Abstract
Despite its success in treating hematologic malignancies, chimeric antigen receptor (CAR) T cell therapy faces two major challenges which hinder its broader applications: the limited effectiveness against solid tumors and the nonspecific toxicities. To address these concerns, researchers have used synthetic biology approaches to develop optimization strategies. In this review, we discuss recent improvements on the CAR and other non-CAR molecules aimed to enhance CAR T cell efficacy and safety. We also highlight the development of different types of inducible CAR T cells that can be controlled by environmental cues and/or external stimuli. These advancements are bringing CAR T therapy one step closer to safer and wider applications, especially for solid tumors.
INTRODUCTION
Chimeric antigen receptor (CAR) T cell therapy has advanced as one of the most promising cancer treatments during the past decade especially for blood tumors.1–5 CAR T therapy involves ex vivo genetic engineering of the patients' T cells with the CAR molecule, which equips the T cells with redirected specificity against target tumor cells, and the subsequent infusion of the CAR T cells back into the patients for cancer treatment. As of September 2021, five CAR T products have been approved by the Food and Drug Administration (FDA) in the United States, targeting leukemia, lymphoma, and multiple myeloma.
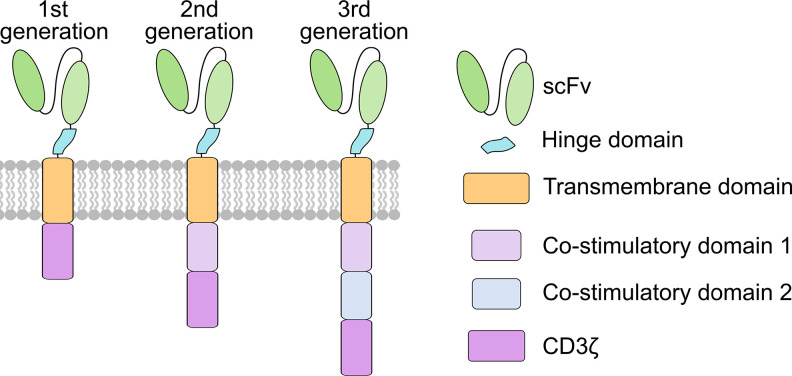
In fact, synthetic chimeric molecules composed of antibody-like variable regions fused to T cell receptor (TCR)-derived constant regions were first reported approximately three decades ago.6–9 These were later referred to as the first generation CAR, typically containing an extracellular single-chain variable fragment (scFv) for antigen recognition, hinge (H), and transmembrane (TM) domains for signal transduction, and an intracellular CD3z for activation (Fig. 1). Despite the antigen-specific activation and cytotoxicity, the first generation CAR T cells showed low proliferation in vivo. This led to the development of the second generation CAR,10–12 where a co-stimulatory domain (e.g., CD28) was added between the transmembrane and the CD3z domains and was shown to resolve low proliferation issues associated with the first generation CAR T cells (Fig. 1).13–16 In 2011, clinical trials of second generation CAR T cells in chronic lymphocytic leukemia (CLL) and B-cell acute lymphoblastic leukemia (B-ALL) patients achieved unprecedented results including complete remission.1,2,17 Since then, CAR T therapy has revolutionized the field of cell-based immunotherapy, especially for hematologic malignances. The third generation CAR, characterized by the incorporation of two co-stimulatory domains (e.g., CD28 and OX40) between the transmembrane and the CD3z domains, was also developed and shown to further augment CAR T cell performance (Fig. 1).18,19
FIG. 1.
Evolution of different generations of CARs. Structures of the first, second, and third generations of CARs.
However, most of CAR T therapy's successes were in hematologic cancers. When facing solid tumors, CAR T cells exhibited limited therapeutic efficacies, mostly attributed to difficulties in homing, infiltration, and survival in the immune-suppressive tumor microenvironment (TME), as well as tumor antigen escape and heterogeneity.20–22 Additionally, side effects associated with CAR T therapy have been reported, including on-target off-tumor toxicity, neurologic toxicity, cytokine release syndrome (CRS), etc.23–25 There is hence an urgent need for the development of the so-called next generation CAR T cells, with the engineering objectives to (a) enhance the efficacy of CAR T cells to overcome issues regarding the ineffectiveness of CAR T therapy in solid tumors, and (b) improve the safety of CAR T cells to mitigate and/or minimize the adverse toxicities associated with previous CAR T products.26–29 Tremendous efforts have been made toward these directions. Herein, we provide a review of recent novel strategies in CAR T cell designs that aim to improve the efficacy and safety of CAR T therapy. First, we discuss modifications on the CAR molecule at the ectodomain, transmembrane domain, and endodomain, as well as those involving multiple domains. We then review modifications on non-CAR molecules, either as “add-ons” to enhance CAR T cell performance, or as knock-out of negative regulators. In addition, we introduce inducible CAR T designs that allow spatial and temporal control over CAR expression or T cell activation. Finally, we discuss the applications of CAR in other types of immune cells. These innovations should aid the advancement of CAR T therapy particularly for treating solid tumors.
MODIFICATIONS ON THE CAR MOLECULE
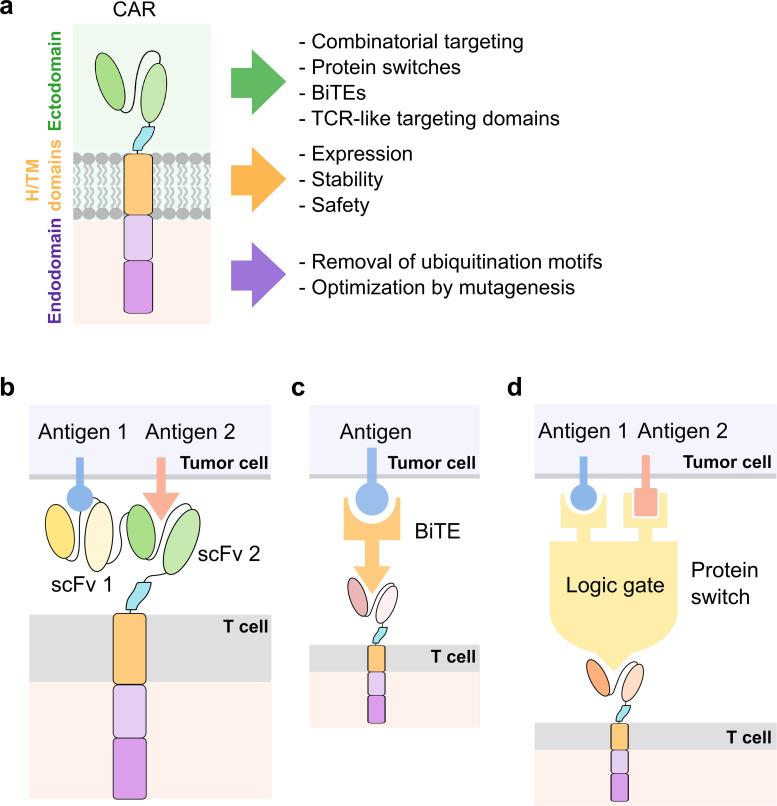
The CAR molecule typically consists of the ectodomain, the hinge (H) and transmembrane (TM) domains, and the endodomain. The ectodomain is a key region responsible for the recognition of target antigens; the H/TM domains transmit antigen recognition signals to the endodomain where signaling occurs; and the endodomain is responsible for co-stimulatory signals that promote T cell survival and proliferation and stimulatory signals required for cytotoxic T cell responses. Multiple strategies of modifications and optimizations have been developed and applied to a constitutively expressed CAR. This section focuses on enhancements that occur in one or more domains in the CAR molecule [Fig. 2(a)].
FIG. 2.
Engineering the CAR molecule. (a) Modifications on the ectodomain, hinge (H), and transmembrane (TM) domains, and endodomain of the CAR molecule. (b) Design of bispecific CARs. (c) Design of CARs utilizing bispecific T cell engagers (BiTEs) as adaptors between CAR T and tumor cells. (d) Design of CARs implementing protein switch-based logic gates to incorporate inputs from two or more tumor antigens.
The ectodomain
Researchers have developed strategies to engineer antigen recognition to enhance CAR T targeting. For example, bispecific CARs containing two tandem ligand-binding domains have been shown to effectively implement an OR logic gate in CAR T signaling, where only two different types of antigens are capable of activating the CAR receptor [Fig. 2(b)]. A BCMA/CS1 bispecific CAR was shown to outperform T cells co-expressing separate BCMA and CS1 monospecific CARs.30 Thus, the enhanced avidity for the targeted cancer cells imparted by the bispecific CAR appears to enhance the immunotherapy and help avoid antigen escape that can occur with heterogeneous tumors. This strategy is generalizable to other combinations of antigens as shown by ongoing research in the area that reports comparable results.31–33 Some researchers have also explored universal CAR designs that utilize bispecific T cell engagers (BiTEs) as a bridge between CAR T and tumor cells [Fig. 2(c)]. For example, Kim et al. developed CAR T cells targeting fluorescein isothiocyanate (FITC), and used bispecific adapters consisting of FITC-conjugated folate to redirect the anti-FITC CAR T cells to tumor cells expressing folate receptors.34 Lee et al. further characterized FITC-folate mediated CAR T cells in vivo and demonstrated their ability in mitigating CRS.35 Rodgers et al. developed peptide-specific switchable CAR T cells (sCAR-T) recognizing peptide neo-epitopes (PNE) inserted in a tumor-antigen-specific antibody and demonstrated PNE dose-dependent activation of sCAR-T.36 Viaud et al. further optimized sCAR-T and characterized their antitumor ability in a syngeneic murine tumor model.37 Using similar principles, Paul et al. showed that a bispecific antibody targeting TRB5–5 and TRBV12 could specifically lyse malignant T cell lines in mouse models.38 Cho et al. developed a split, universal, and programmable (SUPRA) CAR system composed of a universal receptor (zipCAR) expressed on T cells, where a tumor-targeting scFv adaptor (zipFv) enabled the switch of targets in tumor cells and the response to multiple antigens using different adaptors without reengineering the T cells.39 These innovations provide solutions to antigen limitations in conventional CARs.
Despite the enhanced performance of bispecific CARs, for some applications, an OR-gate contributes to increased levels of on-target off-tumor toxicity leading to a lower therapeutic index. As it is difficult to identify surface antigens unique to cancer cells, CAR T cells are expected to kill normal cells expressing target antigens. For applications where such overlap in antigen expression occurs between cancerous and healthy tissues, more complex logic gates may be required. AND logic gates can require multiple antigens to be expressed on a cell before a T cell response can be triggered, and NOT logic gates can prevent CAR T activation when certain antigens are expressed on normal tissues/organs. Toward the implementation of more complex logic gates, Lajoie et al. developed a CAR that targets a non-native epitope that exists in a colocalization-dependent protein switch called Co-LOCKR [Fig. 2(d)].40 This switch can be toggled between a conformation where the epitope is exposed and another conformation where the epitope is hidden depending on co-localization of switch components. While this strategy has been shown to work in vitro to implement complex combinations of AND, OR, and NOT logic gates, such a strategy would require intravenous administration of the switch components.40 Thus, pharmacokinetics and immunogenicity of the colocalization-dependent protein switch may present future challenges for this approach. Other groups have also worked toward introducing Boolean logic gates in CAR T cells. For example, Salzer et al. have developed an avidity-based system where T cells are transduced with multiple CARs that bind weakly to targeted antigen. Thus, on their own, an individual CAR will be insufficient to mount a significant immune response. Then, by further engineering intermolecular interactions between such low-affinity CARs, the researchers were able to develop AND logic gates by introducing a dimerization domain.41
The hinge and transmembrane domains
CARs can also benefit from optimizations in the H/TM domains. It has been shown that different H/TM domains can affect the expression and stability of the CAR molecules as well as the efficiency of signal transmitting.42 Some H/TM domains also appear associated with neurologic toxicity in CAR T therapies. Brudno et al. reported that the occurrence of neurologic toxicity is significantly lower in patients treated with Hu19-CD828Z CAR T cells than those treated with FMC63-28Z CAR T cells.43 As Hu19-CD828Z CAR differs from FMC63-28Z CAR in that it contains a human scFv as opposed to a mouse scFv, and H/TM domains from CD8α as opposed to from CD28, the authors hypothesized that the structural characteristics and the intramolecular and intermolecular binding of the H/TM domains caused the differences in cytokine release and neurologic toxicity between the two types of CAR T cells.43 Muller et al. also noted that the CD28 TM domain can promote heterodimerization with endogenous integrins.44 In another study, Ying et al. set off to optimize the CD19-BBz CAR used in the FDA-approved CAR T therapy medication CTL019 (Kymriah), as CTL019 was shown to cause toxic side effects in patients.45 By varying the length of the H/TM domains, they identified CD19-BBz(86) CAR as the top candidate and showed that CD19-BBz(86) CAR T cells were of similar potency but significantly reduced toxicities in a phase 1 trial.45 Thus, optimization of the H/TM domains can enhance the function and safety of CAR T cells.
The endodomain
As the CAR endodomain is responsible for intracellular signaling, CAR function will be very dependent on the design of the endodomain. For example, Li et al. noticed that upon tumor engagement, expression of CARs in T cells would decrease over time. Specifically, they noticed that ubiquitination of the CAR led to endosomal recycling and loss of CAR expression.46 To counteract this observation, Li et al. mutated lysines in the endodomain to prevent ubiquitination. The end result was a CAR that outperformed the un-optimized CAR. Surprisingly, the ubiquitination-resistant CAR was able to achieve this with lower surface expression than the ubiquitination-susceptible CAR.46 Other researchers have taken a reductionist approach to remove components from the CAR and determine which domains are required for a T cell response. For example, Feucht et al. mutated immunoreceptor tyrosine-based activation motifs (ITAM) in CD3ζ to evaluate which ITAMs are essential for cytotoxicity. The results revealed that mutating the tyrosines to phenylalanines in ITAMs 2–3 resulted in strong effector responses and outperformed the standard 1928z CAR.47 This optimization enhanced the persistence of the CAR while retaining CAR function. Further still, Wu et al. have identified additional CD3 domains that may be useful for CAR T immunotherapy. Specifically, they showed that a CD3ε domain with its intracellular tail and the potential binding partners was capable of improving the cytotoxic activity of a second generation CAR.48
Other modifications
Some researchers proposed to compartmentalize the co-stimulatory and activation signaling domains, which are commonly integrated into the same CAR molecule in second and third generation CAR designs. By separating CD3z signaling and CD28-mediated co-stimulatory signaling, Wilkie et al. developed dual antigen targeting CAR T cells whose proliferation required stimulation of both tumor antigens 1 and 2 (ErbB and MUC1).49 Kloss et al. engineered T cells expressing a CAR that only provides suboptimal activation signaling upon target antigen binding of prostate stem cell antigen (PSCA), and a chimeric co-stimulatory receptor that recognizes a different tumor antigen prostate-specific membrane antigen (PSMA). These engineered T cells only destroy prostate tumors expressing both PSCA and PSMA antigens but not single-antigen positive tumors.50 These combinatorial strategies that require the presence of dual or multiple tumor antigens to unleash the full potentials of engineered T cells can greatly reduce the on-target off-tumor side effects and broaden the applicability of tumor-associated antigen (TAA)-targeted T cell therapies.51,52
Some researchers have decided to forgo the typical CAR architecture altogether. For example, Liu et al. developed a chimeric receptor that utilizes an immunoglobulin heavy chain fused to TCR-Cα and an immunoglobulin light chain fused to TCR-Cβ.53 They showed that this double chain design mimicking the TCR architecture lacks the tonic signaling that poses challenges for some CAR designs. Additionally, the TCR-like scheme showed higher sensitivity toward targeted antigens than traditional CAR designs.53 Walseng et al. showed similar results in T cells and natural killer (NK) cells with their version of a TCR CAR that introduced a cysteine to enhance TCR dimer stability.54 Although promising in mouse models, safety concerns still exist for both TCR-based and CAR-based designs. In general, improving sensitivity toward a targeted antigen may result in a therapy with excessive on-target off-tumor toxicity due to overlap in antigen expression between healthy and cancerous tissues.
MODIFICATIONS ON NON-CAR MOLECULES
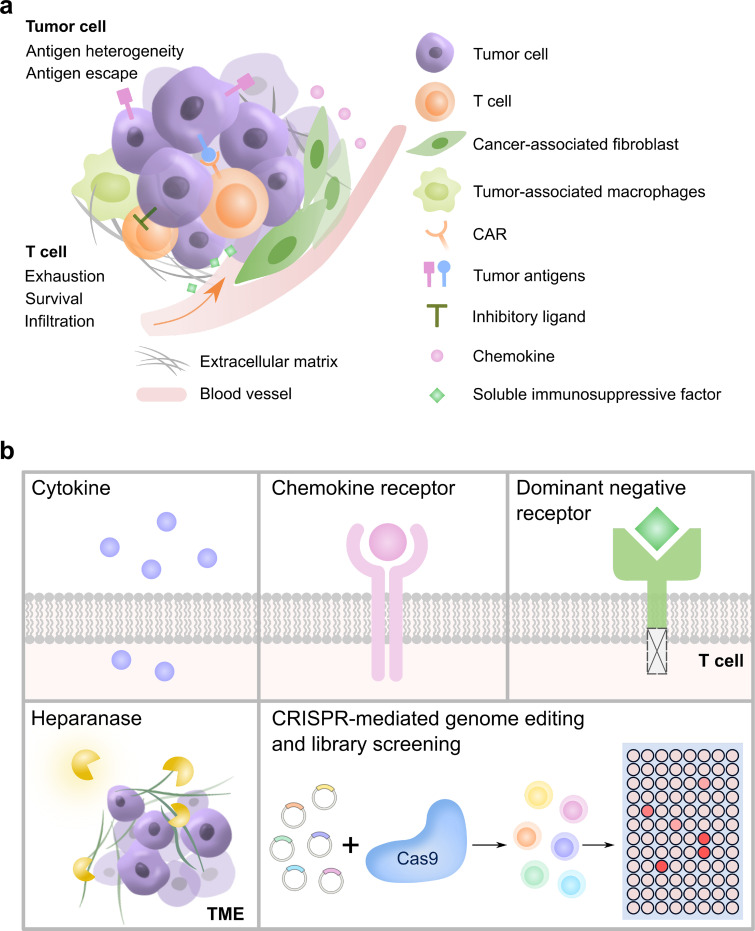
Although CAR T cells have shown great success in the treatment of hematological malignancies, this approach has limited efficacy toward solid tumors. The immunosuppressive TME hinders the infiltration of the T cells, accelerates their exhaustion, and threatens their survival [Fig. 3(a)].20 For instance, the TME of solid tumors is enriched with extracellular matrix (ECM) proteins blocking the infiltration of T cells from blood vessels following intravenous administration. Another prominent example is the elevated expression of inhibitory ligands (e.g., PD-L1) on tumor cell surface that triggers inhibitory signaling pathways in T cells to compromise T cell function. Other challenges such as tumor antigen escape and antigen heterogeneity remain as well. Efforts have been attempted to engineer the so-called armored CAR T cells which can enhance the CAR T cell function in TME via the co-expression or knock-out of non-CAR molecules (see review in Ref. 39).55 Below we highlight some examples of these modifications [Fig. 3(b)].
FIG. 3.
Engineering of armored CAR T cells through modifications on non-CAR molecules. (a) The immunosuppressive TME of a solid tumor faced by CAR T cells. (b) Representative approaches aimed to help CAR T cell fight the immunosuppressive TME, including expressions of cytokines, chemokine receptors, dominant-negative receptors, heparanases, and CRISPR-mediated genome editing and library screening in T cells.
Cytokine secretion
Recombinant interleukin (IL)-12 has been used clinically to treat multiple types of solid tumors. Yeku et al. developed anti-ovarian tumor CAR T cells capable of constitutively secreting IL-12, and observed enhanced survival of these armored CAR T cells in the inhibitory TME of murine ovarian peritoneal carcinomatosis.56 It has also been reported that CAR T cells cultured in IL-15 can preserve a less-differentiated stem cell memory (Tscm) phenotype with reduced exhaustion and enhanced proliferation upon antigen engagement.57 In fact, multiple cell types, including macrophages and dendritic cells, can produce IL-15, which can stimulate CD8+ T cells and NK cells with increased proliferation and tumor cytotoxicity.58 Incorporating IL-15 production within GD2-targeting CAR T cells has demonstrated superior antitumor activity both in vitro and in vivo compared to conventional CAR T cells.59 Cytokines that are essential for T cell zone formation and maintenance in lymphoid organs can also be incorporated to enhance the therapeutic effects of CAR T cells. Adachi et al. engineered CAR T cells expressing both IL-7 and CCL19 (7 × 19 CAR T) and demonstrated complete regression of solid tumors in mice with prolonged survival as compared to conventional CAR T therapy.60 More recently, Luo et al. further engineered CAR T cells expressing IL-7 and CCL21 (7 × 21 CAR T), which revealed superior therapeutic efficacy against solid tumors to conventional CAR or 7 × 19 CAR.61
Chemokine receptor
Insufficient tumor-directed trafficking is another key factor that limits cell-based immunotherapy against solid tumors. To overcome this, scientists have engineered CAR T cells that can utilize the tumor-secreted chemokines for their homing to the tumor sites. For instance, Hodgkin lymphoma can produce chemokine CCL17 and CCL22 which are attractants for the CCR4-expressing T helper 2 (Th2) and regulatory T (Treg) cells, but not for the CD8+ T cells, which lack CCR4 expression. Based on that, researchers have engineered CCR4-expressing CAR T cells, which have shown improved homing and antitumor activity when infused intravenously in mice engrafted with human Hodgkin lymphoma.62 Similarly, IL-8 has also been reported as a chemoattractant for neutrophils and myeloid-derived suppressive cells which contribute to the immunosuppressive nature of TME. Co-expression of IL-8 receptor CXCR2 in the CAR T cells has also achieved promising outcomes including enhanced tumor homing and efficacy in multiple solid tumor models.63–65 Besides, co-expression of other chemokine receptors, including CCR2b,66 CXCR3,67 CXCR4,68 and CCR6,69 have also shown promising results in solid tumor treatment.
Heparanase
Endogenous T cells can produce enzymes that degrade the ECM of solid tumors, one of the barriers for T cell infiltration; however, it has been found that CAR T cells can lose such capability of degrading ECM during the ex vivo engineering process. To improve the tumor infiltration of CAR T cells, Caruana et al. reequiped the anti-GD2 CAR T cells with the enzyme heparanase (HPSE), which degrades heparan sulfate proteoglycans, the main components of ECM.70 Based on their results, these CAR T cells indeed demonstrated improved infiltration into xenograft tumors in mice and prolonged survival as compared to CAR T cells lacking heparanase expression.
Dominant-negative receptors
Another approach to overcome the immunosuppressive signals within TME is to block the immunosuppressive pathways in CAR T cells. Transforming growth factor β (TGF-β), secreted by many tumors including prostate cancer, is known to potently suppress the immune system, creating an immunosuppressive milieu within solid tumors. Studies have demonstrated that the TGF-β signaling can be blocked by expressing dominant-negative TGFBRII, which lacks the intracellular domain for downstream signaling. Using this as an add-on, Kloss et al. demonstrated that the potency of PSMA-directed CAR T cells can be greatly enhanced, with increased T cell proliferation, cytokine secretion, resistance to exhaustion, and long-term in vivo persistence.71 Similarly, co-expressing PD-1 dominant-negative receptor, which blocks the PD-1 pathway, has also demonstrated augmented efficacy for various CAR T therapies targeting CD19, mesothelin, and HIV-1.72–74 Compared with antibody-based PD-1 blockade, the genetic-engineering approach can provide more sustainable and tumor-limited effects and has provided opportunities to treat different types of solid tumors.
High-throughput pooled knock-in
The add-on expression of endogenous or exogenous genes has shown great promise for cancer immunotherapy, and in fact decades of studies on T cell signaling and function have suggested numerous candidates for such applications. However, a high-throughput method for screening the gene candidates that most potently enhance the performance of cell therapies is still needed. Recently, Roth et al. developed a high-throughput platform to assess the functional effects of pooled library of knock-in gene templates in the same locus through CRISPR targeting, with which they demonstrated the rapid screening of a barcoded 36-member library that included dominant-negative receptors, synthetic switch receptors, transcription factors, and metabolic regulators/receptors.75 Using a human melanoma mouse model allowed the direct comparison of T cells knocked in with the pooled library and identified subsets of knock-in constructs that promoted in vivo tumor infiltration. Particularly, the TGF-βR2–41BB chimeric receptor, one of the candidates in the library, has been shown to enhance T cell fitness and promote expression of key effector cytokines, and improve solid tumor clearance in vivo, suggesting that the pooled knock-in technology can be a powerful tool in identifying potential lead constructs from large libraries.
Knock-out of inhibitory surface receptors
Alternatively, researchers have investigated the deletion or disruption of genes that negatively regulate T cell performance. Immune checkpoints such as PD-1 and CTLA-4 are inhibitory receptors that can suppress T cell activation and promote T cell exhaustion and dysfunction.76,77 Immune checkpoint blockade therapies utilizing monoclonal antibodies against the checkpoint receptors have shown promising clinical results.76,77 Investigators have also applied immune checkpoint blockade in CAR T therapy by combining CAR T treatment with PD-1 blocking antibody administration,78 rewiring PD-1 or CTLA-4-based inhibitory signals to CAR T activation (iCARs),79 or engineering CAR T cells with constitutive anti-PD-1 scFv expression and secretion.80 Moreover, recent advancement in gene editing technology has allowed the manipulation of endogenous genes. Using the CRISPR/Cas9 system, Su et al. performed PD-1 gene knock-out in patient-derived T cells and observed enhanced cytokine production and cytotoxicity in vitro.81 Rupp et al. further applied CRISPR/Cas9-mediated PD-1 knock-out in anti-CD19 CAR T cells and demonstrated improved clearance of PD-L1+ tumor xenografts in vivo.82 Hu et al. observed similar results with PD-1 knock-out in anti-mesothelin CAR T cells.83 Furthermore, Ren et al. performed multiplex genome editing to simultaneously knock-out TCR, HLA class I molecule, and PD-1, generating universal allogeneic PD-1 deficient CAR T cells with enhanced antitumor activity.84 Disruption of other inhibitory receptors such as CTLA-4 has also been studied in CAR T cells and achieved promising results.85,86 A list of potential immunoinhibitory receptor targets for genome editing in CAR T cells has been reviewed elsewhere.87
Knock-out of negative regulators
Meanwhile, systematic screening methods have been developed to identify key negative regulators in T cells. Shifrut et al. performed CRISPR/Cas9-based genome-wide loss-of-function screens and identified negative regulators of proliferation following stimulation in primary human T cells in vitro.88 Wei et al. developed a pooled CRISPR/Cas9 mutagenesis screening approach and identified REGNASE-1 as a major negative regulator of T cell antitumor activity among 3017 metabolism-associated factors.89 REGASE-1 knock-out T cells demonstrated improved accumulation and persistence in an adoptive cell therapy model in vivo.89 In another case report, Fraietta et al. discovered unintended disruption of the methylcytosine dioxygenase TET2 gene by CAR lentiviral integration in a CAR T cell administrated into a chronic lymphocytic leukemia (CLL) patient, in addition to a hypomorphic variant in the other TET2 allele.90 The TET2-disrupted CAR T cell underwent massive in vivo expansion and became the dominant population (94% of the CD8+ CAR T cell repertoire at the peak of response), leading to complete remission in this patient.90 Further analysis revealed epigenetic reprogramming and central memory phenotype of the TET2-disrupted CAR T cells, whose potency-enhancing effect was recapitulated by experimental knockdown of TET2.90 While TET2 is a tumor suppressor gene and extreme caution should be used when disrupting such genes, these reports highlighted the potential of endogenous gene silencing and genome/epigenome editing in enhancing the efficacy of CAR T therapy.
Off-the-shelf CAR T cells
An additional hurdle that CAR T based therapies must overcome involves the manufacture and delivery of autologous CAR T cells. FDA-approved therapies require taking a patient's own cells, reprogramming them, and allowing them to proliferate prior to injecting the modified cells back into the patients. The whole process may need around two weeks. For many patients who failed prior to front-line therapies, they may not survive long enough to receive and benefit from the reprogrammed cells. Thus, another field of research involves developing off-the-shelf allogeneic CAR T cells that do not require a two-week waiting period.
Allogenic donor CAR T cells may attack recipient tissues and cause graft-vs-host disease (GVHD) mediated by the TCR expression on donor T cells.91 One solution is to knock-out TCR in allogenic donor CAR T cells. Poirot et al. utilized transcription activator-like effector nuclease (TALEN)-mediated gene editing approach to knockdown TCR and CD52 (a protein targeted by chemotherapeutic agent) in CD19CAR T cells, and demonstrated their high efficacy in a blood cancer mouse model.92 This universal CD19CAR T product (later termed UCART19) was later used to treat B-ALL in two infants,93 followed up by two phase 1 studies in pediatric and adult patients.94 Other attempts to generate universal CAR T cells include CRISPR/Cas9-mediated triple knock-out of TCR, HLA, and PD1,84 as well as the integration of CAR into the TCR alpha constant (TRAC) locus.95,96 In the event that GVHD or other serious adverse events occur that require termination of the CAR T therapy, researchers are actively developing safety switches to kill or inactivate the allogeneic CAR T cells.97–99
At the same time, host-vs-graft (HVG) activities triggered by the infusion of allogeneic T cells, including the host T- and NK-cell responses to eliminate foreign cells, may limit the persistence of infused CAR T cells, and should also be minimized to achieve full therapeutic benefits. Ayuk et al. reported that for some patients, allogeneic CAR T therapy may be feasible as CAR T cells from an HLA-matched donor were able to proliferate and persist during immunotherapy.100 In addition, the allogeneic T cell-activated host lymphocytes would upregulate surface receptors, such as 4–1BB (CD137), which can serve as markers to distinguish the activated cytotoxic effectors from the unstimulated populations. Based on that, Mo et al. engineered the chimeric 4–1BB-specific alloimmune defense receptor (ADR) which selectively eliminates the activated host lymphocytes by the allogeneic CAR T cells while sparing the resting lymphocytes.101 They showed that the ADR-expressing CAR T cells can successfully evade immune rejection and achieve sustained tumor eradication in mouse models of allogeneic T cell therapy of hematopoietic (CD19 CAR) and solid (GD2 CAR) cancers.
STIMULI-INDUCIBLE CAR T CELLS
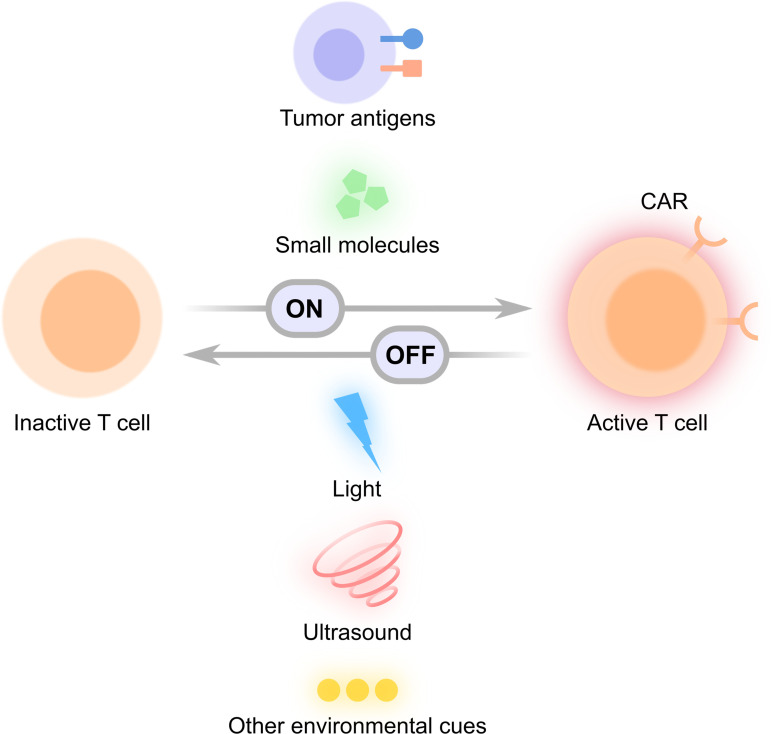
Toxicity remains a major concern in CAR T therapy. CRS is a potentially life-threatening side effect characterized by systemic elevations of cytokines such as IL-6 and interferon γ (IFN γ). CRS is one of the most common adverse effects in CAR T therapy caused by infusion of large amounts of active CAR T cells and is commonly treated with IL-6 receptor blockade.102–104 Another potentially lethal side effect is the “on-target off-tumor” toxicity, where CAR T cells attack normal tissues expressing low levels of the target antigen. An infusion of 1010 anti-ERBB2 CAR T cells attempted to treat a patient with metastatic ERBB2+ cancer caused acute respiratory distress and the subsequent death of the patient.105 The serious toxicity was later believed to be caused by the recognition of low levels of ERBB2 on lung epithelial cells by the infused CAR T cells.105 There is hence an urgent need to engineer safer CAR T cells whose activation can be tightly controlled spatiotemporally to prevent CRS, on-target off-tumor toxicity, and other adverse effects. To this end, researchers have developed stimuli-inducible CAR T cells, where functional CAR expression is activated or deactivated in a controllable manner by environmental cues such as tumor antigens, small molecules, light, ultrasound, and other stimulations (Fig. 4). These designs provide enhanced controllability of CAR T cells, bringing the field one step closer to safer CAR T therapy.
FIG. 4.
Conceptual illustration of stimuli-inducible CAR T cells. Various ON- and OFF-switches controlled by external stimuli such as tumor antigens, small molecules, light, ultrasound, and other environmental cues have been utilized to activate functional CAR expression (ON-switch) or deactivate CAR-expressing T cells (OFF-switch).
Automated CAR T cells
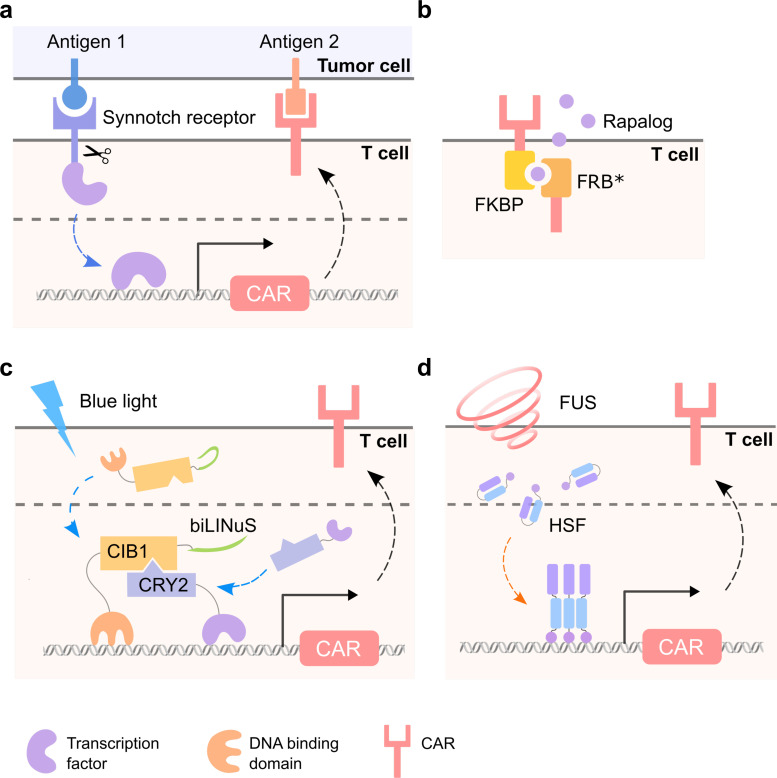
The engineering of AND-gate T cells based on the synthetic Notch receptor (synNotch) system which is activated only by dual antigen recognition allows precise control of CAR T cell signaling [Fig. 5(a)]. In the synNotch design, the CAR-like synNotch receptor on the T cells senses antigen 1 presented on the tumor cells, which induces the expression of a CAR targeting tumor antigen 2; thus, only the tumor cells expressing dual antigens are eliminated.106 This AND-gate circuit expands the antigen sets on solid tumors that can be targeted safely with CAR T cells.107 For instance, the synNotch CAR T cells can sense ROR1 protein and induce the expression of CAR molecules specific for EpCAM or B7-H3, which are expressed on ROR1+ tumor cells but not ROR1+ stromal cells; therefore, this system can overcome the lethal toxicity of constitutive ROR1-CAR T cells.108 Furthermore, the synNotch CAR T cells that recognize the combination of alkaline phosphatase placental-like 2 (ALPPL2) and the tumor-associated antigen-melanoma cell adhesion molecule (MCAM), mesothelin, or HER2 were demonstrated to more precisely guide the T cells to target the tumors.109 Recently, T cells with multiple synNotch receptors as flexible regulatory connectors were developed, which could achieve precise tumor recognition by integrating up to three different antigens while ignoring related two-antigen tumors.110 Similar approaches also allowed the integration of signals from the recognition of multiple imperfect but complementary antigens for the design of T cell killing functions.111 Mechanistically, in addition to the precise targeting of specific tumor cells, the synNotch-regulated CAR expression has also been shown to avert tonic signaling and exhaustion, maintain a higher fraction of the T cells in a naïve/stem cell memory state, thus leading to more efficient tumor killing than conventional CAR T cell.109,111
FIG. 5.
Representative designs of stimuli-inducible CAR T cells. (a) Synnotch CAR T cells. (b) Rapalog-inducible CAR T cells. (c) Blue-light-controllable CAR T cells with the LINTAD system. (d) FUS-controllable heat-sensitive CAR T cells.
Another approach of automated conditionally expressed CARs is driven by tumor microenvironment-associated factors such as hypoxia. Using quantitatively characterized hypoxia-responsive element (HRE) together with a panel of core promoters, Ede et al. found the synthetic promoter, YB_TATA, to have the most prominent contrast in inducing reporter gene expression between normal and hypoxia conditions.112 These regulatory elements were further applied to engineer hypoxia-inducible CAR T cells that can be conditionally activated in the hypoxia tumor microenvironment.112 Later, instead of inducible expression of CARs directly by the hypoxia-responsive element, the oxygen-dependent degradation domain (ODD) of HIF1a was also utilized to induce the degradation of the fused CARs under normoxia condition and thus achieving T cell activation and tumor killing only under hypoxia conditions.113 Recently, a dual oxygen-sensing CAR T cell was developed by appending the ODD domain onto the CAR, whose expression is driven by nine consecutive HREs.114 This design allows the inducible expression of CAR under hypoxia and the degradation of CAR expression leakage under normoxia environment.
Small molecule-controllable CAR T cells
Chemical controllable CAR is an alternative way to control the dose and timing of activated T cells to mitigate side effects such as CRS. A robust ON-switch split-CD19CAR design based on FKBP-FRB* dimerization was proposed, in which the functional CAR molecules are formed only after the addition of dimerization small molecule rapamycin analog AP21967 (rapalog), producing titratable, reversible, and temporally controllable CAR T cells115 [Fig. 5(b)]. This study provides an elegant example of designing safer therapeutic cells by integrating autonomous and user controls. Similar methods can be extended to a broader range of small molecule-inducible dimerization systems116,117 or other CAR molecules such as the epidermal growth factor receptor variant III (EGFRvIII) CAR.118 Another ON-switch design utilizing the tetracycline (Tet)-On system, an inducible gene expression system for mammalian cells, was also used to control CAR expression with a small molecule doxycycline (Dox) and achieved low background expression and an equivalent killing efficiency to the conventional CAR in the presence of Dox.119 Yang et al. designed an inducible gene expression system based on a dietary molecule resveratrol (RES) to regulate CAR expression and demonstrated that RES-activated CAR T cells achieved in vivo cytotoxicity comparable to that of conventional CAR T cells.120
In addition to the ON-switches, the inducible suicide switch (OFF) has been applied to control CAR T cells,121–126 especially when these cells have adverse effects on human patients. One of the most successful suicide genes is the inducible caspase-9 (iCasp9) gene, where the dimerization of iCasp9 by a small molecule compound can trigger the apoptosis of 90% of the modified T cells within 30 min in GVHD patients and end GVHD without recurrence.124 However, the suicide switch approach is irreversible, eliminating the entire CAR-positive T cell population. Yang et al. showed that RES-based inducible gene expression system can also be used to reversibly repress CAR expression, serving as an OFF switch.120 An alternative approach is an inducible degradation of CARs in T cells using the ligand-induced degradation (LID) domain such as degron127,128 and dihydrofolate reductase (DHFR) destabilizing domain.128 The addition of a small molecule ligand of degron-shield-1 promotes the proteasomal degradation of the CAR-LID fusion protein.127 In contrast, CAR-DHFR can be stabilized by the FDA-approved antibiotic trimethoprim (TMP),128 achieving the drug-dependent control of CAR expression and activity both in vitro and in vivo. Jan et al. also developed an OFF-switch CAR using the clinically approved drug lenalidomide to trigger the degradation of CAR proteins.117 Additionally, the proteolysis-targeting chimera (PROTAC) compound was also applied to control the CAR molecules.129 PROTACs are small bifunctional molecules that are able to bind to target protein ligands and E3 ubiquitin ligase, which resultantly degrades the target protein through the ubiquitin-proteasome system.130 The CD19 CAR molecule linked with the bromodomain (BD) from BRD4 protein can be degraded by the E3 ligases after the addition of PROTACs; however, the problem of PROTACs is the degradation of endogenous proteins as well as CAR protein, which may be toxic to CAR T cells.130
In addition to their usage in inducible CARs, small molecules were also applied to pre-condition the CAR T cells before transplantation to enhance therapeutic efficacy. Dasatinib, an FDA-approved tyrosine kinase inhibitor that was initially used to treat chronic myeloid leukemia (CML), was found to be effective in temporarily inactivating CAR T cells. The treatment of dasatinib reduced the acute toxicity, inhibited the tonic CAR signaling, and reinvigorated exhausted CAR T cells, thus resulting in superior antitumor responses in vivo.128,131,132 Similarly, after low dose treatment of Decitabine, a DNA methyltransferase inhibitor that is FDA-approved for treating myelodysplastic syndromes (MDS), CAR T cells showed higher expressions of memory-, proliferation-, and cytokine production-associated genes, and enhanced their persistent antitumor capacity in vivo133 through epigenome reprogramming. Since these small molecules are FDA-approved and have demonstrated safety in humans, implementing these drugs as an on/off control in CAR T cell immunotherapy should be straightforward.
Light-controllable CAR T cells
Optogenetics, where optical and genetic methods are combined to control biological processes with high spatiotemporal precision, was mainly applied in neurobiology in the early stage.134 With the development of genetically encoded light-sensitive proteins, optogenetics has become an increasingly popular tool for the remote control of cellular functions.135 Kennedy et al. demonstrated the application of blue-light-controllable dimerizers cryptochrome 2 (CRY2) and CIB1 in protein translocation, transcription, and Cre-mediated DNA recombination, opening doors for the remote control of cellular functions using a new generation of optogenetic tools.135,136 Recently, researchers have explored the application of optogenetics in CAR T therapy as it may offer enhanced safety and controllability. Huang et al. developed a light-inducible nuclear translocation and dimerization (LINTAD) gene activation system by integrating the CRY2-CIB1 dimerizer and the LOV2-based light-inducible nuclear localization signal (biLINuS) [Fig. 5(c)]. The LINTAD system was applied to regulate CAR expression in T cells and achieved blue-light-controllable tumor killing in vivo.137 Zhang et al. engineered a photoswitchable CAR based on the FITC-folate mediated CAR (reviewed above), where they inserted a photocleavable linker between FITC and folate moieties in the bispecific adaptor, allowing the deactivating of CAR T cells by light.138 O'Donoghue et al. engineered an optoCAR system by fusing one part of a split CAR to the improved light-inducible dimer (iLID) and another to the iLID binding partner SspB, allowing the reconstitution of a functional intact CAR upon illumination.139 Similarly, He et al. also engineered optoCAR based on an optical dimerization system with a circularly permuted LOV2 (cpLOV2), and demonstrated the photoinducible antitumor activity of the optoCAR T cells in vivo.140 While light offers precise spatial and temporal control, its limited tissue penetration depth may prevent further applications in human patients. To overcome this, technologies involving upconversion nanoparticles, implantable LEDs, near-infrared (NIR) light, and optical fibers have been developed.141–143 For example, Nguyen et al. utilized upconversion nanoplates and demonstrated antitumor responses of light-switchable CAR (LiCAR) T cells activated by NIR light.144
Ultrasound and/or heat-controllable CAR T cells
Ultrasound can penetrate deep into biological tissues. In addition to its traditional usage as an imaging tool for diagnosis, ultrasound has been applied to regulate cellular functions for therapeutic purposes. Pan et al. utilized ultrasound to mechanically perturb microbubble-coupled cells, activating the mechanosensitive ion channel Piezo1 and subsequent molecular events including calcium influx, nuclear factor of activated T cells (NFAT) translation, and NFAT-mediated gene expression. They applied this system in CAR T cells and showed ultrasound-inducible tumor cell killing in vitro.145
In addition to direct mechanical stimulation, ultrasound can also cause local hyperthermia when the deposited mechanical energy is converted to thermal energy via internal friction. Focused ultrasound (FUS), capable of causing temperature elevation in a confined region, has been widely used to ablate tumors in the clinics. Inspired by the endogenous heat shock response where heat (or other stresses) activates the heat shock promoter (Hsp) through heat shock factors (HSFs) to drive the expression of heat shock proteins, researchers have employed FUS to activate Hsp-driven transgene expressions by generating mild hyperthermia in vitro and in vivo.146–149 Recently, Wu et al. developed FUS-CAR T cells containing an Hsp-driven Cre-lox switch that can be controlled by FUS to activate CAR expression.150 They further engineered a reversible FUS-CAR T cell where CAR production is directly driven by the Hsp [Fig. 5(d)]. The FUS-activated FUS-CAR T cells demonstrated antitumor efficacies in two subcutaneous tumor models; more importantly, the FUS-CAR T cells were shown to cause significantly lower on-target off-tumor toxicity compared with standard constitutive CAR T cells.150 In another Hsp-based design, Miller et al. utilized plasmic gold nanorods to convert near infrared (NIR) light to heat, activating Hsp-driven IL15 superagonist in constitutive CAR T cells. Their results revealed that NIR-activated IL15-expression enhanced the antitumor activity of CAR T cells in vivo.151
CELL THERAPY BASED ON MACROPHAGES AND NATURAL KILLER CELLS
CAR T therapy remains less effective for solid tumors, mainly attributed to the antigen heterogeneity as well as the physical barrier and immune-suppressive tumor microenvironment.152 Solid tumor microenvironment (SME) can attract myeloid cells including macrophages through chemokines secreted by tumor or stroma cells.153 As such, there is abundant number of macrophages in SME. Typically, these macrophages are polarized toward M2 phenotype, which promotes tumors and resist therapeutic treatments by providing physical barrier and suppressive microenvironment.154 Approaches have been designed to deplete these suppressive M2 tumor macrophages to enhance the therapy efficacy.155 Recently, reengineering these tumor macrophages with CAR (CAR-M) has rendered impressive therapeutic efficacy against solid tumors.156 CAR-M cells had a tendency polarizing toward M1 antitumor phenotypes and also indirectly recruited host immunity to eradicate target tumors including ovarian cancer.157 In an alternative approach, integrated sensing and activating protein (iSNAP), integrating the functionality of both protein-based biosensors and activators, was designed to rewire the negative “don't eat me” CD47/SIRPα pathway into activating and pro-phagocytic signaling in macrophages. The results showed that the iSNAP-rewired macrophages possessed a strong capability of negating the inhibitory CD47 signaling and eradicating tumors including non-Hodgkin's lymphoma (NHL) and colon cancer.158 Given the abundance of macrophages at SME, it becomes an attractive topic to convert the phenotypes and functions of these macrophages via genetic engineering for immunotherapy against solid tumors.
Nature killer (NK) cells are lymphocytes playing key roles in the innate immunity. About 10% mononuclear cells are NK cells in peripheral blood samples.159 NK cells are in general, insensitive to antigens presented by major histocompatibility (MHC) molecules. As such, NK cells can be engineered to develop allogenic therapeutic cell products, avoiding GVHD.160 Initial tests showed that CAR-NK rarely causes CRS. This is particularly appealing for the development of “off-the-shelf” therapeutic products.161 In fact, CAR-NK cells have been developed as potent tools against tumors.162–165 NK cells derived from human iPSCs were engineered to express CAR and demonstrate strong antitumor activity against ovarian cancers.166 Further engineering of these iPSC-derived NK-CAR cells by knocking out the gene encoding cytokine-inducible SH2-containing protein which regulates the IL-15 and JAK-STAT pathways, significantly enhanced the metabolic fitness of NK-CAR cells for more efficient toxicity against multiple cancer types.167 However, compared to T cells, NK cells are relatively more difficult to engineer genetically and to maintain expansion and persistence.159 More research activities will hence be warranted to further improve NK-CAR functionality.
In summary, CAR-M is advantageous over CAR T in terms of SME infiltration, but lacks the ability to proliferate, making cell number a limiting factor in CAR-M based therapies. CAR-NK has the advantages of causing less toxicities and significantly reduced risk of GVHD, rendering it a safer and more universal therapy. Its antigen-independent killing capability may also be harnessed to tackle the issue of antigen escape in some tumors. However, CAR-NK also suffers from difficulties in gene delivery and cell expansion. More optimizations are needed to make CAR-M and CAR-NK alternative and complementary treatments to CAR T therapy.
CONCLUSIONS AND PERSPECTIVES
The fast development of CAR T therapy in the past decades is mainly driven by the interrelated feedback loops between clinical trials and laboratory research. Researchers gain new insights into CAR efficacy, toxicity, and resistance from the “bedside” and create new designs of CAR at the “bench” and then reapply these new CARs to the bedside. Multiple cutting-edge frontiers have shifted CAR designs from simply changing co-stimulatory domains to more sophisticated strategies.
The difficulties of CAR T therapy mainly lie in the treatment of solid tumors, especially in the balance between the reduction of on-target off-tumor toxicity and the persistence of T cells in the immunosuppressive tumor microenvironment.152 Modifications of players in key signaling pathways in CAR T cells have shown promising results, including increased trafficking and fitness of T cells.89,168 Meanwhile, controllable designs have demonstrated spatiotemporal precisions in the efficient regulation of T cells at the tumor site.137,150,151 CAR designs applied to other cell types such as macrophages157 and NK cells166 to circumvent the limitations of T cells are also leading to promising results.
However, most of the engineering strategies only focus on improving individual features. Presumably, combinations of multiple designs can equip the T cells with multiple layers of capabilities and may achieve better therapy results. For example, a combination of knock-out or knock-in with controllable T cells will increase T cell homing and fitness and confine T cell activity at the tumor site, thus reducing the off-tumor toxicities. In the future, high-throughput CRISPR-based screening in human T cells88,169 tailored for the controllable system may shift the CAR engineering direction and outcomes. Furthermore, due to the complicated biological crosstalk among different immune cells in the body, the combinations of different cell types for tumor treatment may lead to novel approaches with high efficiency and specificity. For instance, mixing engineered T cells with NK cells has shown promising antitumor efficacy in a multiple myeloma model.170 As new technologies and engineering approaches are rapidly evolving, it is envisioned that cell-based immunotherapy integrating engineering tools and synthetic biology will provide safe, efficient, and precise therapeutic options for a broad range of solid tumors.
ACKNOWLEDGMENTS
This work was supported in part by grants from NIH under Nos. HL121365, R01EB029122, and R35GM140929 (Y. Wang).
Contributor Information
Yiqian Wu, Email: mailto:y5wu@eng.ucsd.edu.
Yingxiao Wang, Email: mailto:yiw015@eng.ucsd.edu.
AUTHOR DECLARATIONS
Conflict of Interest
Y. Wang is a scientific co-founder of Cell E&G Inc and Acoustic Cell Therapy Inc. These financial interest do not affect the writing of this review.
Ethical Statement
Ethics approval was not required.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Porter D. L., Levine B. L., Kalos M., Bagg A., and June C. H., “ Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia,” N. Engl. J. Med. 365, 725–733 (2011). 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brentjens R. J. et al. , “ Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias,” Blood 118, 4817–4828 (2011). 10.1182/blood-2011-04-348540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gill S., Maus M. V., and Porter D. L., “ Chimeric antigen receptor T cell therapy: 25 years in the making,” Blood Rev. 30, 157–167 (2016). 10.1016/j.blre.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 4. Gill S. and June C. H., “ Going viral: Chimeric antigen receptor T-cell therapy for hematological malignancies,” Immunol. Rev. 263, 68–89 (2015). 10.1111/imr.12243 [DOI] [PubMed] [Google Scholar]
- 5. Sermer D. and Brentjens R., “ CAR T-cell therapy: Full speed ahead,” Hematol. Oncol. 37(Suppl. 1), 95–100 (2019). 10.1002/hon.2591 [DOI] [PubMed] [Google Scholar]
- 6. Kuwana Y. et al. , “ Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions,” Biochem. Biophys. Res. Commun. 149, 960–968 (1987). 10.1016/0006-291X(87)90502-X [DOI] [PubMed] [Google Scholar]
- 7. Irving B. A. and Weiss A., “ The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways,” Cell 64, 891–901 (1991). 10.1016/0092-8674(91)90314-O [DOI] [PubMed] [Google Scholar]
- 8. Eshhar Z., Waks T., Gross G., and Schindler D. G., “ Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors,” Proc. Natl. Acad. Sci. U. S. A. 90, 720–724 (1993). 10.1073/pnas.90.2.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gross G., Waks T., and Eshhar Z., “ Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity,” Proc. Natl. Acad. Sci. U. S. A. 86, 10024–10028 (1989). 10.1073/pnas.86.24.10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hwu P. et al. , “ In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes,” Cancer Res. 55, 3369–3373 (1995). [PubMed] [Google Scholar]
- 11. Moritz D., Wels W., Mattern J., and Groner B., “ Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells,” Proc. Natl. Acad. Sci. U. S. A. 91, 4318–4322 (1994). 10.1073/pnas.91.10.4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brocker T., “ Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells,” Blood 96, 1999–2001 (2000). 10.1182/blood.V96.5.1999 [DOI] [PubMed] [Google Scholar]
- 13. Vandenberghe P. et al. , “ Antibody and B7/BB1-mediated ligation of the CD28 receptor induces tyrosine phosphorylation in human T cells,” J. Exp. Med. 175, 951–960 (1992). 10.1084/jem.175.4.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krause A. et al. , “ Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes,” J. Exp. Med. 188, 619–626 (1998). 10.1084/jem.188.4.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hombach A. et al. , “ Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule,” J. Immunol. 167, 6123–6131 (2001). 10.4049/jimmunol.167.11.6123 [DOI] [PubMed] [Google Scholar]
- 16. Finney H. M., Lawson A. D., Bebbington C. R., and Weir A. N., “ Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product,” J. Immunol. 161, 2791–2797 (1998). [PubMed] [Google Scholar]
- 17. Kalos M. et al. , “ T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia,” Sci. Transl. Med. 3, 95ra73 (2011). 10.1126/scitranslmed.3002842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pule M. A. et al. , “ A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells,” Mol. Ther. 12, 933–941 (2005). 10.1016/j.ymthe.2005.04.016 [DOI] [PubMed] [Google Scholar]
- 19. Tammana S. et al. , “ 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies,” Hum. Gene Ther. 21, 75–86 (2010). 10.1089/hum.2009.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jo Y., Ali L. A., Shim J. A., Lee B. H., and Hong C., “ Innovative CAR-T cell therapy for solid tumor; current duel between CAR-T spear and tumor shield,” Cancers 12, 2087 (2020). 10.3390/cancers12082087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinez M. and Moon E. K., “ CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment,” Front. Immunol. 10, 128 (2019). 10.3389/fimmu.2019.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodriguez-Garcia A., Palazon A., Noguera-Ortega E., D. J. Powell, Jr. , and Guedan S., “ CAR-T cells hit the tumor microenvironment: Strategies to overcome tumor escape,” Front. Immunol. 11, 1109 (2020). 10.3389/fimmu.2020.01109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brudno J. N. and Kochenderfer J. N., “ Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management,” Blood Rev. 34, 45–55 (2019). 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neelapu S. S., “ Managing the toxicities of CAR T-cell therapy,” Hematol. Oncol. 37(Suppl. 1), 48–52 (2019). 10.1002/hon.2595 [DOI] [PubMed] [Google Scholar]
- 25. Neelapu S. S. et al. , “ Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities,” Nat. Rev. Clin. Oncol. 15, 47–62 (2018). 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu S., Yi M., Qin S., and Wu K., “ Next generation chimeric antigen receptor T cells: Safety strategies to overcome toxicity,” Mol. Cancer 18, 125 (2019). 10.1186/s12943-019-1057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hong M., Clubb J. D., and Chen Y. Y., “ Engineering CAR-T cells for next-generation cancer therapy,” Cancer Cell 38, 473–488 (2020). 10.1016/j.ccell.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 28. Andrea A. E., Chiron A., Bessoles S., and Hacein-Bey-Abina S., “ Engineering next-generation CAR-T cells for better toxicity management,” Int. J. Mol. Sci. 21, 8620 (2020). 10.3390/ijms21228620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tian Y., Li Y., Shao Y., and Zhang Y., “ Gene modification strategies for next-generation CAR T cells against solid cancers,” J. Hematol. Oncol. 13, 54 (2020). 10.1186/s13045-020-00890-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zah E. et al. , “ Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma,” Nat. Commun. 11, 2283 (2020). 10.1038/s41467-020-16160-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang Y. et al. , “ Bispecific CAR T Cells against EpCAM and inducible ICAM-1 overcome antigen heterogeneity and generate superior antitumor responses,” Cancer Immunol. Res. 9, 1158 (2021). 10.1158/2326-6066.CIR-21-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dai H. et al. , “ Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia,” J. Hematol. Oncol. 13, 30 (2020). 10.1186/s13045-020-00856-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah N. N. et al. , “ Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial,” Nat. Med. 26, 1569–1575 (2020). 10.1038/s41591-020-1081-3 [DOI] [PubMed] [Google Scholar]
- 34. Kim M. S. et al. , “ Redirection of genetically engineered CAR-T cells using bifunctional small molecules,” J. Am. Chem. Soc. 137, 2832–2835 (2015). 10.1021/jacs.5b00106 [DOI] [PubMed] [Google Scholar]
- 35. Lee Y. G. et al. , “ Use of a single CAR T cell and several bispecific adapters facilitates eradication of multiple antigenically different solid tumors,” Cancer Res. 79, 387–396 (2019). 10.1158/0008-5472.CAN-18-1834 [DOI] [PubMed] [Google Scholar]
- 36. Rodgers D. T. et al. , “ Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies,” Proc. Natl. Acad. Sci. U. S. A. 113, E459–68 (2016). 10.1073/pnas.1524155113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Viaud S. et al. , “ Switchable control over in vivo CAR T expansion, B cell depletion, and induction of memory,” Proc. Natl. Acad. Sci. U. S. A. 115, E10898 (2018). 10.1073/pnas.1810060115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paul S. et al. , “ TCR beta chain-directed bispecific antibodies for the treatment of T cell cancers,” Sci. Transl. Med. 13, eabd3595 (2021). 10.1126/scitranslmed.abd3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho J. H., Collins J. J., and Wong W. W., “ Universal chimeric antigen receptors for multiplexed and logical control of T cell responses,” Cell 173, 1426–1438 (2018). 10.1016/j.cell.2018.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lajoie M. J. et al. , “ Designed protein logic to target cells with precise combinations of surface antigens,” Science 369, 1637–1643 (2020). 10.1126/science.aba6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salzer B. et al. , “ Engineering AvidCARs for combinatorial antigen recognition and reversible control of CAR function,” Nat. Commun. 11, 4166 (2020). 10.1038/s41467-020-17970-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujiwara K. et al. , “ Hinge and transmembrane domains of chimeric antigen receptor regulate receptor expression and signaling threshold,” Cells 9, 1182 (2020). 10.3390/cells9051182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brudno J. N. et al. , “ Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma,” Nat. Med. 26, 270–280 (2020). 10.1038/s41591-019-0737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muller Y. D. et al. , “ The CD28-transmembrane domain mediates chimeric antigen receptor heterodimerization with CD28,” Front. Immunol. 12, 639818 (2021). 10.3389/fimmu.2021.639818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ying Z. et al. , “ A safe and potent anti-CD19 CAR T cell therapy,” Nat. Med. 25, 947–953 (2019). 10.1038/s41591-019-0421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li W. et al. , “ Chimeric antigen receptor designed to prevent ubiquitination and downregulation showed durable antitumor efficacy,” Immunity 53, 456–470 (2020). 10.1016/j.immuni.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 47. Feucht J. et al. , “ Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency,” Nat. Med. 25, 82–88 (2019). 10.1038/s41591-018-0290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu W. et al. , “ Multiple signaling roles of CD3ε and its application in CAR-T cell therapy,” Cell 182, 855–871 e23 (2020). 10.1016/j.cell.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 49. Wilkie S. et al. , “ Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling,” J. Clin. Immunol. 32, 1059–1070 (2012). 10.1007/s10875-012-9689-9 [DOI] [PubMed] [Google Scholar]
- 50. Kloss C. C., Condomines M., Cartellieri M., Bachmann M., and Sadelain M., “ Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells,” Nat. Biotechnol. 31, 71–75 (2013). 10.1038/nbt.2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lanitis E. et al. , “ Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo,” Cancer Immunol. Res. 1, 43–53 (2013). 10.1158/2326-6066.CIR-13-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sukumaran S. et al. , “ Enhancing the potency and specificity of engineered T cells for cancer treatment,” Cancer Discov. 8, 972–987 (2018). 10.1158/2159-8290.CD-17-1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu Y. et al. , “ Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors,” Sci. Transl. Med. 13, eabb5191 (2021). 10.1126/scitranslmed.abb5191 [DOI] [PubMed] [Google Scholar]
- 54. Walseng E. et al. , “ A TCR-based chimeric antigen receptor,” Sci. Rep. 7, 10713 (2017). 10.1038/s41598-017-11126-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hawkins E. R., D'Souza R. R., and Klampatsa A., “ Armored CAR T-cells: The next chapter in T-cell cancer immunotherapy,” Biologics 15, 95–105 (2021). 10.2147/BTT.S291768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yeku O. O., Purdon T. J., Koneru M., Spriggs D., and Brentjens R. J., “ Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment,” Sci. Rep. 7, 10541 (2017). 10.1038/s41598-017-10940-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alizadeh D. et al. , “ IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype,” Cancer Immunol. Res. 7, 759–772 (2019). 10.1158/2326-6066.CIR-18-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Santana Carrero R. M. et al. , “ IL-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses,” Proc. Natl. Acad. Sci. U. S. A. 116, 599–608 (2019). 10.1073/pnas.1814642116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen Y. et al. , “ Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15,” Clin. Cancer Res. 25, 2915–2924 (2019). 10.1158/1078-0432.CCR-18-1811 [DOI] [PubMed] [Google Scholar]
- 60. Adachi K. et al. , “ IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor,” Nat. Biotechnol. 36, 346–351 (2018). 10.1038/nbt.4086 [DOI] [PubMed] [Google Scholar]
- 61. Luo H. et al. , “ Coexpression of IL7 and CCL21 increases efficacy of CAR-T cells in solid tumors without requiring preconditioned lymphodepletion,” Clin. Cancer Res. 26, 5494–5505 (2020). 10.1158/1078-0432.CCR-20-0777 [DOI] [PubMed] [Google Scholar]
- 62. Di Stasi A. et al. , “ T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model,” Blood 113, 6392–6402 (2009). 10.1182/blood-2009-03-209650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Whilding L. M. et al. , “ CAR T-cells targeting the integrin ανβ6 and co-expressing the chemokine receptor CXCR2 demonstrate enhanced homing and efficacy against several solid malignancies,” Cancers 11, 674 (2019). 10.3390/cancers11050674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu G. et al. , “ CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma,” Eur. J. Immunol. 50, 712–724 (2020). 10.1002/eji.201948457 [DOI] [PubMed] [Google Scholar]
- 65. Jin L. et al. , “ CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors,” Nat. Commun. 10, 4016 (2019). 10.1038/s41467-019-11869-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moon E. K. et al. , “ Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor,” Clin. Cancer Res. 17, 4719–4730 (2011). 10.1158/1078-0432.CCR-11-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mikucki M. E. et al. , “ Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints,” Nat. Commun. 6, 7458 (2015). 10.1038/ncomms8458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Itoh-Nakadai A. et al. , “ CXCR4-expressing anti-CD25 CAR T-cells effectively eliminate human AML cells in vivo,” Blood 136, 35–36 (2020). 10.1182/blood-2020-142228 [DOI] [Google Scholar]
- 69. Jin L. Y. et al. , “ Enhance anti-lung tumor efficacy of chimeric antigen receptor-T cells by ectopic expression of C–C motif chemokine receptor 6,” Sci. Bull. 66, 803–812 (2021). 10.1016/j.scib.2020.12.027 [DOI] [PubMed] [Google Scholar]
- 70. Caruana I. et al. , “ Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes,” Nat. Med. 21, 524–529 (2015). 10.1038/nm.3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kloss C. C. et al. , “ Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication,” Mol. Ther. 26, 1855–1866 (2018). 10.1016/j.ymthe.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kiesgen S. et al. , “ Regional delivery of clinical-grade mesothelin-targeted CAR T cells with cell-intrinsic PD-1 checkpoint blockade: Translation to a phase I trial,” Cancer Res. 80, LB–378 (2020). 10.1158/1538-7445.AM2020-LB-378 [DOI] [Google Scholar]
- 73. Chen N., Morello A., Tano Z., and Adusumilli P. S., “ CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy,” Oncoimmunology 6, e1273302 (2017). 10.1080/2162402X.2016.1273302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jiang Z. et al. , “ HIV-1-specific CAR-T cells with cell-intrinsic PD-1 checkpoint blockade enhance anti-HIV efficacy in vivo,” Front. Microbiol. 12, 684016 (2021). 10.3389/fmicb.2021.684016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roth T. L. et al. , “ Pooled knockin targeting for genome engineering of cellular immunotherapies,” Cell 181, 728–744 (2020). 10.1016/j.cell.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pardoll D. M., “ The blockade of immune checkpoints in cancer immunotherapy,” Nat. Rev. Cancer 12, 252–264 (2012). 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hargadon K. M., Johnson C. E., and Williams C. J., “ Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors,” Int. Immunopharmacol. 62, 29–39 (2018). 10.1016/j.intimp.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 78. John L. B. et al. , “ Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells,” Clin. Cancer Res. 19, 5636–5646 (2013). 10.1158/1078-0432.CCR-13-0458 [DOI] [PubMed] [Google Scholar]
- 79. Fedorov V. D., Themeli M., and Sadelain M., “ PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses,” Sci. Transl. Med. 5, 215ra172 (2013). 10.1126/scitranslmed.3006597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li S. et al. , “ Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors,” Clin. Cancer Res. 23, 6982–6992 (2017). 10.1158/1078-0432.CCR-17-0867 [DOI] [PubMed] [Google Scholar]
- 81. Su S. et al. , “ CRISPR-Cas9 mediated efficient PD-1 disruption on human primary T cells from cancer patients,” Sci. Rep. 6, 20070 (2016). 10.1038/srep20070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rupp L. J. et al. , “ CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells,” Sci. Rep. 7, 737 (2017). 10.1038/s41598-017-00462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hu W. et al. , “ CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions,” Cancer Immunol. Immunother. 68, 365–377 (2019). 10.1007/s00262-018-2281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ren J. et al. , “ Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition,” Clin. Cancer Res. 23, 2255–2266 (2017). 10.1158/1078-0432.CCR-16-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Condomines M. et al. , “ Tumor-targeted human T cells expressing CD28-based chimeric antigen receptors circumvent CTLA-4 inhibition,” PLoS One 10, e0130518 (2015). 10.1371/journal.pone.0130518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ren J. et al. , “ A versatile system for rapid multiplex genome-edited CAR T cell generation,” Oncotarget 8, 17002–17011 (2017). 10.18632/oncotarget.15218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mirzaei H. R. et al. , “ Gene-knocked out chimeric antigen receptor (CAR) T cells: Tuning up for the next generation cancer immunotherapy,” Cancer Lett. 423, 95–104 (2018). 10.1016/j.canlet.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 88. Shifrut E. et al. , “ Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function,” Cell 175, 1958–1971 (2018). 10.1016/j.cell.2018.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wei J. et al. , “ Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy,” Nature 576, 471–476 (2019). 10.1038/s41586-019-1821-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fraietta J. A. et al. , “ Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells,” Nature 558, 307–312 (2018). 10.1038/s41586-018-0178-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ruella M. and Kenderian S. S., “ Next-generation chimeric antigen receptor T-cell therapy: Going off the shelf,” BioDrugs 31, 473–481 (2017). 10.1007/s40259-017-0247-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Poirot L. et al. , “ Multiplex genome-edited t-cell manufacturing platform for ‘off-the-shelf’ adoptive T-cell immunotherapies,” Cancer Res. 75, 3853–3864 (2015). 10.1158/0008-5472.CAN-14-3321 [DOI] [PubMed] [Google Scholar]
- 93. Qasim W. et al. , “ Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells,” Sci. Transl. Med. 9, eaaj2013 (2017). 10.1126/scitranslmed.aaj2013 [DOI] [PubMed] [Google Scholar]
- 94. Benjamin R. et al. , “ Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: Results of two phase 1 studies,” Lancet 396, 1885–1894 (2020). 10.1016/S0140-6736(20)32334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Eyquem J. et al. , “ Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection,” Nature 543, 113–117 (2017). 10.1038/nature21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. MacLeod D. T. et al. , “ Integration of a CD19 CAR into the TCR alpha chain locus streamlines production of allogeneic gene-edited CAR T cells,” Mol. Ther. 25, 949–961 (2017). 10.1016/j.ymthe.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sommer C. et al. , “ Allogeneic FLT3 CAR T Cells with an off-switch exhibit potent activity against AML and can be depleted to expedite bone marrow recovery,” Mol. Ther. 28, 2237–2251 (2020). 10.1016/j.ymthe.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Giordano-Attianese G. et al. , “ A computationally designed chimeric antigen receptor provides a small-molecule safety switch for T-cell therapy,” Nat. Biotechnol. 38, 426–432 (2020). 10.1038/s41587-019-0403-9 [DOI] [PubMed] [Google Scholar]
- 99. Gargett T. and Brown M. P., “ The inducible caspase-9 suicide gene system as a ‘safety switch’ to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells,” Front. Pharmacol. 5, 235 (2014). 10.3389/fphar.2014.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ayuk F. et al. , “ Excellent proliferation and persistence of allogeneic donor-derived 41-BB based CAR-T cells despite immunosuppression with cyclosporine A,” Haematologica 105, 322–324 (2020). 10.3324/haematol.2019.245969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mo F. et al. , “ Engineered off-the-shelf therapeutic T cells resist host immune rejection,” Nat. Biotechnol. 39, 56–63 (2021). 10.1038/s41587-020-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kochenderfer J. N. et al. , “ B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells,” Blood 119, 2709–2720 (2012). 10.1182/blood-2011-10-384388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee D. W. et al. , “ Current concepts in the diagnosis and management of cytokine release syndrome,” Blood 124, 188–195 (2014). 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brudno J. N. and Kochenderfer J. N., “ Toxicities of chimeric antigen receptor T cells: Recognition and management,” Blood 127, 3321–3330 (2016). 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Morgan R. A. et al. , “ Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2,” Mol. Ther. 18, 843–851 (2010). 10.1038/mt.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Roybal K. T. et al. , “ Precision tumor recognition by T cells with combinatorial antigen-sensing circuits,” Cell 164, 770–779 (2016). 10.1016/j.cell.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Themeli M. and Sadelain M., “ Combinatorial antigen targeting: ideal T-cell sensing and anti-tumor response,” Trends Mol. Med. 22, 271–273 (2016). 10.1016/j.molmed.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Srivastava S. et al. , “ Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting,” Cancer Cell 35, 489–503 (2019). 10.1016/j.ccell.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hyrenius-Wittsten A. et al. , “ SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models,” Sci. Transl. Med. 13, eabd8836 (2021). 10.1126/scitranslmed.abd8836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Williams J. Z. et al. , “ Precise T cell recognition programs designed by transcriptionally linking multiple receptors,” Science 370, 1099–1104 (2020). 10.1126/science.abc6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Choe J. H. et al. , “ SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma,” Sci. Transl. Med. 13, eabe7378 (2021). 10.1126/scitranslmed.abe7378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ede C., Chen X., Lin M. Y., and Chen Y. Y., “ Quantitative analyses of core promoters enable precise engineering of regulated gene expression in mammalian cells,” ACS Synth. Biol. 5, 395–404 (2016). 10.1021/acssynbio.5b00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Juillerat A. et al. , “ An oxygen sensitive self-decision making engineered CAR T-cell,” Sci. Rep. 7, 39833 (2017). 10.1038/srep39833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kosti P. et al. , “ Hypoxia-sensing CAR T cells provide safety and efficacy in treating solid tumors,” Cell Rep. Med. 2, 100227 (2021). 10.1016/j.xcrm.2021.100227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wu C. Y., Roybal K. T., Puchner E. M., Onuffer J., and Lim W. A., “ Remote control of therapeutic T cells through a small molecule-gated chimeric receptor,” Science 350, aab4077 (2015). 10.1126/science.aab4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Voss S., Klewer L., and Wu Y. W., “ Chemically induced dimerization: Reversible and spatiotemporal control of protein function in cells,” Curr. Opin. Chem. Biol. 28, 194–201 (2015). 10.1016/j.cbpa.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 117. Jan M. et al. , “ Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide,” Sci. Transl. Med. 13, eabb6295 (2021). 10.1126/scitranslmed.abb6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zheng Y. et al. , “ Generation of regulable EGFRvIII targeted chimeric antigen receptor T cells for adoptive cell therapy of glioblastoma,” Biochem. Biophys. Res. Commun. 507, 59–66 (2018). 10.1016/j.bbrc.2018.10.151 [DOI] [PubMed] [Google Scholar]
- 119. Sakemura R. et al. , “ A tet-on inducible system for controlling CD19-chimeric antigen receptor expression upon drug administration,” Cancer Immunol. Res. 4, 658–668 (2016). 10.1158/2326-6066.CIR-16-0043 [DOI] [PubMed] [Google Scholar]
- 120. Yang L. et al. , “ Engineering genetic devices for in vivo control of therapeutic T cell activity triggered by the dietary molecule resveratrol,” Proc. Natl. Acad. Sci. U. S. A. 118, e2106612118 (2021). 10.1073/pnas.2106612118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Budde L. E. et al. , “ Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma,” PLoS One 8, e82742 (2013). 10.1371/journal.pone.0082742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Straathof K. C. et al. , “ An inducible caspase 9 safety switch for T-cell therapy,” Blood 105, 4247–4254 (2005). 10.1182/blood-2004-11-4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Diaconu I. et al. , “ Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells,” Mol. Ther. 25, 580–592 (2017). 10.1016/j.ymthe.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Di Stasi A. et al. , “ Inducible apoptosis as a safety switch for adoptive cell therapy,” N. Engl. J. Med. 365, 1673–1683 (2011). 10.1056/NEJMoa1106152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhou X. O. et al. , “ Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene,” Blood 123, 3895–3905 (2014). 10.1182/blood-2014-01-551671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhou X. et al. , “ Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation,” Blood 125, 4103–4113 (2015). 10.1182/blood-2015-02-628354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Richman S. A. et al. , “ Ligand-induced degradation of a CAR permits reversible remote control of CAR T cell activity in vitro and in vivo,” Mol. Ther. 28, 1600–1613 (2020). 10.1016/j.ymthe.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Weber E. W. et al. , “ Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling,” Science 372, eaba1786 (2021). 10.1126/science.aba1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lee S. M. et al. , “ A chemical switch system to modulate chimeric antigen receptor T cell activity through proteolysis-targeting chimaera technology,” ACS Synth. Biol. 9, 987–992 (2020). 10.1021/acssynbio.9b00476 [DOI] [PubMed] [Google Scholar]
- 130. Gao H., Sun X., and Rao Y., “ PROTAC technology: Opportunities and challenges,” ACS Med. Chem. Lett. 11, 237–240 (2020). 10.1021/acsmedchemlett.9b00597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mestermann K. et al. , “ The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells,” Sci. Transl. Med. 11, eaau5907 (2019). 10.1126/scitranslmed.aau5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Weber E. W. et al. , “ Pharmacologic control of CAR-T cell function using dasatinib,” Blood Adv. 3, 711–717 (2019). 10.1182/bloodadvances.2018028720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang Y. et al. , “ Low-dose decitabine priming endows CAR T cells with enhanced and persistent antitumour potential via epigenetic reprogramming,” Nat. Commun. 12, 409 (2021). 10.1038/s41467-020-20696-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Deisseroth K., “ Optogenetics,” Nat. Methods 8, 26–29 (2011). 10.1038/nmeth.f.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pathak G. P., Vrana J. D., and Tucker C. L., “ Optogenetic control of cell function using engineered photoreceptors,” Biol. Cell 105, 59–72 (2013). 10.1111/boc.201200056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kennedy M. J. et al. , “ Rapid blue-light-mediated induction of protein interactions in living cells,” Nat. Methods 7, 973–975 (2010). 10.1038/nmeth.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Huang Z. et al. , “ Engineering light-controllable CAR T cells for cancer immunotherapy,” Sci. Adv. 6, eaay9209 (2020). 10.1126/sciadv.aay9209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang B. et al. , “ Photoswitchable CAR-T cell function in vitro and in vivo via a cleavable mediator,” Cell Chem Biol 28, 60–69 (2021). 10.1016/j.chembiol.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 139. O'Donoghue G. P. et al. , “ T cells selectively filter oscillatory signals on the minutes timescale,” Proc. Natl. Acad. Sci. U. S. A. 118, e2019285118 (2021). 10.1073/pnas.2019285118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. He L. et al. , “ Circularly permuted LOV2 as a modular photoswitch for optogenetic engineering,” Nat. Chem. Biol. 17, 915–923 (2021). 10.1038/s41589-021-00792-9 [DOI] [PubMed] [Google Scholar]
- 141. Chen S. et al. , “ Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics,” Science 359, 679–684 (2018). 10.1126/science.aaq1144 [DOI] [PubMed] [Google Scholar]
- 142. Park S. I. et al. , “ Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics,” Nat. Biotechnol. 33, 1280–1286 (2015). 10.1038/nbt.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jeong J. W. et al. , “ Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics,” Cell 162, 662–674 (2015). 10.1016/j.cell.2015.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Nguyen N. T. et al. , “ Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety,” Nat. Nanotechnol. 16, 1424 (2021). 10.1038/s41565-021-00982-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pan Y. et al. , “ Mechanogenetics for the remote and noninvasive control of cancer immunotherapy,” Proc. Natl. Acad. Sci. U. S. A. 115, 992–997 (2018). 10.1073/pnas.1714900115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Smith R. C., Machluf M., Bromley P., Atala A., and Walsh K., “ Spatial and temporal control of transgene expression through ultrasound-mediated induction of the heat shock protein 70B promoter in vivo,” Hum. Gene Ther. 13, 697–706 (2002). 10.1089/104303402317322267 [DOI] [PubMed] [Google Scholar]
- 147. Plathow C. et al. , “ Focal gene induction in the liver of rats by a heat-inducible promoter using focused ultrasound hyperthermia: Preliminary results,” Invest. Radiol. 40, 729–735 (2005). 10.1097/01.rli.0000184763.62578.06 [DOI] [PubMed] [Google Scholar]
- 148. Liu Y., Kon T., Li C., and Zhong P., “ High intensity focused ultrasound-induced gene activation in solid tumors,” J. Acoust. Soc. Am. 120, 492–501 (2006). 10.1121/1.2205129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Deckers R. et al. , “ Image-guided, noninvasive, spatiotemporal control of gene expression,” Proc. Natl. Acad. Sci. U. S. A. 106, 1175–1180 (2009). 10.1073/pnas.0806936106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wu Y. et al. , “ Control of the activity of CAR-T cells within tumours via focused ultrasound,” Nat. Biomed. Eng. 5, 1336–1347 (2021). 10.1038/s41551-021-00779-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Miller I. C. et al. , “ Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control,” Nat. Biomed. Eng. 5, 1348–1359 (2021). 10.1038/s41551-021-00781-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hou A. J., Chen L. C., and Chen Y. Y., “ Navigating CAR-T cells through the solid-tumour microenvironment,” Nat. Rev. Drug Discov. 20, 531–550 (2021). 10.1038/s41573-021-00189-2 [DOI] [PubMed] [Google Scholar]
- 153. Biswas S. K., Allavena P., and Mantovani A., “ Tumor-associated macrophages: Functional diversity, clinical significance, and open questions,” Semin. Immunopathol. 35, 585–600 (2013). 10.1007/s00281-013-0367-7 [DOI] [PubMed] [Google Scholar]
- 154. Gabrilovich D. I., Ostrand-Rosenberg S., and Bronte V., “ Coordinated regulation of myeloid cells by tumours,” Nat. Rev. Immunol. 12, 253–268 (2012). 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mantovani A., Marchesi F., Malesci A., Laghi L., and Allavena P., “ Tumour-associated macrophages as treatment targets in oncology,” Nat. Rev. Clin. Oncol. 14, 399–416 (2017). 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chen Y. et al. , “ CAR-macrophage: A new immunotherapy candidate against solid tumors,” Biomed. Pharmacother. 139, 111605 (2021). 10.1016/j.biopha.2021.111605 [DOI] [PubMed] [Google Scholar]
- 157. Klichinsky M. et al. , “ Human chimeric antigen receptor macrophages for cancer immunotherapy,” Nat. Biotechnol. 38, 947–953 (2020). 10.1038/s41587-020-0462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sun J. et al. , “ Engineered proteins with sensing and activating modules for automated reprogramming of cellular functions,” Nat. Commun. 8, 477 (2017). 10.1038/s41467-017-00569-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Schmidt P., Raftery M. J., and Pecher G., “ Engineering NK cells for CAR therapy-recent advances in Gene Transfer Methodology,” Front. Immunol. 11, 611163 (2020). 10.3389/fimmu.2020.611163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gill S., Olson J. A., and Negrin R. S., “ Natural killer cells in allogeneic transplantation: Effect on engraftment, graft- versus-tumor, and graft-versus-host responses,” Biol. Blood Marrow Transplant. 15, 765–776 (2009). 10.1016/j.bbmt.2009.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Goldenson B. H. et al. , “ Umbilical cord blood and iPSC-derived natural killer cells demonstrate key differences in cytotoxic activity and KIR profiles,” Front. Immunol. 11, 561553 (2020). 10.3389/fimmu.2020.561553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Xie G. et al. , “ CAR-NK cells: A promising cellular immunotherapy for cancer,” EBioMedicine 59, 102975 (2020). 10.1016/j.ebiom.2020.102975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Marofi F. et al. , “ CAR-NK cell: A new paradigm in tumor immunotherapy,” Front. Oncol. 11, 673276 (2021). 10.3389/fonc.2021.673276 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 164. Gong Y., Klein Wolterink R. G. J., Wang J., Bos G. M. J., and Germeraad W. T. V., “ Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy,” J. Hematol. Oncol. 14, 73 (2021). 10.1186/s13045-021-01083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Basar R., Daher M., and Rezvani K., “ Next-generation cell therapies: The emerging role of CAR-NK cells,” Blood Adv. 4, 5868–5876 (2020). 10.1182/bloodadvances.2020002547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Li Y., Hermanson D. L., Moriarity B. S., and Kaufman D. S., “ Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity,” Cell Stem Cell 23, 181–192 (2018). 10.1016/j.stem.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhu H. et al. , “ Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity,” Cell Stem Cell 27, 224–237 (2020). 10.1016/j.stem.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Seo H. et al. , “ BATF and IRF4 cooperate to counter exhaustion in tumor-infiltrating CAR T cells,” Nat. Immunol. 22, 983–995 (2021). 10.1038/s41590-021-00964-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wang D. et al. , “ CRISPR screening of CAR T cells and cancer stem cells reveals critical dependencies for cell-based therapies,” Cancer Discov. 11, 1192–1211 (2021). 10.1158/2159-8290.CD-20-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bachiller M. et al. , “ NK cells enhance CAR-T cell antitumor efficacy by enhancing immune/tumor cells cluster formation and improving CAR-T cell fitness,” J. Immunother. Cancer 9, e002866 (2021). 10.1136/jitc-2021-002866 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.