Abstract
Magnetic microfluidics has been gradually recognized as an area of its own. Both conventional microfluidic platforms have incorporated magnetic actuation for microfluidic operation and microscale object manipulation. Nonetheless, there is still much room for improvement after decades of development. In this Perspective, we first provide a quick review of existing magnetic microfluidic platforms with a focus on the magnetic tools and actuation mechanisms. Next, we discuss several emerging technologies, including magnetic microrobots, additive manufacture, and artificial intelligence, and their potential application in the future development of magnetic microfluidics. We believe that these technologies can eventually inspire highly functional magnetic tools for microfluidic manipulation and coordinated microfluidic control at the system level, which eventually drives magnetic microfluidics into an intelligent system for automated experimentation.
INTRODUCTION
After decades of development, microfluidics has become a mature technology widely used in many industries and fundamental research fields. Many mechanisms have been explored and optimized to manipulate fluids and microscale objects in microfluidic systems,1 from the most common pneumatic actuation2 to more specialized magnetic,3 acoustic,4 piezoelectric,5 thermal,6 centrifugal,7 electrokinetic,8 and optical actuation.9 Besides these active actuation methods, surface tension10 and capillary force11 have also been employed to passively drive fluids through microchannels and porous media. Quite frequently, several of these actuation mechanisms are combined to accomplish a certain task.12,13 Among them all, magnetic actuation presents unique advantages. First of all, magnetic actuation allows remote control of fluids and objects in the microfluidic network that is inaccessible by other means. Furthermore, the most common magnetic tool employed for magnetic actuation is magnetic particles, and these magnetic particles could serve multiple purposes. For example, the surface of magnetic particles can be functionalized with biomolecules for cell tagging.14 They can also be coated with a silica or polymer layer and serve as the substrate for solid phase extraction.15,16 A magnetic particle, whether as a separate solid phase in the fluid or in the form of a colloidally stable ferrofluid, is probably the simplest form of magnetic tool for microfluidics.
For more intricate manipulation of fluids and microscale objects, more structurally complex and functionally versatile magnetic tools have been designed. They are usually made of either rigid magnetic alloy (e.g., NiFe) by conventional microengineering methods17 or soft magnetic composite (e.g., Fe3O4 or NdFeB particle-infused silicone rubber) by soft lithography and molding.18,19 These magnetic microtools are able to accelerate fluid mixing,20 regulate flow characteristics,21,22 and sort particles and cells.23 Recently, magnetic soft microrobots with shape-morphing capability have attracted considerable attention in microfluidics. These magnetic microrobots are usually made of soft magnetic composite and then magnetized according to the designed magnetic profile. When exposing to a magnetic field, the body of the magnetic microrobots may move and reshape into a different configuration according to the strength and direction of the external magnetic field for locomotion and objective manipulation. Therefore, these microrobots are capable of dexterous maneuver in fluids by magnetic control, and they have been shown to actuate fluids by mimicking the motion of cilia.24,25 Besides robotics, other advanced technologies, such as additive manufacturing (3D printing)26 and artificial intelligence (AI),27,28 have also been widely adopted in the field of microfluidics, but they have yet to incorporate magnetic elements.
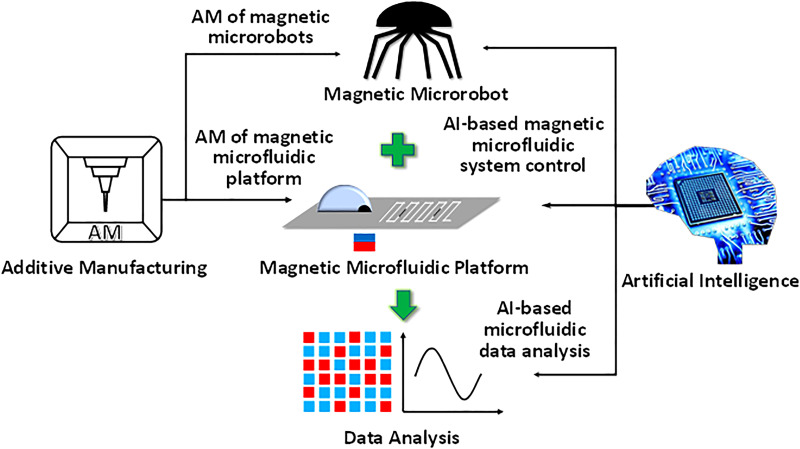
In this Perspective, we first provide a brief review of several representative magnetic tools used for microfluidic manipulation, both in the conventional closed-channel microfluidic platform and open-surface digital microfluidic platform. Next, we examine several magnetic microrobots for their potential in microfluidic manipulation. Finally, we share our opinions on the role of advanced manufacturing and AI technologies in the future development of magnetic microfluidics (Fig. 1).
FIG. 1.
Conceptual illustration of the intelligent magnetic microfluidic system.
MAGNETIC ACTUATION IN CONVENTIONAL CLOSED-CHANNEL MAGNETIC MICROFLUIDIC PLATFORM
In closed-channel microfluidic systems, magnetic particles are primarily used to tag molecules or cells for separation and sorting. For example, when tagged with magnetic particles, specific types of cells or molecules could move across the laminar layer in a magnetic field and concentrate in a particular region of a microfluidic channel, a process known as magnetophoresis. Magnetophoresis has been applied to sorting cancer cells,29 selecting hematopoietic progenitor cells,30 and extracting nucleic acids.31 More in-depth surveys of magnetic separation are available in several review articles published earlier.32–34 Nguyen has made a good summary of different types of interactions between magnetic particles and fluids.35 Depending on the particle size, magnetic fluidic interactions were categorized into ferrohydrodynamics, magnetorheology, and magnetophoresis. Two interesting scenarios are worth special mentioning. The first type is the self-assembly of magnetic particles. When an external magnetic field is applied, magnetic particles can self-assemble into a short chain that rotates together with the applied field.36,37 This rotational motion is used to enhance the efficiency of mixing in the microfluidic system.38,39 Magnetic particles can also be assembled into more complex structures such as rings and pyramids with the help of diamagnetic materials that create patterns with a combination of strong and weak magnetic domains.40,41 Although these large magnetic assemblies do not demonstrate any microfluidic applications yet, such features can potentially be exploited in the future. The second interesting type is ferrofluids that are colloidally stable magnetic nanoparticles with the ability to maintain a homogeneous suspension in a hydrocarbon-based solution.42 Because ferrofluids are immiscible with water and easily deformable, they serve as an excellent actuator of the aqueous solution in convoluted microfluidic networks. For instance, Hatch et al. demonstrated a ferrofluid-enabled rotary micropump.43 This micropump consisted of a circular microchannel partially filled with a ferrofluid plug. The ferrofluid plug was controlled by two permanent magnets. To pump the fluid, the ferrofluid plug was split into two. Initially, all the ferrofluid stayed as a single large plug at the inlet/outlet and a magnet was placed nearby to fix the big ferrofluid plug in place. Another magnet can move freely along the circular channel. When the second passed by the ferrofluid, part of the ferrofluid was dragged out to from a small plug. The parent plug blocked the backflow, while the daughter plug moved in the circular channel to push fluids through the other inlet/outlet. Nguyen further optimized this design by replacing the permanent magnet-based actuation mechanism with an electromagnet array that drove the ferrofluid plug in a way similar to the step motor.44 Ferrofluid was also used as the roller of microperistaltic pumps.45,46 The ferrofluid plug was anchored to the bottom of the microfluidic channel by permanent magnets. An array of electromagnets was placed close to the ceiling of the microfluidic channel. The ferrofluid plug deformed and completely blocked the microfluidic channel once the electromagnet above it was activated. By activating the electromagnet in sequence, the liquid was pumped through the microfluidic channel in a peristaltic manner.
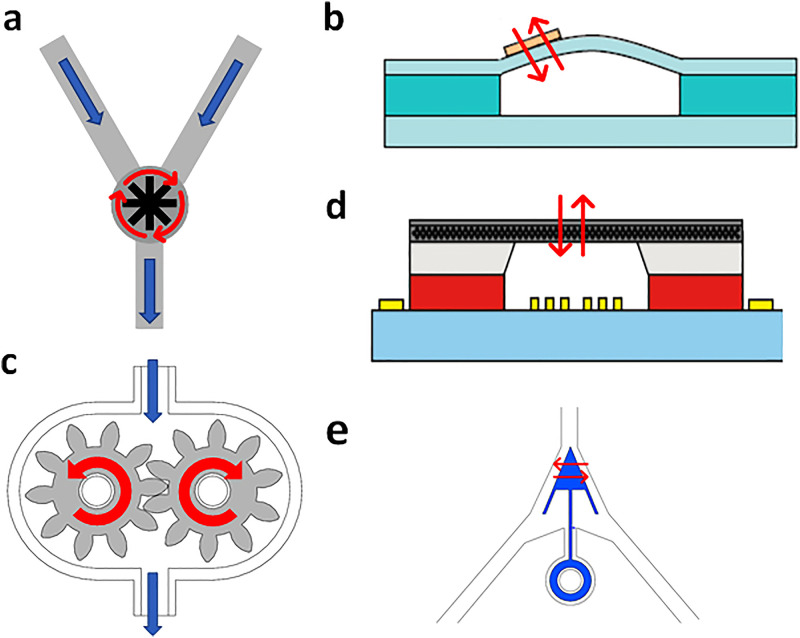
More complex magnetic tools have been introduced to microfluidic systems for intricate microfluidic operations such as mixing, particle sorting, and flow regulation. In silicon-based microfluidic chips, these magnetic tools are usually made of magnetic metals (e.g., iron, cobalt, and nickel) by micromachining techniques such as chemical etching, electroplating, or laser ablation.47 A typical example is a micromagnetic impeller. The impellers are usually located at a junction where two or more fluids converge [Fig. 2(a)]. A rotating magnetic field is required to drive the impeller for microfluidic mixing.48–50 Other types of micromagnetic tools are usually made by depositing magnetic materials to functional components.51,52 For example, Ni et al. deposited the Permalloy on a membrane and controlled the fluctuation of the membrane for microfluidic pumping22 [Fig. 2(b)]. Magnetic shape memory alloy is also used for magnetic microfluidic actuation. Several groups created microfluidic pumps53,54 by making the sidewall of microfluidic channels out of the magnetic shape memory alloy. The microfluidic channels narrowed and sucked in the fluid and returned to its original shape once the magnetic field was removed. With this mechanism, the magnetic microfluidic platform was able to pump the fluid in a peristaltic manner. In polymer-based microfluidic chips, magnetic tools are often made of composites comprising silicone rubber and magnetic particles by soft lithography and molding. A typical application of the soft magnetic tools is micropumps. For instance, a pair of microgears made of magnetic composite is engaged to each other and placed in an enclosed chamber [Fig. 2(c)]. When the gears start to rotate in a rotating magnetic field, the engaging and disengaging the gear teeth lead to a pressure difference for microfluidic pumping.19 Such micromagnetic gears are also used to load microparticles one by one into the microfluidic channels.18,55 Moreover, the flexibility of the polymer allows these micromagnetic tools to undergo reversible deformation, which is a useful feature for membrane-based micropumps56,57 [Fig. 2(d)] or microvalves.58 Another useful application of the soft micromagnetic tool is sorting in microfluidic channels. In the work done by Yamanishi et al., an arrow made of magnetic composite with one end fixed to a Y-junction of the microfluidic channel [Fig. 2(e)] can move left and right under magnetic control to sort microparticles of different sizes.59,60
FIG. 2.
Various magnetic tools used in the conventional closed-channel microfluidic platform. (a) Magnetic metal impeller at the Y-junction of the microfluidic channel. (b) Membrane micropump driven by the attached magnetic metal piece. Reproduced with permission from Ni et al., Microelectron. Eng. 117, 35–40 (2014). Copyright 2014 Elsevier. (c) Micro-gear pump made of magnetic composites. (d) Magnetic soft membrane pump. Reproduced with permission from Said et al., Sens. Actuators, A 245, 85–96 (2016). Copyright 2016 Elsevier. (e) A magnetic composite particle sorter.
MAGNETIC ACTUATION IN MAGNETIC DIGITAL MICROFLUIDIC PLATFORM
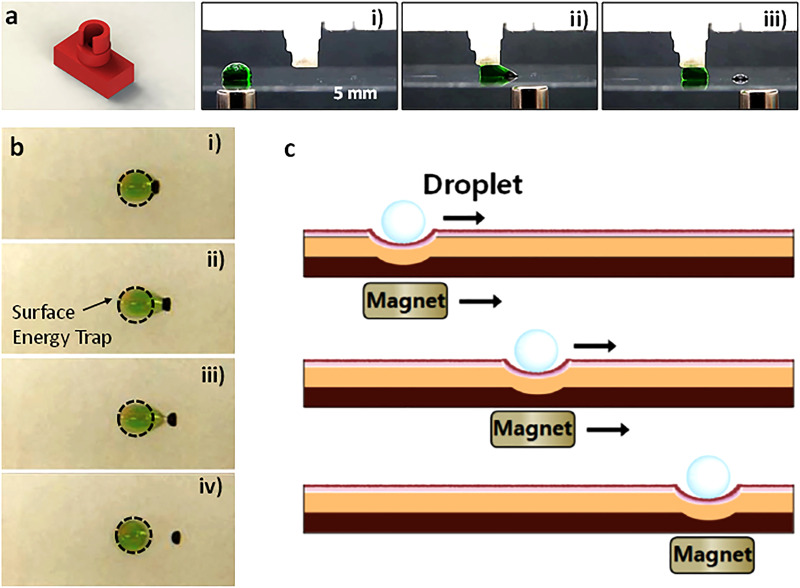
Digital microfluidic systems control liquid in the form of discrete droplets (i.e., digitalized fluid) on an open surface through a number of actuation mechanisms.61 The digital microfluidic system that uses magnetic force as the actuation mechanism is known as magnetic digital microfluidics.3,62 Magnetic particle is the most common form of magnetic actuator in magnetic digital microfluidics that is similar to the conventional magnetic microfluidics. These magnetic particles are often added to the droplets that sit on an open surface. When a magnet below the surface approaches the droplet, the magnetic particles aggregate into a cluster and move with the magnet. Due to surface tension, the magnetic particle cluster can drag the droplet to the desired location. Depending on the speed of the magnetic cluster and droplet-to-particle ratio, or with the help of assistive features such as micropillars and surface energy traps, the magnetic particle cluster could overcome the surface tension and break away from the droplet [Figs. 3(a) and 3(b)]63–67 Another important function of magnetic particles is molecule binding. The surface of magnetic particles can be functionalized to bind proteins, nucleic acids, and other biomolecules for solid phase bioreactions. This unique feature makes magnetic digital microfluidics ideally suited for heterogeneous solid phase assays.16,68–70 Ferrofluid has also been used in magnetic digital microfluidics to actuate droplets in a way similar to magnetic particles except that the ferrofluid remains colloidally stable suspension in the aqueous droplet instead of forming a cluster.71–73 Because ferrofluid is immiscible with the aqueous droplet, it cannot serve as the solid substrate for bioreactions. If the surface of magnetic particles is rendered hydrophobic, the particles would self-assemble on the surface of the aqueous droplet and form a magnetic liquid marble, which moves together with the magnet below the surface.74–76 However, if the magnetic field is too strong,77 all magnetic particles would be stripped off the droplet surface. Similar to ferrofluid, magnetic particles in the liquid marble could not interact with molecules in the aqueous droplet for bioreactions. In some cases, particles may interfere with the bioreactions and signal readout, which is also a problem in the conventional magnetic microfluidics. Therefore, both systems attempted avoid the particle effect by trapping magnetic particles in a polymer matrix. A new type of particle-free magnetic actuation method that uses a flexible substrate impregnated with magnetic materials to manipulate droplets has been proposed [Fig. 3(c)]. A strong permanent magnet below the substrate pulls down a small portion of the substrate to form a dent. If a droplet is nearby, the droplet slips into the dents. The dent follows the movements of the magnet with the droplet trapped inside. Upon the removal of the magnet, the surface becomes flat again due to its flexibility.78,79 The substrate may also take the form of a magnetic pillar array, which manipulates droplets in a way similar to the flat magnetic substrate.80,81 Other magnetic tools such as microspinners are also used in digital microfluidics. A micromagnetic spinner made of polymer-particle composite accelerated mixing in sessile droplets by acting like a gyroscope on the droplet surface.82 It was able to maintain balance on the curved droplet surface due to the conservation of angular momentum while spinning.
FIG. 3.
Various types of magnetic digital microfluidics. (a) Microstructure-assisted magnetic droplet manipulation. Reproduced Kanitthamniyom et al., Microsys. Nanoeng. 6, 48 (2020). Copyright 2020 Author(s), licensed under the Creative Common Attribution (CC BY) License. (b) Surface tension energy trap-assisted magnetic droplet manipulation. Reproduced with permission from Zhang et al., Lab Chip 13, 4827–4831 (2013). Copyright 2013 John Wiley and Sons. (c) Magnetic substrate for droplet manipulation. Reproduced with the permission from Seo et al., Polym. Adv. Technol. 24, 1075–1080 (2013). Copyright 2013 John Wiley and Sons.
So far, quite a number of magnetic tools have been developed for conventional magnetic microfluidics while there are only limited forms of tools for magnetic digital microfluidics. Conventional microfluidics is more mature, and many methods are available for efficient microfluidic manipulation in conventional systems. Therefore, it may be more advantageous to use magnetic tools for the manipulation of micro-objects in conventional systems. In comparison, magnetic actuation is one primary method used for manipulation of digital microfluidics. As digital microfluidics shows many unique advantages in biomedical applications, we believe that more and more technologies will be incorporated in magnetic digital microfluidics that allow precise and automatic manipulation of droplets.
FUTURE DEVELOPMENT OF MAGNETIC ACTUATION FOR MICROFLUIDICS
The aforementioned works have proven the effectiveness and unique advantages of magnetic microfluidics. However, there are limitations to existing magnetic tools that present hurdles to the future development of magnetic microfluidics. The most challenging difficult is manufacturing technology since the size of the magnetic tools is at the scale of millimeter or even sub-millimeter, which usually requires tedious processing steps. As a result, most existing magnetic tools only have simple structures that are not functionally adequate for dexterous maneuver and complex fluidic operations. Although soft lithography seems to solve the problem to some extent, the ability of precise fabrication is constrained to a 2D plane. Therefore, there is demanding work to be done to implement new manufacturing technologies in order to catch up with the development of magnetic microfluidics. Potential methods could be additive manufacturing (here, specifically refer to 3D printing). 3D printing provides a promising way of fabricating complex and intricate structures in high resolution (<1 mm) that match well with the requirements in the fabrication of current magnetic microfluidics. Another critical challenge is that current magnetic microfluidic platform lacks feedback for closed-loop control and system-level integration. The existing control methods for magnetic microfluidics are still based on manual operations by experienced personnel that lacks efficiency and accuracy. Future efforts in this area could be invested in the development of more functionally elaborate magnetic tools such as magnetic microrobots by using 3D printing and AI-based control systems such as computer vision to accomplish intelligent magnetic microfluidics. As we are in the era of AI, future magnetic microfluidics would inevitably comprise an integrated system that includes certain AI components. Advanced fabrication technologies ensure the successful realization of structurally complex magnetic tools that could provide versatile functions. Various control algorithm empowered by the AI allows rapid and accurate results of the experiments conducted by magnetic microfluidics (Fig. 1)
Magnetic microrobots
Magnetic microrobots have demonstrated the capability of handling small objects and dexterous maneuver in confined space.83,84 They can be remotely controlled with high accuracy by adjusting the applied external magnetic field. Many of them are designed to operate in liquid environments, which is ideal for microfluidic applications.85–87 For example, a spherical rigid magnetic microrobot showed the ability to travel against blood flow and deliver drugs to the desired location.88 This microrobot consisted of a Janus microsphere, half of which was coated with magnetic materials and the rest half was functionalized with active compounds for cancer treatment and antibodies for target recognition. The microrobot rolls along well-defined paths and releases the active compound upon light activation once the designated tumor site was reached. This type of magnetic microrobot is useful for the manipulation of microscale objections in conventional closed-channel microfluidic platform as it can be remotely guided to desired locations in the microfluidic network that are hard to access by other means. In addition, its motion is not dictated by the flow streamline. Magnetic microrobots may also find their place in digital microfluidics. Many of these microrobots have shown the ability to lift, carry, and transport small objects with their shape-morphing capability and 6 degree-of-freedom motion.84,89,90 Once rendered hydrophobic, they are envisioned to be able to manipulate liquid droplets not only on a 2D planar surface by also in a 3D space for inter-platform liquid handling. This ability would substantially expand the applicability of magnetic digital microfluidics by enabling the integration of multiple platforms for complex droplet manipulation and bioreactions. Another interesting microrobot appears in the form of a cilia array with carefully designed magnetization profile. By applying a programmable magnetic field, the swing pattern can be well controlled to generate metachronal waves that can transport particles or pump fluid.91 Nonetheless, it must be realized that it is not easy to control many individual magnetic microrobots or other tools in an integrative system as they could affect each other if they are closely spaced. One solution to this problem is to enlarge the space between magnetic components, and another solution is to have only one magnetic component on the platform at a time; other magnetic components are brought to the platform only after the previous magnetic component is removed.
Additive manufacturing of magnetic microrobot
Conventional magnetic microrobots are mostly made by molding. Those microrobots with more intricate geometries are made by assembling pre-fabricated parts, but such an assembly process may prove difficult for microscale components. Advanced manufacturing technologies, such as additive manufacturing (3D printing), offer a potential solution to this problem.92,93 3D printing produces physical parts from digital computer-aided design (CAD) by adding materials layer by layer. It bypasses many design constraints imposed on conventional manufacturing processes and is able to produce complex 3D geometry monolithically and near net shape. In fact, several 3D printing platforms have already been developed to print magnetic composite materials comprising a polymer matrix and a magnetic filler.94,95 Majority of these platforms are based on extrusion with either a viscous fluid or a molten plastic filament as the magnetic “ink”96–98 and some have already been used to fabricate magnetic robots. These robots are typically on the millimeter or centimeter scale99 and show dexterous locomotion enabled by their shape-morphing ability. They also demonstrate the capability of carrying and moving small objects on a planar surface. Although these soft robots are able to transform into 3D structures, they are printed in their respective 2.5D (pseudo-3D) initial configurations. It is relatively easy to develop customized magnetic composite “ink” for extrusion-based 3D printing platforms. However, the accuracy and resolution of extrusion-based printing are not as great as some other 3D printing platforms, such as vat photopolymerization, due to the moving printing head. Vat photopolymerization creates 3D geometries by selectively solidifying UV-curable resins layer by layer with a UV light source and is able to reach sub-micrometer resolution with a two-photon light source.100 Several groups have successfully fabricated micrometer scale magnetic robots with vat photopolymerization.101–103 For example, a magnetic enzyme-degradable swimming microrobot was fabricated by 3D printing a UV-curable gelatin-based resin with two-photon polymerization.104 The microrobot was rendered magnetic by adding Fe3O4 nanoparticles to the resin. Under magnetic control, it was able to swim to the target location and release contrast agent for imaging upon enzymatic degradation. A similar magnetic microswimmer was 3D printed by two-photon polymerization with Zwitterionic resins supplemented with magnetic particles.105 It was able to navigate through complex bioenvironment by tricking the immune system with its Zwitterionic body and deliver various drugs to the target site. Compared to conventional molding method and extrusion-based 3D printing, the magnetic particle loading in microrobots fabricated by vat photopolymerization is substantially lower. The main reason is due to the strong interference of magnetic nanoparticles with the UV light source used to cure the resin.106 Although larger particles reduce the light interference, their rapid sedimentation in the liquid resin may result in non-uniform distribution of magnetic particles in the printed parts or cause printing failure.107 Despite these limitations, vat photopolymerization is likely the most promising tool that may replace certain microfabrication processes.
As the tasks performed on magnetic microfluidic platforms become more and more demanding, magnetic actuation tools required to meet these demands would likely experience growing complexity. More functional magnetic microrobots will need to be developed to provide a full range of fluidic operations. Indeed, their role may go beyond fluidic manipulation and serve more versatile functions that enable experiment automation in microfluidic platforms. One major trend in future fabrication technology is digital manufacturing represented by technologies such as 3D printing. This is because 3D printing lifts the design constraints and enables near-net-shape fabrication of complex geometries in a fully automated manner. We envision that 3D printing would eventually become one mainstream method of fabricating magnetic microfluidic platforms in the future. However, more effort needs to be invested in the development of 3D printing-compatible magnetic composite materials, especially those for vat photopolymerization, in order to fabricate magnetic microrobots and other micro-actuation tools with accurate microfeatures and strong magnetic response.
AI for magnetic microfluidics
AI has been a main driving force for technology advancement in recent years. It is basically computer programs that think and act like humans. Propelled by the accelerating computational speed, deep learning algorithms, such as artificial neuron network (ANN), have become the core of AI systems nowadays.108 A vast range of intelligent systems have been proposed by incorporating AI in certain ways, and microfluidics is no exception. Many existing microfluidic platforms employ AI, primarily, weak AI that focuses on specific tasks such as chip design, system control, data analysis, and outcome prediction. For instance, an AI-empowered design tool for droplet microfluidics, known as DAFD, was developed to automate the design of microfluidic droplet generator chip by correctly predicting the droplet diameter and droplet generation rate with an error of 4.2% and 11.5%, respectively.109 AI has become a popular tool to control and predict the outcome of synthesis processes in microfluidic platforms. An ANN model was trained to predict the size of poly(d,l-lactide-co-glycolide) (PLGA) microparticles synthesized by several different microfluidic systems with a high accuracy based on the polymer concentration and the flow rate.27 AI was also used to generate de novo chemical candidates for microfluidic drug screening.110 Computer vision AI models are especially helpful for droplet microfluidics as they can recognize and track droplets and extract useful information such as droplet composition, flow rate, and other fluidic properties based on a video in real time.111 AI velocimetry was developed to analyze blood flow in a microfluidic aneurysm-on-chip model.112 The ANN-based AI model was able to deduce the flow field based on the images of blood flow in the microfluidic chip without knowing the boundary conditions at the inlet and outlet. Microfluidic systems are known for their ability to facilitate high-throughput and high-content analysis, which could provide abundant clean data to train AI models for diagnostics and other bioanalyses. For instance, by coupling microfluidics with ANN for object detection, a microfluidic imaging flow cytometer was capable of identifying and sorting blood cells at 100 cell/s without the need of staining.113 A similar imaging flow cytometer with optimized efficient ANN was able to analyze >340 images/s with an accuracy over 99%.114 An AI-assisted microfluidic single-cell analysis chip was demonstrated for real-time cell classification based on impedance signals115 and was envisioned to have potential applications in tumor heterogeneity studies and point-of-care cancer diagnostics.
AI has gradually become commonplace in microfluidics. Nonetheless, magnetic microfluidic platforms have yet to take the full advantage of AI. In fact, magnetic microfluidics has some unique features that would facilitate AI implementation. One such feature is the visually identifiable microfluidic components such as magnetic particles and droplets. Most magnetic materials used for magnetic actuation are either black or brown in color with a strong contrast against the background and, hence, are easily identifiable by computer vision models. Droplets appear as discrete features that can also be easily recognized by AIs. Furthermore, magnetic microfluidics could take the full advantage of AI for much needed system-level control. Conventional microfluidic systems can be controlled by just one parameter (i.e., pressure or flow rate. The rest flow profile is dictated by the microfluidic network.), magnetic microfluidics requires additional parameters to control magnetic tools for fluidic actuation and object manipulation. At the moment, there lacks coordinated control of these parameters at the system-level, partly, due to the lack of closed-loop feedback in our opinion. AI can be trained to automatically identify magnetic tools and droplets and determine their locations and configurations, thereby providing closed-loop control based on these data to realize experiment automation.
CONCLUSION
In this Perspective, we share our view on the possible future development of magnetic microfluidics. First, we have reviewed existing technologies for magnetic microfluidics, particularly magnetic actuation mechanisms, for both conventional closed-channel microfluidics and digital microfluidics. Next, we have examined several emerging technologies, including magnetic microrobot, 3D printing, and AI, which promise great potential of improving functionality of magnetic microfluidic platforms. In our humble opinion, we believe that magnetic microfluidics will evolve into an intelligent system that implements digital manufacturing, robotic manipulation, AI-enabled system control, and data analysis for fully automated device fabrication and experimentation, which will significantly broaden the accessibility and applicability of magnetic microfluidic technology in chemical synthesis, clinical diagnostics, high-throughput screening, and other impactful areas. We encourage the scientific community to work together toward this direction and hope what we envision will turn into a reality in a very near future. However, we should at the same time be aware that AI and 3D printing are probably at the peak of the inflated expectation in Gartner's hype cycle. Therefore, one must be fully aware of the limitations of these technologies and implement them only when it makes sense.
ACKNOWLEDGMENTS
Yi Zhang would like to acknowledge the funding support from the University of Electronic Science and Technology of China.
Contributor Information
Yi Zhang, Email: mailto:yi_zhang@uestc.edu.cn.
Guo Zhan Lum, Email: mailto:gzlum@ntu.edu.sg.
Xiaosheng Zhang, Email: mailto:zhangxs@uestc.edu.cn.
AUTHOR DECLARATIONS
Conflict of Interest
Yi Zhang and Songlin Chen declare equity interest in DropLab Scientific (Singapore) Pvt. Ltd and Guangzhou DropLab Scientific Co., Ltd. The rest of the authors declare no conflict of interest.
Author Contributions
Y.Z. and A.Z. contributed equally to this work.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Rezk A., Friend J., Yeo L., and Zhou Y., Microfluidic Devices for Biomedical Applications (Elsevier, 2021), pp. 125–162. [Google Scholar]
- 2.Hess J. F. et al. , “Review on pneumatic operations in centrifugal microfluidics,” Lab Chip 19, 3745–3770 (2019). 10.1039/C9LC00441F [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y. and Nguyen N. T., “Magnetic digital microfluidics—A review,” Lab Chip 17, 994–1008 (2017). 10.1039/C7LC00025A [DOI] [PubMed] [Google Scholar]
- 4.Ding X. et al. , “Surface acoustic wave microfluidics,” Lab Chip 13, 3626–3649 (2013). 10.1039/c3lc50361e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shemesh J., Bransky A., Khoury M., and Levenberg S., “Advanced microfluidic droplet manipulation based on piezoelectric actuation,” Biomed. Microdevices 12, 907–914 (2010). 10.1007/s10544-010-9445-y [DOI] [PubMed] [Google Scholar]
- 6.Vigolo D., Rusconi R., Stone H. A., and Piazza R., “Thermophoresis microfluidics characterization and separation,” Soft Matter 6, 3489 (2010). 10.1039/c002057e [DOI] [Google Scholar]
- 7.Gorkin R. et al. , “Centrifugal microfluidics for biomedical applications,” Lab Chip 10, 1758–1773 (2010). 10.1039/b924109d [DOI] [PubMed] [Google Scholar]
- 8.Chang C.-C. and Yang R.-J., “Electrokinetic mixing in microfluidic systems,” Microfluidics Nanofluidics 3, 501–525 (2007). 10.1007/s10404-007-0178-z [DOI] [Google Scholar]
- 9.Enger J., Goksor M., Ramser K., Hagberg P., and Hanstorp D., “Optical tweezers applied to a microfluidic system,” Lab Chip 4, 196–200 (2004). 10.1039/B307960K [DOI] [PubMed] [Google Scholar]
- 10.You I., Yun N., and Lee H., “Surface-tension-confined microfluidics and their applications,” Chemphyschem 14, 471–481 (2013). 10.1002/cphc.201200929 [DOI] [PubMed] [Google Scholar]
- 11.Berthier E., Dostie A. M., Lee U. N., Berthier J., and Theberge A. B., “Open microfluidic capillary systems,” Anal. Chem. 91, 8739–8750 (2019). 10.1021/acs.analchem.9b01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godino N., Comaskey E., Gorkin R., and Ducrée J., in 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS) (IEEE, 2012), 1017–1020. [Google Scholar]
- 13.Chen H. et al. , “Multiplexed detection of cancer biomarkers using a microfluidic platform integrating single bead trapping and acoustic mixing techniques,” Nanoscale 10, 20196–20206 (2018). 10.1039/C8NR06367B [DOI] [PubMed] [Google Scholar]
- 14.Alnaimat F., Dagher S., Mathew B., Hilal-Alnqbi A., and Khashan S., “Microfluidics based magnetophoresis: A review,” Chem. Rec. 18, 1596–1612 (2018). 10.1002/tcr.201800018 [DOI] [PubMed] [Google Scholar]
- 15.Pereiro I. et al. , “Magnetic fluidized bed for solid phase extraction in microfluidic systems,” Lab Chip 17, 1603–1615 (2017). 10.1039/C7LC00063D [DOI] [PubMed] [Google Scholar]
- 16.Lee H. et al. , “Sample preparation of chemical warfare agent simulants on a digital microfluidic (DMF) device using magnetic bead-based solid-phase extraction,” Microfluidics Nanofluidics 21, 1–11 (2017), 10.1007/s10404-017-1976-6 [DOI] [Google Scholar]
- 17.Capanu M., Boyd J. G., and Hesketh P. J., “Design, fabrication, and testing of a bistable electromagnetically actuated microvalve,” J. Microelectromech. Syst. 9, 181–189 (2000). 10.1109/84.846698 [DOI] [Google Scholar]
- 18.Yoko Y., Yu-Ching L., and Fumihito A., in 2007 IEEE/RSJ International Conference on Intelligent Robots and Systems (IEEE, 2007), pp. 753–758. [Google Scholar]
- 19.Waldschik A. and Büttgenbach S., “Micro gear pump with internal electromagnetic drive,” Microsyst. Technol. 16, 1581–1587 (2010). 10.1007/s00542-010-1028-6 [DOI] [Google Scholar]
- 20.Shanko E. S., van de Burgt Y., Anderson P. D., and den Toonder J. M. J., “Microfluidic magnetic mixing at low Reynolds numbers and in stagnant fluids,” Micromachines (Basel) 10, 731 (2019), 10.3390/mi10110731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casals-Terré J. et al. , “Design, fabrication and characterization of an externally actuated ON/OFF microvalve,” Sens. Actuators, A 147, 600–606 (2008). 10.1016/j.sna.2008.06.022 [DOI] [Google Scholar]
- 22.Ni J., Wang B., Chang S., and Lin Q., “An integrated planar magnetic micropump,” Microelectron. Eng. 117, 35–40 (2014). 10.1016/j.mee.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert D. et al. , “Cell sorting by endocytotic capacity in a microfluidic magnetophoresis device,” Lab Chip 11, 1902–1910 (2011). 10.1039/c0lc00656d [DOI] [PubMed] [Google Scholar]
- 24.Hanasoge S., Hesketh P. J., and Alexeev A., “Microfluidic pumping using artificial magnetic cilia,” Microsyst. Nanoeng. 4, 11 (2018). 10.1038/s41378-018-0010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.den Toonder J. M. and Onck P. R., “Microfluidic manipulation with artificial/bioinspired cilia,” Trends Biotechnol. 31, 85–91 (2013). 10.1016/j.tibtech.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharjee N., Urrios A., Kang S., and Folch A., “The upcoming 3D-printing revolution in microfluidics,” Lab Chip 16, 1720–1742 (2016). 10.1039/C6LC00163G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damiati S. A., Rossi D., Joensson H. N., and Damiati S., “Artificial intelligence application for rapid fabrication of size-tunable PLGA microparticles in microfluidics,” Sci. Rep. 10, 19517 (2020). 10.1038/s41598-020-76477-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galan E. A. et al. , “Intelligent microfluidics: The convergence of machine learning and microfluidics in materials science and biomedicine,” Matter 3, 1893–1922 (2020). 10.1016/j.matt.2020.08.034 [DOI] [Google Scholar]
- 29.Pamme N. and Wilhelm C., “Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis,” Lab Chip 6, 974–980 (2006). 10.1039/b604542a [DOI] [PubMed] [Google Scholar]
- 30.Jing Y. et al. , “Negative selection of hematopoietic progenitor cells by continuous magnetophoresis,” Exp. Hematol. 35, 662–672 (2007). 10.1016/j.exphem.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 31.Karle M. et al. , “Continuous microfluidic DNA extraction using phase-transfer magnetophoresis,” Lab Chip 10, 3284–3290 (2010). 10.1039/c0lc00129e [DOI] [PubMed] [Google Scholar]
- 32.Surendran A. N., Zhou R., and Lin Y., “Microfluidic devices for magnetic separation of biological particles: A review,” J. Med. Devices 15, 024001 (2020), 10.1115/1.4048912 [DOI] [Google Scholar]
- 33.Sajeesh P. and Sen A. K., “Particle separation and sorting in microfluidic devices: A review,” Microfluidics Nanofluidics 17, 1–52 (2013). 10.1007/s10404-013-1291-9 [DOI] [Google Scholar]
- 34.Hejazian M., Li W., and Nguyen N. T., “Lab on a chip for continuous-flow magnetic cell separation,” Lab Chip 15, 959–970 (2015). 10.1039/C4LC01422G [DOI] [PubMed] [Google Scholar]
- 35.Nguyen N.-T., “Micro-magnetofluidics: Interactions between magnetism and fluid flow on the microscale,” Microfluidics Nanofluidics 12, 1–16 (2011). 10.1007/s10404-011-0903-5 [DOI] [Google Scholar]
- 36.Roy T., Sinha A., Chakraborty S., Ganguly R., and Puri I. K., “Magnetic microsphere-based mixers for microdroplets,” Phys. Fluids 21, 027101 (2009), 10.1063/1.3072602 [DOI] [Google Scholar]
- 37.Petousis I., Homburg E., Derks R., and Dietzel A., “Transient behaviour of magnetic micro-bead chains rotating in a fluid by external fields,” Lab Chip 7, 1746–1751 (2007). 10.1039/b713735b [DOI] [PubMed] [Google Scholar]
- 38.Chong W. H., Huang Y., Wong T. N., Ooi K. T., and Zhu G.-P., “Magnetic nanorobots, generating vortexes inside nanoliter droplets for effective mixing,” Adv. Mater. Technol. 3, 1700312 (2018). 10.1002/admt.201700312 [DOI] [Google Scholar]
- 39.Lee S. H., van Noort D., Lee J. Y., Zhang B. T., and Park T. H., “Effective mixing in a microfluidic chip using magnetic particles,” Lab Chip 9, 479–482 (2009). 10.1039/B814371D [DOI] [PubMed] [Google Scholar]
- 40.Helseth L. E., “Self-Assembly of colloidal pyramids in magnetic fields,” Langmuir 21, 7276–7279 (2005). 10.1021/la051140v [DOI] [PubMed] [Google Scholar]
- 41.Helseth L. E., Muruganathan R. M., Zhang Y., and Fischer T. M., “Colloidal rings in a liquid mixture,” Langmuir 21, 7271–7275 (2005). 10.1021/la050247f [DOI] [PubMed] [Google Scholar]
- 42.Jamshaid T. et al. , “Magnetic particles: From preparation to lab-on-a-chip, biosensors, microsystems and microfluidics applications,” TrAC, Trends Anal. Chem. 79, 344–362 (2016). 10.1016/j.trac.2015.10.022 [DOI] [Google Scholar]
- 43.Hatch A., Kamholz A. E., Holman G., Yager P., and Bohringer K. F., “A ferrofluidic magnetic micropump,” J. Microelectromech. Syst. 10, 215–221 (2001). 10.1109/84.925748 [DOI] [Google Scholar]
- 44.Nguyen N.-T. and Chai M.-F., “A stepper micropump for ferrofluid driven microfluidic systems,” Micro Nanosyst. 1, 17–21 (2009). 10.2174/1876402910901010017 [DOI] [Google Scholar]
- 45.Andò B., Ascia A., Baglio S., and Beninato A., “The ‘One drop’ ferrofluidic pump with analog control,” Sens. Actuators, A 156, 251–256 (2009). 10.1016/j.sna.2009.05.006 [DOI] [Google Scholar]
- 46.Ando B., Ascia A., Baglio S., and Pitrone N., “Ferrofluidic pumps: A valuable implementation without moving parts,” IEEE Trans. Instrum. Meas. 58, 3232–3237 (2009). 10.1109/TIM.2009.2017167 [DOI] [Google Scholar]
- 47.Niculescu A. G., Chircov C., Birca A. C., and Grumezescu A. M., “Fabrication and applications of microfluidic devices: A review,” Int. J. Mol. Sci. 22, 2011 (2021). 10.3390/ijms22042011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mensing G. A., Pearce T. M., Graham M. D., and Beebe D. J., “An externally driven magnetic microstirrer,” Philos. Trans. R. Soc. London, A 362, 1059–1068 (2004). 10.1098/rsta.2003.1362 [DOI] [PubMed] [Google Scholar]
- 49.Liang-Hsuan L., Kee Suk R., and Chang L., “A magnetic microstirrer and array for microfluidic mixing,” J. Microelectromech. Syst. 11, 462–469 (2002). 10.1109/JMEMS.2002.802899 [DOI] [Google Scholar]
- 50.Ryu K. S., Shaikh K., Goluch E., Fan Z., and Liu C., “Micro magnetic stir-bar mixer integrated with parylene microfluidic channels,” Lab Chip 4, 608–613 (2004). 10.1039/b403305a [DOI] [PubMed] [Google Scholar]
- 51.Pradeep A., Vineeth Raj S., Stanley J., Nair B. G., and Babu T. G. S., “Automated and programmable electromagnetically actuated valves for microfluidic applications,” Sens. Actuators, A 283, 79–86 (2018). 10.1016/j.sna.2018.09.024 [DOI] [Google Scholar]
- 52.Shen M., Dovat L., and Gijs M. A. M., “Magnetic active-valve micropump actuated by a rotating magnetic assembly,” Sens. Actuators, B 154, 52–58 (2011). 10.1016/j.snb.2009.10.033 [DOI] [Google Scholar]
- 53.Ullakko K., Wendell L., Smith A., Müllner P., and Hampikian G., “A magnetic shape memory micropump: Contact-free, and compatible with PCR and human DNA profiling,” Smart Mater. Struct. 21, 115020 (2012). 10.1088/0964-1726/21/11/115020 [DOI] [Google Scholar]
- 54.Saren A., Smith A. R., and Ullakko K., “Integratable magnetic shape memory micropump for high-pressure, precision microfluidic applications,” Microfluidics Nanofluidics 22, 1–10 (2018). 10.1007/s10404-018-2058-0 [DOI] [Google Scholar]
- 55.Yamanishi Y., Sakuma S., Kihara Y., and Arai F., “Fabrication and application of 3-D magnetically driven microtools,” J. Microelectromech. Syst. 19, 350–356 (2010). 10.1109/JMEMS.2010.2041188 [DOI] [Google Scholar]
- 56.Wu J. H., Wang C. H., Ma Y. D., and Lee G. B., “A nitrocellulose membrane-based integrated microfluidic system for bacterial detection utilizing magnetic-composite membrane microdevices and bacteria-specific aptamers,” Lab Chip 18, 1633–1640 (2018). 10.1039/C8LC00251G [DOI] [PubMed] [Google Scholar]
- 57.Said M. M., Yunas J., Pawinanto R. E., Majlis B. Y., and Bais B., “PDMS based electromagnetic actuator membrane with embedded magnetic particles in polymer composite,” Sens. Actuators, A 245, 85–96 (2016). 10.1016/j.sna.2016.05.007 [DOI] [Google Scholar]
- 58.Singh A., Hirsinger L., Delobelle P., and Khan-Malek C., “Rapid prototyping of magnetic valve based on nanocomposite Co/PDMS membrane,” Microsyst. Technol. 20, 427–436 (2013). 10.1007/s00542-013-1972-z [DOI] [Google Scholar]
- 59.Yamanishi Y., Sakuma S., Onda K., and Arai F., “Powerful actuation of magnetized microtools by focused magnetic field for particle sorting in a chip,” Biomed. Microdevices 12, 745–752 (2010). 10.1007/s10544-010-9428-z [DOI] [PubMed] [Google Scholar]
- 60.Yamanishi Y., Sakuma S., Onda K., and Arai F., “Biocompatible polymeric magnetically driven microtool for particle sorting,” J. Micro-Nano Mechatron. 4, 49–57 (2008). 10.1007/s12213-008-0009-7 [DOI] [Google Scholar]
- 61.Choi K., Ng A. H., Fobel R., and Wheeler A. R., “Digital microfluidics,” Annu. Rev. Anal. Chem. (Palo Alto Calif) 5, 413–440 (2012). 10.1146/annurev-anchem-062011-143028 [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y., “Magnetic digital microfluidics for point-of-care testing: Where are we now?,” Curr. Med. Chem. 28, 6323 (2020), 10.2174/0929867327666200903115448 [DOI] [PubMed] [Google Scholar]
- 63.Long Z., Shetty A. M., Solomon M. J., and Larson R. G., “Fundamentals of magnet-actuated droplet manipulation on an open hydrophobic surface,” Lab Chip 9, 1567–1575 (2009). 10.1039/b819818g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanitthamniyom P. and Zhang Y., “Magnetic digital microfluidics on a bioinspired surface for point-of-care diagnostics of infectious disease,” Electrophoresis 40, 1178–1185 (2019). 10.1002/elps.201900074 [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y., Shin D. J., and Wang T. H., “Serial dilution via surface energy trap-assisted magnetic droplet manipulation,” Lab Chip 13, 4827–4831 (2013). 10.1039/c3lc50915j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanitthamniyom P. et al. , “A 3D-printed modular magnetic digital microfluidic architecture for on-demand bioanalysis,” Microsyst. Nanoeng. 6, 48 (2020). 10.1038/s41378-020-0152-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y. and Wang T. H., “Full-range magnetic manipulation of droplets via surface energy traps enables complex bioassays,” Adv. Mater. 25, 2903–2908 (2013). 10.1002/adma.201300383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pipper J., Zhang Y., Neuzil P., and Hsieh T.-M., “Clockwork PCR including sample preparation,” Angew. Chem. 120, 3964–3968 (2008). 10.1002/ange.200705016 [DOI] [PubMed] [Google Scholar]
- 69.Chiou C. H., Jin Shin D., Zhang Y., and Wang T. H., “Topography-assisted electromagnetic platform for blood-to-PCR in a droplet,” Biosens. Bioelectron. 50, 91–99 (2013). 10.1016/j.bios.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin D. J., Zhang Y., and Wang T.-H., “A droplet microfluidic approach to single-stream nucleic acid isolation and mutation detection,” Microfluidics Nanofluidics 17, 425–430 (2014). 10.1007/s10404-013-1305-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan S.-H., Nguyen N.-T., Yobas L., and Kang T. G., “Formation and manipulation of ferrofluid droplets at a microfluidic T-junction,” J. Micromech. Microeng. 20, 045004 (2010). 10.1088/0960-1317/20/4/045004 [DOI] [Google Scholar]
- 72.Latikka M. et al. , “Ferrofluid microdroplet splitting for population-based microfluidics and interfacial tensiometry,” Adv. Sci. (Weinh) 7, 2000359 (2020). 10.1002/advs.202000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bijarchi M. A., Favakeh A., Sedighi E., and Shafii M. B., “Ferrofluid droplet manipulation using an adjustable alternating magnetic field,” Sens. Actuators, A 301, 111753 (2020). 10.1016/j.sna.2019.111753 [DOI] [Google Scholar]
- 74.Zhao Y. et al. , “Magnetic liquid marbles, their manipulation and application in optical probing,” Microfluidics Nanofluidics 13, 555–564 (2012). 10.1007/s10404-012-0976-9 [DOI] [Google Scholar]
- 75.Zhang L., Cha D., and Wang P., “Remotely controllable liquid marbles,” Adv. Mater. 24, 4756–4760 (2012). 10.1002/adma.201201885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue Y. et al. , “Magnetic liquid marbles: A ‘precise’ miniature reactor,” Adv. Mater. 22, 4814–4818 (2010). 10.1002/adma.201001898 [DOI] [PubMed] [Google Scholar]
- 77.Zhao Y., Fang J., Wang H., Wang X., and Lin T., “Magnetic liquid marbles: Manipulation of liquid droplets using highly hydrophobic Fe3O4 nanoparticles,” Adv. Mater. 22, 707–710 (2010). 10.1002/adma.200902512 [DOI] [PubMed] [Google Scholar]
- 78.Biswas S., Pomeau Y., and Chaudhury M. K., “New drop fluidics enabled by magnetic-field-mediated elastocapillary transduction,” Langmuir 32, 6860–6870 (2016). 10.1021/acs.langmuir.6b01782 [DOI] [PubMed] [Google Scholar]
- 79.Seo K. S., Wi R., Im S. G., and Kim D. H., “A superhydrophobic magnetic elastomer actuator for droplet motion control,” Polym. Adv. Technol. 24, 1075–1080 (2013). 10.1002/pat.3190 [DOI] [Google Scholar]
- 80.Zhou Q., Ristenpart W. D., and Stroeve P., “Magnetically induced decrease in droplet contact angle on nanostructured surfaces,” Langmuir 27, 11747–11751 (2011). 10.1021/la2024633 [DOI] [PubMed] [Google Scholar]
- 81.Wang L. et al. , “Dynamic magnetic responsive wall array with droplet shedding-off properties,” Sci. Rep. 5, 11209 (2015). 10.1038/srep11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y. and Wang T. H., “Micro magnetic gyromixer for speeding up reactions in droplets,” Microfluid Nanofluidics 12, 787–794 (2012). 10.1007/s10404-011-0922-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lum G. Z. et al. , “Shape-programmable magnetic soft matter,” Proc. Natl. Acad. Sci. U.S.A. 113, E6007–E6015 (2016). 10.1073/pnas.1608193113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu W., Lum G. Z., Mastrangeli M., and Sitti M., “Small-scale soft-bodied robot with multimodal locomotion,” Nature 554, 81–85 (2018). 10.1038/nature25443 [DOI] [PubMed] [Google Scholar]
- 85.Fan X. et al. , “Automated noncontact micromanipulation using magnetic swimming microrobots,” IEEE Trans. Nanotechnol. 17, 666–669 (2018). 10.1109/TNANO.2018.2797325 [DOI] [Google Scholar]
- 86.Peyer K. E., Zhang L., and Nelson B. J., “Bio-inspired magnetic swimming microrobots for biomedical applications,” Nanoscale 5, 1259–1272 (2013). 10.1039/C2NR32554C [DOI] [PubMed] [Google Scholar]
- 87.Li D., Liu Y., Yang Y., and Shen Y., “A fast and powerful swimming microrobot with a serrated tail enhanced propulsion interface,” Nanoscale 10, 19673–19677 (2018). 10.1039/C8NR04907F [DOI] [PubMed] [Google Scholar]
- 88.Alapan Y., Bozuyuk U., Erkoc P., Karacakol C. A., and Sitti M., “Multifunctional surface microrollers for targeted cargo delivery in physiological blood flow,” Sci. Robot. 5, eaba5726 (2020). 10.1126/scirobotics.aba5726 [DOI] [PubMed] [Google Scholar]
- 89.Xu C., Yang Z., and Lum G. Z., “Magnetic robots: Small-scale magnetic actuators with optimal six degrees-of-freedom (Adv. Mater. 23/2021),” Adv. Mater. 33, 2170176 (2021). 10.1002/adma.202170176 [DOI] [PubMed] [Google Scholar]
- 90.Sitti M. et al. , “Biomedical applications of untethered mobile milli/microrobots,” Proc. IEEE 103, 205–224 (2015). 10.1109/JPROC.2014.2385105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dong X. et al. , “Bioinspired cilia arrays with programmable nonreciprocal motion and metachronal coordination,” Sci. Adv. 6, eabc9323 (2020). 10.1126/sciadv.abc9323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J. and Pumera M., “3D printing of functional microrobots,” Chem. Soc. Rev. 50, 2794–2838 (2021). 10.1039/D0CS01062F [DOI] [PubMed] [Google Scholar]
- 93.Gul J. Z. et al. , “3D printing for soft robotics—A review,” Sci. Technol. Adv. Mater. 19, 243–262 (2018). 10.1080/14686996.2018.1431862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang F., Wang L., Zheng Z., Liu Y., and Leng J., “Magnetic programming of 4D printed shape memory composite structures,” Compos. Part A: Appl. Sci. Manuf. 125, 105571 (2019), 10.1016/j.compositesa.2019.105571 [DOI] [Google Scholar]
- 95.Zhu P. et al. , “4D printing of complex structures with a fast response time to magnetic stimulus,” ACS Appl. Mater. Interfaces 10, 36435–36442 (2018). 10.1021/acsami.8b12853 [DOI] [PubMed] [Google Scholar]
- 96.Saleh E. et al. , “3D inkjet-printed UV-curable inks for multi-functional electromagnetic applications,” Addit. Manuf. 13, 143–148 (2017). 10.1016/j.addma.2016.10.002 [DOI] [Google Scholar]
- 97.Wang Y., Castles F., and Grant P. S., “3D printing of NiZn ferrite/ABS magnetic composites for electromagnetic devices,” MRS Proc. 1788, 29–35 (2015). 10.1557/opl.2015.661 [DOI] [Google Scholar]
- 98.Palmero E. M. et al. , “Development of permanent magnet MnAlC/polymer composites and flexible filament for bonding and 3D-printing technologies,” Sci. Technol. Adv. Mater. 19, 465–473 (2018). 10.1080/14686996.2018.1471321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim Y., Yuk H., Zhao R., Chester S. A., and Zhao X., “Printing ferromagnetic domains for untethered fast-transforming soft materials,” Nature 558, 274–279 (2018). 10.1038/s41586-018-0185-0 [DOI] [PubMed] [Google Scholar]
- 100.Zhou X., Hou Y., and Lin J., “A review on the processing accuracy of two-photon polymerization,” AIP Adv. 5, 030701 (2015). 10.1063/1.4916886 [DOI] [Google Scholar]
- 101.Suter M. et al. , “Superparamagnetic microrobots: Fabrication by two-photon polymerization and biocompatibility,” Biomed. Microdevices 15, 997–1003 (2013). 10.1007/s10544-013-9791-7 [DOI] [PubMed] [Google Scholar]
- 102.Xia H. et al. , “Ferrofluids for fabrication of remotely controllable micro-nanomachines by two-photon polymerization,” Adv. Mater. 22, 3204–3207 (2010). 10.1002/adma.201000542 [DOI] [PubMed] [Google Scholar]
- 103.Lin Y. and Xu J., “Microstructures fabricated by two-photon polymerization and their remote manipulation techniques: Toward 3D printing of micromachines,” Adv. Opt. Mater. 6, 1701359 (2018), 10.1002/adom.201701359 [DOI] [Google Scholar]
- 104.Ceylan H. et al. , “3D-Printed biodegradable microswimmer for theranostic cargo delivery and release,” ACS Nano 13, 3353–3362 (2019). 10.1021/acsnano.8b09233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cabanach P. et al. , “Zwitterionic 3D-printed non-immunogenic stealth microrobots,” Adv. Mater. 32, 2003013 (2020). 10.1002/adma.202003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Niskanen I. et al. , “Determination of nanoparticle size using Rayleigh approximation and Mie theory,” Chem. Eng. Sci. 201, 222–229 (2019). 10.1016/j.ces.2019.02.020 [DOI] [Google Scholar]
- 107.Löwa N. et al. , “3D-printing of novel magnetic composites based on magnetic nanoparticles and photopolymers,” J. Magn. Magn. Mater. 469, 456–460 (2019). 10.1016/j.jmmm.2018.08.073 [DOI] [Google Scholar]
- 108.Brunette E. S., Flemmer R. C., and Flemmer C. L., in 2009 4th International Conference on Autonomous Robots and Agents (IEEE, Wellington, New Zealand), pp. 385–392. [Google Scholar]
- 109.Lashkaripour A. et al. , “Machine learning enables design automation of microfluidic flow-focusing droplet generation,” Nat. Commun. 12, 25 (2021). 10.1038/s41467-020-20284-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grisoni F. et al. , “Combining generative artificial intelligence and on-chip synthesis for de novo drug design,” Sci. Adv. 7, eabg3338 (2021). 10.1126/sciadv.abg3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hadikhani P., Borhani N., H. Hashemi S. M., and Psaltis D., “Learning from droplet flows in microfluidic channels using deep neural networks,” Sci. Rep. 9, 8114 (2019). 10.1038/s41598-019-44556-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cai S. et al. , “Artificial intelligence velocimetry and microaneurysm-on-a-chip for three-dimensional analysis of blood flow in physiology and disease,” Proc. Natl. Acad. Sci. U.S.A. 118, e2100697118 (2021), 10.1073/pnas.2100697118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herbig M. et al. , “Image-based cell sorting using artificial intelligence,” Proc. SPIE 11250, 112500J (2020). 10.1117/12.2544809 [DOI] [Google Scholar]
- 114.Luo S. et al. , “Deep learning-enabled imaging flow cytometry for high-speed Cryptosporidium and Giardia detection,” Cytometry A 99, 1123 (2021), 10.1002/cyto.a.24321 [DOI] [PubMed] [Google Scholar]
- 115.Joshi K. et al. , “A machine learning-assisted nanoparticle-printed biochip for real-time single cancer cell analysis,” Adv. Biosyst. 4, 2000160 (2020). 10.1002/adbi.202000160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.