Abstract
Wnt signaling is critical to many aspects of skeletal regulation, but the importance of Wnt ligands in the bone anabolic response to mechanical loading is not well established. Recent transcriptome profiling studies by our lab and others show that mechanical loading potently induces genes encoding Wnt ligands, including Wnt1 and Wnt7b. Based on these findings, we hypothesized that mechanical loading stimulates adult bone formation by inducing Wnt ligand expression. To test this hypothesis, we inhibited Wnt ligand secretion in adult (5-mo) mice using a systemic (drug) and a bone-targeted (conditional gene knockout) approach, and subjected them to axial tibial loading to induce lamellar bone formation. Mice treated with the Wnt secretion inhibitor WNT974 exhibited a decrease in bone formation in non-loaded bones as well as a 54% decline in the periosteal bone formation response to tibial loading. Next, osteoblast-specific Wnt secretion was inhibited by dosing 5-month old Osx-CreERT2; WlsF/F mice with tamoxifen. Within 1–2 weeks of Wls deletion, skeletal homeostasis was altered with decreased bone formation and increased resorption, and the anabolic response to loading was reduced 65% compared to control (WlsF/F). Together, these findings show that Wnt ligand secretion is required for adult bone homeostasis, and furthermore establish a role for osteoblast-derived Wnts in mediating the bone anabolic response to tibial loading.
Keywords: Biomechanics < ORTHOPAEDICS, Genetic animal models < ANIMAL MODELS, Osteoblasts < CELLS OF BONE, Wnt/Beta-catenin/LRPs < CELL/TISSUE SIGNALING - Paracrine Pathways
Introduction
Bone formation is regulated by Wnt signaling, which is activated by the interaction of secreted Wnt ligands with Fzd and Lrp cell surface receptors. Loss of Wnt ligands or Fzd and Lrp receptors cause profound deficits in bone formation during development, while loss-of-function mutations in the Wnt pathway inhibitor Sclerostin underlie high bone mass disorders in humans (Maupin et al, 2013) (Balemans et al, 2001).
Bone formation is also regulated by mechanical loading, which appears to stimulate osteogenesis at least partially through the Wnt/Lrp signaling pathway (Robinson et al., 2006). Higher bone formation is observed in the loaded bones of mice subjected to unilateral tibial loading, relative to contralateral non-loaded bones, concomitant with increased expression of genes encoding Wnt ligands and reduced expression of Wnt pathway inhibitors Sost and Dkk1 (Holguin et al. 2016). Additionally, mice lacking the Wnt co-receptor LRP5 exhibit reduced osteogenic responsiveness to mechanical loading, while loading-induced downregulation of Wnt antagonists Sclerostin (Sost) and Dkk1 has been shown to favor bone formation (Lara-Castillo et al., 2015; Robling et al., 2008; Tu et al., 2012).
Reports by several groups demonstrate that loading induces the expression of Wnt genes in bones of mice. Robinson et al. assayed several Wnt-related genes and reported elevated Wnt10b expression 4 h after loading (Robinson et al., 2006). More recently, Kelly et al. used RNAseq and identified Wnt1, Wnt7b and Wnt10b as genes elevated early after axial tibial loading (Kelly et al., 2016), findings corroborated by others (Galea et al., 2017; Holguin et al., 2016). We recently reported, based on RNAseq, that Wnt1 and Wnt7b were two of only eight transcripts significantly upregulated in cortical bone 4 h after 1, 3 and 5 daily bouts of axial tibial loading in 5-mo old mice (Chermside-Scabbo et al., 2020). Wergedal et al. (Wergedal et al., 2015) reported an increase in Wnt16 expression with tibial bending in wildtype mice, and a diminished periosteal response to tibial bending in 10-wk old Wnt16 knockout mice, indicating a functional role of Wnt ligand regulation in the bone response to loading in young mice. (Wergedal et al., 2015)
Wnt protein secretion into the extracellular environment depends on the actions of two intracellular molecules, Porcupine and Wntless, both of which are required for survival in mice (Barrott et al., 2011; Ching & Nusse, 2006; Fu et al., 2009). Porcupine and Wntless are required for the secretion of all Wnt ligands. Porcupine (Porcn) catalyzes the addition of a fatty acid to newly synthesized Wnt proteins in the endoplasmic reticulum (Kadowaki et al., 1996; Willert et al., 2003). The modified proteins can then interact with Wntless (Wls/GPR177), the Wnt-specific transporter that shuttles vesicle-bound Wnt proteins to the cell surface (Banziger et al., 2006). Inhibition of porcupine using WNT974 blocks bone formation and increases bone resorption in young (8–12 wk old) mice (Funck-Brentano et al., 2018; Madan et al., 2018). Moreover, constitutive deletion of Wntless (Wls) in early osteoblasts (Osx-Cre) leads to severely impaired skeletal developmental and perinatal lethality (Osx-Cre) (Tan et al., 2014), while constitutive deletion in mature osteoblasts (Ocn-Cre) leads to osteopenia and spontaneous fractures by 2-mo age (Zhong et al., 2012). Further, results of genome-wide association studies (GWAS) identify 89 variants in the human WLS gene as being significantly associated with bone mineral density (Morris et al., 2019; “Musculoskeletal Knowledge Portal,”). While these studies show a requirement for Wnt ligand secretion by osteoblasts during skeletal development and maturation, it remains unclear whether Wnt secretion by osteoblasts is required for bone homeostasis or the bone anabolic response to mechanical loading.
Our objective was to test the hypothesis that Wnt secretion by osteoblasts is essential for homeostasis in the adult skeleton, and also for the anabolic response to mechanical loading. We dosed adult (5-mo) mice with the porcupine inhibitor WNT974 and observed a decrease in bone formation and increase in resorption, as well as a marked decline in the anabolic response to loading. We then conditionally deleted Wls in osteoblasts in adult mice using a tamoxifen inducible Cre and found that within 1 week, skeletal homeostasis was altered and the anabolic response to loading was greatly impaired. Our findings indicate that secretion of Wnt ligands by osteoblasts is required for adult bone homeostasis and for the anabolic response to mechanical loading.
Methods
Animal models
Studies were approved by the Washington University IACUC. Mice were housed up to five per cage and given ad libitum access to normal chow and water. Mice were euthanized by CO2 asphyxiation.
Porcupine inhibition.
A pharmacological approach was used to systemically inhibit Wnt secretion in 5-month old C57BL/6 female mice (Charles River). Mice were assigned randomly to two treatment groups (vehicle vs drug). Porcupine inhibitor WNT974 (aka Lgk974; Active Biochemicals, Wan Chai, Hong Kong) was prepared as previously described (Zhang and Lawrence, 2016) and delivered daily by oral gavage at a dose of 6mg/kg/day. Control mice received an equivalent volume of vehicle (0.15ml, 5% carboxy-methylcellulose (w/v) in water). Mice were dosed in the morning 1 hour before loading; the reported half-life of WNT974 is 5–8 hrs (Rodon et al., 2018).
Osteoblast-specific Wls deletion.
An inducible Cre/LoxP approach was used to conditionally delete Wls in Osterix-expressing cells, from early osteoblasts to osteocytes. OsxCreERT2 sires (derived from breeders gifted from Dr. Henry Kronenberg) were mated to dams that were homozygous for the conditional floxed Wls allele (JAX #012888) to generate OsxCreERT2; WlsF/F experimental (Wls cKO/WLSΔ) and WlsF/F control littermates. Mice were genotyped by Transnetyx (Transnetyx, Inc. Cordova, TN, USA) using tail snip DNA with probes for wildtype Wls (Gpr177–1 WT), conditional Wls (Gpr177–1 FL), and OsxCreERT2 (Cre). After genotyping, mice were assigned to cKO or control groups as available until the desired sample sizes for each outcome were attained (see below), with similar numbers of littermates of each genotype included in each experimental batch. Tamoxifen was dissolved in corn oil to a final concentration of 10mg/ml and was delivered by oral gavage at a dose of 50mg/kg/day for 3 consecutive days to induce gene knockout in 5-month old male and female mice. Tamoxifen-treated WlsF/F mice served as genotype controls for all experiments. The inducible Ai9 reporter transgene (JAX #007909) was introduced into a subset of mice to generate OsxCreERT2; WlsF/F; Ai9 mice, which were used to assess Cre specificity. All mouse strains have been previously described (Carpenter et al., 2013; Maes et al., 2007; Madisen et al., 2010).
Tibial loading
In vivo tibial compression was used to stimulate cortical bone formation in 5-month old mice. As described previously, mice were loaded for 5 consecutive days (Sun et al., 2019; Main et al., 2020). Briefly, an Electropulse 1000 system (Instron, USA) was used to apply 60 cycles/day (4Hz) of axial compression on the right tibias of anesthetized mice (mouse prone/tibia vertical); contralateral left tibias served as non-loaded control. Wild-type female C57Bl/6 mice treated with vehicle and WNT974 were loaded to −8N (−2200 peak strain), a magnitude reported to induce robust lamellar bone formation in similar C57Bl/6 mice (Sun et al., 2019; Holguin et al., 2014). For Wls control and cKO mice, we performed a priori strain gage measurements (Suppl Fig S7) and in vivo loading pilot studies and determined that a peak strain of −3500 stimulated a strong lamellar response in control mice (whereas −2200ue did not). Peak forces to engender this target strain did not differ between genotypes: −8N in WLSΔ and control females, and −11N in WLSΔ and control males.
Mice designated for gene expression analysis were sacrificed 4 h after the final bout of loading. Mice designated for dynamic histomorphometry received calcein (10mg/kg; Sigma) by IP injection on the last day of loading, followed by alizarin complexone (30mg/kg; Sigma) 5 days later.
Fluorescent histology
Dynamic histomorphometry.
Bilateral tibias were plastic-embedded as described (Brodt and Silva 2010) and sectioned transversely at the 37% location to a thickness of 100 μm. Images at a resolution of 2048×2048 were captured on a Leica confocal microscope (see below). Fluorophore-labeled surfaces were analyzed using commercial software (Osteo II, Bioquant) to calculate standard indices of bone formation, including percent mineralizing surface (MS/BS), mineral apposition rate (MAR), and bone formation rate (BFR/BS) (Dempster et al,. 2013). Values were determined on total periosteal (Ps) and endocortical (Ec) surfaces separately. In instances where no double-labeled surface could be detected, or where the inter-label distance was too small to measure, a value of 0.3μm/day was assigned (Dempster et al., 2013). In addition to absolute values for loaded and non-loaded tibias, relative bone formation rate (BFRLoaded – BFRNon-Loaded) was calculated as a measure of the anabolic response to loading. For all quantitative histomorphometry, the user was blinded to group assignment prior to analysis.
Cre reporter analysis.
Ten μm thick transverse sections from the non-loaded tibias of tamoxifen-treated OsxCreERT2; RosaAi9/+; WlsF/F mice were DAPI-counterstained and imaged on a Leica confocal microscope to analyze endogenous Ai9 reporter expression 3 days after the final dose of tamoxifen.
EdU and TUNEL analyses.
A commercially available kit (Click-iT EdU Cell Proliferation Kit, ThermoFisher) was used to label proliferating cells with thymidine analog 5-ethynyl-2’-deoxyuridine (EdU). Briefly, EdU (0.2mg/ml) dissolved in 5% sucrose water (w/v) was delivered daily to the mice via drinking water during the loading period until sacrifice. Tibias harvested 3 days after loading were fixed in 4% paraformaldehyde (PFA), de-calcified in 14% EDTA, incubated in 30% sucrose/PBS overnight, and embedded in OCT (optimal cutting temperature) medium. Twenty μm thick sections sectioned on a cryostat were stained for EdU following manufacturer instructions. Sections were DAPI-counterstained and imaged on a confocal microscope. Percent proliferation was defined as the ratio between EdU-positive nuclei and total (DAPI-positive) cells on the bone surface. Cell death was analyzed similarly using a commercially available kit that labels fragmented DNA from apoptotic cells (DeadEnd Fluorometric TUNEL System, Promega).
All fluorescence imaging was done on a Leica inverted laser scanning confocal microscope (Leica DMi8/TCS SPE) using a 10μm Z-stack.
Gene expression
Sample preparation.
Gene expression was analyzed by qPCR as described previously (Holguin et al., 2016). Briefly, mice were sacrificed on the last (5th) day of loading, 4 h after tibial compression. Bilateral tibias were dissected out and carefully stripped of muscle, and cut 2mm distal to the tibial plateau and at the tibiofibular junction (TFJ). Bones were microcentrifuged to remove bone marrow, then placed in liquid nitrogen. Adherent muscle was carefully removed to leave the periosteum intact; thus, gene expression represents expression of both surface and embedded cells (osteoblasts and osteocytes). Samples were pulverized with a Mikro Dismembrator (Braun) and lysed in TRIzol. Total RNA was extracted and purified using the Qiagen RNeasy Mini Kit. Complementary DNA (cDNA) was prepared with the iScript cDNA Synthesis Kit (BioRad).
RT-qPCR.
Gene expression was analyzed by Sybr- and Taqman-based qPCR. Sybr-based qPCR was run on a StepOne Plus Machine (Applied Biosystems). Taqman-based qPCR was run on a BioMark HD System in collaboration with the Genome Technology Access Center (GTAC) at Washington University in St. Louis. Genes were chosen that broadly reflect osteogenesis (Bmp2, Col1a1, Sp7, Bglap, Pdpn (E11), Dmp1), osteoclast activity (Opg, Rankl, Rank, Ctsk) and Wnt signaling (Axin2, Ccnd1, Ctnnb1, Dkk1, Dkk2, Lrp5, Lrp6, Nkd2, Sfrp1, Sost, Wnt1, Wnt7b). mRNA expression was reported as relative expression (2−ΔCt), normalized to Tbp.
Wls knockdown validation
First, genomic DNA (gDNA) was extracted from the skeletal and extra-skeletal tissues (DNeasy kit, Qiagen) of 5-month old Wls mice to evaluate DNA recombination after tamoxifen induction. Primers up- and downstream of the floxed locus in the Wls transgene (exon 1) were used to amplify wildtype (1625bp) and mutant (140bp) Wls DNA. P1: CTTCCCTGCTTCTTTAAGCGTC; P4: CTCAGAACTCCCTTCTTGAAGC. PCR conditions were as described previously (Carpenter, Rao et al. 2010). Second, tamoxifen-treated mice were sacrificed 3 days after tamoxifen induction, and tibias were harvested and processed for qPCR as described under Gene Expression. Primers were used to amplify Wls exon 1, which is excised upon Cre-induced recombination. Thus, expression levels reflect the abundance of wild-type Wls RNA in the bone. Expression was normalized to Tbp. Primer sequences were: WLS-F: CAAATCGTTGCCTTTCTGGTG; WLS-R: TTGTCACACTTGTTAGGTCCC; TBP-F: CTGAATAGGCTGTGGAGTAAGTC; TBP-R: CTGAAGAAAGGGAGAATCATGGA (Carpenter, et al., 2010).
Immunohistochemistry
Tissue preparation.
Standard procedures were used to prepare paraffin-embedded tissue sections for immunohistochemistry. Briefly, bones were fixed in 4% PFA overnight at 4°C, followed by de-calcification for 7–10 days in 14% EDTA. Paraffin-embedded tissues were sectioned to a thickness of 10 μm on a microtome and mounted onto glass slides using a flotation water bath system.
Immunostaining.
Heat-induced epitope retrieval in sodium citrate buffer was used for antigen retrieval. Blocking buffer comprised of 3% BSA in PBST was used to block tissues for 1 h at room temperature. Primary antibodies, which were diluted 1:1000 in blocking buffer, included anti-Gpr177 (Wls, Evi) antibody RLAB-177 (Seven Hill Bioreagents) and anti-Sclerostin antibody AF1589 (R&D Systems). Tissues were incubated in primary antibody overnight at 4°C. Secondary reagents included VectaStain Elite ABC HRP kits PK-6101 and PK-6105, and ImmPact DAB peroxidase (HRP) substrate kit SK-4105 (Vector Labs). Tissues were counterstained with hematoxylin.
Serum markers
A separate cohort of Wls control and cKO mice were tamoxifen-treated for 3 consecutive days, then sacrificed 1 week later for serum harvest (without loading). Commercially available ELISA kits from IDA Immunologistics were used to assay serum P1NP and CTX-1. Two-factor ANOVA indicated that sex was not a significant variable, and consequently, female and male data were pooled.
Western blot
Bilateral tibias were collected from control and Wls knockout mice 3 days after tamoxifen induction; mice were not subjected to loading. Tibias were harvested as described above (in Gene Expression), then flushed with ice-cold PBS to remove residual marrow. Protein was extracted from bone and the abundance of phosphorylated LRP6 (pLRP6, CST #2568) and total LRP6 (tLRP6, CST #3395) were assessed by western blot. The ratio of pLRP6/tLRP6 was used as an indicator of Wnt signaling. (See Suppl. Fig. S6.)
Data Analysis
Group sample sizes (n) were determined based on a priori power analysis and are indicated in the Results. Two-factor analysis of variance (ANOVA) was used to study the main effects of loading (non-loaded vs. loaded), and treatment (vehicle vs. WNT974) or genotype (control vs. WLSΔ), and their interaction, on bone formation and gene expression outcomes with Sidak’s multiple comparisons test for pairwise comparisons (Prism, Graphpad 7.0). In this analysis, the significance of the interaction term is critical, because it indicates whether the effect of loading differed between treatment/genotype groups. For dynamic histomorphometry outcomes, we also computed relative (r) values as a net measure of the effect of loading within each animal, where r = Loaded – Non-Loaded, and performed one-factor ANOVA (treatment or genotype) as previously described (Turner et al., 1994; Forwood and Turner, 1994). For bone formation outcomes in the Wls deletion study, male and female mice were analyzed separately. One-factor ANOVA was used for outcomes where loading was not a factor (e.g., serum markers). Significance was defined at p<0.05. Individual data points and the mean ± standard deviation are plotted.
Results
Loading-induced bone formation is reduced in WNT974-treated mice
To investigate the role of Wnt proteins in the anabolic effect of skeletal loading, 5-month old C57BL/6 female mice were subjected to 5 days of cyclic compressive loading on the right tibia while contralateral left tibias served as control. Mice were treated with Porcupine inhibitor WNT974 to block Wnt secretion or vehicle (Fig. 1A). Tibial loading stimulated periosteal bone formation in both treatment groups (Fig. 1B); indices of periosteal bone formation were significantly greater in the loaded vs contralateral non-loaded tibias (Fig. 1C–E). However, the periosteal loading response was diminished in WNT974-treated mice. Two-factor ANOVA indicated that loading and treatment had significant effects on Ps.MS/BS, Ps.MAR, and Ps.BFR/BS. Importantly, there was a significant (p<0.05) or near-significant (p=0.083) loading-treatment interaction for Ps.BFR/BS and Ps.MS/BS, respectively, indicating that the effect of loading on bone formation indices differed between treatment groups. In loaded tibias Ps.MS/BS was 41% lower in WNT974-treated mice relative to vehicle-treated mice (p<0.001, Fig. 1C), and Ps.MAR and Ps.BFR/BS were 40% (p<0.05, Fig. 1D) and 55% (p<0.001, Fig. 1E) lower. Similarly, when the effect of loading is expressed as a relative measure (i.e., loaded minus non-loaded control), periosteal bone formation was reduced by 54% in the WNT974-treated group (Fig. 1F). Together, these data confirm that loading potently induces periosteal bone formation in the adult skeleton, and indicate that the periosteal loading response depends on the secretion of Wnt proteins.
Figure 1.
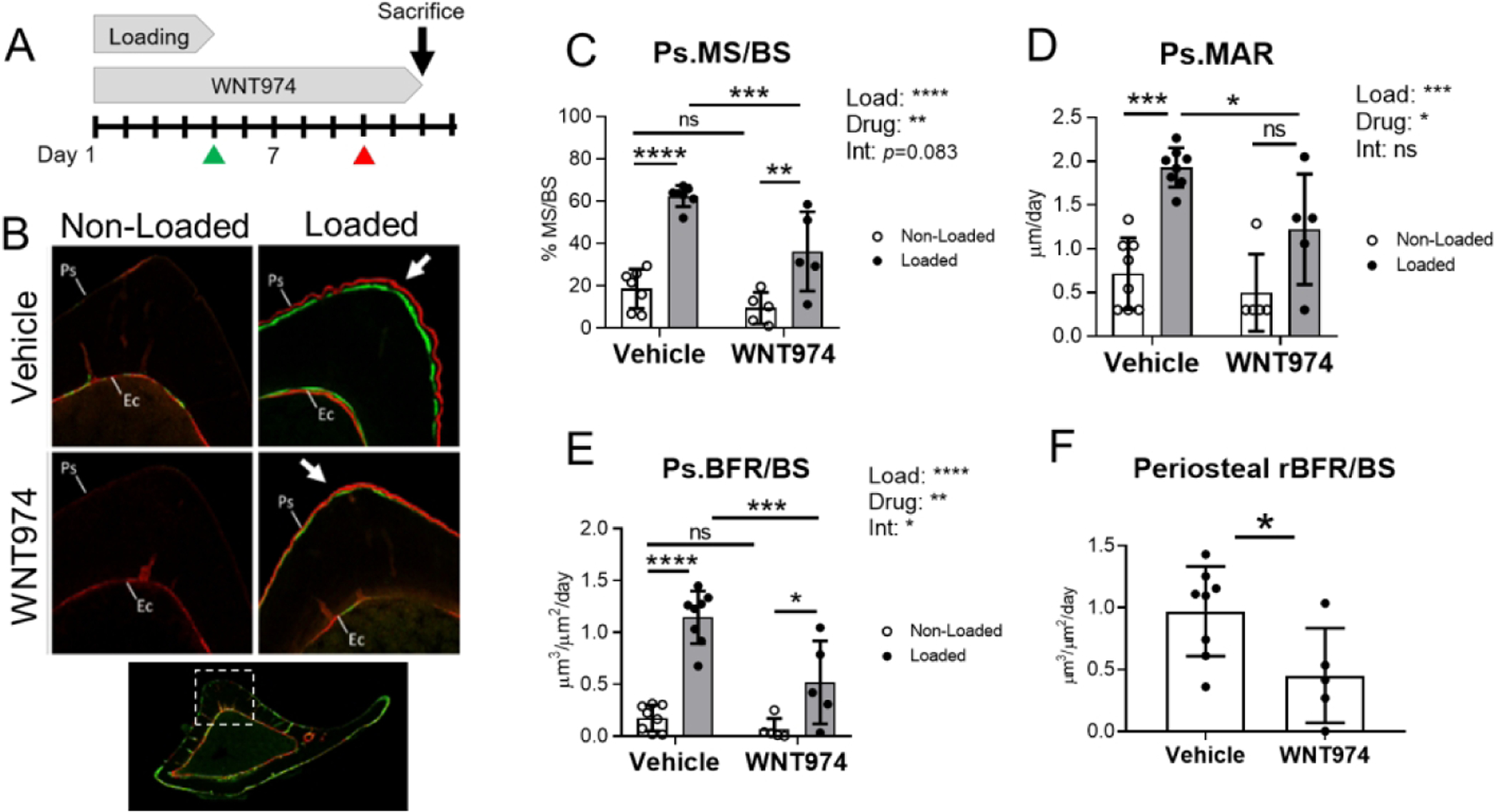
Loading-induced periosteal bone formation is diminished in WNT974-treated mice. (A) Five-month-old C57BL/6 female mice were subjected to 5 days of tibial loading, and concurrently treated with WNT974, to systemically inhibit Wnt secretion, or vehicle. Calcein and alizarin were given on days 5 and 10, respectively (green and red arrowheads) to label actively mineralizing surfaces. (B) Representative transverse images from the mid-diaphysis of non-loaded and loaded tibias in each group showing the region of peak compressive strain, where double-labeled surface is seen in the loaded bones (arrows). Ps= periosteum, Ec=endocortex. (C–F) Indices of periosteal bone formation show that loading stimulated bone formation in both treatment groups, albeit significantly less in WNT974-treated mice. Two-factor ANOVA was used to evaluate the effects of loading (“Load”) and WNT974 treatment (“Drug”), and their interactions (“Int”), with Sidak’s multiple comparisons test for pairwise comparisons (C–E). One-factor ANOVA was used to compare rBFR/BS (F). Bars depict mean ± SD, with individual data points shown (n=5–8 per treatment group). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant (p>0.05).
On the endocortical surface, which experiences a lower strain magnitude than the periosteal surface, loading had a negligible effect on bone formation (Suppl. Fig. S1). Two-factor ANOVA indicated that loading did not affect Ec.MS/BS and Ec.BFR/BS and only marginally increased Ec.MAR. In contrast, WNT974 significantly reduced indices of bone formation, independent of loading (i.e., interaction not significant). In particular, Ec.MS/BS, Ec.MAR, and Ec.BFR/BS were all significantly lower in the non-loaded tibias of WNT974-treated mice compared to vehicle-treated mice. Therefore, independent of loading, Wnt secretion is required for the maintenance of basal endocortical bone formation in 5-month old C57BL/6 mice.
Loading-induced upregulation of osteogenic genes is reduced in WNT974-treated mice
To better understand the mechanisms underlying the osteo-anabolic deficits in WNT974-treated mice, tibial gene expression was assessed by qPCR after day 5 of loading. In vehicle-treated mice, loading potently stimulated expression of genes associated with collagen synthesis (Col1a1), osteoblast differentiation (Sp7/Osterix), and osteoblast activity (Bglap/Osteocalcin), whereas in WNT974-treated mice, loading failed to significantly upregulate expression of Col1a1 or Bglap, and upregulation of Sp7 was blunted (Fig. 2A–C). Moreover, two-factor ANOVA indicated that the expression of Col1a1 and Bglap was significantly reduced by WNT974 treatment in both non-loaded and loaded tibias, with a significant load-treatment interaction for Col1a1. In contrast, neither loading nor WNT974 treatment affected expression of the late osteoblast/osteocyte marker Dmp1 (Fig. 2D). In summary, consistent with periosteal bone formation results, loading induced the upregulation of osteogenic genes, while treatment with WNT974 strongly diminished the osteo-inductive effects of loading.
Figure 2.
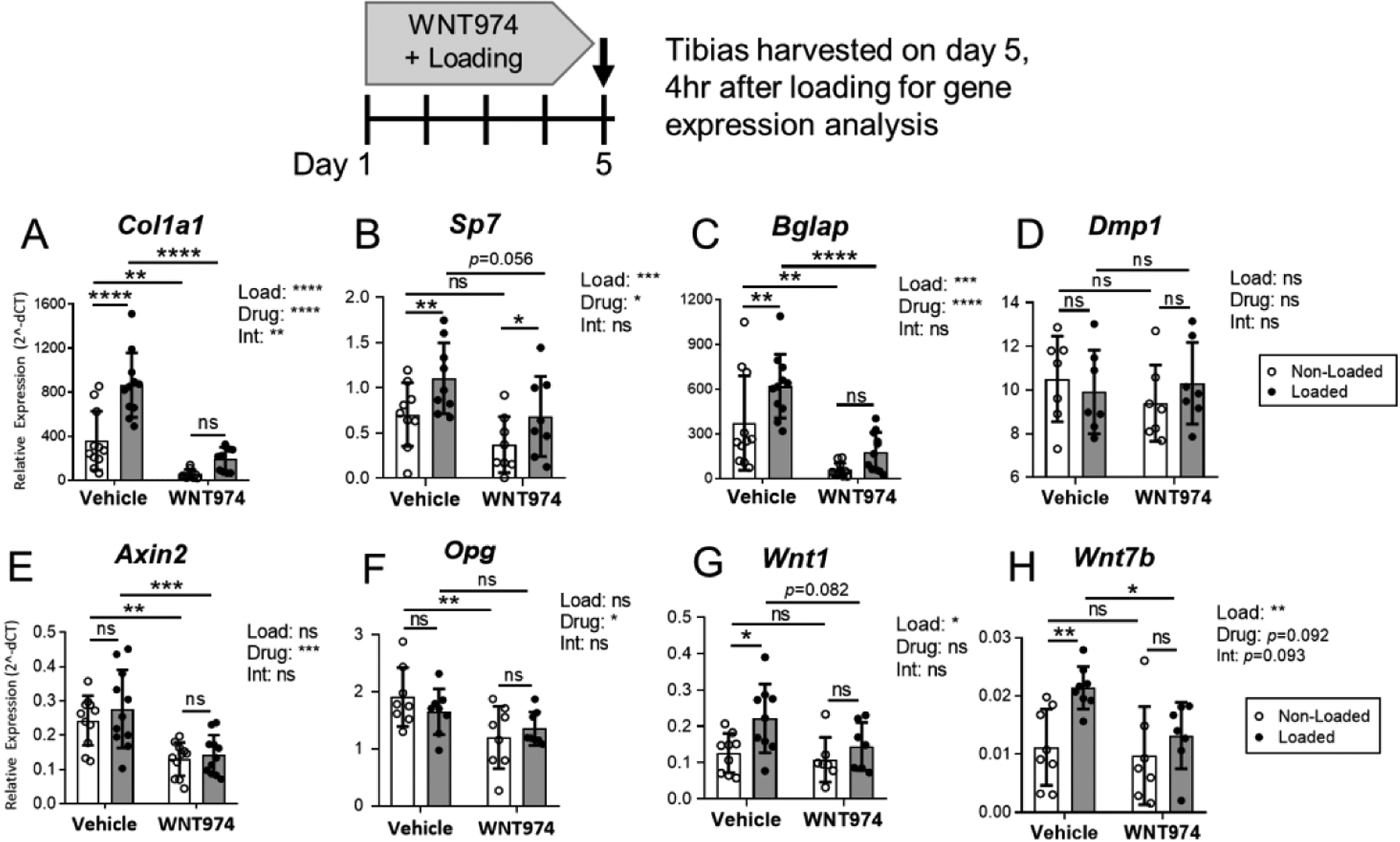
Loading-induced gene expression is altered in WNT974-treated mice. Gene expression in tibias was analyzed by qPCR to evaluate the effect of 5 days of loading and WNT974 treatment. (A–D) Tibial loading had a potent inductive effect on osteogenic gene expression. In vehicle-treated mice, Col1a1, Sp7, and Bglap were significantly higher in loaded versus non-loaded tibias. By contrast, in WNT974-treated mice tibial loading failed to significantly increase Col1a1 and Bglap expression. Dmp1 was not affected by loading in either group. (E–F) Wnt target genes Axin2 and Opg were not significantly regulated by loading but were significantly lower in the bones of WNT974-treated mice (G–H). Wnt1 and Wnt7b were responsive to loading but not WNT974 treatment. n=7–11/group. Data analysis as described in Figure 1. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant (p>0.05).
We also surveyed a panel of Wnt pathway-related genes. Loading had a negligible effect on tibial expression of the Wnt target genes Axin2 and Opg, whereas WNT974 significantly decreased their expression (Fig. 2E–F). Axin2 and Opg expression were reduced by 47% and 37%, respectively, in the non-loaded tibias of WNT974-treated mice compared to vehicle-treated mice. In contrast, two-way ANOVA indicated that Wnt1 and Wnt7b were not affected by WNT974 treatment but were significantly up-regulated by loading (Fig. 2G–H). Other Wnt pathway related genes were not consistently regulated by loading or WNT974 treatment (Suppl. Fig. S2).
Loading-induced bone formation is reduced in osteoblast-specific Wls knockout mice
To investigate the role of Wnts secreted by osteoblasts in loading-induced bone formation, we conditionally deleted the intracellular Wnt transporter Wntless (Wls), in Osterix-expressing cells in 5-month old female and male mice. A fluorescent Cre reporter bred into a subset of tamoxifen-inducible OsxCreERT2;WlsF/F mice showed robust Cre activity in tibial osteoblasts and osteocytes following a 3-day regimen of tamoxifen (Suppl. Fig. S3). PCR of genomic DNA showed that Wls deletion was specific to bone (Suppl. Fig. S4). Based on these results, a 3-day tamoxifen dosing regimen was used to conditionally delete Wls in adult mice, followed by loading 3 days later (Fig. 3A). Body weight did not differ between control and Wls knockouts (not shown). Wls mRNA expression was 53% lower in knockout tibias relative to controls at the start of loading (p<0.001, Fig. 3B), and likewise immunostaining for Wls protein showed reduced expression in osteoblasts and osteocytes in bones of knockout mice (Suppl. Fig. S5). In addition, western blot analysis showed reduced phosphorylated-Lrp6 in knockout bones, confirming diminished activation of Wnt/Lrp signaling (Suppl. Fig. S6).
Figure 3.
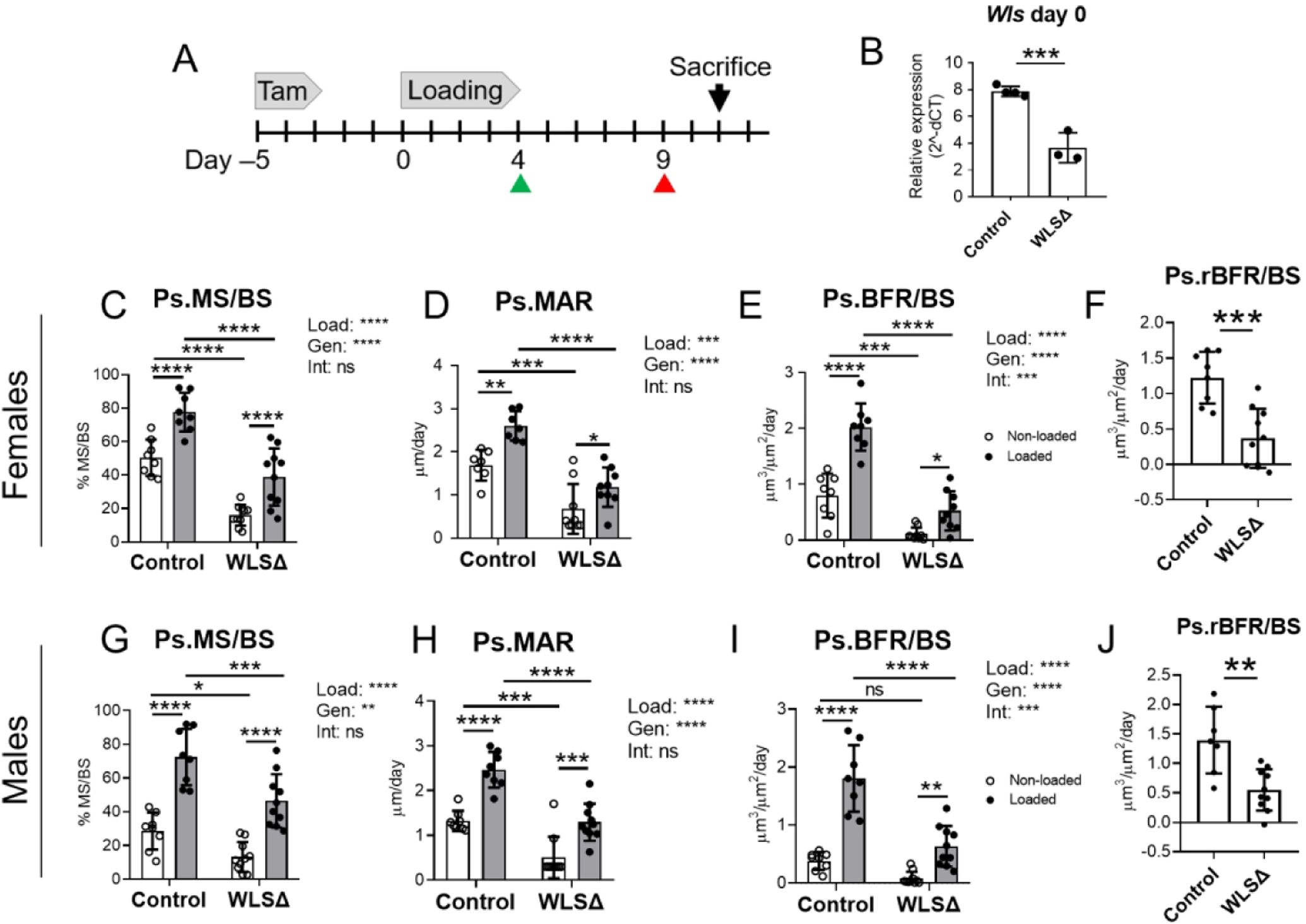
Loading-induced periosteal bone formation is impaired in Wntless knockout mice. (A) Male and female mice were given tamoxifen to induce Wls knockout at 5-months old, then subjected to 5 days of loading on the right tibia to stimulate bone formation. (B) Tibial Wls expression was 53% lower in Wls cKO mice (WLSΔ) compared to controls (n=3–4) on day 0. Mice received calcein and alizarin (green and red arrowheads, respectively), in preparation for dynamic histomorphometry. (C–F) Indices of periosteal bone formation in Wls cKO females (n=7–10). (G–J) Indices of periosteal bone formation in Wls cKO males (n=8–11). Two-factor ANOVA was used to evaluate the effects of loading (“Load”) and genotype (“Gen”), and loading-genotype interactions (“Int”), with Sidak’s multiple comparisons test for pairwise comparisons. Oneway ANOVA was used to compare relative bone formation rate (BFR/BSLoaded minus BFR/BS Non-Loaded), which was used as an index of loading-induced bone formation (F and J). Bars depict mean ± SD, with individual data points shown (n=5–8 per treatment group). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant (p>0.05).
Next, we subjected control and Wls cKO mice to tibial loading to stimulate lamellar bone formation. In both loaded and non-loaded bones, periosteal and endocortical bone formation indices were significantly lower in Wls knockouts (Figures 3 and 4). In females, Ps.BFR/BS and Ec.BFR/BS were 85% and 82% lower, respectively, in the non-loaded tibias of knockouts relative to controls (Figs. 3E and 4C). Similarly, in males Ps.BFR/BS and Ec.BFR/BS were 78% and 87% lower, respectively, in the non-loaded bones of knockouts relative to controls (Fig. 3I and 4G). In a separate cohort of mice not subjected to loading, serum analysis by ELISA showed that P1NP was 60% lower in Wls cKO mice compared to controls (p<0.001, Suppl. Fig S8). Together, these data support a role for osteoblast-derived Wnts in maintaining basal-level bone formation in the adult skeleton.
Figure 4.
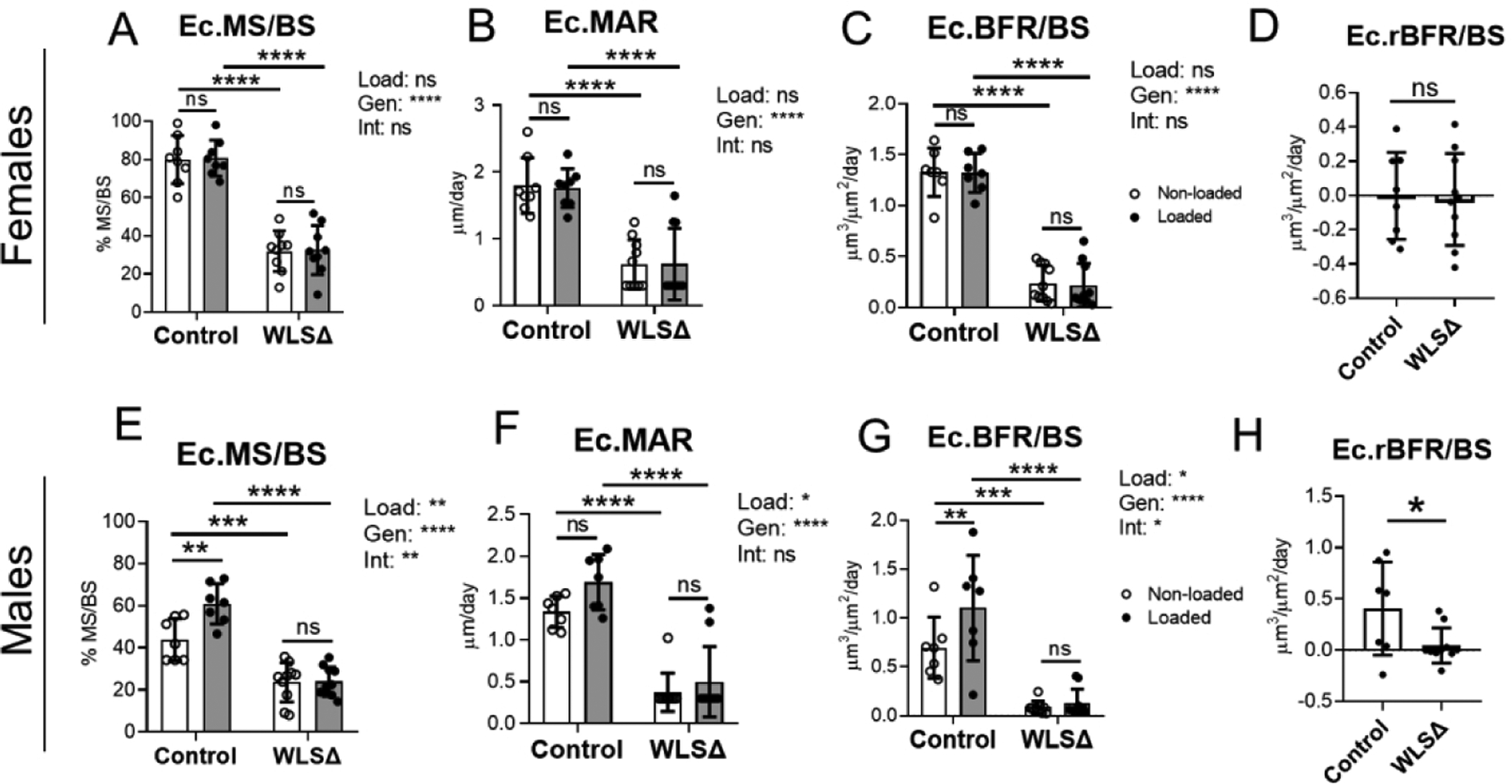
Endocortical bone formation is reduced in Wls knockouts. Indices of endocortical bone formation in Wls knockout females (n=7–10) (A–D) and Wls knockout males (n=8–11) (E–H). In females, loading had a negligible effect on endocortical bone formation (A–C). In males, loading enhanced Ec.MS/BS and Ec.BFR/BS in controls, but not in knockouts (E–G). Independent of loading, endocortical bone formation indices were lower in Wls knockout mice relative to controls. Data analysis as described in Figure 3. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant (p>0.05).
In both males and females, loading enhanced periosteal bone formation in both genotypes, but bone formation indices were significantly lower in the loaded bones of knockout mice compared to sex-matched controls. In females, Ps.MS/BS was 50% lower in loaded knockout bones relative to controls, while Ps.MAR and Ps.BFR/BS were 55% and 74% lower, respectively (p<0.0001, Fig. 3C–E). Similarly, in males Ps.MS/BS, Ps.MAR, and Ps.BFR/BS were 36%, 48%, and 65% lower, respectively, in loaded bones of knockouts vs controls (p<0.001, Fig. 3G–3I). Importantly, a highly significant loading-genotype interaction was detected for Ps.BFR/BS in both sexes, indicating that the effect of loading on periosteal bone formation differed between knockout and control mice (p<0.001, Figs. 3E and 3I). This difference between genotypes was further illustrated by a significant reduction in the relative (loaded minus non-loaded) periosteal bone formation rate in Wls knockouts compared to controls (Fig. 3F and 3J).
In contrast to the highly anabolic effect of loading on the periosteal surface, loading had a negligible effect on endocortical bone formation in females (Fig. 4A–D), and a modest effect in males (Fig. 4E–H). In males, loading significantly increased Ec.MS/BS and Ec.BFR/BS in control but not knockout mice, and two-factor ANOVA indicated a significant loading-genotype interaction for Ec.MS/BS and Ec.BFR/BS (Figs. 4E and 4G). Taken together with the periosteal results, these data demonstrate that osteoblast-derived Wnts contribute to loading-induced bone formation.
Recent studies have demonstrated a role for periosteal cell proliferation in the anabolic response to loading (Zannit and Silva, 2019) (Zannit, et al 2020). To test whether the impaired loading response in Wls cKO mice was due to diminished cell proliferation, we dosed mice with EdU throughout the 5-day loading period and for 3 additional days until sacrifice (Suppl. Fig. S9A). Loading caused an increase in the EdU+ periosteal surface, to a similar level (~30%) in control and Wls knockout mice. Thus, loading-induced periosteal cell proliferation appeared normal in Wls cKO mice (Suppl Fig S9). Similarly, we did not observe any evidence of increased TUNEL+ staining in Wls knockout bones, suggesting that their impaired anabolism was not due to increased apoptosis (Suppl. Fig. S10).
Loading-induced expression of osteogenic and Wnt target genes is impaired in osteoblast-specific Wls knockout mice
To further characterize the effects of Wls deletion in osteoblasts, expression of genes related to bone formation and Wnt signaling was surveyed by qPCR (Figures 5 and 6). Wls deletion reduced osteoblastic gene expression; in non-loaded bones, Col1a1, Sp7, Bglap, and Pdpn were 35–55% lower in Wls cKO mice relative to controls (p<0.05, Fig. 5B–E).
Figure 5.
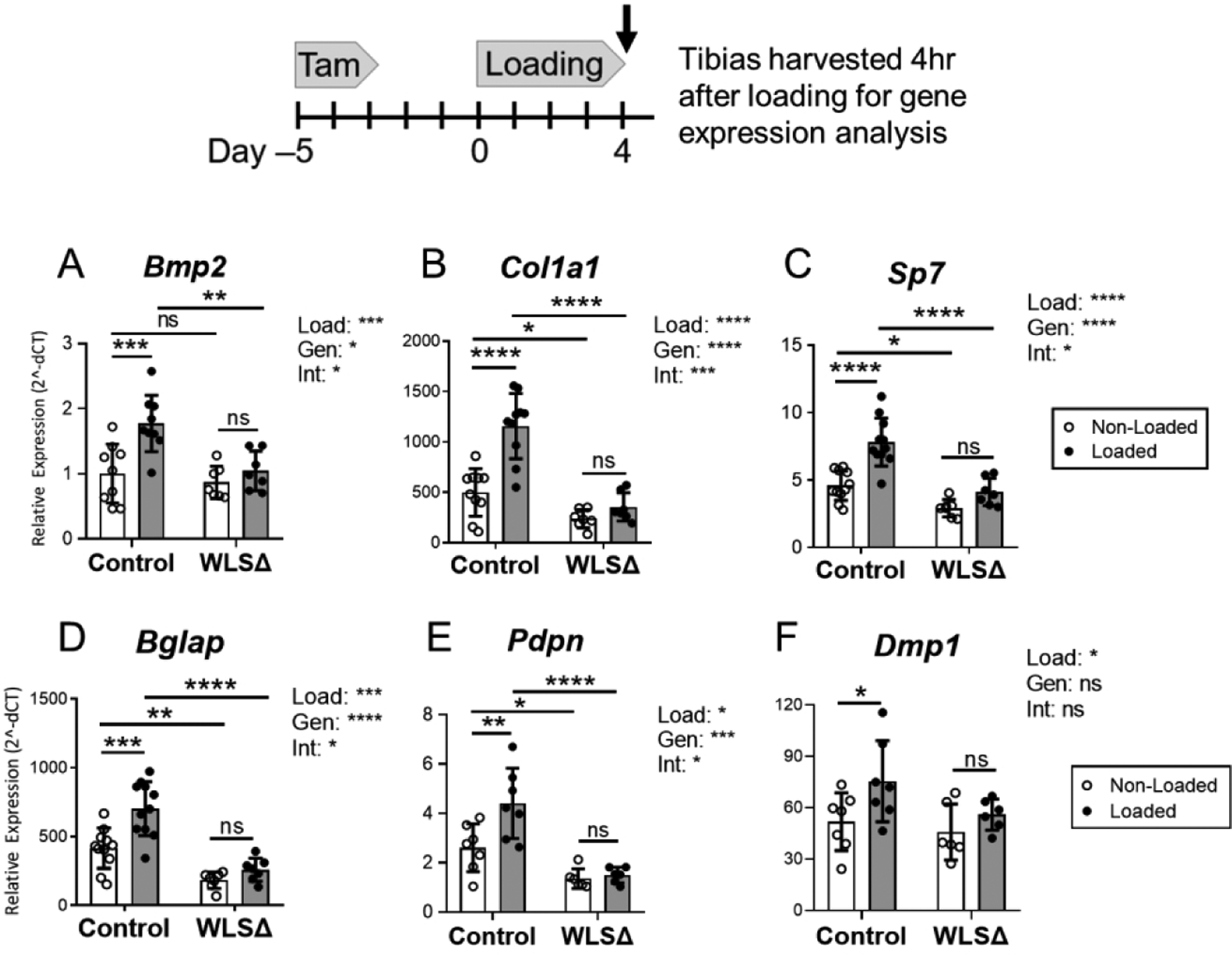
Loading-induced upregulation of osteogenic genes is impaired in Wntless knockout mice. qPCR was used to evaluate gene expression after 5 days of loading in control and Wls cKO (WLSD) mice. Expression of genes related to osteogenesis was significantly higher in the loaded versus non-loaded bones in control mice, whereas loading failed to significantly upregulate osteogenic gene expression in the tibias of knockout mice. Two-factor ANOVA identified a significant loading-genotype interaction for Bmp2, Col1a1, Sp7, Bglap, and Pdpn, indicating that osteoblast-specific Wls expression was required for the regulation of these genes by loading. Data analysis as described in Figure 3. (n=8–11) *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant (p>0.05).
Figure 6.
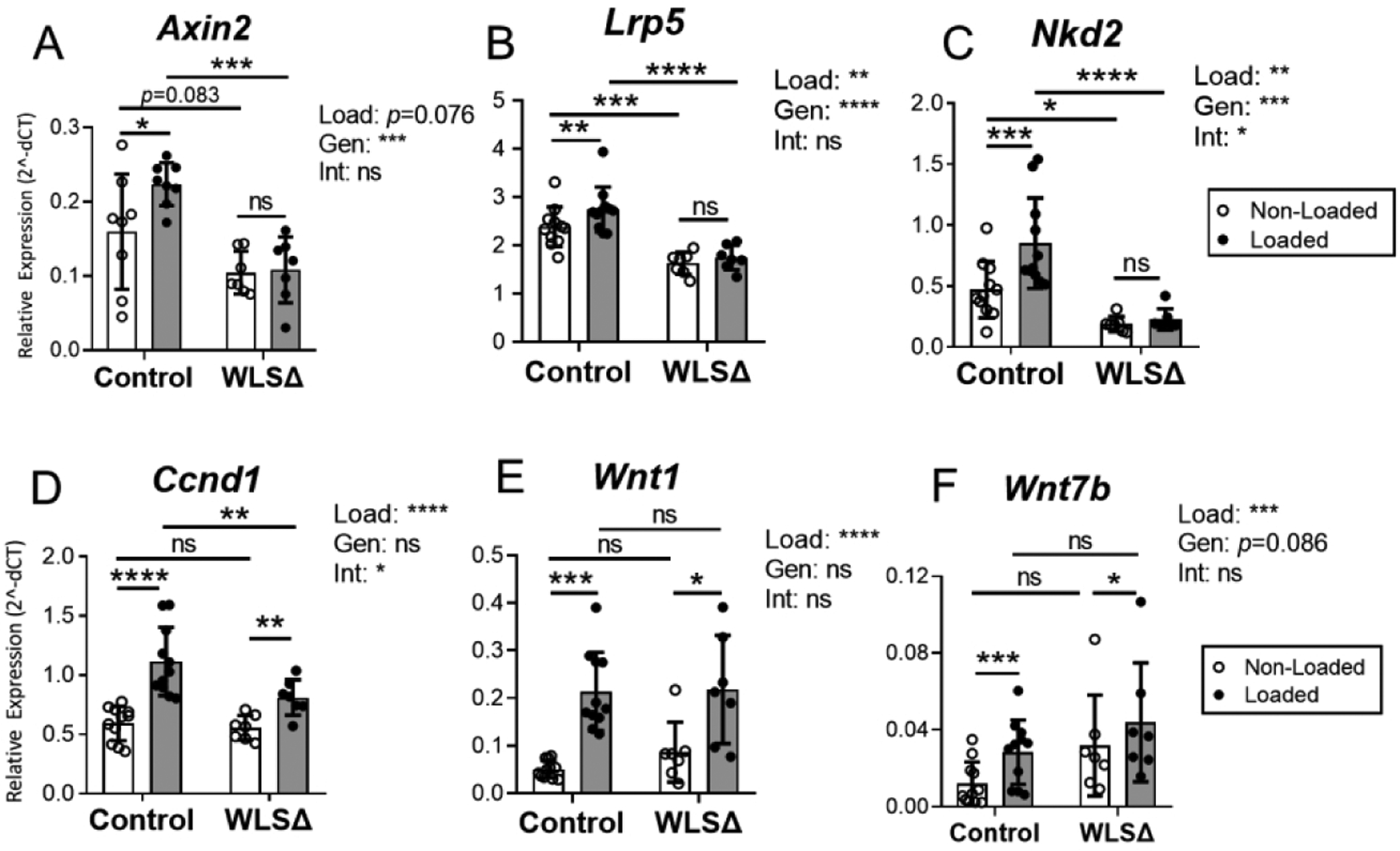
Wnt target gene expression was blunted in Wls knockouts. Expression of Wnt target genes (A–D) and Wnt ligand genes (E–F) in the bone was evaluated by qPCR after 5 days of loading. Wnt target genes Axin2, Lrp5, and Nkd2 were significantly upregulated by loading in control but not Wls cKO (WLSΔ) mice (A–C). Loading-induced upregulation of Wnt1 and Wnt7b was observed in both groups (E–F). Data analysis as described in Figure 3. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant (p>0.05).
In control mice, tibial loading enhanced osteogenic marker expression. Bmp2, Col1a1, Sp7, Bglap, Pdpn, and Dmp1 were all significantly higher in the loaded vs. non-loaded bones of control mice (Fig. 5A–F). In stark contrast, loading failed to significantly enhance the expression of these same genes in Wls knockouts. Importantly, two-factor ANOVA indicated a significant loading-genotype interaction for Bmp2, Col1a1, Sp7, Bglap, and Pdpn (p<0.05, Fig. 5A–E), demonstrating that loading-induced upregulation of these osteogenic factors was different between genotypes.
Next, a survey of several Wnt target genes showed that Wls deletion blunted their expression in non-loaded and loaded bones. In non-loaded tibias, Axin2 expression was marginally lower in knockout versus control mice (−32%, p=0.083; Fig. 6A), while Lrp5 and Nkd2 were significantly lower (−32% and −60%, respectively, p<0.0001; Fig. 6B–C). Notably, expression of these three genes was significantly higher in loaded bones of control mice, but was not elevated in loaded bones of knockouts (Fig. 6A–C). In partial contrast, Ccnd1 expression was increased by loading in both genotypes, although its expression was reduced in the loaded bones of knockout compared to control mice (Fig. 6D). Thus, Wls deletion in osteoblasts reduced expression of Wnt target genes in bone and their induction by mechanical loading.
Recent studies report that Wnt1 and Wnt7b are osteogenic (Luther, Yorgan et al. 2018) (Chen et al, 2014; Song et al. 2020), and that loading potently stimulates their expression (Kelly, et al, 2016; Chermside-Scabbo et al, 2020). Consistent with these latter reports, Wnt1 expression was 4.2-fold higher in the loaded versus non-loaded limbs of control mice, while Wnt7b was 2.6-fold higher (Fig. 6E–F). In knockout mice, Wnt1 and Wnt7b also increased significantly with loading (2.5 and 1.4-fold higher, respectively) and there was no significant loading-genotype interaction, indicating that the upregulation of these two ligands was not different between genotypes. Together, these data confirm that loading potently induces Wnt1 and Wnt7b, and demonstrate that loading-associated regulation of Wnt1 and Wnt7b in the bone does not require Wnt protein secretion by osteoblasts.
Lastly, we examined the expression of several Wnt antagonists to determine whether they were modulated by Wls deletion. In control mice, loading reduced Dkk1 expression, while Dkk2 and sFRP1 increased in response to loading (Suppl. Fig. S11). By comparison, the effect of loading on Dkk1, Dkk2, and sFRP1 expression was negligible in Wls cKO mice. Sost expression was not affected by loading in either genotype (Suppl. Fig. S11). On the other hand, we used immunohistochemistry to assess osteocyte-specific Sclerostin protein expression and found that tibial loading reduced the number of SOST+ osteocytes at the mid-diaphysis by 10% in controls (p=0.026) but not significantly in Wls knockouts (4%, p=0.423) (Suppl. Fig. S12).
Wls deletion in osteoblast lineage cells enhances bone resorption in adult mice
Wnts produced by osteoblasts modulate bone resorption indirectly by regulating the expression of the osteoclast inhibitor Opg, and directly by regulating the activity of osteoclast progenitors (Moverare-Skrtic et al, 2012). In mice, constitutive Wls deletion in Ocn-expressing cells increases markers of bone resorption (Zhong, et al., 2012). To assess the short-term impact of Wls deletion in osteoblasts in adult animals, qPCR was used to analyze expression of several osteoclast regulators in the tibias of control and knockout mice. Additionally, a separate group of naïve 5-month old mice were tamoxifen-treated and sacrificed 1 week later for serum analysis.
Opg expression in bones of Wls knockout cKO was marginally decreased (−25%, p=0.052; Fig. 7A). Loading had a modest but significant stimulatory effect on Opg expression in bones of control but not knockout mice (p<0.05 versus p=0.46, Fig. 7A). In addition, Wls deletion increased expression of the pro-osteoclast factor Rankl in bone (Fig. 7B). Notably, the Rankl / Opg ratio was 2-fold higher in Wls knockout bones relative to controls (p<0.001), indicating that loss of Wnt secretion by osteoblasts favors osteoclastogenesis.
Figure 7.
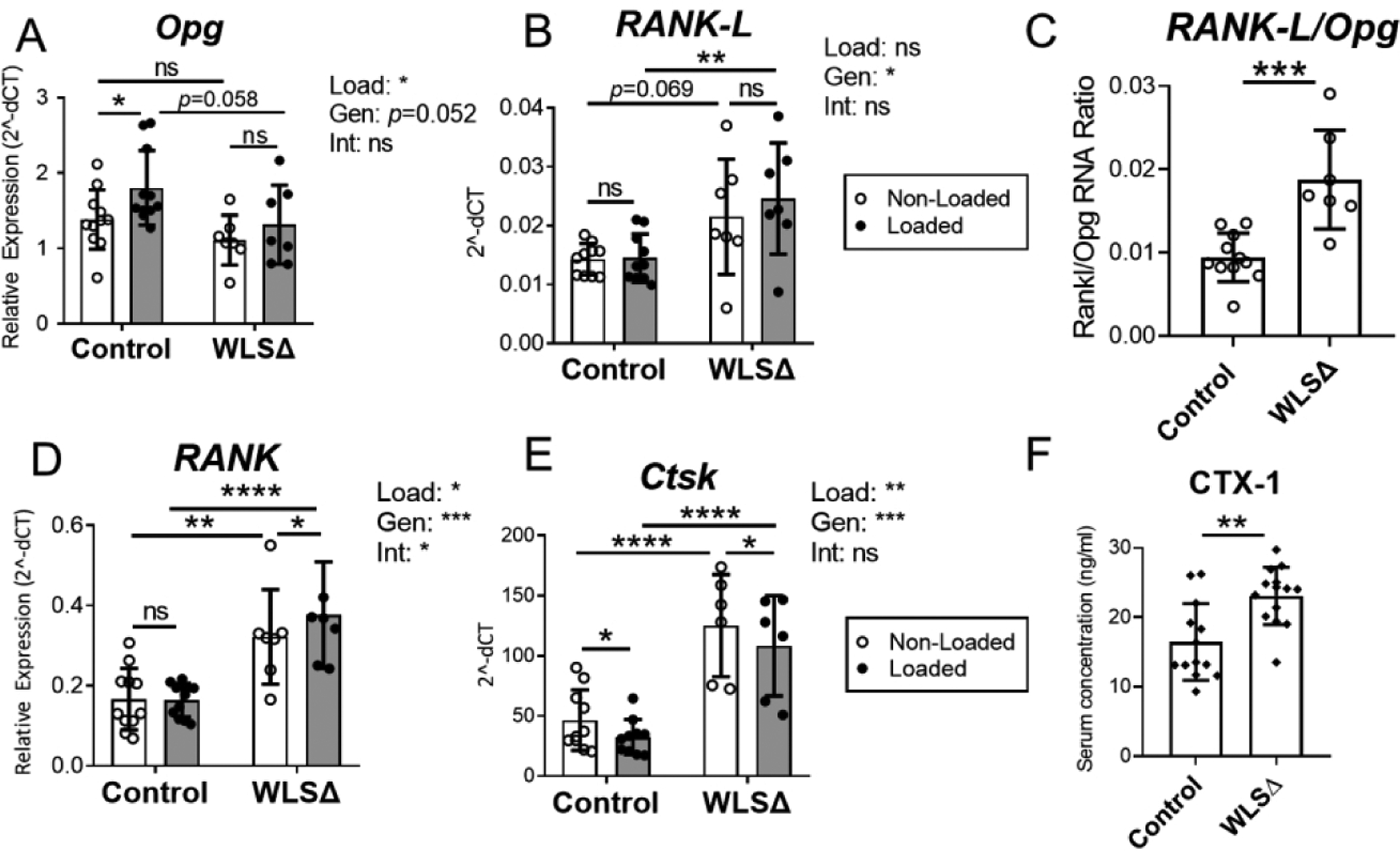
Wls deletion in osteoblasts increased bone resorption in adult mice. (A–E) Genes expressed by osteoblasts (Opg, Rankl) and osteoclasts (RANK, Ctsk) were analyzed by qPCR (n=6–10), and two-factor ANOVA was used to evaluate the effects of loading (“Load”) and genotype (“Gen”) on gene expression. Two-factor ANOVA indicated that loading had a modest but significant role in regulating Opg, RANK, and Ctsk in the bone, while genotype was a significant factor regulating RANKL, RANK, and Ctsk. (C) The ratio between RANK-L RNA and Opg RNA was used as a measure of local bone resorption dynamics. In non-loaded tibias, the RANK-L to Opg ratio was significantly higher in Wls cKO (WLSΔ) bones, indicating a shift toward increased bone resorption in Wls knockout mice. (F) Serum collected from naïve (not loaded) mice showed that Wls deletion significantly increased the bone resorption marker CTX-1 one week after knockout (n=13–14). Data analysis as described in Figure 3. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant (p>0.05).
Expression of RANK and Ctsk, which are predominantly expressed by osteoclast lineage cells, was also elevated in Wls cKO bones. RANK expression was 93% greater in the non-loaded bones of knockouts versus controls (p<0.01), while Ctsk was 260% higher (p<0.0001) (Fig. 7D–E). Finally, serum CTX-1 was 40% higher in naïve (non-loaded) Wls cKO mice compared to controls (p<0.01, Fig. 7F). Together these data indicate that even brief interruption of Wnt secretion in osteoblasts has a significant impact on bone turnover in the adult skeleton.
Discussion
Wnt signaling is critical to many aspects of skeletal regulation, including homeostasis of the adult skeleton and the adaptive response of the skeleton to mechanical loading. However, the importance of loading-associated Wnt ligand induction in the osteo-anabolic response to loading is not well documented. To investigate the role of Wnt ligands, collectively, in the bone anabolic response to loading, we inhibited Wnt ligand secretion in adult (5-mo) mice using a systemic (drug) and a bone-targeted (genetic) approach, and subjected them to axial tibial loading. Mice treated with the porcupine inhibitor WNT974 exhibited a decrease in bone formation in non-loaded limbs as well as a marked decline in the anabolic response to tibial loading. Similarly, within 1–2 weeks of Wls deletion in osteoblasts, skeletal homeostasis was altered with decreased bone formation and increased resorption, and the anabolic response to loading was greatly impaired. These findings establish a requirement for Wnt ligand secretion by osteoblasts for adult bone homeostasis and the anabolic response to mechanical loading.
Previous reports have established a requirement for Wnt ligand secretion in skeletal development and maturation. Treatment of 8–12 wk old mice using the porcupine inhibitor WNT974 reduces bone mass within 3–4 weeks (Funck-Brentano et al., 2018; Madan et al., 2018). Furthermore, Wls deletion in osteoblasts severely impairs skeletal development leading to perinatal lethality in Osx-Cre;Wls cKO mice (Tan et al., 2014), and severe osteopenia and skeletal fragility within 2 months postnatally in Ocn-Cre;Wls cKO mice (Zhong et al., 2012). Our results extend these findings to adult (5-mo) mice. Porcupine inhibition led to reduced bone formation (independent of mechanical loading), evident by a significantly lower expression of osteogenic genes (e.g, Col1a1, Sp7) and rates of endocortical bone formation in the non-loaded limbs of WNT974 treated mice. Furthermore, when we deleted Wls in osteoblasts of 5-mo old mice using the inducible Osx-CreERT2 driver, within 1–2 weeks we observed reduced osteogenic gene expression in bone, a lower serum level of the bone formation marker P1NP, an increase in the Rankl/Opg ratio in bone and in the serum marker of bone resorption CTX-1, along with reduced periosteal and endocortical bone formation rates in non-loaded bones of Wls cKO mice. Collectively, these results establish that secretion of Wnt ligands by osteoblasts is essential for skeletal homeostasis in adult mice, and that short-term inhibition of Wnt secretion causes rapid, deleterious changes in bone turnover.
The ability of the bone to adapt to mechanical stimuli is a key principal of skeletal physiology. A main objective of this study was to assess the requirement of Wnt ligand secretion by osteoblast-lineage cells for the anabolic response to mechanical loading. The importance of the Wnt signaling pathway in bone’s loading response has been demonstrated in LRP5 mutant mice (Niziolek et al., 2012; Robinson et al., 2006; Sawakami et al., 2006; L. Zhao et al., 2013), and with the finding that overexpression of the Wnt antagonist SOST (normally downregulated by loading (Robling et al., 2008)) blocks the anabolic loading response (Tu et al., 2012). Several studies show that loading upregulates the expression of Wnt genes including Wnt1, Wnt7b, Wnt10b and Wnt16 (Chermside-Scabbo et al., 2020; Galea et al., 2017; Holguin et al., 2016; Kelly et al., 2016; Robinson et al., 2006; Wergedal et al., 2015), suggesting a functional role for increased ligand abundance in the bone formation response. Notably, Wergedal et al. (Wergedal et al., 2015) reported that 10-wk old female mice with global deletion of Wnt16 had a diminished periosteal response to tibial bending (Wergedal et al., 2015). Here, we extend these studies to show that Wnt ligands produced by Osx-expressing cells (osteoblasts and osteocytes) are, collectively, essential for both the periosteal and endocortical anabolic response to axial tibial loading in adult mice. First, we observed an increase in osteogenic gene expression after 5 days of loading in control mice, which is a normal precedent to increased bone formation (Chermside-Scabbo et al., 2020; Holguin et al., 2016; Mantila Roosa et al., 2011). But this loading-induced increase in osteogenic gene expression was diminished in both WNT974 treated and Wls cKO mice. For example, loading increased the expression of type I collagen transcript Col1a1 by 2.3-fold in control mice, but this effect was absent in Wls cKO mice. Moreover, tibial loading significantly increased periosteal bone formation rate in control mice, an effect which was significantly diminished in both WNT974 treated and Wls cKO mice. Loading also increased endocortical bone formation in male control mice but not in Wls cKO mice. Taken together, these results demonstrate a requirement for Wnt ligand secretion by osteoblasts in the anabolic response to loading. Moreover, these results motivate follow-up studies targeting osteoblast expression of individual Wnt ligands to identify which ones are important in the bone anabolic response. The role of Wnt1 and Wnt7b induction, in particular, is worth exploring in light of recent evidence that Wnt1 and Wnt7b potently induce bone formation when constitutively expressed in vivo (Joeng et al, 2017) (Chen et al., 2014).
Results from Wls cKO mice offer insights into the regulation of Wnt related gene expression by Wnt signaling in osteoblasts. Non-loaded bones from Wls cKO mice had reduced expression of the canonical Wnt target gene Axin2 and also showed a trend for lower levels of phosphorylated LRP6, consistent with reduced Wnt signaling (Clevers & Nusse, 2012). Wls cKO led to reduced expression of Wnt pathway antagonists Dkk1, Sost and Nkd2 (Burgers & Williams, 2013; Canalis, 2013; S. Zhao et al., 2015), suggesting that Wnt signaling positively regulates these genes as part of a feedback loop. Notably, expression of Wnt1 and Wnt7b was not significantly affected by Wls deletion. Moreover, these ligands were upregulated by loading in both control mice (consistent with previous reports (Chermside-Scabbo et al., 2020; Holguin et al., 2016; Kelly et al., 2016)), and also in Wls cKO mice. This finding suggests that load-induced upregulation of these two ligands is not regulated by Wnt signaling in osteoblasts. Additional work is needed to clarify the mechanisms whereby Wnt ligands in bone are induced by mechanical loading, although one recent study found that the upregulation of Wnt1 in osteocytes was regulated by the mechanosensitive Piezo1 channel, possibly via YAP/TAZ (Li et al., 2019).
Sost is regulated by mechanical loading (Robling et al, 2008). A near-significant reduction in Sost expression (−30%) was observed in the loaded tibias of wild-type C57BL/6 mice after 5 days of loading (p=0.061; Suppl Fig S2C). In Wntless mice Sost expression was 17% lower in the loaded vs non-loaded tibias of control mice, although the difference was not significant (p=0.23; Suppl Fig S11D). The lack of Sost downregulation here may be related to the fact that tibias were harvested after 5 days of loading. Therefore our data reflects gene expression dynamics during active bone formation rather than an acute transcriptional response to one bout of loading (Chermside-Scabbo et al., 2020). Analysis of protein expression by immunohistochemistry showed that after 5 bouts of loading, the percentage of Sclerostin-positive osteocytes at the mid-diaphysis was reduced by 10% in the loaded vs non-loaded tibias of control mice (p=0.026; Suppl Fig S12B).
Our findings highlight the functional importance of Wnt ligand secretion in skeletal regulation. Recent genome-wide association study (GWAS) results identified 89 variants in the human WLS gene as being significantly associated (either positively or negatively) with estimated bone mineral density (eBMD) in the UK Biobank dataset (Morris et al., 2019; “Musculoskeletal Knowledge Portal,”). In contrast, there were no variants in PORCN that were significantly associated with eBMD. The association of WLS variants with bone mass in humans, in combination with the current and previous loss of function studies in mice (Zhong, et al. 2012; Tan et al., 2014), suggests that minor dysregulation in Wnt ligand secretion may influence bone mass accrual in response to anabolic cues, including mechanical loading.
While responses to loading were diminished by targeting Wnt secretion, they were not absent. Periosteal bone formation indices were significantly higher in loaded vs. non-loaded limbs of WNT974 treated as well as Wls cKO mice, a result that might be due to two factors. First, non-Wnt pathways are also important in loading-induced bone formation, as noted in Sost and Dkk1 knockout mice (Pflanz et al., 2017; Morse et al., 2020), and second, Wnt secretion was not completely blocked in our studies. We cannot distinguish the relative importance of these two by factors, but note that even a ~50% reduction in Wls expression in bone of cKO mice (Fig. 3B) is sufficient to reduce their relative loading-induced periosteal bone formation rate by 60–70% (Suppl. Fig. S9).
In summary, we used pharmacological (WNT974) and genetic (Wls cKO) approaches in adult mice to disrupt Porcupine and Wntless, two factors required for Wnt ligand secretion. Mice were subjected to unilateral axial tibial loading, an established model for induction of bone formation. Local bone responses were assessed by qPCR and dynamic histomorphometry, while systemic responses were assessed by serum markers of bone turnover. Results from WNT974 treated mice and Wls cKO mice were consistent, showing sharply diminished measures of bone formation in both non-loaded and loaded bones compared to control mice. In particular, the normal induction of bone formation by mechanical loading seen in control mice was significantly blunted in mice with inhibition of Wnt ligand secretion. We conclude that secretion of Wnt ligands by osteoblasts is required for homeostasis and the full anabolic response to mechanical loading in the adult skeleton.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 AR047867, T32 AR060719 and the Washington University Musculoskeletal Research Center (P30 AR074992). We thank Crystal Idleburg and Samantha Coleman for histology support. We thank the Genome Technology Access Center (supported by P30 CA91842 and UL1 TR002345) for help with gene expression assays. We thank the Core Laboratory for Clinical Studies for their help with serum assays. Finally, we thank Roberto Civitelli for advice and comments on the study.
Footnotes
Disclosures
LYL, MDB, NM, CCS, and RP have no financial or non-financial competing interests to disclose. MJS is on the editorial board at Bone, Journal of Orthopaedic Research, and Calcified Tissue International, and serves on the board of directors at the Orthopaedic Research Society.
References
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2001) Increased bone density in Sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol. Genet 10(5):537–43. doi: 10.1093/hmg/10.5.5367 [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, & Basler K (2006). Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell, 125(3), 509–522. doi: 10.1016/j.cell.2006.02.049 [DOI] [PubMed] [Google Scholar]
- Barrott JJ, Cash GM, Smith AP, Barrow JR, & Murtaugh LC (2011). Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A, 108(31), 12752–12757. doi: 10.1073/pnas.1006437108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers TA, & Williams BO (2013). Regulation of Wnt/beta-catenin signaling within and from osteocytes. Bone, 54(2), 244–249. doi: 10.1016/j.bone.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E (2013). Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol, 9(10), 575–583. doi: 10.1038/nrendo.2013.154 [DOI] [PubMed] [Google Scholar]
- Chen J, Tu X, Esen E, Joeng KS, Lin C, Arbeit JM, Ruegg MA, Hall MN, Ma L, Long F (2014). WNT7B Promote Bone Formation in part through mTORC1. PLoS Genet. 10(1):e1004145. doi: 10.1371/pgen.1004145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermside-Scabbo CJ, Harris TL, Brodt MD, Braenne I, Zhang B, Farber CR, & Silva MJ (2020). Old Mice Have Less Transcriptional Activation But Similar Periosteal Cell Proliferation Compared to Young-Adult Mice in Response to in vivo Mechanical Loading. J Bone Miner Res, 35(9), 1751–1764. doi: 10.1002/jbmr.4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, & Nusse R (2006). A dedicated Wnt secretion factor. Cell, 125(3), 432–433. doi: 10.1016/j.cell.2006.04.018 [DOI] [PubMed] [Google Scholar]
- Clevers H, & Nusse R (2012). Wnt/beta-catenin signaling and disease. Cell, 149(6), 1192–1205. doi: 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Forwood MR, & Turner CH (1994). The response of rat tibiae to incremental bouts of mechanical loading: a quantum concept for bone formation. Bone, 15(6), 603–609. [DOI] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, & Hsu W (2009). Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci U S A, 106(44), 18598–18603. doi: 10.1073/pnas.0904894106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funck-Brentano T, Nilsson KH, Brommage R, Henning P, Lerner UH, Koskela A, … Ohlsson C (2018). Porcupine inhibitors impair trabecular and cortical bone mass and strength in mice. J Endocrinol, 238(1), 13–23. doi: 10.1530/JOE-18-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea GL, Meakin LB, Harris MA, Delisser PJ, Lanyon LE, Harris SE, & Price JS (2017). Old age and the associated impairment of bones’ adaptation to loading are associated with transcriptomic changes in cellular metabolism, cell-matrix interactions and the cell cycle. Gene, 599, 36–52. doi: 10.1016/j.gene.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin N, Brodt MD, Sanchez ME, & Silva MJ (2014). Aging diminishes lamellar and woven bone formation induced by tibial compression in adult C57BL/6. Bone, 65, 83–91. doi: 10.1016/j.bone.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin N, Brodt MD, & Silva MJ (2016). Activation of Wnt Signaling by Mechanical Loading Is Impaired in the Bone of Old Mice. J Bone Miner Res, 31(12), 2215–2226. doi: 10.1002/jbmr.2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeng KS, Lee Y, Lim J, Chen Y, Jiang M, Munivez E, Ambrose C, Lee BH (2017) Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis. J Clin Invest. 127(7):2678–2688. doi: 10.1172/JCI92617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, & Perrimon N (1996). The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev, 10(24), 3116–3128. doi: 10.1101/gad.10.24.3116 [DOI] [PubMed] [Google Scholar]
- Kelly NH, Schimenti JC, Ross FP, & van der Meulen MC (2016). Transcriptional profiling of cortical versus cancellous bone from mechanically-loaded murine tibiae reveals differential gene expression. Bone, 86, 22–29. doi: 10.1016/j.bone.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Castillo N, Kim-Weroha NA, Kamel MA, Javaheri B, Ellies DL, Krumlauf RE, … Johnson ML (2015). In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone, 76, 58–66. doi: 10.1016/j.bone.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, & Xiong J (2019). Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife, 8. doi: 10.7554/eLife.49631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan B, McDonald MJ, Foxa GE, Diegel CR, Williams BO, & Virshup DM (2018). Bone loss from Wnt inhibition mitigated by concurrent alendronate therapy. Bone Res, 6, 17. doi: 10.1038/s41413-018-0017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantila Roosa SM, Liu Y, & Turner CH (2011). Gene expression patterns in bone following mechanical loading. J Bone Miner Res, 26(1), 100–112. doi: 10.1002/jbmr.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin KA, Droscha CJ, Williams BO (2013) A Comprehensive Overview of Skeletal Phenotypes Associated with Alterations in Wnt/β-Catenin Signaling in Humans and Mice. Bone Res. 1(1):27–71. doi: 10.4248/BR201301004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, … Richards JB (2019). An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet, 51(2), 258–266. doi: 10.1038/s41588-018-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musculoskeletal Knowledge Portal. Retrieved 2019 Dec 9 https://msk.hugeamp.org/phenotype.html?phenotype=eBMD
- Niziolek PJ, Warman ML, & Robling AG (2012). Mechanotransduction in bone tissue: The A214V and G171V mutations in Lrp5 enhance load-induced osteogenesis in a surface-selective manner. Bone, 51(3), 459–465. doi: 10.1016/j.bone.2012.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, … Bex FJ (2006). Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem, 281(42), 31720–31728. doi: 10.1074/jbc.M602308200 [DOI] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, … Turner CH (2008). Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem, 283(9), 5866–5875.doi: 10.1074/jbc.M705092200 [DOI] [PubMed] [Google Scholar]
- Rodon J, Argilés G, Connolly RM, Vaishampayan U, de Jonge M, Garralda E, Giannakis M, Smith DC, Dobson JR, McLaughlin ME, Seroutou A, Ji Y, Morawiak J, Moody SE, Janku F. Phase 1 study of single-agent WNT974, a first-in-class Porcupine inhibitor, in patients with advanced solid tumours. Br J Cancer. 2021. Jul;125(1):28–37. doi: 10.1038/s41416-021-01389-8. Epub 2021 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, … Turner CH (2006). The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem, 281(33), 23698–23711. doi: 10.1074/jbc.M601000200 [DOI] [PubMed] [Google Scholar]
- Tan SH, Senarath-Yapa K, Chung MT, Longaker MT, Wu JY, & Nusse R (2014). Wnts produced by Osterix-expressing osteolineage cells regulate their proliferation and differentiation. Proc Natl Acad Sci U S A, 111(49), E5262–5271. doi: 10.1073/pnas.1420463111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, … Bellido T (2012). Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone, 50(1), 209–217. doi: 10.1016/j.bone.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CH, Forwood MR, Rho JY, & Yoshikawa T (1994). Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res, 9(1), 87–97. doi: 10.1002/jbmr.5650090113 [DOI] [PubMed] [Google Scholar]
- Wergedal JE, Kesavan C, Brommage R, Das S, & Mohan S (2015). Role of WNT16 in the regulation of periosteal bone formation in female mice. Endocrinology, 156(3), 1023–1032. doi: 10.1210/en.2014-1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, … Nusse R (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature, 423(6938), 448–452. doi: 10.1038/nature01611 [DOI] [PubMed] [Google Scholar]
- Zannit HM, Silva MJ. Proliferation and Activation of Osterix-Lineage Cells Contribute to Loading-Induced Periosteal Bone Formation in Mice. JBMR Plus. 2019. Sep 11;3(11):e10227. doi: 10.1002/jbm4.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannit HM, Brodt MD, Silva MJ. Proliferating osteoblasts are necessary for maximal bone anabolic response to loading in mice. FASEB J. 2020;34(9):12739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Shim JW, Dodge TR, Robling AG, & Yokota H (2013). Inactivation of Lrp5 in osteocytes reduces young’s modulus and responsiveness to the mechanical loading. Bone, 54(1), 35–43. doi: 10.1016/j.bone.2013.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Kurenbekova L, Gao Y, Roos A, Creighton CJ, Rao P, … Yustein JT (2015). NKD2, a negative regulator of Wnt signaling, suppresses tumor growth and metastasis in osteosarcoma. Oncogene, 34(39), 5069–5079. doi: 10.1038/onc.2014.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, … Williams BO (2012). Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A, 109(33), E2197–2204. doi: 10.1073/pnas.1120407109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


