Abstract
The unexpected emergence of the new Coronavirus disease (COVID-19) has affected more than three hundred million individuals and resulted in more than five million deaths worldwide. The ongoing pandemic has underscored the urgent need for effective preventive and therapeutic measures to develop anti-viral therapy. The natural compounds possess various pharmaceutical properties and are reported as effective anti-virals. The interest to develop an anti-viral drug against the novel severe acute respiratory syndrome Coronavirus (SARS-CoV-2) from natural compounds has increased globally. Here, we investigated the anti-viral potential of selected promising natural products. Sources of data for this paper are current literature published in the context of therapeutic uses of phytoconstituents and their mechanism of action published in various reputed peer-reviewed journals. An extensive literature survey was done and data were critically analyzed to get deeper insights into the mechanism of action of a few important phytoconstituents. The consumption of natural products such as thymoquinone, quercetin, caffeic acid, ursolic acid, ellagic acid, vanillin, thymol, and rosmarinic acid could improve our immune response and thus possesses excellent therapeutic potential. This review focuses on the anti-viral functions of various phytoconstituent and alkaloids and their potential therapeutic implications against SARS-CoV-2. Our comprehensive analysis provides mechanistic insights into phytoconstituents to restrain viral infection and provide a better solution through natural, therapeutically active agents.
Keywords: SARS-CoV-2, Phytoconstituent, COVID-19 therapy, Natural anti-viral products, Polyphenols, Alkaloids
Graphical Abstract
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an emerging strain of coronavirus (CoV), has affected millions of people worldwide. The coronavirus infection has rapidly spread all over, affecting 210 countries and territories across the globe. According to the surveillance statistics reported by the World corona tracker, by January 19, 2022, the pandemic has caused more than 336 million number of confirmed infection cases, with 5.5 million worldwide deaths (https://www.worldometers.info/coronavirus). COVID-19 has marked the history with the third life-threatening coronavirus epidemic in the human population during the 21st century [1], [2], [3], [4], [5]. This emerging health crisis calls for the urgent development of specific therapeutics against COVID-19 to potentially reduce the burden of this emerging pandemic [6]. Chinese scientists released the sequence of the SARS-CoV-2 genome to understand viral physiology and develop new diagnostic tools, treatment options and anti-viral vaccines [7], [8].
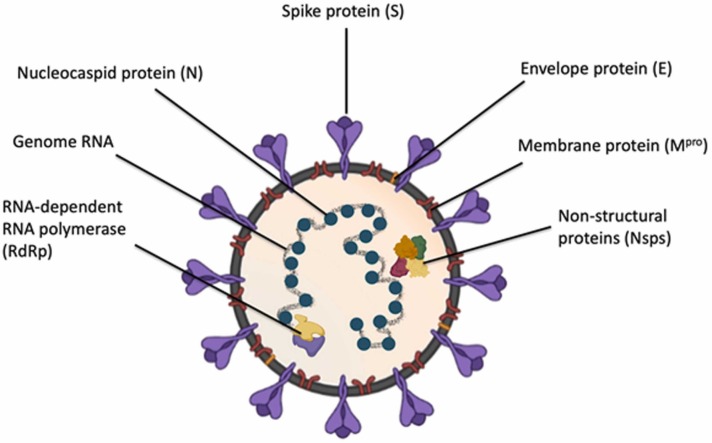
A total of six different human infecting coronavirus strains, SARS-CoV, MERS-CoV, H-CoV-229E, H-CoV-OC43, H-CoV-NL63, H-CoV-HKU1, have been reported at present [9]. Except for SARS-CoV and MERS-CoV strains responsible for the respiratory infections in China during 2002–2003 and in the Middle East in 2012, respectively. The other four strains are common and cause the common cold in healthy individuals. The novel coronavirus, including six other species in the coronavirus genus of the coronavirus family, distributed broadly among birds, humans and other mammals, have been reported [10], [11], [12], [13]. The genome length of coronaviruses ranged from ~27 kb to ~31 kb, establishing the genome of coronaviruses as the largest found in any RNA viruses ( Fig. 1).
Fig. 1.
Structural representation of SARS-CoV-2.
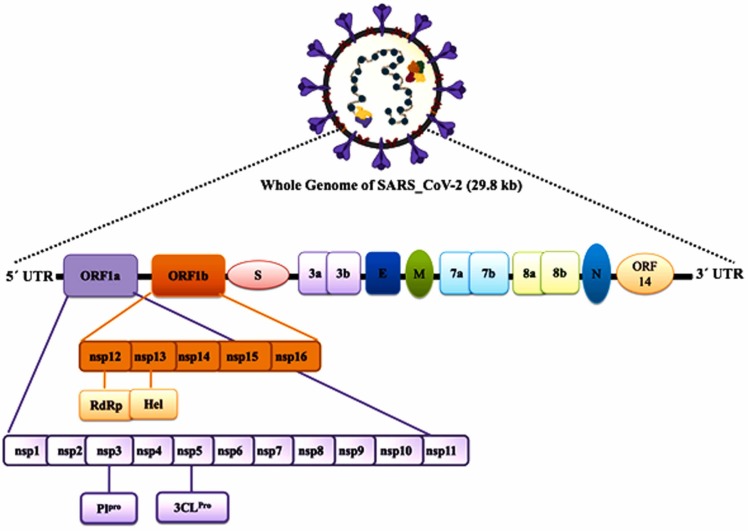
The SARS-CoV-2 is an enveloped, positive sense-RNA β-coronavirus with around 30 kb genome [14]. The complex genome of SARS-CoV-2 encodes for several non-structural proteins (Nsps) and structural proteins [15]. A major part of the genome consisting of replicase ORF1ab encompassing Nsps, encodes for pp1a and pp1ab, the two overlapping polyproteins (pp) of SARS-CoV-2 [16]. The main protease (Mpro), ~306 amino acids long of the virus, is encoded by these polypeptides pp1a and pp1ab [17]. The Mpro of the virus cleaves the polypeptides at multiple conserved sites resulting in 16 functional viral Nsps. These digested Nsps perform various viral enzymatic activities and are involved primarily in the replication process [15], [16], [18]. An important SARS-CoV-2 protein, Nsp15, is a crucial endoribonuclease required for viral intervention during an innate immune response [15]. Considering the importance of Mpro and Nsp15 in viral replication and survival, these viral proteins could be attractive drug targets to develop effective COVID-19 therapy ( Fig. 2).
Fig. 2.
Schematic diagram of the SARS-CoV-2 genome organization and the different proteins encoded by various genes.
Other than Nsps, the coronavirus genome comprises several genes encoding for the structural protein such as including the S (spike) gene, E gene (viral envelope protein), and N (nucleocapsid protein) gene. The spike protein of the virus plays an important role in the viral entry into the human cells by attaching to the human angiotensin-converting enzyme 2 (ACE2) receptor through which the virus fuses with the target membrane [19]. The affinity for the human ACE-2 was found much more in the novel strain of coronavirus SARS-CoV-2, as compared to the SARS-CoV strain, indicating the SARS-CoV-2 fusion mechanism as a novel and attractive target for coronavirus inhibition. The S1 submit of spike protein binds the ACE2 receptor, whereas the S2 subunit forms the fusion core, bringing viral and host cell membranes closer for effective fusion following infection [20]. Therefore, the S2 submit of spike protein is considered a potential target for viral fusion inhibition [21], [22].
This global calamity has posed a difficult time throughout the world, impeding the normal lifestyle. It has become urgent to develop a new vaccine or drugs against this deadly infectious disease at the earliest [23], [24], [25]. The scientific community worldwide has reported various preliminary studies on the pathophysiology of COVID-19 patients and provided some clues to treat this pandemic [26]. Unfortunately, currently, no FDA-approved drugs are available and the development of new therapeutic moieties and vaccines remains a costly and time-consuming affair with high failure chances, too [27], [28]. Considering this pervasive situation, it is essential to apply different strategies to tackle the present scenario. The plants with medicinal properties have provided an excellent and widespread therapeutic alternative since ancient times against various infectious diseases owing to their safer, cheaper, and less toxicity profile [29], [30], [31], [32], [33]. Phytoconstituents are important compounds for drug discovery against various human diseases [34], [35], [36]. Recent studies have suggested the potential of polyphenols, alkaloids to combat COVID-19 with their chemical properties are listed in Table 1.
Table 1.
Phytoconstituents from natural sources with their chemical properties.
| S.N. | Compound | Structure | Chemical Formula | Molecular Mass |
|---|---|---|---|---|
| 1 | Thymoquinone | 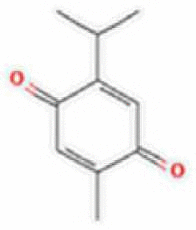 |
C10H12O2 | 164.201 g/mol |
| 2 | Quercetin | 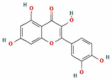 |
C15H10O7 | 302.236 g/mol |
| 3 | Caffeic Acid | 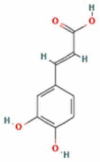 |
C9H8O4 | 180.16 g/mol |
| 4 | Ursolic acid | 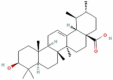 |
C30H48O3 | 456.71 g/mol |
| 5 | Ellagic acid | 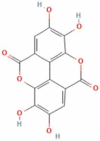 |
C14H6O8 | 302.197 g/mol |
| 6 | Vanillin | 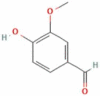 |
C8H8O3 | 152.15 g/mol |
| 7 | Thymol | 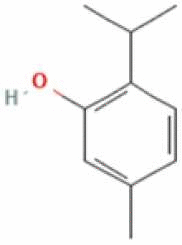 |
C10H14O | 150.22 g/mol |
| 8 | Rosmarinic Acid | 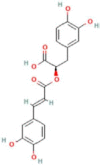 |
C18H16O8 | 360.31 g/mol |
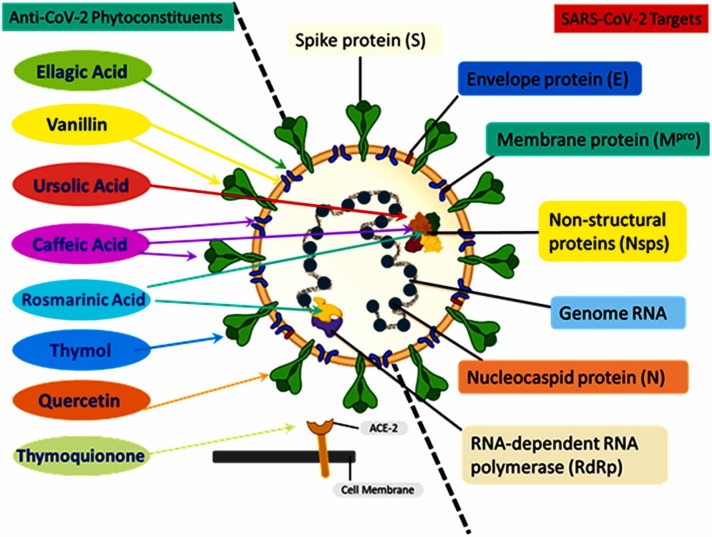
Phytoconstituents like polyphenols, flavonoids, and alkaloids are found in ample amounts plants and are abundant in a broad range of human diet [37], [38], [39], [40], [41], [42]. The best-characterized complex class, all the structurally diverse members, comprise a central oxygenated heterocycle, a three-ring structure with two aromatic center [43], [44]. Presently, these phytoconstituents are of great interest for their antioxidative, anti-inflammatory, anti-pathogenic, cardioprotective, and anti-carcinogenic properties [45], [46], [47], [48], [49]. These polyphenolic compounds, alkaloids, comprise multiple essential characteristics against viral diseases like immune system alteration [50], suppression of viral replication [51], and decrease in viral uptake by host target membrane [52]. Moreover, studies have suggested the anti-viral activity of phytoconstituents against rabies virus, HIV, chandipura virus, Japanese Encephalitis Virus, Enterovirus, Influenza A/H1N1, other influenza viruses, and SARS-CoV-2 [30], [53], [54], [55]. Phytoconstituents show beneficial impact on COVID-19 therapy are listed in Table 2, and their sites of action are illustrated in Fig. 3 .
Table 2.
A list of different phytoconstituents shows anti-viral activity against SARS-CoV‐2.
| Compound | Biological Role | Activity against Covid-19 | Natural source | Ref. |
|---|---|---|---|---|
| Thymoquinone | Anti-oxidant immune-regulatory, anti-inflammatory, and anti-oxidant benefits | Prevent the SARS-CoV-2 entry; inhibits viral replication | Nigella sativa | [26], [56], [57], [58], [59], [60], [61] |
| Quercetin | Anti-oxidant, anti-inflammatory, anti-cancerous, anti-viral, anti-bacterial, and immunomodulatory | Inhibition of 3CL protease activity and viral entry inside the host cell | Apples, Honey, Raspberries, Onions, Red Grapes, Cherries, Citrus Fruits, And Green Leafy Vegetables | [49], [62], [63], [64], [65], [66], [67], [68] |
| Caffeic Acid | Anti-oxidant, anti-inflammatory, anti-bacterial and anti-viral | Inhibit the virus attachment to the host cell; Binds 3CL protease inhibits the viral replication |
Blueberries, Kiwis, Coffee, Cherries, Apples, Oils, And Tea | [69], [70], [71], [72], [73], [74], [75] |
| Ursolic Acid | Anti-inflammatory, anti-bacterial effects, anti-oxidant, anti-cancer, and anti-diabetic, | Potently block the Mproenzyme | Mimusopscaffra, Ilex paraguarieni,and Glechoma hederacea | [76], [77], [78], [79], [80] |
| Ellagic Acid | Anti-oxidant and anti-proliferative, Inhibit fibrosis, oxidative stress, and inflammation in the diabetic liver | Inhibits the Mpro and RdRp; Prevent viral attachment and internalization to the host cell |
Raspberries, Strawberries, Pomegranate, Persimmon, Grapes, Black Currants, Plumes, Mango, Guava, Walnuts, Almonds, Longan seeds, Green Tea, and Momordica charantia | [81], [82], [83], [84], [85] |
| Vanillin | Anti-clastogenic, anti-microbial agent, anti-oxidant | MPro inhibition | Vanilla bean | [86], [87], [88], [89], [90] |
| Thymol | Anti-oxidant, local anesthetic, anti-carcinogenesis, anti-nociceptive, cicatrizing, antiseptic, as well as a potential as a growth enhancer and immunomodulator | Inhibit the viral spike protein; prevent the SARS-CoV-2 entry, potent disinfectants. | Thymus vulgaris, Ocimum, Origanum, Monarda genera, members of Verbenaceae, Scrophulariaceae, Ranunculaceae, and Apiaceae families | [91], [92], [93] |
| Rosmarinic Acid | Antispasmodic, analgesic, anti-rheumatic, diuretic, and antiepileptic agent food flavoring agent, | Inhibition of viral entry, replication | Rosemary, Perilla, Sage, Mint, and Basil. | [94], [95], [96], [97] |
Fig. 3.
Schematic representation of SARS-CoV-2 structure showing the viral genome and important viral proteins (S protein, N protein, E protein, Mpro protein, Nsps, and RdRp,). Natural phytochemicals showed therapeutic potential against the SARS-CoV-2 via binding to these proteins followed by inhibiting their functions. The phytochemicals such as thymoquinone interact with ACE-2 receptors to block the entry; thymol interacts with S protein; quercetin interacts with 3CL protease; vanillin interacts with both the 3CL protease and S1 proteins; rosmarinic acid interacts with NSP-15; ursolic acid interacts with NSP15 and M proteins; ellagic acid interacts with M proteins and caffeic acid, and its derivatives interact with M proteins, NSP-15, and spike protein.
This review summarizes the therapeutic properties of essential phytoconstituents, polyphenols, alkaloids, like thymoquinone, quercetin, caffeic acid, ursolic acid, etc., and further discusses their therapeutic potential in COVID-19.
2. Thymoquinone inhibits the CoV-2 entry and replication
Nigella sativa consists of a wide range of bioactive constituents such as thymoquinone and nigellimine offer a range of beneficial spectra for the COVID-19 treatment by blocking the virus introduction to host pneumocytes; by furnishing ionophores to improve zinc consumption, thus enhancing the host immune response to coronavirus-2, and further suppressing the viral replication. Thymoquinone (2-Isopropyl-5-methyl-1, 4-benzoquinone) is the main active ingredient of Nigella sativa. El primarily extracted by Dakhakhny and subsequently found to exhibit numerous therapeutic properties as immune-regulatory, anti‐inflammatory, anti-oxidant, antimicrobial, antitumor, analgesic, anti-Alzheimer, and hepatoprotective [98], [99], [100], [101]. Thymoquinone-mediated inhibition of 5-lipoxygenase, leukotriene B4, C4, and Th2 cytokines in bronchoalveolar lavage fluid was reported with a remarkable increase in immune cell numbers in lung tissue [102], [103]. The anti-inflammatory activities of thymoquinone are regulated by the higher production of hem oxygenase 1 (HO-1) in HaCaT (human keratinocyte) cells [101]. Furthermore, the anti-oxidant properties of thymoquinone are linked with the redox activities of quinone structure and unrestricted competence of thymoquinone to cross substantial barriers to cellular niche [26], [104].
Nigella sativa extract and thymoquinone were highly effective in avian influenza virus (H9N2 AIV) infection model [105]. Intriguingly, Nigella sativa extract treatment of the cells before infection with coronavirus reduces the division and survival of the virus inside the cell [106] . Additionally, thymoquinone regulates nitric oxide (NO), reactive oxygen species (ROS), and transforming growth factor β1 (TGF-β1) production and protects the multiple organ dysfunction syndromes (MODS) [56], [58], [107], [108], [109].
A molecular docking‐based study has identified nigellidine and α‐hederin among the compounds against SARS-CoV‐2 [110]. N. sativa extract decelerates COVID-19 infection and might provide better results [26]. The beneficial effects of thymoquinone could be enhanced by using a zinc supplement because zinc during any pathogenic virus or bacterial infection may improve innate and adaptive immunity. The effectiveness of the zinc salt supplement in combination with thymoquinone could also be augmented as it functions as an ionophore to allow Zn2+ to enter pneumocytes, the target cell for SARS-CoV-2 [57]. Bioactive compounds of N. sativa seed, especially thymoquinone, α-hederin, and nigellidine, could be alternative and promising herbal drugs to combat COVID-19 infection [56]. Thymoquinone and other active ingredients of N. sativa seed could influence the immune response and thus protect from COVID-19 [26].
3. Quercetin mediated inhibition of 3CL protease activity, and viral entry
Quercetin (3,3′,4′5,7-pentahydroxyflavone), classified as a flavonoid, is found in many plants and foods consumed by humans such as apples, berries, grape, onions, green tea, and Ginkgo biloba. A polyphenolic compound, quercetin, presents a variety of physiological benefits, including anti-oxidant, anti-inflammatory, anti-cancerous, anti-viral, anti-bacterial, and immunomodulatory [49], [111], [112], [113], [114], [115]. Studies including in vitro system and in vivo animal model have shown the immune-modulating effects of quercetin, such as an increase in the chemotaxis motion of neutrophil cells, phagocytosis, lytic activity and proliferation of different immune cells. Additionally, the cytokines expressing genes are regulated by quercetin [111]. The quercetin treatment to cell cultures inhibited influenza strains and preventing H5N1 viral entry [116]. Health workers and doctors recommend using quercetin to improve healthy immune function. The use of quercetin supplements in the common people and sports professionals has been reported. It is proven that severe physical activities are responsible for a short-term decline in the immune, which enhances the risk of infection [66], [111]. Furthermore, the use of quercetin has been reported in viral infection owing to its potential anti-viral effects in inhibiting viral proteases, reverse transcriptase, polymerases, reverse transcriptase, binding viral capsid proteins, and suppressing DNA gyrase [117], [118], [119], [120], [121], [122], [123], [124].
The anti-viral activities of quercetin have been well established. In cultured cells, the administration of quercetin inhibits the growth of various respiratory viruses [121], [125]. Quercetin significantly restrained the cytomegalovirus replication inoculated HeLa cells with an IC50 (half inhibitory concentration) of 0.8 μM [126]. The treatment of quercetin inhibits replication of dengue virus type 2 in Vero cells with an IC50 of 35.7 μg/mL, resulting in a 67%reduction of dengue virus RNA [127].
The anti-viral effects of quercetin were studied on various associates of the Corona viridae family [128]. Quercetin binds and inhibits the proteolytic activity of the 3CL protease of SARS-CoV-2 with an IC50 of 4.95 μM [129]. The hydroxyl group of quercetin regulates the inhibition of 3CL protease. Mutational modeling analysis of Q189A identified the Gln189 as an essential position on 3CL protease responsible for the quercetin binding. Additionally, treatment of Vero E6 cells with quercetin interrupts viral entry with an EC50 (half-effective concentration) of 83.4 μM and with low CC50 (cytotoxicity) of 3.32 mM [128].
4. Caffeic acid regulates the CoV-2 attachment to the host cell
Caffeic acid (3,4-dihydroxycinnamic acid) is a polyphenol, belongs to the phenolic acid family. It is one of the potent and abundantly found in nature hydroxycinnamic acids [69]. The chemical formulation of caffeic acid is [(E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid] with a molecular mass of 180.16 g/mol[130], [131], [132]. Caffeic acid is the main hydroxycinnamic acid abundantly present in blueberries, kiwis, coffee, cherries, apples, oils, and tea [70], [71], [72]. Besides the mentioned foodstuffs, caffeic acid is also found in propolis, a mixture used in medicine [133]. In the last few years, several studies reported the biological properties of caffeic acid, caffeic acid derivatives (CAFDs), and the Caffeic Acid Phenethyl Ester (CAPE) that have shown various immune-modulatory properties such as; anti-oxidant, anti-inflammatory, anti-bacterial and anti-viral effects [134], [135], [136], [137], [138], [139], [140], [141]. Caffeic acid derivatives have a high potential for treating and preventing various human disorders [142], [143], [144]. Caffeic acid with advantageous biological properties, accompanied by acceptable safety characteristics, makes them suitable candidates for clinical studies [143]. The role of caffeic acid as a potential anti-viral agent has been reported against the influenza virus, herpes simplex virus, and severe fever with thrombocytopenia syndrome (SFTS) virus [75], [145]. Furthermore, caffeic acid, CAPE, CAFDs as a potential antiviral agent for the treatment of a number of viruses such as HCV, HIV, human sarcoma, polio, and influenza A viruses. The scientific basic and antiviral mechanisms of CA, CAPE, and CAFDs might be same and/or different depending on the type of the virus.
Anti-viral activity of caffeic acid has previously been reported against the human sarcoma, polio, and influenza A viruses [73], [146]. CAPE has anti-viral activity against HIV and hepatitis C virus [141], [147]. A library consisting of CAFDs was screened to identify the novel therapeutic natural compounds as an anti-COVID19 agent. The important drug targets of SARS-CoV-2, such as spike ectodomain (open), spike glycoprotein (closed), Nsp15 endoribonuclease, Mpro (6LU7), and S2 subunit (6LXT), have been subjected to the study. The analysis has identified several CAFDs as modulators of SARS-CoV-2 drug targets, in particular, khainaoside C as Mpro modulator, khainaoside B as SARS-CoV-2 fusion protein, 6-O-Caffeoylarbutin as Nsp15, khainaoside C as spike (open), and vitexfolin A as spike (closed) modulator [22]. The effect of ethanolic extracts of Sambucus formosana (elderberry) stems from a variant HCoVNL63 of the human coronavirus was studied. A very high efficacy was reported (EC50 of 1.17 ± 0.75 μg/mL). Subsequent analysis of the phenolic constituents of the extracts identified caffeic acid as the most potent compound (EC50 of 3.54 ± 0.77 μM; or ∼0.64 ± 0.14 μg/mL) in anti-viral assays [148]. Caffeic acid has also been found to inhibit the virus attachment to the host cell [149]. Caffeic acid, CAPE, galangin, and chrysin have a high potential to suppress the viral 3CL protease enzyme and prevent viral replication [150].
The host cell surface heat shock protein A5 (HSPA5) during viral infection is upregulated and subjected to be recognized by the SARS-CoV-2 spike protein. Molecular docking and molecular dynamics simulations studies were performed to observe the binding potential of natural compounds to HSPA5 substrate-binding domain β (SBDβ). The results showed high to a moderate binding affinity for several phytoestrogens, including caffeic acid, CAPE, and thymoquinone, to the HSPA5 SBDβ and indicated the potential of these compounds as anti-COVID-19 agents [61]. In addition, docking studies revealed that CAPE showed the highest affinity to both 3CL-protease and S1 spike protein [121]. Furthermore, hydrogen bond formation between the catalytic site residue Lys50 of Mpro and caffeic acid was reported. Caffeic acid forms hydrogen bonds with the catalytic site residues of both E and N protein with a docking score of −6.1 kcal/mol and −7.4 kcal/mol [151], [152].
5. Ursolic acid, a potent inhibitor of viral Mpro enzyme
Ursolic acid (3-β-hydroxy-urs-12-en-28-oic acid) is a naturally occurring pentacyclic triterpenoid of isoprenoid units [76], [77], [78]. Ursolic acid is highly soluble in alcoholic NaOH and glacial acetic acid and low soluble in an aqueous medium [153], [154]. The biosynthesis of ursolic acid occurs by folding and cycling squalene from a dammarenyl cation, making the third ursolic acid ring expand and generate an additional ring [76], [155]. Ursolic acid, majorly extracted from medicinal plants Mimusopscaffra, Ilex paraguarieni, and Glechoma hederacea, show anti-inflammatory, anti-bacterial, anti-oxidant, anti-cancer, and anti-diabetic potential. Still, its bioavailability and solubility limit its clinical application [76], [79], [80], [156], [157], [158], [159].
Ursolic acid has been reported to effective anti-viral response against the SARS-CoV-2. The studies have reported that ursolic acid modulates the Mpro activity of SARS-CoV-2, which is required to process replicase-transcriptase machinery for viral replication and particle assembly. Ursolic acid, including other natural metabolites, was reported as a potential inhibitor against Mpro [160]. Furthermore, in a study using molecular docking and molecular dynamic simulations, three ligands bound to protease during 50 ns of MD simulations [161]. To review the ethnobotanical knowledge of medicinal plants traditionally used to treat different viral diseases by the Ethiopian people, Tegen et al. [162] suggested those plants consisting of active anti-viral components, including ursolic acid, are promising to treat COVID-19. The bioactive constituents of herbal origin, ursolic acid, with a few more compounds, bind and potentially block the Mpro enzyme of SARS-CoV-2. The ursolic acid showed the highest docking score (−8.7 kcal/mol) with the Mpro followed by other compounds tested, suggesting the potential binding and inhibitory effects of ursolic acid [163]. Ursolic acid showed a high binding affinity (−9.7 kcal/mol) for papain-like protease PLpro of SARS-CoV-2 and forms hydrogen bonding at amino acid residues Asp108 and attaches at Ala107, Pro248 and Tyr264 with alkyl, π-alkyl interaction [164].
6. Ellagic acid mediated binding of viral Mpro and RdRp enzymes
Ellagic acid (2,3,7,8-tetrahydroxy[1]-benzopyranol [5,4,3-cde] benzopyran-5,10-dione), discovered by Braconnotin 1831 and found in numerous fruits and vegetables, is a naturally occurring polyphenolic compound [81], [165]. Ellagic acid with a molecular weight of 302.197 g mol−1 and a melting point of 350 °C is a highly thermostable molecule [166]. The structural feature of ellagic acid represents four lipophilic domains that form hydrogen-bond sides, four phenolic groups that function as electron acceptors, and two lactones that denote the hydrophilic domain [167], [168]. Ellagic acid is found in the forms of hydrolyzable tannins called ellagitannins in many fruits, in particular raspberries, strawberries, pomegranate, persimmon, grapes, black currants, plums, mango, guava, walnuts, almonds, longan seeds and green tea [82], [83], [84]. The anti-oxidant and anti-proliferative biological activities of ellagic acid have encouraged research towards the potential health benefits of the compound [169], [170]. Ellagic acid can obstruct tumor cell migration, extracellular matrix invasion and angiogenesis, important for infiltrative tumor behavior and metastatic function [171], [172], [173], [174]. Additionally, ellagic acid enhances the tumor susceptibility to radio- and chemo-therapies [175]. Ellagic acid extracted from Momordica charantiawas reported to inhibit fibrosis, oxidative stress, and inflammation in the diabetic rat liver. It performs a favorable function in improving several health conditions [85], [176], [177].
In several studies, small molecule inhibitors have targeted the main protease (Mpro) of SARS-CoV-2 to develop a promising treatment option for the infectious disease COVID-19 [32], [178], [179]. Ellagic acid has shown remarkable binding with the catalytic site of the Mpro enzyme with noteworthy interaction with the prime catalytic site residue Cys145 [180], thereby representing their potential to act as drug candidates for COVID-19 therapeutic. Shaldam et al. [181] studied the binding affinity of 14 selected phenolics and terpenes against the Mpro and RNA-dependent RNA polymerase (RdRp) enzymes of the SARS-CoV-2 virus using molecular docking. They reported that the ellagic acid, quercetin, with two more compounds, interacts most with the SARS-CoV-2 target enzymes. Another molecular docking analysis has shown that ellagic acid, gallic acid, geraniin, kaempferitrin, kaempferol, and quercetin with significant binding affinity for a receptor-binding domain (RBD) and (GRP78) of SARS-CoV-2 [182]. Furthermore, the constituents of Moringa oliefera consisting of ellagic acid were investigated for the interactions against two crucial proteins of SARS-CoV-2 using quantum chemical, molecular docking and dynamic methods. The analysis has shown that the ellagic acid possesses the highest binding affinities of −7.1 kcal mol-1 against nsp9 and −6.9 kcal mol-1 against nsp10 [183].
7. Potential beneficial effects of vanillin as an inhibitor of CoV-2Mpro inhibitor
Vanillin (4-hydroxy-3-methoxybenzaldehyde) is a naturally occurring organic compound, commonly used as a flavoring and aromatic agent in various foods, perfumes, and pharmaceuticals. The vanillin consists of aldehyde, ether, and hydroxyl with a molecular formula of C8H8O3. Primarily extracted from the vanilla bean, it is generally used as an intermediate chemical molecule to produce crucial pharmaceutical formulations and other agents. Vanillin is of interest because of its medicinal uses as an anticlastogenic, antimicrobial agent and its biogenetic relationship to the phenylpropanoid pathway and other molecules of physiological significance, notably salicylate [41], [184]. Following the expression of the HCHL (4-hydroxycinnamoyl-CoA hydratase/lyase) enzyme of the Pseudomonas, efforts have been made to establish a direct capacity for vanillin production into plants by deviation of the phenylpropanoid pathway [86], [87]. The therapeutic potential of vanillin and its main metabolites as an anti-oxidant and antimicrobial for treating inflammatory diseases and their actions on redox status using molecular docking evaluation have been reported [88], [89], [90], [185], [186], [187]. Law et al. [188] subjected vanillin derived 20-compounds together with monolaurin and tetrodotoxin as test sets and evaluated their potential as SARS-CoV-2 inhibitors.
8. Thymol as a spike protein inhibitor
Thymol (2-isopropyl-5-methylphenol) is the phenolic monoterpene in thyme species and the main constituent of thyme essential oils. Other than the medicinal plant thyme (Thymus vulgaris), thymol is also extracted from plants such as Ocimum, Origanum, Monarda genera, and other plants, for instance, the members of Verbenaceae, Scrophulariaceae, Ranunculaceae, and Apiaceae families. Thymol has been used in traditional medicine as an expectorant, and antiseptic agent, primarily as a treatment option for the upper respiratory system, coughs, headaches, and diarrhea. The current search of medicinal plant compounds as a therapeutic option for various human diseases has involved thymol. Recent reports have suggested the multi-functional role of thymol as an anti-viral, anti-bacterial, antibiofilm, antifungal, anti-inflammatory, antileishmanial, and anti-cancer agent. The novel development of nano-capsules comprised of the thymol compounds expanded their medicinal use [93]. Several studies have shown additional biological properties of thymol, in particular anti-oxidant, local anesthetic, anti-carcinogenesis, anti-nociceptive, cicatrizing, antiseptic, as well as a potential as a growth enhancer, and immunomodulator [91], [92], [189], [190].
Kulkarni et al. [191] have docked the major components of several essential oils against the S1 receptor binding domain of the spike glycoprotein. The group has observed several phytochemicals, including thymol, are effective anti-viral agents that inhibit the viral spike protein [191]. An in-silico study was performed by selecting eighteen well-reported anti-viral phytochemicals to find out whether they can prevent SARS-CoV-2 infection. The structure of a host protein, TMPRSS2 (transmembrane protease serine 2), was predicted, which cleaves the spike protein of SARS-CoV-2, thereby aiding the viral internalization. Subsequently, the catalytic domain of TMPRSS2 was docked against the eighteen selected phytochemicals. Following it, the target-inhibitor complex's stability was analyzed using molecular dynamic simulation, which indicated thymol as a better inhibitor due to their stable binding with TMPRSS2, inducing subtle modification in the spatial arrangement of the catalytic triad residues [192].
Using an in silico approach, it was suggested that unique phytocompounds thymol could physically bind SARSCoV-2 spike glycoproteins (6VXX and 6VYB), SARS-CoV-2 B.1.351 South Africa variant of spike glycoprotein (7NXA), and ACE2 to prevent the SARS-CoV-2 binding to the host ACE2, TMPRSS2 and neutrapilin-1 receptors [193]. Qazi et al. [194] recently elucidated the epigenetic mechanism of SARS-CoV-2 and its impact on the environment. Thus, inactivating it from the surfaces when sprayed and are not harmful to the biological environment.
9. Rosmarinic acid-meditated inhibition of CoV-2 entry and replication
Rosmarinic acid (3,4-dihydroxyphenyllactic acid) is a polyphenol molecule commonly found in many culinary herbs, such as rosemary, perilla, sage, mint, and basil. It is found to be slightly soluble in an aqueous medium whereas very well in most organic solvents. This phytoconstituent comprises several compelling biological and pharmacological properties, particularly anti-oxidant, anti-inflammatory, anti-viral, anti-bacterial, hepatoprotective, and anti-nociceptive. The rosmarinic acid functions as a defense molecule in the plants, whereas the rosmarinic acid-containing medicinal plants, herbs and spices have beneficial and health-promoting effects and rheumatic, diuretic, and antiepileptic agents. Additionally, rosmarinic acid has remarkable carcinogenesis progression [195]. The L-phenylalanine and L-tyrosine, the primary amino acids, together with several eight enzymes and other co-factors, are required for rosmarinic acid biosynthesis [94], [95], [96].
Tegen et al. [162] reviewed the ethnobotanical knowledge of medicinal plants used as a treatment option for various viral infections since ancient times. They predicted the use of phyto-compounds as COVID-19 therapeutics. Medicinal plants containing rosmarinic acid, ursolic acid, and some others are a few promising compounds for treating COVID-19. In another report, natural compounds, including rosmarinic acid and a few other compounds, have been displayed the potential to enhance the expression of ACE-2. They could exasperate SARS-CoV-2 infection by degrading the host receptors aiding viral endocytosis [97].
Furthermore, a molecular docking study has analyzed the binding affinities of various phytocompounds with the Nsp15 protein. It represented rosmarinic acid, ursolic acid interacting successfully with Nsp15 viral protein suggesting their potential role in inhibiting the SARS-CoV-2 replication [196]. The RdRp of SARS CoV-2 has been subjected to molecular docking analysis with compounds from Plectranthusamboinicus consisting of rosmarinic acid. The interaction profiling of rosmarinic acid with the target protein SARS-CoV-2 linked RdRp has been reported for further consideration [197].
10. Conclusion and future prospects
In present unfavorable conditions of COVID-19 across the world, there is an urgent need to develop antiviral drugs or therapeutic alternatives. The identification and development of novel drugs are time-consuming, and further validation and clinical trials of such novel drugs/targets are mandatory to check the efficacy and effectiveness. Additionally, the absence of COVID-19 specific treatment and drugs encourages the scientific community across the globe to look for other options to successfully combat the current disease scenario.
In this view, the medically important plants containing specific phytoconstituents could provide a wide scope as therapeutic against COVID-19. The medically critical phytochemicals that naturally possess a wide range of therapeutic beneficial and dynamic resources of chemical constituents show anti-viral characteristics [198], [199]. Numerous docking simulations studies have recommended using these compounds to improve COVID-19 therapy. The phytoconstituents reviewed in this article include thymoquinone, quercetin, caffeic acid, ellagic acid, ursolic acid, thymol, vanillin, rosmarinic acid. These phytoconstituents represent a promising option for treating infections of coronavirus disease by targeting viral protein and inhibiting viral replication or endocytosis. However, the dosage of these compounds at higher concentrations may be toxic beyond a certain level.
It is needless to mention that remarkable in vitro and in vivo studies are required to determine each compound's safe and therapeutic concentration before the clinical trials in humans to be carried out. To develop effective COVID-19 therapy, initial studies involve those molecules that have already been FDA-approved or considered safe for drug use, as in the case of polyphenolic constituents. It is anticipated that the phytoconstituents discussed in this report will aid the development of an effective and safe anti-SARS-CoV-2 treatment option from naturally procured compounds.
Funding
This work is support and funded through the Indian Council of Medical Research (Grant No. BMI/11(39)/2020).
CRediT authorship contribution statement
Sabeeha Ali: Conceptualization, Writing – original draft, Data curation, Investigation, Methodology. Manzar Alam: Conceptualization, Writing – original draft, Methodology. Fatima Khatoon: Writing – original draft. Urooj Fatima: Writing – original draft. Abdelbaset Mohamed Elasbali: Data curation, Investigation, Methodology, Project administration. Mohd Adnan: Data curation, Investigation, Methodology. Asimul Islam: Data curation, Investigation, Methodology. Md. Imtaiyaz Hassan: Conceptualization, Writing – original draft, Investigation, Supervision. Mejdi Snoussi: Data curation, Investigation, Writing – review & editing. Vincenzo De Feo: Conceptualization, Writing – original draft, Investigation, Supervision, Project administration.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Acknowledgments
AME extends his appreciation to the Deanship of Scientific Research at Jouf University for funding his work through Research Grant Number (DSR-2021-01-0365). MA expresses thanks to the Indian Council of Medical Research for financial support (Grant No. 45/6/2020-DDI/BMS). MIH, SA and MA sincerely thank the Department of Science and Technology, Government of India, for the FIST support (FIST program No. SR/FST/LSII/2020/782).
Data Availability
The information that supports the findings of this study is available in this article.
References
- 1.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., Guo G.Y., Du J., Zheng C.L., Zhu Q., Hu M., Li X.Y., Peng P., Shi H.Z. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020;55:5. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asrani P., Eapen M.S., Hassan M.I., Sohal S.S. Implications of the second wave of COVID-19 in India. Lancet Respir. Med. 2021;9(9):e93–e94. doi: 10.1016/S2213-2600(21)00312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asrani P., Hasan G.M., Sohal S.S., Hassan M.I. Molecular basis of pathogenesis of coronaviruses: a comparative genomics approach to planetary health to prevent zoonotic outbreaks in the 21st century. OMICS. 2020;24(11):634–644. doi: 10.1089/omi.2020.0131. [DOI] [PubMed] [Google Scholar]
- 6.Asrani P., Eapen M.S., Hassan M.I., Sohal S.S. Implications of the second wave of COVID-19 in India. Lancet Respir. Med. 2021;9:e93–e94. doi: 10.1016/S2213-2600(21)00312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asrani P., Hassan M.I. SARS-CoV-2 mediated lung inflammatory responses in host: targeting the cytokine storm for therapeutic interventions. Mol. Cell Biochem. 2021;476(2):675–687. doi: 10.1007/s11010-020-03935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kin N., Miszczak F., Lin W., Gouilh M.A., Vabret A., EPICOREM C. Genomic analysis of 15 human coronaviruses OC43 (HCoV-OC43s) circulating in france from 2001 to 2013 reveals a high intra-specific diversity with new recombinant genotypes. Viruses. 2015;7(5):2358–2377. doi: 10.3390/v7052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brian D.A., Baric R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866(10) doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumari P., Singh A., Ngasainao M.R., Shakeel I., Kumar S., Lal S., Singhal A., Sohal S.S., Singh I.K., Hassan M.I. Potential diagnostics and therapeutic approaches in COVID-19. Clin. Chim. Acta. 2020;510:488–497. doi: 10.1016/j.cca.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naqvi A.A.T., Anjum F., Shafie A., Badar S., Elasbali A.M., Yadav D.K., Hassan M.I. Investigating host-virus interaction mechanism and phylogenetic analysis of viral proteins involved in the pathogenesis. PLoS ONE. 2021;16:12. doi: 10.1371/journal.pone.0261497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y., Jedrzejczak R., Maltseva N.I., Wilamowski M., Endres M., Godzik A., Michalska K., Joachimiak A. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. Protein Sci. 2020;29(7):1596–1605. doi: 10.1002/pro.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amoretti M., Amsler C., Bonomi G., Bouchta A., Bowe P., Carraro C., Cesar C.L., Charlton M., Collier M.J.T., Doser M., Filippini V., Fine K.S., Fontana A., Fujiwara M.C., Funakoshi R., Genova P., Hangst J.S., Hayano R.S., Holzscheiter M.H., Jørgensen L.V., Lagomarsino V., Landua R., Lindelöf D., Rizzini E.L., Macrì M., Madsen N., Manuzio G., Marchesotti M., Montagna P., Pruys H., Regenfus C., Riedler P., Rochet J., Rotondi A., Rouleau G., Testera G., Variola A., Watson T.L., van der Werf D.P. production and detection of cold antihydrogen atoms. Nature. 2002;419(6906):456–459. doi: 10.1038/nature01096. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Wang X.J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genom. 2020;47(2):119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AlAjmi M.F., Khan S., Choudhury A., Mohammad T., Noor S., Hussain A., Lu W., Eapen M.S., Chimankar V., Hansbro P.M., Sohal S.S., Elasbali A.M., Hassan M.I. Impact of deleterious mutations on structure, function and stability of serum/glucocorticoid regulated kinase 1: a gene to diseases correlation. Front. Mol. Biosci. 2021;8:1073. doi: 10.3389/fmolb.2021.780284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain M., Jabeen N., Raza F., Shabbir S., Baig A.A., Amanullah A., Aziz B. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J. Med. Virol. 2020;92(9):1580–1586. doi: 10.1002/jmv.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adem Ş., Eyupoglu V., Sarfraz I., Rasul A., Zahoor A.F., Ali M., Abdalla M., Ibrahim I.M., Elfiky A.A. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine. 2020;85 doi: 10.1016/j.phymed.2020.153310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asrani P., Tiwari K., Eapen M.S., McAlinden K.D., Haug G., Johansen M.D., Hansbro P.M., Flanagan K.L., Hassan M.I., Sohal S.S. Clinical features and mechanistic insights into drug repurposing for combating COVID-19. Int. J. Biochem. Cell Biol. 2022;142(106114):5. doi: 10.1016/j.biocel.2021.106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asrani P., Eapen M.S., Chia C., Haug G., Weber H.C., Hassan M.I., Sohal S.S. Diagnostic approaches in COVID-19: clinical updates. Expert Rev. Respir. Med. 2021;15(2):197–212. doi: 10.1080/17476348.2021.1823833. [DOI] [PubMed] [Google Scholar]
- 25.Asrani P., Hussain A., Nasreen K., AlAjmi M.F., Amir S., Sohal S.S., Hassan M.I. Guidelines and safety considerations in the laboratory diagnosis of sars-cov-2 infection: a prerequisite study for health professionals. Risk Manag. Healthc. Policy. 2021;14:379–389. doi: 10.2147/RMHP.S284473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulyar M.F., Li R., Mehmood K., Waqas M., Li K., Li J. Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: a hope to decelerate the COVID-19 pandemic. Phytomedicine. 2020;85 doi: 10.1016/j.phymed.2020.153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abd El-Aziz T.M., Stockand J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infect. Genet. Evol. 2020;83 doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamsi A., Mohammad T., Anwar S., AlAjmi M.F., Hussain A., Rehman M.T., Islam A., Hassan M.I. Glecaprevir and Maraviroc are high-affinity inhibitors of SARS-CoV-2 main protease: possible implication in COVID-19 therapy. Biosci. Rep. 2020;40(6) doi: 10.1042/BSR20201256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotula J.J., Balakumar N., Khan D., Patel B. COVID-19 and therapeutic drugs repurposing in hand: the need for collaborative efforts. Le Pharm. Hosp. Clin. 2021;56(1):3–11. [Google Scholar]
- 30.Das A., Pandita D., Jain G.K., Agarwal P., Grewal A.S., Khar R.K., Lather V. role of phytoconstituents in the management of COVID-19. Chem. Biol. Interact. 2021;341 doi: 10.1016/j.cbi.2021.109449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumari P., Singh A., Ngasainao M.R., Shakeel I., Kumar S., Lal S., Singhal A., Sohal S., Singh I.K., Hassan M. Potential diagnostics and therapeutic approaches in COVID-19. Clin. Chim. Acta. 2020;510:488–497. doi: 10.1016/j.cca.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammad T., Shamsi A., Anwar S., Umair M., Hussain A., Rehman M.T., AlAjmi M.F., Islam A., Hassan M.I. Identification of high-affinity inhibitors of SARS-CoV-2 main protease: towards the development of effective COVID-19 therapy. Virus Res. 2020;288:288. doi: 10.1016/j.virusres.2020.198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jairajpuri D.S., Hussain A., Nasreen K., Mohammad T., Anjum F., Tabish Rehman M., Mustafa Hasan G., Alajmi M.F., Imtaiyaz Hassan M. Identification of natural compounds as potent inhibitors of SARS-CoV-2 main protease using combined docking and molecular dynamics simulations. Saudi J. Biol. Sci. 2021;28(4):2423–2431. doi: 10.1016/j.sjbs.2021.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dos Santos C.N., Menezes R., Stewart D. Polyphenols as new leads in drug discovery: biological activity and mechanisms. Curr. Pharm. Des. 2018;24(19):2041–2042. doi: 10.2174/138161282419180924094610. [DOI] [PubMed] [Google Scholar]
- 35.Ali S., Alam M., Hasan G.M., Hassan M.I. Potential therapeutic targets of Klebsiella pneumoniae: a multi-omics review perspective. Brief. Funct. Genom. 2021 doi: 10.1093/bfgp/elab038. [DOI] [PubMed] [Google Scholar]
- 36.Alam M., Ali S., Ashraf G.M., Bilgrami A.L., Yadav D.K., Hassan M.I. Epigallocatechin 3-gallate: from green tea to cancer therapeutics. Food Chem. 2022;379 doi: 10.1016/j.foodchem.2022.132135. [DOI] [PubMed] [Google Scholar]
- 37.Abian O., Ortega-Alarcon D., Jimenez-Alesanco A., Ceballos-Laita L., Vega S., Reyburn H.T., Rizzuti B., Velazquez-Campoy A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020;164:1693–1703. doi: 10.1016/j.ijbiomac.2020.07.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan P., Rahman S., Queen A., Manzoor S., Naz F., Hasan G.M., Luqman S., Kim J., Islam A., Ahmad F., Hassan M.I. Elucidation of dietary polyphenolics as potential inhibitor of microtubule affinity regulating kinase 4: in silico and in vitro studies. Sci. Rep. 2017;7(1):1–15. doi: 10.1038/s41598-017-09941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammad T., Khan F.I., Lobb K.A., Islam A., Ahmad F., Hassan M.I. Identification and evaluation of bioactive natural products as potential inhibitors of human microtubule affinity-regulating kinase 4 (MARK4) J. Biomol. Struct. Dyn. 2019;37(7):1813–1829. doi: 10.1080/07391102.2018.1468282. [DOI] [PubMed] [Google Scholar]
- 40.Naz H., Khan P., Tarique M., Rahman S., Meena A., Ahamad S., Luqman S., Islam A., Ahmad F., Hassan M.I. Binding studies and biological evaluation of β-carotene as a potential inhibitor of human calcium/calmodulin-dependent protein kinase IV. Int. J. Biol. Macromol. 2017;96:161–170. doi: 10.1016/j.ijbiomac.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 41.Naz H., Tarique M., Khan P., Luqman S., Ahamad S., Islam A., Ahmad F., Hassan M.I. Evidence of vanillin binding to CAMKIV explains the anti-cancer mechanism in human hepatic carcinoma and neuroblastoma cells. Mol. Cell. Biochem. 2018;438(1–2):35–45. doi: 10.1007/s11010-017-3111-0. [DOI] [PubMed] [Google Scholar]
- 42.Qayyum S., Mohammad T., Slominski R.M., Hassan M.I., Tuckey R.C., Raman C., Slominski A.T. Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes. Am. J. Physiol. Endocrinol. Metab. 2021;321(2):E246–E251. doi: 10.1152/ajpendo.00174.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 44.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81(Suppl. 1):S230–S242. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 45.Vujic I., Moore L.B., LeVeen H.H. Recanalization of occluded superior vena cava for replacement of LeVeen shunt catheter. Radiology. 1987;164(1):270–272. doi: 10.1148/radiology.164.1.2954184. [DOI] [PubMed] [Google Scholar]
- 46.Kempuraj D., Castellani M.L., Petrarca C., Frydas S., Conti P., Theoharides T.C., Vecchiet J. Inhibitory effect of quercetin on tryptase and interleukin-6 release, and histidine decarboxylase mRNA transcription by human mast cell-1 cell line. Clin. Exp. Med. 2006;6(4):150–156. doi: 10.1007/s10238-006-0114-7. [DOI] [PubMed] [Google Scholar]
- 47.Mink P.J., Scrafford C.G., Barraj L.M., Harnack L., Hong C.P., Nettleton J.A., Jacobs DR Jr. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007;85(3):895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 48.Nair M.P., Mahajan S., Reynolds J.L., Aalinkeel R., Nair H., Schwartz S.A., Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin. Vaccin. Immunol. 2006;13(3):319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nieman D.C., Henson D.A., Gross S.J., Jenkins D.P., Davis J.M., Murphy E.A., Carmichael M.D., Dumke C.L., Utter A.C., McAnulty S.R., McAnulty L.S., Mayer E.P. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007;39(9):1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- 50.Jasso-Miranda C., Herrera-Camacho I., Flores-Mendoza L.K., Dominguez F., Vallejo-Ruiz V., Sanchez-Burgos G.G., Pando-Robles V., Santos-Lopez G., Reyes-Leyva J. Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infect. Drug Resist. 2019;12:1833–1852. doi: 10.2147/IDR.S210890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.S. Lalani, C.L. Poh, Flavonoids as antiviral agents for enterovirus A71 (EV-A71), Viruses, 12(2). [DOI] [PMC free article] [PubMed]
- 52.Vázquez-Calvo Á., Jiménez de Oya N., Martín-Acebes M.A., Garcia-Moruno E., Saiz J.C. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017;8:1314. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirzaie A., Halaji M., Dehkordi F.S., Ranjbar R., Noorbazargan H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19) Complement. Ther. Clin. Pract. 2020;40 doi: 10.1016/j.ctcp.2020.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganjhu R.K., Mudgal P.P., Maity H., Dowarha D., Devadiga S., Nag S., Arunkumar G. Herbal plants and plant preparations as remedial approach for viral diseases. Virusdisease. 2015;26(4):225–236. doi: 10.1007/s13337-015-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020;25(4):668–688. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Islam M.N., Hossain K.S., Sarker P.P., Ferdous J., Hannan M.A., Rahman M.M., Chu D.T., Uddin M.J. Revisiting pharmacological potentials of Nigella sativa seed: a promising option for COVID-19 prevention and cure. Phytother. Res. 2020;35(3):1329–1344. doi: 10.1002/ptr.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahman M.T. Potential benefits of combination of Nigella sativa and Zn supplements to treat COVID-19. J. Herb. Med. 2020;23 doi: 10.1016/j.hermed.2020.100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmad A., Rehman M.U., Ahmad P., Alkharfy K.M. Covid-19 and thymoquinone: connecting the dots. Phytother. Res. 2020;34(11):2786–2789. doi: 10.1002/ptr.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badary O.A., Hamza M.S., Tikamdas R. Thymoquinone: a promising natural compound with potential benefits for COVID-19 prevention and cure. Drug Des. Dev. Ther. 2021;15:1819–1833. doi: 10.2147/DDDT.S308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan M.A., Younus H. Potential implications of black seed and its principal constituent thymoquinone in the treatment of COVID-19 patients. Curr. Pharm. Biotechnol. 2020;22(10):1315–1324. doi: 10.2174/1389201021999201110205048. [DOI] [PubMed] [Google Scholar]
- 61.Elfiky A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2020;39(9):3194–3203. doi: 10.1080/07391102.2020.1761881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Derosa G., Maffioli P., D’Angelo A., Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19) Phytother. Res. 2020;35(3):1230–1236. doi: 10.1002/ptr.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front. Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diniz L.R.L., Souza M., Duarte A., Sousa D.P. Mechanistic aspects and therapeutic potential of quercetin against COVID-19-associated acute kidney injury. Molecules. 2020;25:23. doi: 10.3390/molecules25235772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bastaminejad S., Bakhtiyari S. Quercetin and its relative therapeutic potential against COVID-19: a retrospective review and prospective overview. Curr. Mol. Med. 2020;21(5):385–391. doi: 10.2174/1566524020999200918150630. [DOI] [PubMed] [Google Scholar]
- 66.Aucoin M., Cooley K., Saunders P.R., Cardozo V., Remy D., Cramer H., Neyre Abad C., Hannan N. The effect of quercetin on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: a rapid review. Adv. Integr. Med. 2020;7(4):247–251. doi: 10.1016/j.aimed.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williamson G., Kerimi A. Testing of natural products in clinical trials targeting the SARS-CoV-2 (Covid-19) viral spike protein-angiotensin converting enzyme-2 (ACE2) interaction. Biochem. Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grochowicz U., Kułakowski P., Jurgiel R., Budaj A., Dłuzniewski M. Susceptibility to the development of supraventricular arrhythmia in patients with mitral valve prolapse syndrome. Pol. Tyg. Lek. 1988;43(41):1323–1324. [PubMed] [Google Scholar]
- 69.Khan F.A., Maalik A., Murtaza G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2017;24(4):695–702. doi: 10.1016/j.jfda.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Seedi H.R., El-Said A.M., Khalifa S.A., Göransson U., Bohlin L., Borg-Karlson A.K., Verpoorte R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012;60(44):10877–10895. doi: 10.1021/jf301807g. [DOI] [PubMed] [Google Scholar]
- 71.Del Rio D., Rodriguez-Mateos A., Spencer J.P., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2012;18(14):1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sova M., Saso L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients. 2020;12:8. doi: 10.3390/nu12082190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Utsunomiya H., Ichinose M., Ikeda K., Uozaki M., Morishita J., Kuwahara T., Koyama A.H., Yamasaki H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014;34(4):1020–1024. doi: 10.3892/ijmm.2014.1859. [DOI] [PubMed] [Google Scholar]
- 74.Langland J., Jacobs B., Wagner C.E., Ruiz G., Cahill T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antivir. Res. 2018;160:143–150. doi: 10.1016/j.antiviral.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 75.Ogawa M., Shirasago Y., Ando S., Shimojima M., Saijo M., Fukasawa M. Caffeic acid, a coffee-related organic acid, inhibits infection by severe fever with thrombocytopenia syndrome virus in vitro. J. Infect. Chemother. 2018;24(8):597–601. doi: 10.1016/j.jiac.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Pironi A.M., de Araújo P.R., Fernandes M.A., Salgado H., Chorilli M. Characteristics, biological properties and analytical methods of ursolic acid: a review. Crit. Rev. Anal. Chem. 2017;48(1):86–93. doi: 10.1080/10408347.2017.1390425. [DOI] [PubMed] [Google Scholar]
- 77.Shanmugam M.K., Dai X., Kumar A.P., Tan B.K.H., Sethi G., Bishayee A. Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013;85(11):1579–1587. doi: 10.1016/j.bcp.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 78.López-Hortas L., Pérez-Larrán P., González-Muñoz M.J., Falqué E., Domínguez H. Recent developments on the extraction and application of ursolic acid. A review. Food Res. Int. 2018;103:130–149. doi: 10.1016/j.foodres.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 79.Mlala S., Oyedeji A.O., Gondwe M., Oyedeji O.O. Ursolic acid and its derivatives as bioactive agents. Molecules. 2019;24:15. doi: 10.3390/molecules24152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Son J., Lee S.Y. Therapeutic potential of ursonic acid: comparison with ursolic acid. Biomolecules. 2020;10:11. doi: 10.3390/biom10111505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evtyugin D.D., Magina S., Evtuguin D.V. Recent advances in the production and applications of ellagic acid and its derivatives. A review. Molecules. 2020;25:12. doi: 10.3390/molecules25122745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Türk G., Sönmez M., Çeribaşı A.O., Yüce A., Ateşşahin A. Attenuation of cyclosporine A-induced testicular and spermatozoal damages associated with oxidative stress by ellagic acid. Int. Immunopharmacol. 2009;10(2):177–182. doi: 10.1016/j.intimp.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Rangkadilok N., Worasuttayangkurn L., Bennett R.N., Satayavivad J. Identification and quantification of polyphenolic compounds in Longan (Euphoria longana Lam.) fruit. J. Agric. Food Chem. 2005;53(5):1387–1392. doi: 10.1021/jf0403484. [DOI] [PubMed] [Google Scholar]
- 84.Plundrich N., Grace M.H., Raskin I., Ann Lila M. Bioactive polyphenols from muscadine grape and blackcurrant stably concentrated onto protein-rich matrices for topical applications. Int. J. Cosmet. Sci. 2013;35(4):394–401. doi: 10.1111/ics.12057. [DOI] [PubMed] [Google Scholar]
- 85.Ríos J.L., Giner R.M., Marín M., Recio M.C. A pharmacological update of ellagic acid. Planta Med. 2018;84(15):1068–1093. doi: 10.1055/a-0633-9492. [DOI] [PubMed] [Google Scholar]
- 86.Walton N.J., Mayer M.J., Narbad A. Vanillin. Phytochemistry. 2003;63(5):505–515. doi: 10.1016/s0031-9422(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 87.Banerjee G., Chattopadhyay P. Vanillin biotechnology: the perspectives and future. J. Sci. Food Agric. 2018;99(2):499–506. doi: 10.1002/jsfa.9303. [DOI] [PubMed] [Google Scholar]
- 88.Bezerra-Filho C.S.M., Barboza J.N., Souza M., Sabry P., Ismail N., de Sousa D.P. Therapeutic potential of vanillin and its main metabolites to regulate the inflammatory response and oxidative stress. Mini Rev. Med. Chem. 2019;19(20):1681–1693. doi: 10.2174/1389557519666190312164355. [DOI] [PubMed] [Google Scholar]
- 89.Tai A., Sawano T., Yazama F. Anti-oxidant properties of ethyl vanillin in vitro and in vivo. Biosci. Biotechnol. Biochem. 2011;75(12):2346–2350. doi: 10.1271/bbb.110524. [DOI] [PubMed] [Google Scholar]
- 90.Yadav R., Saini D., Yadav D. Synthesis and evaluation of vanillin derivatives as antimicrobial agents. Turk. J. Pharm. Sci. 2018;15(1):57–62. doi: 10.4274/tjps.97752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marchese A., Orhan I.E., Daglia M., Barbieri R., Di Lorenzo A., Nabavi S.F., Gortzi O., Izadi M., Nabavi S.M. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem. 2016;210:402–414. doi: 10.1016/j.foodchem.2016.04.111. [DOI] [PubMed] [Google Scholar]
- 92.Salehi B., Mishra A.P., Shukla I., Sharifi-Rad M., Contreras M., Segura-Carretero A., Fathi H., Nasrabadi N.N., Kobarfard F., Sharifi-Rad J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018;32(9):1688–1706. doi: 10.1002/ptr.6109. [DOI] [PubMed] [Google Scholar]
- 93.Kowalczyk A., Przychodna M., Sopata S., Bodalska A., Fecka I. Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules. 2020;25:18. doi: 10.3390/molecules25184125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petersen M., Simmonds M.S. Rosmarinic acid. Phytochemistry. 2003;62(2):121–125. doi: 10.1016/s0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 95.Colica C., Di Renzo L., Aiello V., De Lorenzo A., Abenavoli L. Rosmarinic acid as potential anti-inflammatory agent. Rev. Recent Clin. Trials. 2018;13(4):240–242. doi: 10.2174/157488711304180911095818. [DOI] [PubMed] [Google Scholar]
- 96.Hitl M., Kladar N., Gavarić N., Božin B. Rosmarinic acid-human pharmacokinetics and health benefits. Planta Med. 2020;87(4):273–282. doi: 10.1055/a-1301-8648. [DOI] [PubMed] [Google Scholar]
- 97.Junior A.G., Tolouei S., Dos Reis Lívero F.A., Gasparotto F., Boeing T., de Souza P. Natural agents modulating ACE-2: a review of compounds with potential against SARS-CoV-2 infections. Curr. Pharm. Des. 2021;27(13):1588–1596. doi: 10.2174/1381612827666210114150607. [DOI] [PubMed] [Google Scholar]
- 98.Banerjee S., Kaseb A.O., Wang Z., Kong D., Mohammad M., Padhye S., Sarkar F.H., Mohammad R.M. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69(13):5575–5583. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- 99.Chaieb K., Kouidhi B., Jrah H., Mahdouani K., Bakhrouf A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011;11:29. doi: 10.1186/1472-6882-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmad A., Mishra R.K., Vyawahare A., Kumar A., Rehman M.U., Qamar W., Khan A.Q., Khan R. Thymoquinone (2-Isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 2019;27(8):1113–1126. doi: 10.1016/j.jsps.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khader M., Eckl P.M. Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci. 2015;17(12):950–957. [PMC free article] [PubMed] [Google Scholar]
- 102.El Gazzar M., El Mezayen R., Marecki J.C., Nicolls M.R., Canastar A., Dreskin S.C. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int. Immunopharmacol. 2006;6(7):1135–1142. doi: 10.1016/j.intimp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 103.Rana R., Rathi V., Chauhan K., Jain K., Chhabra S.S., Acharya R., Kalra S.K., Gupta A., Jain S., Ganguly N.K., Yadav D.K. Exploring the role of epidermal growth factor receptor variant III in meningeal tumors. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0255133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Darakhshan S., Bidmeshki Pour A., Hosseinzadeh Colagar A., Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharm. Res. 2015;95–96:138–158. doi: 10.1016/j.phrs.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 105.Salem M.L., Hossain M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int. J. Immunopharmacol. 2000;22(9):729–740. doi: 10.1016/s0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 106.Ulasli M., Gurses S.A., Bayraktar R., Yumrutas O., Oztuzcu S., Igci M., Igci Y.Z., Cakmak E.A., Arslan A. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol. Biol. Rep. 2014;41(3):1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galley H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011;107(1):57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 108.Ichinose F., Buys E.S., Neilan T.G., Furutani E.M., Morgan J.G., Jassal D.S., Graveline A.R., Searles R.J., Lim C.C., Kaneki M., Picard M.H., Scherrer-Crosbie M., Janssens S., Liao R., Bloch K.D. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ. Res. 2007;100(1):130–139. doi: 10.1161/01.RES.0000253888.09574.7a. [DOI] [PubMed] [Google Scholar]
- 109.Ammar E.S.M., Gameil N.M., Shawky N.M., Nader M.A. Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. Int. Immunopharmacol. 2011;11(12):2232–2236. doi: 10.1016/j.intimp.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 110.Xu H., Liu B., Xiao Z., Zhou M., Ge L., Jia F., Liu Y., Jin H., Zhu X., Gao J., Akhtar J., Xiang B., Tan K., Wang G. Computational and experimental studies reveal that thymoquinone blocks the entry of coronaviruses into in vitro cells. Infect. Dis. Ther. 2021;10(1):483–494. doi: 10.1007/s40121-021-00400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Henson D., Nieman D., Davis J.M., Dumke C., Gross S., Murphy A., Carmichael M., Jenkins D.P., Quindry J., McAnulty S., McAnulty L., Utter A., Mayer E. Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int. J. Sports Med. 2008;29(10):856–863. doi: 10.1055/s-2007-989424. [DOI] [PubMed] [Google Scholar]
- 112.Alam M., Hasan G.M., Hassan M.I. A review on the role of TANK-binding kinase 1 signaling in cancer. Int. J. Biol. Macromol. 2021;183:2364–2375. doi: 10.1016/j.ijbiomac.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 113.Heinz S.A., Henson D.A., Austin M.D., Jin F., Nieman D.C. Quercetin supplementation and upper respiratory tract infection: a randomized community clinical trial. Pharmacol. Res. 2010;62(3):237–242. doi: 10.1016/j.phrs.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dahiya R., Mohammad T., Roy S., Anwar S., Gupta P., Haque A., Khan P., Kazim S.N., Islam A., Ahmad F., Hassan M.I. Investigation of inhibitory potential of quercetin to the pyruvate dehydrogenase kinase 3: towards implications in anticancer therapy. Int. J. Biol. Macromol. 2019;136:1076–1085. doi: 10.1016/j.ijbiomac.2019.06.158. [DOI] [PubMed] [Google Scholar]
- 115.Yousuf M., Khan P., Shamsi A., Shahbaaz M., Hasan G.M., Haque Q., Christoffels A., Islam A., Hassan M.I. Inhibiting CDK6 activity by quercetin is an attractive strategy for cancer therapy. ACS Omega. 2020;5(42):27480–27491. doi: 10.1021/acsomega.0c03975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu W., Li R., Li X., He J., Jiang S., Liu S., Yang J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses. 2015;8(1) doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shinozuka K., Kikuchi Y., Nishino C., Mori A., Tawata S. Inhibitory effect of flavonoids on DNA-dependent DNA and RNA polymerases. Experientia. 1988;44(10):882–885. doi: 10.1007/BF01941188. [DOI] [PubMed] [Google Scholar]
- 118.Bachmetov L., Gal-Tanamy M., Shapira A., Vorobeychik M., Giterman-Galam T., Sathiyamoorthy P., Golan-Goldhirsh A., Benhar I., Tur-Kaspa R., Zemel R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012;19(2):e81–e88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 119.Spedding G., Ratty A., Middleton E., Jr. Inhibition of reverse transcriptases by flavonoids. Antivir. Res. 1989;12(2):99–110. doi: 10.1016/0166-3542(89)90073-9. [DOI] [PubMed] [Google Scholar]
- 120.Cushnie T.P., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Refaat H., Mady F.M., Sarhan H.A., Rateb H.S., Alaaeldin E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int. J. Pharm. 2020;592 doi: 10.1016/j.ijpharm.2020.120028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deepak Singh D., Han I., Choi E.H., Yadav D.K. CRISPR/Cas9 based genome editing for targeted transcriptional control in triple-negative breast cancer. Comput. Struct. Biotechnol. J. 2021;19:2384–2397. doi: 10.1016/j.csbj.2021.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singh D.D., Verma R., Parimoo P., Sahu A., Kumar V., Upadhyay E., Yadav D.K. Potential therapeutic relevance of CRISPR/Cas9 guided epigenetic regulations for neuropsychiatric disorders. Curr. Top. Med. Chem. 2021;21(10):878–894. doi: 10.2174/1568026621666210317154502. [DOI] [PubMed] [Google Scholar]
- 124.Kumar S., Rana R., Yadav D.K. Atomic-scale modeling of the effect of lipid peroxidation on the permeability of reactive species. J. Biomol. Struct. Dyn. 2020;39(4):1284–1294. doi: 10.1080/07391102.2020.1730971. [DOI] [PubMed] [Google Scholar]
- 125.De Palma A.M., Vliegen I., De Clercq E., Neyts J. Selective inhibitors of picornavirus replication. Med. Res. Rev. 2008;28(6):823–884. doi: 10.1002/med.20125. [DOI] [PubMed] [Google Scholar]
- 126.Evers D.L., Chao C.F., Wang X., Zhang Z., Huong S.M., Huang E.S. Human cytomegalovirus-inhibitory flavonoids: studies on antiviral activity and mechanism of action. Antivir. Res. 2005;68(3):124–134. doi: 10.1016/j.antiviral.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zandi K., Teoh B.T., Sam S.S., Wong P.F., Mustafa M.R., Abubakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011;8:560. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78(20):11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front. Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Son S., Lewis B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J. Agric. Food Chem. 2002;50(3):468–472. doi: 10.1021/jf010830b. [DOI] [PubMed] [Google Scholar]
- 131.Kudugunti S.K., Vad N.M., Whiteside A.J., Naik B.U., Yusuf M.A., Srivenugopal K.S., Moridani M.Y. Biochemical mechanism of caffeic acid phenylethyl ester (CAPE) selective toxicity towards melanoma cell lines. Chem. Biol. Interact. 2010;188(1):1–14. doi: 10.1016/j.cbi.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paracatu L.C., Faria C.M., Quinello C., Rennó C., Palmeira P., Zeraik M.L., da Fonseca L.M., Ximenes V.F. Caffeic Acid phenethyl ester: consequences of its hydrophobicity in the oxidative functions and cytokine release by leukocytes. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/793629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Silva T., Oliveira C., Borges F. Caffeic acid derivatives, analogs and applications: a patent review (2009-2013) Expert Opin. Ther. Pat. 2014;24(11):1257–1270. doi: 10.1517/13543776.2014.959492. [DOI] [PubMed] [Google Scholar]
- 134.Sud’ina G.F., Mirzoeva O.K., Pushkareva M.A., Korshunova G.A., Sumbatyan N.V., Varfolomeev S.D. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with anti-oxidant properties. FEBS Lett. 1993;329(1–2):21–24. doi: 10.1016/0014-5793(93)80184-v. [DOI] [PubMed] [Google Scholar]
- 135.Russo A., Longo R., Vanella A. Anti-oxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73 Suppl. 1:S21–S29. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 136.Jung W.K., Choi I., Lee D.Y., Yea S.S., Choi Y.H., Kim M.M., Park S.G., Seo S.K., Lee S.W., Lee C.M., Park Y.M., Choi I.W. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-kappaB pathways. Int. J. Biochem. Cell Biol. 2008;40(11):2572–2582. doi: 10.1016/j.biocel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 137.Doiron J.A., Leblanc L.M., Hébert M.J., Levesque N.A., Paré A.F., Jean-François J., Cormier M., Surette M.E., Touaibia M. Structure-activity relationship of caffeic acid phenethyl ester analogs as new 5-lipoxygenase inhibitors. Chem. Biol. Drug Des. 2016;89(4):514–528. doi: 10.1111/cbdd.12874. [DOI] [PubMed] [Google Scholar]
- 138.Natarajan K., Singh S., Burke TR Jr, Grunberger D., Aggarwal B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA. 1996;93(17):9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ali S., Ehtram A., Arora N., Manjunath P., Roy D., Ehtesham N.Z., Hasnain S.E. The M. tuberculosis Rv1523 methyltransferase promotes drug resistance through methylation-mediated cell wall remodeling and modulates macrophages immune responses. Front. Cell Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.622487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arasoglu T., Derman S., Mansuroglu B. Comparative evaluation of antibacterial activity of caffeic acid phenethyl ester and PLGA nanoparticle formulation by different methods. Nanotechnology. 2015;27(2) doi: 10.1088/0957-4484/27/2/025103. [DOI] [PubMed] [Google Scholar]
- 141.Erdemli H.K., Akyol S., Armutcu F., Akyol O. Antiviral properties of caffeic acid phenethyl ester and its potential application. J. Intercult. Ethnopharmacol. 2015;4(4):344–347. doi: 10.5455/jice.20151012013034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pittalà V., Salerno L., Romeo G., Acquaviva R., Di Giacomo C., Sorrenti V. Therapeutic potential of caffeic acid phenethyl ester (CAPE) in diabetes. Curr. Med. Chem. 2016;25(37):4827–4836. doi: 10.2174/0929867324666161118120908. [DOI] [PubMed] [Google Scholar]
- 143.Silva H., Lopes N.M.F. Cardiovascular effects of caffeic acid and its derivatives: a comprehensive review. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.595516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rana R., Joon S., Chauhan K., Rathi V., Ganguly N.K., Kumari C., Yadav D.K. Role of extracellular vesicles in glioma progression: deciphering cellular biological processes to clinical applications. Curr. Top. Med. Chem. 2021;21(8):696–704. doi: 10.2174/1568026620666201207100139. [DOI] [PubMed] [Google Scholar]
- 145.H. Utsunomiya, et al., Inhibition by caffeic acid of the influenza A virus multiplication in vitro, Int. J. Mol. Med., 34(4), pp. 1020–1024. [DOI] [PubMed]
- 146.Ikeda K., Tsujimoto K., Uozaki M., Nishide M., Suzuki Y., Koyama A.H., Yamasaki H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011;28(4):595–598. doi: 10.3892/ijmm.2011.739. [DOI] [PubMed] [Google Scholar]
- 147.Meyer J.J., Afolayan A.J., Taylor M.B., Erasmus D. Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens. J. Ethnopharmacol. 1997;56(2):165–169. doi: 10.1016/s0378-8741(97)01514-6. [DOI] [PubMed] [Google Scholar]
- 148.Weng J.R., Lin C.S., Lai H.C., Lin Y.P., Wang C.Y., Tsai Y.C., Wu K.C., Huang S.H., Lin C.W. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 2019;273 doi: 10.1016/j.virusres.2019.197767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang G.F., Shi L.P., Ren Y.D., Liu Q.F., Liu H.F., Zhang R.J., Li Z., Zhu F.H., He P.L., Tang W., Tao P.Z., Li C., Zhao W.M., Zuo J.P. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009;83(2):186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 150.Al-Hatamleh M.A.I., Hatmal M.M., Sattar K., Ahmad S., Mustafa M.Z., Bittencourt M.D.C., Mohamud R. Antiviral and immunomodulatory effects of phytochemicals from honey against COVID-19: potential mechanisms of action and future directions. Molecules. 2020;25:21. doi: 10.3390/molecules25215017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bhowmik D., Nandi R., Jagadeesan R., Kumar N., Prakash A., Kumar D. Identification of potential inhibitors against SARS-CoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and pharmacokinetics approaches. Infect. Genet. Evol. 2020;84 doi: 10.1016/j.meegid.2020.104451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kumar V., Dhanjal J.K., Kaul S.C., Wadhwa R., Sundar D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (M(pro)) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020;39(11):3842–3854. doi: 10.1080/07391102.2020.1772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kashyap D., Tuli H.S., Sharma A.K. Ursolic acid (UA): a metabolite with promising therapeutic potential. Life Sci. 2016;146:201–213. doi: 10.1016/j.lfs.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 154.Hussain H., Green I.R., Ali I., Khan I.A., Ali Z., Al-Sadi A.M., Ahmed I. Ursolic acid derivatives for pharmaceutical use: a patent review (2012-2016) Expert Opin. Ther. Pat. 2017;27(9):1061–1072. doi: 10.1080/13543776.2017.1344219. [DOI] [PubMed] [Google Scholar]
- 155.Seo D.Y., Lee S.R., Heo J.W., No M.H., Rhee B.D., Ko K.S., Kwak H.B., Han J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018;22(3):235–248. doi: 10.4196/kjpp.2018.22.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Singh D.D., Verma R., Tripathi S.K., Sahu R., Trivedi P., Yadav D.K. Breast cancer transcriptional regulatory network reprogramming by using the CRISPR/Cas9 system: an oncogenetics perspective. Curr. Top. Med. Chem. 2021;21:2800–2813. doi: 10.2174/1568026621666210902120754. [DOI] [PubMed] [Google Scholar]
- 157.Dwivedi G.R., Rai R., Pratap R., Singh K., Pati S., Sahu S.N., Kant R., Darokar M.P., Yadav D.K. Drug resistance reversal potential of multifunctional thieno[3,2-c]pyran via potentiation of antibiotics in MDR P. aeruginosa. Biomed. Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.112084. [DOI] [PubMed] [Google Scholar]
- 158.Alam M., Ali S., Mohammad T., Hasan G.M., Yadav D.K., Hassan M.I. B Cell lymphoma 2: a potential therapeutic target for cancer therapy. Int. J. Mol. Sci. 2021;22(19):10442. doi: 10.3390/ijms221910442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alam M., Ali S., Ahmed S., Elasbali A.M., Adnan M., Islam A., Hassan M.I., Yadav D.K. Therapeutic potential of ursolic acid in cancer and diabetic neuropathy diseases. Int. J. Mol. Sci. 2021;22(22):12162. doi: 10.3390/ijms222212162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mishra P., Sohrab S., Mishra S.K. A review on the phytochemical and pharmacological properties of Hyptis suaveolens (L.) Poit. Future J. Pharm. Sci. 2021;7(1):65. doi: 10.1186/s43094-021-00219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kumar A., Choudhir G., Shukla S.K., Sharma M., Tyagi P., Bhushan A., Rathore M. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J. Biomol. Struct. Dyn. 2020;39(10):3760–3770. doi: 10.1080/07391102.2020.1772112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tegen D., Dessie K., Damtie D. Candidate anti-COVID-19 medicinal plants from ethiopia: a review of plants traditionally used to treat viral diseases. Evid. Based Complement. Altern. Med. 2021;2021 doi: 10.1155/2021/6622410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.P. Halder, et al., Evaluation of potency of the selected bioactive molecules from Indian medicinal plants with M(Pro) of SARS-CoV-2 through in silico analysis, J. Ayurveda Integr. Med., 2021. [DOI] [PMC free article] [PubMed]
- 164.Mitra D., Verma D., Mahakur B., Kamboj A., Srivastava R., Gupta S., Pandey A., Arora B., Pant K., Panneerselvam P., Ghosh A., Barik D.P., Mohapatra P. Molecular docking and simulation studies of natural compounds of Vitex negundo L. against papain-like protease (PL(pro)) of SARS CoV-2 (coronavirus) to conquer the pandemic situation in the world. J. Biomol. Struct. Dyn. 2021:1–22. doi: 10.1080/07391102.2021.1873185. [DOI] [PubMed] [Google Scholar]
- 165.Malini P., Kanchana G., Rajadurai M. Antibiabetic efficacy of ellagic acid in streptozotocin-induced diabetes mellitus in albino wistar rats. Asian J. Pharm. Clin. Res. 2011;4(3):124–128. [Google Scholar]
- 166.Saeed M., Naveed M., BiBi J., Kamboh A.A., Arain M.A., Shah Q.A., Alagawany M., El-Hack M., Abdel-Latif M.A., Yatoo M.I., Tiwari R., Chakraborty S., Dhama K. The promising pharmacological effects and therapeutic/medicinal applications of Punica granatum L. (Pomegranate) as a functional food in humans and animals. Recent Pat. Inflamm. Allergy Drug Discov. 2018;12(1):24–38. doi: 10.2174/1872213X12666180221154713. [DOI] [PubMed] [Google Scholar]
- 167.Al-Shar’i N.A., Al-Balas Q.A., Hassan M.A., El-Elimat T.M., Aljabal G.A., Almaaytah A.M. Ellagic acid: a potent glyoxalase-I inhibitor with a unique scaffold. Acta Pharm. 2020;71(1):115–130. doi: 10.2478/acph-2021-0005. [DOI] [PubMed] [Google Scholar]
- 168.Shakeri A., Zirak M.R., Sahebkar A. Ellagic acid: a logical lead for drug development? Curr. Pharm. Des. 2017;24(2):106–122. doi: 10.2174/1381612823666171115094557. [DOI] [PubMed] [Google Scholar]
- 169.Garcia-Nino W.R., Zazueta C. Ellagic acid: pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015;97:84–103. doi: 10.1016/j.phrs.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 170.Derosa G., Maffioli P., Sahebkar A. Ellagic acid and its role in chronic diseases. Adv. Exp. Med. Biol. 2016;928:473–479. doi: 10.1007/978-3-319-41334-1_20. [DOI] [PubMed] [Google Scholar]
- 171.Dahiya R., Mohammad T., Gupta P., Haque A., Alajmi M.F., Hussain A., Hassan M.I. Molecular interaction studies on ellagic acid for its anticancer potential targeting pyruvate dehydrogenase kinase 3. RSC Adv. 2019;9(40):23302–23315. doi: 10.1039/c9ra02864a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gulzar M., Syed S.B., Khan F.I., Khan P., Ali S., Hasan G.M., Taneja P., Hassan M.I. Elucidation of interaction mechanism of ellagic acid to the integrin linked kinase. Int. J. Biol. Macromol. 2019;122:1297–1304. doi: 10.1016/j.ijbiomac.2018.09.089. [DOI] [PubMed] [Google Scholar]
- 173.Gupta P., Mohammad T., Khan P., Alajmi M.F., Hussain A., Rehman M.T., Hassan M.I. Evaluation of ellagic acid as an inhibitor of sphingosine kinase 1: a targeted approach towards anticancer therapy. Biomed. Pharmacother. 2019;118:118. doi: 10.1016/j.biopha.2019.109245. [DOI] [PubMed] [Google Scholar]
- 174.Yousuf M., Shamsi A., Khan P., Shahbaaz M., AlAjmi M.F., Hussain A., Hassan G.M., Islam A., Rizwanul Haque Q.M., Hassan M.I. Ellagic acid controls cell proliferation and induces apoptosis in breast cancer cells via inhibition of cyclin-dependent kinase 6. Int. J. Mol. Sci. 2020;21:10. doi: 10.3390/ijms21103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ceci C., Lacal P.M., Tentori L., De Martino M.G., Miano R., Graziani G. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients. 2018;10:11. doi: 10.3390/nu10111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vattem D.A., Shetty K. Biological functionality of ellagic acid: a review. J. Food Biochem. 2005;29(3):234–266. [Google Scholar]
- 177.Zeb A. Ellagic acid in suppressing in vivo and in vitro oxidative stresses. Mol. Cell Biochem. 2018;448(1–2):27–41. doi: 10.1007/s11010-018-3310-3. [DOI] [PubMed] [Google Scholar]
- 178.Khan S., Fakhar Z., Hussain A., Ahmad A., Jairajpuri D.S., Alajmi M.F., Hassan M.I. Structure-based identification of potential SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1848634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shamsi A., Mohammad T., Anwar S., Amani S., Khan M.S., Husain F.M., Rehman M.T., Islam A., Hassan M.I. Potential drug targets of SARS-CoV-2: From genomics to therapeutics. Int. J. Biol. Macromol. 2021;177:1–9. doi: 10.1016/j.ijbiomac.2021.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pandey A.K., Verma S. An in-silico evaluation of dietary components for structural inhibition of SARS-Cov-2 main protease. J. Biomol. Struct. Dyn. 2020:1–7. doi: 10.1080/07391102.2020.1809522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.M.A. Shaldam, et al., In silico screening of potent bioactive compounds from honeybee products against COVID-19 target enzymes, Environ. Sci. Pollut. Res. Int., 28(30), pp. 40507–40514. [DOI] [PMC free article] [PubMed]
- 182.Arokiyaraj S., Stalin A., Kannan B.S., Shin H. Geranii herba as a potential inhibitor of SARS-CoV-2 main 3CL(pro), spike RBD, and regulation of unfolded protein response: an in silico approach. Antibiotics. 2020;9(12) doi: 10.3390/antibiotics9120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Muhammad S., Hassan S.H., Al-Sehemi A.G., Shakir H.A., Khan M., Irfan M., Iqbal J. Exploring the new potential antiviral constituents of Moringa oliefera for SARS-COV-2 pathogenesis: an in silico molecular docking and dynamic studies. Chem. Phys. Lett. 2021;767 doi: 10.1016/j.cplett.2021.138379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yousuf M., Shamsi A., Queen A., Shahbaaz M., Khan P., Hussain A., Alajmi M.F., Rizwanul Haque Q.M., Imtaiyaz Hassan M. Targeting cyclin-dependent kinase 6 by vanillin inhibits proliferation of breast and lung cancer cells: Combined computational and biochemical studies. J. Cell. Biochem. 2021;122(8):897–910. doi: 10.1002/jcb.29921. [DOI] [PubMed] [Google Scholar]
- 185.Tai A., Sawano T., Yazama F., Ito H. Evaluation of anti-oxidant activity of vanillin by using multiple anti-oxidant assays. Biochim. Biophys. Acta. 2010;1810(2):170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 186.Yadav D.K. Recent advances on small molecule medicinal chemistry to treat human diseases. Curr. Top. Med. Chem. 2021;21(8):684–685. doi: 10.2174/156802662108210319145541. [DOI] [PubMed] [Google Scholar]
- 187.Yadav D.K. Recent advances on small molecule medicinal chemistry to treat human diseases-part II. Curr. Top. Med. Chem. 2021;21(10):849–850. doi: 10.2174/156802662110210601102135. [DOI] [PubMed] [Google Scholar]
- 188.Law W.Y., Asaruddin M.R., Bhawani S.A., Mohamad S. Pharmacophore modelling of vanillin derivatives, favipiravir, chloroquine, hydroxychloroquine, monolaurin and tetrodotoxin as M(Pro) inhibitors of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) BMC Res. Notes. 2020;13(1):527. doi: 10.1186/s13104-020-05379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh D.D., Yadav D.K. TNBC: potential targeting of multiple receptors for a therapeutic breakthrough, nanomedicine, and immunotherapy. Biomedicines. 2021;9:8. doi: 10.3390/biomedicines9080876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Turab Naqvi A.A., Hasan G.M., Hassan M.I. Targeting tau hyperphosphorylation via kinase inhibition: strategy to address Alzheimer’s disease. Curr. Top. Med. Chem. 2020;20(12):1059–1073. doi: 10.2174/1568026620666200106125910. [DOI] [PubMed] [Google Scholar]
- 191.Kulkarni S.A., Nagarajan S.K., Ramesh V., Palaniyandi V., Selvam S.P., Madhavan T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020;1221 doi: 10.1016/j.molstruc.2020.128823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yadav P.K., Jaiswal A., Singh R.K. In silico study on spice-derived antiviral phytochemicals against SARS-CoV-2 TMPRSS2 target. J. Biomol. Struct. Dyn. 2021:1–11. doi: 10.1080/07391102.2021.1965658. [DOI] [PubMed] [Google Scholar]
- 193.Rolta R., Salaria D., Sharma P., Sharma B., Kumar V., Rathi B., Verma M., Sourirajan A., Baumler D.J., Dev K. Phytocompounds of Rheum emodi, Thymus serpyllum, and Artemisia annua inhibit spike protein of SARS-CoV-2 binding to ACE2 receptor: in silico approach. Curr. Pharmacol. Rep. 2021:1–15. doi: 10.1007/s40495-021-00259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Qazi S., Sheikh K., Raza K. In silico approach to understand the epigenetic mechanism of SARS-CoV-2 and its impact on the environment. Virusdisease. 2021:1–12. doi: 10.1007/s13337-021-00655-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ghasemzadeh Rahbardar M., Hosseinzadeh H. Effects of rosmarinic acid on nervous system disorders: an updated review. Naunyn Schmiede Arch. Pharmacol. 2020;393(10):1779–1795. doi: 10.1007/s00210-020-01935-w. [DOI] [PubMed] [Google Scholar]
- 196.Kumar S., Kashyap P., Chowdhury S., Kumar S., Panwar A., Kumar A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine. 2020;85 doi: 10.1016/j.phymed.2020.153317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Selvaraj J., Rekha U.V., Jh S.F., Sivabalan V., Ponnulakshmi R., Vishnupriya V., Kullappan M., Sreekandan R.N., Mohan S.K. Molecular docking analysis of SARS-CoV-2 linked RNA dependent RNA polymerase (RdRp) with compounds from Plectranthus amboinicus. Bioinformation. 2021;17(1):167–170. doi: 10.6026/97320630017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Singh D.D., Han I., Choi E.H., Yadav D.K. Recent advances in pathophysiology, drug development and future perspectives of SARS-CoV-2. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.580202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Singh D.D., Han I., Choi E.H., Yadav D.K. Immunopathology, host-virus genome interactions, and effective vaccine development in SARS-CoV-2. Comput. Struct. Biotechnol. J. 2020;18:3774–3787. doi: 10.1016/j.csbj.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The information that supports the findings of this study is available in this article.