ABSTRACT
Molecular diagnostic methods improve the detection of Shigella, yet their ability to detect Shigella drug resistance on direct stool specimens is less clear. We tested 673 stool specimens from a Shigella treatment study in Bangladesh, including 154 culture-positive stool specimens and their paired Shigella isolates. We utilized a TaqMan array card that included quantitative PCR (qPCR) assays for 24 enteropathogens and 36 antimicrobial resistance (AMR) genes. Shigella was detected by culture in 23% of stool specimens (154/673), while qPCR detected Shigella at diarrhea-associated quantities in 49% (329/673; P < 0.05). qPCR for AMR genes on the Shigella isolates yielded >94% sensitivity and specificity compared with the phenotypic susceptibility results for azithromycin and ampicillin. The performance for trimethoprim-sulfamethoxazole susceptibility was less robust, and the assessment of ciprofloxacin was limited because most isolates were resistant. The detection of AMR genes in direct stool specimens generally yielded low specificities for predicting the resistance of the paired isolate, whereas the sensitivity and negative predictive values for predicting susceptibility were often higher. For example, the detection of ermB or mphA in stool yielded a specificity of 56% but a sensitivity of 91% and a negative predictive value of 91% versus the paired isolate’s azithromycin resistance result. Patients who received azithromycin prior to presentation were universally culture negative (0/112); however, qPCR still detected Shigella at diarrhea-associated quantities in 34/112 (30%). In sum, molecular diagnostics on direct stool specimens greatly increase the diagnostic yield for Shigella, including in the setting of prior antibiotics. The molecular detection of drug resistance genes in direct stool specimens had low specificity for confirming resistance but could potentially “rule out” macrolide resistance.
KEYWORDS: Shigella, molecular methods, phenotypic resistance
INTRODUCTION
Shigella is a leading cause of diarrheal disease (1). Antimicrobial treatment for patients with shigellosis has been recommended because of its severity (2, 3). However, antibiotic resistance is increasing, including multidrug-resistant (MDR) Shigella, particularly among Shigella sonnei isolates (3–5). Therefore, antimicrobial susceptibility testing (AST) is important to guide local regimens and for individual patients (6).
Traditional antimicrobial susceptibility testing is culture dependent. This is problematic for Shigella as it is a fastidious organism, and culture has been clearly demonstrated to be an insensitive detection method compared with nucleic acid amplification (7–9). In one large childhood diarrhea study, 50% of Shigella isolates were missed by culture (7). A general problem with nucleic acid amplification tests, however, is that antibiotic susceptibility information is usually unavailable. Many resistance-associated genes have been identified, but since these are frequently carried by plasmids and are not species specific, resistance genes detected in stool cannot be readily assigned to a specific organism. For example, we have found that the detection of a broad panel of resistance-associated genes in stool yields only 75% specificity for the phenotypic resistance pattern of cultured Escherichia coli isolates (10).
In this study, we reanalyzed 673 stool specimens and 154 paired Shigella isolates that were collected from patients with inflammatory diarrhea and presumed shigellosis in Bangladesh, all of whom were empirically treated with azithromycin according to the local standard of care. The primary outcomes of this study have been reported previously and showed that individuals infected with Shigella isolates with higher azithromycin MICs had worse clinical outcomes (11), providing the basis for clinical breakpoints for azithromycin and Shigella (12). Here, we utilized molecular diagnostics on this cohort to examine the rates of Shigella missed by culture and assessed the ability of molecular diagnosis on stool specimens to predict the phenotypic susceptibility pattern of Shigella.
MATERIALS AND METHODS
Bacterial isolates and stool specimens.
One hundred fifty-four Shigella species isolates and their paired stool specimens as well as 519 Shigella species culture-negative stool specimens were obtained from a previous study performed at the International Centre for Diarrhoeal Diseases and Research, Bangladesh (icddr,b) (11). The enrollment period was January to September 2019. Stool specimens were obtained from patients presenting with diarrhea for <96 h, reporting mucus in the stool, with complaints of abdominal pain or cramps, and with ≥10 white blood cells and any number of red blood cells per high-power field. Stool underwent culture within hours, followed by species identification, serotyping, and antimicrobial susceptibility testing (AST) at icddr,b. Stool specimens and bacterial isolates were later shipped on dry ice from icddr,b to the University of Virginia and stored at −80°C upon arrival until testing in June 2020. The icddr,b and University of Virginia Institutional Review Boards approved this work.
Nucleic acid extraction.
The DNA of stool specimens was extracted using a QIAamp Fast DNA stool minikit (Qiagen, Hilden, Germany) as previously described (13). Briefly, 200-mg stool specimens were added to the tube containing acid-washed glass beads (Sigma-Aldrich, St. Louis, MO, USA) and suspended with 1 mL InhibitEX buffer, followed by bead beating for 2 min. Stool specimens were then incubated at 95°C for 5 min, followed by centrifugation at 20,000 × g for 1 min. The supernatant (600 μL) was transferred to a new tube containing 25 μL proteinase K followed by 600 μL lysis buffer AL, mixed, and incubated at 70°C for 10 min. Six hundred microliters of ethanol was added to the lysate and mixed vigorously. The lysate was then purified through a QIAamp minispin column according to the manufacturer’s instructions and then eluted into 200 μL, and the eluate was stored at −20°C to be used as a DNA template. For bacterial isolates, 200 μL at a 0.5 McFarland standard prepared as an inoculum for antimicrobial susceptibility testing was centrifuged at 8,000 × g for 10 min. The bacterial pellet was resuspended with 200 μL TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) and heated at 95°C for 15 min, followed by centrifugation at 8,000 × g for 5 min, and the supernatant was stored at −20°C for use as a DNA template.
Detection of enteropathogens and antimicrobial resistance genes from stool specimens.
We utilized a custom TaqMan array card (TAC) (Applied Biosystems, Thermo Fisher Scientific, Foster City, CA, USA) containing our previously developed antimicrobial resistance (AMR) genes (10), enteropathogens (14), and S. flexneri serotyping assays (15). The TAC layout is shown in Fig. S1 in the supplemental material. The procedure for performing the TAC assay was described previously (10). Cycling conditions included reverse transcription at 45°C for 20 min and an initial denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min.
Detection of antimicrobial resistance genes in culture isolates.
The AMR genes were detected by performing quantitative PCR (qPCR) on 384-well PCR plates using AMR assays identical to the ones described above. One microliter of input DNA was mixed with 2.5 μL of 2× PCR buffer, 0.2 μL of 25× PCR enzyme from the AgPath-ID-PCR kit (Applied Biosystems), 0.25 μL of 20× primer/probe assay mix (Applied Biosystems), and 1.05 μL of nuclease-free water to yield a 5-μL final volume. qPCR was performed on a ViiA 7 instrument (Applied Biosystems) using the same cycling conditions as the ones described above.
Antimicrobial susceptibility testing.
The Shigella species isolates were tested for susceptibility at icddr,b with broth microdilution using the Sensititre NARMS Gram-negative plate (catalog number CMV4AGNF; Thermo Fisher Scientific, Waltham, MA, USA). Procedures were performed according to Clinical and Laboratory Standards Institute guidelines (12, 16).
Statistical analysis.
On the basis of previous studies that documented the correlation between enteropathogen quantity and diarrheal etiology, we utilized these previously reported pathogen-specific quantities to ascribe etiologies, including shigellosis (7, 17) (see Table S1 in the supplemental material). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of genotypic methods were analyzed against phenotypic methods as the gold standard. Receiver operating characteristic (ROC) analysis was performed with SPSS statistics software version 27 to define a cycle threshold (CT) ΔCT value between the resistance gene and the Shigella detection gene (ipaH) that optimized sensitivity and specificity according to Youden’s index. The statistical significance of the chi-square test or Fisher’s exact test was used to determine differences in proportions.
RESULTS
Prevalence of Shigella by PCR.
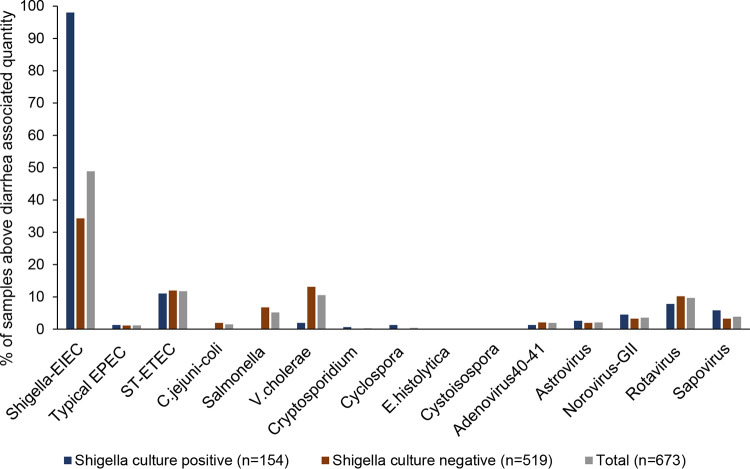
In the original study, there were 737 stool specimens, of which 154 were culture positive. Here, we tested these 154 paired stool specimens and the 519 available culture-negative stool specimens. These 673 stool specimens were tested for a panel of enteropathogens using our custom-designed TaqMan array card (see Fig. S1 in the supplemental material). Previously utilized pathogen-specific quantities (i.e., qPCR CT values; see Table S1) (7, 17) were used to ascribe likely etiologies. Whereas Shigella was detected by culture in 23% of stool specimens (154/673), qPCR detected Shigella at diarrhea-associated quantities in 49% (329/673; P < 0.05) (Fig. 1). The next most prevalent pathogens detected at diarrhea-associated quantities were, in descending order, heat-stabile toxin-producing enterotoxigenic E. coli (ST-ETEC), Vibrio cholerae, rotavirus, Salmonella spp., norovirus GII, sapovirus, adenovirus 40-41, astrovirus, typical enteropathogenic E. coli (EPEC), and Campylobacter jejuni-C. coli (Fig. 1). Overall, 54% of samples contained one pathogen at diarrhea-associated quantities, 15% contained two pathogens, 4% contained three pathogens, 1% contained four or more pathogens, and 26% revealed no pathogen at diarrhea-associated quantities.
FIG 1.
Prevalence of diarrhea-associated pathogens. Six hundred seventy-three diarrhea stool specimens were tested for 24 enteropathogens by qPCR, and likely diarrhea-associated pathogens were assessed using previously reported qPCR cycle threshold values (7, 17). EIEC, enteroinvasive E. coli; EPEC, enteropathogenic E. coli; ST-ETEC, heat-stabile toxin-producing enterotoxigenic E. coli; E.histolytica, Entamoeba histolytica.
Performance of genotypic antimicrobial susceptibility testing on Shigella species isolates.
Interpretive criteria exist for susceptibility testing of Shigella for ampicillin, azithromycin, ciprofloxacin, and trimethoprim-sulfamethoxazole (SXT) (18). In this work, we examined the ability of 36 genotypic assays to predict resistance for Shigella (Fig. S1). All 154 enrollment Shigella isolates along with 20 isolates that were obtained at patient follow-up were tested by qPCR for these genes, and the results were compared to previously reported broth microdilution antibiotic susceptibility results (11). Figure S2 shows the qPCR CT values of the relevant resistance genes versus ipaH as an inoculum control. Youden’s index revealed that a resistance gene qPCR CT value of 25 was optimally discriminatory for phenotypic susceptibility and resistance (Fig. S2). Using this cutoff, the macrolide resistance-associated genes ermB and mphA yielded 98% sensitivity and 99% specificity versus phenotypic results (Table 1). Fourteen ampicillin resistance-associated genes were assayed, of which blaTEM, the blaCTX-M1 group, blaDHA, and blaOXA-1 were found in 29%, 19%, 20%, and 36% of ampicillin-resistant isolates, respectively. Combining the presence of any of these four genes yielded 95% sensitivity and 94% specificity against the phenotypic gold standard. For ciprofloxacin, there were limited numbers of ciprofloxacin-susceptible isolates from this study, limiting this analysis. The gyrA and parC mutations exhibited 96 to 100% sensitivity for ciprofloxacin-resistant isolates. parC exhibited 93% (13/14) specificity; however, the gyrA mutations yielded only 36% (5/14) specificity. For SXT, resistance requires both dfrA and sul genes. The dfrA genes were found in 100% of SXT-resistant isolates, while sul genes were found in 74%, and the combination yielded 74% sensitivity and 86% specificity. Multidrug-resistant Shigella, defined as resistance to 3 or more classes of antibiotics, was seen in 60/149 (40%) of the strains in Table 1. Genotypic testing yielded 80 to 86% sensitivity and 91 to 92% specificity to detect MDR Shigella.
TABLE 1.
Correlation between phenotypic and genotypic susceptibility testing on Shigella species isolates
| Antimicrobial agent and isolate genotypea | No. of isolates with phenotypeb |
Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|
| R | I | S | |||
| Azithromycin (n = 154) | |||||
| ermB | |||||
| Pos | 52 | 0 | 0 | 90 | 100 |
| Neg | 6 | 5 | 91 | ||
| mphA | |||||
| Pos | 57 | 5 | 1 | 98 | 99 |
| Neg | 1 | 0 | 90 | ||
| ermB and mphA | |||||
| Pos | 52 | 0 | 0 | 90 | 100 |
| Neg | 6 | 5 | 91 | ||
| ermB or mphA | |||||
| Pos | 57 | 5 | 1 | 98 | 99 |
| Neg | 1 | 0 | 90 | ||
| Ampicillin (n = 149) | |||||
| bla TEM | |||||
| Pos | 17 | 0 | 4 | 29 | 96 |
| Neg | 42 | 0 | 86 | ||
| bla CTX-M1 | |||||
| Pos | 11 | 0 | 0 | 19 | 100 |
| Neg | 48 | 0 | 90 | ||
| bla DHA | |||||
| Pos | 12 | 0 | 0 | 20 | 100 |
| Neg | 47 | 0 | 90 | ||
| bla OXA-1 | |||||
| Pos | 21 | 0 | 1 | 36 | 99 |
| Neg | 38 | 0 | 89 | ||
| Any β-lactamase | |||||
| Pos | 56 | 0 | 5 | 95 | 94 |
| Neg | 3 | 0 | 85 | ||
| Ciprofloxacin (n = 149) | |||||
| gyrA | |||||
| MT | 124 | 2 | 9 | 100 | 36 |
| WT | 0 | 9 | 5 | ||
| parC | |||||
| MT | 119 | 0 | 1 | 96 | 93 |
| WT | 5 | 11 | 13 | ||
| gyrA and parC | |||||
| MT | 119 | 0 | 1 | 96 | 93 |
| WT | 5 | 11 | 13 | ||
| gyrA or parC | |||||
| MT | 124 | 2 | 9 | 100 | 36 |
| WT | 0 | 9 | 5 | ||
| Trimethoprim-sulfamethoxazole (n = 149) | |||||
| dfrA | |||||
| Pos | 73 | 0 | 64 | 100 | 16 |
| Neg | 0 | 0 | 12 | ||
| sul | |||||
| Pos | 54 | 0 | 11 | 74 | 86 |
| Neg | 19 | 0 | 65 | ||
| dfrA and sul | |||||
| Pos | 54 | 0 | 11 | 74 | 86 |
| Neg | 19 | 0 | 65 | ||
| dfrA or sul | |||||
| Pos | 73 | 0 | 64 | 100 | 16 |
| Neg | 0 | 0 | 12 | ||
| MDR (n = 149) | |||||
| Resistance gene of ≥3 classes | |||||
| Pos | 48 | 0 | 8 | 80 | 91 |
| Neg | 12 | 0 | 81 | ||
| Resistance gene of all 4 classes | |||||
| Pos | 18 | 0 | 10 | 86 | 92 |
| Neg | 3 | 0 | 118 | ||
Phenotypic susceptibility results were not available for ampicillin, ciprofloxacin, and trimethoprim-sulfamethoxazole for 5 isolates. Pos, positive; Neg, negative; MT, mutant; WT, wild type.
S, susceptible; I, intermediate; R, resistant (excluded intermediate from the analysis).
Prevalence of antimicrobial resistance genes in Shigella flexneri versus S. sonnei.
We then compared the prevalences of resistance genes between Shigella flexneri (n = 87) and S. sonnei (n = 58) and whether multiple mutations conferred higher MICs. Unsurprisingly, there was generally more resistance in S. sonnei, as we previously reported that 88% of the S. sonnei strains from this study were MDR, versus 45% for S. flexneri (11). The cooccurrence of ermB and mphA was higher (43/44; 98%) in azithromycin-resistant S. sonnei than in S. flexneri isolates (9/14; 64% [P = 0.002]). In other words, 36% (5/14) of azithromycin-resistant S. flexneri isolates carried mphA only, versus 0% (0/44) of S. sonnei isolates (P < 0.001) (Fig. S3A). For ampicillin-resistant isolates, the most frequent resistance genes were blaOXA-1 for S. flexneri and the blaCTX-M1 group for S. sonnei (Fig. S3B). Both mutation 83L of the gyrA gene and mutation 80I of the parC gene were present in most ciprofloxacin-resistant Shigella isolates (Fig. S3C). Most S. flexneri isolates were susceptible to SXT and contained the dfrA1 gene, while 96% of S. sonnei isolates were resistant and included both dfrA and sul genes (Fig. S3D).
Performance of genotypic antimicrobial resistance detection on Shigella culture-positive stool specimens.
We then explored how well the detection of these AMR genes in stool predicted the susceptibility pattern of the paired isolates. There were 154 stool specimens with paired cultured isolates for which susceptibility testing results were available. The stool qPCR CT values of the resistance genes and Shigella-specific gene (ipaH) were examined, yet there was no discernible stool CT cutoff to define resistant or susceptible isolates (Fig. S4). To try to better associate the presence of resistance genes with the Shigella ipaH target, we examined the ΔCT (CT of the resistance gene − CT of ipaH) and determined the ΔCT cutoff with the highest Youden index. The optimal ΔCT cutoffs were a ΔCT of >3 to predict the susceptibility of the cultured Shigella isolates to azithromycin, a ΔCT of >5 for ampicillin and trimethoprim-sulfamethoxazole, and a ΔCT of >6 for ciprofloxacin (Fig. 2). Using these cutoffs, the specificities for detecting these resistance genes in stool were 8% to 79% for predicting the resistance pattern of the Shigella isolate, yielding positive predictive values (PPVs) of 49% to 97%. In contrast, the sensitivities and negative predictive values (NPVs) were often higher; in other words, the absence of a resistance gene could potentially predict susceptibility (Table 2). Notably, ermB or mphA yielded a sensitivity of 91% and an NPV of 91%. The detection of any β-lactamase in stool yielded a sensitivity of 92% and an NPV of 86%. The detection of dfrA and sul yielded a sensitivity of 82% and an NPV of 70%. For ciprofloxacin, the optimal molecular test was the detection of parC mutations, which yielded 77% sensitivity and 79% specificity; however, this analysis was limited by the low numbers of susceptible Shigella isolates. In addition to this stool genotype-isolate phenotype comparison, we also examined the stool genotype-isolate genotype comparison and noted similar results with high sensitivities and low specificities (Table S2).
FIG 2.
Detection of AMR genes in direct stool specimens compared with Shigella phenotypic susceptibility results. One hundred fifty-four Shigella species culture-positive stool specimens were tested for antimicrobial resistance genes. The ΔCT (CT of the resistance gene − CT of ipaH) was determined and plotted against the phenotypic susceptibility results of the paired cultured isolates. ROC analysis and Youden’s index were used to optimize ΔCT cutoffs to differentiate susceptible and resistant isolates.
TABLE 2.
Correlation between phenotypic and genotypic susceptibility results on direct stool specimens
| Antimicrobial agent and isolate genotypea | No. of isolates with phenotypeb |
Sensitivity (%) | Specificity (%) | PPVc (%) | NPVd (%) | ||
|---|---|---|---|---|---|---|---|
| R | I | S | |||||
| Azithromycin (n = 154; ΔCT ≤ 3) | |||||||
| ermB | |||||||
| Pos | 50 | 5 | 30 | 86 | 67 | 63 | 88 |
| Neg | 8 | 0 | 61 | ||||
| mphA | |||||||
| Pos | 49 | 5 | 38 | 84 | 58 | 56 | 85 |
| Neg | 9 | 0 | 53 | ||||
| ermB and mphA | |||||||
| Pos | 46 | 5 | 28 | 79 | 69 | 62 | 84 |
| Neg | 12 | 0 | 63 | ||||
| ermB or mphA | |||||||
| Pos | 53 | 5 | 40 | 91 | 56 | 57 | 91 |
| Neg | 5 | 0 | 51 | ||||
| Ampicillin (n = 149; ΔCT ≤ 5) | |||||||
| bla TEM | |||||||
| Pos | 44 | 0 | 54 | 75 | 40 | 45 | 71 |
| Neg | 15 | 0 | 36 | ||||
| bla CTX-M1 | |||||||
| Pos | 26 | 0 | 27 | 44 | 70 | 49 | 66 |
| Neg | 33 | 0 | 63 | ||||
| bla DHA | |||||||
| Pos | 25 | 0 | 22 | 42 | 76 | 53 | 67 |
| Neg | 34 | 0 | 68 | ||||
| bla OXA-1 | |||||||
| Pos | 20 | 0 | 11 | 34 | 88 | 65 | 67 |
| Neg | 39 | 0 | 79 | ||||
| Other β-lactamasee | |||||||
| Pos | 16 | 0 | 21 | 27 | 77 | 43 | 62 |
| Neg | 43 | 0 | 69 | ||||
| Any β-lactamase | |||||||
| Pos | 54 | 0 | 59 | 92 | 34 | 48 | 86 |
| Neg | 5 | 0 | 31 | ||||
| Ciprofloxacin (n = 149; ΔCT ≤ 6) | |||||||
| gyrA | |||||||
| MT | 120 | 4 | 12 | 97 | 14 | 91 | 33 |
| WT | 4 | 7 | 2 | ||||
| parC | |||||||
| MT | 95 | 1 | 3 | 77 | 79 | 97 | 28 |
| WT | 29 | 10 | 11 | ||||
| gyrA and parC | |||||||
| MT | 95 | 1 | 3 | 77 | 79 | 97 | 28 |
| WT | 29 | 10 | 11 | ||||
| gyrA or parC | |||||||
| MT | 120 | 4 | 12 | 97 | 14 | 91 | 33 |
| WT | 4 | 7 | 2 | ||||
| Trimethoprim-sulfamethoxazole (n = 149; ΔCT ≤ 5) | |||||||
| dfrA | |||||||
| Pos | 73 | 0 | 70 | 100 | 8 | 51 | 100 |
| Neg | 0 | 0 | 6 | ||||
| sul | |||||||
| Pos | 60 | 0 | 49 | 82 | 36 | 55 | 68 |
| Neg | 13 | 0 | 27 | ||||
| dfrA and sul | |||||||
| Pos | 60 | 0 | 46 | 82 | 39 | 57 | 70 |
| Neg | 13 | 0 | 30 | ||||
| dfrA or sul | |||||||
| Pos | 73 | 0 | 73 | 100 | 4 | 50 | 100 |
| Neg | 0 | 0 | 3 | ||||
| MDR (n = 149; ΔCT ≤ 6) | |||||||
| Resistance gene of ≥3 classes | |||||||
| Pos | 53 | 0 | 66 | 88 | 26 | 45 | 77 |
| Neg | 7 | 0 | 23 | ||||
| Resistance gene of all 4 classes | |||||||
| Pos | 20 | 0 | 76 | 95 | 41 | 21 | 98 |
| Neg | 1 | 0 | 52 | ||||
Phenotypic susceptibility results were not available for ampicillin, ciprofloxacin, and trimethoprim-sulfamethoxazole for 5 samples. Pos, positive; Neg, negative; MT, mutant; WT, wild type.
S, susceptible; I, intermediate; R, resistant (excluded intermediate from analysis).
PPV, positive predictive value.
NPV, negative predictive value.
These included blaSHV, blaCTX-M2, blaCTX-M8, blaCTX-M9, blaACT-MIR, blaCMY2-LAT, blaNDM, blaKPC, blaOXA-48, and blaOXA-9.
Effect of prior antibiotics on Shigella detection.
We next examined the impact of prior antibiotic administration on the detection of Shigella. We had complete antibiotic data on 666 participants, of whom 241/666 (36%) participants self-reported taking antibiotics prior to presentation. The main antibiotics reported were azithromycin (17%), metronidazole (12%), and ciprofloxacin (9%), with only 4 individuals reporting any other antibiotic. The rate of Shigella culture positivity was higher in those who reported not receiving antibiotics prior to enrollment (27% versus 13% in those reporting receiving antibiotics [P < 0.001]) (Fig. 3A). Receiving prior antibiotics was also associated with lower rates of molecularly detected Shigella (91/241 [38%] versus 238/425 [56%] of those receiving no antibiotic [P < 0.001]) (Fig. 3A). This lower rate was driven by azithromycin and not ciprofloxacin or metronidazole (Fig. 3B to D).
FIG 3.
Effect of prior antibiotics on Shigella culture positivity. Self-reported antibiotics received prior to enrollment into the study were assessed for their impact on Shigella culture positivity. The impacts of receiving any antibiotic (A), azithromycin (B), fluoroquinolone (C), and metronidazole (D) on the positive culture rate of Shigella spp. are shown. *, P < 0.05. Note that 20 individuals received multiple antibiotics and are included in both groups for this analysis.
Effect of prior antibiotics on drug-resistant Shigella.
Finally, we assessed the impact of prior antibiotic administration on Shigella drug resistance. As mentioned above, there were no positive cultures among individuals who reported receiving azithromycin prior to enrollment, so the effects of azithromycin on phenotypic drug resistance could not be examined. However, individuals with a molecular diagnosis of Shigella who reported receiving azithromycin prior to enrollment had higher rates of ermB and mphA in stool than those who did not receive azithromycin (Fig. S5A) (P < 0.05), whereas gyrA or parC was not affected. Similarly, parC mutations were more common in those who reported receiving ciprofloxacin prior to presentation, while ermB and mphA were not affected (Fig. S5B).
DISCUSSION
This molecular reanalysis of an azithromycin Shigella treatment study revealed several interesting features. First, we found that Shigella was the leading cause of invasive diarrhea in Dhaka, Bangladesh, present in about 50% of cases, while culture detected only ∼20%. Therefore, molecular diagnostics more than doubled the diagnostic yield for Shigella, even requiring diarrhea-associated quantities to ascribe shigellosis. One contributor to low culture yields was prior azithromycin administration, whereas molecular detection remained a viable strategy for the diagnosis of Shigella infection. It is worth noting that Shigella culture was performed immediately in this study, so this likely represents the best possible study design to maximize the culture yield. Second, we found that molecular diagnostics on isolates were highly capable of predicting the phenotypic resistance profile of Shigella for azithromycin and ampicillin, with sensitivities or specificities of >94%. Predictions for trimethoprim-sulfamethoxazole were moderate (74 to 86%) and could not be clearly ascertained for ciprofloxacin because of the low number of susceptible Shigella isolates in this setting. Third, in contrast to the genotypic prediction of the susceptibility profile of isolates, direct detection of these same genes in stool specimens was less useful. For most drugs, detection of resistance genes was poorly predictive of resistance; however, the absence of detection of resistance genes was reasonably predictive of susceptibility to some antibiotics, most notably azithromycin. Fourth, the molecular detection of AMR genes revealed that resistance was increased with prior antibiotic exposure.
We were not surprised to see this higher yield of molecular diagnoses of Shigella infection than with culture. We have seen similar 2- to 4-fold increases previously in large multisite studies in Africa, Asia, and South America (7, 17). The overall yield of molecular diagnosis for an etiology in this study was 74%, similar to what we have seen in those other studies. Other causes of diarrhea in this study ascertained by molecular diagnostics were ST-ETEC, Salmonella, cholera, and rotavirus, but clearly, Shigella was dominant, which is no surprise as the inclusion criteria were chosen to enrich for shigellosis.
The accuracy of genotypic methods to predict phenotypic susceptibility for Shigella was as we expected. We found similar results in a previous study of E. coli (10), and the mechanisms of resistance of these two organisms are similar. Other studies have also shown high correlations between genotypic and phenotypic AMR results for Shigella isolates (19).
The imperfect accuracy of genotypic methods in stool specimens to predict the phenotypic susceptibility of Shigella was also not surprising but was important to document as we are not aware of any other studies that have directly measured this. The low specificity of the detection of resistance genes in predicting the Shigella phenotypic result was expected because these are mostly plasmid-borne resistance genes and can be present in any number of bacteria in the stool besides Shigella. Indeed, we previously found a similarly low specificity in stool to predict the phenotypic results for cultured E. coli (10). Even after correlating the quantities of resistance genes to Shigella genes, the ability to detect resistant Shigella remained limited. The absence of detection of resistance genes, in contrast, was predictive of susceptibility, with a reasonably high negative predictive value, at least for azithromycin. Unfortunately, this absence of resistance genes was relatively rare in Dhaka, Bangladesh, presumably because of extensive antibiotic use in the community. For instance, for azithromycin, the absence of resistance genes was found in only 33% (51/154) of cases. So while this result has a 91% predictive value for azithromycin-susceptible Shigella, it is unclear how to interpret the remaining two-thirds of cases where the detection of ermB or mphA occurred, as it is a coin toss (57% positive predictive value) whether that result implicates azithromycin-resistant Shigella. We therefore believe that these direct genotypic diagnostics for AMR are likely not ready for clinical uptake in this setting. It is possible, however, that in other parts of the world where AMR gene carriage and antibiotic-resistant Shigella are less common, these genotypic results on stool specimens could be more clinically useful to “rule out” resistance. Another potential strategy for a clinical laboratory would be to use molecular diagnostics as the screen to detect Shigella and then focus culture efforts on only Shigella PCR-positive specimens to then permit phenotypic AST.
Our study found that prior antibiotic exposure was associated with higher rates of the relevant AMR genes. This is not surprising as a number of studies have shown associations between antimicrobial exposure and the carriage of drug-resistant bacteria (20, 21). A lower yield of stool culture has been noted after the administration of antibiotics (22); however, it was striking to note how potent and selective prior azithromycin exposure affected Shigella culture positivity, as 0% of such individuals were culture positive. This finding was not seen with prior ciprofloxacin or metronidazole exposure, consistent with the high rate of ciprofloxacin-resistant Shigella isolates in this setting and the lack of activity of metronidazole against Shigella. Fortunately, molecular diagnostics remained able to implicate Shigella in many of these azithromycin-treated cases. Moreover, inference of the AMR genes in the stool specimens of the cases suggested a potentially higher rate of azithromycin resistance (versus non-azithromycin-treated cases).
The TaqMan array card approach yielded other important epidemiological information. The most frequent ampicillin resistance gene for S. flexneri was blaOXA-1, while for S. sonnei, they were blaTEM and blaCTX-M1. These results are similar to those in previous reports (23–27). Several reports have suggested that resistance to azithromycin in Shigella species isolates is associated with the presence of mphA (11 to 55%), ermB (1 to 2%), or both genes (43% to 87%) (28–32). In our study, we found a 90% cooccurrence of ermB and mphA in azithromycin-resistant isolates. For ciprofloxacin resistance, we found that the resistance gene pattern of S. flexneri was gyrA83L-gyrA87N-parC80I, which had been reported previously as a pattern of Bangladeshi isolates (33). The S. sonnei resistance pattern was gyrA83L-gyrA87G-parC80I. This pattern has been suggested to have emerged from a single common ancestor, Central Asia lineage III (34, 35).
Limitations of this work include that it was a single-site study, with high antimicrobial resistance, which could impact generalizability. It is possible that other mechanisms of resistance exist that were not probed with our menu of assays, although we tried to be comprehensive. Another limitation is that the pathogen quantity determined by qPCR is quite useful epidemiologically to ascribe etiology but may not perfectly ascertain etiology on an individual-case basis (nor can any diagnostic). Prior antibiotic administration was based on patient recall, which could be subject to bias. Finally, testing was not contemporaneous: phenotypic susceptibility information was obtained in real time during the study in 2019, while the molecular testing of stool specimens occurred in 2020 using frozen materials.
In summary, Shigella and MDR Shigella are common causes of diarrhea in Dhaka. Prior receipt of azithromycin will greatly affect the culture yield, yet molecular detection methods can still detect Shigella. The detection of AMR genes in stool appears to have limited predictive power to confirm antibiotic resistance; however, the absence of AMR genes has good negative predictive power to predict azithromycin-susceptible Shigella.
ACKNOWLEDGMENTS
The study was supported by National Institutes of Health (NIH) grant K24 AI102972 (to E.R.H.), and the original study was supported by the Centers for Disease Control and Prevention (contract number 75D30118C02910 to E.R.H.).
We thank icddr,b, which provided material and shared data; additionally, we thank patients who participated in the project for their essential contributions.
Footnotes
Supplemental material is available online only.
Contributor Information
Eric R. Houpt, Email: erh6k@virginia.edu.
Nathan A. Ledeboer, Medical College of Wisconsin
REFERENCES
- 1.Puzari M, Sharma M, Chetia P. 2018. Emergence of antibiotic resistant Shigella species: a matter of concern. J Infect Public Health 11:451–454. 10.1016/j.jiph.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2005. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Rahman M, Shoma S, Rashid H, El Arifeen S, Baqui AH, Siddique AK, Nair GB, Sack DA. 2007. Increasing spectrum in antimicrobial resistance of Shigella isolates in Bangladesh: resistance to azithromycin and ceftriaxone and decreased susceptibility to ciprofloxacin. J Health Popul Nutr 25:158–167. [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson CN, Duy PT, Baker S. 2015. The rising dominance of Shigella sonnei: an intercontinental shift in the etiology of bacillary dysentery. PLoS Negl Trop Dis 9:e0003708. 10.1371/journal.pntd.0003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya D, Bhattacharya H, Sayi DS, Bharadwaj AP, Singhania M, Sugunan AP, Roy S. 2015. Changing patterns and widening of antibiotic resistance in Shigella spp. over a decade (2000-2011), Andaman Islands, India. Epidemiol Infect 143:470–477. 10.1017/S0950268814000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis 65:e45–e80. 10.1093/cid/cix669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque ASG, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388:1291–1301. 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vu DT, Sethabutr O, Von Seidlein L, Tran VT, Do GC, Bui TC, Le HT, Lee H, Houng HS, Hale TL, Clemens JD, Mason C, Dang DT. 2004. Detection of Shigella by a PCR assay targeting the ipaH gene suggests increased prevalence of shigellosis in Nha Trang, Vietnam. J Clin Microbiol 42:2031–2035. 10.1128/JCM.42.5.2031-2035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsay B, Ochieng JB, Ikumapayi UN, Toure A, Ahmed D, Li S, Panchalingam S, Levine MM, Kotloff K, Rasko DA, Morris CR, Juma J, Fields BS, Dione M, Malle D, Becker SM, Houpt ER, Nataro JP, Sommerfelt H, Pop M, Oundo J, Antonio M, Hossain A, Tamboura B, Stine OC. 2013. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J Clin Microbiol 51:1740–1746. 10.1128/JCM.02713-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pholwat S, Liu J, Taniuchi M, Chinli R, Pongpan T, Thaipisutikul I, Ratanakorn P, Platts-Mills JA, Fleece M, Stroup S, Gratz J, Mduma E, Mujaga B, Walongo T, Nshama R, Kimathi C, Foongladda S, Houpt ER. 2019. Genotypic antimicrobial resistance assays for use on E. coli isolates and stool specimens. PLoS One 14:e0216747. 10.1371/journal.pone.0216747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houpt ER, Ferdous T, Ara R, Ibrahim M, Alam MM, Kabir M, Platts-Mills J, Ahmed T, Faruque ASG, Taniuchi M, Haque R. 2021. Clinical outcomes of drug-resistant shigellosis treated with azithromycin in Bangladesh. Clin Infect Dis 72:1793–1798. 10.1093/cid/ciaa363. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing, CLSI supplement M100, 31st ed. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pholwat S, Pongpan T, Chinli R, Rogawski McQuade ET, Thaipisuttikul I, Ratanakorn P, Liu J, Taniuchi M, Houpt ER, Foongladda S. 2020. Antimicrobial resistance in swine fecal specimens across different farm management systems. Front Microbiol 11:1238. 10.3389/fmicb.2020.01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. 2013. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51:472–480. 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Pholwat S, Zhang J, Taniuchi M, Haque R, Alam M, Ochieng JB, Jones JA, Platts-Mills JA, Tennant SM, Houpt E. 2021. Evaluation of molecular serotyping assays for Shigella flexneri directly on stool samples. J Clin Microbiol 59:e02455-20. 10.1128/JCM.02455-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial disk susceptibility test, CLSI standard M02, 13th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, McMurry TL, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, McCormick BJJ, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, Houpt ER, MAL-ED Network Investigators. 2018. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 6:e1309–e1318. 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing, CLSI supplement M100, 29th ed. Clinical and laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Terry LM, Barker CR, Day MR, Greig DR, Dallman TJ, Jenkins C. 2018. Antimicrobial resistance profiles of Shigella dysenteriae isolated from travellers returning to the UK, 2004-2017. J Med Microbiol 67:1022–1030. 10.1099/jmm.0.000779. [DOI] [PubMed] [Google Scholar]
- 20.Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, Grobusch MP, Lashof AMO, Molhoek N, Schultsz C, Stobberingh EE, Verbrugh HA, de Jong MD, Melles DC, Penders J. 2017. Import and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis 17:78–85. 10.1016/S1473-3099(16)30319-X. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Matsui Y, Riley LW. 2020. Risk factors for fecal carriage of drug-resistant Escherichia coli: a systematic review and meta-analysis. Antimicrob Resist Infect Control 9:31. 10.1186/s13756-020-0691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitkara YK. 2005. Limited value of routine stool cultures in patients receiving antibiotic therapy. Am J Clin Pathol 123:92–95. 10.1309/eqp21kembb6ehg9b. [DOI] [PubMed] [Google Scholar]
- 23.Cui X, Wang J, Yang C, Liang B, Ma Q, Yi S, Li H, Liu H, Li P, Wu Z, Xie J, Jia L, Hao R, Wang L, Hua Y, Qiu S, Song H. 2015. Prevalence and antimicrobial resistance of Shigella flexneri serotype 2 variant in China. Front Microbiol 6:435. 10.3389/fmicb.2015.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamanlou S, Ahangarzadeh Rezaee M, Aghazadeh M, Ghotaslou R, Babaie F, Khalili Y. 2018. Characterization of integrons, extended-spectrum beta-lactamases, AmpC cephalosporinase, quinolone resistance, and molecular typing of Shigella spp. from Iran. Infect Dis (Lond) 50:616–624. 10.1080/23744235.2018.1455222. [DOI] [PubMed] [Google Scholar]
- 25.Toro CS, Farfan M, Contreras I, Flores O, Navarro N, Mora GC, Prado V. 2005. Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol Infect 133:81–86. 10.1017/s0950268804003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranjbar R, Farahani A. 2019. Shigella: antibiotic-resistance mechanisms and new horizons for treatment. Infect Drug Resist 12:3137–3167. 10.2147/IDR.S219755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JS, Kim J, Jeon SE, Kim SJ, Kim NO, Hong S, Kang YH, Han S, Chung GT. 2014. Complete nucleotide sequence of the IncI1 plasmid pSH4469 encoding CTX-M-15 extended-spectrum beta-lactamase in a clinical isolate of Shigella sonnei from an outbreak in the Republic of Korea. Int J Antimicrob Agents 44:533–537. 10.1016/j.ijantimicag.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Gaudreau C, Barkati S, Leduc JM, Pilon PA, Favreau J, Bekal S. 2014. Shigella spp. with reduced azithromycin susceptibility, Quebec, Canada, 2012-2013. Emerg Infect Dis 20:854–856. 10.3201/eid2005.130966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darton TC, Tuyen HT, The HC, Newton PN, Dance DAB, Phetsouvanh R, Davong V, Campbell JI, Hoang NVM, Thwaites GE, Parry CM, Thanh DP, Baker S. 2018. Azithromycin resistance in Shigella spp. in Southeast Asia. Antimicrob Agents Chemother 62:e01748-17. 10.1128/AAC.01748-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Li H, Lv N, Zhang Y, Xu X, Ye Y, Gao Y, Li J. 2020. Prevalence of plasmid-mediated determinants with decreased susceptibility to azithromycin among Shigella isolates in Anhui, China. Front Microbiol 11:1181. 10.3389/fmicb.2020.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yousfi K, Gaudreau C, Pilon PA, Lefebvre B, Walker M, Fournier É, Doualla Bell F, Martineau C, Longtin J, Bekal S. 2019. Genetic mechanisms behind the spread of reduced susceptibility to azithromycin in Shigella strains isolated from men who have sex with men in Québec, Canada. Antimicrob Agents Chemother 63:e01679-18. 10.1128/AAC.01679-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell D, Bowen A, Bhatnagar A, McCullough A, Grass J, Chen J, Folster JP. 2020. Identification and characterization of Shigella with decreased susceptibility to azithromycin in the United States, 2005 to 2014. J Glob Antimicrob Resist 21:417–419. 10.1016/j.jgar.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azmi IJ, Khajanchi BK, Akter F, Hasan TN, Shahnaij M, Akter M, Banik A, Sultana H, Hossain MA, Ahmed MK, Faruque SM, Talukder KA. 2014. Fluoroquinolone resistance mechanisms of Shigella flexneri isolated in Bangladesh. PLoS One 9:e102533. 10.1371/journal.pone.0102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung The H, Boinett C, Pham Thanh D, Jenkins C, Weill FX, Howden BP, Valcanis M, De Lappe N, Cormican M, Wangchuk S, Bodhidatta L, Mason CJ, Nguyen TNT, Ha Thanh T, Voong VP, Duong VT, Nguyen PHL, Turner P, Wick R, Ceyssens PJ, Thwaites G, Holt KE, Thomson NR, Rabaa MA, Baker S. 2019. Dissecting the molecular evolution of fluoroquinolone-resistant Shigella sonnei. Nat Commun 10:4828. 10.1038/s41467-019-12823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung The H, Rabaa MA, Pham Thanh D, De Lappe N, Cormican M, Valcanis M, Howden BP, Wangchuk S, Bodhidatta L, Mason CJ, Nguyen Thi Nguyen T, Vu Thuy D, Thompson CN, Phu Huong Lan N, Voong Vinh P, Ha Thanh T, Turner P, Sar P, Thwaites G, Thomson NR, Holt KE, Baker S. 2016. South Asia as a reservoir for the global spread of ciprofloxacin-resistant Shigella sonnei: a cross-sectional study. PLoS Med 13:e1002055. 10.1371/journal.pmed.1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2. Download JCM.01774-21-s0001.pdf, PDF file, 0.1 MB (112.3KB, pdf)
Fig. S1 to S5. Download JCM.01774-21-s0002.pdf, PDF file, 0.6 MB (621.4KB, pdf)