Abstract
Since influenza viruses can cause severe illness, timely diagnosis is important for an adequate intervention. The available rapid detection methods either lack sensitivity or require complex laboratory manipulation. This study describes a rapid, sensitive detection method that can be easily applied to routine diagnosis. This method simultaneously detects influenza viruses A and B in specimens of patients with respiratory infections using a TaqMan-based real-time PCR assay. Primers and probes were selected from highly conserved regions of the matrix protein gene of influenza virus A and the hemagglutinin gene segment of influenza virus B. The applicability of this multiplex PCR was evaluated with 27 influenza virus A and 9 influenza virus B reference strains and isolates. In addition, the specificity of the assay was assessed using eight reference strains of other respiratory viruses (parainfluenza viruses 1 to 3, respiratory syncytial virus Long strain, rhinoviruses 1A and 14, and coronaviruses OC43 and 229E) and 30 combined nose and throat swabs from asymptomatic subjects. Electron microscopy-counted stocks of influenza viruses A and B were used to develop a quantitative PCR format. Thirteen copies of viral RNA were detected for influenza virus A, and 11 copies were detected for influenza virus B, equaling 0.02 and 0.006 50% tissue culture infective doses, respectively. The diagnostic efficacy of the multiplex TaqMan-based PCR was determined by testing 98 clinical samples. This real-time PCR technique was found to be more sensitive than the combination of conventional viral culturing and shell vial culturing.
Influenza virus infection is a highly contagious respiratory disease that can spread easily and that is responsible for considerable morbidity and mortality each year. Elderly and compromised individuals are especially at risk of developing severe illness and complications. Therefore, rapid diagnosis is important not only for timely therapeutic intervention but also for the identification of a beginning influenza outbreak. Recently published results of clinical trials using new anti-influenza virus compounds, the neuraminidase inhibitors, demonstrated that these drugs are effective against influenza viruses A and B and are most effective when administered early, when symptoms first emerge (6, 8, 12). With the development of such new treatment options, rapid detection methods become even more desirable.
Virus isolation via cell culturing, shell vial culturing, antigen detection, and serologic analysis are the methods currently used for the laboratory diagnosis of influenza viruses. Each of these methods, however, has its limitations. For example, although virus isolation via cell culturing can be a robust and sensitive method for the detection of limited numbers of viable virions, it is labor-intensive and depends on optimal sample transport for sensitive virus isolation. Moreover, since the concentrations of viable virus can decline rapidly after the first few days of the infection, the virus can become undetectable by culturing in the later course of the infection (7). Finally, the results from cell culturing generally are obtained too late for adequate intervention.
Alternative diagnostic techniques, such as viral antigen detection (immunofluorescence and enzyme immunoassay techniques) and shell vial culturing, on the other hand, provide results much more quickly but generally are less sensitive than conventional cell culturing (4, 11, 15, 18, 20).
To overcome this lack of sensitivity and to obtain rapid diagnostic results, PCR techniques have been developed for the specific detection and subtyping of influenza viruses. They have proven to be very sensitive and specific but, unfortunately, are often difficult to implement in a routine diagnostic setting and still require time-consuming sample handling and post-PCR analysis (1, 3, 5). Needless to say, better techniques are still needed.
Here, we describe a multiplex TaqMan-based real-time PCR assay for the rapid and simultaneous detection of influenza viruses (influenza virus A, influenza virus B, or both) in clinical specimens. We also compare this real-time PCR assay to conventional culturing methods and to an in-house nested PCR assay. The method can generate results within 4 to 5 h and does not require any post-PCR processing (9, 10, 14). Moreover, the assay can be used for direct virus quantification and can be easily implemented in routine viral diagnostic testing.
MATERIALS AND METHODS
Virus stocks.
Influenza virus A/Port Chalmers/1/73 (H3N2), influenza virus B/Lee/40, and parainfluenza viruses 1 to 3 were obtained from the American Type Culture Collection (Manassas, Va.). Influenza virus A and B reference strains and isolates and reference strains of rhinovirus 1A, rhinovirus 14, respiratory syncytial virus (Long strain), coronavirus OC43, and coronavirus 229E were kindly provided by the Laboratory for Virology, National Institute of Public Health and the Environment (Bilthoven, The Netherlands).
Virus particle counts.
Purified human influenza virus A/PR/8 (H1N1) (virus particles were counted by electron microscopy [EM]) was obtained from Advanced Biotechnologies Incorporated (ABI), Columbia, Md. Influenza virus A/Texas/36/91 (H1N1), influenza virus A/Port Chalmers/1/73 (H3N2), and influenza virus B/Lee/40 were propagated at 33°C on tertiary rhesus monkey kidney cells pretreated with Eagle minimal essential medium (BioWhittaker) supplemented with streptomycin, penicillin, amphotericin B, and 0.01% trypsin. After the development of a cytopathic effect, cells and supernatant were harvested and frozen at −70°C. The virus particle count of each stock was then determined by quantitative EM.
Clinical specimens.
Combined nose and throat swab specimens or nasal washes were obtained from individuals with upper or lower respiratory tract symptoms. Some of these specimens were obtained at regional general practices participating in a study to evaluate the efficacy of influenza vaccination. The other clinical samples were obtained from patients with respiratory illnesses at the University Medical Center Utrecht in 1998 and 1999. Routine diagnostic logistics were used for sample transportation from the general practices to the laboratory as well as for sample transportation from the outpatient clinic to the laboratory. The samples that were sent by mail were left at room temperature for a maximum of 24 h. The samples from the outpatient clinic were sent to the laboratory within 2 h. All of the samples were transported in 5 ml of virus transport medium. Nasal wash specimens and swab specimens were vortexed for 10 s and centrifuged at 2,000 × g for 15 min. One milliliter of the supernatant was used directly for virus culturing. The remaining material was stored at −70°C until RNA extraction.
Virus isolation and growth.
Confluent tertiary rhesus monkey kidney cells were inoculated with 100 μl of each clinical sample. After absorption for 1 h at room temperature, the inoculum was removed and 5 ml of fresh Eagle minimal essential medium supplemented with 0.02 M HEPES, 0.075% bicarbonate, 100 U each of penicillin and streptomycin per ml, 25 U of nystatin (Gibco) per ml, 0.2 M glutamine (SVM [Foundation for the Advancement of Public Health and Environment {Stichting Volksgezondheid en Milieu in Dutch}]) and 0.01% trypsin (SVM) was added. The cultures were then incubated at 33°C on roller drums and examined twice weekly for 10 days for a cytopathic effect. Regular testing for hemadsorption was performed using a 0.25% guinea pig erythrocyte suspension. Positive cultures were identified by immunofluorescence with commercial monoclonal antibodies (Dako Imagen) for influenza viruses A and B and parainfluenza viruses 1 to 3. Further subtyping of the strains was performed at the National Reference Center for Influenza, Rotterdam, The Netherlands.
After 2 days of culturing, usually before a cytopathic effect was noticed, rapid antigen testing was performed by immunofluorescence with commercial monoclonal antibodies for influenza viruses A and B (shell vial culturing). The supernatants of the clinical specimens were also cultured on other tissue cell lines (R-HeLa cells and HEp-2c cells) for the detection of other respiratory viruses.
Viral genomic RNA isolation and cDNA synthesis.
RNA extraction was performed according to the method described by Boom et al. (2). Briefly, 10 to 100 μl of respiratory specimen, tissue culture supernatant, or EM-counted virus stock was mixed with 900 μl of lysis buffer and 50 μl of silica and incubated for 10 min at room temperature in order to bind the nucleic acid to the silica particles. Unbound material was removed by several washing steps. The RNA was then eluted either in 100 μl of 40-ng/μl poly(A) RNA before one-tube reverse transcription (RT)-PCR (13) or in 100 μl of RNase-free water before cDNA synthesis.
cDNA was synthesized by using MultiScribe reverse transcriptase and random hexamers (both from PE Applied Biosystems). Each 50-μl reaction mixture contained 10 μl of eluted RNA, 5 μl of 10× RT buffer, 5.5 mM MgCl2, 500 μM each deoxynucleoside triphosphate, 2.5 μM random hexamer, and 0.4 U of RNase inhibitor per μl (all from PE Applied Biosystems). After incubation for 10 min at 25°C, RT was carried out for 30 min at 48°C, followed by RT inactivation for 5 min at 95°C. The cDNA was stored at −70°C before further use.
Qualitative PCR.
A multiplex nested PCR was performed for influenza viruses A and B. A one-tube RT-PCR was followed by a second (nested) amplification. First-round amplification primers and nested primers were selected from conserved regions of the gene for the matrix protein of influenza virus A (first-round primer set: FLU-1, 5′ CAGAGACTTGAAGATGTCTTTGC 3′, and FLU-2, 5′ GGCAAGTGCACCAGCAGAATAACT 3′; second-round primer set: FLU-3, 5′ GACCRATCCTGTCACCTCTGACT 3′, and FLU-4, 5′ ATTTCTTTGGCCCCATGGAATGT 3′) and the hemagglutinin gene segment of influenza virus B (FLUB-5, 5′ GAATCTGCACTGGGATAACATC 3′, and FLUB-8, 5′ TTTGTTCTGTCRATGCATTATAGG 3′; inner primer set: FLUB-2, 5′ TCTCATTTTGCAAATCTCAAAGG 3′, and FLUB-3, 5′ TCRTGGAGTATTGAARCTTTTGC 3′). The RT-PCR and nested PCR conditions that we applied were those described by Nijhuis et al. (13); we used a PE 9600 Thermocycler (Perkin-Elmer). PCR products were visualized on an ethidium bromide-stained agarose gel using UV illumination. A 100-bp marker (5-μl) was used as a control for fragment lengths.
Real-time quantitative PCR.
Primers and probes for influenza viruses A and B were selected using Primer Express software (PE Applied Biosystems) and were based on genomic regions highly conserved in various subtypes and genotypes of influenza virus A (matrix protein gene) and influenza virus B (hemagglutinin gene segment). The exact primers and probes were chosen after a sequence comparison of 39 influenza virus A strains and 44 influenza virus B strains. Probes were obtained without runs of identical nucleotides to avoid nonspecific interactions, with no G's at the 5′ end, and with a melting temperature of 69°C (10°C above the melting temperature of the primers to ensure full hybridization of the probes during primer extension). Moreover, primers and probes were tested for possible interactions to make sure that they could be used together in a multiplex assay. The forward and reverse primers (INFA-1, INFA-2, INFA-3, INFB-1, and INFB-2) and probes (INFAp1/3 and INFBp1/2) used are shown in Table 1. For influenza virus A, two forward primers with different nucleotides at base 4 at the 5′ end were selected to ensure that all strains of influenza virus A could be detected. Both fluorogenic probes for influenza viruses A and B consisted of oligonucleotides with the 5′ reporter dye 6-carboxyfluorescein (FAM) and the 3′ quencher dye 6-carboxytetramethylrhodamine (TAMRA). A 25-μl PCR was performed using 5 μl of cDNA, 12.5 μl of TaqMan universal PCR master mix containing ROX as a passive reference (PE Applied Biosystems), 900 nM each influenza virus A primer, 300 nM each influenza virus B primer, and 100 nM each probe. Amplification and detection were performed with an ABI Prism 7700 sequence detection system under the following conditions: 2 min at 50°C to require optimal AmpErase uracil-N-glycosylase activity, 10 min at 95°C to activate AmpliTaq Gold DNA polymerase, and 45 cycles of 15 s at 95°C and 1 min at 60°C.
TABLE 1.
Selected primers and probes for TaqMan amplification of viral RNA from influenza viruses A and B
| Influenza virus type (target) | Primer or probe | Sequence | Nucleotide positionsa |
|---|---|---|---|
| A (M gene) | INFA-1 | 5′ GGACTGCAGCGTAGACGCTT | 217–236 |
| INFA-2 | 5′ CATCCTGTTGTATATGAGGCCCAT | 382–405 | |
| INFA-3 | 5′ CATTCTGTTGTATATGAGGCCCAT | 277–300 | |
| INFA probe | 5′ CTCAGTTATTCTGCTGGTGCACTTGCCA | 349–376 | |
| B (HA gene) | INFB-1 | 5′ AAATACGGTGGATTAAATAAAAGCAA | 970–995 |
| INFB-2 | 5′ CCAGCAATAGCTCCGAAGAAA | 1119–1139 | |
| INFB probe | 5′ CACCCATATTGGGCAATTTCCTATGGC | 1024–1050 |
Primer and probe positions for influenza virus A correspond to the matrix (M) gene of A/Port Chalmers/1/73 (H3N2) and A/Texas/36/91 (H1N1), and those for influenza virus B correspond to the hemagglutinin (HA) gene of B/Lee/40.
During amplification, the ABI Prism sequence detector monitored real-time PCR amplification by quantitatively analyzing fluorescence emissions. The reporter dye (FAM) signal was measured against the internal reference dye (ROX) signal to normalize for non-PCR-related fluorescence fluctuations occurring from well to well. The threshold cycle represented the refraction cycle number at which a positive amplification reaction was measured and was set at 10 times the standard deviation of the mean baseline emission calculated for PCR cycles 3 to 15.
RESULTS
Sensitivity.
The sensitivity of the multiplex assay was determined in two ways: (i) by a virus infectivity assay and (ii) by counting the viral particles using EM. Influenza virus A/PR/8/34 (sucrose gradient purified) and influenza virus B/Lee/40 were first counted by EM and subsequently titrated by serial dilution. The 50% tissue culture infective doses (TCID50) for the two strains, calculated by the Kärber formula, were 1.8 × 109 and 2.0 × 109/ml, respectively, corresponding to 9 × 1011 and 3.3 × 1012 viral particles, respectively.
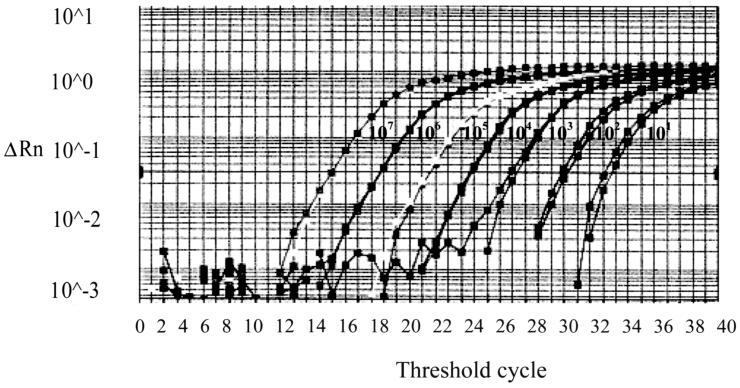
The 10-fold serially diluted concentrations of the two strains were then amplified using the multiplex TaqMan assay. Eleven viral particles of influenza virus B/Lee/40 and 13 viral particles of influenza virus A/PR/8/34 could be detected by both the multiplex TaqMan assay and the separate TaqMan assays for influenza viruses A and B (Fig. 1). This level of sensitivity correlated with 0.02 TCID50 of influenza virus A/PR/8/34 and 0.006 TCID50 of influenza virus B/Lee/40.
FIG. 1.
Standardization of influenza virus B in the multiplex TaqMan assay. Serial dilutions were made using the EM-counted influenza virus B/Lee/40 stock. A minimum of ±10 copies of RNA could be detected after 40 cycles. The intensity of fluorescence is given on the y axis (ΔRn = reporter signal [FAM]/passive reference signal [ROX]).
Specificity.
The specificity of the multiplex TaqMan PCR was assessed by testing reference strains of subtypes of influenza virus A H1N1 (A/Singapore/6/86, A/Taiwan/1/86, A/Texas/36/91, A/Bayern/7/95, A/PR/8/34, and NIB-39rec Bayern), H2N2 (A/Singapore/1/57, A/Japan/307/57, and A/England/1/66), and H3N2 (A/Hongkong/1/68, A/Philadelphia/2/82, A/Shangdong/9/93, A/RESVIR, A/Sydney/5/97, and A/Port Chalmers/1/73); influenza virus B (B/Yamagata/16/88, B/Lee/40, B/Panama/45/90, and B/Singapore/222/79); and a variety of other respiratory viruses (rhinovirus 1A, rhinovirus 14, respiratory syncytial virus [Long strain], coronaviruses OC43 and 229E, and parainfluenza viruses 1 to 3). Five H1N1, seven H3N2, and five influenza virus B isolates from patients were also tested. All of the influenza virus strains but none of the other respiratory viruses were detected. In addition, nose and throat swab specimens obtained from 30 asymptomatic subjects during the winter season were analyzed by the multiplex TaqMan PCR to assess the possibility of false-positive results; none of the samples gave a positive signal.
Comparison of TaqMan PCR, shell vial culturing, and conventional culturing to nested RT-PCR for clinical specimens.
A total of 98 clinical specimens were collected during the 1998-1999 and 1999-2000 winter seasons. Eighty of the samples were sent by mail at room temperature, whereas 18 of the samples were transported to the laboratory immediately at 4°C. The samples were analyzed for influenza viruses A and B using multiplex nested PCR, multiplex TaqMan PCR, cell culturing, and shell vial culturing (Table 2). All of the nested RT-PCR-positive samples were subsequently used in a sensitivity analysis. When the results of the multiplex TaqMan PCR and the combined results of conventional cell culturing and shell vial culturing were compared with those of the nested PCR, overall sensitivities of 88 and 51%, respectively, were found. For the 18 samples that were transported at 4°C, sensitivities were 83% for multiplex TaqMan PCR and 44% for conventional culturing and/or shell vial culturing. For the 80 samples that were sent by mail at room temperature, sensitivities were 96% for multiplex TaqMan PCR and 57% for conventional culturing and/or shell vial culturing.
TABLE 2.
Comparison of conventional culturing or shell vial culturing, multiplex TaqMan PCR, and nested multiplex PCR for the detection of influenza viruses A and B in 98 clinical specimens
| Method | No. (%) of samples that were:
|
|
|---|---|---|
| Positive | Negative | |
| Conventional culturing or shell vial culturing | 22 (12) | 76 (88) |
| Multiplex TaqMan PCR | 40 (41) | 58 (59) |
| Influenza virus A | 36 (37) | 62 (63) |
| Influenza virus B | 4 (4) | 94 (96) |
| Nested multiplex PCR | 44 (45) | 54 (55) |
Longitudinal follow-up.
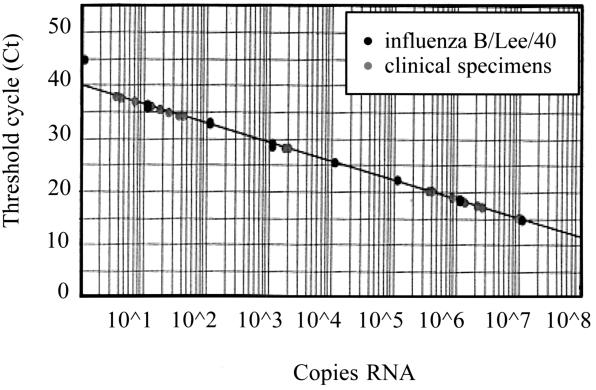
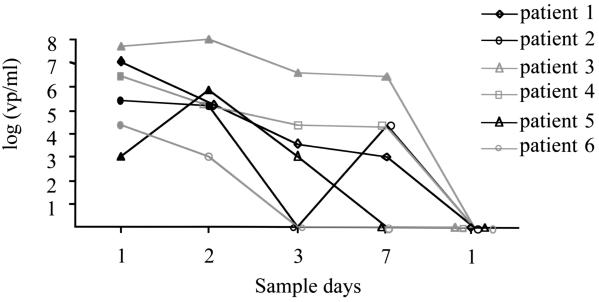
Six patients infected with influenza virus (two with influenza virus B and four with influenza virus A [H3N2]) were monitored during their infections. A total of 30 nasal washes were obtained on days 1 to 3, 7, and 14 after the presentation of influenza-like symptoms. The number of viral RNA copies in the clinical samples was determined by extrapolation to a standard curve generated upon amplification of serial dilutions of the EM-counted virus stocks (A/PR/8/34 and B/Lee/40) (Fig. 2). Using the multiplex TaqMan PCR, we were able to detect and quantify influenza virus in nasal washes up to 7 days after the initial presentation of influenza-like symptoms in four patients, as shown in Fig. 3. Using conventional culturing, we could detect virus on day 7 only in one patient. The multiplex TaqMan PCR was also much more sensitive for the detection of influenza viruses A and B than conventional culturing and/or shell vial culturing: 20 of 30 specimens (66%) were positive with the multiplex TaqMan PCR, while 11 of 30 specimens (35%) were positive with tissue cell culturing and/or shell vial culturing.
FIG. 2.
Standard curve generated by the analysis of known amounts of viral RNA of influenza virus B/Lee/40 with the multiplex TaqMan PCR. Unknown quantities of virus in clinical specimens are plotted against the standard curve.
FIG. 3.
Longitudinal follow-up of six patients with either influenza virus A (patients 3 to 6) or virus B infection (patients 1 and 2). Quantitative analysis was performed using the multiplex TaqMan PCR. The clinical samples were plotted against the standard curve. The filled symbols represent the clinical specimens that were also found positive by conventional culturing and/or shell vial culturing. vp, virus particles.
DISCUSSION
Our findings demonstrate that the multiplex TaqMan PCR is a sensitive and specific method for the simultaneous rapid detection of influenza viruses A and B. In fact, we were able to detect as little as 0.02 TCID50 for influenza virus A and 0.006 TCID50 for influenza virus B, corresponding to approximately 10 viral RNA copies.
For epidemiological reasons, it is important to type and subtype influenza virus strains. In recent studies, typing and subtyping of influenza virus strains have been performed using (multiplex) RT-PCR (5, 16, 19). This type of analysis, however, is time-consuming, either because (sub)type-specific PCRs need to be performed or because the post-PCR analysis is complicated.
The multiplex TaqMan PCR described here allows extremely rapid and accurate diagnosis of both types of influenza viruses within 4 to 5 h. Our type-specific probes, for example, can be labeled with different fluorogenic dyes to distinguish between influenza viruses A and B, because the ABI Prism 7700 sequence detection system has the capability of detecting multiple dyes with distinct emission wavelengths (17). Then, sequential TaqMan PCRs using subtype-specific primers can be performed to subtype influenza virus A in detail (16).
Besides being rapid, this method also has the advantage of a standardized protocol that can be applied easily to other respiratory viruses; the TaqMan PCR can be performed under uniform amplification conditions, thereby allowing the use of target-specific primer and probe sets. In addition, the procedure is less complicated than other RT-PCR methods, and the chances of contamination are minimized because there is no post-PCR processing of the samples.
The multiplex TaqMan PCR was more sensitive than standard conventional culturing or shell vial culturing; i.e., the multiplex TaqMan PCR detected influenza viruses at lower concentrations. The low recovery rate with culture techniques is usually explained by viral inactivation caused by the transportation of samples. However, in this study, the transport conditions did not affect the sensitivity of conventional culturing, although the number of tested clinical specimens was small.
In order to correct for false-positive results, we obtained samples not only from symptomatic patients but also from asymptomatic individuals during the same influenza season. Since none of the latter samples contained influenza virus RNA, the positive results obtained with the multiplex TaqMan PCR, which were confirmed by nested PCR, can be considered true positives.
Follow-up of the six symptomatic patients showed that influenza virus could be detected up to 7 days after infection using the multiplex TaqMan PCR, a period when most of the patients were still clinically ill. In contrast, influenza virus could be isolated by conventional culturing only during the first 1 or 2 days for the majority of these patients.
We were able to quantify the results of our PCR technique using serial dilutions of EM-counted stocks of influenza viruses A and B. A standard curve could be generated with the multiplex TaqMan PCR, creating a quantitative format for the assay. Even though influenza virus infection usually persists for only 1 week, quantification might be a useful tool for evaluating the effects of antiviral therapy.
In conclusion, we have developed a rapid, highly sensitive and specific quantitative real-time PCR for the simultaneous detection of influenza viruses A and B. Results can be obtained within a few hours, thus allowing time for adequate clinical management and the evaluation of antiviral therapy.
ACKNOWLEDGMENTS
We thank Charles Boucher, Department of Virology, University Medical Center Utrecht, for critically reading the manuscript. We also thank Eric Claas, Department of Virology, University Medical Center Leiden, for the gift of A/Japan/307/57 virus and A/England/1/66 virus.
REFERENCES
- 1.Atmar R L, Baxter B D, Dominguez E A, Taber L H. Comparison of reverse transcription-PCR with tissue culture and other rapid diagnostic assays for detection of type A influenza virus. J Clin Microbiol. 1996;34:2604–2606. doi: 10.1128/jcm.34.10.2604-2606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boom R, Sol C J, Salimans M M, Jansen C L, Wertheim-van Dillen P M, van der Noorda J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claas E C, van Milaan A J, Sprenger M J, Ruiten-Stuiver M, Arron G I, Rothbarth P H, Masurel N. Prospective application of reverse transcriptase polymerase chain reaction for diagnosing influenza infections in respiratory samples from a children's hospital. J Clin Microbiol. 1993;31:2218–2221. doi: 10.1128/jcm.31.8.2218-2221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doller G, Schuy W, Tjhen K Y, Stekeler B, Gerth H J. Direct detection of influenza virus antigen in nasopharyngeal specimens by direct enzyme immunoassay in comparison with quantitating virus shedding. J Clin Microbiol. 1992;30:866–869. doi: 10.1128/jcm.30.4.866-869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis J S, Fleming D M, Zambon M C. Multiplex reverse transcription-PCR for surveillance of influenza A and B viruses in England and Wales in 1995 and 1996. J Clin Microbiol. 1997;35:2076–2082. doi: 10.1128/jcm.35.8.2076-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayden F G, Atmar R L, Schilling M, Johnson C, Poretz D, Paar D, Huson L, Ward P, Mills R G. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–1343. doi: 10.1056/NEJM199910283411802. [DOI] [PubMed] [Google Scholar]
- 7.Hayden F G, Osterhaus A D M E, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 8.Hayden F G, Treanor J J, Fritz R S, Lobo M, Betts R F, Miller M, Kinnersley N, Mills R G, Ward P, Straus S E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 9.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 10.Kato T, Mizokami M, Mukaide M, Orito E, Ohno T, Nakano T, Tanaka Y, Kato H, Sugauchi F, Ueda R, Hirashima N, Shimamatsu K, Kage M, Kojiro M. Development of a TT virus DNA quantification system using real-time detection PCR. J Clin Microbiol. 2000;38:94–98. doi: 10.1128/jcm.38.1.94-98.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kok J, Mickan L, Burrell C J. Routine diagnosis of seven respiratory viruses and Mycoplasma pneumoniae by enzyme immunoassay. J Virol Methods. 1994;50:87–100. doi: 10.1016/0166-0934(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 12.Management of Influenza in the Southern Hemisphere Trialists Study Group. Randomised trial of efficacy and safety of inhaled zanamavir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- 13.Nijhuis M, Boucher C A, Schuurman R. Sensitive procedure for the amplification of HIV-1 RNA using a combined reverse-transcription and amplification reaction. BioTechniques. 1995;19:178–180. , 182. [PubMed] [Google Scholar]
- 14.Pongers-Willemse M J, Verhagen O J, Tibbe G J, Wijkhuijs A J, de Haas V, Roovers E, van der Schoot C E, van Dongen J J. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia. 1998;12:2006–2014. doi: 10.1038/sj.leu.2401246. [DOI] [PubMed] [Google Scholar]
- 15.Schmid M L, Kudesia G, Wake S, Read R C. Prospective comparative study of culture specimens and methods in diagnosing influenza in adults. Br Med J. 1998;316:275. doi: 10.1136/bmj.316.7127.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweiger B, Zadow I, Heckler R, Timm H, Pauli G. Application of a fluorogenic PCR assay for typing and subtyping of influenza viruses in respiratory samples. J Clin Microbiol. 2000;38:1552–1558. doi: 10.1128/jcm.38.4.1552-1558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vet J A, Majithia A R, Marras S A, Tyagi S, Dube S, Poiesz B J, Kramer F R. Multiplex detection of four pathogenic retroviruses using molecular beacons. Proc Natl Acad Sci USA. 1999;96:6394–6399. doi: 10.1073/pnas.96.11.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiselka M. Influenza: diagnosis, management, and prophylaxis. Br Med J. 1994;308:1341–1345. doi: 10.1136/bmj.308.6940.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright K E, Wilson G A, Novosad D, Dimock C, Tan D, Weber J M. Typing and subtyping of influenza viruses in clinical samples by PCR. J Clin Microbiol. 1995;33:1180–1184. doi: 10.1128/jcm.33.5.1180-1184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler T, Hall H, Sanchez-Fauquier A, Gamble W, Cos N. Type and subtype specific detection of influenza viruses in clinical specimens by rapid culture assay. J Clin Microbiol. 1995;33:318–322. doi: 10.1128/jcm.33.2.318-321.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]