Abstract
Interactions between anti-hypertensive agents (ACEI), comorbidities, inflammation, and stress status may impact hospital stay duration in COVID-19 patients. This retrospective study analyzed epidemiological data, comorbidities, metabolic/inflammatory markers, and clinical information from 165 SARS-CoV-2 positive patients. In a multiple linear regression model, an IL-6 higher than 100 mg/L, glucose at admission (baseline levels at the hospital entry), and the interaction between ACEI administration and LDH predicted the days of hospital admission (P < 0.001). In conclusion, hypertensive patients suffering more severe inflammatory condition assessed by LDH levels clinically benefited more and reduced the hospital stay when prescribed ACEI agents than those with lower systemic baseline inflammation at admission.
1. Introduction
The coronavirus disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), outreached in China at the end of 2019, with a later rapid worldwide extension, and a later surge in the case lethality ratio, pathogenesis and transmissibility, in most countries [1]. The viral infectious incidence and severity depend on the presence of associated comorbidities or metabolic and inflammatory-related diseases [2,3]. In this context, obesity and hypertension (HT) are associated with unfavorable evolution of the COVID-19 disease, with a high probability to develop severe pneumonia and impaired inflammatory reactions, in addition to organ and tissue damage. These outcomes were also related to increases in stay length, intensive care unit (ICU) stay, and mortality, as described elsewhere [4,5]. Covid-19 patients display inflammatory complications that have been related to a “cytokine storm” involving exacerbated blood interleukin 6 (IL-6), C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), neutrophil/lymphocyte index (NLI), D-Dimer, and red cell distribution width (RDW) levels. Furthermore, consistent associations between hospital stay days and ICU admission implications concerning prescribed therapies and clinical outcomes were found as described elsewhere [6]. During the COVID-19 pandemic, several therapeutic approaches have been tested to fight against this viral disease [7], including the frequent administration of drugs belonging to Angiotensin-converting enzyme inhibitors (ACEI) group. Given the high prevalence of hypertension in severe cases of COVID-19 and the known clinical associations of pathogenic and inflammatory mechanisms accompanying SARS-CoV-2 infection, which may interfere with the administration of anti-hypertensive pharmacological agents (i.e. ACEI), the current research targeted in analyzing interactions of stay duration days at the hospital (dependent variable) with obesity, hypertension, the inflammatory status at admission based on IL-6 and LDH measurements and the metabolic stress status based on glycemia values (independent variables).
2. Materials and methods
This retrospective study was based on a series of 165 subjects consecutively admitted at the Puerta de Hierro Majadahonda University Hospital (Madrid, Spain) between March 15 and April 15, 2020. The following inclusion criteria were considered: patients >18 years old with positive PCR for SARS-CoV-2, moderate/severe pneumonia according to WHO guidelines, oxygen saturation rate < 94%, and respiratory rate > 22 breaths per minute. Patients with COVID-19 at 90 days before or those with incomplete clinical/metabolic information were excluded from the analyses. The variables recorded included administrative and epidemiological data, comorbidities, diagnosis examinations and information about the evolution of the disease following accepted standards [8]. Comorbidities and complications during hospital stay were defined as diagnoses included in the clinical history at admission and at discharge. Systolic and diastolic blood pressures (SBP and DBP, respectively) were recorded by the center's triage system upon arrival at the emergency room. HT was established as: SBP >140 mmHg and DBP >90 mmHg. Baseline blood cholesterol, glucose, inflammatory markers (IL-6, CRP, LDH), and liver enzymes (AST: aspartate aminotransferase; ALT: alanine transaminase) were collected from the first analysis performed during hospital admission. Height and weight were self-reported in all cases, with subsequent calculation of the Body Mass Index (BMI: kg/m2). Obesity was setup as BMI ≥30 kg/m2. The data record was developed through information collected in the electronic medical record (SELENE System, Cerner Iberia, S.L.U, Madrid, Spain) filling in a template form previously established by the main investigators of this study. The laboratory analyzes were carried out by the Biochemistry Service of the center, according to validated analytical protocols. Triage was performed according to the Manchester scale [9], as a standard procedure in our center. Implementation was reviewed by two expert investigators to ensure the validity of the protocol. The Research Ethics Committee of the Puerta de Hierro Majadahonda University Hospital approved the study (PI 94/20). All the participating subjects gave their informed consent prior to its inclusion. This work respects the guidelines of Spanish and European Laws, as well as the Declaration of Helsinki, declaring no conflicts of interests or external sources of funding concerning this investigation. Quantitative variables are expressed as means and standard deviations, while qualitative variables are expressed as frequencies and proportions. Clinical and phenotypical characteristics of COVID-19 patients were compared by categorical variables or by the median values of continuous variables. For this last purpose, the reference values of IL-6 and LDH considered in our design are compatible with previous reports exploring laboratory predictors and prognostic factors of severe COVID-19 disease concerning respiratory functions [10,11] as well as pharmacological prescription making decision criteria [12]. Moreover, Chi-square and unpaired Student's t-tests were applied for the univariate analysis. Multivariate regression analyses were used to predict stay duration days at the hospital, which were adjusted for baseline characteristics of the population and the day of extraction and including appropriate product terms. Eventual statistical interactions between clinical, inflammatory, and pharmacological markers were evaluated by multivariate regression analyses using product-term variables in the model. Statistical analyses were performed in the STATA software (version 12.1 for Windows, Texas, USA). P values (two-tailed) below 0.05 were considered statistically significant. P values (two-tailed) below 0.10 were considered as marginal significance.
3. Results
The clinical and phenotypical characteristics of COVID-19 patients based on the presence or absence of obesity, HT, and ACEI administration are reported (Table 1 ). As expected, patients with obesity underwent higher frequencies of ICU stay/mortality as well as greater blood levels of glucose at admission than their lean counterparts (Table 1). Moreover, statistical tendencies (P < 0.10) to display elevated levels of LDH, CRP and higher frequencies of increased IL-6 were found in subjects with obesity, although these were at a marginal significance (P < 0.10). Meanwhile, patients with diagnosed HT were statistically older and had a higher AST:ALT ratio as well as marginally greater serum levels of total cholesterol, CRP, DBP, ALT, and elevated IL-6 than normotensive individuals (Table 1). Instead, no statistical differences were found in liver markers neither inflammatory features when compared HT patients with ACEI prescription that those who were not under this treatment.
Table 1.
Clinical and phenotypical characteristics of COVID-19 patients based on obesity, hypertension and ACEI administration.
| Obesity |
HT |
HT + ACEI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | No (n = 103) | Yes (n = 51) | P | No (n = 99) | Yes (n = 66) | P | No (n = 17) | Yes (n = 49) | P |
| Age | 63.7 ± 11.5 | 61.4 ± 11.9 | 0.259 | 59.1 ± 11.2 | 68.4 ± 9.9 | <0.001 | 69.4 ± 9.0 | 68.0 ± 10.3 | 0.612 |
| Sex (F/M) | 36/67 | 18/33 | 0.967 | 39/60 | 17/49 | 0.070 | 5/12 | 12/37 | 0.689 |
| Days of admission | 3.90 ± 2.76 | 3.42 ± 2.53 | 0.307 | 3.76 ± 2.60 | 3.98 ± 2.87 | 0.616 | 4.31 ± 3.16 | 3.87 ± 2.79 | 0.599 |
| No ICU/No mortality | 88 (85.4) | 35 (68.6) | 0.014 | 80 (80.8) | 50 (75.8) | 0.437 | 13 (76.5) | 37 (75.5) | 0.937 |
| ICU/mortality | 15 (14.6) | 16 (31.4) | 19 (19.2) | 16 (24.2) | 4 (23.5) | 12 (24.5) | |||
| Glucose at admission (mg/dL) | 118.7 ± 48.6 | 145.2 ± 64.7 | 0.007 | 124.9 ± 56.8 | 137.5 ± 61.7 | 0.200 | 125.1 ± 65.7 | 141.7 ± 60.5 | 0.355 |
| Total cholesterol (mg/dL) | 138.1 ± 31.4 | 145.4 ± 34.2 | 0.270 | 144.5 ± 34.7 | 132.9 ± 25.8 | 0.053 | 133.4 ± 34.9 | 132.7 ± 23.2 | 0.942 |
| LDH (IU/L) | 346.1 ± 134.0 | 378.1 ± 148.8 | 0.097 | 352.3 ± 153.4 | 366.3 ± 117.6 | 0.539 | 387.0 ± 128.1 | 358.8 ± 114.1 | 0.402 |
| CRP (mg/L) | 120.4 ± 109.4 | 158.2 ± 205.6 | 0.073 | 118.1 ± 106.2 | 155.4 ± 186.2 | 0.055 | 159.5 ± 69.6 | 154.1 ± 210.5 | 0.923 |
| IL-6 (0–100 mg/L) | 74 (71.8) | 31 (60.8) | 0.083 | 70 (70.7) | 40 (60.6) | 0.089 | 10 (58.8) | 30 (61.2) | 0.861 |
| IL-6 (>100 mg/L) | 29 (28.2) | 20 (39.2) | 29 (29.3) | 26 (39.4) | 7 (41.2) | 19 (38.8) | |||
| SBP (mmHg) | 138.9 ± 21.3 | 137.7 ± 22.4 | 0.748 | 136.7 ± 20.4 | 139.6 ± 23.9 | 0.402 | 145.7 ± 19.3 | 137.5 ± 25.2 | 0.224 |
| DBP (mmHg) | 79.3 ± 14.2 | 79.0 ± 13.9 | 0.895 | 80.3 ± 13.4 | 77.3 ± 15.2 | 0.094 | 79.0 ± 10.9 | 76.7 ± 16.4 | 0.593 |
| D-dimer (μg/mL) | 1.86 ± 6.85 | 1.55 ± 2.01 | 0.757 | 1.93 ± 7.00 | 1.43 ± 1.78 | 0.580 | 1.28 ± 0.77 | 1.48 ± 2.03 | 0.683 |
| AST (IU/L) | 53.4 ± 35.2 | 46.9 ± 19.4 | 0.223 | 52.5 ± 33.0 | 48.9 ± 25.8 | 0.456 | 53.0 ± 28.1 | 47.5 ± 25.2 | 0.467 |
| ALT (IU/L) | 42.9 ± 34.4 | 37.7 ± 21.1 | 0.333 | 43.6 ± 32.3 | 35.9 ± 25.4 | 0.055 | 36.8 ± 21.9 | 35.6 ± 26.6 | 0.879 |
| AST:ALT ratio | 1.46 ± 0.84 | 1.40 ± 0.56 | 0.640 | 1.35 ± 0.45 | 1.61 ± 1.02 | 0.029 | 1.49 ± 0.31 | 1.64 ± 1.17 | 0.609 |
Values are expressed as means ± standard deviations. P values were calculated by Student's T-tests. Bold numbers indicate P value lower than 0.05. F: female; M: male; HT: hypertension; ACEI: Angiotensin Converting Enzyme Inhibitors; LDH: lactate dehydrogenase; IL-6: interleukin 6; CRP: C-reactive protein; SBP: systolic blood pressure; DBP: diastolic blood pressure; AST: aspartate aminotransferase; ALT: alanine transaminase.
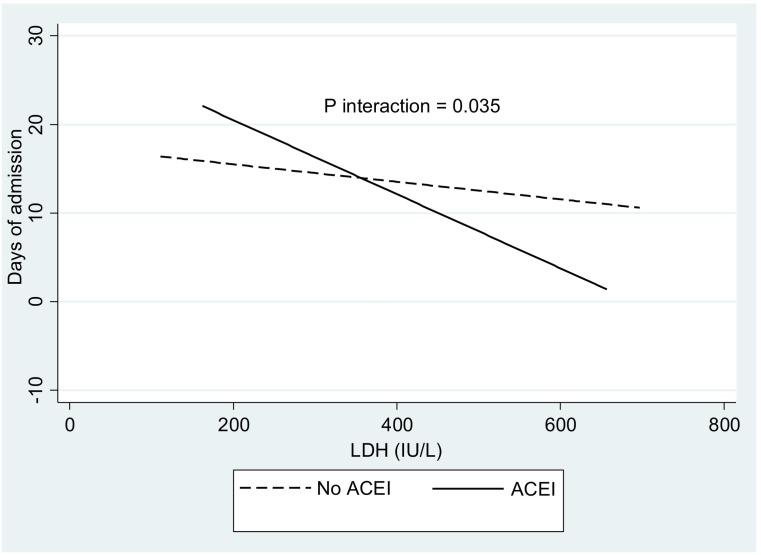
The demographic, clinical and biochemical characteristics of the total COVID-19 sample based on cutoffs of IL-6 (clinical criteria) and median values of LDH (statistical criteria) are reported (Table 2 ). Patients with blood levels of IL-6 greater than 100 mg/L had significantly shorter days of admission, but higher frequencies of ICU stay/mortality than those with IL-6 concentrations equal or lower than 100 mg/L (Table 2). In addition, increased levels of glucose at admission, CRP and ALT were identified in subjects with elevated IL-6 (Table 2). Similarly, older age and greater AST:ALT ratio were found in this same group, although at marginal (P < 0.10) significance (Table 2). Patients with LDH values above the median (332.5 IU/L) presented higher levels of LDH, AST, AST:ALT ratio as well as a tendency (P < 0.10) to have elevated glucose at admission, DBP, D-dimer, and ALT than individuals below the median value. About 75% of patients had been treated with corticoids at hospital admission, with an increasing proportional trend in those with higher IL-6 concentrations (about 63% in patients with 0–100 mg/dL and 96% in those with >100 mg/dL). Interestingly, regression analyses including corticoid therapy demonstrated the same statistical trends. The multiple linear regression model that predicted the days of admission in COVID-19 patients using clinical, inflammatory, and pharmacological variables is reported (Table 3 ). Noteworthy, increased IL-6 (>100 mg/L), glucose at admission, obesity (at a marginal significance), and the interaction between ACEI and LDH predicted the days of admission in approximately 27%. The LDH * ACEI interaction is depicted (Fig. 1 ). Patients who were under ACEI treatment and presented elevated LDH stayed lower days of admission, which was not observed in those without ACEI treatment (Fig. 1).
Table 2.
Clinical and phenotypical characteristics of COVID-19 patients based on cut-offs of IL-6 and median values of LDH.
| All |
IL-6 |
LDH |
|||||
|---|---|---|---|---|---|---|---|
| Variable | n = 165 | 0–100 mg/L (n = 110) | >100 mg/L (n = 55) | P | <332.5 IU/L (n = 77) | ≥332.5 IU/L (n = 77) | P |
| Age | 62.8 ± 11.6 | 61.5 ± 11.3 | 65.3 ± 11.9 | 0.051 | 63.1 ± 12.3 | 63.1 ± 10.9 | 0.983 |
| Sex (F/M) | 56/109 | 38/72 | 18/37 | 0.816 | 29/48 | 23/54 | 0.307 |
| Days of admission | 3.85 ± 2.70 | 4.27 ± 2.76 | 2.96 ± 2.35 | 0.004 | 3.74 ± 2.57 | 3.88 ± 2.88 | 0.758 |
| No ICU/No mortality | 130 (78.8) | 101 (91.8) | 29 (52.7) | <0.001 | 61 (79.2) | 59 (76.6) | 0.698 |
| ICU/mortality | 35 (21.2) | 9 (8.2) | 26 (47.3) | 16 (20.8) | 18 (23.4) | ||
| Glucose at admission (mg/dL) | 130.2 ± 59.1 | 119.9 ± 42.7 | 148.1 ± 77.0 | 0.004 | 121.2 ± 53.7 | 138.4 ± 64.1 | 0.087 |
| Total cholesterol (mg/dL) | 140.1 ± 32.1 | 142.6 ± 31.3 | 135.3 ± 33.2 | 0.246 | 141.5 ± 30.5 | 135.2 ± 32.0 | 0.298 |
| LDH (IU/L) | 358.1 ± 139.4 | 362.4 ± 150.2 | 350.1 ± 117.6 | 0.603 | 253.2 ± 57.3 | 463.1 ± 116.2 | <0.001 |
| CRP (mg/L) | 132.8 ± 143.7 | 107.7 ± 73.9 | 182.1 ± 217.8 | 0.002 | 127.2 ± 193.9 | 150.0 ± 70.7 | 0.342 |
| SBP (mmHg) | 137.8 ± 21.9 | 137.2 ± 20.7 | 139.2 ± 24.2 | 0.578 | 136.4 ± 22.7 | 139.1 ± 21.2 | 0.439 |
| DBP (mmHg) | 79.1 ± 14.1 | 79.3 ± 14.0 | 78.6 ± 14.5 | 0.749 | 77.6 ± 14.4 | 80.6 ± 12.4 | 0.080 |
| D-dimer (μg/mL) | 1.71 ± 5.46 | 1.78 ± 6.58 | 1.60 ± 2.11 | 0.846 | 0.96 ± 0.98 | 2.53 ± 7.68 | 0.084 |
| AST (IU/L) | 51.1 ± 30.3 | 53.1 ± 34.4 | 47.2 ± 20.1 | 0.240 | 41.1 ± 23.3 | 59.7 ± 30.7 | <0.001 |
| ALT (IU/L) | 40.6 ± 29.9 | 45.0 ± 34.5 | 32.0 ± 15.0 | 0.009 | 35.0 ± 25.6 | 43.6 ± 30.3 | 0.059 |
| AST:ALT ratio | 1.45 ± 0.74 | 1.38 ± 0.83 | 1.58 ± 0.53 | 0.057 | 1.31 ± 0.39 | 1.63 ± 0.97 | 0.009 |
Values are expressed as means ± standard deviations. P values were calculated by Student's T-tests. Bold numbers indicate P value lower than 0.05. F: female; M: male; LDH: lactate dehydrogenase; IL-6: interleukin 6; CRP: C-reactive protein; SBP: systolic blood pressure; DBP: diastolic blood pressure; AST: aspartate aminotransferase; ALT: alanine transaminase.
Table 3.
Multiple linear regression models deeming clinical, inflammatory, and pharmacological variables as important predictors of stay duration days at the hospital as the main outcome.
| Variable | β coefficients (CI 95%) | P |
|---|---|---|
| IL6 (>100 mg/L) | 5.1995 (2.8072, 7.5917) | <0.001 |
| Obesity (yes) | 0.4117 (−0.0168, 0.8402) | 0.060 |
| Glucose at admission | 0.0353 (0.0002, 0.0703) | 0.049 |
| HT (yes) | 2.2986 (−3.7035, 8.3006) | 0.450 |
| ACEI * LDH | −0.0361 (−0.0695, −0.0025) | 0.035 |
| Adjusted R2 | 0.2683 | <0.001 |
Bold numbers indicate P < 0.05.
Fig. 1.
Interaction between ACEI and LDH concerning days of admission in COVID-19 patients.
4. Discussion
The time of hospitalization in COVID patients diagnosed with hypertension and treated with ACE2 antagonists was dependent on the baseline inflammatory condition as assessed via IL-6 levels, and circulating glucose concentrations at admission. The most relevant finding of this study was that a negative interaction between LDH and ACEI prescription was statistically demonstrated concerning hospital stay, which means a protective role of these antagonists/blockers on those patients with high LDH values as a marker of tissue damage and inflammation. This information is compatible with the differential responses found in some patients and allow to understand and predict personalized outcomes concerning clinical stays of COVID-19 patients within precision medicine endeavors.
COVID-19 not only affects the pulmonary tissue, but also induces harms in multiple organs, particularly the cardiovascular system [13]. This infectious condition may lead to myocardial injuries, endothelial dysfunctions and microvascular spasms, usually requiring preventive or pharmacological measures including ACE2 antagonist's administration [14]. These findings warrant specific analyses considering factors with potential impact on the clinical and stay outcomes. In this context, available mortality figures suggest that COVID-19 is more lethal in aged patients with comorbid conditions including hypertension [15]. The SARS-CoV-2 pathogenesis is initiated by the binding of viral spike protein with the target receptor ACE2 facilitating virus internalization within host cells [16]. Mechanistically, SARS-CoV-2, following serine protease cleavage of the S protein, binds to the transmembrane ACE2 to penetrate into type 2 pneumocytes, macrophages, perivascular pericytes, and cardiomyocytes, whose entry may be blocked by ACE2 antagonists/blockers [17]. Although there were a debate about whether the use of ACEI are useful or hazardous in patients with COVID-19, several reviews and analyses now suggest that ACEI and angiotensin receptor blockers (ARBs) administration should not be discontinued in COVID-19 patients with hypertension since that there was no evidence that both pharmacological agents affected the risk COVID-19 incidence and mortality [[18], [19], [20], [21], [22]]. However, it appears that phenotypical interactions and confounding factors such as the baseline inflammatory status, concurrent metabolic diseases and complications (obesity, diabetes dyslipidemia), hyperglycemia associated to morbid stress, age or sex need to be individually accounted for precision management. These features may explain clinical discrepancies concerning clinical outcomes and duration stays among COVID 19 patients administered ACE2 antagonists as found in current analyses, where inflammation markers (LDH/IL-6), circulating glucose and ACEI were involved concerning clinical outcomes.
Given that accumulating evidence suggests that SARS-CoV2 is associated with a hyperinflammation condition characterized by excessive release of pro-inflammatory cytokines, anti-viral agents alone will not provide the much required therapeutic effect [23]. Hence, the need to combine anti-inflammatory agents such as interferons, ACE2 inhibitors, IL-6, and Janus kinase (JAK) family inhibitors, anticoagulants and other agents involved in inflammation resolution needs to conjointly examine the inflammatory status in these patients by measuring inflammatory markers such as IL-6 or LDH [24]. Using meta-regression tests, neutrophils, lymphocytes, IL-6, ferritin, C-reactive protein, D-dimer and LDH demonstrated that hyperinflammation, blunted adaptive immune response and intravascular coagulation, playing key roles in the pathogenesis of COVID-19 [25]. Indeed, LDH and IL-6 are valid inflammation markers and reliable surrogates for inflammation categorization in COVID-19 patients regardless of age or sex [24], and support the inclusion of these inflammation proxies in our regression analyses to explain the hospital stay duration implications. In fact, IL-6, LDH, C-reactive protein, and lymphocytes are key inflammatory markers in COVID-19 patients with hypertension receiving ACEI therapy, who had a lower rate of severe diseases and a trend toward a lower level of IL-6 in peripheral blood, which supports the benefit of using ACEIs to potentially contribute to the improvement of clinical outcomes of COVID-19 patients with inflammation features and hypertension [26].
A limitation of this analysis is the retrospective nature of the design, but the results were plausible according to available evidences and the hypothesis of this research. Although the analyzed sample was relatively small, it is comparable to larger series such as the COVID-DATA-SAFE-LIFES cohort [27]. Also, initial stages of COVID-19 hampered to collect some clinical information. This research should be considered as a proof of principle with valuable results and actual clinical applications, although type I and type II errors cannot be discarded given the relative small sample size. As positive strengths are that an interaction of ACE2 antagonist with the baseline LDH status has been demonstrated after adjusted by relevant inflammation variables and confounding factors. In this regard, alternative linear regression models with corticoids administration as covariable revealed that the use of corticoids did not affected the ACEI*LDH interaction (data not shown). Interestingly, those with higher IL-6 values were more prone to be treated with corticoids, which provide additional support to the need to appraise the inflammatory status in hypertensive patients.
5. Conclusion
In conclusion, the interaction between ACEI administration and the inflammatory marker LDH influenced the stay duration (days) at the hospital, which could contribute to improve the clinical/pharmacological management of COVID-19 disease under a personalized medicine approach, where patients with a more severe inflammatory status may probably benefit more specifically by ACEI treatment. This investigation allowed to define some factors that discriminate some divergent personalized clinical outcomes with emphasis on factors explaining clinical interindividual differences for a precision vascular pharmacology in COVID-19 patients.
Declaration of Competing Interest
We guarantee that there is no conflict of interest in our paper.
References
- 1.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with COVID-19. SN Compr. Clin. Med. 2020 Jun;25:1–8. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez Urbistondo M., Mora Vargas A., Expósito Palomo E., Aparicio De Miguel M., Castejón Díaz R., Daimiel L., Ramos López O., San Cristóbal R., Martínez J.A., Vargas Núñez J.A. Evolution of patients infected with SARS-CoV-2 according to previous metabolic status. Nutr. Hosp. 2021 doi: 10.20960/nh.03469. Spanish. [DOI] [PubMed] [Google Scholar]
- 4.Popkin B.M., Du S., Green W.D., Beck M.A., Algaith T., Herbst C.H., Alsukait R.F., Alluhidan M., Alazemi N., Shekar M. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes. Rev. 2020;21(11) doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S., Wang J., Liu F., Liu J., Cao G., Yang C., Liu W., Tu C., Zhu M., Xiong B. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens. Res. 2020;43(8):824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Urbistondo M., Mora-Vargas A., Expósito-Palomo E., Castejón R., Citores M.J., Rosado S., de Mendoza C., Baños I., Fernández-Cruz A., Daimiel L., San-Cristóbal R., Vargas J.A., Martinez J.A. Inflammatory-related clinical and metabolic outcomes in COVID-19 patients. Mediat. Inflamm. 2020;2020:2914275. doi: 10.1155/2020/2914275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casucci G., Acanfora D., Incalzi R.A. The cross-talk between age, hypertension and inflammation in COVID-19 patients: therapeutic targets. Drugs Aging. 2020;37(11):779–785. doi: 10.1007/s40266-020-00808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alaa A., Qian Z., Rashbass J., Benger J., van der Schaar M. Retrospective cohort study of admission timing and mortality following COVID-19 infection in England. BMJ Open. 2020 Nov 23;10(11) doi: 10.1136/bmjopen-2020-042712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zachariasse J.M., Seiger N., Rood P.P., Alves C.F., Freitas P., Smit F.J., Roukema G.R., Moll H.A. Validity of the Manchester triage system in emergency care: a prospective observational study. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0170811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan M., Wang R.R., Chen X., Han J., Li Q., Miao M., Rao J., Huang J., Yu L., Xu Y., Li L., Shao Q., Ma H., Han M., Fan X. Laboratory predictors of severe coronavirus disease 2019 and lung function in followed-up. Clin. Respir. J. 2021 Aug;15(8):904–914. doi: 10.1111/crj.13381. [DOI] [PubMed] [Google Scholar]
- 11.Martha J.W., Wibowo A., Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis. Postgrad. Med. J. 2021 Jan 15 doi: 10.1136/postgradmedj-2020-139542. postgradmedj-2020-139542. [DOI] [PubMed] [Google Scholar]
- 12.Fujino M., Ishii M., Taniguchi T., Chiba H., Kimata M., Hitosugi M. The value of Interleukin-6 among several inflammatory markers as a predictor of respiratory failure in COVID-19 patients. Diagnostics (Basel) 2021 Jul 23;11(8):1327. doi: 10.3390/diagnostics11081327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D’Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Wang D.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbosa L.C., Gonçalves T.L., de Araujo L.P., Rosario L.V.O., Ferrer V.P. Endothelial cells and SARS-CoV-2: an intimate relationship. Vasc. Pharmacol. 2021;137 doi: 10.1016/j.vph.2021.106829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan S.H., Zaidi S.K. Review of evidence on using ACEi and ARBs in patients with hypertension and COVID-19. Drugs Ther. Perspect. 2020 Jun 9:1–4. doi: 10.1007/s40267-020-00750-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luzi L., Bucciarelli L., Ferrulli A., Terruzzi I., Massarini S. Obesity and COVID-19: the ominous duet affecting the renin-angiotensin system. Minerva Endocrinol. (Torino) 2021;46(2):193–201. doi: 10.23736/S2724-6507.20.03402-1. [DOI] [PubMed] [Google Scholar]
- 17.Albini A., Di Guardo G., Noonan D.M., Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020;15(5):759–766. doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurado A., Martín M.C., Abad-Molina C., Orduña A., Martínez A., Ocaña E., Yarce O., Navas A.M., Trujillo A., Fernández L., Vergara E., Rodríguez B., Quirant B., Martínez-Cáceres E., Hernández M., Perurena-Prieto J., Gil J., Cantenys S., González-Martínez G., Martínez-Saavedra M.T., Rojo R., Marco F.M., Mora S., Ontañón J., López-Hoyos M., Ocejo-Vinyals G., Melero J., Aguilar M., Almeida D., Medina S., Vegas M.C., Jiménez Y., Prada Á., Monzón D., Boix F., Cunill V., Molina J. COVID-19: age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study. Immun. Ageing. 2020;17:22. doi: 10.1186/s12979-020-00194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong Y., Zhao L., Wu G., Hu C., Wu C., Xu M., Dong H., Zhang Q., Wang G., Yu B., Lv J., Wu C., Zhang S., Cao C., Shu L., Pan Y., Liu X., Wu F. Impact of renin-angiotensin system inhibitors use on mortality in severe COVID-19 patients with hypertension: a retrospective observational study. J. Int. Med. Res. 2020;48(12) doi: 10.1177/0300060520979151. 300060520979151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020;43(7):648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COVID-19 RISk and Treatments (CORIST) Collaboration RAAS inhibitors are not associated with mortality in COVID-19 patients: findings from an observational multicenter study in Italy and a meta-analysis of 19 studies. Vasc. Pharmacol. 2020;135:106805. doi: 10.1016/j.vph.2020.106805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chukwuma I.F., Apeh V.O., Chiletugo O.F. Mechanisms and potential therapeutic targets of hyperinflammatory responses in SARS-CoV-2. Acta Virol. 2021;65(1):3–9. doi: 10.4149/av_2021_102. [DOI] [PubMed] [Google Scholar]
- 24.Fei F., Smith J.A., Cao L. Clinical laboratory characteristics in patients with suspected COVID-19: one single-institution experience. J. Med. Virol. 2021;93(3):1665–1671. doi: 10.1002/jmv.26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khinda J., Janjua N.Z., Cheng S., van den Heuvel E.R., Bhatti P., Darvishian M. Association between markers of immune response at hospital admission and COVID-19 disease severity and mortality: a meta-analysis and meta-regression. J. Med. Virol. 2021;93(2):1078–1098. doi: 10.1002/jmv.26411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos-Lopez O., San-Cristobal R., Martinez-Urbistondo D., Micó V., Colmenarejo G., Villares-Fernandez P., Daimiel L., Martinez J.A. Proinflammatory and hepatic features related to morbidity and fatal outcomes in COVID-19 patients. J. Clin. Med. 2021;10(14):3112. doi: 10.3390/jcm10143112. [DOI] [PMC free article] [PubMed] [Google Scholar]