Abstract
The emergence of infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter spp. has necessitated the search for alternative parenteral agents such as the polymyxins. The National Committee for Clinical Laboratory Standards (NCCLS) documents do not currently provide interpretative criteria for the testing of the polymyxins, colistin and polymyxin B. Therefore, an evaluation of the antimicrobial activity of colistin and polymyxin B was initiated using 200 bloodstream infection pathogens collected through the SENTRY Antimicrobial Surveillance Program. All susceptibility tests were performed according to the NCCLS recommendations. Polymyxin B and colistin displayed a nearly identical spectrum of activity, exhibiting excellent potency against P. aeruginosa (MIC90, 2 μg/ml) and Acinetobacter sp. (MIC90, 2 μg/ml). In contrast, they showed limited activity against some other nonfermentative bacilli such as Burkholderia cepacia (MIC90, ≥128 μg/ml). Excellent correlation was achieved between broth microdilution and agar dilution tests (r = 0.96 to 0.98); 94.3% of the results were ±1 log2 dilution between the methods used for both compounds. At a resistance breakpoint of ≥4 μg/ml for both agents, unacceptable false-susceptible or very major errors were noted for colistin (5%) and polymyxin B (6%). Modified zone criteria for colistin (≤11 and ≥14 mm) and polymyxin B (≤10 and ≥14 mm) were suggested, but some degree of error persisted (≥3.5%). It is recommended that all susceptible disk diffusion results be confirmed by MIC tests using the preferred reference NCCLS method. The quality control (QC) ranges listed in the product package insert require an adjusted range by approximately 3 mm for both NCCLS gram-negative quality control strains. This evaluation of in vitro susceptibility test methods for the polymyxin class drugs confirmed continued serious testing error with the disk diffusion method, the possible need for breakpoint adjustments, and the recalculation of disk diffusion QC ranges. Clinical laboratories should exclusively use MIC methods to assist the therapeutic application of colistin or polymyxin B until disk diffusion test modifications are sanctioned and published by the NCCLS.
Polymyxins are a group of polycationic peptides naturally synthesized by Bacillus polymyxa, a nonactinomycete bacterium (7). Members of this class (colistin and polymyxin B) act primarily on the gram-negative bacterial cell wall, leading to rapid permeability changes in the cytoplasmic membrane and ultimately to cell death. These drugs cross the bacterial outer membrane (self-promoted pathway) by competitive divalent cation displacement by the bulky polycations, which noncovalently cross the bridge adjacent to the polysaccharide component. Consequently, the bacterial outer membrane becomes distorted and more permeable, permitting increased uptake of the permeabilizing compounds (10, 19). Of the five recognized polymyxins (A to E), only polymyxins B and E (colistin) have advanced to therapeutic use.
Polymyxin B and colistin have been demonstrated to be active against Pseudomonas aeruginosa. Four decades ago, polymyxins were among the few treatments available for serious P. aeruginosa infections. Since 1980 other, less-toxic antimicrobial agents have become available, and the clinical use of polymyxins has been limited to topical formulations for the treatment of skin, ear, and ocular diseases. Polymyxin B topical formulations also have been used prophylatically for the prevention of infection in neutropenic or cystic fibrosis patients (6, 11). Recently, the emergence of multidrug-resistant P. aeruginosa and Acinetobacter spp. isolates causing life-threatening infections has restored the potential therapeutic indication for the parenteral use of polymyxins (1, 12, 20), usually colistin in North American medical centers. Consequently, clinical microbiology laboratories should be able to perform reliable susceptibility testing for drugs in this class.
The National Committee on Clinical Laboratory Standards (NCCLS) does not provide guidance for the testing of polymyxins. In fact, the resistance breakpoint criteria for polymyxins were last available in the 1981 NCCLS Approved Standard M2-A2 S2 (15); however, with the very restricted use of polymyxins, the published information was later withdrawn.
In this study, the antimicrobial activities of the polymyxins were evaluated against selected contemporary bacterial pathogens. The correlation of the broth microdilution, disk diffusion, and agar dilution methods was performed to determine the susceptibility criteria for polymyxins. Disk diffusion quality controls for Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were also carried out to validate method control and the interpretation criteria only found in the disk product package insert.
MATERIALS AND METHODS
Bacterial strains.
Bloodstream isolates collected from distinct geographic areas during 1998 through the SENTRY Antimicrobial Surveillance Program were evaluated in this study. Only one isolate per patient was included. The distribution of species was as follows: Acinetobacter spp. (60), Burkholderia cepacia (12), Enterobacter spp. (5), Klebsiella pneumoniae (9), Morganella morganii (2), Proteus mirabilis (2), Providencia rettgeri (2), Pseudomonas aeruginosa (80), Serratia marcescens (5), and Stenotrophomonas maltophilia (23). The bacterial identifications were confirmed by routine laboratory methods in laboratories at the University of Iowa College of Medicine (Iowa City, Iowa). The isolates were selected at random, but nearly 20% of the P. aeruginosa and Acinetobacter spp. isolates were chosen to be resistant to carbapenems (imipenem, meropenem), and all K. pneumoniae were extended-spectrum β-lactamases (ESBL)-producing strains.
Antimicrobial agents.
Polymyxin B and colistin sulfate powders were obtained from Sigma Chemical (St. Louis, Mo.). Other tested drugs were obtained commercially or provided by their respective manufacturers.
Susceptibility testing.
Antimicrobial susceptibility testing was performed as recommended by the NCCLS (16, 17). All bacterial isolates were tested by broth microdilution and disk diffusion tests, while only 35 isolates representing all the species were tested by agar dilution to provide a comparison to its broth microdilution test results. These organisms were selected to provide a wide range of polymyxin MICs (Fig. 1). The susceptibility test medium was Mueller-Hinton agar or broth according to the test performed. Microdilution broth trays were prepared by PML Microbiologics (Wilsonville, Oreg.) and stored at −70°C until used. E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control (QC) strains.
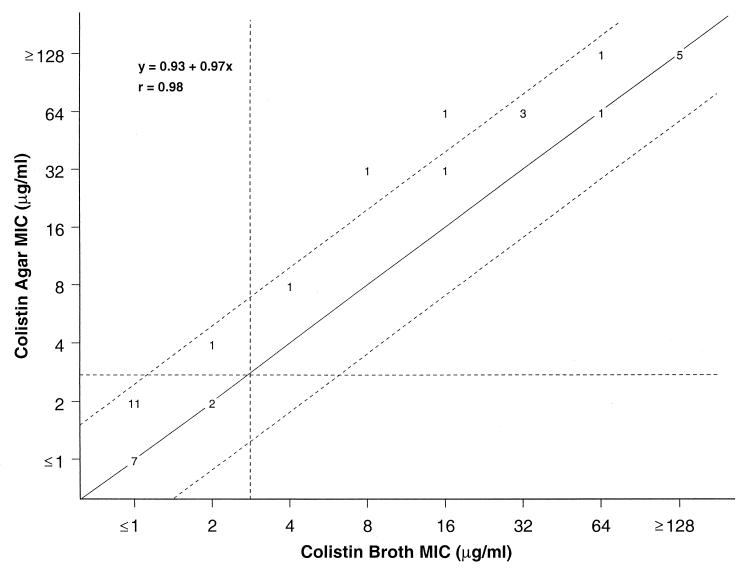
FIG. 1.
Scattergram results for colistin comparing broth microdilution MICs with the reference agar dilution method against 35 recent clinical isolates selected to possess a wide range of MIC values. The diagonal line represents complete agreement, and the numbers represent the occurrences observed at each point. The broken lines represent ±1-log2 MIC agreement limits between test results. Horizontal and vertical broken lines indicate the resistant MIC breakpoints (≥4 μg/ml).
QC study.
Determination of the disk diffusion QC results for E. coli ATCC 25922 and P. aeruginosa ATCC 27853 was done by testing two or three 300-U polymyxin B disks from two different lots (BD Microbiology Systems, Cockeysville, Md.; Difco Laboratories, Detroit, Mich.). Three 10-μg colistin disks were tested from a single lot (BD Microbiology Systems). The tests were replicated 10 times and read by four different observers. This study produced at least 20 replicate zone diameters for each disk and 200 and 120 zones overall for polymyxin B and colistin, respectively.
Statistical methods.
The MIC results of the broth microdilution and agar dilution methods were compared by regression analysis. Essential agreement was defined to be when the broth microdilution results agreed within ±1-log2 dilution of the reference agar dilution test. A result was determined to be discrepant if there was a ≥2-log2 dilution difference between test results. Broth microdilution results were also compared to the zones of inhibition produced by the polymyxin disk diffusion tests also using the method of least squares as applied to computers.
Categorical agreement was defined if the test results were within the same susceptibility category, and errors were determined by methods published in NCCLS M23-A2 (18) and ranked as follows: very major error, false-susceptible result by the disk diffusion test; major error, false-resistant result produced by the disk diffusion test; and minor error, intermediate result by the disk diffusion method and a resistant or susceptible category for the dilution test (only two categories have been suggested).
RESULTS
Polymyxin antimicrobial activity.
Tables 1 and 2 (colistin only) summarize the activities of the polymyxins against 200 contemporary gram-negative clinical isolates. Polymyxin B and colistin displayed nearly identical spectrums of activity, exhibiting usable potency against P. aeruginosa (MIC90, 2 μg/ml) and Acinetobacter sp. (MIC90, 2 μg/ml). In contrast, they showed no activity against some other nonfermentative bacilli such as B. cepacia (MIC90, ≥128 μg/ml). Nearly three-quarters of the S. maltophilia isolates were inhibited at 4 μg/ml of both compounds. The polymyxins (MIC50, ≤1 μg/ml) were eightfold more potent than ceftazidime (MIC50, 8 μg/ml) and cefepime (MIC50, 8 μg/ml) and showed similar activity to that displayed by the carbapenems against Acinetobacter sp. strains. Only three carbapenem-resistant Acinetobacter sp. isolates were not inhibited at polymyxin MICs of ≤2 μg/ml. Although levofloxacin and doxycycline exhibited similar potency against Acinetobacter sp., only doxycycline showed a susceptibility rate greater than 90.0%.
TABLE 1.
Antimicrobial activity of colistin and polymyxin B compared to other antimicrobial agents against 200 contemporary bacterial isolates
| Organism (no. of isolates tested) | Antimicrobial agentb | MIC (μg/ml)
|
% Susceptible | ||
|---|---|---|---|---|---|
| 50% | 90% | Range | |||
| Acinetobacter spp. (60) | Polymyxin B | ≤1 | 2 | ≤1–8 | 95.0a |
| Colistin | ≤1 | 2 | ≤1–32 | 96.7a | |
| Ceftazidime | 8 | >32 | ≤0.25–>32 | 55.0 | |
| Cefepime | 8 | >32 | ≤0.25–>32 | 55.0 | |
| Imipenem | 0.5 | >16 | ≤0.12–>16 | 80.0 | |
| Meropenem | 1 | >16 | ≤0.12–>16 | 80.0 | |
| Ciprofloxacin | 0.5 | >4 | ≤0.03–>4 | 58.3 | |
| Levofloxacin | 0.25 | >8 | ≤0.06–>8 | 60.0 | |
| Amikacin | 16 | >64 | ≤1–>64 | 51.7 | |
| Tobramycin | 8 | >16 | 0.5–>16 | 48.3 | |
| Doxycycline | 0.25 | 4 | ≤0.12–>16 | 91.7 | |
| TMP-SMX | 2 | >4 | ≤0.06–>4 | 50.0 | |
| B. cepacia (12) | Polymyxin B | >128 | >128 | >128 | 0.0a |
| Colistin | >128 | >128 | >128 | 0.0a | |
| Ceftazidime | 4 | 32 | 2–>32 | 66.7 | |
| Cefepime | 32 | >32 | 4–>32 | 8.3 | |
| Imipenem | 8 | >16 | 0.25–>16 | 16.7 | |
| Meropenem | 2 | 16 | 0.25–16 | 75.0 | |
| Ciprofloxacin | 1 | 2 | 0.5–4 | 58.3 | |
| Levofloxacin | 2 | 8 | 1–8 | 75.0 | |
| Amikacin | >64 | >64 | 32–>64 | 0.0 | |
| Tobramycin | >16 | >16 | 2–>16 | 8.3 | |
| Doxycycline | 4 | >16 | 1–>16 | 50.0 | |
| TMP-SMX | 1 | 2 | 0.5–2 | 100.0 | |
| K. pneumoniae (9) | Polymyxin B | ≤1 | ≤1–2 | 100.0a | |
| Colistin | ≤1 | ≤1–2 | 100.0a | ||
| Ceftazidime | 4 | 1–16 | 77.8 | ||
| Cefepime | 2 | ≤0.25–>32 | 66.7 | ||
| Imipenem | 0.25 | ≤0.12–0.5 | 100.0 | ||
| Meropenem | ≤0.12 | ≤0.12–0.25 | 100.0 | ||
| Ciprofloxacin | ≤0.03 | ≤0.03–0.25 | 100.0 | ||
| Levofloxacin | ≤0.06 | ≤0.06–0.25 | 100.0 | ||
| Amikacin | 8 | ≤1–64 | 88.9 | ||
| Tobramycin | >16 | ≤0.25–>16 | 44.4 | ||
| Doxycycline | 1 | 0.5–>16 | 88.9 | ||
| TMP-SMX | 1 | 0.25–>4 | 77.8 | ||
| P. aeruginosa (80) | Polymyxin B | ≤1 | 2 | ≤1–2 | 100.0a |
| Colistin | ≤1 | ≤1 | ≤1–2 | 100.0a | |
| Ceftazidime | 2 | >32 | ≤0.25–>32 | 68.8 | |
| Cefepime | 2 | 16 | ≤0.25–>32 | 80.0 | |
| Imipenem | 2 | >16 | ≤0.12–>16 | 78.8 | |
| Meropenem | 0.5 | 8 | ≤0.12–>16 | 85.0 | |
| Ciprofloxacin | 0.12 | 4 | ≤0.03–>4 | 81.3 | |
| Levofloxacin | 0.5 | >8 | ≤0.06–>8 | 78.8 | |
| Amikacin | 2 | 8 | ≤1–>64 | 93.8 | |
| Tobramycin | 0.5 | 2 | ≤0.25–>16 | 91.3 | |
| Doxycycline | 16 | >16 | 0.5–>16 | 7.5 | |
| TMP-SMX | >4 | >4 | 0.12–>4 | 5.0 | |
| S. maltophilia (23) | Polymyxin B | 2 | 8 | ≤1–64 | 73.9a |
| Colistin | ≤1 | 32 | ≤1–64 | 73.9a | |
| Ceftazidime | 8 | 32 | 1–>32 | 65.2 | |
| Cefepime | 16 | 32 | 1–>32 | 26.1 | |
| Imipenem | >16 | >16 | >16 | 0.0 | |
| Meropenem | >16 | >16 | 8–>16 | 0.0 | |
| Ciprofloxacin | 1 | >4 | 0.25–>4 | 52.2 | |
| Levofloxacin | 0.5 | 4 | 0.12–>8 | 87.0 | |
| Amikacin | >64 | >64 | 8–>64 | 4.3 | |
| Tobramycin | >16 | >16 | 2–.16 | 8.7 | |
| Doxycycline | 1 | 2 | ≤0.12–8 | 95.7 | |
| TMP-SMX | 0.5 | >4 | 0.12–>4 | 73.9 | |
| Othersc (16) | Polymyxin B | 64 | >128 | ≤1–>128 | 37.5a |
| Colistin | 64 | >128 | ≤1–>128 | 37.5a | |
| Ceftazidime | ≤0.25 | 32 | ≤0.25–>32 | 62.5 | |
| Cefepime | ≤0.25 | 2 | ≤0.25–>32 | 93.8 | |
| Imipenem | 0.5 | 4 | 0.25–4 | 100.0 | |
| Meropenem | ≤0.12 | ≤0.12 | ≤0.12 | 100.0 | |
| Ciprofloxacin | ≤0.03 | 4 | ≤0.03–>4 | 81.3 | |
| Levofloxacin | ≤0.06 | 4 | ≤0.06–>8 | 81.3 | |
| Amikacin | 4 | 8 | ≤1–8 | 100.0 | |
| Tobramycin | 1 | 8 | 0.5–>16 | 87.5 | |
| Doxycycline | 2 | >16 | 0.25–>16 | 62.5 | |
| TMP-SMX | 0.5 | >4 | 0.12–>4 | 68.8 | |
Resistant strains with colistin and polymyxin B MICs of ≥4 μg/ml.
TMP-SMX, trimethoprim-sulfamethoxazole.
E. aerogenes (four strains), E. cloacae (one strain), M. morganii (two strains), P. mirabilis (two strains), P. rettgeri (two strains), and S. marcescens (five strains).
TABLE 2.
Colistin MIC frequency distribution tested against a diverse sample of bacterial species
| Organism (no. of isolates tested) | No. of isolates (%) inhibited at MIC (μg/ml) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ≤1 | 2 | 4 | 8 | 16 | 32 | 64 | ≥128 | |
| Acinetobacter spp. (60) | 54 (90.0) | 3 (95.0) | 1 (96.7) | 1 (98.3) | 1 (100.0) | |||
| B. cepacia (12) | 12 (100.0) | |||||||
| Enterobacter spp. (5) | 4 (80.0)a | 1 (100.0)b | ||||||
| Indole-positive group (4) | 2 (50.0)c | 2 (100.0)d | ||||||
| K. pneumoniae (9) | 8 (88.9) | 1 (100.0) | ||||||
| Proteus mirabilis (2) | 1 (50.0) | 1 (100.0) | ||||||
| P. aeruginosa (80) | 79 (98.8) | 1 (100.0) | ||||||
| S. marcescens (5) | 5 (100.0) | |||||||
| S. maltophilia (23) | 13 (56.5) | 3 (69.6) | 1 (73.9) | 1 (78.3) | 2 (87.0) | 2 (95.7) | 1 (100.0) | |
| All strains (200) | 160 (80.0) | 8 (84.0) | 2 (85.0) | 2 (86.0) | 2 (87.0) | 4 (89.0) | 2 (90.0) | 20 (100.0) |
E. aerogenes (four strains)
E. cloacae (one strain).
M. morganii (two strains)
P. rettgeri (two strains).
Against P. aeruginosa, colistin and polymyxin B were at least twofold more potent than ceftazidime, cefepime, and amikacin (MICs, 2 μg/ml). In contrast, polymyxin B and colistin were not active against B. cepacia (MICs, ≥128 μg/ml). Against this pathogen, the most active compounds based on potency and susceptibility rate were: trimethoprim-sulfamethoxazole (MIC50, 1 μg/ml), 100.0% susceptible) > meropenem = levofloxacin (MIC50, 1 μg/ml, 75.0%). Ceftazidime and meropenem were fourfold more potent than comparative drugs in their classes, cefepime and imipenem, respectively. The aminoglycosides showed very poor activity against these isolates.
Among the selected S. maltophilia isolates, 26.2% isolates were resistant to polymyxins. Doxycycline showed the highest susceptibility rate (95.7%) for S. maltophilia, followed by levofloxacin (87.0%), and the usual “drug of choice” trimethoprim-sulfamethoxazole (73.9%). Carbapenems and aminoglycosides were not active against this pathogen.
Polymyxin B and colistin also demonstrated excellent activity against the ESBL-producing K. pneumoniae isolates (MIC50, ≤1 μg/ml). Although many ESBL-producing isolates show resistance to fluoroquinolones, the isolates tested in this study were very susceptible to this class of agents. Carbapenems were also potent, exhibiting 100.0% susceptibility rates.
Against AmpC-cephalosporinase producers, the activities of polymyxin B and colistin varied from excellent (Enterobacter aerogenes and M. morganii MICs, ≤1 μg/ml) to poor (S. marcescens and P. rettgeri MICs, ≥128 μg/ml). Carbapenems and cefepime were the only agents with complete activity and spectrum versus these Enterobacteriaceae (except one S. marcescens isolate with a cefepime MIC of ≥32 μg/ml).
Comparisons of broth microdilution and agar dilution methods.
Figure 1 shows the comparative analysis between the reference dilution tests for colistin using a subset of 35 strains having a wide range of polymyxins MICs. Polymyxin B (data not shown) and colistin broth microdilution MICs showed excellent correlation with agar dilution test results, exhibiting an essential agreement of 94.3% (±1-log2 dilution) for both compounds (r = 0.96 to 0.98). Only two organisms showed agar dilution MIC values at fourfold higher than broth microdilution MICs for each compound. A trend toward higher MIC results with the agar method was observed. The discrepant results between the reference MIC tests would result in a single change in the susceptibility category (one dilution variation; Fig. 1), if the resistance breakpoint of ≥4 μg/ml was applied.
Comparison of disk diffusion zones to reference broth microdilution MICs.
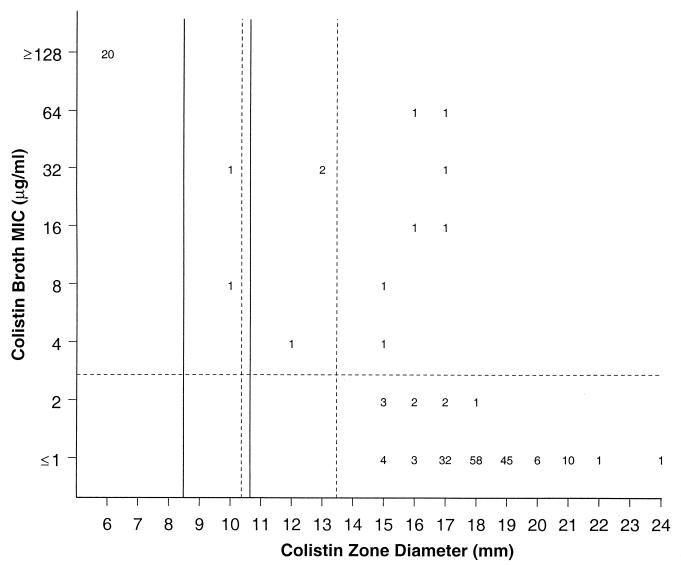
Figures 2 and 3 present the scattergram for 200 bacterial strains tested by broth microdilution and standardized disk diffusion methods against colistin and polymyxin B, respectively. For both polymyxins, two distinct large bacterial populations exhibiting MICs of ≤2 and ≥128 μg/ml were detected. When the previously suggested interpretative resistance criteria for colistin (MICs, ≥4 μg/ml; zone, ≤8 mm) were applied to these data, 1 and 5% of minor and very major errors (false susceptible) were detected. Modifying the colistin disk diffusion resistant and susceptible zone diameters to ≤10 and ≥14 mm slightly improved error rates by decreasing very major errors to 3.5% but increased the minor error rate to 1.5%. All but one error observed occurred among Acinetobacter spp. (two) and S. maltophilia isolates (seven).
FIG. 2.
Scattergram comparing broth microdilution MICs and 10-μg disk zone diameters for colistin tested against 200 contemporary clinical isolates. The solid lines represent the breakpoint values contained in reference 15, while the broken lines show the proposed new breakpoint values for colistin.
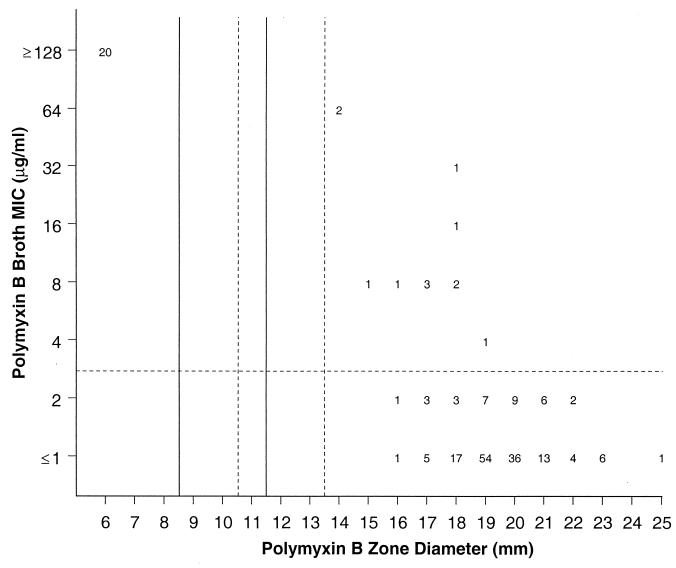
FIG. 3.
Scattergram comparing broth microdilution MICs and 300-U disk zone diameters for polymyxin B tested against 200 contemporary clinical isolates. The solid lines represent the breakpoint values contained in reference 15, while the broken lines show the proposed new breakpoint values for colistin (see Fig. 2).
Based on the long-established polymyxin B susceptibility interpretative criteria (MICs, ≥4 μg/ml; zones, ≥8 mm), 12 strains would present discrepant results between test methods (Fig. 3). All errors represented false-susceptible results (6.0%; very major errors). Most errors occurred with S. maltophilia (seven) and Acinetobacter spp. (three). Even if the zone diameters were modified as suggested for colistin, the percentage of false-susceptible results would not be diminished.
Determination of polymyxin QC.
The disk diffusion QC results for the colistin and polymyxin B disks tested against E. coli ATCC 25922 and P. aeruginosa ATCC 27853 are shown in Table 3. The number of occurrences at each zone diameter determined by various readers were compared and demonstrated minimal variation between technologists. For the 120 colistin zone diameters produced (three disks, four observers, and 10 replicates), the modal zone diameters were 18 and 17 mm for E. coli ATCC 25922 and P. aeruginosa ATCC 27853, respectively. The interval derived from calculating the two standard deviations above and below the mean (17.8 mm) contains 95% of the results, and the modified QC ranges of zones suggested would be 16 to 20 mm and 15 to 19 mm for the E. coli ATCC 25922 and P. aeruginosa ATCC 27853 strains, respectively.
TABLE 3.
Colistin and polymyxin disk diffusion QC results for E. coli ATCC 25922 and P. aeruginosa ATCC 27853 (120 to 200 observations)
| Antimicrobial disk and QC strain | No. of positive isolates at zone diam (mm) of:
|
% isolates in range
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | PIa | Proposedb | |
| Colistin | ||||||||||
| E. coli ATCC 25922 | 0 | 4a | 33b | 70b | 12b | 1b | 0 | 0 | 0.0 | 100.0 |
| P. aeruginosa ATCC 27853 | 8b | 36b | 70b | 6b | 0b | 0 | 0 | 0 | NDc | 100.0 |
| Polymyxin B | ||||||||||
| E. coli ATCC 25922 | 0 | 1a | 9b | 79b | 89b | 22b | 0 | 0 | 0.5 | 99.5 |
| P. aeruginosa ATCC 27853 | 0 | 0 | 3b | 35b | 90b | 64b | 7b | 1 | ND | 99.5 |
PI, package insert range. Current PI ranges were: for colistin, E. coli ATCC 25922 at 11 to 15 mm, and for polymyxin B, E. coli ATCC 25922 at 12 to 16 mm.
The proposed QC ranges from cited experiments were: for colistin, E. coli ATCC 25922 at 16 to 20 mm, and P. aeruginosa ATCC 27853 at 15 to 19 mm; for polymyxin B, E. coli ATCC 25922 at 17 to 20 mm, and for P. aeruginosa ATCC 27853 at 17 to 21 mm.
PI zone diameter ranges were not defined (ND) for P. aeruginosa ATCC 27853.
The 300-U polymyxin B disk zone diameters ranged from 17 to 20 mm for the E. coli ATCC 25922, with a mean and mode at 19 mm. Although the polymyxin B zones varied from 17 to 22 mm for P. aeruginosa ATCC 27853, the mean and mode was the same as that observed for the E. coli strain (19 mm). Using the same statistical method applied to the colistin disk tests, the modified QC ranges suggested would be 17 to 20 mm (4 mm) for E. coli ATCC 25922 and 17 to 21 mm (5 mm) for P. aeruginosa ATCC 27853. The proposed ranges would include nearly all (≥99.5%) values obtained within the limits. These ranges were significantly larger than those listed in the disk product package insert.
DISCUSSION
Emerging resistance in gram-negative bacilli has produced the necessity for the parenteral use of polymyxins associated with the need for reliable susceptibility methods to predict the clinical response. In the 1970s, the NCCLS published the breakpoints of susceptibility for colistin and polymyxin B (15). However, at that time the procedures for standardization of susceptibility testing, the establishment of interpretative breakpoints, and the definition of QC strains guidelines were less rigorous (18). With the introduction of aminoglycosides and broad-spectrum β-lactams into clinical practice for the treatment of gram-negative infections, the use of polymyxins became very uncommon and was generally restricted to topical indications. This led to the NCCLS removal of these agents from the list of drugs suggested for testing and reporting (16).
The activity of polymyxins against P. aeruginosa isolates has been widely known. One of the objectives of this study; however, was to observe if the polymyxins remain effective against routine contemporary clinical isolates of P. aeruginosa and also against multiresistant nonfermentative isolates such as Acinetobacter spp. and S. maltophilia. Results proved that both polymyxin B and colistin were very active against recent P. aeruginosa and Acinetobacter spp. isolates, and our observations agreed with other published reports (1, 4, 13). All P. aeruginosa isolates, including the carbapenem nonsusceptible isolates, were inhibited at ≤2 μg/ml concentrations of colistin and polymyxin B. Although the polymyxins had also been very active against Acinetobacter spp., with nearly 95.0% of isolates being inhibited by MICs of ≤2 μg/ml, three carbapenem-resistant Acinetobacter isolates also showed reduced susceptibility to colistin and polymyxin B (MICs of ≥4 for colistin and polymyxin B). These isolates were obtained from infected patients in the United States and Brazil. The clinical significance of the reduced susceptibility to polymyxins observed among these isolates is currently under investigation. If the reduced susceptibility to polymyxins correlates with a poor clinical response, the situation will be disastrous, leaving no efficacious drug for the treatment of serious infections caused by multidrug-resistant Acinetobacter spp. strains.
Although P. aeruginosa strains resistant to polymyxins were not detected in this study, polymyxin-resistant isolates have been described (9, 14). Polymyxin B and colistin show near-complete cross-resistance (6, 7), and two types of resistance to polymyxin B have been observed in P. aeruginosa: low-level, transmissible mutations and high-level stepwise resistance, that is unstable without the presence of polymyxin (9, 14). Nikas and Hancock proposed that the P. aeruginosa outer membrane protein OprH blocks the self-promoted uptake pathway. Thus, the overexpression of OprH caused by mutation or as a result of adaptation to an Mg2+-deficient medium can be associated with resistance to polymyxin B, aminoglycosides, and EDTA (19, 20).
When the polymyxins were tested by dilution methods, two distinct bacterial populations (very susceptible and resistant) could be easily distinguished. Based on the bacterial population distribution, we suggest that the long-used resistance breakpoint applied to colistin and polymyxin B remain at ≥4 μg/ml, a level easily achievable in serum following intravenous administration (6, 7, 11). The agar dilution and broth microdilution methods showed excellent agreement for testing colistin and polymyxin B. Only three bacterial isolates showed discords (≥2-log2 dilutions) between the dilution methods, but these differences resulted in very limited one-dilution error.
The correlation between the MICs and disk diffusion zone diameters can be expressed mathematically by a regression line analysis or compared by error rate bounding. The regression line and acceptable correlation coefficients can be misleading if applied from data that fails to disperse evenly among the entire range of MIC values observed for the colistin and polymyxin B data (2). Using previously published (15) or the modified (≤10- and ≥14-mm) disk diffusion zones proposed here for the polymyxins, the percentages of false-susceptible errors for colistin (5.0 or 3.5%) and polymyxin B (6.0%) were clearly and reproducibly unacceptable. The majority of false-susceptible errors occurred among S. maltophilia isolates. These results were influenced in part by the fact that the disk diffusion test has been determined to be an unreliable method for S. maltophilia testing. Furthermore, false-susceptible errors were also detected among other species such as Acinetobacter spp. and, rarely, Enterobacter cloacae. Therefore, we suggest that results of the disk diffusion test should be confirmed with a dilution method, especially when polymyxin use is required for the treatment of serious systemic infections caused by species other than P. aeruginosa. The disk testing problem was further complicated by erroneous QC zone diameter ranges found in product package inserts for gram-negative strains suggested by the NCCLS (16–18).
Due to its ability to bind strongly to the lipopolysaccharides of gram-negative bacteria and membranes, formulations such as polymyxin B-dextran 70 and covalent polymyxin B conjugated with human immunoglobulin G have been used as an adjuvant therapy of septic shock (3, 5, 8). These combination agents reduce the polymyxin toxicity profiles. However, these “sepsis treatment” agents have negligible antimicrobial activity, even against highly susceptible gram-negative species such as P. aeruginosa.
Further studies evaluating contemporary pharmacokinetics and pharmacodynamics, toxicity, and the clinical response of parenteral polymyxin regimens appear warranted. To perform such studies, the restandardization of antimicrobial susceptibility methods for polymyxins becomes essential (18). Meanwhile, the parenteral use of polymyxins should be restricted to the treatment of infections caused by P. aeruginosa or possibly Acinetobacter spp. strains resistant to less-toxic antimicrobial agents. It appears that the footnote warning found in reference 15 still applies: “colistin and polymyxin B diffuse poorly in agar, and the diffusion method is thus less accurate. Resistance is always significant, but when treatment of infections caused by a susceptible strain is being considered, results of a diffusion test should be confirmed with a dilution method. MIC correlates cannot be calculated reliably from regression analysis.”
ACKNOWLEDGMENTS
We thank the following persons for their significant contributions to the manuscript: K. Meyer, M. Stiwell, D. M. Johnson, and M. Barrett.
REFERENCES
- 1.Appleman M D, Belzberg H, Citron D M, Heseltine P N, Yellin A E, Murray J, Berne T V. In vitro activities of nontraditional antimicrobials against multiresistant Acinetobacter baumannii strains isolated in an intensive care unit outbreak. Antimicrob Agents Chemother. 2000;44:1035–1040. doi: 10.1128/aac.44.4.1035-1040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry A L. The antimicrobic susceptibility test: principle and practices. New York, N.Y: Lea & Febiger; 1976. Establishment of zone-size interpretative criteria; p. 196. [Google Scholar]
- 3.Bucklin S E, Lake P, Logdberg L, Morrison D C. Therapeutic efficacy of a polymyxin B-dextran 70 conjugate in experimental model of endotoxemia. Antimicrob Agents Chemother. 1995;39:1462–1466. doi: 10.1128/aac.39.7.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catchpole C R, Andrews J M, Brenwald N, Wise R. A reassessment of the in vitro activity of colistin sulphomethate. J Antimicrob Chemother. 1997;39:255–260. doi: 10.1093/jac/39.2.255. [DOI] [PubMed] [Google Scholar]
- 5.Evans M E, Feola D J, Rapp R P. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960–967. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- 6.Drabick J J, Bhattacharjee A K, Hoover D L, Siber G E, Morales V E, Young L D, Brown S L, Cross A S. Covalent polymyxin B conjugate with human immunoglobulin G as an antiendotoxin reagent. Antimicrob Agents Chemother. 1998;42:583–588. doi: 10.1128/aac.42.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fekety R. Polymyxins. In: Mandell G L, Douglast R G Jr, Bennett J E, editors. Principles and practice of infectious diseases. 3rd ed. New York, N.Y: Churchill Livingstone, Inc.; 1990. pp. 323–325. [Google Scholar]
- 8.Fuchs P C, Barry A L, Brown S D. PMX-622 (polymyxin B-dextran 70) does not alter in vitro activities of 11 antimicrobial agents. Antimicrob Agents Chemother. 1998;42:2765–2767. doi: 10.1128/aac.42.10.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groisman E A, Kayser J, Soncini F C. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol. 1997;179:7040–7045. doi: 10.1128/jb.179.22.7040-7045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock R E W, Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1988;7:713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- 11.Horton J, Pankey G A. Polymyxin B, colistin, and sodium colistimethate. Med Clin Am. 1982;66:135–142. doi: 10.1016/s0025-7125(16)31447-x. [DOI] [PubMed] [Google Scholar]
- 12.Levin A S, Barone A A, Penco J, Santos M V, Marinho I S, Arruda E A, Manrique E I, Costa S F. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–1011. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- 13.MacGowan A P, Rynn C, Wotton M, Bowker K E, Holt H A, Reeves D S. In vitro assessment of colistin's antipseudomonal antimicrobial interactions with other antibiotics. Clin Microbiol Infect. 1999;5:32–36. doi: 10.1111/j.1469-0691.1999.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 14.Moore R A, Woodruff W A, Hancock R E W. Evidence for two distinct mechanisms of resistance to polymyxin B in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;26:536–545. doi: 10.1128/aac.26.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobic disc susceptibility tests. Approved standard M2–A2 S2. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1981. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 5th ed. Approved standard M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Performance standard for antimicrobial susceptibility testing. Document M100–S10. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Development of in vitro susceptibility testing criteria and quality control parameters. Document M23–A2. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 19.Nicas T I, Hancock R E W. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980;143:872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young M L, Bains M, Bell A, Hancock R W. Role of Pseudomonas aeruginosa outer membrane protein OprH in polymyxin and gentamicin resistance: isolation of an OprH-deficient mutant by gene replacement techniques. Antimicrob Agents Chemother. 1992;36:2566–2568. doi: 10.1128/aac.36.11.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]