Abstract
Background & Aims
In the context of the Italian severe acute respiratory syndrome coronavirus 2 vaccination program, liver transplant (LT) recipients were prioritized for vaccine administration, although the lower response to vaccines is a well-known problem in this population. We aimed to evaluate immunogenicity of BNT162b2 mRNA vaccine in LT recipients and healthy controls and to identify factors associated with negative response to vaccine.
Methods
In a cohort of adult patients with LT, we prospectively evaluated the humoral response (with anti-Spike protein IgG-LIAISON SARS-CoV-2 S1/S2-IgG chemiluminescent assay) at 1 and 3 months after 2-dose vaccination. A group of 307 vaccinated health care workers, matched by age and sex, served as controls.
Results
Overall, 492 LT patients were enrolled (75.41% male; median age, 64.85 years). Detectable antibodies were observed in the 75% of patients, with a median value of 73.9 AU/mL after 3 months from 2-dose vaccination. At multivariable analysis, older age (>40 years; P = .016), shorter time from liver transplantation (<5 years; P = .004), and immunosuppression with antimetabolites (P = .029) were significantly associated with non-response to vaccination. Moreover, the LT recipients showed antibody titers statistically lower than the control group (103 vs 261 AU/mL; P < .0001). Finally, in both controls and LT patients, we found a trend of inverse correlation between age and antibody titers (correlation coefficients: −0.2023 and −0.2345, respectively).
Conclusions
Three months after vaccination, LT recipients showed humoral response in 75% of cases. Older age, shorter time from transplantation, and use of antimetabolites were factors associated with non-response to vaccination, and LT recipients at risk of non-response to vaccination needed to be kept under close monitoring.
Keywords: COVID-19, Immunosuppression, Liver Transplantation, SARS-CoV-2 Vaccination
Abbreviations used in this paper: COVID-19, coronavirus disease 2019; HCC, hepatocellular carcinoma; IQR, interquartile range; LT, liver transplant; SARS-CoV 2, severe acute respiratory syndrome coronavirus 2
What You Need to Know.
Background
Liver transplant recipients showed poor humoral response to coronavirus disease 2019 vaccination when compared with healthy subjects.
Findings
The factors associated with a non-response response to coronavirus disease 2019 vaccination are older age, shorter time from liver transplantation, and immunosuppression regimens including antimetabolites.
Implications for patient care
This study identified liver transplant recipients at risk of non-response to vaccination to be kept under close monitoring.
The coronavirus disease 2019 (COVID-19) pandemic rapidly spread in the first months of 2020, and at the end of 2020, an mRNA vaccine (BNT162b2) was approved.1 , 2 Solid organ transplant recipients are considered a vulnerable group at increased risk of severe disease and death in case of infection.3 For this reason, this group of patients has been prioritized in the timeline schedule of the National Vaccination Program. Data about immunogenicity of vaccination in liver transplant (LT) recipients are poor because this population was excluded from clinical trials. Additionally, lower response to vaccination is a well-known problem in immunocompromised solid organ recipients due to the chronic immunosuppressive state.4 Nonetheless, national and international transplant societies have recommended to prioritize vaccination for all recipients with a follow-up of more than 3 months post-liver transplant.5, 6, 7
Previous studies in the setting of influenza vaccination suggest that, despite lower antibody and cell-mediated immunity elicited by solid organ transplant recipients, vaccination has been significantly associated with lower rates of severe infection and complications.7
Concordant low immunogenicity has been reported by different recent studies conducted in LT recipients analyzing small series of patients with a great heterogeneity in terms of timing of post-vaccine response assessment, of vaccine administered (Moderna, Pfizer, Astra-Zeneca, or Johnson & Johnson), and of type of response (humoral and/or cellular) analyzed.8, 9, 10, 11, 12, 13, 14, 15, 16
In this scenario, the primary objective of the present study was to evaluate immunogenicity and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination with Pfizer-BioNTech in a cohort of LT recipients by quantifying the humoral response after 1 and 3 months. Secondary objectives were: (1) to compare the antibody response in LT recipients with a group of healthy subjects; (2) to explore the impact of different factors (ie, age, type of immunosuppression, steroid treatment, comorbidities, etc) on elicited humoral immune responses to better stratify those at-risk for seronegative response; and (3) to study clinical reactogenicity of Pfizer-BioNTech as well as breakthrough infections in vaccinated LT recipients.
Methods
Study Design and Target Population
This is a prospective study evaluating the effectiveness of SARS-CoV-2 vaccination with Pfizer-BioNTech in a cohort of adult LT recipients regularly followed up at 2 referral hospitals in Southern Italy (Cardarelli Hospital and Federico II Academic Hospital) in accordance with the Declaration of Helsinki.
The protocol was approved by the local ethic board of the promoting center (Federico II University of Naples, n 214/2021). All patients and controls involved in the study provided written informed consent to participate.
From March 2021, transplant recipients were prioritzed in Italy to receive the Pfizer-BioNTech BNT162b2 vaccine, administered in 2 doses given 3 weeks apart, in the context of the national COVID-19 vaccination program launched in January 2021. The administration of vaccine was conducted according to the vaccination schedule specified in the BNT162b2 vaccine summary of product characteristics.17
In this prospective cohort, all consecutive LT patients who completed the 2-dose BNT162b2 vaccine series before May 20, 2021 were included and followed-up until August 15, 2021.
After signing the informed consent, blood samples were collected at 3 time points: prior to first dose and 1 month and 3 months after the second dose of the BNT162b2 vaccine for detecting anti-Spike protein IgG (DiaSorin, Italy).18
Exclusion criteria for receiving the vaccine and entering the study included age <18 years, transplantation within the last 3 months, and active SARS-CoV-2 infection. Of note, LT patients with simultaneous kidney (or other solid organ) transplantation have been excluded according to study design.
History of previous COVID-19 was not an exclusion criterion, and patients were considered for vaccination 3 months after the infection.
Clinical data was obtained from patients’ medical records and routine blood tests up to 3 months prior to the day of first dose administration. In particular, the following data were collected for analysis: age, gender, body mass index, comorbidities, transplant date, immunosuppressive drugs, use of steroids, and history of retransplantation.
Control Group
Health care workers of the Cardarelli Hospital and Federico II Academic Hospital who were offered the vaccination with BNT162b2 in-hospital served as controls. History of previous COVID-19 was registered. None of them received immunosuppressive treatment or had major comorbidities. Because the 2 populations were not matched for age and sex, a subgroup within the transplanted patients was selected and matched in a 1:1 ratio by sex and age class with controls. Accordingly, we selected 2 subpopulation consisting of 307 cases (LT recipients) and 307 controls (health care workers).
Details about quantification of humoral response, previous COVID-19 exposure, safety report, breakthrough COVID-19 infections, and statistical analysis are reported in the Supplementary Materials (including Supplementary Methods, Supplementary Results, and Supplementary Tables 1 and 2).
Results
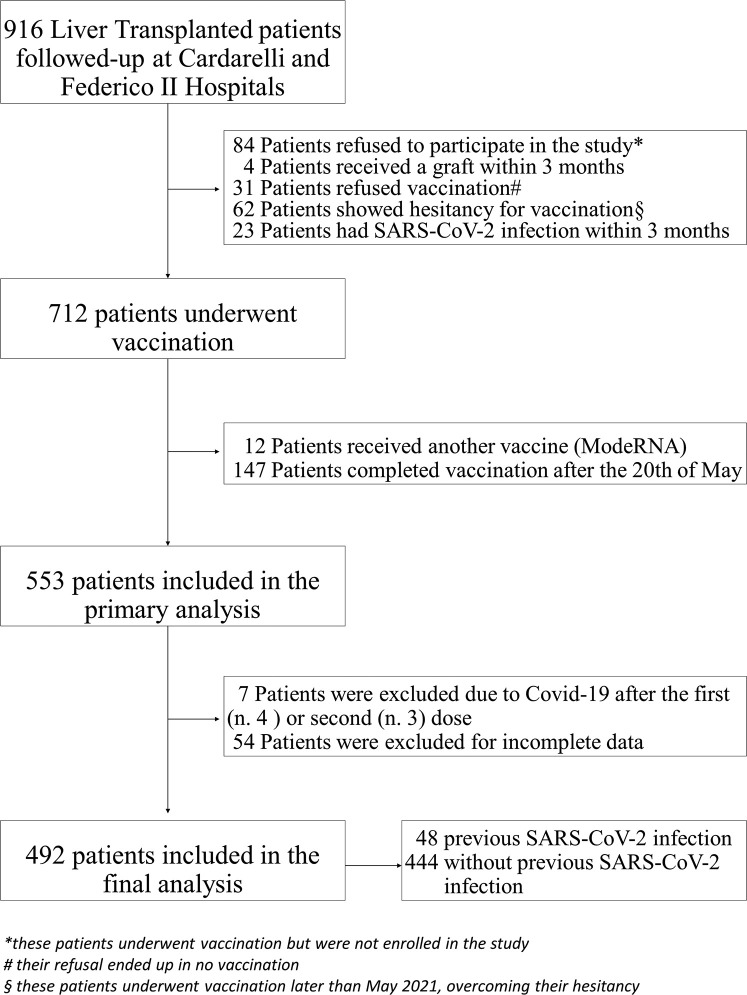
A total of 916 patients who were actively followed at Cardarelli Hospital and Federico II Academic Hospital after liver transplantation were initially screened for BNT162b2 vaccine and the final population included 492 patients. All of these patients received the 2 doses within the 20th of May, and they were prospectively and consecutively included in the study. A study flowchart is depicted in Figure 1 .
Figure 1.
Study flowchart.
Characteristics of LT Recipients
Characteristics of the 492 enrolled patients are described in Table 1 . Overall, 371 LT recipients (75.41%) were male, and the median age at the time of vaccination was 64.85 years (interquartile range [IQR], 57.2–70.09 years). One hundred patients (20.33%) were obese, according to body mass index ≥30 kg/m2. All patients had stable graft function prior to the vaccination. Fourteen patients had a history of retransplantation.
Table 1.
Characteristics, Medications, and Comorbidities of LT Recipients, Overall Population, and Stratified by Subsequent Humoral Response to 2-dose BNT162b2 Vaccine or Previous SARS-CoV-2 Exposure
| Characteristics | Overall (n = 492) | Negative serology to SARS-CoV-2 vaccination (No previous COVID-19) (n = 108) |
Positive serology to SARS-CoV-2 vaccination (No previous COVID-19) (n = 336) |
Previous COVID-19 (n = 48) |
|---|---|---|---|---|
| Age, y | 64.85 (57.20–70.09) | 65.57 (59.40–70.96) | 65.17 (56.93–70.13) | 63.51 (51.25–67.48) |
| <40 | 41 (8.33) | 1 (0.91) | 31 (9.22) | 9 (18.75) |
| 40–65 | 206 (41.87) | 50 (46.29) | 136 (40.47) | 20 (41.67) |
| >65 | 245 (49.80) | 57 (52.80) | 169 (50.31) | 19 (39.58) |
| Male sex | 371 (75.41%) | 83 (76.85) | 249 (74.1) | 39 (81.25%) |
| BMI, kg/m2 | 26.50 (24–29) | 26 (23–28.50) | 26.25 (24–29) | 27.25 (25.25–30) |
| 25–30 | 224 (45.53) | 52 (48.15) | 147 (43.75) | 25 (52.08) |
| ≥30 | 100 (20.33) | 17 (15.78) | 70 (20.83) | 13 (27.68) |
| Smoke history | 122 (24.95) | 33 (30.55) | 74 (22.02) | 15 (31.25) |
| Indication to LT | ||||
| HCC | 192 (39.02) | 53 (49.07) | 120 (35.71) | 19 (39.58) |
| Advanced chronic liver disease | 300 (60.98) | 55 (50.93) | 216 (64.29) | 29 (60.42) |
| Etiology of liver diseasea | ||||
| Viral infection | 376 (76.42) | 85 (78.7) | 257 (76.49) | 34 (70.83) |
| ALD | 38 (7.72) | 9 (8.33) | 25 (7.44) | 4 (8.33) |
| NAFLD | 8 (1.63) | 3 (2.78) | 5 (1.49) | 0 (0.00) |
| Autoimmune liver diseases | 22 (4.47) | 4 (3.70) | 14 (4.17) | 4 (8.33) |
| Other | 50 (10.16) | 6 (5.56) | 37 (11.01) | 7 (14.58) |
| History of re-transplantation | 14 (2.85) | 3 (2.77) | 10 (2.97) | 1 (2.08) |
| History of rejection | 113 (22.97) | 28 (25.93) | 73 (21.73) | 12 (25) |
| Time from transplantation, y | 14.08 (5.71–20.07) | 9.96 (3.65–16.74) | 15.17 (6.56–20.84) | 13.96 (5.85–21.57) |
| <1 | 13 (2.64) | 7 (6.49) | 5 (1.48) | 1 (2.08) |
| 1–5 | 93 (18.9) | 28 (25.92) | 55 (16.36) | 10 (20.83) |
| 6–10 | 80 (16.26) | 19 (17.59) | 55 (16.36) | 6 (12.50) |
| >10 | 306 (62.20) | 54 (50) | 221 (65.8) | 31 (64.58) |
| Immunosuppressive drugs | ||||
| Calcineurin inhibitor | 395 (80.28) | 87 (80.56) | 270 (80.36) | 38 (79.17) |
| Antimetabolite | 168 (34.15) | 53 (49.07) | 98 (29.17) | 17 (35.42) |
| mTor inhibitor | 137 (27.85) | 36 (33.33) | 82 (24.40) | 19 (39.58) |
| Single drug | 293 (59.55) | 43 (39.81) | 225 (66.96) | 25 (52.08) |
| Two or more drugs | 199 (40.45) | 65 (60.19) | 111 (33.04) | 23 (47.92) |
| Steroids | 35 (7.11) | 9 (8.33) | 19 (5.65) | 7 (14.58) |
| Comorbidities | ||||
| Cardiovascular disease | 79 (16.12) | 25 (23.14) | 47 (13.98) | 7 (14.58) |
| Diabetes mellitus | 107 (21.75) | 26 (24.07) | 73 (21.72) | 8 (16.67) |
| Active cancer | 16 (3.25) | 3 (2.77) | 10 (2.97) | 3 (6.25) |
| Previous cancer | 71 (14.43) | 15 (13.88) | 46 (13.69) | 10 (20.83) |
| Chronic kidney disease | 51 (10.39) | 12 (11.11) | 34 (10.11) | 5 (10.42) |
| Respiratory disease | 10 (2.03) | 3 (2.77) | 6 (1.78) | 1 (2.08) |
| Two or more comorbidities | 75 (15.24) | 18 (16.66) | 51 (15.17) | 6 (12.50) |
Note: Data are presented as number (percent) or median (interquartile range).
ALD, Alcoholic liver disease; BMI, body mass index; COVID-19, coronavirus disease 2019; HCC, hepatocellular carcinoma; LT, liver transplant; NAFLD, nonalcoholic fatty liver disease; SARS-CoV 2, severe acute respiratory syndrome coronavirus 2.
One patient can have more than one cause of liver disease, so the sum of etiologies did not necessarily sum to 100%.
The major indication for LT was viral infection (76.4%), followed by hepatocellular carcinoma (HCC) (39%). Overall, 293 patients (59.55%) received a single immunosuppressant agent, with calcineurin inhibitors as the predominant immunosuppressants (80.28%). In particular, 286 patients (58.13%) were on tacrolimus, alone or in combination, 109 patients (22.15%) were on cyclosporine A alone or in combination, 168 patients (34.15%) were on mycofenolate mofetil alone or in combination, and 137 patients (27.85%) were on mTOR inhibitors (everolimus or sirolimus), alone or in combination.
The median time from LT to COVID-19 vaccination was 14.08 years (IQR, 5.71–20.07 years), and 386 patients (78.45%) had been transplanted more than 5 years before vaccination, whereas 13 patients (2.64%) received transplantation within 1 year. No patient transplanted during the past year (during the COVID-19 pandemic) received livers from SARS-CoV-2 positive donors; however, there was no information about previous SARS-CoV-2 infection in 3 donors.
In relation to comorbidities, 79 patients (16.12%) had cardiovascular disease, 51 patients (10.39%) had chronic kidney disease, and 107 patients (21.75%) had diabetes. Multiple comorbidities were not very frequent, with 75 patients (15.24%) having 2 or more comorbidities (Table 1).
Humoral Response of LT Recipients
Detectable levels of antibodies were observed in 333 of 444 patients (75%), with a median value of 99.05 AU/mL (IQR, 25.05–278 AU/mL) at 1 month (median, 28 days; IQR, 28–31 days), and in 336 of 444 patients (75.67%) with a median value of 73.9 AU/mL (IQR, 25.75–156 AU/mL) at 3 months (median, 88 days; IQR, 86–91 days) after 2-dose vaccination, respectively.
Among patients with detectable antibody titer at 1 month, 16 of 333 patients (4.8%) fell below the threshold of positivity at 3 months, whereas antibody titer increased in 20 of 333 patients (6%), decreased in 142 of 333 patients (42.64%), and remained stable in 155 of 333 patients (46.54%). On the other hand, among those with undetectable titer at 1 month, 19 of 111 patients (17.1%) developed detectable antibody titers at 3 months.
Among those with a high-positive titer at 1 month (>400 AU/mL), 17 of 83 patients (20.48%) dropped to a titer below 200 AU/mL, and 38/83 (45.78%) remained strongly positive at 3 months.
Data from the 48 patients who were seropositive at timepoint 0, indicating previous SARS-CoV-2 infection, were analyzed separately.
Factors Associated With Negative Serology Among LT Recipients
At univariable analysis, comparing patients with negative vs positive serology to SARS-CoV-2 vaccination, the factors statistically significant associated with absence of humoral response were older age (>40 years; P = .016), history of HCC as indication for LT (P = .014), shorter time from liver transplantation (<5 years; P = .004), presence of cardiovascular disease as comorbidity (P = .028), immunosuppressive regimens with multiple drugs (P < .001), and antimetabolite therapy (P < .001) (Table 2 ). At multivariable analysis, only age, baseline immunosuppression, and time from LT were significantly associated with vaccination non-response (Table 2).
Table 2.
Univariable and Multivariable Analysis on Factors Associated With Positive Serology to BNT162b2 Vaccine in LT Recipients According to Antibody Titers 3 Months After 2-dose Vaccination (Dependent Variable: Positive Serology)
| Characteristics | Univariable, OR (95% CI) | P value | Multivariable, OR (95% CI) | P value |
|---|---|---|---|---|
| Age, y (continuous) | 0.98 (0.95–0.99) | .016 | ||
| <40 | Ref | Ref | ||
| 40–65 | 0.09 (0.01–0.66) | .018 | 0.12 (0.02–0.99) | .049 |
| >65 | 0.09 (0.01–0.72) | .022 | 0.11 (0.01–0.86) | .036 |
| Male sex | 0.86 (0.52–1.43) | .568 | ||
| BMI, kg/m2 (continuous) | 1.03 (0.97–1.08) | .307 | ||
| <25 | ref | |||
| 25–30 | 0.93 (0.57–1.49) | .755 | ||
| ≥30 | 1.35 (0.71–2.56) | .36 | ||
| Smoke history, yes | 0.64 (0.39–1.04) | .069 | ||
| Indication to LT | ||||
| HCC | 192 (39.02) | .014 | 0.77 (0.46–1.28) | .327 |
| Advanced chronic liver disease | 300 (60.98) | .014 | Omitteda | |
| Etiology of liver disease | ||||
| Viral infection | 0.88 (0.52–1.48) | .634 | ||
| ALD | 0.88 (0.39–1.96) | .762 | ||
| NAFLD | 0.53 (1.12–2.25) | .388 | ||
| Autoimmune liver diseases | 1.13 (0.36–3.51) | .832 | ||
| Other | 2.10 (0.86–5.13) | .102 | ||
| History of re-transplantation | 1.07 (0.29–3.97) | .915 | ||
| History of rejection | 0.79 (0.48–1.31) | .366 | ||
| Time from transplantation, y | ||||
| <1 | Ref | Ref | ||
| 1–5 | 2.75 (0.80–9.45) | .108 | 2.33 (0.64–8.43) | .195 |
| 5–10 | 4.05 (1.15–14.29) | .03 | 3.53 (0.93–13.39) | .063 |
| >10 | 5.73 (1.75–18.75) | .004 | 4.55 (1.24–16.60) | .022 |
| Immunosuppressive drugs | ||||
| Calcineurin inhibitor | 0.98 (0.57–1.70) | .964 | ||
| Antimetabolite | 0.42 (0.27–0.66) | < .00 | 0.51 (0.28–0.93) | .029 |
| mTor inhibitor | 0.64 (0.40–1.03) | .069 | ||
| Single drug | Ref | |||
| Two or more drugs | 0.32 (0.20–0.51) | < .00 | 0.58 (0.31–1.03) | .082 |
| Steroids | 0.66 (0.29–1.50) | .322 | ||
| Comorbidities | ||||
| Cardiovascular disease | 0.54 (0.31–0.93) | .028 | 0.58 (0.32–1.03) | .065 |
| Diabetes mellitus | 0.87 (0.52–1.46) | .61 | ||
| Active cancer | 1.07 (0.29–3.97) | .915 | ||
| Previous cancer | 0.98 (0.52–1.84) | .958 | ||
| Chronic kidney disease | 0.90 (0.45–1.81) | .776 | ||
| Respiratory disease | 0.63 (0.15–2.58) | .528 | ||
| Two or more comorbidities | 0.89 (0.49–1.60) | .71 |
Note: Estimates were derived from multivariable logistic regression models adjusted for all the outcome variables. Only the variables found to be significant in the monovariate analysis were added in the multivariate.
Note: Boldface P values indicate statistical significance.
ALD, Alcoholic liver disease; BMI, body mass index; CI, confidence interval; HCC, hepatocellular carcinoma; LT, liver transplant; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio.
Omitted because of collinearity
Additional analysis was separately conducted in patients with positive serology to vaccine comparing strongly positive patients (antibody titer >400 AU/mL) vs other positive patients (antibody titer between 25 and 400 AU/mL) to identify factors associated with higher humoral response. At univariable analysis, the factors statistically significant associated with a stronger humoral response were younger age (<40 years; P > .001), and indication to LT (P = .029 for viral infection and P = .005 for “other indication” to LT [ie, indication not including viral infection, alcoholic and non-alcoholic liver disease, or HCC]). At multivariable analysis, only younger age was still significantly associated with strong response to the vaccine.
Humoral Response of Controls
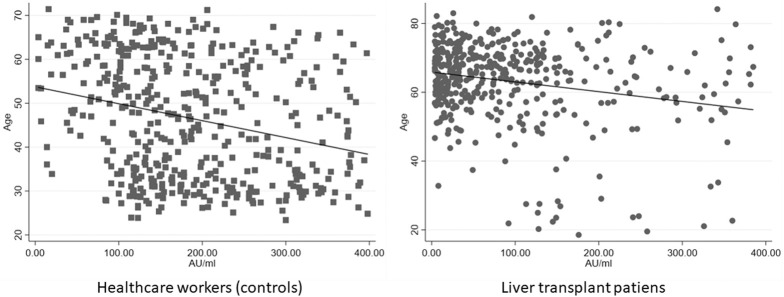
A subpopulation consisting of 307 cases and 307 controls without prior infection with SARS-CoV-2, matched in a 1:1 ratio by sex and age class, was further analyzed. We compared the serology of the selected 307 LT patients with a control group of 307 health care workers with no major comorbidities matched for age class and gender (median age, 59.45 years; IQR, 51.91–64.64 years; 69.38% male). In the control group, only 7 of 307 patients (2.28%) showed negative serology 12 weeks after 2-dose vaccination, whereas in the LT group, 70 of 307 patients (22.80%) showed negative serology (P < .0001). After 3 months from the 2-dose vaccination, antibody titers of controls showed a median value of 261 AU/mL (IQR, 128–401 AU/mL), statistically higher than the LT patients (103 AU/mL; IQR, 28.6–246 AU/mL; P < .0001) (Figure 2 ). In the entire population, both in the control and LT group, we found a trend of inverse correlation between age and antibody titers (correlation coefficient: −0.2023; P < .0001 and −0.2345; P < .0001, respectively) (Figure 3 ). Finally, a multivariate analysis showed that factors associated with a seronegative response to vaccination were older age and liver transplantation (Supplementary Table 2).
Figure 2.
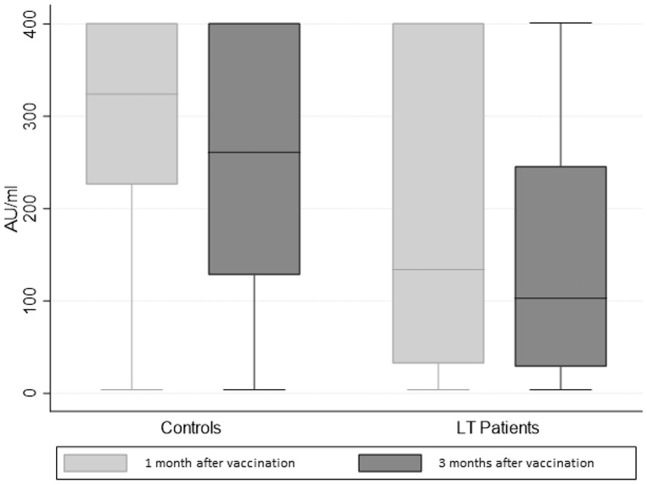
Humoral response to 2-dose BNT162b2 vaccine after 1 and 3 months in LT patients and health care workers (controls).
Figure 3.
Scatterplot correlation between age and antibody titers in LT recipients (right panel) and health care workers as controls (left panel).
Data about characteristics and humoral response of patients with previous SARS-CoV-2 infection, breakthrough infections after vaccine, tolerability and safety of BNT162b2 vaccination are reported in the Supplementary Results.
Discussion
To our knowledge, this is the first study prospectively evaluating humoral response to SARS-CoV-2 BNT162b2 vaccination in a large cohort of liver transplanted patients (492 LT patients) followed-up for 3 months after the completion of the schedule. Our results showed a less frequent positive humoral response in LT patients when compared with healthy controls (77% vs 98%) and identified the most vulnerable individuals among them (ie, older age, shorter time from LT, and immunosuppression regimens including antimetabolites).
Although knowledge is limited due to the absence of well-defined clinical trials in this special population, it is well-established that the immune response elicited by vaccines is multi-faceted and that usually antibody- and cell-mediated responses in solid organ transplanted subjects are less pronounced. In a systematic review, response varied in relation to the type of vaccine, age, and type of organ transplanted,4 whereas some studies showed that additional doses or higher doses are useful to increase immunogenicity.19 Nonetheless, it is important to underline that the interpretation of immunological response to vaccination in transplanted subjects is complex and needs to consider several variables such as the organ transplanted, timing from transplantation, age, current or past treatment, and comorbidities. According to this data, international and national societies recommended SARS-CoV-2 vaccination in all solid organ transplant recipients (12 years and older) as well as priority vaccination of their household members and caregivers to reduce infection risk for these vulnerable patients.5 , 6
The results of the present study are in line with Boyarsky, showing that 67% of solid organ transplanted patients mounted a positive humoral response to SARS-CoV-2 vaccine after 3 months from the second dose.10 Similarly, Strauss reported 81% of seropositivity after 1 month from the 2-dose vaccination in a cohort of 161 LT patients,14 in contrast to initial results published by Rabinovich, which showed a 47.5% rate after 1 month in a cohort of 80 LT subjects.8 Additionally, they observed negative serologic response in patients of older age (>63 years), the use of high-dose prednisone in the past 12 months, and in treatment with regimens including antimetabolites and triple immunosuppressive therapy.8 Our results confirm that older age and use of antimetabolite are factors associated with absence of humoral response to the vaccine, but we also identified the shorter time from liver transplantation as an additional risk factor, confirming data reported by Strauss,14 Herrera,13 and Mazzola.12 It is well-known that the “immunosenescence” (ie, an age-related dysregulation and decline of the immune system) has been associated with a weak response to vaccinations in older subjects.20 We showed a correlation between age and antibody titer confirming the effect of immunosenescence. The increased risk of absent humoral response to SARS-CoV-2 vaccination in the first years after transplantation can be explained because of a higher level of immunosuppression during induction and in the immediate post-transplant period. Moreover, we showed a poor antibody response in LT patients undergoing immunosuppressive regimes containing antimetabolites. This finding has been observed in different settings because mycophenolic acid inhibits de novo purine biosynthesis in lymphocytes suppressing antibody production and cell-mediated immune activity.21, 22, 23 Finally, when compared with health care workers, LT patients showed a significantly reduced level of humoral response (103 AU/mL vs 260 AU/mL), confirming data reported by Rabinovich.8
Furthermore, our results confirmed that 2-dose vaccination with BNT162b2 elicits a vigorous immune response in previously COVID-19-exposed LT patients compared with SARS-CoV-2-naïve patients, as has already been reported in the literature.24
Moreover, our 3 anecdotic cases of breakthrough infection after 2-dose vaccination sustain the evidence that BNT162b2 vaccine was effective in preventing symptomatic COVID-19, according to data demonstrating protection ranging between 70% and 95% in healthy individuals.25 Therefore, we would strongly recommend COVID-19 vaccination in LT recipients, but we would like to highlight the relevance of vaccinating also their close contacts (family and caregivers) to reduce the risk of exposure. Additionally, even if LT patients showed a weak response to vaccination, they benefited in terms of morbidity and mortality compared with unvaccinated LT recipients.26 However, LT patients need to be aware that they have a higher risk to be unprotected after vaccination compared with healthy individuals, and thus, they should continue to practice strict COVID-19 precautions, such as social distancing and wearing masks. Furthermore, given that many LT patients develop weak SARS-CoV-2 antibody responses after 2 doses of an mRNA-based vaccine, third doses have been authorized for immunocompromised subjects, improving the immunogenicity of the vaccine in this vulnerable group, as showed by recent papers.27 , 28
Finally, we also showed that the BNT162b2 vaccine was well tolerated by LT patients, and none reported organ rejection or compromised graft function, even if there has also been concern that COVID-19 vaccination might contribute to organ rejection and graft dysfunction.29
The strengths of this study include its novelty. It is the first published report about SARS-CoV-2 vaccination in a large cohort of LT recipients (492 patients) undergoing the same vaccination schedule (BNT162b2). Secondly, to our knowledge, our data shows for the first time the humoral response after 1 and 3 months from 2-dose vaccination in LT recipients in comparison with a large control group matched for age and sex.
Nonetheless, our study has some limitations. First, it did not assess cell-mediated immune response to vaccination. The response to vaccination relies on the development of both humoral and cellular response. Furthermore, cellular immunity, in the absence of positive antibody titers, can prevent severe disease or death from COVID-19, as suggested by some other reports.30 , 31 The lack of exploration of the cellular immune response precludes us to address the full spectrum of vaccine immunogenicity. Secondly, the short follow-up period (3 months) is not sufficient to draw definitive conclusions. Longer follow-up for clinical outcomes and also the assessment of the cellular response in this special setting are needed.
In summary, even if the immunological response to vaccination is more complex beyond measuring antibody titers, in this large cohort of LT recipients followed-up for 3 months, we showed that antibody response to completed SARS-CoV- 2 vaccination is more consistent than that reported in preliminary studies evaluating it after only 4 weeks (75% vs 47%).
Acknowledgment
The authors thank the co-authors of the UniNa (University of Naples)-Collaborating Group: Giuseppina Pontillo, Luca Pignata, Maria Rosaria Attanasio, Raffaele Lieto, Francesco Cutolo, Marianna Cuomo - Department of Clinical Medicine and Surgery, Gastroenterology and Hepatology Unit, University of Naples “Federico II,” Naples, Italy. The authors also thank CRT Campania (Centro Regionale Trapianti) for its constant support in the management of liver transplanted patients in Campania Region.
CRediT Authorship Contributions
Maria Guarino (Conceptualization: Lead; Writing – review & editing: Lead)
Ilaria Esposito (Conceptualization: Lead; Writing – review & editing: Lead)
Giuseppe Portella (Supervision: Equal)
Valentina Cossiga (Data curation: Equal)
Ilaria Loperto (Methodology: Equal)
Raffaella Tortora (Data curation: Equal)
Michele Cennamo (Methodology: Equal)
Mario Capasso (Data curation: Equal)
Daniela Terracciano (Methodology: Equal)
Alfonso Galeota Lanza (Data curation: Equal)
Sarah Di Somma (Methodology: Equal)
Francesco Paolo Picciotto (Supervision: Lead)
Filomena Morisco (Supervision: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2022.01.012.
Contributor Information
UniNa Collaborating Group:
Giuseppina Pontillo, Luca Pignata, Maria Rosaria Attanasio, Raffaele Lieto, Francesco Cutolo, and Marianna Cuomo
Supplementary Methods
Quantification of Humoral Response
Freshly collected blood in clot activator and gel tube was centrifuged at 4500 rpm for 10 minutes. The sera were separated and stored at −20 °C for the subsequent analysis.
Collected samples were analyzed for detecting anti-Spike protein IgG using LIAISON severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S1/S2-IgG chemiluminescent assay (DiaSorin, Italy) (range < 3.8 to >400 AU/mL).18 Of note, the manufacturer proposed cutoffs were slightly modified based on the Federico II laboratory experience (in-house standard operating procedure). Accordingly, results below 18.0 AU/mL were considered negative, those ranging between 18.0 and 25.0 AU/mL were considered as sub-optimal, and those >25 AU/mL were considered as positive. For the purpose of the analysis, borderline results (a total of 30 patients) were considered as negative. Additionally, antibody titers at 3 months after full vaccination were considered stable, in case of a titer change of less than 10% at 1 month.
Previous Coronavirus Disease 2019 (COVID-19) Exposure
During the 7 days preceding first vaccination dose injection, all patients were interviewed about previous COVID-19 infection. Furthermore, baseline serology test to detect IgG-antibodies against the nucleocapsid protein subunit to ensure a SARS-CoV-2 negative status was performed with chemiluminescent assays (positive test was defined as an index signal ≥1.4 [Abbot Diagnostics, Libertyville, IL]). Patients with positive anti-SARS-CoV-2 antibody status at baseline were included in the study but were analyzed and presented separately.
Safety Report
After each dose, patients were asked with a phone interview questionnaire if they experienced any local symptoms such as pain, redness, swelling, and regional lymphadenopathy, or any systemic symptoms such as fever, chills, headache, fatigue, myalgia, arthralgia, nausea, vomiting, or diarrhea. Additionally, they were asked to report any development of anaphylaxis requiring epinephrine, or any other medical illnesses, in addition to those specifically asked.
Patients were asked to rate their symptoms on a semiquantitative scale as none, mild, moderate, or severe. In particular, mild symptoms were defined as symptoms that did not interfere with daily activities, whereas moderate symptoms were defined as those that caused some interference with daily activity, and severe symptoms were defined as those that prevented daily activity.
Breakthrough COVID-19 Infection
Polymerase chain reaction-confirmed SARS-CoV-2 diagnosis of COVID-19 after full vaccination (both doses) was classified as a breakthrough infection.
Statistical Analysis
The Shapiro-Wilk test was performed to analyze normal distribution. Continuous variables were expressed as median and interquartile range. Categorical variables were described as absolute frequency and percentage. Descriptive statistics of the study variables were performed using the Kruskal-Wallis equality-of-populations rank test, the χ2, and the Fisher exact test, as appropriate. The correlation between continuous variables was investigated using the Spearman rank correlation test. Antibody titers were compared between patient groups using the Mann-Whitney U test, as appropriate.
Estimation of vaccination non-response risk was assessed by odds ratio and their 95% confidence intervals by means of univariate logistic regression models considering, for the liver transplant (LT) population, the following independent variables: age, sex, body mass index, active smoking, indication to LT, comorbidities, time from transplantation, and regimens of immunosuppression. To identify independent factors predicting non-response to vaccination, variables associated with the chosen outcome with a P ≤ .05 were finally entered into a multivariable logistic model.
To evaluate the association between response to vaccination and liver transplantation, a univariate logistic analysis comparing data of LT recipients and health care workers (controls) was performed. The multivariate logistic analysis was performed by inserting as covariates: age group, sex, and LT. The binary dependent variable considered was the serological response to BNT162b2 mRNA vaccine.
Statistical analyses were performed using STATA 15 statistical software (StataCorp, College Station, TX).
Supplementary Results
Characteristics and Humoral Response of Patients With Previous Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection
All liver transplant (LT) patients were tested for anti-SARS-CoV-2 antibodies (IgG anti-nucleocapsid) before vaccination. Among all 492 enrolled patients, 48 (9.7%) tested positive for previous SARS-CoV-2 exposure (see Table 1 for characteristics). None of them were diagnosed by chance based on the results of serology testing: they had a known history of coronavirus disease 2019 (COVID-19). For the 48 patients with known previous COVID-19 infection, the median interval between infection and 2-dose vaccination was 149 days (interquartile range [IQR], 112−187 days). In terms of clinical presentation, 27 patients were asymptomatic, whereas 16 had mild disease and were managed at home. Five patients were hospitalized (3 with moderate COVID-19 and 2 with critical/severe disease).
All these patients underwent BNT162b2 vaccination with the standard schedule.
Detectable antibody titers were detected in 42 of 48 patients (87.5%) at 1 month (median, 29 days; IQR, 27–31 days) and at 3 months (median, 89 days; IQR, 86–92 days) after 2-dose vaccination, with median values of 401 AU/mL (IQR, 152.5–401 AU/mL) and 383.5 AU/mL (IQR, 05.5–401 AU/mL), respectively (statistically significantly higher than COVID-19 unexposed patients; P < .001). Among the 6 patients with undetectable titer at 1 month, none developed detectable antibody titers at 3 months.
Breakthrough Infections After 2-dose Vaccination
During follow-up (median, 109 days since full vaccination; IQR, 84–116 days), 3 (0.6%) of 492 vaccinated LT recipients were diagnosed with COVID-19 (polymerase chain reaction-confirmed SARS-CoV-2 infections). All of them were asymptomatic. The 3 patients experienced a close contact with a positive COVID-19 case (a family member) and, therefore, they were subjected to nasopharyngeal swab that tested positive after full vaccination (median, 43 days; IQR, 38–46 days). Notably, only 1 of the 3 patients with breakthrough infection had undetectable antibody titers 1 month after full vaccination, whereas the other 2 patients showed positive antibody titers (32.1AU/mL and 70.5 AU/mL). The patient with seronegative response to vaccination showed 2 of the 3 factors associated with negative antibody response (age >65 years and immunosuppressive regimen with antimetabolite). Of note, these 3 patients were not included in the previous statistical analysis.
Tolerability and Safety of BNT162b2 mRNA Vaccine
The vaccination was well-tolerated. No major adverse events occurred in all enrolled patients.
There were no significant differences between the first and the second dose for all the reported side effects. In particular, the most commonly reported side effect after both doses were local pain in the injection site (334/492; 67.8%), followed by fatigue (188/492; 38.3%), headache (196/492; 39.8%), and myalgia/arthralgia (104/492; 21.1%), consistent with the pivotal trial. Overall local and systemic symptoms developed in 378 (76.8%) and 251 (51.1%) patients, respectively. There was a similar rate of reported effects between seropositive and seronegative LT patients (77.4% vs 76.1%), as well as between health care workers and LT patients (72.1% vs 76.8%). See Supplementary Table 1 for details.
No suspected or confirmed graft rejection, compromised graft function, neurological events, or severe allergic reaction were observed in the LT population during the follow-up period (12 weeks after the second dose of vaccination).
Supplementary Table 1.
Evaluation of Reactogenity (Local and Systemic Symptoms) After SARS-CoV-2 Vaccination in LT Patients and Health Care Workers
| Overall LT patients (n = 492) | Negative serology to SARS-CoV-2 vaccination (n = 108) | Positive serology to SARS-CoV-2 vaccination (n = 336) | Previous SARS-CoV-2 infection (n = 48) | Health care workers (n = 307) | |
|---|---|---|---|---|---|
| Reported local side effects | |||||
| After 1st dose | |||||
| Pain | 368 | 76 | 257 | 35 | 287 |
| Mild | 274 | 45 | 206 | 23 | 231 |
| Moderate | 93 | 31 | 50 | 12 | 56 |
| Severe | 1 | 0 | 1 | 0 | 0 |
| Redness | 44 | 12 | 27 | 5 | 24 |
| Mild | 31 | 9 | 18 | 4 | 18 |
| Moderate | 13 | 3 | 9 | 1 | 6 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Swelling | 31 | 6 | 22 | 3 | 32 |
| Mild | 19 | 2 | 14 | 3 | 28 |
| Moderate | 12 | 4 | 8 | 0 | 4 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Regional lymphadenopathy | 1 | 0 | 1 | 0 | 0 |
| After 2nd dose | |||||
| Pain | 301 | 52 | 226 | 23 | 271 |
| Mild | 223 | 35 | 176 | 12 | 243 |
| Moderate | 78 | 17 | 50 | 11 | 27 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Redness | 35 | 8 | 21 | 6 | 21 |
| Mild | 25 | 5 | 16 | 4 | 19 |
| Moderate | 10 | 3 | 5 | 2 | 2 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Swelling | 27 | 4 | 19 | 4 | 24 |
| Mild | 19 | 4 | 12 | 3 | 19 |
| Moderate | 8 | 0 | 7 | 1 | 5 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Regional lymphadenopathy | 0 | 0 | 0 | 0 | 0 |
| Reported systemic side effects | |||||
| After 1st dose | |||||
| Fatigue | 216 | 51 | 147 | 18 | 259 |
| Mild | 104 | 23 | 70 | 11 | 184 |
| Moderate | 89 | 22 | 61 | 6 | 63 |
| Severe | 23 | 6 | 16 | 1 | 12 |
| Myalgia/arthralgia | 103 | 21 | 73 | 9 | 87 |
| Mild | 64 | 12 | 47 | 5 | 71 |
| Moderate | 36 | 8 | 24 | 4 | 15 |
| Severe | 3 | 1 | 2 | 0 | 1 |
| Headache | 185 | 32 | 129 | 24 | 174 |
| Mild | 140 | 23 | 98 | 19 | 135 |
| Moderate | 44 | 9 | 30 | 5 | 39 |
| Severe | 1 | 0 | 1 | 0 | 0 |
| Fever/chills | 63 | 12 | 46 | 5 | 41 |
| Mild | 42 | 7 | 32 | 3 | 28 |
| Moderate | 19 | 4 | 13 | 2 | 12 |
| Severe | 2 | 1 | 1 | 0 | 1 |
| Nausea/vomiting | 3 | 0 | 2 | 1 | 1 |
| Diarrhea | 8 | 2 | 4 | 2 | 2 |
| After 2nd dose | |||||
| Fatigue | 161 | 43 | 99 | 19 | 201 |
| Mild | 57 | 19 | 33 | 5 | 123 |
| Moderate | 74 | 13 | 51 | 10 | 75 |
| Severe | 30 | 11 | 15 | 4 | 3 |
| Myalgia/arthralgia | 105 | 20 | 74 | 11 | 74 |
| Mild | 53 | 8 | 40 | 5 | 61 |
| Moderate | 48 | 11 | 31 | 6 | 13 |
| Severe | 4 | 1 | 3 | 0 | 0 |
| Headache | 207 | 41 | 140 | 26 | 171 |
| Mild | 102 | 17 | 74 | 11 | 138 |
| Moderate | 102 | 23 | 64 | 15 | 33 |
| Severe | 3 | 1 | 2 | 0 | 0 |
| Fever/chills | 71 | 14 | 50 | 7 | 58 |
| Mild | 29 | 4 | 23 | 2 | 34 |
| Moderate | 39 | 9 | 25 | 5 | 23 |
| Severe | 3 | 1 | 2 | 0 | 1 |
| Nausea/vomiting | 1 | 0 | 1 | 0 | 0 |
| Diarrhea | 4 | 1 | 2 | 1 | 1 |
Note: Data are presented as number.
LT, Liver transplant; SARS-CoV 2, severe acute respiratory syndrome coronavirus 2.
Supplementary Table 2.
Univariable and Multivariable Analysis on Factors Associated With Positive Serology to BNT162b2 mRNA Vaccine in LT Recipients and Health Care Workers (Controls) According to Antibody Titers After 3 Months From the 2-dose Vaccine (Dependent Variable: Positive Serology)
| Characteristics | Univariable, OR (95% CI) | P value | Multivariable, OR (95% CI) | P value |
|---|---|---|---|---|
| Age, y | ||||
| <40 | Ref | Ref | ||
| 40–65 | 0.07 (0.02–0.23) | < .00 | 0.16 (0.05–0.53) | .003 |
| >65 | 0.03 (0.00–0.09) | < .00 | 0.17 (0.05–0.58) | .005 |
| Male sex | 0.37 (0.24–0.58) | < .00 | 0.86 (0.53–1.39) | .55 |
| LT | 0.04 (0.02–0.08) | < .00 | 0.07 (0.03–0.13) | < .00 |
Note: Estimates were derived from multivariable logistic regression models adjusted for all the outcome variables.
Note: Boldface P values indicate statistical significance.
CI, Confidence interval; LT, liver transplant; OR, odds ratio.
References
- 1.Mulligan M.J., Lyke K.E., Kitchin N., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira M.R., Mohan S., Cohen D.J., et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckerle I., Rosenberger K.D., Zwahlen M., et al. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornberg M., Buti M., Eberhardt C.S., et al. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo F.P., Piano S., Bruno R., et al. Italian Association for the Study of the Liver. Italian Association for the Study of the Liver position statement on SARS-CoV2 vaccination. Dig Liver Dis. 2021;53:677–681. doi: 10.1016/j.dld.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar D., Ferreira V.H., Blumberg E., et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis. 2018;67:1322–1329. doi: 10.1093/cid/ciy294. [DOI] [PubMed] [Google Scholar]
- 8.Rabinowich L., Grupper A., Baruch R., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyarsky B.J., Chiang T.P., Teles A.T., et al. Antibody kinetics and durability in SARS-CoV-2 mRNA vaccinated solid organ transplant recipients. Transplantation. 2021;105:e137–e138. doi: 10.1097/TP.0000000000003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miele M., Busà R., Russelli G., et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant. 2021;21:2919–2921. doi: 10.1111/ajt.16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzola A., Todesco E., Drouin S., et al. Poor antibody response after two doses of SARS-CoV-2 vaccine in transplant recipients. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera S., Colmenero J., Pascal M., et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021;21:3971–3979. doi: 10.1111/ajt.16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss A.T., Hallett A.M., Boyarsky B.J., et al. Antibody response to SARS-CoV-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl. 2021;27:1852–1856. doi: 10.1002/lt.26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thuluvath P.J., Robarts P., Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruether D.F., Schaub G.M., Duengelhoef P.M., et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2022;20:162–172.e9. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency Comirnaty summary of product characteristics, 2021. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf Available at:
- 18.DiaSorin. LIAISON SARS-CoV-2 S1/S2 IgG. 2020. Available at: https://www.diasorin.com/sites/default/files/allegati/liaisonr_sars-cov-2_s1s2_igg_brochure.pdf.pdf. Accessed April 14, 2021.
- 19.Natori Y., Shiotsuka M., Slomovic J., et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66:1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
- 20.Crooke S.N., Ovsyannikova I.G., Poland G.A., et al. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rentenaar R.J., van Diepen F.N.J., Meijer R.T., et al. Immune responsiveness in renal transplant recipients: mycophenolic acid severely depresses humoral immunity in vivo. Kidney Int. 2002;62:319–328. doi: 10.1046/j.1523-1755.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- 22.Prendecki M., Clarke C., Edwards H., et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allison A.C., Eugui E.M. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 24.Lombardi A., Consonni D., Oggioni M., et al. SARS-CoV-2 anti-spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J Infect Public Health. 2021;14:1120–1122. doi: 10.1016/j.jiph.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aslam S., Adler E., Mekeel K., Little S.J. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021;23 doi: 10.1111/tid.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall V.G., Ferreira V.H., Ku T., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaba A.H., Zhu X., Liang T., et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2021 doi: 10.1111/ajt.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyarsky B.J., Ou M.T., Greenberg R.S., et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105:e56–e57. doi: 10.1097/TP.0000000000003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cucchiari D., Egri N., Bodro M., et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt T., Klemis V., Schub D., et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21:3990–4002. doi: 10.1111/ajt.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]