Abstract
To date the coronavirus family is composed of seven different viruses which were commonly known as cold viruses until the appearance of the severe acute respiratory coronavirus (SARS-CoV) in 2002, the middle east respiratory syndrome coronavirus (MERS) in 2012 and the severe acute respiratory coronavirus 2 (SARS-CoV-2) which caused the COVID-19 global pandemic in 2019. Using bioinformatic approaches we tested the potential interactions of human miRNAs, expressed in pulmonary epithelial cells, with the available coronavirus genomes. Putative miRNA binding sites were then compared between pathogenic and non pathogenic virus groups. The pathogenic group shares 6 miRNA binding sites that can be potentially involved in the sequestration of miRNAs already known to be associated with deep vein thrombosis. We then analysed ∼100k SARS-CoV-2 variant genomes for their potential interaction with human miRNAs and this study highlighted a group of 97 miRNA binding sites which is present in all the analysed genomes. Among these, we identified 6 miRNA binding sites specific for SARS-CoV-2 and the other two pathogenic viruses whose down-regulation has been seen associated with deep vein thrombosis and cardiovascular diseases. Interestingly, one of these miRNAs, namely miR-20a-5p, whose expression decreases with advancing age, is involved in cytokine signaling, cell differentiation and/or proliferation. We hypothesize that depletion of poorly expressed miRNA could be related with disease severity.
Keywords: SARS-CoV-2, miRNA sponge, Non-coding RNA, COVID-19 genome variant, Virus
1. Introduction
In December 2019 a new severe respiratory disease was reported in Wuhan, capital of the Hubei province of China. It was soon discovered that this atypical respiratory infection is caused by a novel coronavirus [1,2]. This new virus was named Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) because of its homology with the SARS coronavirus (SARS-CoV) [3], which was responsible for acute respiratory disease and high mortality during 2002–2003 [4]. To date, SARS-CoV-2 is the seventh known coronavirus that infects humans [5]. It is known that the virus is transmitted via respiratory droplets and aerosols from person to person and, once inside the body, it can bind specific host receptors and enters host cells through endocytosis or membrane fusion [6,7]. A better understanding of how the virus interacts with the cellular machinery could spur the development of more effective therapies.
Recent studies explored repositioning of FDA-approved drugs in order to identify treatments against SARS-CoV-2 [8,9]. In another study the authors demonstrated the existence of target motifs in the SARS-CoV-2 genome suitable for specific binding with endogenous human micro and long non-coding RNAs (miRNAs and lncRNAs, respectively), which hints at the potential for miRNA-based drugs against the virus [10].
Multiple viruses such as influenza, HIV-1 and dengue are known to produce their own miRNAs. However the role of miRNAs in the biology of single stranded RNA viruses (e.g. Coronaviridae family) is controversial, as they replicate in the cytoplasm and do not access the nuclear miRNAs' apparatus. Some viruses may benefit from using their own or the host's miRNAs to inhibit antiviral responses or to facilitate viral replication [11]. In particular, it was described how the hepatitis C virus is able to de-repress host miR-122 targets by sealing the endogenous miRNA, promoting the virus infection [12]. Moreover, cellular stress responses, cell-type and age can affect host miRNA expression profiles [[13], [14], [15]]. Accumulating evidence suggests that there is a global reduction of miRNA expression following endoplasmic reticulum stress (ER stress) [16,17]. ER stress and the activation of stress response have been associated with coronavirus infection [18,19] and they have been proposed as a mechanism that facilitates SARS-CoV replication [20]. Comparison of peripheral blood miRNA's expression profiles between SARS-CoV-2 patients and healthy control donors shows the existence of a class of miRNAs differentially expressed between the two conditions, these miRNAs appear to be involved in protein kinase expression regulation [21]. In another study a set of differentially expressed miRNAs between moderate and severe SARS-CoV-2 patients has been spotlighted, functional enrichment analysis for predicted targets of miRNAs shows an involvement in processes such as virus binding and defense response to virus [22]. Given these observations, it is reasonable to think that the miRNA-based interplay between the host and SARS-CoV-2 may be the cause that virus accesses and attacks the host cells [23]. Moreover, it is possible that individual differences in miRNA expression profiles, caused by e.g. age or previous diseases, could affect the severity of infection or the effectiveness of an antiviral treatment. Considering the role that miRNAs can play in the replication and defense evasion mechanisms made by viruses inside the cell, we investigate here the possible role of the SARS-CoV-2 genome and known variants as potential sponge for the miRNAs expressed in the infected cells.
2. Results and discussion
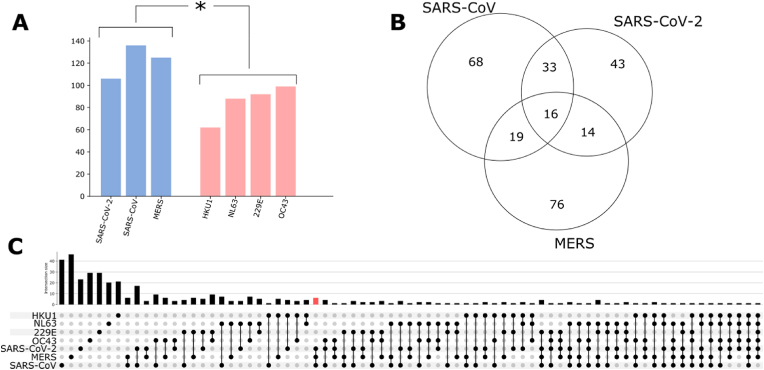
We analyzed and compared the potential sponge effect of seven human coronaviruses (HCoV) on human endogenous miRNAs. Sequencing data for three pathogenic strains (SARS-CoV-2 (NC_045512.2), SARS-CoV (NC_004718.3), and MERS-CoV (NC_019843.3)) and four non-pathogenic strains (HCoV-OC43 (KU131570.1), HCoV-229E (NC_002645.1), HCoV-HKU1 (KF686346.1), and HCoV-NL63 (NC_005831.2)) are currently available and were used in this study. We tested these virus sequences against the set of 940 mature human miRNAs known to be expressed in pulmonary epithelial cells, selected from the RNA Atlas database (see Methods). On average we found 122 (±12) miRNAs which can potentially interact with the genome of viruses in the pathogenic group (Fig. 1A blu bars) vs 85 (±13) miRNAs for the non-pathogenic group (Fig. 1A red bars). This analysis shows how all the analyzed virus sequences can potentially act as sponge for hundreds of endogenous human miRNAs and also that there are more predicted binding sites in the pathogenic group compared with the non-pathogenic one (Mann-Whitney P < 0.05).
Fig. 1.
A) Number of putative miRNA binding sites identified in three pathogenic and four non-pathogenic Coronavirus genomic sequences, reported in blue and red, respectively; B) Intersection of the miRNA binding sites found in viruses of the pathogenic group; C) Intersection of the miRNA binding sites for all the seven genomes analyzed, the red bar highlights the 6 miRNA binding sites present only in the pathogenic group.
Despite the high sequence identity between SARS-CoV-2 and SARS-CoV (∼80%) only 50% of SARS-CoV-2 miRNA's binding sites are shared with SARS-CoV (Fig. 1B), while a lower percentage of SARS-CoV-2 miRNA's binding sites is shared with MERS (Fig. 1B).
Notably, 16 miRNA binding sites appear to be in common among the three pathogenic coronaviruses (Figure 1B), 6 of these are specific for the pathogenic group and do not appear in the non-pathogenic one (miR-10394-5p, miR-103a-1-5p, miR-103a-2-5p, miR-193a-5p, miR-4717-3p, miR-6740-5p) (Fig. 1C red bar).
Interestingly, downregulation of the exosomal miR-103a in SARS-CoV-2 has been observed when comparing SARS-CoV-2 infected patients with high vs low serum level of the D-dimer [24]. Moreover low levels of miR-103 have also been associated with deep vein thrombosis [25,26]. Furthermore downregulation of miR-193a has been observed in SARS-CoV-2 patients [27] but no relationship between miR-193a expression and disease severity has been reported to date.
No associations between the other four miRNAs and SARS-CoV-2 infection have been reported, however their downregulation has been associated with various other diseases. The downregulation of miR-4717 and the resulting upregulation of its target SOCS3 has been associated with type 1 diabetes [28].
Finally, the downregulation of miR-10394 and miR-6740 has been associated with venous thrombosis in colorectal cancer and cardiovascular disease respectively [29].
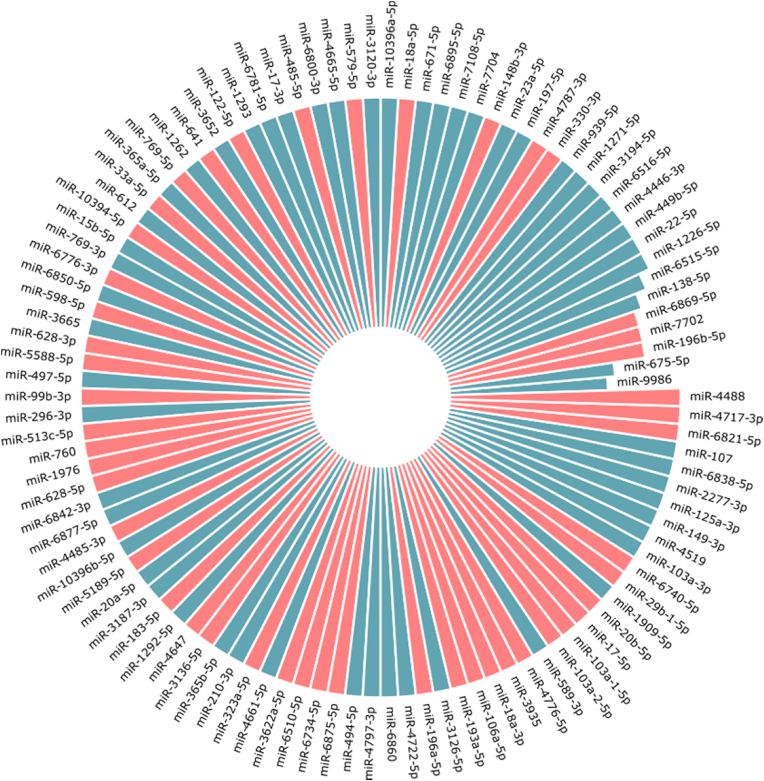
Given the impact that SARS-CoV-2 variants has on transmissibility, severity, and/or immunity, we investigated the distribution of putative miRNA binding sites in ∼100k unique SARS-CoV-2 variant genomes in order to evaluate possible "sponge" effects. Globally, we found 580 binding sites for unique miRNAs across all of the analyzed genomes, 97 unique miRNA binding sites were found to be present in more than 99% of the genomes (Fig. 2).
Fig. 2.
The circular barplot shows the 106 miRNAs that can be sequestered by more than 30% of the SARS-CoV-2 variant genomes used in this study, 97 of which are shared between more than 99% genomes. ∼50% of the miRNA binding sites, highlighted in red, are specific for SARS-CoV-2 and not present in the non-pathogenic strains.
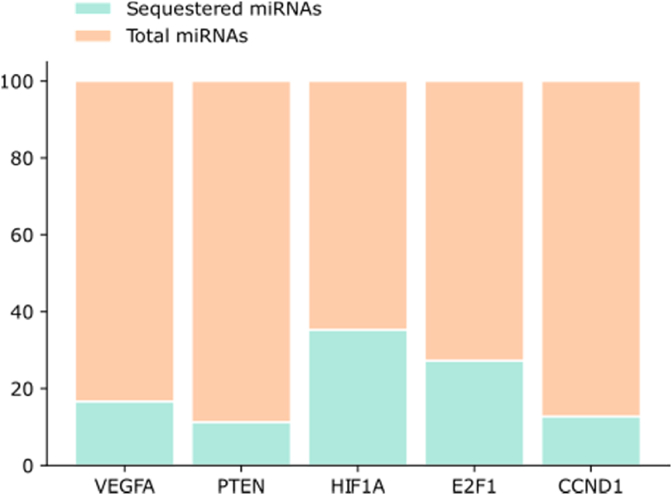
There are 439 mRNAs that can be targeted by the core set of miRNAs whose binding sites are shared by more than 99% of the SARS-Cov-2 sequences. All the identified mRNAs with the associated miRNAs are listed in the supplementary S1. We then focused our attention on the top 5 mRNAs that are regulated by the highest number of the putative sequestered miRNAs (Fig. 3).
Fig. 3.
The number of miRNAs that potentially can be sequestered by SARS-CoV-2 sequence (green) out of the total number of miRNAs that regulate each single mRNA (orange).
The miRNAs potentially sequestered by the SARS-CoV-2 sequence, could no longer be able to downregulate their targets, resulting in their upregulation. Analyzing the 5 top mRNA we found 7 different miRNAs for VEGF-A, PTEN and 6 different miRNAs for HIF1A, E2F1 and CCND1.
In agreement with our hypothesis, Ackermann and collaborators reported an up-regulation of VEGF-A in patients who died from SARS-CoV-2 [30], data also supported by clinical results demonstrating an increase in VEGF-A levels in alveolar bronchial lavage fluid from COVID-19 patients [31,32]. In another study, the PTEN regulation pathway showed the highest association with other activated pathways in human bronchial epithelial cells infected with SARS-CoV, moreover, it is also known how PTEN is involved in the activation of dendritic cells and secretion of proinflammatory mediators promoting the cytokine storm in the SARS-CoV-2 patients [33]. Cytokine storm and hypoxia are a prelude to multiple organ failure and lethality, in this context HIF1A plays a dual role, if on one hand it suppresses the angiotensin-converting enzyme 2 (ACE2) on the other hand it is involved in the activation of pro-inflammatory cytokine expression and the subsequent inflammatory process [34]. Moreover, the vascular endothelial growth factor (VEGF) is transcriptionally upregulated by HIF1A and accumulates under hypoxia enhancing cytokine storm. VEGFA can increase cytokine expression and pulmonary vascular permeability [35] and, notably, the endothelial cells are essential contributors to the initiation and propagation of severe forms of SARS-CoV-2 infection [36]. We also identified the transcription factor E2F1 which is known to be involved in virally-mediated acute respiratory diseases [37]. Finally, there are no studies regarding the involvement of CCND1 in the SARS-CoV-2 infection but its overexpression has been associated many times with bronchial epithelial diseases such as cancer. CCND1 is overexpressed in premalignant versus normal or hyperplastic epithelial cells [38,39], thus highlighting its contribution to an unhealthy state of the tissue.
Finally, the association between the putative SARS-CoV-2 sponge activity and the severity of the disease was investigated. The miRNAs expression, as well as that of the genes and the proteins, can be influenced by several factors such as sex, ethnicity, health/disease state, or age.
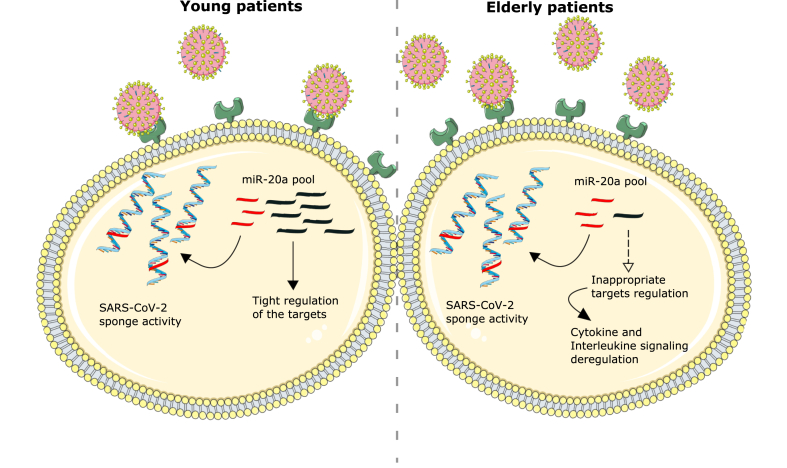
Aging is known to be related with changes in pulmonary physiology, pathology and function, during the period of lung infection. Therefore, there are several age-related differences in severity and tolerance that lead to worse clinical outcomes in SARS-CoV-2 infected elderly individuals [40]. To this purpose, the list of age-related miRNAs in bronchial human healthy biopsies released by Brandsma and collaborators has been used [15]. The authors highlighted 27 differentially expressed miRNAs (13 miRNAs with lower expression levels and 14 with higher expression levels with increasing age). Interestingly, we found that miR-20a-5p, which shows a lower expression level in older subjects, can be sequestered by 99% of all mutated SARS-CoV-2 sequences used in this analysis (Fig. 4). Compared with the healthy controls, miR-20a is downregulated in the peripheral blood of human patients with SARS-CoV-2 [21] and, moreover, its downregulation has been reported to play a role in respiratory diseases influenza A and respiratory syncytial virus [41,42]. miR-20a is involved in the regulation of several targets enriched in biological processes such as: regulation of cell differentiation, cell proliferation and response to endogenous stimulus. Furthermore, the mRNA targets are enriched in pathways such as: cytokine signaling in the immune system, interleukin signaling, cyclin D associated events in G1. Pathways or biological processes that are widely associated with SARS-CoV-2 infection and that could be at the basis of the differences in the degree of the virus lethality in different age groups.
Fig. 4.
SARS-CoV-2 infection and potential miRNA sponge activity of viral RNA.
Schematic representation of viral RNA sponge activity in young (left) and elderly patients (right). miR-20a shows a lower expression in the elderly patients group. Viral RNA sponge activity could drastically reduce the miRNA intracellular concentration, thus interfering with the processes in which the miRNA is involved. The schematic art pieces used in this figure were provided by Servier Medical art (https://smart.servier.com) [43]. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
3. Materials and methods
3.1. Genome sequences collection and pre-processing
SARS-CoV-2 genome sequences and metadata were downloaded from the COVID-19 Data Portal (last access: 2021 June 11). Only the genomes with Homo sapiens host and coverage 100% were downloaded in FASTA format. To discard incomplete genomes, only sequences longer than 25kbps and without unresolved nucleotides were considered and deduplicated, obtaining a total of 103763 unique genomes. Each genome was aligned to the SARS-CoV-2 reference genome (NCBI accession ID: NC_045512.2) by applying the Multiple Alignment using Fast Fourier Transform (MAFFT) sequence aligner [44]. Variants were called by parsing the MAFFT alignment with a custom Python script.
3.2. Pulmonary epithelial cell's miRNA and gene selection
The experimentally verified mature miRNAs and genes expressed in the pulmonary epithelial cells have been extracted from the RNA Atlas database [45].
3.3. Age-related miRNA in human bronchial biopsies
miRNAs whose expression was shown to be significantly related to age were selected from the work of Brandsma and collaborators [15]. The Authors highlighted 27 miRNAs differentially expressed between young and elder subjects. These included 13 miRNAs with lower expression levels and 14 with higher expression levels with increasing age.
3.4. RNA-RNA hybridization
The putative RNA-RNA hybridizations between the host miRNAs and virus RNA genomic sequence were calculated using the RNA hybrid tool [46]. We selected bulged hybridizations, i.e. hybridizations showing a perfect complementarity between the miRNA seed sequence and the viral genome, followed by 3 or 4 mismatches and then again followed by another stretch of perfect complementarity. This model was proposed and tested by Lavenniah and collaborators who have shown how the bulged hybridization can evade the degradation via RISC-mediated endonucleolytic cleavage upon miRNA binding [47].
3.5. miRNA-target interaction
The miRNA-mRNA target interactions were selected using the RAID v2.0 database; only human experimentally validated interactions were used for this analysis.
3.6. Functional enrichment analysis
Gene Ontology (GO) classification and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed using the online tools Gene Set Enrichment Analysis (GSEA) [48].
4. Conclusions and future perspectives
In this study, we performed a bioinformatic analysis to investigate the potential sponge role of SARS-CoV-2 sequence on the miRNAs expressed in human pulmonary epithelial cells. We first compared putative binding sites of these human miRNA within a set of seven HCoV RNA genomes. The pathogenic group (MERS, SARS-CoV, SARS-CoV-2) showed a higher number of binding sites compared with the non-pathogenic one (OC43, HKU1, 229E, NL63). In this analysis, we identified 6 miRNA binding sites present only in the pathogenic group, namely miR-10394-5p, miR-103a-1-5p, miR-103a-2-5p, miR-193a-5p, miR-4717-3p, miR-6740-5p. Interestingly, the down-regulation of these miRNAs has been seen to be associated with deep vein thrombosis and cardiovascular diseases. We then analysed the potential sponge effect of about 100 thousand SARS-CoV-2 genome variants and we identified a total of 439 miRNA binding sites, 97 of which are in common among more than 99% of the genomes analyzed. These miRNAs, potentially seized by the virus genome, may not carry out their regulatory action The physiological expression level of a selected number of miRNAs shows a correlation with age and can also be influenced by previous pathologies. For this reason, the possible SARS-CoV-2 sponge effect on these miRNAs could be related with the disease severity. SARS-CoV-2 could seize the low expressed miRNAs causing a more severe disease in a certain portion of the population. This is the case of the miR-20a-5p which shows a lower expression level in older subjects and it is known to be involved in pathways such as cytokine and interleukin signaling.
These results could be a starting point for the development of new strategies for the treatment of SARS-CoV-2 infection, based on an enhanced autogenous miRNA production or exogenous administration. Moreover, the altered concentration in the bloodstream of specific miRNAs, caused by the SARS-CoV-2 sponge activity inside the cells, could be helpful to follow the disease progression. Finally, understanding the biological differences underlying the severity of SARS-CoV-2 is a key point for developing more accurate antiviral therapies.
Funding
AIRC project [to MHC] (grant number IG 23539).
Data availability statement
SARS-CoV-2 genome identifiers (NCBI accession ID: NC_045512.2), datasets and scripts used in this manuscript are available upon request.
CRediT authorship contribution statement
G. Pepe: Conceptualization, Writing – original draft, Supervision. A. Guarracino: Investigation. F. Ballesio: Investigation. L. Parca: Supervision. G. Ausiello: Supervision. M. Helmer-Citterich: Funding acquisition, Supervision.
Declaration of competing interest
None declared.
Acknowledgments
We thank prof PF Gherardini for insightful discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ncrna.2022.01.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Noor R. A comparative review of pathogenesis and host innate immunity evasion strategies among the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) Arch. Microbiol. 2021;203:1943–1951. doi: 10.1007/s00203-021-02265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 pathophysiology: a review. Clin. Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment, postgrad. Med. J. 2021;97:312–320. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rota P.A. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 5.Chams N., Chams S., Badran R., Shams A., Araji A., Raad M., Mukhopadhyay S., Stroberg E., Duval E.J., Barton L.M., Hajj Hussein I. COVID-19: a multidisciplinary review. Front. Public Health. 2020;8:383. doi: 10.3389/fpubh.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong M., Zhang J., Ma X., Tan J., Chen L., Liu S., Xin Y., Zhuang L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19, Biomed. Pharmacother. 2020;131:110678. doi: 10.1016/j.biopha.2020.110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan S., Chan J.F.W., Chik K.K.H., Chan C.C.Y., Tsang J.O.L., Liang R., Cao J., Tang K., Chen L.-L., Wen K., Cai J.-P., Ye Z.-W., Lu G., Chu H., Jin D.-Y., Yuen K.-Y. Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system. Pharmacol. Res. 2020;159:104960. doi: 10.1016/j.phrs.2020.104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natarelli L., Parca L., Mazza T., Weber C., Virgili F., Fratantonio D. MicroRNAs and long non-coding RNAs as potential candidates to target specific motifs of SARS-CoV-2. Noncoding RNA. 2021;7 doi: 10.3390/ncrna7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbu M.G., Condrat C.E., Thompson D.C., Bugnar O.L., Cretoiu D., Toader O.D., Suciu N., Voinea S.C. MicroRNA involvement in signaling pathways during viral infection. Front. Cell Dev. Biol. 2020;8:143. doi: 10.3389/fcell.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luna J.M., Scheel T.K.H., Danino T., Shaw K.S., Mele A., Fak J.J., Nishiuchi E., Takacs C.N., Catanese M.T., de Jong Y.P., Jacobson I.M., Rice C.M., Darnell R.B. Hepatitis C virus RNA functionally sequesters miR-122. Cell. 2015;160:1099–1110. doi: 10.1016/j.cell.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartoszewski R., Sikorski A.F. Editorial focus: understanding off-target effects as the key to successful RNAi therapy, Cell. Mol. Biol. Lett. 2019;24:69. doi: 10.1186/s11658-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartoszewski R., Sikorski A.F. Editorial focus: entering into the non-coding RNA era. Cell. Mol. Biol. Lett. 2018;23:45. doi: 10.1186/s11658-018-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong J., Woldhuis R.R., Boudewijn I.M., van den Berg A., Kluiver J., Kok K., Terpstra M.M., Guryev V., de Vries M., Vermeulen C.J., Timens W., van den Berge M., Brandsma C.A. Age-related gene and miRNA expression changes in airways of healthy individuals. Sci. Rep. 2019;9:3765. doi: 10.1038/s41598-019-39873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebert M., Bartoszewska S., Janaszak-Jasiecka A., Moszyńska A., Cabaj A., Króliczewski J., Madanecki P., Ochocka R.J., Crossman D.K., Collawn J.F., Bartoszewski R. PIWI proteins contribute to apoptosis during the UPR in human airway epithelial cells. Sci. Rep. 2018;8:16431. doi: 10.1038/s41598-018-34861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omidkhoda N., Wallace Hayes A., Reiter R.J., Karimi G. The role of MicroRNAs on endoplasmic reticulum stress in myocardial ischemia and cardiac hypertrophy. Pharmacol. Res. 2019;150:104516. doi: 10.1016/j.phrs.2019.104516. [DOI] [PubMed] [Google Scholar]
- 18.Chan S.-W. Frontiers Media SA; 2015. The Unfolded Protein Response in Virus Infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan C.-P., Siu K.-L., Chin K.-T., Yuen K.-Y., Zheng B., Jin D.-Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2006;80:9279–9287. doi: 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C., Hu X., Li L., Li J. Differential microRNA expression in the peripheral blood from human patients with COVID-19. J. Clin. Lab. Anal. 2020;34 doi: 10.1002/jcla.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang H., Gao Y., Li Z., Miao Y., Huang Z., Liu X., Xie L., Li H., Wen W., Zheng Y., Su W. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 2020;10 doi: 10.1002/ctm2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S., Amahong K., Sun X., Lian X., Liu J., Sun H., Lou Y., Zhu F., Qiu Y. The miRNA: a small but powerful RNA for COVID-19. Briefings Bioinf. 2021;22:1137–1149. doi: 10.1093/bib/bbab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gambardella J., Sardu C., Morelli M.B., Messina V., Castellanos V., Marfella R., Maggi P., Paolisso G., Wang X., Santulli G. medRxiv; 2020. Exosomal microRNAs Drive Thrombosis in COVID-19. 2020.06.16.20133256. [Google Scholar]
- 25.Santulli G. 2015. MicroRNA: Basic Science: from Molecular Biology to Clinical Practice. [Google Scholar]
- 26.Rodriguez-Rius A., Lopez S., Martinez-Perez A., Souto J.C., Soria J.M. Identification of a plasma MicroRNA profile Associated with venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2020;40:1392–1399. doi: 10.1161/ATVBAHA.120.314092. [DOI] [PubMed] [Google Scholar]
- 27.Pierce J.B., Simion V., Icli B., Pérez-Cremades D., Cheng H.S., Feinberg M.W. Computational analysis of targeting SARS-CoV-2, viral entry proteins ACE2 and TMPRSS2, and interferon genes by host MicroRNAs. Genes. 2020;11 doi: 10.3390/genes11111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori H., Shichita T., Yu Q., Yoshida R., Hashimoto M., Okamoto F., Torisu T., Nakaya M., Kobayashi T., Takaesu G., Yoshimura A. Suppression of SOCS3 expression in the pancreatic β-cell leads to resistance to type 1 diabetes. Biochem. Biophys. Res. Commun. 2007;359:952–958. doi: 10.1016/j.bbrc.2007.05.198. [DOI] [PubMed] [Google Scholar]
- 29.Song X., Sun Z., Chen G., Shang P., You G., Zhao J., Liu S., Han D., Zhou H. Matrix stiffening induces endothelial dysfunction via the TRPV4/microRNA-6740/endothelin-1 mechanotransduction pathway. Acta Biomater. 2019;100:52–60. doi: 10.1016/j.actbio.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray P., Wangzhou A., Ghneim N., Yousuf M., Paige C., Tavares-Ferreira D., Mwirigi J., Shiers S., Sankaranarayanan I., McFarland A., Neerukonda S., Davidson S., Dussor G., Burton M., Price T. SSRN.; 2020. A Pharmacological Interactome between COVID-19 Patient Samples and Human Sensory Neurons Reveals Potential Drivers of Neurogenic Pulmonary Dysfunction; p. 3581446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., Hu J.-L., Xu W., Zhang Y., Lv F.-J., Su K., Zhang F., Gong J., Wu B., Liu X.-M., Li J.-J., Qiu J.-F., Chen J., Huang A.-L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 33.Zolfaghari Emameh R., Nosrati H., Eftekhari M., Falak R., Khoshmirsafa M. Expansion of single cell transcriptomics data of SARS-CoV infection in human bronchial epithelial cells to COVID-19. Biol. Proced. Online. 2020;22:16. doi: 10.1186/s12575-020-00127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serebrovska Z.O., Chong E.Y., Serebrovska T.V., Tumanovska L.V., Xi L. Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sin. 2020;41:1539–1546. doi: 10.1038/s41401-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisht K., Brunck M.E., Matsumoto T., McGirr C., Nowlan B., Fleming W., Keech T., Magor G., Perkins A.C., Davies J., Walkinshaw G., Flippin L., Winkler I.G., Levesque J.-P. HIF prolyl hydroxylase inhibitor FG-4497 enhances mouse hematopoietic stem cell mobilization via VEGFR2/KDR. Blood Adv. 2019;3:406–418. doi: 10.1182/bloodadvances.2018017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nain Z., Rana H.K., Liò P., Islam S.M.S., Summers M.A., Moni M.A. Pathogenetic profiling of COVID-19 and SARS-like viruses. Briefings Bioinf. 2021;22:1175–1196. doi: 10.1093/bib/bbaa173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratschiller D., Heighway J., Gugger M., Kappeler A., Pirnia F., Schmid R.A., Borner M.M., Betticher D.C. Cyclin D1 overexpression in bronchial epithelia of patients with lung cancer is associated with smoking and predicts survival. J. Clin. Oncol. 2003;21:2085–2093. doi: 10.1200/JCO.2003.03.103. [DOI] [PubMed] [Google Scholar]
- 39.Sun C., Huang C., Li S., Yang C., Xi Y., Wang L., Zhang F., Fu Y., Li D. Hsa-miR-326 targets CCND1 and inhibits non-small cell lung cancer development. Oncotarget. 2016;7:8341–8359. doi: 10.18632/oncotarget.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Mao B., Liang S., Yang J.-W., Lu H.-W., Chai Y.-H., Wang L., Zhang L., Li Q.-H., Zhao L., He Y., Gu X.-L., Ji X.-B., Li L., Jie Z.-J., Li Q., Li X.-Y., Lu H.-Z., Zhang W.-H., Song Y.-L., Qu J.-M., Xu J.-F. Shanghai clinical treatment experts group for COVID-19, association between age and clinical characteristics and outcomes of COVID-19. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leon-Icaza S.A., Zeng M., Rosas-Taraco A.G. microRNAs in viral acute respiratory infections: immune regulation, biomarkers, therapy, and vaccines. ExRNA. 2019;1:1–7. doi: 10.1186/s41544-018-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potential biomarkers for the early prediction of SARS-COV-2 disease outcome. Microb. Pathog. 2021;158:105057. doi: 10.1016/j.micpath.2021.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.SMART Servier medical art. 2016. https://smart.servier.com/
- 44.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorenzi L., Chiu H.-S., Cobos F.A., Gross S., Volders P.-J., Cannoodt R., Nuytens J., Vanderheyden K., Anckaert J., Lefever S., Goovaerts T., Hansen T.B., Kuersten S., Nijs N., Taghon T., Vermaelen K., Bracke K.R., Saeys Y., De Meyer T., Deshpande N., Anande G., Chen T.-W., Wilkins M.R., Unnikrishnan A., De Preter K., Kjems J., Koster J., Schroth G.P., Vandesompele J., Sumazin P., Mestdagh P. bioRxiv; 2019. The RNA Atlas, a Single Nucleotide Resolution Map of the Human Transcriptome; p. 807529. [DOI] [Google Scholar]
- 46.Rehmsmeier M., Steffen P., Hochsmann M., Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavenniah A., Luu T.D.A., Li Y.P., Lim T.B., Jiang J., Ackers-Johnson M., Foo R.S.-Y. Engineered circular RNA sponges act as miRNA inhibitors to attenuate pressure overload-induced cardiac hypertrophy. Mol. Ther. 2020;28:1506–1517. doi: 10.1016/j.ymthe.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SARS-CoV-2 genome identifiers (NCBI accession ID: NC_045512.2), datasets and scripts used in this manuscript are available upon request.