Abstract
Background
Methadone is a highly effective treatment for opioid use disorder. Its use in the United States is highly regulated at both the federal and state level. The regulations related to take-home doses were loosened because of the 2019 Novel Coronavirus public health emergency declaration. The aim was to assess the effect of loosened regulations on methadone-related exposures reported to poison control centers.
Methods
Retrospective analysis of population-based intentional methadone exposures (in persons 18 years of age and older) reported to the American Association of Poison Control Centers’ National Poison Data System. A quasi-experimental design looking at one year before and after the March 16, 2020 loosening of methadone take-home regulations. Severity of exposure was assessed by: disposition (discharged from emergency department, admitted to non-critical care versus critical care units), medical treatments received, and medical outcomes (no effect, minor effect, moderate effect, major effect, death). One tail Student t-test and Chi Square were used; p significance was <0.05.
Results
The number of adult intentional exposures involving methadone increased by 5.3% in the year following the change in federal regulations (p<0.05). There was no statistically significant difference in distribution of age, gender, whether exposures involved methadone-only or methadone plus other substances, therapies administered or hospitalizations. There was no difference in overall distribution of medical outcomes, including deaths.
Conclusions
Although the number of exposures involving methadone increased post-regulation change, the severity of exposures remained unchanged. Various additional factors (Medicare and Medicaid expansion; increased number of opioid treatment programs) may have also contributed to this increase. As federal officials consider possible permanent changes to the methadone regulations, it is important to evaluate potential related risks and benefits. This study lends support to the consideration that loosening of methadone regulations does not necessarily lead to a substantial increase in severity of exposures.
Keywords: Methadone, Opioid use disorder, Opioid treatment program, Agonist treatment
Background
Methadone has been used in the United States as a highly effective medication for the treatment of Opioid Use Disorder (OUD) since the late 1960s. Its use is strictly regulated by the Drug Enforcement Administration (DEA) under the Narcotic Addict Treatment Act of 1974 (and related federal regulation, 42CFR8.12), the Substance Abuse and Mental Health Services Administration (SAMHSA), as well as individual state regulations. For the treatment of OUD, it must be dispensed by an opioid treatment programs (OTP) which must be registered with the DEA, certified by SAMHSA, accredited by an independent accrediting body (approved by SAMHSA), and licensed by the state in which it operates. The federal regulations outline strict requirements for all aspects of the use of methadone including efforts to limit the potential for diversion. A major tool used for this purpose is the stringent requirement for patients to “earn” unsupervised (take-home; take-away; “carry”) doses of methadone other than for days when the clinic is closed (e.g. Sundays/holidays). The regulation stipulates that a patient who is doing well may receive one additional weekly take-home dose every 90 days during the first 270 days of treatment, with a two-week supply allowed after one year and a four-week supply after two years.1 Programs are encouraged to use eight criteria “in determining whether a patient is responsible in handling opioid drugs for unsupervised use.” These include such somewhat vague items as “absence of recent abuse of drugs”, “stability of the patient's home environment and social relationships”, and “whether the rehabilitative benefit …outweighs the potential risks of diversion” (HHS, 2001).
Despite this concern for public safety, there is little evidence in the scientific literature that relaxing regulations and allowing increased take-home doses leads to more diversion or jeopardizes public safety. Conversely, there is evidence that stringent take-home policies negatively impact patient engagement and retention in treatment and hinder the ability of OTPs to provide true patient-centered care (Amiri et al., 2018; Deering et al., 2011; Gerra et al., 2011; Kourounis et al., 2016; Pani, Pirastu, Ricci & Gessa, 1996).
On January 31, 2020, the Secretary of Health and Human Services (HHS) declared a public health emergency for the 2019 Novel Coronavirus. As the pandemic worsened and more states prepared to implement Stay-at-Home orders, SAMHSA released an ‘Opioid Treatment Program (OTP) Guidance’ on March 16, 2020 allowing states to “…request blanket exceptions for all stable patients…to receive 28 days of Take-Home doses …and up to 14 days of Take-Home medication for those patients who are less stable but who the OTP believes can safely handle this level of Take-Home medication.” (SAMHSA, 2020a) This decision potentially impacted more than 400,000 individuals who receive methadone through an OTP (SAMHSA, 2020b).
The COVID-19 public health emergency declaration provided a naturalistic experiment to look at outcomes related to the loosening of methadone regulations. Several studies looking at the effect of increased take-home doses during the COVID-19 pandemic have reported minimal diversion of methadone and no increases in methadone-related overdoses (Amram, Amiri, Thorn, Lutz & Joudrey, 2021; Brothers, Viera & Heimer, 2021; Figgatt, Salazar, Day, Vincent & Dasgupta, 2021; Joseph, Torres-Lockhart, Stein, Mund & Nahvi, 2021; Levander, Pytell, Stoller, Korthuis & Chander, 2021; Meteliuk et al., 2021). Most of the studies thus far have looked at data collected from a single program (Amram et al., 2021) or a group of programs in a single area (Figgatt et al., 2021; Joseph et al., 2021) or a single state (Brothers et al., 2021), or other countries (Meteliuk et al., 2021). Others suffer from substantial biases such as reliance almost entirely on patient self-report (Figgatt et al., 2021) or program self-report with low response rates (Levander et al., 2021).
On November 18, 2021, SAMHSA announced that it would be “extending the take-home flexibilities for one year, effective upon the eventual expiration of the COVID-19 Public Health Emergency” and that “SAMHSA is also considering mechanisms to make this flexibility permanent” (SAMHSA, 2021a, 2021b) further highlighting the need for relevant data to help guide the proposed changes. The objective of the current study is to look at changes in exposures involving methadone reported to poison control centers across the entire U.S. before and after the loosening of regulations.
Methods
We conducted a retrospective observational analysis of the National Poison Data System (NPDS), a large unified, near real-time registry from 55 poison control centers that cover the entire U.S. and its territories (AAPCC, 2021a, 2021b). Specialists in Poison Information (SPI; usually a nurse or pharmacist with extensive additional training in toxicology) respond over the phone, in real time, to calls originating from the public and health care professionals regarding exposure to various substances. Exposures are defined in NPDS as “actual or suspected contact with any substance which has been ingested, inhaled, absorbed, applied to, or injected into the body.” “Poisoning” (International Classification of Diseases-10), “intoxication” (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) and “overdose” are all classified as exposures in NPDS. However, not all exposures in NPDS necessarily meet ICD-10 criteria for poisoning or DSM-5 criteria for intoxication. For example, a clinician may call with a medication overdose in a patient who takes methadone appropriately.
The determination of intentional vs unintentional is made by the SPI making an assessment based on the information provided by the caller or the health care provider. Intentional exposures are defined as exposures resulting from purposeful action. These exposures are further classified as: Abuse- exposure resulting from the intentional improper or incorrect use of a substance where the patient was likely attempting to gain a high, euphoric effect or some other psychotropic effect, including recreational use of a substance for any effect; Misuse- exposure resulting from intentional improper or incorrect use of a substance for reasons other than the pursuit of psychotropic effect; Suspected Suicide or Unknown. Abuse and misuse were grouped together under non-medical use for the purposes of this study.
Medical outcomes are classified as: no effect- no symptoms; minor effect- symptoms that resolved rapidly; moderate effect- symptoms that were more pronounced, more prolonged, or more of a systemic nature; major effect- symptoms which were life threatening or resulted in significant residual disability (e.g. cardiac arrest, respiratory arrest). A unique feature of poison control center exposure management is the use of follow up calls to monitor progress, provide on-going treatment recommendations, and determine final medical outcome for each exposure incident. Data collected by each poison control center are uploaded in near-real time (every 8–15 min) to NPDS.
NPDS was queried for human exposures involving methadone from March 19,2019 to March 15, 2021 (2 years). The query included all ages, all exposure reasons, and all associated medical outcomes. The pre-period was March 19, 2019 to March 16, 2020 (364 days) and the post-period was March 17, 2020 to March 15, 2021 (364 days). Inclusion criteria: intentional exposures. Exclusion criteria: non-intentional exposures; age less than 18 years; indirect reports. For comparison, all intentional exposures to all substances in persons over 18 years of age in NPDS were used. One tail Student t-test was used for normally distributed data and Chi Square was used for categorical data comparison in pre- vs post- groups; p significance was <0.05. The study used existing de-identified data and was exempt from institutional review board review in accordance with the Common Rule.
Results
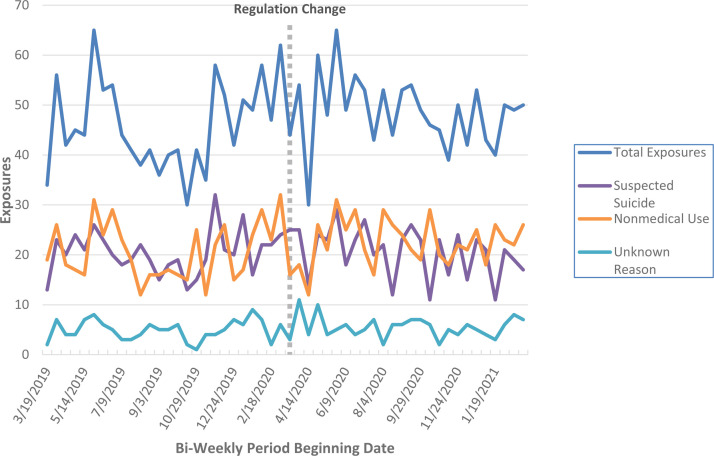
Exposures were reported from all states in the U.S. Fig. 1 illustrates exposures over time. A total of 2461 exposures were included in the study (Fig. 2; Supplementary Material). Counts ranged from 71 to 110 methadone exposures per 4-weeks in the pre- to 84 to 113 in the post-period. The 4-week mean increased from 92.2 (95% CI= 84.6–99.8) exposures involving methadone pre- to 97.1 (95% CI=93–101.2) in the post-period.
Fig. 1.
Intentional exposures involving methadone.
Reported to National Poison Data System (NPDS) per two weeks.
During the study period, total methadone exposures increased by 5.3% from 1199 pre- to 1262 in the post-period. At the same time, total intentional exposures in persons over age 18 years captured in NPDS decreased by 9.17% from total 306,871 pre- to 278,729 post-period. The increase in methadone exposures was statistically significant when compared to total intentional exposures (p<0.05).
There was no statistically significant difference in distribution of age, gender, whether exposures involved methadone-only or methadone plus other substances, or therapies administered in the pre- vs post-period. There was no difference in the proportion of patients admitted to the hospital. A total of 20 and 13 deaths were reported in the pre- vs post-period. There was no statistically significant difference in overall distribution of medical outcomes, including deaths (Table 1 ).
Table 1.
Characteristics of adult exposures involving methadone reported to the National Poison Data System Pre- vs Post-intervention (March 16, 2020).
| Nonmedical useN = 1123 (%) |
P value | Suspected attempted suicideN = 1067 (%) |
P value | Unknown reason N = 271 (%) |
P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre- | Post- | Pre- | Post- | Pre- | Post- | ||||
| N | 539 | 584 | 0.14 | 532 | 535 | 0.02 | 128 | 143 | 0.45 |
| Median age (years) | 39 | 39 | 0.80 | 42 | 41 | 0.65 | 45 | 41 | 0.26 |
| Male | 321(59.6) | 365 (62.5) | 0.31 | 254 (47.7) | 242 (45.2) | 0.41 | 60 (46.9) | 75 (52.4) | 0.36 |
| Methadone-only | 216(40.1) | 237 (40.6) | 0.86 | 121 (22.7) | 139 (26.0) | 0.22 | 54 (42.2) | 61 (42.7) | 0.94 |
| THERAPIES | |||||||||
| Naloxone administered | 295(54.7) | 301 (51.5) | 0.29 | 250 (47.0) | 252 (47.1) | 0.97 | 80 (62.5) | 73 (51) | 0.06 |
| Intubation/ventilator | 36 (6.7) | 34 (5.8) | 0.55 | 82 (15.4) | 69 (12.9) | 0.24 | 28 (21.9) | 24 (16.8) | 0.29 |
| Vasopressors administered | 10 (1.9) | 15 (2.6) | 0.42 | 22 (4.1) | 20 (3.7) | 0.74 | 5 (3.9) | 4 (2.8) | 0.61 |
| DISPOSITIONS | |||||||||
| Treated & released from ED | 154 (28.6) | 165 (28.3) | 102 (19.2) | 88 (16.4) | 15 (11.7) | 24 (16.8) | |||
| Admitted non-critical care | 107 (19.9) | 142 (24.3) | 104 (19.5) | 135 (25.2) | 21 (16.4) | 34 (23.8) | |||
| Admitted critical care | 158 (29.3) | 148 (25.3) | 235 (44.2) | 210 (39.3) | 67 (52.3) | 53 (37.1) | |||
| Admitted psychiatry | 16 (3.0) | 8 (1.4) | 57 (10.7) | 69 (12.9) | 9 (7) | 2 (1.4) | |||
| Lost to follow up | 104 (19.3) | 121 (20.7) | 0.10 | 34 (6.4%) | 33 (6.2%) | 0.10 | 16 (12.5) | 30 (20.1) | <0.05 |
| MEDICAL OUTCOMES | |||||||||
| Not followed/no effect | 48 (8.9) | 55 (9.4) | 45 (8.5) | 52 (9.7) | 10 (7.8) | 10 (7.0) | |||
| Minor effect | 60 (11.1) | 72 (12.3) | 65 (12.2) | 66 (12.3) | 9 (7) | 7 (4.9) | |||
| Moderate effect | 201(37.3) | 218 (37.3) | 238 (44.7) | 228 (42.6) | 58 (45.3) | 58 (40.6) | |||
| Major effect | 162(30.1) | 177 (30.3) | 151 (28.4) | 163 (30.5) | 43 (33.6) | 45 (31.5) | |||
| Death | 9 (1.7) | 7 (1.2) | 11 (2.1) | 3 (0.6) | 0 (0) | 3 (2.1) | |||
| Unknown outcome | 59 (10.9) | 55 (9.4) | 0.92 | 22 (4.1) | 23 (4.3) | 0.22 | 8 (6.3) | 20 (14) | 0.44 |
Nonmedical use: exposure resulting from the intentional improper or incorrect use of a substance where the patient was likely attempting to gain a high, euphoric effect or some other psychotropic effect, including recreational use of a substance for any effect (e.g. using recreational drugs) OR exposure resulting from intentional improper or incorrect use of a substance for reasons other than the pursuit of psychotropic effect (e.g. person deliberately increases the dosage of a medication to enhance therapeutic effect, overuse of a substance to perform better).
Conclusions
The continued increase in drug overdose fatalities in 2021 (with over 100,000 in the 12-month period ending April 2021; greater than 75% involving opioids) (Ahmad, Rossen & Sutton, 2021) has added further urgency to the need to increase access to care for individuals with OUD across the country, especially in more rural areas where distances can be a major impediment to treatment. Support for extension of the loosened methadone take-home regulations (Trujols et al., 2020) and the November 2021 announcement that SAMHSA plans to “make this flexibility permanent” (SAMHSA, 2021a) have highlighted the importance of assessing the potential benefits and harms of changing this decades old, highly restrictive system.
This study found a small but statistically significant increase in intentional exposures involving methadone in the year after methadone regulations were loosened though with no increase in severity of exposures, including fatalities. This result is important to contextualize since it occurred in the setting of various concurrent changes that, collectively, could have increased methadone exposures considerably. First, methadone regulations were loosened allowing for more take-home doses. A late-2020 report from the HHS Office of Inspector General stated that 89% of randomly-selected OTPs surveyed had implemented some level of increased take-home doses (HHS, 2021). The loosening of regulations was “permissive” and not mandatory, resulting in wide variability in how states and individual programs actually implemented the allowed changes. Individual states had to request the exception from SAMHSA (through their State Opioid Treatment Authority) and then grant the exception to individual programs that also had to explicitly request the exception prior to implementing changes. There was little guidance from SAMHSA as to the specifics of how to implement changes or the duration (all states had begun to lift Stay-at-Home orders by May 20, 2020 but the public health emergency was continually renewed through 2021 allowing for the loosened methadone regulations to continue for that period). Insurance payment also likely significantly affected state and individual program implementation given that Medicaid does not reimburse for unsupervised dosing in a number of states, even during the pandemic.
A second factor that may have impacted the amount of methadone being administered and the number of patients in treatment relates to coverage of OTP services by Medicare and Medicaid. On January 1, 2020, Medicare, which had not previously covered treatment through an OTP, expanded coverage for OUD treatment services furnished by OTPs and sent letters urging OTPs to enroll Medicare patients immediately (HHS, 2019, 2020a). Similarly, although Medicaid coverage for methadone has expanded over the past several decades (partially related to expansion related to the Affordable Care Act), only 42 of the 53 states and territories provided coverage for methadone through OTPs as of 2018 (SAMHSA, 2018). In October of 2018, the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT Act) was signed, requiring that all states provide Medicaid coverage for methadone by October 1, 2020 (HHS, 2020b; SUPPORT, 2018). In the absence of a centralized, real-time data-base, it is difficult to get an accurate number of patients receiving methadone through an OTP, but it is likely that this number increased in the past two years given the expansion of coverage by Medicare and Medicaid.
A third factor that likely impacted the number of individuals in treatment for OUD is the increase in the number of OTPs that have opened over the past decade with a total of more than 1830 OTPs (up from 1200 in 2010) in 49 states, the District of Columbia and Puerto Rico. During the study period, 85 OTPs opened in the pre- vs 106 in the post-period (SAMHSA, 2021c). Thus, the increase in allowable take-home doses, the increase in Medicare and Medicaid coverage, and the increase in OTPs, collectively, likely increased the number of individuals receiving treatment for OUD in 2020 and 2021. Related to this, it is also possible that lack of familiarity with methadone resulted in increased reporting from providers in areas where methadone was previously not available.
Fig. 1 illustrates exposures over time. The short-lived decrease in exposures immediately following March 17, 2020, is likely due to state-issued Stay-at-Home orders (which began in California March 19, 2020 and existed in 32 states by March 31, 2020). This paralleled the decrease in emergency department and Emergency Medical System (EMS) encounters observed nationally during this time period (Lerner, Newgard & Mann, 2020).
The severity of exposures is a unique feature of NPDS data. It can be measured in final medical outcome, therapies administered, and disposition (admitted to hospital vs not admitted). Severity of exposures in all three areas was not statistically different (Table 1). This is reassuring given the potential for significant increased severity resulting from higher quantities of methadone potentially available to non-tolerant individuals.
Limitations include selection bias resulting from underreporting of exposures in NPDS, where reporting is voluntary. The degree to which poison control centers are utilized by the general public and healthcare professionals is extremely variable and difficult to measure. Importantly, reporting practices to poison centers did not change over the course of the study making comparisons pre- and post- valid. NPDS data are also subject to disruptions in care and competing priorities experienced by providers. Informational bias could result from missing data due to the retrospective nature of the study. Additionally, the data are limited by the fact that NPDS does not distinguish between methadone prescribed for pain management and methadone dispensed from an OTP for the treatment of OUD. Similarly, NPDS is not always able to determine whether the methadone was originally prescribed/dispensed to the patient or to someone else (diverted).
Restrictions on the provision of methadone to treat OUD must balance concerns regarding patient and public safety with treatment engagement, retention, autonomy, and acceptability. In light of provisional data from the National Vital Statistics System (NVSS) which shows an increase in drug overdose deaths involving methadone starting in March 2020 (up from 2743 in February 2020 to 3780 in March 2021) (Ahmad et al., 2021), it is more important than ever that various sources of data be analyzed to more carefully evaluate the risks and benefits of loosening methadone regulations. This study lends support to the notion that the potential for increased unregulated methadone does not necessarily lead to a substantial increase in the severity of related methadone exposures. Data from other sources will be important to fully evaluate the potential impact of this regulatory change.
Ethics approval
The authors declare that the work reported herein did not require ethics approval because it did not involve animal or human participation.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.drugpo.2022.103591.
Appendix. Supplementary materials
References
- Ahmad F.B., Rossen L.M., Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. 2021 https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm Retrieved from. [Google Scholar]
- American Association of Poison Control Center (AAPCC). (2021b). National Poison Data System (NPDS) data dashboard tracking opioid trends. Retrieved from https://aapcc.org/track/opioids.
- American Association of Poison Control Centers (AAPCC). (2021a). National Poison Data System (NPDS) data dictionary. version 2016.07.11. Retrieved from https://aapcc.org/data-sytem.
- Amiri S., Lutz R., Socías M.E., McDonell M.G., Roll J.M., Amram O. Increased distance was associated with lower daily attendance to an opioid treatment program in Spokane County Washington. Journal of Substance Abuse Treatment. 2018;93:26–30. doi: 10.1016/j.jsat.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Amram O., Amiri S., Thorn E.L., Lutz R., Joudrey P.J. Changes in methadone take home dosing before and after COVID-19. Journal of Substance Abuse Treatment. 2021 doi: 10.1016/j.jsat.2021.108552. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers S., Viera A., Heimer R. Changes in methadone program practices and fatal methadone overdose rates in Connecticut during COVID-19. Journal of Substance Abuse Treatment. 2021;131 doi: 10.1016/j.jsat.2021.108449. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering D., Sheridan J., Sellman J.D., Adamson S.J., Pooley S., Robertson R., et al. Consumer and treatment provider perspectives on reducing barriers to opioid substitution treatment and improving treatment attractiveness. Addictive Behaviors. 2011;36(6):636–642. doi: 10.1016/j.addbeh.2011.01.00410. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services (HHS) Code of federal regulations- title 42: 8- Medication assisted treatment for opioid use disorder. Federal Register. 2001;66(11):4090–4102. January 17, 2001: Retrieved from https://www.govinfo.gov/content/pkg/FR-2001-01-17/pdf/01-723.pdf#page=15. [Google Scholar]
- Department of Health and Human Services (HHS) Guidance to state medicaid agencies on dually eligible beneficiaries receiving medicare opioid treatment services effective January 1, 2020. 2019. Centers for medicare and medicaid services. CMCS informational bulletin.https://www.hhs.gov/guidance/document/guidance-state-medicaid-agencies-dually-eligible-beneficiaries-receiving-medicare-opioid (Dec. 17, 2019), Retrieved from. [Google Scholar]
- Department of Health and Human Services (HHS). (2020a). Centers for medicare and medicaid services. Opioid treatment programs (OTP). Dear opioid treatment program sponsors and state opioid treatment authorities. Retrieved from https://www.cms.gov/files/document/letter-otp-program-sponsors-and-state-opioid-treatment-authorities-sotas-pdf.pdf.
- Department of Health and Human Services (HHS). (2020b). Centers for medicare and medicaid services. Mandatory medicaid state plan coverage of medication assisted treatment. Retrieved from https://www.medicaid.gov/federal-policy-guidance/downloads/sho20005.pdf.
- Department of Health and Human Services- Office of the Inspector General (HSS). (2021). Opioid treatment programs reported challenges encountered during the COVID-19 pandemic and actions taken to address them. A-09-20-01001; November 2020. Retrieved from https://oig.hhs.gov/oas/reports/region9/92001001.pdf.
- Figgatt M.C., Salazar Z., Day E., Vincent L., Dasgupta N. Take-home dosing experiences among persons receiving methadone maintenance treatment during COVID-19. Journal of Substance Abuse Treatment. 2021;123 doi: 10.1016/j.jsat.2021.108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G., Saenz E., Busse A., Maremmani I., Ciccocioppo R., Zaimovic A., et al. Supervised daily consumption, contingent take-home incentive and non-contingent take-home in methadone maintenance. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35(2):483–489. doi: 10.1016/j.pnpbp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Joseph G., Torres-Lockhart K., Stein M.R., Mund P.A., Nahvi S. Reimagining patient-centered care in opioid treatment programs: Lessons from the Bronx during COVID-19. Journal of Substance Abuse Treatment. 2021;122 doi: 10.1016/j.jsat.2020.108219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourounis G., Richards B.D., Kyprianou E., Symeonidou E., Malliori M.M., Samartzis L. Opioid substitution therapy: Lowering the treatment thresholds. Drug and Alcohol Dependence. 2016;161:1–8. doi: 10.1016/j.drugalcdep.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Lerner E.B., Newgard C.D., Mann N.C. Effect of the coronavirus disease 2019 (COVID-19) pandemic on the U.S. emergency medical services system: A preliminary report. Academic Emergency Medicine: Official Journal of the Society for Academic Emergency Medicine. 2020;27(8):693–699. doi: 10.1111/acem.14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levander X.A., Pytell J.D., Stoller K.B., Korthuis P.T., Chander G. COVID-19-related policy changes for methadone take-home dosing: A multistate survey of opioid treatment program leadership. Substance Abuse. 2021:1–7. doi: 10.1080/08897077.2021.1986768. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteliuk A., Galvez de Leon S.J., Madden L.M., Pykalo I., Fomenko T., Filippovych M., et al. Rapid transitional response to the COVID-19 pandemic by opioid agonist treatment programs in Ukraine. Journal of Substance Abuse Treatment. 2021;121 doi: 10.1016/j.jsat.2020.108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani P.P., Pirastu R., Ricci A., Gessa G.L. Prohibition of take-home dosages: Negative consequences on methadone maintenance treatment. Drug and Alcohol Dependence. 1996;41(1):81–84. doi: 10.1016/0376-8716(96)01240-9. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Substance Abuse and Mental Health Services Administration; Rockville, MD: 2018. Medicaid coverage of medication-assisted treatment for alcohol and opioid use disorders and of medication for the reversal of opioid overdose. HHS publication no. SMA-18-5093.https://store.samhsa.gov/sites/default/files/d7/priv/medicaidfinancingmatreport_0.pdf Retrieved from. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2020a). FAQs: provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. Retrieved from https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf.
- Substance Abuse and Mental Health Services Administration (SAMHSA) Substance Abuse and Mental Health Services Administration; Rockville, MD: 2020. National survey of substance abuse treatment services (N-SSATS): 2019. Data on substance abuse treatment facilities; p. 2020. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2021a). SAMHSA extends the methadone take-home flexibility for one year while working toward a permanent solution. Retrieved from https://www.samhsa.gov/newsroom/press-announcements/202111181000.
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2021b). Methadone take-home flexibilities extension guidance. Retrieved from https://www.samhsa.gov/medication-assisted-treatment/statutes-regulations-guidelines/methadone-guidance.
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2021c). Opioid treatment program directory. Retrieved from https://dpt2.samhsa.gov/treatment/directory.aspx.
- SUPPORT for Patients and Communities Act, Pub. L. No. 115–271. (2018). Retrieved from https://www.congress.gov/115/plaws/publ271/PLAW-115publ271.pdf.
- Trujols J., Larrabeiti A., Sànchez O., Madrid M., De Andrés S., Duran-Sindreu S. Increased flexibility in methadone take-home scheduling during the COVID-19 pandemic: Should this practice be incorporated into routine clinical care? Journal of Substance Abuse Treatment. 2020;119 doi: 10.1016/j.jsat.2020.108154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.