Abstract
Infection with SARS-CoV-2 has dominated discussion and caused global healthcare and economic crisis over the past 18 months. Coronavirus disease 19 (COVID-19) causes mild-to-moderate symptoms in most individuals. However, rapid deterioration to severe disease with or without acute respiratory distress syndrome (ARDS) can occur within 1–2 weeks from the onset of symptoms in a proportion of patients. Early identification by risk stratifying such patients who are at risk of severe complications of COVID-19 is of great clinical importance. Computed tomography (CT) is widely available and offers the potential for fast triage, robust, rapid, and minimally invasive diagnosis: Ground glass opacities (GGO), crazy-paving pattern (GGO with superimposed septal thickening), and consolidation are the most common chest CT findings in COVID pneumonia. There is growing interest in the prognostic value of baseline chest CT since an early risk stratification of patients with COVID-19 would allow for better resource allocation and could help improve outcomes. Recent studies have demonstrated the utility of baseline chest CT to predict intensive care unit (ICU) admission in patients with COVID-19. Furthermore, developments and progress integrating artificial intelligence (AI) with computer-aided design (CAD) software for diagnostic imaging allow for objective, unbiased, and rapid assessment of CT images.
Keywords: COVID-19, Chest CT, ICU admission, Artificial intelligence
Introduction
Infection with SARS-CoV-2 has dominated discussion and caused global healthcare and economic crisis over the past 18 months. The effects of the virus were first observed in Wuhan, China, in December 2019. The virus rapidly spread across the globe, and in March 2020, the World Health Organization (WHO) declared coronavirus disease 19 (COVID-19) a pandemic. COVID-19 causes mild-to-moderate symptoms in most, but rapid deterioration to severe disease with or without acute respiratory distress syndrome (ARDS) can occur within 1–2 weeks from the onset of symptoms in a proportion of patients [1]. Patients with severe disease often require treatment in intensive care units; therefore, the early identification of such patients who are at risk of severe complications of COVID-19 is of great clinical importance.
The prevalence of severe COVID-19 is reported to be 15.7–26.1% among hospitalized patients. These cases were often associated with abnormal chest computed tomography (CT) findings and clinical laboratory data, e.g., age, comorbidities, and symptoms [2]. There is growing interest in the prognostic value of chest CT from the time of initial presentation with suspected COVID-19. Expedient and early risk stratification of patients with COVID-19 would allow for better resource allocation and could help improve outcomes.
Furthermore, developments and progress integrating artificial intelligence (AI) with computer-aided design (CAD) software for diagnostic imaging allow for objective, unbiased, and rapid assessment of CT images [3].
In this comprehensive review, we will discuss the role and prognostic value of the baseline chest CT in COVID-19 patients, as well as future directions in this field.
Chest CT in the diagnosis of COVID-19 pneumonia
Patients with a suspected diagnosis of COVID-19 pneumonia usually undergo non-contrast material-enhanced chest CT [4], performed with a high-resolution technique (using thin sections < 1.5 mm) and high-spatial-resolution kernel to enhance visualization of lung parenchyma anatomy. Sometimes, contrast medium injection is required to rule out pulmonary embolism as COVID-19 thrombotic and thromboembolic complications may be suspected [5].
Ground glass opacities (GGO), crazy-paving pattern (GGO with superimposed septal thickening), and consolidation are the most common chest CT findings in COVID-19 pneumonia (Fig. 1) [6]. These findings are usually bilateral and multilobar, mostly distributed in the subpleural/peripheral and posterior regions of the lungs [7], but occasionally they can show a bronchovascular distribution or a combination of both.
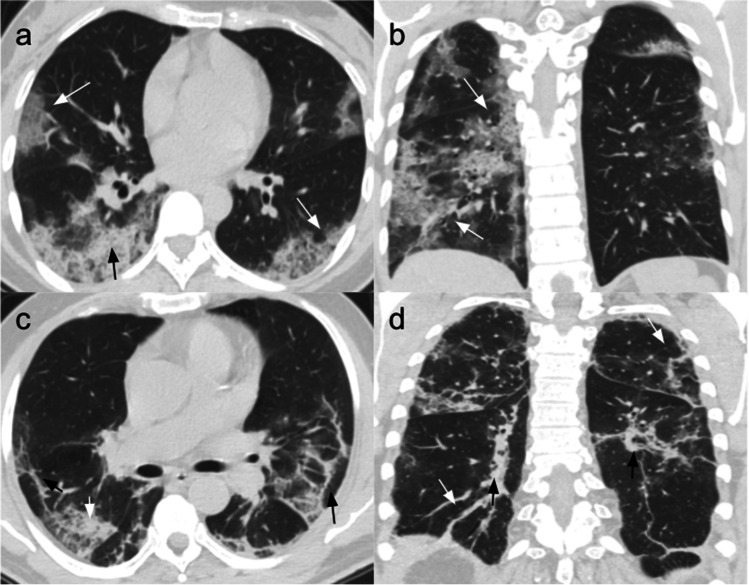
Fig. 1.
Common chest CT findings in COVID-19 pneumonia. Patient 1: CT scans of a 47-year-old woman affected by COVID-19 pneumonia and hospitalized for 6 days without ICU admission. She was treated with antiviral and antibiotic therapy, hydroxychloroquine, and low flow nasal cannula (2 ml/min). (a) Non-contrast CT scan, axial plane, performed at admission showing bilateral crazy-paving opacities (white arrows) and right posterior consolidation (black arrow). (b) Non-contrast coronal plane showing bilateral asymmetric GGOs and crazy-paving areas (white arrows), mostly in the posterior subpleural lung regions. Patient 2: CT scans of a 73-year-old man with COVID-19 pneumonia, hospitalized for 12 days without ICU admission. He was treated with a low flow nasal cannula (ranging from 2 to 4 ml/min), antibiotics, and IV fluids. (c) Non-contrast CT scan, axial plane, performed at admission showing bilateral GGOs with superimposed interlobular and intralobular septal thickening (white arrow), and architectural distortion appearing in the peripheral areas (black arrows). (d) Non-contrast coronal plane showing architectural distortion with bilateral subpleural lines (white arrows) and traction bronchiectasis (black arrows)
During the course of the disease, GGOs rapidly increase and become consolidated and/or associated with a crazy-paving pattern until the peak of CT lung involvement, which is usually observed 9–13 days after the symptoms onset. The severity of the findings slowly reduces in the absorption phase [8].
Pleural effusion, lung cavitation, lymphadenopathy, and calcification are not typically seen [9, 10].
Centrilobular nodules with the tree-in-bud pattern are not distinctive and likely indicate other causes of pneumonia [11].
Chest CT as a prognostic tool in COVID-19 patients
Prognostic value of chest CT
Resource allocation toward diagnosing and managing COVID-19 is still a critical issue. Understanding the prognostic value of a baseline CT to assess the outcome of the disease in the earliest phases of onset could help lead to improved resource distribution.
A parameter that has proven to be fundamental for patient risk stratification is the timing of the scan. The sensitivity of the CT scan is highest when it is performed within the first two to three weeks from the onset of the symptoms [12, 13]. Li et al. demonstrated that the prognostic value of chest CT increased if performed at least 6 days after onset of symptoms. In the first 5 days (first period), there is no significant difference between severe and non-severe patients, while, starting from the second period (6–10 days), CT images contain more prognostic elements: While survivors reach the severity peak up to 10 days, the severity of non-survivors CT progressively increases up to 20 days after symptoms onset [14].
Furthermore, there have been numerous attempts made to standardize reporting of chest CT for suspected COVID-19 as a grading system of chest CT findings in COVID-19 patients may facilitate both the communication and significance of results as well as more efficient diagnosis of disease. Higher CT scores from such grading systems are associated with worse outcomes, including a higher mortality risk, showing the importance of imaging when managing patients and evaluating their prognosis [15–17].
Pulmonary prognostic findings for ICU admission
The type and the extent of initial pulmonary lesions in intensive care unit (ICU) patients have been observed to differ from those seen in less severely ill patients [18–20].
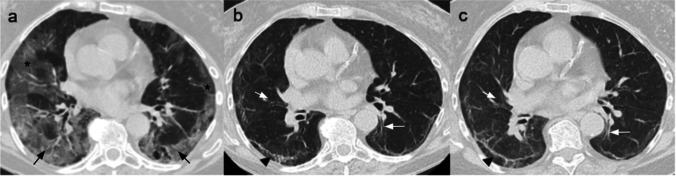
More severe disease is associated with the scattered bilateral distribution of lesions either in the subpleural and centro-parenchymal areas, a higher number of involved lobes, a higher percentage of the involved lung parenchyma, and exhibit the features typical of the progressive stage of the disease. The latter include the coexistence of diffuse GGO and consolidations (Fig. 2) [21, 22].
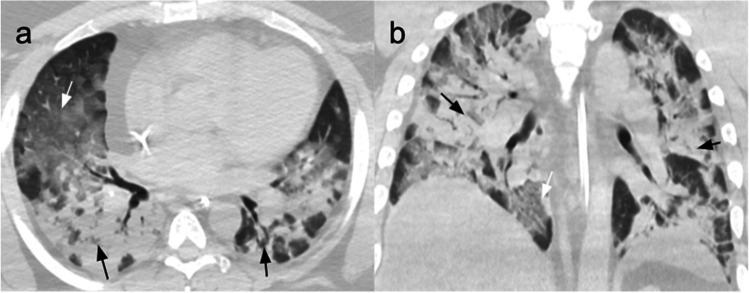
Fig. 2.
CT scans of a 36-year-old man affected by severe COVID-19 pneumonia and hospitalized for 11 days with ICU admission on the second day, after being treated with CPAP. In ICU, he went through seven cycles of pronation with progressive improvement of lung distress. (a) Non-contrast CT scan performed on the first day in ICU, axial plane, showing GGOs (white arrow) and consolidation (black arrows) in all the lobes, with only a few areas of normal parenchyma. (b) Non-contrast coronal plane CT scan showing diffuse bilateral consolidation crazy-paving pattern involving the majority of the lung parenchyma
Different patterns from chest CT portray different prognostic roles, as demonstrated by Liang et al. They showed that mixed and reticular patterns may be indicative of a better outcome as they represent resolving of inflammation. The absence of mixed and reticular patterns, on the other hand, was linked to a worse prognosis, as the pneumonia was shifted toward a worsening disease pattern rather than toward resolution [23].
Erturk et al. added that, in patients that died from COVID-19 or were admitted to ICU, crazy paving, bronchus distortion, bronchiectasis, air trapping, and enlargement of mediastinal-hilar nodes were more common and significantly correlated with prolonged hospitalization [24].
The extent of lesions has been used by Aydemir et al. to classify patients into 4 groups and study the relationship with adverse events. They found a positive correlation between pulmonary involvement and rate of ICU transfer with significant differences among the different groups: 2.2% for group 0 (no lesions), 5.6% for group 1 (unilateral and few lesions in one segment or lobe), 13.5% for group 2 (≤ 3 lesions in multiple lobes or segments), and 17.7% for group 3 (diffuse and bilateral lesions) [25].
The use of pattern categorization of CT findings has been tested by Jin et al. and compared with clinical outcomes, i.e., ICU, mechanical ventilation (MV), or death.
They divided their series of CT scans acquired within 2 weeks after symptom onset into 4 groups: pattern 0 (negative), pattern 1 (bronchopneumonia pattern), pattern 2 (organizing pneumonia pattern), pattern 3 (progressive organizing pneumonia pattern), and pattern 4 (diffuse alveolar damage pattern). They found that this CT pattern correlates can play a prognostic role in the stratification of these patients, helping in the decision-making process and in the allocation of healthcare resources. Particularly, they observed that patients with CT pattern 4 were those who shared a higher risk of admission to ICU/mechanical ventilation/death, while patterns 3 and 4 could correlate with pulmonary residuals on CT [26].
In a study by Chon et al., pleural effusions were not typical features for COVID-19 pneumonia but were nonetheless a better prognostic factor: Their presence increases the risk (OR 19.41) for critical events (ICU admission or death) with a 19.41 odds ratio (OR), higher than those of parenchymal lesions (OR 7.15 for crazy paving) [27]. Abkhoo et al. included pleural effusion in the significant factors able to predict mortality in patients admitted to ICU, together with cardiomegaly and pericardial effusion. They also considered hypertension and low oxygen saturation as predisposing factors for mortality, creating a model with 90.0% PPV [28].
Furthermore, specific vascular changes (VCs) can predict disease progression due to their relation with respiratory distress, increasing the risk for hospitalization and ICU need. The considered VCs were thinning or enlargement, irregular course due to angulation or traction, vessel wall irregularity, bronchovascular ectasia, and annular segmental concentric contraction (vascular knuckle) that correlated with the diameter and the location of the lesions, especially if central and in the middle lobe [29].
The prognostic role of pulmonary findings has been further investigated by Hegazu et al. in a cohort of 168 ICU patients with COVID-19. The majority of patients had multifocal and bilateral GGOs, significantly correlated with SOFA score on admission and with specific comorbidities, mainly cardiovascular disease and obesity. No significant correlation has been observed between radiographic findings and mortality, despite a higher incidence of multifocal and bilateral consolidations in death patients [30].
Table 1 provides a summary of included papers focused on the evaluation of pulmonary prognostic findings for ICU admission.
Table 1.
Pulmonary prognostic findings for ICU admission
| Reference | Author | Year | CT findings | Prognostic value |
|---|---|---|---|---|
| [18] | Meiler | 2020 |
Significantly higher incidence in patients with a negative outcome (33/64): - consolidation (88%) - crazy paving (42%) - geographic shape of opacification (55%) - bronchial dilatation (27%) - air bronchogram (82%) - pleural effusion (30%) - vessel enlargement (64%) - bilateral involvement (100%) - RML involvement (100%) - extent of parenchymal opacifications > 66% of lung volume (39%) |
Independent predictors of a negative outcome (mechanical ventilation, ICU admission, extracorporeal membrane oxygenation, death): crazy paving – OR 8.9, extent of parenchymal opacifications > 66% of lung volume – OR 6.04 OR– negative outcome: 3.39 – dyspnea |
| [19] | Parry | 2020 |
Significantly higher incidence in clinically unstable patients (20/89): - consolidation (80%) - crazy paving (70%) - vessel enlargement (90%) - air bronchogram (65%) - peripheral and central distribution (85%) - anteroposterior distribution (70%) - bilateral involvement (95%) - percentage of total lung involvement (median 39.1%) |
Increased frequency of specific CT findings in clinically unstable patients (ICU admission or death) – indicators of poor short-term prognosis Higher frequency in clinically unstable patients: - older age (median age 63.6 vs. 44.6) |
| [20] | Tabatabaei | 2020 |
Significantly higher incidence in ICU patients (11/120): - consolidation (82%) - crazy paving (45%) - air bronchogram (45%) - peripheral and central involvement (82%) - percentage of total lung involvement (median 36.52, combined with death group) - pleural effusion (45%) |
Increased frequency of specific CT findings in ICU patients – indicators of poor short-term prognosis |
| [21] | Cau | 2021 |
Significantly higher incidence in ICU patients (23/218): - consolidation - mixed lesions - bilateral opacities - extensive involvement (GGO + consolidations) |
Higher frequency in ICU patients: - male sex - comorbidities (cancer) - abnormal laboratory values: high CRP and LDH - high risk of mortality |
| [22] | Tekcan Sanli | 2020 |
Significantly higher incidence in ICU patients (20/231): - consolidation (65%) - affected lobe number (median 5) - affected lung parenchyma percentage (median 50%) - total number of lesions (median 13.5) - mediastinal lymphadenopathy (25%) - pleural effusion (50%) - pleural thickening (25%) - air bronchogram (40%) |
Higher risk of ICU admission with consolidations in RML/RUL/LUP, increased number of affected lober, and percentage of affected parenchymal involvement Higher frequency in clinically unstable patients: - older age (median age 65.0) - comorbidities: diabetes (50%), hypertension (70%), COPD (30%) - PaO2 < 93% or respiratory rate > 20 (90%) - abnormal laboratory values: low lymphocyte count (80%), N/L ratio > 3 (68.4%), high CRP (89.5%), elevated D-dimer (93.3%) |
| [23] | Liang | 2020 |
Higher incidence in discharged severe patients (26/47): - first week: GGOs (79.2%), consolidation (16.7%) - second week: GGOs (45.5%), consolidation (15.2%), reticular pattern (6.1%), mixed pattern (33.3%) - from the third week: GGOs (29%), consolidation (2%), reticular pattern (33%), mixed pattern (37%) Higher incidence in death severe patients (21/47): - first week: GGOs (90%), consolidation (10%) - second week: GGOs (92%), consolidation (8%) - from the third week: GGOs (73%), consolidations (27%) |
Significantly higher frequency in non-survivors: - older age (median 77 years) - comorbidities (cerebrovascular disease, diabetes mellitus, and kidney disease) - clinical syndromes (sepsis and septic shock) - abnormal laboratory values: CRP, ALT, lymphocyte count, and O2 saturation Significant difference in discharged and dead patients: - CT pattern within the second week - CT pattern within the third week - CT distribution within the third week (100% diffuse in the death group) |
| [24] | Erturk | 2020 |
Significantly higher incidence in ICU patients (25/262): - crazy paving (64%) - air bronchogram (44%) - bronchus distortion (68%) - bronchiectasis (80%) - air trapping (52%) - pleural thickening (60%) - mediastinal/hilar lymph nodes enlargement (52%) - number of involved lobes (median 5) |
Increased frequency of specific CT findings in ICU patients – indicators of poor short-term prognosis Higher frequency in ICU patients: - older age (median age 64.56 vs. 53.89) |
| [25] | Aydemire | 2021 |
Significantly higher incidence in ICU patients (47/477): - presence of lesions (96%) - extension of lung involvement (4% – group 0, 19% – group 1, 26% – group 2, 51% – group 3) |
Correlation between the extent of radiographic involvement and ICU admission Correlation with increased lung involvement: - abnormal laboratory values: increased D-dimer/Ferritin/LDH/CRP/ESR/ALT and decreased lymphocyte count |
| [26] | Jin | 2020 |
Significantly higher incidence in patients with adverse outcomes (13/94): - diffuse lesions distribution in the entire lungs - consolidation mixed with or without GGO |
Independent risk factor for adverse outcome: pattern 4– diffuse alveolar damage – HR 18.90 Correlation with adverse outcomes: - age ≥ 65 years – HR 9.39 - comorbidity – HR 4.14 - severe or critical illness – HR 4.62 - presence of fatigue – HR 3.62, chest congestion and/or shortness of breath – HR 3.81 – abnormal laboratory value: neutrophil percentage > 75% – HR 14.12 |
| [27] | Chon | 2020 |
Significantly higher incidence in severe patients (36/281): - mixed consolidations and GGO (50%) - crazy paving appearance(38.9%) - pleural effusion (27.8%) - similar lower and upper lobe distribution (22.2%) - peripheral predominant distribution (72.2%) - higher number of lobe involvement (median 3.5) and segment involvement(median 8) |
Independent risk factor for critical events: pleural effusion – OR 19.41, crazy-paving appearance – OR 7.15 Correlation with critical events: - age > 77 years – OR 16.26 - comorbidities: neurologic disease – OR 11.18, malignancy – OR 8.41 - abnormal laboratory value: absolute lymphocyte count, < 1320 cells/μL – OR 4.19, CRP > 0.5 mg/dL – OR 19.69, LDH > 474 U/L OR 5.05 |
| [28] | Abkhoo | 2021 |
Higher incidence in ICU patients (121/121): - GGOs (71.9%) - peripheral (38.8%) and bilateral (98.3%) distribution - lower lobe predominance (94.2%) - cardiomegaly (63.6%) - parenchymal bands (47.9%) - crazy-paving pattern (44.4%) |
Significantly higher frequency in non-survivors: - pleural and pericardial effusion - older age - lower O2 saturation - hypertension, low diastolic blood pressure Predictive model (pericardial effusion – OR 6.56, SpO2 – OR 0.91, hypertension – OR 4.11) – mortality: sensitivity 78.7%, specificity 61.1%, PPV 90.0%, accuracy 75.5% |
| [29] | Tekcan Sanli | 2021 |
Correlation with the presence of specific vascular changes: - lesions diameter > 5 cm - crazy-paving pattern - peripheral and central involvement - higher risk of RML and LUL involvement - involvement of > 2 lobes - involvement of > 50% of lung parenchyma |
Increased frequency of vascular changes in ICU patients – indicators of poor short-term prognosis Correlation with the presence of specific vascular changes: - PaO2 < 93% or respiratory rate > 20 - smoking rate – OR: 3.5 - abnormal laboratory values: increased CRP (median 5.7 mg/L) and LDH |
| [30] | Hejazi | 2021 | Higher incidence in ICU patients (168/168): multifocal (58%) and bilateral (60%) GGO |
Significant correlation in ICU patients: - multifocal GGO and SOFA score on day 1 - bilateral GGO and SOFA score on day 1 - multifocal bilateral GGO and SOFA score on day 1 - multifocal bilateral GGO and SOFA score on day 5 - unilateral/bilateral GGO and CRP - unifocal/unilateral/bilateral GGO patterns and overweight/obesity - multifocal/bilateral GGO and heart failure - unifocal/multifocal/unilateral/bilateral or multifocal bilateral GGO and cardiovascular diseases - unifocal/unilateral GGO and malignancy |
Extrapulmonary prognostic findings for ICU admission
In addition to estimation of lung parenchyma involvement, chest CT can provide extrapulmonary data about body composition to predict COVID-19 severity. It has already been demonstrated that obesity is a risk factor for poor clinical outcomes [31–33] and Pediconi et al. further assessed the relationship between adipose tissue and severity of lung disease. They retrospectively calculated the area of subcutaneous and visceral adipose tissue (SAT and VAT, respectively) with a manual segmentation at the L3 vertebral level, testing them in multiple models for prediction of ICU admission. At univariate analysis, VAT and SAT areas significantly correlated with lung disease severity (total score as the sum of each lobe score). Moreover, after multivariate logistic regression, VAT score (< 100 cm2 normal weight – score 0; 100–129 cm2 overweight – score 1; > 130 cm2 obesity – score 2) was identified as the best predictor (OR 4.307–12.842) without the significant contribution of comorbidities at ROC analysis (0.834 vs. 0.821 for the CT-based model) [34]. In addition to VAT and SAT, whose ratio resulted in being a predictor of poor outcome (HR 1.30), Bunnell et al. focused on the intermuscular adipose tissue (IMAT), which is already considered as an independent risk factor for impaired lipid profile, glucose tolerance, and muscle quality [35]. They performed body segmentation from axial CT images at the L4 level, and after model adjustment for clinical variables, higher IMAT was associated with adverse outcome (HR 1.44), i.e., reduced time to ICU admission or in-hospital death [36].
Other authors evaluated the relationship between cardiac adipose tissue and COVID-19 pneumonia extent to enhance outcome prediction. Grodecki et al. quantified the extent of epicardial adipose tissue (EAT), significantly higher in patients with adverse outcomes, i.e., ICU admission, need of mechanical ventilation or vasopressor therapy, and death (median 132.2 mL vs. 84.9 mL); EAT resulted in being positively correlated with pneumonia extent (r = 0.29). Moreover, after multivariate logistic regression analysis, authors concluded that EAT was an independent predictor of adverse outcome (volume – OR 5.1 per doubling, attenuation – OR 3.4 per 5 HU increase) and pneumonia severity (OR 2.5) [37]. Phan et al. evaluated epicardial and pericardial adipose tissue, determining cardiac adipose tissue volumes indexed to body surface area (CATi), in diabetic COVID-19 patients to estimate short-term outcomes, i.e., ICU requirement or death, in the first 21 days from admission. The proposed risk score included CATi and IL-6 measurement as significant prognostic variables to predict adverse events with AUC 0.76 [38]. A different approach focused on the fat-to-muscle ratio (FMR) as an early biomarker for outcomes within a follow-up period of 22 days after the initial CT scan. They extracted the fat mass according to the axial waist circumference and the average muscle area of the bilateral spine muscles at the T12 vertebral level. In the multivariate logistic regression, high FMR and age significantly predicted the necessity for ICU treatment [39].
This finding was confirmed in another study which demonstrated that a lower muscle mass was independently associated with poor clinical outcome, i.e., ICU admission (OR 4.3) and in-hospital mortality (OR 2.3). In this study, axial CT images at T5 or T12 vertebral levels were chosen to measure bilateral paravertebral skeletal muscle mass (SMM), estimating patients’ height by vertebral size. Their combined model of CT-derived data incorporating muscle mass and lung involvement predicted ICU admission and death (AUC 0.83 and 0.81, respectively), and the addition of clinical data did not significantly improve predictive performance [40].
Also, Giraudo et al. estimated the prognostic role of muscle mass reduction, defined as the presence of a CT density HU < 30 of the right paravertebral muscle at T12 level. ICU patients had a significantly lower muscle attenuation (29.0 vs. 39.4), and after logistic regression analysis, muscle mass was confirmed to be a predictor of ICU admission, reaching a sensitivity of 71.15 with a cut-off value of 34 HU as the initial sign of muscle loss. However, this parameter did not statistically influence the overall outcome of COVID-19 patients and was not associated with higher mortality risk [41].
Kottlors et al. tested bone mineral density (BMD) of T9-T12 vertebral levels as a predictor of ICU admission within the 22 days after the initial CT scan. The assessed parameters were mean bone density (mg/mL), Z-score, and T-score. BMD alone proved to be a significant predictor of ICU admission with a risk > 75% if BMD < 80 mg/mL. After the addition of age and gender in the multivariate regression model, BMD could no longer be considered as a significant indicator for a high linear correlation between age and BMD [42].
Different from the previous study, Tahtabasi et al. measured BMD at L1 level, using a cut-off value of 100 HU to define low density. In the lower BMD group, they observed a significantly higher rate of ICU admission (33.4% vs. 21.2%) as a result of univariate analysis [43].
Table 2 provides a summary of included papers focused on the evaluation of extra-pulmonary prognostic findings for ICU admission.
Table 2.
Extrapulmonary prognostic findings for ICU admission
| Reference | Author | Year | CT findings | Prognostic value |
|---|---|---|---|---|
| [34] | Pediconi | 2021 |
Significantly higher incidence in ICU patients (26/62): - higher lung disease severity score (median 16) - VAT area (median 258.3 cm2) - VAT score |
Independent predictor of ICU admission: VAT score – OR 4.307–12.842 AUC – ICU: 0.834 (VAT, SAT, lung disease severity, and comorbidities) |
| [36] | Bunnell | 2021 |
- Median VAT/SAT ratio 0.51 (median SAT 269.9 cm2, median VAT 145.6 cm2) - Median IMAT 12.1 cm2 - Median paraspinal and abdominal muscle 134.5 cm2 |
Independent predictor of ICU admission or death: VAT/SAT – OR 1.30, higher IMAT – HR 1.44 |
| [37] | Grodecki | 2021 | Significantly higher EAT volume in patients with clinical deterioration (median 132.2 mL – 23/109) |
Positive correlation between EAT volume and total pneumonia burden (r = 0.29) Independent predictors of clinical deterioration: EAT volume – OR 5.1, EAT attenuation – OR 3.4 |
| [38] | Phen | 2021 | Significantly higher CATi in patients with an adverse event at 21 days (20/41) | AUC CATi*IL-6 – adverse events at 21 days: 0.76 |
| [39] | Kottlors (1) | 2020 | Higher FMR according to the respective degree of medical care (median ICU 6.2 – 26/58 patients) | Independent predictor of ICU admission: FMR ≥ 7 – increased probability to about 80% |
| [40] | Schiaffino | 2021 | Significantly lower T5 and T12 paravertebral muscle mass in ICU patients (92/552) |
Independent predictors of ICU admission: SMM T5 – OR 3.3, SMM T12 – OR 1.9 AUC– ICU: 0.834 (muscle status, chest CT features + / − clinical features) |
| [41] | Giraudo | 2021 | Significantly lower attenuation of right paravertebral muscles in ICU patients (median HU 29.0 – 36/150) | Performances HU – ICU (cut-off 34 HU): accuracy 62.9%, sensitivity 71.1%, specificity 53% |
| [42] | Kottlors (2) | 2020 | Significantly lower BMD in ICU patients: (median ICU 6.2 – 26/58 patients) |
Independent predictor of ICU admission: BMD < 80 mg/ml – increased probability to about 75% AUC – ICU: 0.824 (only age in the regression model, no advantages from BMD) |
| [43] | Tahtabasi | 2021 | Lower BMD (≤ 100 HU) in ICU patients (52/209) |
Significantly higher rate of ICU admission in patients with lower BMD (33.4% vs. 21.2%) Significant correlation between clinical classification and lower BMD (r = 0.152) |
Semi-quantitative analysis of lung for prognostic features
Some authors used semi-quantitative methods (assigning specific scores according to the percentage of involved parenchyma) to assess the prognostic value of baseline chest CT (Fig. 3) [44].
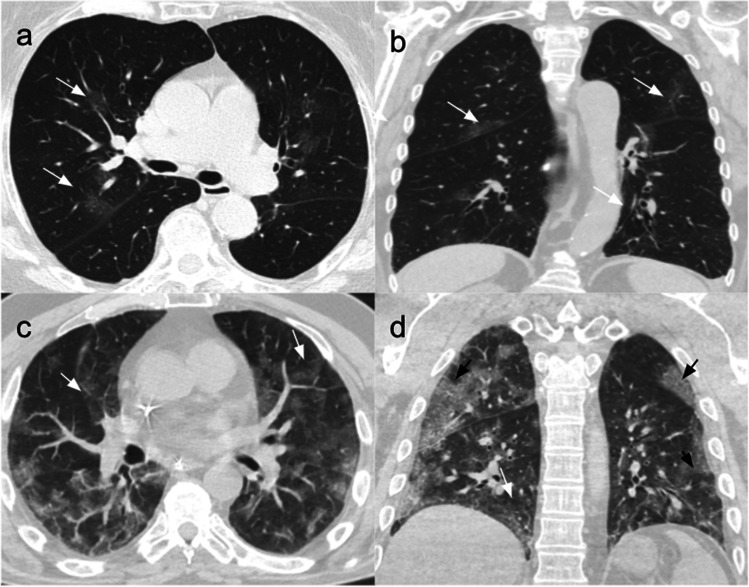
Fig. 3.
Use of semi-quantitative methods to predict the outcome of COVID-19 patients, assigning specific scores according to the percentage of involved parenchyma at chest CT scan. Patient 1 – mild disease: CT scans of an 80-year-old woman affected by COVID-19 pneumonia, hospitalized for 11 days without ICU admission and treated with low flow nasal cannula (2 ml/min) and antibiotics. (a) Non-contrast CT scan, axial plane, performed at admission showing bilateral GGOs in the centro-parenchymal areas (white arrows). (b) Non-contrast coronal plane showing bilateral GGOs in the posterior subpleural areas (white arrows). Patient 2 – severe disease: CT scans of a 68-year-old man affected by COVID-19 pneumonia and hospitalized for 25 days, with ICU admission on the fifth day due to progressive deterioration of respiratory function. He was treated with IV antibiotic and antiviral therapy and heparin. His stay in ICU was complicated with multiple urinary tract infections that led to stage two AKI, thus prolonging his total hospitalization days. (a) Non-contrast CT scan, axial plane, performed at admission showing bilateral and diffuse GGO areas in both lungs (white arrows). (b) Non-contrast coronal plane showing bilateral GGOs (white arrows) and subpleural areas with interlobular and intralobular septal thickening (black arrows)
Indeed, the visual quantification of lung lesions has been tested by Ruch et al., who stratified patients into 6 classes (normal, 0–10%, 11–25%, 26–50%, 51–75%, and > 75%) on the basis of pulmonary parenchymal involvement.
The extent of lesions at baseline CT scan was independently associated with prognosis. Furthermore, 69.5% of patients (66/95) with > 50% lung involvement developed the severe disease, i.e., ICU admission or death, in the 7 days after hospital admission [45]. Similarly, in another multicenter study by Luo et al., a pulmonary opacity score ≥ 41% at admission resulted in being independently associated with severe COVID-19 disease (OR 15.58), specifically ICU admission (OR 6.26), and respiratory failure (OR 19.49) [46].
The CT severity score (CTSS) has been reported to correlate with disease severity and is used to quantify pulmonary involvement in patients with a COVID-19 Reporting and Data System (CO-RADS) ≥ 3 (indeterminate, high, very high, or RT-PCR + for COVID-19).
It is based on a visual assessment of lobar parenchymal involvement and consists of the sum of the scores given to the radiologists to each lobe on the basis of their involvement in the disease (ranging from 0 (no involvement) to 25 (maximum involvement)). After adjustment for confounding variables, CTSS at presentation was associated with ICU admission (OR 1.23) and also with hospital admission and 30-day mortality. Specifically, a CTSS value ≥ 15 predicted ICU admission with specificity ≥ 90%, while a CTSS value ≤ 9 excluded ICU admission with sensitivity ≥ 90% [47].
Büttner et al. described a further semi-quantitative method for estimation of pulmonary involvement.
Their method included the measurement of affected lung areas at three levels (aortic arch, tracheal bifurcation, inferior end of the xiphoid) divided by the total lung area at the same level, followed by an average of the three images. They found that the median percentage of involved parenchyma was higher in patients admitted to the ICU or in those intubated and a 10% increase in affected lung area significantly increased the instantaneous risk of intubation (HR 2.00) and ICU necessity (HR 173). Statistical analyses identified 17.6% as a cut-off of parenchymal involvement for ICU treatment with a specificity of 80.0%, a sensitivity of 77.8%, and an area under the curve (AUC) for the endpoint of 85.6% [48].
Using the same method for the semi-quantitative analysis, Hosse et al. confirmed that a 10% increase in the affected parenchyma increased the risk for ICU admission (OR 1.68) and for invasive ventilation (OR 1.58), improving early detection of patients at risk for poor outcome compared to subjective assessment [49]. In their analysis of serial CT features of COVID-19 patients, Li et al. stated that critical patients had higher CT scores from the second to the fourth week after onset of symptoms with a peak value of all the features in the third week. The overall lung involvement score on the second week had the highest predictive value for whole-course clinical severity with a sensitivity of 81% and a specificity of 69.2% [50].
Other authors combined semi-quantitative CT analysis with clinical laboratory data to identify risk factors for ICU admission and in-hospital death. Among the evaluated predictors, high values of CT scores, older age (≥ 53 years), lower oxygen (O2) saturation ≤ 90%, erythrocyte sedimentation rate (ESR) ≥ 60 mm/h, and white blood cells (WBC) ≥ 8000 × 103/μL were found to be significant [51–53].
Based on similar findings, Salahshour et al. considered patients with age ≥ 53, SpO2 ≤ 91, and CT score ≥ 8 at high risk for poor outcome, i.e., ICU admission and death, creating a predictive model with 81.95% accuracy. Their semi-quantitative score evaluated the extension of pulmonary involvement (PI) as the sum of GGOs and consolidation, with a maximum total score of 35 [54].
Table 3 provides a summary of included papers focused on the semi-quantitative analysis of lungs for prognostic features.
Table 3.
Semi-quantitative analysis of lung for prognostic features
| Reference | Author | Year | CT findings | Results |
|---|---|---|---|---|
| [44] | Baysal | 2021 |
Higher incidence in ICU patients (39/405): - GGO (87.2%) - consolidations (79.5%) - air bronchogram (53%) - reticular pattern (48.7%) - pleural effusions (31%) - number of involved lobes (median IQR 5) |
Higher median CT score in ICU patients: - median IQR 13 vs. 4 - AUC – ICU: 0.71–0.75 Higher frequency in ICU patients: - older age (median age 65) - comorbidities: hypertension (57%), chronic kidney disease (17%) |
| [45] | Ruch | 2020 |
Significantly higher incidence in severe patients (95/572): - GGO (98.9%) - consolidations (74.7%) - pulmonary embolism (16.8%) - bilateral involvement (100%) |
Association between lung involvement > 50% and early severe disease (ICU or death) OR: 2.35 Higher frequency in severe patients: - male sex (80%) - dyspnea (86.3%) - lower SpO2 (median 90%) - abnormal laboratory tests: higher CRP (median 154 mg/L), higher neutrophil count (median 6375 cells/mm3), lower lymphocyte count (median 740 cells/mm3), higher lactate (median 1.2 mmol/L) |
| [46] | Luo | 2021 | Significantly higher incidence in ICU patients (64/496): pulmonary opacity score ≥ 41% (59%) | Association between pulmonary opacity score ≥ 41% and ICU admission: OR 2.35 |
| [47] | Lieveld | 2020 | CO-RADS scoring system: ≥ 4 as the optimal cut-off for discriminating between a positive and a negative PCR AUC of 0.912 |
Higher median CT score in ICU patients: 14.8 vs. 5.5 – discharge home and 9.4 – hospital admission Association between CTSS and ICU admission - OR: 1.23 - AUC – ICU: 0.81 Higher frequency in severe patients: - comorbidities: CVD (29.1%), COPD (20%), diabetes (23.7%), current malignancy (18.2), hypertension (36.3) |
| [48] | Buttner | 2020 |
Higher incidence in ICU patients (18/28): - consolidation (94.4%) - pleural effusions (16.7%) |
Higher percentage of affected lung area in ICU patients: - 26% vs. 7.8% - 10% increase in the affected lung parenchyma area - increased the instantaneous risk of intubation (HR 2.00) and ICU need (HR 1.73) - AUC – ICU: 0.856 Higher frequency in ICU patients: - younger age (median age 58.2) - female sex (57%) - obesity (22.2%) |
| [49] | Hosse | 2021 |
Higher incidence in ICU patients (137/265): - extensive consolidation (13.1%) - extensive GGOs (27.0%) - posterolateral involvement (37.2%) |
Higher percentage of affected lung area in ICU patients: - 25.5% vs. 5.4% - 10% increase in the affected lung parenchyma area - increased the instantaneous risk of intubation (HR 1.35) and ICU need (HR 1.68) - AUC – ICU: 0.735 Higher frequency in ICU patients: - older age (median age 75.0) OR – ICU: 1.27 - male sex, OR – ICU: 1.20 - chronic lung disease, OR – ICU: 1.68 |
| [50] | Li | 2021 |
Higher incidence in severe/critical patients (35/53): - peak of CT scores in the third week (vs. the second week of moderate patients) - higher overall lung involvement score (from the second to the fourth week) |
AUC – severe/critical disease: overall lung involvement score (2nd week) 0.747, ground glass opacity score (2nd week) – 0.744 AUC combined models – severe/critical disease: overall lung involvement score (2nd week) + CURB65 0.808, overall lung involvement score (2nd week) + qSOFA 0.810 Higher frequency in ICU patients: - cough - higher qSOFA and CURB65 at admission |
| [51] | Shayganfar | 2021 | Significantly higher incidence in ICU/death (38/176): bilateral lung involvement (97.4%) |
Higher median CT score in ICU /death: - 14.39 vs. 9.53 - AUC – ICU: 0.732 - OR – CT score ≥ 11: 4.38 OR – ICU/death: 4.38 – age ≥ 60, 2.78 – O2 saturation of ≤ 90.5% |
| [52] | Mozafari | 2021 |
Significantly higher incidence in ICU patients (32/213): - consolidation (100%) - crazy paving (71.87%) - linear opacities (78.1%) - air bronchogram (78.1%) - bilateral distribution (100%) - peripheral and central involvement (96.75%) - pleural effusion (15.6%) - higher number of involved lobes (87.5% – ≥ 5), mainly RUL (96.9%), RML (90.6%), LUL (96.9%) |
Higher median CT score in ICU patients: - median 17.34 vs. 6.78 - home discharge and 10.66 - hospitalized - deceased patients after ICU admission (23/32): higher age – median 62.4 vs. 47.77, higher score – median 20.78 vs. 16.00 Higher frequency in ICU patients: - male sex (71.9%) - non pulmonary pre-existing conditions (43.75%) - lower SpO2 (median 86.78%) - dyspnea (100%) - higher temperature (median 37.02) - abnormal laboratory tests: higher ESR (median 68.50 mm/h), higher WBC (median 10.02 103/μL), lower lymphocyte count (median 11.22%) |
| [53] | Davarpanah | 2020 |
Significantly higher incidence in ICU patients (45/228): - consolidation (31%) - pleural effusion (26%) - bronchial wall thickening (42%) - peripheral and central distribution (24%) |
Higher median CT score in ICU patients: - 11.9 vs. 8.2 - OR – total CT score (per 1 score increase): 1.13 Higher frequency in ICU patients: - older age (median age 65), OR – ICU (per 1-year increase): 1.05 - lower O2 saturation, OR – ICU (O2 saturation ≤ 88%): 3.97 |
| [54] | Salahshour | 2021 |
Significantly higher incidence in ICU patients (72/739): - total pulmonary involvement score (median 15.1) and density index (median 3.3) - peripheral, pleural-based distribution of lesions (41.2%) - bilateral lesions (92.6%) - crazy paving - pleural effusion (bilateral – 16.2%, unilateral 10.3%) - crazy paving (36.8%) - parenchymal band (39.7%) |
AUC PI (cut-off 8) – ICU: 0.77 Predictive model (age ≥ 53, SpO2 ≤ 91, PI score ≥ 8) – ICU: sensitivity 40.98%, specificity 89.11%, NPV 89.63%, accuracy 81.95% Significantly higher frequency in ICU patients: - older age (median age 60.6) - higher respiratory rate (> 24) - lower O2 saturation (< 93%) - higher PCR - higher mortality (27.8%) |
Chest CT and artificial intelligence in COVID-19 patients for the prediction of ICU admission
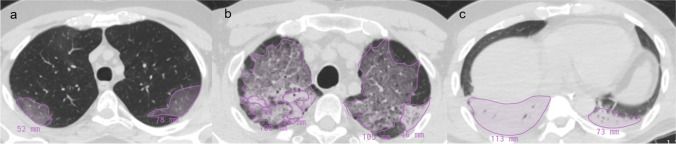
Multiple AI models have been developed and tested on CT images to improve the clinical decision-making process in the management of patients with a suspected diagnosis of COVID-19. They offer promise in identifying COVID-19 pneumonia and its complications [55–59], in the differential diagnosis of pneumonia due to other etiologic agents [60–62], and in risk stratification of patients [63–66]. These tools, usually trained with images labeled by radiologists (Fig. 4), have demonstrated additional advantages including increased diagnostic efficiency and reduced workload on radiologists [67, 68].
Fig. 4.
Labeling of CT images for the training phase of AI algorithms. Each CT finding is manually contoured and labeled with the name of the specific finding related to (a) GGO, (b) crazy paving, and (c) consolidation
A developed deep neural network (DNN) has been tested for automatic quantification of disease severity and prediction of patients’ outcomes, focusing on ICU admission and mortality risk. This AI-based framework employed DNN to segment lung lobes and pulmonary opacities and then to estimate lobar severity score (0–5) according to the volume ratio of pulmonary opacities of each lung lobe and total extent of pulmonary opacities as the sum of lobar scores (0–25). These findings were correlated with the final outcome of included patients, divided into 4 groups (group 1 – length of hospitalization < 7 days; group 2 – length of hospitalization > 7 days; group 3 – ICU admission; group 4 – death). The three analyzed models [support vector machine (SVM), random forest (RF), and logistic regression (LR)] outperformed radiologists in the outcome prediction in group 3 with an AUC of 0.766 for SVM, 0.757 for RF, and 0.766 for LR. The model also outperformed radiologists in group 4, reaching an AUC of 0.655 for SVM, 0.676 for RF, and 0.736 for LR [69].
Chatzitofis et al. built a prediction model without results of laboratory tests immediately after ED admission. Their two-stage data-driven algorithm was able to stratify patients into three groups (moderate, severe, and extreme) according to the possibility of being discharged, hospitalized, or admitted to ICU, respectively. Using the COVID-19_CHDSET Dataset (annotated CT dataset of COVID-19 patients from Milan) as the training set, the developed algorithm with DenseNet201-VoI as backbone model yielded an AUC of 0.97, 0.92, and 1.00 for the three classes, respectively, and accuracy of 88.88%, specificity of 94.73%, and sensitivity of 89.77% [70].
A similar risk stratification model has been used by deep learning (DL)-based fully automatic algorithm by Weikert et al. to extract pulmonary and cardiovascular measures from CT images. Their aim was to test the model performance in the prediction of ICU admission while also considering demographic findings and six laboratory features related to cell damage and inflammation – C-reactive protein (CRP), lactate dehydrogenase (LDH), WBC, procalcitonin, albumin, and D-dimer. CT metrics alone and laboratory findings alone resulted in an AUC of 0.88 and 0.86, respectively, while the combination of CT, laboratory, and demographic data had an AUC of 0.91 [71].
Liu et al. showed that three quantitative volume ratios automatically extracted by CT scans of COVID-19 patients could predict severe illness better than to previous clinical biomarkers. In their study, they segmented lungs and pneumonia lesions by combining a fully convolutional network with adaptive thresholding and morphological operations. This way, they were able to automatically extract the percentages of ground glass opacity (GGO), volume (PGV), semi-consolidation volume (PSV), and consolidation volume (PCV), which was defined as the area of intermediate homogeneous increase in density. They found that these CT findings, calculated on day 0 and day 4 (including their changes from day 0 to day 4), were correlated with the risk prediction of severe illness [72].
Ho et al. also combined CT features with laboratory measurements for the prediction of disease severity in which the DL model was built on an artificial neural network (ANN) for clinical and laboratory features and on a convolutional neural network (CNN) for CT imaging data. They classified patients according to the high (event) or low (event-free) risk of severe progression, i.e., respiratory deterioration (high-flow nasal cannula, mechanical ventilation, ICU admission), renal failure, septic shock, or death. The mixed artificial convolutional neural network (ACNN) model yielded an AUC of 0.916, an accuracy of 93.9%, and a specificity of 96.9%. Among clinical and laboratory features, CRP and WBC demonstrated a strong positive correlation with the outcome; age was considered as a significant risk factor, while female sex and oxygen saturation showed a negative correlation with the endpoint [73].
Another model used a deep CNN to segment CT imaging and to extract 12 laboratory tests that showed the largest change in the two groups of patients, i.e., severe and non-severe groups. The former included those with respiratory failure and need for supplemental oxygen, multi-organ failure, or ICU admission. The prediction model showed an AUC of 0.93, and D-dimer, LDH, and lymphocytes were identified as predictors of higher mortality risk [74].
Artificial intelligence has been applied to perform quantitative CT, a rapid and objective approach to assess disease severity and to predict the clinical outcome: It takes advantage of computer-aided software to segment and quantify lung volumes according to specific Hounsfield unit (HU) intervals [75]. Cai et al. investigated the validity of CT quantification using two U-Net models to segment images and to calculate total lung volume, lesion volume, and non-lesion lung volume. Then, they built random forest models to stratify patients (moderate, severe, critical) and to predict patients’ outcomes, including need and length of ICU stay, that reach an AUC of 0.960 [76]. Similarly, Yan et al. used a U-Net-based architecture to extract quantitative CT parameters, i.e., total opacity/GGO/consolidation volumes and percentages. Despite a significant difference in all the aforementioned CT parameters between ICU and non-ICU patients, multivariate analysis revealed age > 60 years (OR 12.72), comorbidities (OR 5.55), and CT total opacity percentage > 10.5% (TOP – OR 8.0) as predictors for adverse outcome [77].
A machine learning multiparametric model was developed to estimate the need for ICU treatment, including quantitative CT features (affected lung volume) and inflammatory parameters (CRP and IL-6). The RF modeling yielded an AUC of 0.79, an accuracy of 0.80, a sensitivity of 0.72, and a specificity of 0.86, and it demonstrated a major involvement of upper lung in high-risk patients (mean importance 0.184) [78]. A French retrospective study focused on the automated quantification of GGOs, included in the range from − 700 to − 501 HU), and normally restricted parenchyma, included in the range from − 900 to − 701 HU. The latter was significantly lower in patients admitted to ICU, and GGOs were considered as a biomarker of pulmonary injury, considering a significant correlation between measured lung volumes and a respiratory assessment severity score (7 degrees: ranging from 1 (absence of hospitalization and inability to resume normal activity) to 7 (death)) [79].
Other studies focused on the prognostic value of non-affected lung parenchyma, as demonstrated by Colombi et al. They quantified well-aerated parenchyma on admission CT either visually (%V-WAL) or with open-source software (%S-WAL and absolute volume, VOL-WAL) to estimate the occurrence of ICU admission or death. After adjustment for demographics and clinical parameters, a %V-WAL < 73% or %S-WAL < 71% and VOL-WAL < 2.9 L (OR 2.6; 95% CI 1.2–5.8; P < 0.01) was identified as a predictor of adverse outcome [80]. In a similar study, a software-based estimation of the normal lung parenchyma percentage (SQNLP) < 81.1% (sensitivity 86.5% and specificity 86.7%) accurately predicted ICU admission, and an SQNLP < 82.45% was related to severe pneumonia (sensitivity 83.1% and specificity 84.2%) and increased presence of crazy-paving pattern [81].
Radiomics represents a further application of artificial intelligence for prognostic evaluation of COVID-19 patients, eventually integrated by additional DL algorithms for the inclusion of non-imaging features. Wu et al. constructed a radiomic signature (Radscore) of 5 features selected after application of LASSO regression and integrated with clinical risk factors (age, sex, type on admission, comorbidities) for the prediction of poor outcome, i.e., death, MV, and ICU admission. The hybrid of clinical and radiomic models showed an AUC of 0.862 (vs. AUC of 0.816 of the RadScore only) [82]. The radiomic model by Xu et al. achieved an AUC of 0.869 in the prediction of ICU admission that improved up to 0.916 after the introduction of clinical and laboratory features. Moreover, the resulting hybrid model accurately estimated the progression time to ICU need in COVID-19 patients [83].
The proposed holistic model by Chao et al. included imaging data and demographic, clinical, and laboratory findings to predict the need for ICU admission. Features derived from CT scans included hierarchical lobe-wise quantification features, ratio of opacity volume, and whole lung radiomics. The use of an RF classifier allowed to create this hybrid model that achieved an AUC of 0.884 and a sensitivity of 96.1% [84].
Another merged model based on 6 radiomic features and 3D-Resnet-10-based DL scores confirmed the complementarity of the two types of features in the distinction between severe and critical cases of COVID-19 according to the presence of respiratory failure, MV, and organ failure requiring ICU admission. In the test cohort, the model reached an AUC of 0.861 which was higher than the AUC of the single radiomic or DL models (0.838 and 0.787, respectively) [85].
Bartolucci et al. performed either quantitative and texture CT analysis to create and compare different models for the prediction of ICU admission, including also blood laboratory-arterial gas analyses: In the a priori feature selection, the authors included 3 volumes in the radiological model (well-aerated lung, % GGOs, and % consolidations), 86 texture features in the radiomic model, and 6 parameters for the clinical model (age, LDH, D-dimer, PCR, lymphocyte count, P/F ratio). After binomial regression, only relevant features were included in the tested models, and the hybrid radiological model (age, P/F, LDH, % of consolidations) demonstrated the best performance in the validation set with an AUC 0.82 [86].
Table 4 provides a summary of included papers focused on the application of AI for the prediction of ICU admission.
Table 4.
Application of AI for the prediction of ICU admission
| Reference | Author | Year | Predictor | N° patients | Results |
|---|---|---|---|---|---|
| [69] | Fang | 2021 | DL – SVM, RF, LR | 193 COVID + : 105 – dataset A, 88 – dataset B | AUC – ICU: 0.813 |
| [70] | Chatzitofis | 2021 | DL – DenseNet201 | 497 COVID + | AUC – ICU: 0.99 – COVID-19_CHDSETOS, 1.00 – COVID-19_CHDSETUS |
| [71] | Weikert | 2021 | DL – UNet | 120 COVID + | AUC – ICU: 0.91 |
| [72] | Liu | 2020 | QCT | 134 COVID + | AUC – severe disease: 0.93 |
| [73] | Ho | 2021 | DL – ResNet50, Inception V3, DenseNet121 | 297 COVID + | AUC – event: 0.916 |
| [74] | Li | 2020 | DL | 46 COVID + | AUC – severe cases: 0.93 |
| [75] | Ufuk | 2021 | QCT | 76 COVID + | AUC – extensive disease: 0.873 |
| [76] | Cai | 2020 | QCT | 99 COVID + | AUC – ICU: 0.945 |
| [77] | Yan | 2021 | QCT | 221 COVID + | AUC TOP – ICU: 0.88 |
| [78] | Burian | 2020 | QCT | 65 COVID + | AUC – ICU: 0.79 |
| [79] | Noll | 2020 | QCT | 37 COVID + | Correlation with clinical data in ICU and non-ICU patients |
| [80] | Colombi | 2020 | QCT | 236 COVID + | AUC – ICU: 0.86 |
| [81] | Durhan | 2020 | QCT | 90 COVID + | AUC – ICU: 0.944 |
| [82] | Wu | 2020 | Radiomics | 492 COVID + | AUC – poor outcome: 0.862 – early phase group, 0.976 – late-phase group |
| [83] | Xu | 2020 | Radiomics | 3024 COVID + : 1662 – cohort 1, 700 – cohort 2, 662 – cohort 3 | AUC – ICU: 0.916 |
| [84] | Chao | 2020 | Radiomics | 295 COVID + : 113 – dataset A, 125 – dataset B, 57 – dataset C | AUC – ICU: 0.884 |
| [85] | Li | 2020 | DL – Radiomics | 217 COVID + | AUC – poor outcome: 0.861 |
| [86] | Bartolucci | 2021 | QCT, Radiomics | 115 COVID + | AUC hybrid radiological model: 0.82 |
Follow-up chest CT in severe COVID-19 patients
Mid- and long-term follow-up studies have demonstrated that pulmonary fibrosis could be more frequently detected on CT scans of severe and critical COVID-19 patients due to a more extensive lung inflammation during the acute phase (Fig. 5) [87]. In fact, despite a relatively higher persistence of pulmonary lesions in hospitalized patients at short-term follow-up, mainly as GGOs, the difference between severe and non-severe cases regards their dissipation. It is slower in severe patients that had a higher percentage of irreversible lesions, such as fibrous strips [88]. Moreover, ARDS is a known risk factor for the development of fibrotic-like changes, eventually associated with ventilator-induced lung injury after NIV [89, 90].
Fig. 5.
Serial CT scans of a 73-year-old woman affected by severe COVID-19 pneumonia and hospitalized for 34 days. She required ICU admission (total length: 12 days) for progressive respiratory failure, treated with intubation and prone-position ventilation. (a) Non-contrast CT scan at admission showing scattered bilateral GGOs either in the subpleural and centro-parenchymal areas (*), associated with initial thickening of interlobular septa (black arrow). (b) Non-contrast CT scan at 3 months demonstrating absorptions of previous opacifications and appearance of signs of fibrosis, mainly traction bronchiectasis (white arrow) and parenchymal bands (black arrowhead). (c) Non-contrast CT scan at 1 year confirming stable fibrotic sequelae
Han et al. performed a prospective study to assess the presence of lung fibrotic changes at 6 months follow-up, with a further evaluation after 1 year from the beginning of the infection. Using semi-quantitative CT scores to quantify the extent of pulmonary findings, they found that 35% of severe COVID-19 patients demonstrated radiographic features of fibrosis, i.e., traction bronchiectasis, parenchymal bands, and honeycombing, at 6 months follow-up. In addition, their results included multiple independent predictor factors: age > 50 years (OR 8.5), heart rate > 100 bpm (OR 13), or a total chest CT score ≥ 18 (OR 4.2) at admission, length of hospital stay ≥ 17 days (OR 5.5), development of ARDS (OR 13), or need of NIV (OR 6.3) during hospitalization. In their following study, the authors confirmed that all the patients with fibrotic changes at 6-month CT had persistence of those abnormalities at 1 year with a slight severity reduction in 23% of cases; moreover, they did not find significant differences in CT scores of fibrotic patients: These features indicate that late-stage pulmonary fibrosis may be irreversible. From the clinical point of view, 78% of patients with persistent exertional dyspnea and 85% of abnormal pulmonary diffusion had fibrotic changes on CT (negative correlation, r = − 0.35) [91, 92].
Some of the aforementioned radiographic changes typical of fibrosis, i.e., honeycombing and traction bronchiectasis, have been evaluated in another retrospective study, performed in 43 patients admitted to ICU with a minimum follow-up of 6 months. According to Poitevineau et al., they were of limited extent (< 10% of lung parenchyma) and occurred in a minority of patients (28%) with longer ICU stay (median 24 days) or extensive pneumonia at baseline CT (> 50%). In fact, the most common pattern was late organizing pneumonia without fibrotic changes, characterized by residual ground glass and parenchymal bands [93].
Liu et al. found similar results in their follow-up study performed 7 months after recovery from COVID-19. They confirmed that patients were more prone to develop fibrosis after severe disease, especially if older, treated with steroid or mechanical ventilation, and with a longer hospital stay. The fibrosis group of patients demonstrated significant differences in some laboratory values, i.e., lower values lymphocyte count, mainly T cell count, and higher values of D-dimer and LDH at discharge. For the prediction of pulmonary fibrosis at 7 months follow-up, the combined clinical-radiological model reached an AUC of 0.945 [94].
Tabatabaei et al. further confirmed the results of previous studies about the chronic sequela of COVID-19, focusing on a 3-month follow-up; 42.3% of patients included in their cohort (22/52) demonstrated residual lung involvement: GGOs and/or peripheral parenchymal bands in the initial site of infection. Authors also identified the same risk factors, i.e., severe disease (higher rate of ICU admission, longer hospitalization, extensive disease at admission with higher CT scores), higher number of patients with comorbidities, and leukocytosis at admission and during the hospital stay. Considering the possibility to potentially predict the development of specific pulmonary findings at mid-term follow-up, the presence of specific features at admission can suggest the need for follow-up CT scans and specific management choices [95].
Truffaut et al. focused on the correlation between radiological pulmonary findings at admission and 3-month sequelae in ARDS COVID-19 patients, previously admitted ICU discharge. Abnormal lung function test (DLCO and FEV1) and the number of affected lung segments on follow-up CT scan resulted in being significantly correlated with the number of affected lung segments on baseline CT scan, but not with the initial CT pattern. Moreover, a minority of the included patients showed normalization (14%) at follow-up CT scan, while the majority of patients exhibited a decreased number of affected segments (median 8.1 vs. 17.2) with fibrosis (100%) as predominant radiological abnormality [96].
Table 5 provides a summary of included papers focused on the evaluation of pulmonary findings on follow-up CT scans of severe COVID-19 patients.
Table 5.
Pulmonary findings on follow-up CT scan of severe COVID-19 patients
| Reference | Author | Year | Length of follow-up | CT findings | Results |
|---|---|---|---|---|---|
| [91] | Han (1) | 2021 | 6 months | Fibrotic lung changes (40/114): traction bronchiectasis, parenchymal bands, and/or honeycombing | Independent predictors of fibrosis development: age > 50 years – OR 8.5, HR > 100 bpm – OR 13, or a total chest CT score ≥ 18 – OR 4.2 at admission, length of hospital stay ≥ 17 days – OR 5.5, development of ARDS – OR 13, or need of NIV – OR 6.3 during hospitalization |
| [92] | Han (2) | 2021 | 1 year | Stable fibrotic lung changes and traction bronchiectasis (27/35), compared with 6 months follow-up |
Absence of significant differences in CT scores of fibrotic patients at 6 months and 12 months follow-up Negative correlation between the score of fibrotic lung changes and DLCO% (r = − 0.35, p = 0.01) at 1-year follow-up |
| [93] | Poitevineau | 2021 | 6 months |
Fibrotic lung changes in > 10% of lung parenchyma (12/43): traction bronchiectasis within residual GGO (100%), honeycombing and reticulations (42%) Late organizing pneumonia pattern (19/43): residual ground glass, parenchymal bands |
Higher incidence of fibrotic lung changes in patients with longer ICU stay (median 24 days) and pneumonia extent > 50% at baseline CT |
| [94] | Liu | 2021 | 7 months |
Significantly higher incidence in fibrosis group (12/41): - interlobular septal thickening, irregular interface, reticular pattern, parenchymal band, and traction bronchiectasis - higher levels of opacity score, volume of opacity, and percentage of opacity |
Independent predictors of fibrosis development: age > 50 years – OR 1.078, steroid therapy – OR 12.880, opacity score at discharge – OR 1.565, presence of traction bronchiectasis – OR 13.570 Correlation with fibrosis development: - Abnormal laboratory values: increased D-dimer (median 1.02 mg/L vs. 0.31 mg/L)/LDH (median 220.25 IU/L vs. 182.46 IU/L) and decreased lymphocyte count (median 0.97 × 109/L vs. 1.32 × 109/L) - AUC combined model – fibrosis: 0.945 (clinical and CT indicators) |
| [95] | Tabatabaei | 2020 | 3 months |
Higher incidence in patients with residual disease (22/52): - GGOs (54.5%) - mixed GGOs and subpleural parenchymal bands (31.8%) - pure parenchymal bands (13.7%) |
Higher median CT score in fibrosis group: median 10.3 vs. 7.3 Significantly higher incidence in fibrosis group (12/41): longer hospitalization (median 9.3 days vs. 6.9), higher rate of ICU admission with endotracheal intubation (40.9% vs. 6.7%), leukocytosis (median 7211.7 cells/mm3 vs. 5282.6 cells/mm3), higher number of patients with comorbidities (54.4% vs. 16.7%) |
| [96] | Truffaut | 2021 | 3 months | Significantly higher number of affected segments at baseline (median 17.2) in patients with residual disease (19/22) |
Correlation between number of affected segments at baseline and at follow-up Correlation between number of affected segments at baseline and persistent DLCO impairment and low FEV1 at follow-up |
Conclusions
In this paper, we summarized the role that baseline CT scan plays in the prediction of ICU admission in COVID-19 patients.
Such patients’ stratification is of great utility in the current context of the pandemic and in any healthcare setting with resource constraints. The prognostic pulmonary findings for severe disease include a major extent of parenchymal involvement, vascular abnormalities, and the presence of fibrotic features. Furthermore, specific laboratory parameters or features about body composition, mainly high adipose content, can also be associated with a poor outcome.
The prognostic value of CT scans can be improved when integrated into AI systems. Indeed, the combination of imaging and machine learning can provide tools for fast, accurate, and precise disease extent quantification as well as the identification of patients at risk for severe adverse events from COVID-19.
Author contribution
MEL had the idea for the article. MEL, LL, and AA performed the literature search and data analysis. MEL, LL, and AA drafted the work. MEL, MF, AC, and VS critically revised the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethical approval
Not applicable.
Conflict of interest
MEL, AA, LL, DL, MF, and VS declare they have no relevant financial or non-financial interests to disclose. AC has received support from AmGen, Blue Earth Diagnostics, and Novartis as honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events, from Blue Earth Diagnostics, Novartis for participation on a data safety monitoring board or advisory board.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria Elena Laino and Angela Ammirabile contributed equally to this work
Contributor Information
Maria Elena Laino, Email: mariaelena.laino@humanitas.it.
Angela Ammirabile, Email: angela.ammirabile@humanitas.it.
Ludovica Lofino, Email: ludovica.lofino@humanitas.it.
Dara Joseph Lundon, Email: dara.lundon@ucdconnect.ie.
Arturo Chiti, Email: arturo.chiti@hunimed.eu.
Marco Francone, Email: marco.francone@hunimed.eu.
Victor Savevski, Email: victor.savevski@humanitas.it.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos MK, Ferreira Júnior JR, Wada DT, et al. Artificial intelligence, machine learning, computer-aided diagnosis, and radiomics: advances in imaging towards to precision medicine. Radiol Bras. 2019;52:387–396. doi: 10.1590/0100-3984.2019.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues JCL, Hare SS, Edey A, et al. An update on COVID-19 for the radiologist – a British society of thoracic imaging statement. Clin Radiol. 2020;75:323–325. doi: 10.1016/j.crad.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams HJA, Kwee TC, Yakar D, et al. Chest CT imaging signature of coronavirus disease 2019 infection: in pursuit of the scientific evidence. Chest. 2020;158:1885–1895. doi: 10.1016/j.chest.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, Zhong Z, Li H, et al. CT imaging features of 4121 patients with COVID-19: a meta-analysis. J Med Virol. 2020;92:891–902. doi: 10.1002/jmv.25910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanne JP, Little BP, Chung JH, et al. Essentials for radiologists on COVID-19: an update-radiology scientific expert panel. Radiology. 2020;296:E113–E114. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye Z, Zhang Y, Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng M-Y, Lee EYP, Yang J, et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardiothorac Imaging. 2020;2:e200034. doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jajodia A, Ebner L, Heidinger B, et al. Imaging in corona virus disease 2019 (COVID-19) – a scoping review. Eur J Radiol Open. 2020;7:100237. doi: 10.1016/j.ejro.2020.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillo E, Bedmar Gomez I, Dangeard S, et al. COVID-19 pneumonia: diagnostic and prognostic role of CT based on a retrospective analysis of 214 consecutive patients from Paris, France. Eur J Radiol. 2020;131:109209. doi: 10.1016/j.ejrad.2020.109209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Yang Z, Ai T, et al. Association of “initial CT” findings with mortality in older patients with coronavirus disease 2019 (COVID-19) Eur Radiol. 2020;30:6186–6193. doi: 10.1007/s00330-020-06969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Yang L, Gui S, et al. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID-19 in Wuhan, China. Theranostics. 2020;10:6113–6121. doi: 10.7150/thno.46569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei Q, Li G, Ma X, et al. Correlation between CT findings and outcomes in 46 patients with coronavirus disease 2019. Sci Rep. 2021;11:1103. doi: 10.1038/s41598-020-79183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasilewski PG, Mruk B, Mazur S, et al. COVID-19 severity scoring systems in radiological imaging – a review. Pol J Radiol. 2020;85:e361–e368. doi: 10.5114/pjr.2020.98009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meiler S, Schaible J, Poschenrieder F, et al. Can CT performed in the early disease phase predict outcome of patients with COVID 19 pneumonia? Analysis of a cohort of 64 patients from Germany. Eur J Radiol. 2020;131:109256. doi: 10.1016/j.ejrad.2020.109256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parry AH, Wani AH, Shah NN, et al. Chest CT features of coronavirus disease-19 (COVID-19) pneumonia: which findings on initial CT can predict an adverse short-term outcome? BJR Open. 2020;2:20200016. doi: 10.1259/bjro.20200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabatabaei SMH, Talari H, Moghaddas F, Rajebi H. Computed tomographic features and short-term prognosis of coronavirus disease 2019 (COVID-19) pneumonia: a single-center study from Kashan, Iran. Radiol Cardiothorac Imaging. 2020;2:e200130. doi: 10.1148/ryct.2020200130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cau R, Falaschi Z, Paschè A, et al. Computed tomography findings of COVID-19 pneumonia in intensive care unit-patients. J Public Health Res. 2021;10:2270. doi: 10.4081/jphr.2021.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tekcan Sanli DE, Yildirim D, Sanli AN, et al. Predictive value of CT imaging findings in COVID-19 pneumonia at the time of first-screen regarding the need for hospitalization or intensive care unit. Diagn Interv Radiol. 2021;27:599–606. doi: 10.5152/dir.2020.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang B, Xie L, Yang F, et al. CT changes of severe coronavirus disease 2019 based on prognosis. Sci Rep. 2020;10:21849. doi: 10.1038/s41598-020-78965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erturk SM, Durak G, Ayyildiz H, et al. Covid-19: correlation of early chest computed tomography findings with the course of disease. J Comput Assist Tomogr. 2020;44:633–639. doi: 10.1097/RCT.0000000000001073. [DOI] [PubMed] [Google Scholar]
- 25.Aydemir Y, Gündüz Y, Köroğlu M, et al. The relationship of extent of initial radiological involvement with the need of intensive care, mortality rates, and laboratory parameters in Covid-19. Turk J Med Sci. 2021;51:1012–1020. doi: 10.3906/sag-2009-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin C, Tian C, Wang Y, et al. A pattern categorization of CT findings to predict outcome of COVID-19 pneumonia. Front Public Health. 2020;8:567672. doi: 10.3389/fpubh.2020.567672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chon Y, Kim JY, Suh YJ, et al. Adverse initial CT findings associated with poor prognosis of coronavirus disease. J Korean Med Sci. 2020;35:e316. doi: 10.3346/jkms.2020.35.e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abkhoo A, Shaker E, Mehrabinejad M-M, et al. Factors predicting outcome in intensive care unit-admitted COVID-19 patients: using clinical, laboratory, and radiologic characteristics. Crit Care Res Pract. 2021;2021:9941570. doi: 10.1155/2021/9941570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tekcan Şanlı DE, Yıldırım D. A new imaging sign in COVID-19 pneumonia: vascular changes and their correlation with clinical severity of the disease. Diagn Interv Radiol. 2021;27:172–180. doi: 10.5152/dir.2020.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hejazi ME, Malek Mahdavi A, Navarbaf Z, et al (2021) Relationship between chest CT scan findings with SOFA score, CRP, comorbidity, and mortality in ICU patients with COVID-19. Int J Clin Pract e14869. 10.1111/ijcp.14869 [DOI] [PMC free article] [PubMed]
- 31.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng KI, Gao F, Wang X-B, et al. Letter to the editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metab Clin Exp. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Siqueira JVV, Almeida LG, Zica BO, et al. Impact of obesity on hospitalizations and mortality, due to COVID-19: A systematic review. Obes Res Clin Pract. 2020;14:398–403. doi: 10.1016/j.orcp.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pediconi F, Rizzo V, Schiaffino S, et al. Visceral adipose tissue area predicts intensive care unit admission in COVID-19 patients. Obes Res Clin Pract. 2021;15:89–92. doi: 10.1016/j.orcp.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waters DL. Intermuscular adipose tissue: a brief review of etiology, association with physical function and weight loss in older adults. Ann Geriatr Med Res. 2019;23:3–8. doi: 10.4235/agmr.19.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunnell KM, Thaweethai T, Buckless C, et al. Body composition predictors of outcome in patients with COVID-19. Int J Obes (Lond) 2021;45:2238–2243. doi: 10.1038/s41366-021-00907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grodecki K, Lin A, Razipour A, et al. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metab Clin Exp. 2021;115:154436. doi: 10.1016/j.metabol.2020.154436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phan F, Boussouar S, Lucidarme O, et al. Cardiac adipose tissue volume and IL-6 level at admission are complementary predictors of severity and short-term mortality in COVID-19 diabetic patients. Cardiovasc Diabetol. 2021;20:165. doi: 10.1186/s12933-021-01327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kottlors J, Zopfs D, Fervers P, et al. Body composition on low dose chest CT is a significant predictor of poor clinical outcome in COVID-19 disease – a multicenter feasibility study. Eur J Radiol. 2020;132:109274. doi: 10.1016/j.ejrad.2020.109274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiaffino S, Albano D, Cozzi A, et al (2021) CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology 204141. 10.1148/radiol.2021204141 [DOI] [PMC free article] [PubMed]
- 41.Giraudo C, Librizzi G, Fichera G, et al. Reduced muscle mass as predictor of intensive care unit hospitalization in COVID-19 patients. PLoS ONE. 2021;16:e0253433. doi: 10.1371/journal.pone.0253433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kottlors J, Große Hokamp N, Fervers P, et al. Early extrapulmonary prognostic features in chest computed tomography in COVID-19 pneumonia: bone mineral density is a relevant predictor for the clinical outcome – a multicenter feasibility study. Bone. 2021;144:115790. doi: 10.1016/j.bone.2020.115790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tahtabasi M, Kilicaslan N, Akin Y, et al. The prognostic value of vertebral bone density on chest CT in hospitalized COVID-19 patients. J Clin Densitom. 2021 doi: 10.1016/j.jocd.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baysal B, Dogan MB, Gulbay M, et al. Predictive performance of CT for adverse outcomes among COVID-19 suspected patients: a two-center retrospective study. Bosn J Basic Med Sci. 2021 doi: 10.17305/bjbms.2020.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruch Y, Kaeuffer C, Ohana M, et al. CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin Microbiol Infect. 2020;26:1417.e5–1417.e8. doi: 10.1016/j.cmi.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo H, Wang Y, Liu S, et al. Associations between CT pulmonary opacity score on admission and clinical characteristics and outcomes in patients with COVID-19. Intern Emerg Med. 2021 doi: 10.1007/s11739-021-02795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lieveld AWE, Azijli K, Teunissen BP, et al. Chest CT in COVID-19 at the ED: validation of the COVID-19 reporting and data system (CO-RADS) and CT severity score: a prospective, multicenter, observational study. Chest. 2021;159:1126–1135. doi: 10.1016/j.chest.2020.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Büttner L, Aigner A, Fleckenstein FN, et al. Diagnostic value of initial chest CT findings for the need of ICU treatment/intubation in patients with COVID-19. Diagnostics (Basel) 2020;10:929. doi: 10.3390/diagnostics10110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosse C, Büttner L, Fleckenstein FN, et al. CT-based risk stratification for intensive care need and survival in COVID-19 patients – a simple solution. Diagnostics (Basel) 2021;11:1616. doi: 10.3390/diagnostics11091616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Liu S, Wang B, et al. Predictive value of chest CT scoring in COVID-19 patients in Wuhan, China: a retrospective cohort study. Respir Med. 2021;176:106271. doi: 10.1016/j.rmed.2020.106271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shayganfar A, Sami R, Sadeghi S, et al. Risk factors associated with intensive care unit (ICU) admission and in-hospital death among adults hospitalized with COVID-19: a two-center retrospective observational study in tertiary care hospitals. Emerg Radiol. 2021 doi: 10.1007/s10140-021-01903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mozafari A, Miladinia M, Sabri A, et al. The challenge of deciding between home-discharge versus hospitalization in COVID-19 patients: the role of initial imaging and clinicolaboratory data. Clin Epidemiol Glob Health. 2021;10:100673. doi: 10.1016/j.cegh.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davarpanah AH, Asgari R, Moharamzad Y, et al (2020) Risk factors for poor outcome in patients with severe viral pneumonia on chest CT during the COVID-19 outbreak: a perspective from Iran. SN Compr Clin Med 1–11. 10.1007/s42399-020-00445-3 [DOI] [PMC free article] [PubMed]
- 54.Salahshour F, Mehrabinejad M-M, Nassiri Toosi M, et al. Clinical and chest CT features as a predictive tool for COVID-19 clinical progress: introducing a novel semi-quantitative scoring system. Eur Radiol. 2021 doi: 10.1007/s00330-020-07623-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang S, Jiang L, Cao Z, et al. Deep learning for detecting corona virus disease 2019 (COVID-19) on high-resolution computed tomography: a pilot study. Ann Transl Med. 2020;8:450. doi: 10.21037/atm.2020.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harmon SA, Sanford TH, Xu S, et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat Commun. 2020;11:4080. doi: 10.1038/s41467-020-17971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni Q, Sun ZY, Qi L, et al. A deep learning approach to characterize 2019 coronavirus disease (COVID-19) pneumonia in chest CT images. Eur Radiol. 2020;30:6517–6527. doi: 10.1007/s00330-020-07044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lessmann N, Sánchez CI, Beenen L, et al. Automated assessment of COVID-19 reporting and data system and chest CT severity scores in patients suspected of having COVID-19 using artificial intelligence. Radiology. 2021;298:E18–E28. doi: 10.1148/radiol.2020202439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang X, Li X, Bian Y, et al. Radiomics nomogram for the prediction of 2019 novel coronavirus pneumonia caused by SARS-CoV-2. Eur Radiol. 2020;30:6888–6901. doi: 10.1007/s00330-020-07032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Qin L, Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296:E65–E71. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng Q-Q, Zheng KI, Chen J, et al. Radiomics-based model for accurately distinguishing between severe acute respiratory syndrome associated coronavirus 2 (SARS-CoV-2) and influenza A infected pneumonia. MedComm. 2020 doi: 10.1002/mco2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai HX, Wang R, Xiong Z, et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest ct. Radiology. 2020;296:E156–E165. doi: 10.1148/radiol.2020201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao L-S, Li P, Sun F, et al. Development and validation of a deep learning-based model using computed tomography imaging for predicting disease severity of coronavirus disease 2019. Front Bioeng Biotechnol. 2020;8:898. doi: 10.3389/fbioe.2020.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Q, Du S-Y, Gao S, et al. A model based on CT radiomic features for predicting RT-PCR becoming negative in coronavirus disease 2019 (COVID-19) patients. BMC Med Imaging. 2020;20:118. doi: 10.1186/s12880-020-00521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang L, Han R, Ai T, et al. Serial quantitative chest CT assessment of COVID-19: a deep learning approach. Radiol Cardiothorac Imaging. 2020;2:e200075. doi: 10.1148/ryct.2020200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lanza E, Muglia R, Bolengo I, et al. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur Radiol. 2020;30:6770–6778. doi: 10.1007/s00330-020-07013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong D, Tang Z, Wang S, et al. The role of imaging in the detection and management of COVID-19: a review. IEEE Rev Biomed Eng. 2021;14:16–29. doi: 10.1109/RBME.2020.2990959. [DOI] [PubMed] [Google Scholar]
- 68.Laino ME, Ammirabile A, Posa A, et al. The applications of artificial intelligence in chest imaging of COVID-19 patients: a literature review. Diagnostics. 2021;11:1317. doi: 10.3390/diagnostics11081317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang X, Kruger U, Homayounieh F, et al. Association of AI quantified COVID-19 chest CT and patient outcome. Int J Comput Assist Radiol Surg. 2021;16:435–445. doi: 10.1007/s11548-020-02299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chatzitofis A, Cancian P, Gkitsas V, et al. Volume-of-interest aware deep neural networks for rapid chest CT-based COVID-19 patient risk assessment. Int J Environ Res Public Health. 2021;18:2842. doi: 10.3390/ijerph18062842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weikert T, Rapaka S, Grbic S, et al. Prediction of patient management in COVID-19 using deep learning-based fully automated extraction of cardiothoracic CT metrics and laboratory findings. Korean J Radiol. 2021 doi: 10.3348/kjr.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu F, Zhang Q, Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10:5613–5622. doi: 10.7150/thno.45985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho TT, Park J, Kim T, et al. Deep learning models for predicting severe progression in COVID-19-infected patients. JMIR Med Inform. 2021 doi: 10.2196/24973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li D, Zhang Q, Tan Y, et al. Prediction of COVID-19 severity using chest computed tomography and laboratory measurements: evaluation using a machine learning approach. JMIR Med Inform. 2020;8:e21604. doi: 10.2196/21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ufuk F, Demirci M, Uğurlu E, et al. Evaluation of disease severity with quantitative chest CT in COVID-19 patients. Diagn Interv Radiol. 2021;27:164–171. doi: 10.5152/dir.2020.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai W, Liu T, Xue X, et al. CT quantification and machine-learning models for assessment of disease severity and prognosis of COVID-19 patients. Acad Radiol. 2020;27:1665–1678. doi: 10.1016/j.acra.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan C, Chang Y, Yu H, et al. Clinical factors and quantitative CT parameters associated with ICU admission in patients of COVID-19 pneumonia: a multicenter study. Front Public Health. 2021;9:648360. doi: 10.3389/fpubh.2021.648360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burian E, Jungmann F, Kaissis GA, et al (2020) Intensive care risk estimation in COVID-19 pneumonia based on clinical and imaging parameters: experiences from the Munich Cohort. J Clin Med 9:. 10.3390/jcm9051514 [DOI] [PMC free article] [PubMed]
- 79.Noll E, Soler L, Ohana M, et al. A novel, automated, quantification of abnormal lung parenchyma in patients with COVID-19 infection: initial description of feasibility and association with clinical outcome. Anaesth Crit Care Pain Med. 2020;40:100780. doi: 10.1016/j.accpm.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296:E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Durhan G, Ardalı Düzgün S, Başaran Demirkazık F, et al. Visual and software-based quantitative chest CT assessment of COVID-19: correlation with clinical findings. Diagn Interv Radiol. 2020;26:557–564. doi: 10.5152/dir.2020.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu Q, Wang S, Li L, et al. Radiomics analysis of computed tomography helps predict poor prognostic outcome in COVID-19. Theranostics. 2020;10:7231–7244. doi: 10.7150/thno.46428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu Q, Zhan X, Zhou Z, et al. CT-based rapid triage of COVID-19 patients: risk prediction and progression estimation of ICU admission, mechanical ventilation, and death of hospitalized patients. medRxiv. 2020 doi: 10.1101/2020.11.04.20225797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chao H, Fang X, Zhang J, et al. Integrative analysis for COVID-19 patient outcome prediction. Med Image Anal. 2021;67:101844. doi: 10.1016/j.media.2020.101844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li C, Dong D, Li L, et al. Classification of severe and critical Covid-19 using deep learning and radiomics. IEEE J Biomed Health Inform. 2020;24:3585–3594. doi: 10.1109/JBHI.2020.3036722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bartolucci M, Benelli M, Betti M, et al. The incremental value of computed tomography of COVID-19 pneumonia in predicting ICU admission. Sci Rep. 2021;11:15619. doi: 10.1038/s41598-021-95114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou J-N, Sun L, Wang B-R, et al. The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT. PLoS ONE. 2021;16:e0248957. doi: 10.1371/journal.pone.0248957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong L, Zhang S, Wang J, et al. Analysis of chest CT results of coronavirus disease 2019 (COVID-19) patients at first follow-up. Can Respir J. 2020;2020:5328267. doi: 10.1155/2020/5328267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desai SR, Wells AU, Rubens MB, et al. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210:29–35. doi: 10.1148/radiology.210.1.r99ja2629. [DOI] [PubMed] [Google Scholar]
- 90.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 91.Han X, Fan Y, Alwalid O, et al (2021) Fibrotic interstitial lung abnormalities at 1-year follow-up CT after severe COVID-19. Radiology 210972. 10.1148/radiol.2021210972 [DOI] [PMC free article] [PubMed]
- 92.Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poitevineau T, Chassagnon G, Bouam S, et al (2021) Computed tomography after severe COVID-19 pneumonia: findings at 6 months and beyond. ERJ Open Research 7:. 10.1183/23120541.00488-2021 [DOI] [PMC free article] [PubMed]
- 94.Liu M, Lv F, Huang Y, Xiao K. Follow-up study of the chest CT characteristics of COVID-19 survivors seven months after recovery. Front Med (Lausanne) 2021;8:636298. doi: 10.3389/fmed.2021.636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tabatabaei SMH, Rajebi H, Moghaddas F, et al. Chest CT in COVID-19 pneumonia: what are the findings in mid-term follow-up? Emerg Radiol. 2020;27:711–719. doi: 10.1007/s10140-020-01869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Truffaut L, Demey L, Bruyneel AV, et al. Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res. 2021;22:29. doi: 10.1186/s12931-021-01625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.