [Google Scholar]
- Delitala AP, Fanciulli G, Maioli M, Delitala G. Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. Eur J Intern Med. 2017;38:17–24. doi: 10.1016/j.ejim.2016.12.015. Mar. [DOI] [PubMed] [Google Scholar]
- Duntas LH, Jonklaas J. COVID-19 and Thyroid Diseases: A Bidirectional Impact. J Endocr Soc. 2021;5(8):bvab076. doi: 10.1210/jendso/bvab076. Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. Lancet Diabetes Endocrinol. 2015;3(10):816–825. doi: 10.1016/S2213-8587(15)00225-9. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Guo W, Guo Y, Shi M, Dong G, Wang G. al.Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021;44(5):1031–1040. doi: 10.1007/s40618-020-01460-w. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand MJ, Jackson JC, Pan X, Nau D, Pieper D, Denison E, et al. The relationship between cultural tightness-looseness and COVID-19 cases and deaths: a global analysis. Lancet Planet Health. 2021;5(3):e135–e144. doi: 10.1016/S2542-5196(20)30301-6. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias P, Bajo MA, Selgas R, Díez JJ. Thyroid dysfunction and kidney disease: An update. Rev Endocr Metab Disord. 2017;18(1):131–144. doi: 10.1007/s11154-016-9395-7. Maroi: 10.1007/s11154-016-9395-7. [DOI] [PubMed] [Google Scholar]
- Izquierdo JL, Ancochea J, Soriano JB. Clinical Characteristics and Prognostic Factors for Intensive Care Unit Admission of Patients With COVID-19: Retrospective Study Using Machine Learning and Natural Language Processing. J Med Internet Res. 2020;22(10):e21801. doi: 10.2196/21801. Savana COVID-19 Research Group. Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Yoo DM, Min CY, Choi HG. The Effects of Previous Thyroid Disease on the Susceptibility to, Morbidity of, and Mortality Due to COVID-19: A Nationwide Cohort Study in South Korea. J. Clin. Med. 2021;10:3522. doi: 10.3390/jcm10163522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal A, Cortes T, Genere N, Hamidi O, Jasim S, Newman CB, et al. Treatment of Thyroid Dysfunction and Serum Lipids: A Systematic Review and Meta-analysis. J Clin Endocrinol Metab. 2020;105(12):dgaa672. doi: 10.1210/clinem/dgaa672. Dec 1. [DOI] [PubMed] [Google Scholar]
- Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472–474. doi: 10.1164/ajrccm/138.2.472. Aug. [DOI] [PubMed] [Google Scholar]
- Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Role of non-thyroidal illness syndrome in predicting adverse outcomes in COVID-19 patients predominantly of mild-to-moderate severity. Clin Endocrinol (Oxf) 2021;95(3):469–477. doi: 10.1111/cen.14476. Sep(Lui, 2021a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status, and Outcome in 191 Patients with COVID-19. J Clin Endocrinol Metab. 2021;106(2):e926–e935. doi: 10.1210/clinem/dgaa813. Jan 23(Lui, 2021b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik J, Zaidi SMJ, Waqar AU, Khawaja H, Malik A, Ishaq U, et al. Association of hypothyroidism with acute COVID-19: a systematic review. Expert Rev Endocrinol Metab. 2021:1–7. doi: 10.1080/17446651.2021.1968830. Aug 23. [DOI] [PubMed] [Google Scholar]
- Marcolino MS, Pires MC, Ramos LEF, Silva RT, Oliveira LM, Carvalho RLR, et al. ABC2-SPH risk score for in-hospital mortality in COVID-19 patients: development, external validation and comparison with other available scores. Int J Infect Dis. 2021;110:281–308. doi: 10.1016/j.ijid.2021.07.049. Sep(Marcolino, 2021a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcolino MS, Ziegelmann PK, Souza-Silva MVR, Nascimento IJB, Oliveira LM, Monteiro LS, et al. Brazilian COVID-19 Registry Investigators. Clinical characteristics and outcomes of patients hospitalized with COVID-19 in Brazil: Results from the Brazilian COVID-19 registry. Int J Infect Dis. 2021;107:300–310. doi: 10.1016/j.ijid.2021.01.019. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins JRM, Villagelin DGP, Carvalho GA, Vaisman F, Teixeira PFS, Scheffel RS, et al. Management of thyroid disorders during the COVID-19 outbreak: a position statement from the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism (SBEM) Arch Endocrinol Metalab. 2021:368–375. doi: 10.20945/2359-3997000000352. April 265/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía F, Medina C, Cornejo E, Morello E, Vásquez S, Alave J, et al. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One. 2020 Dec 28;15(12):e0244171. doi: 10.1371/journal.pone.0244171. [DOI] [PMC free article] [PubMed]
- Narasaki Y, Sohn P, Rhee CM. The Interplay Between Thyroid Dysfunction and Kidney Disease. Semin Nephrol. 2021;41(2):133–143. doi: 10.1016/j.semnephrol.2021.03.008. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Thyroid disease: assessment and management (NG145). 2019. Available from https://www.nice.org.uk/guidance/ng145 [PubMed]
- Ruggeri RM, Campennì A, Deandreis D, Siracusa M, Tozzoli R, Petranović Ovčariček P, et al. SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol. 2021;17(7):737–759. doi: 10.1080/1744666X.2021.1932467. Jul10.1080/1744666X.2021.1932467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its Impact on Patients with COVID-19. SN Compr Clin Med. 2020:1–8. doi: 10.1007/s42399-020-00363-4. Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Y, Percik R, Oberman B, Yaffe D, Zimlichman E, Tirosh A. Sick Euthyroid Syndrome on Presentation of Patients With COVID-19: A Potential Marker for Disease Severity. Endocr Pract. 2021;27(2):101–109. doi: 10.1016/j.eprac.2021.01.001. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabrawishi M, Al-Gethamy MM, Naser AY, Ghazawi MA, Alsharif GF, Obaid EF, et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237130. Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N. Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189–194. doi: 10.1378/chest.102.1.189. Jul. [DOI] [PubMed] [Google Scholar]
- Soeroto AY, Purwiga A, Emmy H, Pranggono EHP, Roesli RMA. Asthma does not increase COVID-19 mortality and poor outcomes: A systematic review and meta-analysis. Asian Pac J Allergy Immunol. 2021 doi: 10.12932/AP-110920-0955. Apr 18. [DOI] [PubMed] [Google Scholar]
- Sue LY, Leung AM. Levothyroxine for the Treatment of Subclinical Hypothyroidism and Cardiovascular Disease. Front Endocrinol (Lausanne) 2020;11 doi: 10.3389/fendo.2020.591588. Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Korkmaz H, Aydın H. Kumbul Doğuç D. FABP4 levels in hypothyroidism and its relationship with subclinical atherosclerosis. Turk J Med Sci. 2019 Oct 24;49(5):1490–1497. doi: 10.3906/sag-1904-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivgoulis G, Fragkou PC, Karofylakis E, Paneta M, Papathanasiou K, Palaiodimou L, et al. Hypothyroidism is associated with prolonged COVID-19-induced anosmia: a case-control study. J Neurol Neurosurg Psychiatry. 2021 doi: 10.1136/jnnp-2021-326587. Apr 20jnnp-2021-326587. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. Non-thyroidal illness in the ICU: a syndrome with different faces. Thyroid. 2014;24(10):1456–1465. doi: 10.1089/thy.2014.0201. Octdoi: 10.1089/thy.2014.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerwen M, Alsen M, Little C, Barlow J, Naymagon L, Tremblay D, et al. Outcomes of Patients With Hypothyroidism and COVID-19: A Retrospective Cohort Study. Front Endocrinol (Lausanne). 2020 Aug 18;11:565. doi: 10.3389/fendo.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajner SM, Maia AL. New Insights toward the Acute Non-Thyroidal Illness Syndrome. Front Endocrinol (Lausanne). 2012;3:8. doi: 10.3389/fendo.2012.00008. Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Diagnostic testing for SARS-CoV-2. Interim guidance. Online. 11 September 2020. Available at: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2.
- Zhang Y, Lin F, Tu W, Zhang J, Choudhry AA, Ahmed O, et al. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol Cell Endocrinol. 2021;521 doi: 10.1016/j.mce.2020.111097. Feb 5oi: 10.1016/j.mce.2020.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R, Wu C, Zhang S, Wang G, Zhang Q, Yu B, et al. Euthyroid Sick Syndrome in Patients With COVID-19. Front Endocrinol (Lausanne). 2020;11 doi: 10.3389/fendo.2020.566439. Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.