Abstract
SARS-CoV-2 infections mostly lead to mild or even asymptomatic infections in children, but the reasons for this are not fully understood. More efficient local tissue responses, better thymic function, and cross-reactive immunity have all been proposed to explain this. In rare cases of children and young people, but very rarely in adults, post-infectious hyperinflammatory syndromes can develop and be serious. Here, I will discuss our current understanding of SARS-CoV-2 infections in children and hypothesize that a life history and energy allocation perspective might offer an additional explanation to mild infections, viral dynamics, and the higher incidence of rare multisystem inflammatory syndromes in children and young people.
Brodin summarizes the current understanding of immune responses to SARS-CoV-2 infections in children and the possible explanations for the overall milder COVID-19 disease in young versus older individuals. To explain the combined observations to date, Brodin proposes an energy allocation perspective to explain mild disease, viral dynamics, and MIS-C in children and young people.
Introduction
Early after the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first described in the Hubei province of China, it became clear that children were underrepresented among patients presenting to local hospitals with severe coronavirus disease 2019 (COVID-19) (Guan et al., 2020). This demographic pattern has been replicated as the virus spread across the planet (Brodin, 2020; Preston et al., 2021). New variants with greater transmissibility, like delta (B.1.617.2) (Delahoy et al., 2021) and omicron, infect many, but fortunately do not seem to give rise to more severe disease in children. Infections with the related Middle Eastern Respiratory virus (MERS) (Thabet et al., 2015) and SARS-CoV (Zhong and Wong, 2004) have previously been shown to cause milder disease in children as compared with adults, but the underlying reasons for these differences remain elusive. Other infections by herpes family viruses Varicella Zoster and Epstein-Barr virus as well as the flavivirus Dengue (Thai et al., 2011) are more likely to be mild or even asymptomatic in young children as compared with primary infections occurring in adolescents and adults. Not all respiratory viral infections are mild in children and respiratory syncytial virus (RSV), influenza viruses, rhinoviruses, and metapneumovirus can all cause severe disease and are among the leading causes of death in children under 5 years of age (Tregoning and Schwarze, 2010). These viruses also give rise to severe infections in adults although immunity from prior infections offers important protection to many. Multiple hypotheses have been proposed to explain the differences in COVID-19 diseases severity in the young versus the elderly, and in this perspective article, I aim to summarize these different ideas and discuss the evidence for and against each of these. I also discuss alternative disease manifestations and their possible disease mechanisms and conclude with a hypothesis based on life history and resource allocation theory that could explain widely different clinical consequences of SARS-CoV-2 infections in children and young people.
Severe COVID-19 in young individuals
In individuals under 50 years old who developed life-threatening COVID-19, researchers within the COVID Human Genetic Effort consortium (https://www.covidhge.com/) uncovered an enrichment of inborn errors of immunity involving the viral sensor Toll-like receptor 3 (TLR3) or the gene IRF7, a key inducer of Type I interferon (IFN) (Zhang et al., 2020a). Also, patients with deficiencies in the type-I IFN receptor IFNAR1 was reported, and additional patients have since been identified (Khanmohammadi et al., 2021), underscoring the importance of TLR3 and IRF7-dependent type-I IFN responses in determining COVID-19 severity (Zhang et al., 2020b). A different group of researchers failed to replicate such enrichment of patients with inborn errors of type-I IFN immunity (Povysil et al., 2021), a result that could be explained by differences in the age, ancestries, and definition of severe COVID-19 between the cohorts as well as the use of the general population (Povysil et al., 2021) versus asymptomatic/paucisymptomatic COVID-19 cases as controls (Zhang et al., 2021). Also, deficiencies in the X-linked viral sensor TLR7 have been reported in patients with severe COVID-19 (Abolhassani et al., 2021; Asano et al., 2021; Fallerini et al., 2021; Kosmicki et al., 2021; van der Made et al., 2020), and some of these variants have been shown to impair SARS-CoV-2 recognition and induction of IFN-I responses in vitro (Asano et al., 2021). Observations from patients with known inborn errors of immunity infected with SARS-CoV-2 provide additional clues about the determinants of disease severity. Patients with defects in adaptive immune cells, either B cells, T cells, or both, mostly develop mild to asymptomatic COVID-19 (Meyts et al., 2021). Patients with secondary T cell deficiency due to Calcineurin-inhibitor treatment after solid-organ transplantation exhibit mortality rates comparable with the general population, despite significant co-morbidity (Raja et al., 2021). All in all, these observations indicate that robust antiviral type-I IFN immunity early after infection is the most critical determinant of COVID-19 disease severity.
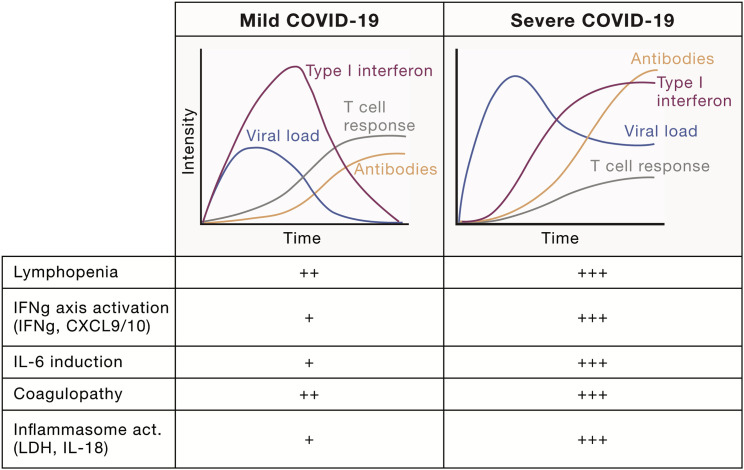
One group of children with inborn errors of immunity that develop very severe COVID-19 is patients with autoimmune polyendocrine syndrome type 1 (APS-1) caused by mutations in the AIRE gene (Bastard et al., 2021a; Beccuti et al., 2020). Such patients carry neutralizing autoantibodies to various cytokines, including type-I IFNs and IL-17 cytokines. To investigate whether neutralizing autoantibodies could phenocopy inborn errors of IFN-I and give rise to severe COVID-19, Bastard and colleagues screened sera from patients with severe COVID-19 and found ∼2.6% of females and 12.5% of male patients had such neutralizing autoantibodies to IFN-I (Bastard et al., 2020). In a larger cohort, autoantibodies to IFN-I were found to increase with age and were present in >6% of individuals above 80 years of age and explain ∼20% of cases of fatal COVID-19 (Bastard et al., 2021b). Children with APS-1 infected with SARS-CoV-2 can be saved by plasma exchange removing neutralizing anti-IFN-I autoantibodies (Bastard et al., 2021a; Lemarquis et al., 2021) or supported by treatment with monoclonal anti-SARS-CoV-2 antibodies (Ferré et al., 2021). The SARS-CoV-2 virus encodes multiple proteins that interfere with the same IFN-I responses (Lei et al., 2020), which further indicates their importance. Severe COVID-19 thus develop when early IFN-I and/or IFN-III responses fail to control viral replication, leading to imbalanced immune activation and systemic hyperinflammation and immunopathology (Brodin, 2021; Carvalho et al., 2021) (Figure 1 A). Inborn errors of type I IFN immunity explain severe COVID-19 pneumonia mostly in young adults, while neutralizing autoantibodies to type I IFN are seen at increasing frequency with age and explain around 20% of total fatal COVID-19 cases (Bastard et al., 2021b). Despite high rates of infections and low rates of vaccination among children, the incidence of fatal COVID-19 remains low, ∼2 per million individuals <18 years of age according to a recent national survey in the UK (Smith et al., 2021). Considering the many possible cases of unrecognized immunodeficiencies in such children, the spectrum of susceptibility is likely quite narrow as indicated also by COVID-19 presentations in patients with known inborn errors of immunity (Bucciol et al., 2021).
Figure 1.
Immune responses in mild and severe COVID-19
Characteristic differences between patients developing mild versus severe COVID-19 disease with delayed and imbalanced IFN-I responses, lymphopenia, and uncontrolled viral replication. Typical signs of mild versus severe COVID-19 in terms of cytokine production, inflammasome activation, and coagulopathy, as seen a number of population studies (Carvalho et al., 2021).
Local airway immune responses to SARS-CoV-2
The immune system of young children encounters many new microbes. After an initial period of protection from maternal antibodies providing passive immunity (Pou et al., 2019), the infant immune system must respond to these novel challenges. In SARS-CoV-2-infected children, innate immune responses in the upper airways have been reported as more pronounced as compared with those in infected adults. This includes both type-I and type-II IFN-responses and inflammasome-dependent pathways (Koch et al., 2021; Loske et al., 2021; Pierce et al., 2021). Differences in basal antiviral gene expression in epithelial cells and differences in tissue immune cell composition between children and adults indicate that airways of young children are better prepared to manage viral infections locally (Loske et al., 2021). It is important to note that the ages of children in these studies were on average 2, 6, and 9 years, respectively, and that airway responses might be different in younger children <6 month of age given the increased susceptiblility to several respiratory viruses, RSV, and influenza in such young children (Tregoning and Schwarze, 2010). The reasons for the differences between adult and pediatric local airway immunity are not known but could be a consequence of more frequent viral infections, local microbiome differences (Reyman et al., 2021), more recent vaccinations, and epigenetic adaptations in innate immune cells, i.e., trained immunity (Zimmermann and Curtis, 2020). Further studies will be needed to explore the relative contribution of these local tissue responses in explaining COVID-19 disease severity among the young and old.
Adaptive immunity to SARS-CoV-2 in children
The innate immune response is usually not sufficient to clear SARS-CoV-2 viruses, and the virus persists for months in immunocompromised individuals, especially those with severe T cell deficiencies (Avanzato et al., 2020; Nakajima et al., 2021). In a mouse model, CD8+ T cells are required and sufficient to clear SARS-CoV-2 viruses, while neutralizing antibodies offer protection from re-infection (Israelow et al., 2021) in line with human data (Addetia et al., 2020). Adaptive immune responses in severe versus mild COVID-19 are characterized by more pronounced T cell lymphopenia and bystander activation of T cells, yet robust B cell activation and production of higher titer neutralizing antibodies as compared with mild disease (Carvalho et al., 2021) (Figures 1A and 1B). In adults with severe COVID-19, SARS-CoV-2-specific immunoglobulin A (IgA)-antibodies that activate neutrophils have been reported, and these were absent in children with mild COVID-19 (Bartsch et al., 2021). The gradual increase in severe COVID-19 with age, even during adulthood, has inspired some researchers to propose that thymic involution might explain this result (Palmer et al., 2021). The known pace of thymic involution correlates well with increased COVID-19 severity beyond 20 years of age but cannot explain the added protection in children below 20 years of age (Palmer et al., 2021). There has been much written about the role of B and/or T cells specific for common cold coronaviruses in offering some level of protection from, or modulation of, SARS-CoV-2 infections and COVID-19 disease. T cells that react to SARS-CoV-2 peptides can be found in samples collected prior to 2019 (Mateus et al., 2020), and cross-reacting cytotoxic T cells are predominantly found in tonsils and not blood (Niessl et al., 2021). As primary infection with several human CoVs (HCoVs) typically occur early in childhood, and children are frequently reinfected with common cold coronaviruses (HCoVs), one could imagine more cross-reactive T cells in children. Niessl and co-workers found relatively low functional responses by tonsillar SARS-CoV-2 cross-reactive T cells from unexposed individuals, with slightly more polyfunctionality in cells from children as compared with adults (Niessl et al., 2021). Cross-reactive antibodies have also been reported, mostly against the S2 domain of the spike-protein, which is more conserved among HCoVs, and titers of such antibodies increase upon SARS-CoV-2 infection (Röltgen et al., 2021). Recent HCoV infection is associated with milder COVID-19 (Sagar et al., 2021). Some have interpreted the decline in antibody titers after adult HCoV infection to mean that immunity is short-lived, yet in a human challenge study, protection from symptomatic disease upon viral challenge was maintained for at least one year in adult volunteers (Callow et al., 1990), and reinfections are common (Hamady et al., 2021). As both adults and young people carry immune memory to HCoV, it is difficult to attribute the milder COVID-19 in young people to this factor, although subtle differences in the quantity or quality of cross-reactive immunity might modulate disease course differently in young and old individuals. Importantly, there is no evidence suggesting that cross-reactive immunity would lead to worse COVID-19 disease through antibody-mediated enhancement (Arvin et al., 2020).
Viral dynamics in children and adults
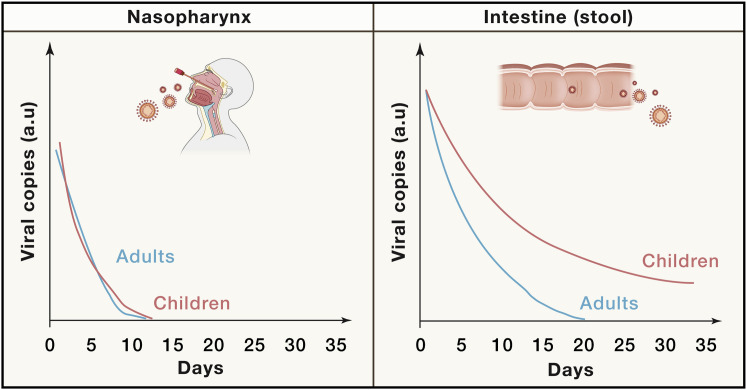
Upon infection, the virus quickly replicates in the airway epithelial cells and reaches high copy numbers in both children and adults. Most studies performed use quantitative PCR methods to infer viral load rather than performing gold standard plaque assays, but with this weakness in mind, the available data suggest similar viral loads in children and adults at the time of presentation (Jones et al., 2021). Several studies have assessed the dynamics of viral replication, shedding, and clearance from different body fluids. One study found culturable virus from lungs and upper airways, but not from stool, despite rather high amounts of stool RNA (Wölfel et al., 2020). Other studies have suggested persistence of virus in lung tissue after mild to moderate COVID-19 (Ceulemans et al., 2021) as well as in the small intestine (duodenum and terminal ileum) (Gaebler et al., 2021). Interestingly, a meta-analysis of 37 studies concluded that in adults, the average time to viral clearance was 14 days for the respiratory tract and 19 days in stool samples (Morone et al., 2020). A couple of smaller studies have analyzed viral persistence in samples from children. Du et al. (2020) found that the average time to viral clearance was 9 days for the respiratory tract and 34 days for stool samples, and Xing et al. (2020) reported three children having positive airway samples 10–15 days, while stool samples remained positive for 23–34 days post diagnoses (Figure 2 ). SARS-CoV-2 infect apical enterocytes but is inactivated when released into the lumen (Zang et al., 2020), making it difficult to extrapolate findings using stool samples to viral dynamics within the intestinal epithelium. Clearly, more studies analyzing virus-infected intestinal cells collected over time would be needed to confirm these findings, but these preliminary results do imply more extended viral persistence in children as compared with adults, despite comparable levels of virus in the respiratory tract at diagnosis.
Figure 2.
Viral dynamics in the young and old
Relative viral load (arbitrary units, a.u.) over time (days from diagnosis) as determined by PCR from either nasopharyngeal or stool samples. A schematic model summarizing the results of multiple studies showing prolonged shedding of viral nucleic acid from stool, particularly in children.
Multisystem inflammatory syndrome in children and young adults (MIS-C/A)
In the spring of 2020, as pediatricians, we began seeing cases of children bearing resemblance with Kawasaki disease, a postinfectious inflammation of medium-sized arteries such as the coronaries (Shulman and Rowley, 2015). Cases of Kawasaki disease are seen every winter in the US and Europe with an incidence of 10–20 cases per 100, 000 children, while the incidence is 10–30 times higher in northeast Asia (Kim, 2019). The cases of suspected Kawasaki disease seen in the spring of 2020 in Europe were atypical in presentation and occurred at a much increased frequency compared with previous years (Riphagen et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020). Immunological analyses revealed a hyperinflammatory syndrome, distinct from Kawasaki disease and different from the cytokine storm seen in severe, acute COVID-19 (Consiglio et al., 2020; Rodriguez et al., 2020). The condition is now termed multisystem inflammatory syndrome in children, MIS-C (and MIS-A in adults) and is a rare disorder following an often asymptomatic or paucisymptomatic SARS-CoV-2 infection in children and young adults with a delay of 1–2 months. The condition also bears clear resemblance with toxic shock syndrome and is treated with steroids and immunoglobulins (McArdle et al., 2021) and, in some cases, targeted immunomodulators such as IL-1RA and anti-TNF. The condition is associated with broadly specific autoantibodies targeting various tissue antigens (Consiglio et al., 2020; Gruber et al., 2020; Ramaswamy et al., 2021), much like what is seen in some acute COVID-19 patients (Wang et al., 2021). To explain the broad immune activation and dysregulation, Cheng and colleagues searched for, and found, a superantigen motif in the SARS-CoV-2 spike protein not found in other related HCoVs and with an ability to bind and cross-link and activate T cells with particular T cell receptor (TCR)-β chains (Cheng et al., 2020). Following this initial study, expansion of T cells carrying the TRBV11-2 gene, in combination with variable alpha chains, a hallmark of superantigen-mediated T cell activation, has been reported in several studies of patients with MIS-C (Hoste et al., 2022; Moreews et al., 2021; Porritt et al., 2021; Ramaswamy et al., 2021). But if this condition is caused by superantigen-mediated activation, why does it occur in children and young people and not in the elderly, and why only in a small fraction of infected youngsters? One possibility is that an additional pathogen carrying a superantigen motif is required in conjunction with the SARS-CoV-2 infection in order to trigger MIS-C. If this pathogen is exclusive to children and young people, that could explain the lack of MIS-C among the elderly. An alternative hypothesis is that the initial immune response triggered by SARS-CoV-2 would differ between children and adults, creating the necessary condition for subsequent superantigen-mediated MIS-C only in children and young people. This will be discussed further below.
The fact that MIS-C only occurs in small fraction of infected children could be explained by additional genetic requirements for disease (Sancho-Shimizu et al., 2021), and one such clue comes from HLA-class I genes recently associated with MIS-C (Porritt et al., 2021). Intriguing observations of MIS-C being very rare in countries such as Japan (Fukuda et al., 2021), despite many reported cases of pediatric COVID-19, is further suggestive of such genetic requirements for MIS-C apart from the necessary age-associated differences. The delayed presentation of MIS-C differs from other superantigen-mediated diseases and could be explained by the requirement for a coinfecting pathogen, yet the precise timing after initial infection is puzzling. Another recent hypothesis to explain this delay states that the gut, rather than the airway mucosa, is the source of superantigens in MIS-C (Yonker et al., 2021). The evidence discussed in the previous section of prolonged viral shedding in the intestines of children, possibly beyond what is seen in adults, together with a recent study showing that the integrity of the intestinal barrier is disrupted in MIS-C patients, collectively provides a possible explanation to these puzzling facts (Yonker et al., 2021). As most children with MIS-C have intestinal symptoms and often show inflammation of the terminal ileum (Morparia et al., 2021), SARS-CoV-2 virus could then be persisting in the intestine of children more so than in adults and, in genetically susceptible individuals, give rise to local superantigen-mediated T cell activation and inflammation, loss of intestinal barrier integrity, and release of superantigens into the bloodstream. This could then potentially explain the systemic hyperinflammatory responses seen in children and young people with MIS-C.
An energy allocation perspective on COVID-19 in children
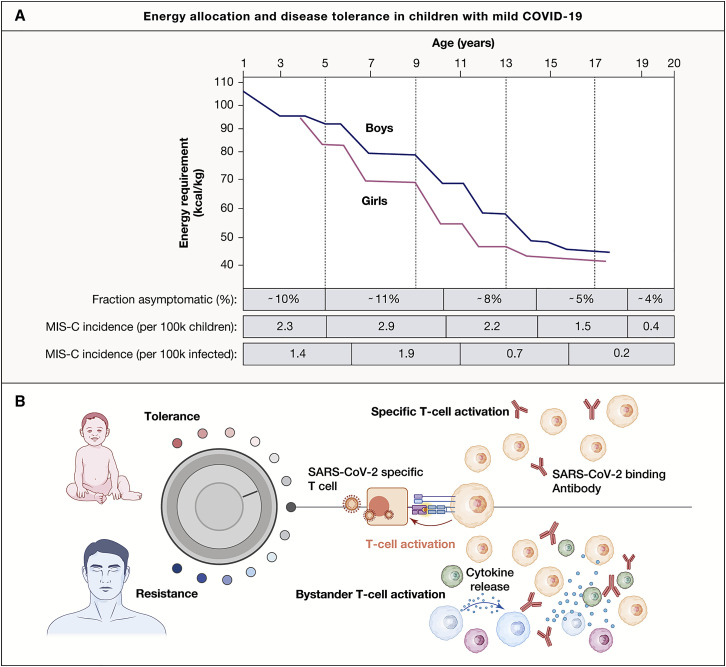
One major physiological difference between children and adults is physical growth. Humans are born immature and defenseless, and there is strong evolutionary pressure promoting rapid growth early in life (Hochberg, 2011). Consequently, energy allocation trade-offs between immune defenses and growth will likely favor the latter unless the pathogen represents a serious threat to the survival and fitness of the child (Stearns, 1992). Disease tolerance is an immune defense strategy used when the immune response to a pathogen is more damaging than the pathogen itself (Medzhitov et al., 2012). The decision to resist or tolerate a particular pathogen is likely to be different in a growing child as compared with an adult, and I hypothesize that growing children are more likely to choose disease tolerance to avoid systemic inflammatory response whenever possible. The energy requirements of children from birth to 20 years of age show a steady decreasing trend with a slightly higher energy requirement in boys versus girls (Figure 3 A). Of all immune system processes, the systemic inflammatory response is the costliest and often lead to weight loss in children during infectious episodes with fever, muscle ache, and other signs of systemic inflammation. Regarding SARS-CoV-2 infections, the highest rates of asymptomatic COVID-19 are seen in children with highest energetic requirements (Leidman et al., 2021) (Figure 3B). Also, other infections such as primary Epstein-Barr virus are mostly asymptomatic in infants but often severe in adolescents, and this is linked to stronger systemic inflammatory responses and bystander activation of T cells (Jayasooriya et al., 2015). Possibly, these phenomena are linked to the propensity of choosing disease tolerance over resistance, a decision coupled to resource allocation trade-offs between growth and immune defense. Mild and asymptomatic COVID-19 in children leads to seroconversion, the development of neutralizing antibodies, and specific T cells but is not associated with systemic inflammation or bystander activation of T cells (Figure 3C). Because T cell mediated responses are important for viral clearance, it is tempting to think that less bystander T cell activation and a milder systemic inflammatory response might increase the likelihood for viral persistence. In support of this, Cotugno and colleagues recently reported similar adaptive T and/or B cell responses but reduced cytokine responses in blood of asymptomatic as compared with symptomatic children infected with SARS-CoV-2 (Cotugno et al., 2021). Given the hypothesis formulated by Yonker et al. (2021) that MIS-C arises because of viral persistence in the gut and subsequent superantigen-mediated T cell activation, the determinants of viral persistence might also be linked to the probability of developing MIS-C. If children and young people are more likely to choose disease tolerance versus resistance because of energy allocation toward growth, this might explain why MIS-C is mostly seen in children and young adults. To illustrate this further, I extracted incidence estimates from two recent reports showing that children with the highest energy requirement also have a higher propensity for asymptomatic infection and the highest incidence of MIS-C (Figure 3B). Boys have a slightly higher energy requirement than girls throughout childhood (Figure 3A), and this should translate into a higher propensity for disease tolerance and more frequent mild or asymptomatic infections among boys according to the energy allocation theory proposed herein. Hospitalization rates are difficult to interpret because they are strongly influenced by co-morbidities and other risk factors, but among children hospitalized with COVID-19, a slight overrepresentation of girls has been reported (Preston et al., 2021). For MIS-C, the opposite is true, and a small, yet reproducible overrepresentation of boys has been reported in several cohorts (Abrams et al., 2021; Feldstein et al., 2021; Kahn et al., 2021; McArdle et al., 2021). Clearly, additional explanations are required, given that only a small fraction of infected children will ever develop MIS-C despite the majority experiencing mild infections, and this is likely the result of rare genetic determinants in combination with viral characteristics and other environmental factors (Sancho-Shimizu et al., 2021).
Figure 3.
An energy allocation theory to explain mild COVID-19 and MIS-C in children based on disease tolerance and viral persistence
(A) Energy requirement (kcal/kg) decrease with age in US children and is slightly higher in boys than girls (adapted from Torun, 2005). The fraction of asymptomatic children among SARS-CoV-2 PCR+ children across the indicated age groups (Leidman et al., 2021), MIS-C incidence in the US per 100,000 children across indicated age groups (Belay et al., 2021), and MIS-C incidence per 1 million cases of COVID-19 across the indicated age groups (Payne et al., 2021).
(B) In growing children, the threshold for energy-expenditure on system inflammatory responses are higher, leading to disease tolerance in most cases and mild to asymptomatic COVID-19, while in the elderly, the obese, and individuals with inadequate type-I IFN-respones, systemic inflammation is triggered, driving bystander T cell activation and immunopathology.
Concluding remarks
There is strong evolutionary pressure on young children to grow, and energy allocation trade-offs are likely to favor growth over expensive systemic inflammatory responses whenever possible. By choosing disease tolerance over maximal resistance, children are more likely to present with mild and even asymptomatic disease but might also be less efficient at viral clearance and, consequently, be more prone to some level of viral persistence and possibly others conditions linked to such viral persistence such as superantigen-mediated immune activation in MIS-C (Figure 3D).
Apart from mild to severe COVID-19 and MIS-C, another outcome after SARS-CoV-2 infection is long COVID or post-acute COVID-19 syndrome (PACS), defined as a multiorgan disease syndrome lasting beyond 12 weeks after initial infection (Brodin, 2021). This poorly understood condition merits further investigation in both children and adults, yet too little is known to date to speculate around long COVID in relation to the energy allocation hypothesis I have described. Also, a careful literature review has found that long COVID in children is likely to be less frequent and less severe as compared with what is reported in adults (Behnood et al., 2021; Nalbandian et al., 2021). The suggestion herein that the propensity for disease tolerance and/or resistance is linked to energy allocation tradeoff between growth and immune defense is not only applicable to growing children, but also to individuals with altered metabolic states. Obesity is associated with more severe COVID-19, which has been attributed to the low-grade inflammation (Brodin, 2021), but could also be explained by higher propensity for systemic inflammatory responses associated with severe COVID-19, as suggested by the energy allocation hypothesis.
As we learn more about the variable disease presentation upon SARS-CoV-2 infection in children and older people, we also learn important unique features of immune systems in children and young people that will help us also understand other diseases that involve the immune system and with different manifestations across the age spectrum. Taking on a physiological and life history perspective will make the unique aspects of immune systems in children more understandable in relation to the demands associated with different phases of life.
Acknowledgments
I thank Dr. Kim Vestö for assistance with figure preparations using BioRender. I am grateful to members of our laboratory as well as colleagues such as Dr. Carrie Lucas (Yale), Prof. Moshe Ardithi (UCLA), Prof. Michael Levin, Dr. Vanessa Sancho Shimitzu, Dr. Elizabeth Whittaker (Imperial College, London), Prof. Yenan Bryceson, and Anna Carin Horne (Karolinska Institutet). Finally, I am grateful to be a member of the COVID Human Genetic Effort consortium and to Prof. Jean-Laurent Casanova (Rockefeller University) and Prof. Helen Su (NIH) for initiating and leading this effort as well as all of the consortium’s members for inspiring discussions and sharing of knowledge and data. I also thank the two reviewers for their constructive input. This work was funded by Garfield Weston foundation, The Swedish Research council (2021-06529), Karolinska Institutet, and donations from Bure Equity AB, and Jonas & Christina af Jochnick Foundation.
Declaration of interests
The authors declare no competing interests.
References
- Abolhassani H., Vosughimotlagh A., Asano T., Landegren N., Boisson B., Delavari S., Bastard P., Aranda-Guillén M., Wang Y., Zuo F., et al. X-Linked TLR7 Deficiency Underlies Critical COVID-19 Pneumonia in a Male Patient with Ataxia-Telangiectasia. J. Clin. Immunol. 2021 doi: 10.1007/s10875-021-01151-y. Published online October 23, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams J.Y., Oster M.E., Godfred-Cato S.E., Bryant B., Datta S.D., Campbell A.P., Leung J.W., Tsang C.A., Pierce T.J., Kennedy J.L., et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc. Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.-L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020;58:e02107-20. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin A.M., Fink K., Schmid M.A., Cathcart A., Spreafico R., Havenar-Daughton C., Lanzavecchia A., Corti D., Virgin H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., Zhang P., Meertens L., Bolze A., Materna M., et al. COVID Human Genetic Effort. COVID-STORM Clinicians. COVID Clinicians. Imagine COVID Group. French COVID Cohort Study Group. CoV-Contact Cohort. Amsterdam UMC Covid- Biobank. NIAID-USUHS COVID Study Group X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021;6:eabl4348. doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., Barbian K., Judson S.D., Fischer E.R., Martens C., et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020;183:1901–1912. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch Y.C., Wang C., Zohar T., Fischinger S., Atyeo C., Burke J.S., Kang J., Edlow A.G., Fasano A., Baden L.R., et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021;27:454–462. doi: 10.1038/s41591-021-01263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Orlova E., Sozaeva L., Lévy R., James A., Schmitt M.M., Ochoa S., Kareva M., Rodina Y., Gervais A., et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 2021;218:e20210554. doi: 10.1084/jem.20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Gervais A., Voyer T.L., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.-H., Eto S., Garcia-Prat M., et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci Immunol. 2021;6:eabl4340. doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccuti G., Ghizzoni L., Cambria V., Codullo V., Sacchi P., Lovati E., Mongodi S., Iotti G.A., Mojoli F. A COVID-19 pneumonia case report of autoimmune polyendocrine syndrome type 1 in Lombardy, Italy: letter to the. J. Endocrinol. Invest. 2020;43:1175–1177. doi: 10.1007/s40618-020-01323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnood S.A., Shafran R., Bennett S.D., Zhang A.X.D., O’Mahoney L.L., Stephenson T.J., Ladhani S.N., De Stavola B.L., Viner R.M., Swann O.V. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. J. Infect. 2021 doi: 10.1016/j.jinf.2021.11.011. Published online November 20, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay E.D., Abrams J., Oster M.E., Giovanni J., Pierce T., Meng L., Prezzato E., Balachandran N., Openshaw J.J., Rosen H.E., et al. Trends in Geographic and Temporal Distribution of US Children With Multisystem Inflammatory Syndrome During the COVID-19 Pandemic. JAMA Pediatr. 2021;175:837–845. doi: 10.1001/jamapediatrics.2021.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. 2020;109:1082–1083. doi: 10.1111/apa.15271. [DOI] [PubMed] [Google Scholar]
- Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- Bucciol G., Tangye S.G., Meyts I. Coronavirus disease 2019 in patients with inborn errors of immunity: lessons learned. Curr. Opin. Pediatr. 2021;33:648–656. doi: 10.1097/MOP.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A.J. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho T., Krammer F., Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat. Rev. Immunol. 2021;21:245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans L.J., Khan M., Yoo S.-J., Zapiec B., Van Gerven L., Van Slambrouck J., Vanstapel A., Van Raemdonck D., Vos R., Wauters E., et al. Persistence of SARS-CoV-2 RNA in lung tissue after mild COVID-19. Lancet Respir. Med. 2021;9:e78–e79. doi: 10.1016/S2213-2600(21)00240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.H., Zhang S., Porritt R.A., Noval Rivas M., Paschold L., Willscher E., Binder M., Arditi M., Bahar I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. USA. 2020;117:25254–25262. doi: 10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., Tan Z., Zicari S., Ruggiero A., Pascucci G.R., et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell. 2020;183:968–981. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotugno N., Ruggiero A., Pascucci G.R., Bonfante F., Petrara M.R., Pighi C., Cifaldi L., Zangari P., Bernardi S., Cursi L., et al. Pediatr Allergy Immu; 2021. Virological and immunological features of SARS-COV-2 infected children with distinct symptomatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahoy M.J., Ujamaa D., Whitaker M., O’Halloran A., Anglin O., Burns E., Cummings C., Holstein R., Kambhampati A.K., Milucky J., et al. COVID-NET Surveillance Team. COVID-NET Surveillance Team Hospitalizations Associated with COVID-19 Among Children and Adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1255–1260. doi: 10.15585/mmwr.mm7036e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Yu J., Liu X., Chen H., Lin L., Li Q. Persistence of SARS-CoV-2 virus RNA in feces: A case series of children. J. Infect. Public Health. 2020;13:926–931. doi: 10.1016/j.jiph.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallerini C., Daga S., Mantovani S., Benetti E., Picchiotti N., Francisci D., Paciosi F., Schiaroli E., Baldassarri M., Fava F., et al. GEN-COVID Multicenter Study Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. eLife. 2021;10:e67569. doi: 10.7554/eLife.67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein L.R., Tenforde M.W., Friedman K.G., Newhams M., Rose E.B., Dapul H., Soma V.L., Maddux A.B., Mourani P.M., Bowens C., et al. Overcoming COVID-19 Investigators Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré E.M.N., Schmitt M.M., Ochoa S., Rosen L.B., Shaw E.R., Burbelo P.D., Stoddard J.L., Rampertaap S., DiMaggio T., Bergerson J.R.E., et al. SARS-CoV-2 Spike Protein-Directed Monoclonal Antibodies May Ameliorate COVID-19 Complications in APECED Patients. Front. Immunol. 2021;12:720205. doi: 10.3389/fimmu.2021.720205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S., Kaneta M., Miyake M., Ohya T., Miyakawa K., Iwamoto M., Ito S. A case of multisystem inflammatory syndrome in children in a Japanese boy: with discussion of cytokine profile. Mod Rheumatol Case Rep. 2021;5:442–447. doi: 10.1080/24725625.2021.1920140. [DOI] [PubMed] [Google Scholar]
- Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., Wilson K.M., Onel K., Geanon D., Tuballes K., et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C) Cell. 2020;183:982–995. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., et al. China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamady A., Lee J., Loboda Z.A. Waning antibody responses in COVID-19: what can we learn from the analysis of other coronaviruses? Infection. 2021 doi: 10.1007/s15010-021-01664-z. Published online July 29, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Z. Evolutionary perspective in child growth. Rambam Maimonides Med. J. 2011;2:e0057. doi: 10.5041/RMMJ.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste L., Roels L., Naesens L., Bosteels V., Vanhee S., Dupont S., Bosteels C., Browaeys R., Vandamme N., Verstaen K., et al. MIS-C Clinicians TIM3+ TRBV11-2 T cells and IFNγ signature in patrolling monocytes and CD16+ NK cells delineate MIS-C. J. Exp. Med. 2022;219:e20211381. doi: 10.1084/jem.20211381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelow B., Mao T., Klein J., Song E., Menasche B., Omer S.B., Iwasaki A. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci Immunol. 2021;6:eabl4509. doi: 10.1126/sciimmunol.abl4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasooriya S., de Silva T.I., Njie-jobe J., Sanyang C., Leese A.M., Bell A.I., McAulay K.A., Yanchun P., Long H.M., Dong T., et al. Early virological and immunological events in asymptomatic Epstein-Barr virus infection in African children. PLoS Pathog. 2015;11:e1004746. doi: 10.1371/journal.ppat.1004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.C., Biele G., Mühlemann B., Veith T., Schneider J., Beheim-Schwarzbach J., Bleicker T., Tesch J., Schmidt M.L., Sander L.E., et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 2021;373:eabi5273. doi: 10.1126/science.abi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R., Berg S., Berntson L., Berthold E., Brodin P., Bäckström F., Compagno M., Fasth A., Framme J.L., Horne A., et al. Population-based study of multisystem inflammatory syndrome associated with COVID-19 found that 36% of children had persistent symptoms. Acta Paediatr. 2021;111:354–362. doi: 10.1111/apa.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanmohammadi S., Rezaei N., Khazaei M., Shirkani A. A Case of Autosomal Recessive Interferon Alpha/Beta Receptor Alpha Chain (IFNAR1) Deficiency with Severe COVID-19. J. Clin. Immunol. 2021 doi: 10.1007/s10875-021-01166-5. Published online October 28, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.B. Reality of Kawasaki disease epidemiology. Korean J. Pediatr. 2019;62:292–296. doi: 10.3345/kjp.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C.M., Prigge A.D., Anekalla K.R., Shukla A., Do-Umehara H.C., Setar L., Chavez J., Abdala-Valencia H., Politanska Y., Markov N.S., et al. Immune response to SARS-CoV-2 in the nasal mucosa in children and adults. medRxiv. 2021 doi: 10.1101/2021.01.26.21250269. [DOI] [Google Scholar]
- Kosmicki J.A., Horowitz J.E., Banerjee N., Lanche R., Marcketta A., Maxwell E., Bai X., Sun D., Backman J.D., Sharma D., et al. Regeneron Genetics Center. UKB Exome Sequencing Consortium Pan-ancestry exome-wide association analyses of COVID-19 outcomes in 586,157 individuals. Am. J. Hum. Genet. 2021;108:1350–1355. doi: 10.1016/j.ajhg.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidman E., Duca L.M., Omura J.D., Proia K., Stephens J.W., Sauber-Schatz E.K. COVID-19 Trends Among Persons Aged 0-24 Years - United States, March 1-December 12, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:88–94. doi: 10.15585/mmwr.mm7003e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarquis A., Campbell T., Aranda-Guillén M., Hennings V., Brodin P., Kämpe O., Blennow K., Zetterberg H., Wennerås C., Eriksson K., et al. J Allergy Clin Immun; 2021. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loske J., Röhmel J., Lukassen S., Stricker S., Magalhães V.G., Liebig J., Chua R.L., Thürmann L., Messingschlager M., Seegebarth A., et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2021 doi: 10.1038/s41587-021-01037-9. Published online August 18, 2021. [DOI] [PubMed] [Google Scholar]
- Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M., Burger Z.C., Rawlings S.A., Smith D.M., Phillips E., et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle A.J., Vito O., Patel H., Seaby E.G., Shah P., Wilson C., Broderick C., Nijman R., Tremoulet A.H., Munblit D., et al. BATS Consortium Treatment of Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2021;385:11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., Lopez-Granados E., Gianelli C., Robles-Marhuenda A., Jeandel P.-Y., et al. IUIS Committee of Inborn Errors of Immunity Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J. Allergy Clin. Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreews M., Gouge K.L., Khaldi-Plassart S., Pescarmona R., Mathieu A.-L., Malcus C., Djebali S., Bellomo A., Dauwalder O., Perret M., et al. Polyclonal expansion of TCR Vbeta 21.3+ CD4+ and CD8+ T cells is a hallmark of Multisystem Inflammatory Syndrome in Children. Sci Immunol. 2021;6:eabh1516. doi: 10.1126/sciimmunol.abh1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morone G., Palomba A., Iosa M., Caporaso T., De Angelis D., Venturiero V., Savo A., Coiro P., Carbone D., Gimigliano F., et al. Incidence and Persistence of Viral Shedding in COVID-19 Post-acute Patients With Negativized Pharyngeal Swab: A Systematic Review. Front. Med. (Lausanne) 2020;7:562. doi: 10.3389/fmed.2020.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morparia K., Park M.J., Kalyanaraman M., McQueen D., Bergel M., Phatak T. Abdominal Imaging Findings in Critically Ill Children With Multisystem Inflammatory Syndrome Associated With COVID-19. Pediatr. Infect. Dis. J. 2021;40:e82–e83. doi: 10.1097/INF.0000000000002967. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Ogai A., Furukawa K., Arai R., Anan R., Nakano Y., Kurihara Y., Shimizu H., Misaki T., Okabe N. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J. Infect. Chemother. 2021;27:387–389. doi: 10.1016/j.jiac.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessl J., Sekine T., Lange J., Konya V., Forkel M., Maric J., Rao A., Mazzurana L., Kokkinou E., Weigel W., et al. Identification of resident memory CD8+ T cells with functional specificity for SARS-CoV-2 in unexposed oropharyngeal lymphoid tissue. Sci Immunol. 2021;6:eabk0894. doi: 10.1126/sciimmunol.abk0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S., Cunniffe N., Donnelly R. COVID-19 hospitalization rates rise exponentially with age, inversely proportional to thymic T-cell production. J. R. Soc. Interface. 2021;18:20200982. doi: 10.1098/rsif.2020.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A.B., Gilani Z., Godfred-Cato S., Belay E.D., Feldstein L.R., Patel M.M., Randolph A.G., Newhams M., Thomas D., Magleby R., et al. Incidence of Multisystem Inflammatory Syndrome in Children Among US Persons Infected With SARS-CoV-2. JAMA Netw Open. 2021;4:e2116420. doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C.A., Sy S., Galen B., Goldstein D.Y., Orner E., Keller M.J., Herold K.C., Herold B.C. Natural mucosal barriers and COVID-19 in children. JCI Insight. 2021;6:e148694. doi: 10.1172/jci.insight.148694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porritt R.A., Paschold L., Rivas M.N., Cheng M.H., Yonker L.M., Chandnani H., Lopez M., Simnica D., Schultheiß C., Santiskulvong C., et al. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J. Clin. Invest. 2021;131:e146614. doi: 10.1172/JCI146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou C., Nkulikiyimfura D., Henckel E., Olin A., Lakshmikanth T., Mikes J., Wang J., Chen Y., Bernhardsson A.K., Gustafsson A., et al. The repertoire of maternal anti-viral antibodies in human newborns. Nat. Med. 2019;25:591–596. doi: 10.1038/s41591-019-0392-8. [DOI] [PubMed] [Google Scholar]
- Povysil G., Butler-Laporte G., Shang N., Wang C., Khan A., Alaamery M., Nakanishi T., Zhou S., Forgetta V., Eveleigh R.J.M., et al. Rare loss-of-function variants in type I IFN immunity genes are not associated with severe COVID-19. J. Clin. Invest. 2021;131:e147834. doi: 10.1172/JCI147834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston L.E., Chevinsky J.R., Kompaniyets L., Lavery A.M., Kimball A., Boehmer T.K., Goodman A.B. Characteristics and Disease Severity of US Children and Adolescents Diagnosed With COVID-19. JAMA Netw. Open. 2021;4:e215298. doi: 10.1001/jamanetworkopen.2021.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja M.A., Mendoza M.A., Villavicencio A., Anjan S., Reynolds J.M., Kittipibul V., Fernandez A., Guerra G., Camargo J.F., Simkins J., et al. COVID-19 in solid organ transplant recipients: A systematic review and meta-analysis of current literature. Transplant. Rev. (Orlando) 2021;35:100588. doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy A., Brodsky N.N., Sumida T.S., Comi M., Asashima H., Hoehn K.B., Li N., Liu Y., Shah A., Ravindra N.G., et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity. 2021;54:1083–1095. doi: 10.1016/j.immuni.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyman M., Clerc M., van Houten M.A., Arp K., Chu M.L.J.N., Hasrat R., Sanders E.A.M., Bogaert D. Microbial community networks across body sites are associated with susceptibility to respiratory infections in infants. Commun Biol. 2021;4:1233. doi: 10.1038/s42003-021-02755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L., Pekkarinen P.T., Lakshmikanth T., Tan Z., Consiglio C.R., Pou C., Chen Y., Mugabo C.H., Nguyen N.A., Nowlan K., et al. Systems-level immunomonitoring from acute to recovery phase of severe COVID-19. Cell Reports Medicine. 2020;1:100078. doi: 10.1016/j.xcrm.2020.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röltgen K., Nielsen S.C.A., Arunachalam P.S., Yang F., Hoh R.A., Wirz O.F., Lee A.S., Gao F., Mallajosyula V., Li C., et al. mRNA vaccination compared to infection elicits an IgG-predominant response with greater SARS-CoV-2 specificity and similar decrease in variant spike recognition. medRxiv. 2021 10/1101/2021.04.05.21254952. [Google Scholar]
- Sagar M., Reifler K., Rossi M., Miller N.S., Sinha P., White L.F., Mizgerd J.P. Recent endemic coronavirus infection is associated with less-severe COVID-19. J. Clin. Invest. 2021;131:131. doi: 10.1172/JCI143380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Shimizu V., Brodin P., Cobat A., Biggs C.M., Toubiana J., Lucas C.L., Henrickson S.E., Belot A., Tangye S.G., Milner J.D., et al. MIS-C@CHGE SARS-CoV-2-related MIS-C: A key to the viral and genetic causes of Kawasaki disease? J. Exp. Med. 2021;218:e20210446. doi: 10.1084/jem.20210446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman S.T., Rowley A.H. Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat. Rev. Rheumatol. 2015;11:475–482. doi: 10.1038/nrrheum.2015.54. [DOI] [PubMed] [Google Scholar]
- Smith C., Odd D., Harwood R., Ward J., Linney M., Clark M., Hargreaves D., Ladhani S.N., Draper E., Davis P.J., et al. Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat. Med. 2021 doi: 10.1038/s41591-021-01578-1. Published online November 11, 2021. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. The Evolution of Life Histories. Oxford University Press; 1992. [Google Scholar]
- Thabet F., Chehab M., Bafaqih H., Al Mohaimeed S. Middle East respiratory syndrome coronavirus in children. Saudi Med. J. 2015;36:484–486. doi: 10.15537/smj.2015.4.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai K.T.D., Nishiura H., Hoang P.L., Tran N.T.T., Phan G.T., Le H.Q., Tran B.Q., Nguyen N.V., de Vries P.J. Age-specificity of clinical dengue during primary and secondary infections. PLoS Negl. Trop. Dis. 2011;5:e1180. doi: 10.1371/journal.pntd.0001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torun B. Energy requirements of children and adolescents. Public Health Nutr. 2005;8:968–993. doi: 10.1079/phn2005791. [DOI] [PubMed] [Google Scholar]
- Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., Debray A., Basmaci R., Salvador E., Biscardi S., et al. Outbreak of Kawasaki disease in children during COVID-19 pandemic: a prospective observational study in Paris, France. medRxiv. 2020 doi: 10.1101/2020.05.10.20097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Made C.I., Simons A., Schuurs-Hoeijmakers J., van den Heuvel G., Mantere T., Kersten S., van Deuren R.C., Steehouwer M., van Reijmersdal S.V., Jaeger M., et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA. 2020;324:663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., et al. Yale IMPACT Team Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., Ramnarayan P., Fraisse A., Miller O., Davies P., et al. PIMS-TS Study Group and EUCLIDS and PERFORM Consortia Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Xing Y.H., Ni W., Wu Q., Li W.J., Li G.J., Wang W.D., Tong J.N., Song X.F., Wong G.W.K., Xing Q.S. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonker L.M., Gilboa T., Ogata A.F., Senussi Y., Lazarovits R., Boribong B.P., Bartsch Y.C., Loiselle M., Rivas M.N., Porritt R.A., et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Invest. 2021;131:e149633. doi: 10.1172/JCI149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R., Castro M.F.G., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B., et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5:eabc3582. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bastard P., Liu Z., Pen J.L., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bastard P., Bolze A., Jouanguy E., Zhang S.-Y., Cobat A., Notarangelo L.D., Su H.C., Abel L., Casanova J.L., COVID Human Genetic Effort Life-Threatening COVID-19: Defective Interferons Unleash Excessive Inflammation. Med (N Y) 2020;1:14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cobat A., Bastard P., Notarangelo L.D., Su H.C., Abel L., Casanova J.-L., COVID Human Genetic Effort (CHGE) Association of rare predicted loss-of-function variants of influenza-related type I IFN genes with critical COVID-19 pneumonia. J. Clin. Invest. 2021;131:e152474. doi: 10.1172/JCI152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N.-S., Wong G.W.K. Epidemiology of severe acute respiratory syndrome (SARS): adults and children. Paediatr. Respir. Rev. 2004;5:270–274. doi: 10.1016/j.prrv.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2020;106:429–439. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]