Abstract
A total of 154 human serum samples (32 acute-phase and 22 convalescent-phase serum samples obtained within a week and between days 8 and 26 after the onset of rash, respectively, and 100 samples drawn from healthy immune adults) were processed by an immunofluorescence assay for the detection of immunoglobulin M (IgM), total immunoglobulin G (IgG), IgG1, IgG2, IgG3, and IgG4 measles virus-specific antibodies. In the acute phase, IgG1 was seen first, followed by IgG2, IgG3, and IgG4 responses, the mean seropositivity of which gradually increased during convalescence, reaching 100% (standard deviation [SD], 84 to 100%), 57% (SD, 34 to 80%), 86% (SD, 66 to 100%), and 86% (SD, 66 to 100%), respectively. IgG2 rose and fell in connection with IgG3 subclass antibodies, showing a rate of detection of IgG2 and/or IgG3 subclass antibodies of 95.5% (range, 100 to 86.5%) in the convalescent phase of infection. The mean percentage of measles IgG2 and IgG3 seropositivity dropped significantly during the memory phase, to 2% (range, 2 to 6%) and 3% (range, 3 to 7%), respectively (P < 0.05); meanwhile IgG1 and IgG4 subclass responses remained relatively unmodified in samples obtained years after infection (mean 100% [SD, 96 to 100%] and 86% [SD, 79 to 93%], respectively). Results obtained defined two highly different immune isotypic response patterns. One pattern is restrictive to IgG2 and/or IgG3 in the convalescent phase and is kinetically similar to the IgM antibody response, so its detection could be referred to as a recent viral activity. On the other hand, IgG1 and IgG4 were detected in both the convalescent and memory phases of the immune response, but their isolated occurrence without IgG2 and IgG3 could be related to the long-lasting immunity.
Measles has been targeted for global eradication by the World Health Organization's Expanded Programme on Immunization; for the effective control and eventual eradication of measles, it is necessary to impair measles transmission by establishing herd immunity. To accomplish this aim, a sensitive surveillance system is essential to detect wild-virus circulation, as well as sensitive and specific diagnostic tests (4, 5). The diagnosis of measles infection is serologically confirmed by the presence of a fourfold rise in antibody titers for paired acute- and convalescent-phase sera or most often by detection of anti-measles virus immunoglobulin M (IgM) antibody. The performance of IgM detection for the differentiation of primary and secondary measles antibody responses depends upon (i) propagation of the virus within the community, (ii) characteristics of the individual immune response, (iii) time of specimen collection, and (iv) assay sensitivity.
Similar to other serological markers, a subclass-restricted response to antigens has been recently demonstrated (8, 9, 10, 12, 18); however, a limited amount of data is available on the virus-specific immunoglobulin G (IgG) subclass responses during the ordinary course of measles viral infection. Narita et al. suggested that the IgG3 response could play a major role in acute-phase immunity during primary infection, while the IgG1 response could be related to maintenance of measles immunity (14).
These data offer early support for the hypothesis that the IgG isotypic immune response could also be a useful serologic tool, in addition to specific measles IgM antibody detection, to eventually distinguish between early and late measles infection.
The present study was undertaken to point out the specific antiviral IgG1, IgG2, IgG3, and IgG4 subclass response patterns elicited during natural infection (acute and convalescent phases) as well as in the long-lasting humoral immunity to measles virus.
The aim of this paper is to contribute to the global understanding of antibody responses to measles virus infections.
MATERIALS AND METHODS
Serum specimens.
A total of 154 positive human serum samples for measles antibodies were used in this study. Serum specimens were classified within two groups according to the characteristics of the measles cases from which they were obtained. In group 1 were 54 serum samples collected during a measles virus outbreak in Argentina in 1998. 32 of these were acute serum samples obtained within a week after the onset of rash (median, 3 days; range, 1 to 7 days) and 22 were convalescent serum samples obtained between days 8 and 26 (median, 17 days) after the onset of rash. The diagnosis was confirmed by the detection of measles-specific IgM antibodies by immunofluorescence assay (IFA) test as a screening method, and it was subsequently ratified by capture enzyme immunoassays (6, 17). In group 2 were 100 serum samples obtained from healthy adults with detectable neutralizing measles antibodies who reported a history of natural, long-past measles infection that occurred at least 10 years earlier.
Antisera.
Mouse monoclonal antibodies to human IgG subclasses were obtained from Sigma Chemical Co., St. Louis, Mo. These antibodies were used at dilutions of 1:100 (IgG1), 1:32 (IgG2), 1:32 (IgG3), and 1:32 (IgG4) according to the manufacturer's instructions. Rabbit monoclonal antibodies to human IgM and total IgG were obtained from CAPPEL and used at dilutions of 1:100 and 1:150, respectively. The optimal dilutions of monoclonal antibodies were determined by titrations against reference positive sera diluted 1:20 (initial dilution of serum for IFA assay).
Preparation of antigen slides.
A suspension of Vero cells (105 cells/ml) was seeded onto a bottle of 25 cm2 and infected with an Edmonston-Schwartz strain of measles virus at a 0.1 multiplicity of infection. Fifty microliters of the infected cells and 50 μl of the uninfected cells were placed on each well slide and were incubated for 48 h at 37°C in a humidified incubator with 5% CO2. Then monolayers were washed twice in phosphate-buffered saline (PBS; pH 7.2) and once with distilled water. The fixation of cells was done with acetone at 4°C for 10 min. Finally, slides were stored at −20°C for later use.
IFA.
Briefly described, 20-fold dilutions of serum samples were incubated with fixed cells for 30 and 90 min for IgG and IgM antibody detection, respectively, at 37°C in a humidified chamber; washed three times with PBS for 10 min per wash; and incubated for 30 min at 37°C with fluorescein isothiocyanate-conjugated anti-human IgM, total IgG, and IgG subclasses. After two 10-min washes with PBS, the slides were mounted with glycerol buffer onto coverslips and then examined under a fluorescence microscope at ×40 magnification.
A serum dilution was considered positive for measles IgM and IgG antibodies if, under a fluorescence intensity of 1+ or more, there was well-defined staining of cytoplasmatic granules in cells coalescing to form multinucleated giant cells.
A serum dilution was considered negative for measles IgM or IgG antibodies if the cells exhibited less than 1+ fluorescence and displayed the reddish-orange counterstain or if the fluorescence observed was not in the specific staining pattern of measles.
IgM antibody capture enzyme-immunoassay.
IgM antibody capture enzyme immunoassay for measles confirmation was carried out in the National Reference Laboratory (Santa Fe, Argentina) according to the method of Erdman et al. (6). Briefly, goat anti-human IgM antibodies diluted in PBS were coated onto microtiter plates for 1 h at 37°C. After the plates were washed, serum that was diluted 1:100 in PBS was added to four consecutive wells, and the plates were incubated for an additional 1 h at 37°C. After the plates were washed, either baculovirus-measles virus nucleoprotein or S9-uninfected cell control lysate diluted in PBS-gelatin/Tween (GT) with 4% normal goat serum was added to duplicate cells. Plates were then incubated for 2 h at 37°C. Plates were washed, biotinylated anti-measles virus was added for each specimen, and the plates were incubated for 1 h at 37°C. The plates were then washed three times, and streptavidin-peroxidase was added and incubated for 20 min at 37°C. Next a solution containing tetramethylbenzidine and hydrogen peroxide was added, and this mixture was incubated for 15 min at room temperature. Color development was stopped by the addition of 1 N sulfuric acid solution, and absorbance was read at 450 nm. For each sample, we calculated the difference between the mean optical densities for the antigen-positive wells (P) and uninfected cell control wells (N). A sample was considered positive if P − N ≥ 0.100.
Data analysis.
Results were expressed with a 95% confidence interval (CI), and the chi-square distribution test was used to analyze data.
RESULTS
A total of 154 human serum samples were processed by IFA for the detection of IgM, total IgG, IgG1, IgG2, IgG3, and IgG4 measles virus-specific antibodies. All serum samples obtained between days 0 and 26 after onset of rash were IgM (100%) and total IgG (100%) seropositive (group 1). In all of the serum samples drawn several years after the primary measles infection (group 2), the specific total IgG response was maintained, and 4 out of 100 (4%) of serum samples revealed IgM equivocal results, that is, a fluorescence pattern so low that it could not be considered significant.
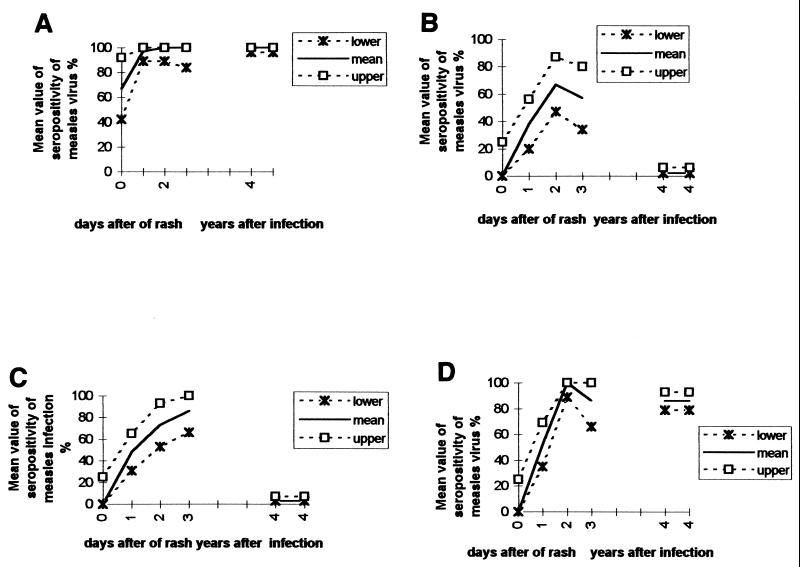
The mean values and standard deviations (95% CI) for the seropositivity of measles virus antibodies IgG1, IgG2, IgG3, and IgG4 of the serum samples from groups 1 and 2 after onset of rash are shown in Fig. 1 and Table 1. The measles virus-specific IgG1 subclass reached its peak in samples drawn on day 8 after onset of the rash and was detected in 100% of the specimens obtained years after measles infection. The daily percentage of measles IgG2, IgG3, and IgG4 seropositivity gradually increased, reaching means of 67 (47 to 87%), 73 (53 to 93%), and 100% (89 to 100%) in the early convalescent phase of infection, respectively. In the late convalescent phase, IgG2 and IgG4 seropositivities dropped slightly at means 57% (34 to 80%) and 86% (66 to 100%), respectively; meanwhile, IgG3 seropositivity reached its peak mean, 86% (range, 66 to 100%). In spite of these slightly different rates, the mean values obtained in the early and late convalescent phases did not reach statistical significance (P > 0.05). On the other hand, the percentage of measles virus-specific IgG2 and IgG3 seropositivity dropped significantly in the memory phase (mean, 2% [range, 2 to 6%] and 3% [range, 3 to 7%], respectively) (P < 0.05); meanwhile, the IgG4 subclass response remained relatively unmodified in samples obtained years after infection (mean, 86%; range, 66 to 100%).
FIG. 1.
Mean values of seropositivity of measles virus-specific IgG1 (A), IgG2 (B), IgG3 (C), and IgG4 (D) in recent and long-lasting immunity. Numbers at the bottom of each panel (x axis) correspond to the following phases: 0, acute phase (samples drawn on days 1 through 7 after onset of rash); 1, early convalescent phase (samples drawn on days 8 through 14 after onset of rash); 3, late convalescent phase (samples drawn on days 15 through 26 after onset of rash); 4, samples drawn 10 to 30 years after measles infection.
TABLE 1.
Values of measles virus-specific IgG isotypes in acute- and convalescent-phase sera and in sera from patients with past infection
| Infection phase | Time after onset of rash | Total Samples (n) | Mean % (range) of isolates seropositive for a:
|
||||
|---|---|---|---|---|---|---|---|
| IgM | IgG1 | IgG2 | IgG3 | IgG4 | |||
| 0 | 3 | 100 | 67 (42–92) | 0 (0–25) | 0 (0–25) | 0 (0–25) | |
| Acute | 1–7 days | 29 | 100 | 97 (89–100) | 38 (20–56) | 48 (31–65) | 52 (35–69) |
| Early convalescence | 8–14 days | 15 | 100 | 100 (89–100) | 67 (47–87) | 73 (53–93) | 100 (89–100) |
| Late convalescence | 15–26 years after measles infection | 7 | 100 | 100 (84–100) | 57 (34–80) | 86 (66–100) | 86 (66–100) |
| Long-lasting immunity | 10–30 years | 100 | 100 (96–100) | 2 (2–6) | 3 (3–7) | 86 (79–93) | |
CI, 95.
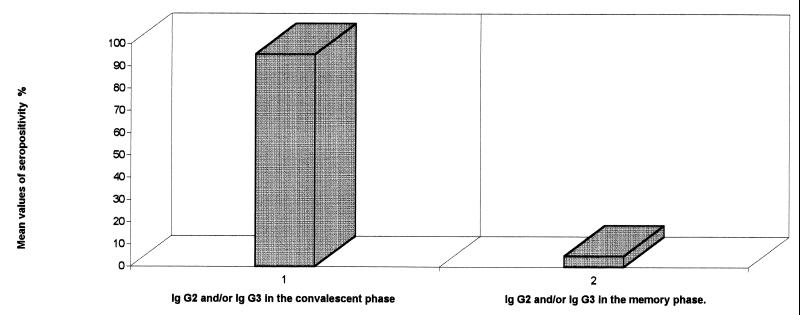
In the convalescent phase of measles virus infection (serum drawn from days 8 through 26), specific IgG2 and/or IgG3 was demonstrated in 21 out of 22 (95.5%; range, 100 to 86.5%). By contrast, only 5 out of 100 (5%; range, 9 to 1%) exhibited the IgG2 and/or IgG3 response many years after primary measles infection (P < 0.05)(Fig. 2).
FIG. 2.
Mean values of seropositivity of measles virus-specific IgG2 and/or IgG3 immune response in convalescent and memory phases.
DISCUSSION
A subclass-restricted response to viral antigens has been demonstrated, but very little is known about the subclasses that react in measles infection.
In the present study, the distribution of measles-specific antibodies among the four subclasses was investigated by IFA assay in patients with natural infections (acute and convalescent phases) and adults with long-lasting humoral immunity to measles. In the acute phase, IgG1 was seen first, followed by IgG2, IgG3, and IgG4 responses, which increased gradually during convalescence. In the memory phase, IgG2 and IgG3 responses decreased significantly, presumably representing markers of acute phase or viral replication, while IgG1 and IgG4 maintained their levels thereafter.
Even though several patterns of IgG subclasses have been reported in response to different viral infections (8, 12, 18), it seems that during the primary infection phase, there is a presence of IgG1 and IgG3 mainly during the convalescent stage. In spite of that, IgG3 was not used, until now, as an IgM-like antibody response, that is, as a serological index of recent measles infection. This could be attributed to a lower rate of IgG3 detection as compared with that reported for IgM antibody in the early immune response (8, 14).
The most interesting finding in our study was the rise and fall of measles virus-specific IgG2 in addition to IgG3 subclass antibodies, showing a subclass restriction characteristic pattern of recent infection. Moreover, the median rate of detection of IgG2 and/or IgG3 subclass antibodies of 95.5% (range, 100 to 86.5%) was similar to the IgM antibody frequency reported in the early stage of measles infection (15).
Different authors have reported low reactivity of IgG2 to varicella-zoster and herpes simplex viruses at any time of infection and to rubella virus infection in the early phase (10, 18). Instead we detected an IgG2 rate of positivity of 67% (range, 47 to 87%) in the early convalescent phase. The discrepancy in reference to IgG2 reactivity could be attributed in part to the methodology used in the test. It is necessary to point out that in viral serology, certain precautions must be taken due to the restricted antiviral response. That is, it cannot be ruled out that certain viral antigens with which IgG2 may react were absent from the antigenic preparation coating enzyme-linked immunosorbent assay microplates. The results reported in the present paper were obtained using an IFA assay and from using fluorescein isothiocyanate-conjugated monoclonal antibodies directed against human IgG isotypes, which in turn reacted against viral antigens expressed in infected cells, displaying the morphological and structural properties of in vivo natural infection.
An IgG1 and IgG4 pattern of antibody memory response was defined. That is, a mean of 86% (range, 93 to 79%) of the samples showed both IgG isotypes (IgG1 and IgG4), thus suggesting that the IgG1 and IgG4 responses play a major role in the maintenance of immunity. This finding is supported by Asano et al. (1), who found high levels of IgG1 and IgG4 antibody activity to varicella-zoster virus in the memory phase of infection. It is likely that the IgG1 subclass plays a leading role in protection against natural infection, because IgG1 consisting mainly of IgG has enough functions of complement fixation and binding to mononuclear cells. Whereas IgG4 is present at the lowest concentrations of any IgG subclass in serum (13), the IgG4 antibody activities were the second highest at 10 years after infection.
The regulation of antibody subclass expression in humans is not well understood, although different authors present evidence that the capacity of a given individual to respond to an antigen by producing antibodies of predominantly one or another IgG subclass may be determined genetically. That is, upon activation, B lymphocytes can change the isotype of the antibody they express by immunoglobulin isotype switch recombination. The isotype switch is mediated by a DNA recombination that moves the variable gene (VDI) from its initial position upstream of the constant region Cu gene, deleting the DNA between the recombination breakpoints (11). The immune response genetic control could explain the concept of individual response to one IgG isotype or another. This notion may account for the fact that 4 out of 100 healthy individuals had a background of measles IgM antibody response, and 2 and 3 out of 100 displayed an IgG2 and IgG3 isotype response, respectively.
Besides, the isotype switch recombination is a highly regulated process controlled by soluble cytokines and by T-cell membrane interaction regulation with the CD40 molecule on the B-cell surface (11). However, cytokine regulation of IgG subtype expression is poorly understood. There are studies in vitro which have demonstrated that interleukins 4 (IL-4) and 3 (IL-3) are responsible for the induction of IgG4 and immunoglobulins E (IgE), and interleukin 12 in the production of IgG2, and interleukin 10 (IL-10) induced only gamma 1 and gamma 3 germ-line mRNA transcripts on B lymphocytes (2, 3, 7, 16).
In summary, in the present study we have defined two highly different immune isotypic response patterns. One of them is restrictive to IgG2 and/or IgG3 in the convalescent phase of natural measles infection, while IgG1 and IgG4 are detected in the convalescent as well as in the memory phases of the immune response. Both IgG2 and IgG3 follow a kinetic pattern similar to that of the IgM antibody response. Thus, their detection could be linked to recent viral activity. In fact, they play a major role only during measles onset. The isolated occurrence of both IgG1 and IgG4 in the absence of IgG2 and IgG3 might be related to long-lasting immunity.
Finally, the IgG isotypic immune responses may contribute to the existing set of serological markers to characterize the measles infection phase, when IgM detection cannot provide a suitable diagnosis.
REFERENCES:
- 1.Asano Y, Hiroishi Y, Itakura N, Hirose S, Kajita Y, Nagai T, Yazaki T, Takahashi M. Immunoglobulin subclass antibodies to varicella-zoster virus. Pediatrics. 1987;80:933–936. [PubMed] [Google Scholar]
- 2.Biere F, Servet-Delprat C, Bridon J M, Saint-Remy J M, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ B cells to secrete Ig G1 and Ig G3. J Exp Med. 1994;179:757–762. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boer B A, Kruize Y C, Yazdanbaksh M. In vitro production of Ig G4 by peripheral blood mononuclear cells (PBMC): the contribution of committed B cells. Clin Exp Immunol. 1998;114:252–257. doi: 10.1046/j.1365-2249.1998.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunell P A. Measles control in the 1990's. Measles serology. Expanded Programme of Immunization, publication no. WHO/EPI/GEN/90.4. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 5.Cutts F T, Henderson R H, Clementes C J, Chen R T, Patriarca P A. Principles of measles control. Bull W H O. 1991;69:1–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Erdman D D, Anderson L J, Adams D R, Stewart J A, Makowitz L, Bellini William. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J Clin Microbiol. 1991;29:1466–1471. doi: 10.1128/jcm.29.7.1466-1471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fjieda S, Saxon A, Zhang K. Direct evidence that gamma 1 and gamma 3 switching in human B cells is interleukin-10 dependent. Mol Immunol. 1996;33:1335–1343. doi: 10.1016/s0161-5890(96)00092-2. [DOI] [PubMed] [Google Scholar]
- 8.Gupta C K, Leszczynski J, Gupta R K, Siber G R. IgG subclass antibodies to human cytomegalovirus (CMV) in normal human plasma samples and immune globulins and their neutralizing activities. Biologicals. 1996;24:117–124. doi: 10.1006/biol.1996.0015. [DOI] [PubMed] [Google Scholar]
- 9.Linde A, Dahl H, Wahren B, Salahuddin F Z, Briberfeld P. IgG antibodies to human herpesvirus-6 in children and adults and in primary Epstein-Barr virus infections and cytomegalovirus infections. J Virol Methods. 1988;21:117–123. doi: 10.1016/0166-0934(88)90058-4. [DOI] [PubMed] [Google Scholar]
- 10.Linde G A. Subclass distribution of rubella virus-specific immunoglobulin G. J Clin Microbiol. 1985;21:117–121. doi: 10.1128/jcm.21.1.117-121.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malisan F, Briere F, Bridon J M, Harindranath N, Molls F, Max E, Banchereau J, Martinez-Valdez H. Interleukin-10 induces immunoglobulin G isotype switch recombination in Huan CD40-activated naive B lymphocytes. J Exp Med. 1996;183:937–947. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathiesen T, Brattstrom C, Anderson J, Linde A, Ljungam P, Wahren B. Immunoglobulin G subclass and lymphocyte stimulatory responses to cytomegalovirus in transplant patients with primary cytomegalovirus infections. J Med Virol. 1992;36:65–69. doi: 10.1002/jmv.1890360113. [DOI] [PubMed] [Google Scholar]
- 13.Meulenbroek A J, Zeijlemaker W P. Human Ig G subclasses: useful diagnostic markers for immunocompetence. Amsterdam, The Netherlands: CLB; 1996. [Google Scholar]
- 14.Narita M, Yamada S, Matsuzono Y, Itakura O, Togashi T, Kikuta H. Measles virus specific immunoglobulin G subclass response in serum and cerebrospinal fluid. Clin Diagn Virol. 1997;8:233–239. doi: 10.1016/s0928-0197(97)10007-1. [DOI] [PubMed] [Google Scholar]
- 15.Norrby E, Gollmar Y. Appearance and persistence of antibodies against different virus components after regular measles infection. Infect Immun. 1972;6:240–247. doi: 10.1128/iai.6.3.240-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punnonen J, Aversa G, Cocks B G, McKenzie A N, Menon S, Zurawski G, de Waal Malefyt R, de Vries J E. Interleukin 13 induces interleukin 4-independent Ig G4 and Ig E synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossier E, Miller H, McCulloch B, Sullivan L, Ward K. Comparison of immunofluorescence and enzyme immunoassay for detection of measles-specific immunoglobulin M antibody. J Clin Microbiol. 1991;29:1069–1071. doi: 10.1128/jcm.29.5.1069-1071.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundqvist V A, Linde A, Wahren B. Virus-specific immunoglobulin G subclass in herpes simplex and varicella-zoster virus infections. J Clin Microbiol. 1984;20:94–98. doi: 10.1128/jcm.20.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]