Abstract
Evidence suggests that Helicobacter pylori plays a role in gastric cancer initiation. However, epidemiologic studies on the specific role of other bacteria in the development of gastric cancer are lacking. We conducted a case-control study of 89 cases with gastric intestinal metaplasia (IM) and 89 matched controls who underwent upper gastrointestinal endoscopy at three sites affiliated with NYU Langone Health. We performed shotgun metagenomic sequencing using oral wash samples from 89 case-control pairs and antral mucosal brushing samples from 55 case-control pairs. We examined the associations of relative abundances of bacterial taxa and functional pathways with IM using conditional logistic regression with and without elastic-net penalty. Compared with controls, oral species Peptostreptococcus stomatis, Johnsonella ignava, Neisseria elongata, and Neisseria flavescens were enriched in cases (odds ratios [ORs] = 1.29–1.50, P = 0.004–0.01) while Lactobacillus gasseri, Streptococcus mutans, S. parasanguinis, and S. sanguinis were under-represented (ORs = 0.66–0.76, P = 0.006–0.042) in cases. Species J. ignava and Filifactor alocis in the gastric microbiota were enriched (ORs = 3.27 and 1.43, P = 0.005 and 0.035, respectively), while S. mutans, S. parasanguinis, and S. sanguinis were under-represented (ORs = 0.61–0.75, P = 0.024–0.046), in cases compared with controls. The lipopolysaccharide and ubiquinol biosynthesis pathways were more abundant in IM, while the sugar degradation pathways were under-represented in IM. The findings suggest potential roles of certain oral and gastric microbiota, which are correlated with regulation of pathways associated with inflammation, in the development of gastric precancerous lesions.
Keywords: oral microbiome, gastric microbiome, gastric intestinal metaplasia, case-control study, shotgun metagenomic sequencing
Introduction
Gastric cancer (GC) is the fifth most common cancer and the fourth leading cause of cancer deaths worldwide, with over 1 million new cases and 769,000 deaths in 20201. Histologically the major type of GC is the intestinal type of non-cardia GC that occurs via a predictable progression from chronic gastritis to atrophic gastritis, intestinal metaplasia (IM), dysplasia, and gastric adenocarcinoma2. Helicobacter pylori (H. pylori), which causes mucosal inflammation and progressive destruction of the hydrochloric acid-secreting glands of the stomach, plays a crucial role in the initial steps of the carcinogenesis cascade3. However, only 3% of H. pylori+ individuals develop GC4, implying that there are other risk factors. In addition, colonization of H. pylori decreases (and is eventually lost) under achlorhydric condition in the precancerous and cancerous lesions5,6. The loss of H. pylori and impairment of acid secretion in later steps of carcinogenesis may allow the stomach to be colonized by oral and intestinal microbes that are not ordinarily present under its normal acidic condition.
Oral health conditions and selected periodontal pathogens have been related to GC and precancerous lesions7,8, suggesting that the oral microbiome may also contribute to the onset and progression of GC. In addition, oral bacteria can reach the stomach through swallowed saliva, nutrients, and drinks and change its microbiota and possibly immune defenses9. However, studies investigating the role of specific oral bacteria in GC development are limited.
Mechanistic studies have suggested that the presence of other bacteria following H. pylori infection promotes GC development10,11. Several studies also have provided evidence that gastric microbiota other than H. pylori is altered during the progression from a healthy gastric mucosa to GC12–14. However, existing studies were mostly based on genetic analysis of 16S rRNA which is limited in characterizing the underlying microbial species and genes that might be involved in carcinogenesis15. Shotgun metagenomic sequencing provides higher-level taxonomic and functional resolution by targeting the entire genomic content of a sample16.
In the present study, we performed shotgun metagenomics on the oral and gastric microbial communities to comprehensively evaluate the compositional and functional changes associated with gastric IM, an established precancerous lesion for GC with shared risk factors17.
Materials and Methods
Study population and sample collection
We invited individuals who were scheduled for upper gastrointestinal endoscopy for clinically indicated reasons at three sites affiliated with NYU Langone Health, including Bellevue Hospital Center, a private group practice at New York City, and NYU Langone Gastroenterology Associates between 2009–2019, following protocols similar to those used in the Bellevue Hospital Center7,8. Exclusion criteria include: 1) prior gastric surgery, 2) use of antimicrobial agents within the prior 2 months, 3) current use of anticoagulants, 4) active gastrointestinal bleeding, and 5) having had or suspected to have esophageal varices. Information on demographic and lifestyle factors was collected with structured questionnaires administered by a trained interviewer. We collected biopsies from the antrum, cardia, corpus, and fundus of the stomach for standard pathology review, blinded to questionnaire data.
We collected stimulated saliva samples from participants recruited between 2009 and 2011, and oral wash samples from those recruited in later years. Briefly, participants were asked to chew a piece of paraffin wax to stimulate saliva production and to gently expectorate 2–5 mL of saliva directly into a sterile sample collection tube, on ice8. Participants recruited in later years were asked to swish with 10 ml saline and to expectorate into a sterile sample collection tube. Both saliva and oral wash samples were vortex-mixed thoroughly, immediately placed into a container with ice, transferred to the laboratory within 1 h, and stored at −80°C for further processing. In 2016, we started to collect a mucosal brushing sample from the gastric antrum during the endoscopy, using an endoscopic cytology brush.
A total of 1198 eligible individuals were approached, and 675 (56%) were recruited, including 348 from the Bellevue Hospital Center, 246 from the private group practice, and 81 from the NYU Langone Gastroenterology Associates. The most common reasons for declining participation and comparison on the distribution of sex, age, and race between participants and non-participants are described in detail in the Supplementary Material. We recruited a total of 125 cases with newly diagnosed IM in the gastric antrum or body/fundus and 550 non-cases with normal gastric histology or superficial gastritis without any atrophy, according to the pathologic review results. Saliva or oral wash samples were collected from 82%, serum samples from 91%, of the participants recruited. Antral brushing samples were collected from 91.5% of those recruited since 2016.
For the 106 IM cases with saliva/oral wash samples, we selected controls that were individually matched on sex, recruitment site, and sample types (stimulated saliva or oral wash), and frequency-matched on age categories (<35, 35–49, 50–64, 65+ years) and recruitment year (± 3 years). Based on the matching criteria, we finalized the selection of 89 IM cases (antrum: n = 84, body/fundus: n = 5) and 89 controls, and antral brushing samples were available for 55 case-control pairs.
H. pylori and CagA seropositivity measurements
Serum samples were available for 170 subjects in the 89 case-control pairs for determination of H. pylori/cytotoxin-associated gene A (CagA) seropositivity using enzyme-linked immunosorbent assay, as described18,19 with slight modification. Briefly, IgG antibodies to H. pylori whole cell antigens or to a recombinant CagA fragment were tested in duplicate and in parallel with known positive controls. The cutoff for H. pylori and CagA positivity was an optical density ratio > 0.6 and > 0.3, respectively.
Shotgun metagenomic sequencing
Bacterial DNA from the 54 saliva samples was isolated using the MasterPure DNA purification kit (Epicentre, Madison, WI). DNA from the 124 oral wash and 110 antral mucosal brushing samples was extracted using the DNeasy PowerLyzer PowerSoil kit (Qiagen, Germantown, MD). Metagenomic DNA samples were quantified using the Qubit dsDNA HS Assay Kit with a Qubit Fluorometer (ThermoFisher Scientific, Waltham, MA) and normalized to a concentration of 5 ng/μl. Shotgun sequencing libraries were constructed using the automated KAPA HyperPrep kit (Roche, Wilmington, MA) and sequenced on an Illumina NovaSeq 6000 System (Illumina, San Diego, LA) at 2×100 bp paired-end with 96 samples pooled in each run by the NYU Genomics Core.
Sequencing data processing
Raw sequencing reads were demultiplexed, and Trimmomatic (v0.36) was used to trim low-quality sequences. Retained reads were first aligned to the human genome (GRCh38) by Bowtie2 (v_2.2.9) and the mapped reads were filtered out. Details on metagenomic sequencing data were summarized in Supplementary Methods and Supplementary Table S1, S2 and S3. The non-human reads were further used for taxonomic classification by Kraken2 (v_ 2.0.8-beta)20 against the eHOMD reference database (http://www.homd.org/), which reduces a shotgun metagenome to a table of relative abundance for each taxon at the level from phylum to species. Gene family and pathway abundance of each sample was determined directly from the processed reads using HUMAnN2 (v_0.11.1)21 with default parameters. HUMAnN2 maps reads to functionally annotated microbial species genomes and performs translated search to align non-human reads to UniRef90 protein clusters (gene families)22. Gene families are then grouped into MetaCyc pathways using MinPath. For a lower level of resolution, we also regrouped UniRef90 gene families into Gene Ontology (GO) categories using the “humann2_regroup_table” script. We removed unintegrated/unmapped/unknown/ungrouped pathways, categories, and gene families prior to calculating relative abundance, using the “humann2_renorm_table” script.
Statistical analyses
α and β diversity
α-diversity (within-subject diversity) was assessed using richness (observed number of species and Chao1) and diversity (the Shannon and Simpson’s diversity index) metrics. Species level read counts were rarefied to the 90% of the minimum sample depth in the dataset (611,771 and 33,287 reads per sample in the oral and gastric microbiota, respectively). We used conditional logistic regression models using matched sets as strata to determine whether α-diversity was associated with gastric IM, adjusting for age and race.
β-diversity (between-subject diversity) was assessed using the Jensen-Shannon Divergence (JSD) on the species level. Principal coordinate analysis (PCoA) was used for visualization. Non-parametric permutational multivariate analysis of variance (PERMANOVA; ‘adonis’ function, ‘vegan’ package, R) with 9999 permutations was used to test the association between community-level bacterial composition and gastric IM, using matched sets as strata and adjusting for age and race.
Identification of taxa associated with gastric IM
We applied the centered log-ratio (clr) transformation23 to the relative abundance of taxa at each level (e.g. phylum, class, etc.) after adding a pseudo relative abundance (the minimal relative abundance in the whole dataset at each level), in order to remove compositional constraints of sequencing. Additionally, we excluded rare taxa with mean relative abundance ≤ 0.01%. These exclusions resulted in inclusion of 9 phyla, 18 classes, 29 orders, 40 families, 65 genera, and 265 species for the oral microbiota, and 9 phyla, 18 classes, 29 orders, 44 families, 78 genera, and 297 species for the gastric microbiota in the analyses. We fit standard conditional logistic regression models to assess the association between the relative abundance of each taxon and gastric IM, using matched pairs as strata and adjusting for age and race. Additional adjustment for ever smoking, ever drinking, and H. pylori/CagA status did not impact effect estimates (data not shown). P values from these models were adjusted for the false discovery rate (FDR)24 at each taxonomic level (i.e., genus, species) separately. Taxa associated with gastric IM were also assessed using conditional logistic regression with elastic-net penalties, to allow selection of a set of representative taxa while considering their correlations25. We conducted leave-one-out cross-validation using the “cv.clogitL1” function in the clogitL1 R package26 and covariates (age and race) were also penalized in each model. We also conducted stratified analyses to assess whether the association between bacterial taxa and gastric IM differed by seropositivity of H. pylori. We further conducted analyses separately for oral wash samples (62 pairs, n = 124) and saliva samples (27 pairs, n = 54), and combined the results using fixed-effect meta-analyses (‘metagen’ function, ‘meta’ package, R). Additional sensitivity analyses were conducted to exclude cases of IM in the gastric body and/or fundus (n = 5 and n = 3 for oral and gastric microbiome analyses, respectively) and their paired controls.
Identification of functional pathways and GO categories associated with gastric IM
We assessed associations of metagenomic functional pathways and GO categories’ relative abundance with gastric IM using standard conditional logistic regression models as described above. Relative abundance of pathways and GO categories was clr transformed. We only considered pathways and GO categories with mean relative abundance > 0.03%27 and largely explained by known species (< 25% unclassified in > 75% of individuals according to the species-specific pathway data)28. For GO categories analyses, in addition to the criteria above, we further focused on GO categories with variance > the 25th percentile of variances27. This resulted in inclusion of 111 and 69 pathways, as well as 266 and 143 GO categories in the oral and gastric microbiome, respectively. We presented pathways and GO categories related to gastric IM with a nominal P < 0.05. We also examined to what extent these pathways and GO categories were driven by specific species by calculating Spearman’s correlation coefficients between pathway/GO relative abundance and species relative abundance and using heatmap for visualization.
Results
Participant characteristics
Compared with controls, cases were more likely to be older or Asians (Table 1, all P < 0.05), and more likely to carry antibody to H. pylori, particularly the CagA-positive strain (P = 0.06). There were no significant differences by case status in terms of educational attainment, smoking status and intensity, and alcohol consumption (all P > 0.05).
Table 1.
Characteristics of study participants by case status of intestinal metaplasia
| Characteristics | Intestinal metaplasia | ORa (95% CI) | P | |
|---|---|---|---|---|
|
| ||||
| Cases (n=89) | Controls (n=89) | |||
|
| ||||
| Women, n (%) | 52 (58.4) | 52 (58.4) | – | – |
| Age (y), mean (SD) | 58.3 (10.9) | 57.3 (10.5) | 1.13 (1.01–1.27) | 0.04 |
| BMI (kg/m2), mean (SD) | 25.0 (4.5) | 26.5 (5.6) | 0.93 (0.86–1.02) | 0.12 |
| Ever smoking, n (%) | 30 (33.7) | 26 (29.2) | 1.64 (0.77–3.47) | 0.20 |
| Pack-years, mean (SD) | 83.1 (183.9) | 77.2 (159.5) | 1.01 (0.99–1.03)b | 0.54 |
| Ever drinking, n (%) | 48 (53.9) | 50 (56.2) | 1.45 (0.68–3.08) | 0.33 |
| Daily alcohol intakec, mean (SD) | 0.2 (0.6) | 0.5 (1.3) | 0.75 (0.51–1.10) | 0.14 |
| Education, n (%) | ||||
| <College | 48 (53.9) | 50 (56.2) | 1.55 (0.46–5.18) | 0.48 |
| College | 28 (31.5) | 19 (21.4) | 2.50 (0.71–8.75) | 0.15 |
| Graduate | 13 (14.6) | 20 (22.5) | Ref | |
| Race, n (%) | ||||
| White | 8 (9.0) | 20 (22.5) | Ref | |
| Black | 5 (5.6) | 16 (18.0) | 0.89 (0.23–3.44) | 0.86 |
| Hispanic | 23 (25.8) | 21 (23.6) | 2.45 (0.63–9.58) | 0.20 |
| Asian | 53 (59.6) | 32 (36.0) | 4.22 (1.48–12.1) | 0.007 |
| H. pylori status (in serum), n (%) | ||||
| Negative | 50 (61.7) | 64 (71.9) | Ref | |
| Positive, CagA negative | 9 (11.1) | 5 (5.6) | 3.51 (0.80–15.4) | 0.10 |
| Positive, CagA positive | 22 (27.2) | 20 (22.5) | 2.80 (0.98–8.04) | 0.06 |
| Recruitment year | – | – | ||
| 2009–2011 | 27 (30.3) | 27 (30.3) | ||
| 2013–2015 | 8 (9.0) | 0 | ||
| 2016–2018 | 54 (60.7) | 62 (69.7) | ||
| Recruitment location | – | – | ||
| Bellevue Hospital | 40 (44.9) | 40 (44.9) | ||
| Private clinic | 46 (51.7) | 46 (51.7) | ||
| NYU Ambulatory Center | 3 (3.4) | 3 (3.4) | ||
ORs were calculated using logistic regression conditional on matching factors including sex, recruitment site (Bellevue Hospital, Private clinic, Ambulatory Care Center), age categories (<35, 35–49, 50–64, 65+ years), and recruitment year (± 3 years), adjusting for age (continuous) and race (White, African American, Hispanic, Asian).
Per 10 pack-years.
Daily consumption of total standard drinks of alcoholic beverages (a 12-oz can of beer, 4-oz glass of wine, and 1.5-oz shot of hard liquor).
Overall oral and gastric microbiota diversity in relation to gastric IM
Cases did not differ significantly from matched controls in oral and gastric α-diversity (all P > 0.05; data not shown). We found significant differences in overall oral microbial composition between cases and controls (P < 0.01; Supplementary Figure S1A); overall gastric microbial composition was marginally related to gastric IM (P = 0.067; Supplementary Figure S1B).
Oral and gastric taxa associated with gastric IM
We identified 2 phyla, 6 classes, 8 orders, 9 families, 10 genera, and 50 species in the oral microbiota that were nominally associated with gastric IM (FDR-adjusted P = 0.07–0.26, Supplementary Table S4). In leave-one-out cross-validated elastic-net conditional logistic regression models for gastric IM, 2 phyla, 6 classes, 2 orders, 4 families, 5 genera, and 10 species were selected as the most important oral bacterial taxa associated with gastric IM (Table 2). Oribacterium sinus, Peptostreptococcus stomatis, Neisseria elongata, N. flavescens, and SR1 bacterium oral taxon 874 were positively related to gastric IM (ORs = 1.24–1.43, P = 0.004–0.03). The higher-rank taxa of these species had consistent associations with gastric IM and were also retained in the model (Table 2). For instance, in the Clostridia-Clostridiales-Peptostreptococcaceae-Peptostreptococcus-P. stomatis lineage, Clostridia (class), Peptostreptococcaceae (family), Peptostreptococcus (genus), and P. stomatis (species) were all selected in the model. The procedure also selected several species with their relative abundance inversely associated with gastric IM. These species included Lactobacillus gasseri, Streptococcus sanguinis, Shuttleworthia satelles, Achromobacter xylosoxidans, and Kingella oralis (odds ratios [ORs] = 0.66–0.80, P = 0.002–0.046). The higher-rank taxa to which L. gasseri belongs, such as Bacilli (class) and Lactobacillus (genus) were also selected by the model. The association patterns of the aforementioned species with gastric IM remained similar in meta-analyses of participants with oral wash and saliva samples separately (Supplementary Table S5).
Table 2.
Taxa in the oral microbiomea associated with gastric intestinal metaplasia
| Taxon (class; order; family; genus; species) | Mean relative abundance, % | ORb (95% CI) | P c | |
|---|---|---|---|---|
|
| ||||
| Cases | Controls | |||
|
| ||||
| Bacteroidetes | 26.9 | 23.3 | 1.59 (1.03–2.47) | 0.037 |
| Bacteroidia; Bacteroidales; Porphyromonadaceae (family) | 5.34 | 3.60 | 1.56 (1.12–2.18) | 0.009 |
| Firmicutes | ||||
| Bacilli (class) | 17.2 | 21.0 | 0.64 (0.44–0.94) | 0.021 |
| Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus (genus) | 0.07 | 0.19 | 0.56 (0.40–0.80) | 0.001 |
| Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus; Lactobacillus gasseri (species) | 0.003 | 0.04 | 0.66 (0.51–0.86) | 0.002 |
| Bacilli; Lactobacillales; Streptococcaceae; Streptococcus; Streptococcus sanguinis (species) | 0.62 | 0.78 | 0.69 (0.50–0.94) | 0.018 |
| Clostridia (class) | 2.02 | 1.67 | 1.71 (1.00–2.93) | 0.048 |
| Clostridia; Clostridiales; Lachnospiraceae; Oribacterium; Oribacterium sinus (species) | 0.32 | 0.24 | 1.33 (1.03–1.72) | 0.030 |
| Clostridia; Clostridiales; Lachnospiraceae; Shuttleworthia; Shuttleworthia satelles (species) | 0.01 | 0.02 | 0.76 (0.59–0.96) | 0.023 |
| Clostridia; Clostridiales; Peptostreptococcaceae (family) | 0.24 | 0.15 | 1.59 (1.13–2.24) | 0.008 |
| Clostridia; Clostridiales; Peptostreptococcaceae; Peptostreptococcus (genus) | 0.13 | 0.08 | 1.56 (1.16–2.10) | 0.003 |
| Clostridia; Clostridiales; Peptostreptococcaceae; Peptostreptococcus; Peptostreptococcus stomatis (species) | 0.11 | 0.07 | 1.35 (1.08–1.70) | 0.008 |
| Proteobacteria | ||||
| Alphaproteobacteria (class) | 0.03 | 0.05 | 0.81 (0.67–0.99) | 0.043 |
| Betaproteobacteria; Burkholderiales; Alcaligenaceae (family) | 0.27 | 0.29 | 0.74 (0.59–0.92) | 0.008 |
| Betaproteobacteria; Burkholderiales; Alcaligenaceae; Achromobacter (genus) | 0.27 | 0.29 | 0.75 (0.60–0.93) | 0.008 |
| Betaproteobacteria; Burkholderiales; Alcaligenaceae; Achromobacter; Achromobacter xylosoxidans (species) | 0.27 | 0.29 | 0.76 (0.62–0.93) | 0.008 |
| Betaproteobacteria; Neisseriales (order) | 12.3 | 9.66 | 1.27 (1.02–1.57) | 0.033 |
| Betaproteobacteria; Neisseriales; Neisseriaceae (family) | 12.3 | 9.66 | 1.28 (1.03–1.59) | 0.026 |
| Betaproteobacteria; Neisseriales; Neisseriaceae; Kingella; Kingella oralis (species) | 0.10 | 0.23 | 0.80 (0.65–1.00) | 0.046 |
| Betaproteobacteria; Neisseriales; Neisseriaceae; Neisseria (genus) | 12.0 | 9.23 | 1.27 (1.04–1.56) | 0.020 |
| Betaproteobacteria; Neisseriales; Neisseriaceae; Neisseria; Neisseria elongata (species) | 0.49 | 0.27 | 1.43 (1.12–1.83) | 0.004 |
| Betaproteobacteria; Neisseriales; Neisseriaceae; Neisseria; Neisseria flavescens (species) | 3.56 | 2.04 | 1.29 (1.06–1.56) | 0.010 |
| Epsilonproteobacteria (class) | 0.87 | 0.72 | 1.57 (1.01–2.46) | 0.046 |
| Gammaproteobacteria (class) | 7.38 | 7.07 | 1.38 (1.05–1.82) | 0.023 |
| Gammaproteobacteria; Enterobacterales (order) | 0.04 | 0.05 | 0.70 (0.52–0.96) | 0.026 |
| Gammaproteobacteria; Pasteurellales; Pasteurellaceae; Aggregatibacter (genus) | 0.75 | 0.56 | 1.41 (1.09–1.82) | 0.009 |
| Phylum no rank | ||||
| SR1 bacterium oral taxon 874 (species) | 0.05 | 0.03 | 1.24 (1.05–1.47) | 0.010 |
| Synergistetes | 0.07 | 0.07 | 0.81 (0.66–0.99) | 0.038 |
| Synergistia (class) | 0.08 | 0.08 | 0.83 (0.69–1.00) | 0.048 |
Taxa relative abundances were normalized with the clr transformation. All taxa (phyla, classes, orders, families, genera, species) and covariates (age and race) were selected using conditional logistic regression with elastic-net penalties; leave-one-out cross-validation was conducted using the “cv.clogitL1” function in the clogitL1 R package. Taxa selected by the penalized model and with a nominal P < 0.05 based on standard conditional logistic regression models were shown in the table.
ORs were calculated using standard logistic regression models conditional on matching factors including sex, recruitment site (Bellevue Hospital, Private clinic, Ambulatory Care Center), age categories (<35, 35–49, 50–64, 65+ years), and recruitment year (± 3 years), adjusting for age (continuous) and race (White, African American, Hispanic, Asian).
P values from conditional logistic regression models.
We identified 1 class, 1 order, 2 families, 2 genera, and 17 species in the gastric microbiota that were nominally associated with gastric IM, including a highly significant species Johnsonella ignava (P = 0.005; Supplementary Table S6). In leave-one-out cross-validated elastic-net conditional logistic regression models, 9 taxa were selected as the most important taxa related to gastric IM (Table 3). Specifically, higher relative abundance of species Actinomyces sp. oral taxon 448, Prevotella baroniae, Filifactor alocis, Veillonella sp. oral taxon 780, and Leptotrichia goodfellowii was associated with higher odds of gastric IM (ORs = 1.42–1.67, P = 0.015–0.04) while higher relative abundance of L. gasseri and S. mutans was related to lower odds of gastric IM (ORs = 0.75 and 0.61, P = 0.046 and 0.024, respectively). Genus Filifactor to which F. alocis belongs was also selected with consistent association with gastric IM.
Table 3.
Taxa in the gastric microbiomea associated with gastric intestinal metaplasia
| Taxon (class; order; family; genus; species) | Mean relative abundance, % | ORb (95% CI) | P c | |
|---|---|---|---|---|
|
| ||||
| Cases | Controls | |||
|
| ||||
| Actinobacteria | ||||
| Actinobacteria; Actinomycetales; Actinomycetaceae; Actinomyces; Actinomyces sp. oral taxon 448 (species) | 0.11 | 0.10 | 1.42 (1.02–1.99) | 0.040 |
| Bacteroidetes | ||||
| Bacteroidia; Bacteroidales; Prevotellaceae; Prevotella; Prevotella baroniae (species) | 0.20 | 0.09 | 1.61 (1.03–2.51) | 0.037 |
| Firmicutes | ||||
| Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus; Lactobacillus gasseri (species) | 0.02 | 0.04 | 0.75 (0.56–0.99) | 0.046 |
| Bacilli; Lactobacillales; Streptococcaceae; Streptococcus; Streptococcus mutans (species) | 0.03 | 0.03 | 0.61 (0.39–0.94) | 0.024 |
| Clostridia; Clostridiales; Peptostreptococcaceae; Filifactor (genus) | 0.12 | 0.05 | 1.47 (1.03–2.11) | 0.036 |
| Clostridia; Clostridiales; Peptostreptococcaceae; Filifactor; Filifactor alocis (species) | 0.11 | 0.04 | 1.43 (1.03–2.01) | 0.035 |
| Negativicutes; Veillonellales; Veillonellaceae; Veillonella; Veillonella sp. oral taxon 780 (species) | 0.05 | 0.02 | 1.53 (1.09–2.16) | 0.015 |
| Fusobacteria | ||||
| Fusobacteriia; Fusobacteriales; Leptotrichiaceae; Pseudoleptotrichia; Leptotrichia goodfellowii (species) | 0.01 | 0.01 | 1.67 (1.09–2.57) | 0.019 |
| Proteobacteria | ||||
| Deltaproteobacteria; Desulfovibrionales; Desulfomicrobiaceae (family) | 0.02 | 0.01 | 1.50 (1.04–2.17) | 0.031 |
Taxa relative abundances were normalized with the clr transformation. All taxa (phyla, classes, orders, families, genera, species) and covariates (age and race) were selected using conditional logistic regression with elastic-net penalties; leave-one-out cross-validation was conducted using the “cv.clogitL1” function in the clogitL1 R package. Taxa selected by the penalized model and with a nominal P < 0.05 based on standard conditional logistic regression models were shown in the table.
ORs were calculated using standard logistic regression models conditional on matching factors including sex, recruitment site (Bellevue Hospital, Private clinic, Ambulatory Care Center), age categories (<35, 35–49, 50–64, 65+ years), and recruitment year (± 3 years), adjusting for age (continuous) and race (White, African American, Hispanic, Asian).
P values from conditional logistic regression models.
Stratified analyses of the associations between the aforementioned species and gastric IM by serum H. pylori status (Supplementary Table S7) indicated that many of the associations were stronger among those tested positive for H. pylori or CagA antibodies. The associations between the aforementioned species and gastric IM did not materially change with exclusion of cases of IM in the gastric body and/or fundus (Supplementary Table S8).
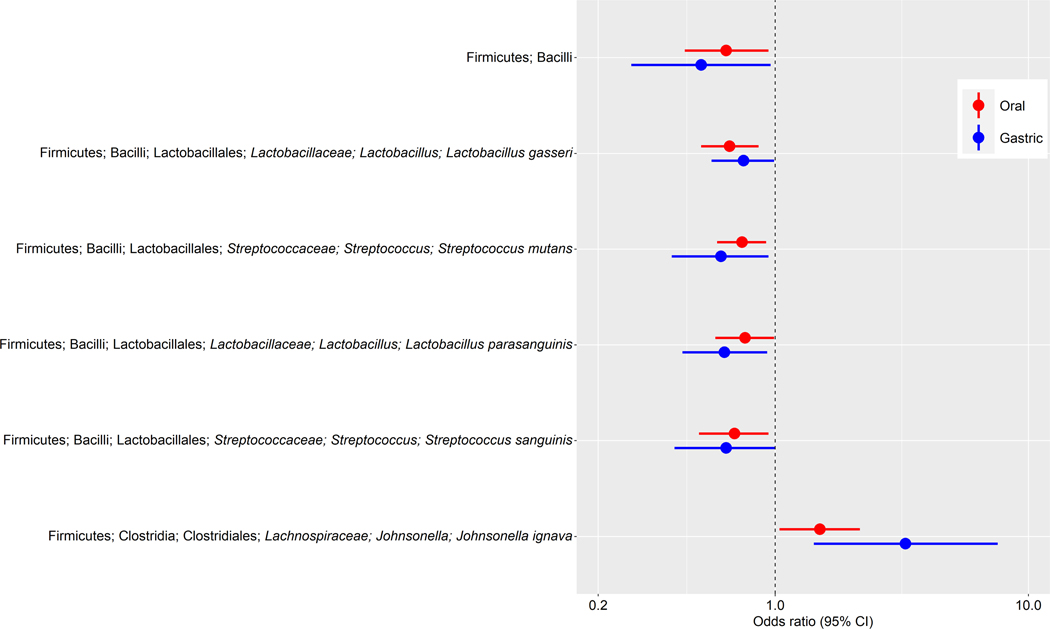
Several taxa in both the oral and gastric microbiota showed consistent associations with gastric IM (Figure 1). These included class Bacilli and species L. gasseri, S. mutans, S. parasanguinis, and S. sanguinis, that were associated with lower odds of gastric IM, as well as species J. ignava, that was associated with higher odds of gastric IM. In addition, P. stomatis, which was positively related to gastric IM in the oral data, was marginally associated with gastric IM in the gastric data (P = 0.058).
Figure 1.
Oral and gastric taxa associated with gastric intestinal metaplasia. Forest plot of odds ratios (ORs) and 95% confidence intervals (95% CI) for associations between clr-transformed taxa relative abundance and gastric IM in standard conditional logistic regression models. These taxa in both the oral and gastric microbiota were consistently associated with gastric IM.
Oral and gastric functional pathways and GO categories associated with gastric IM
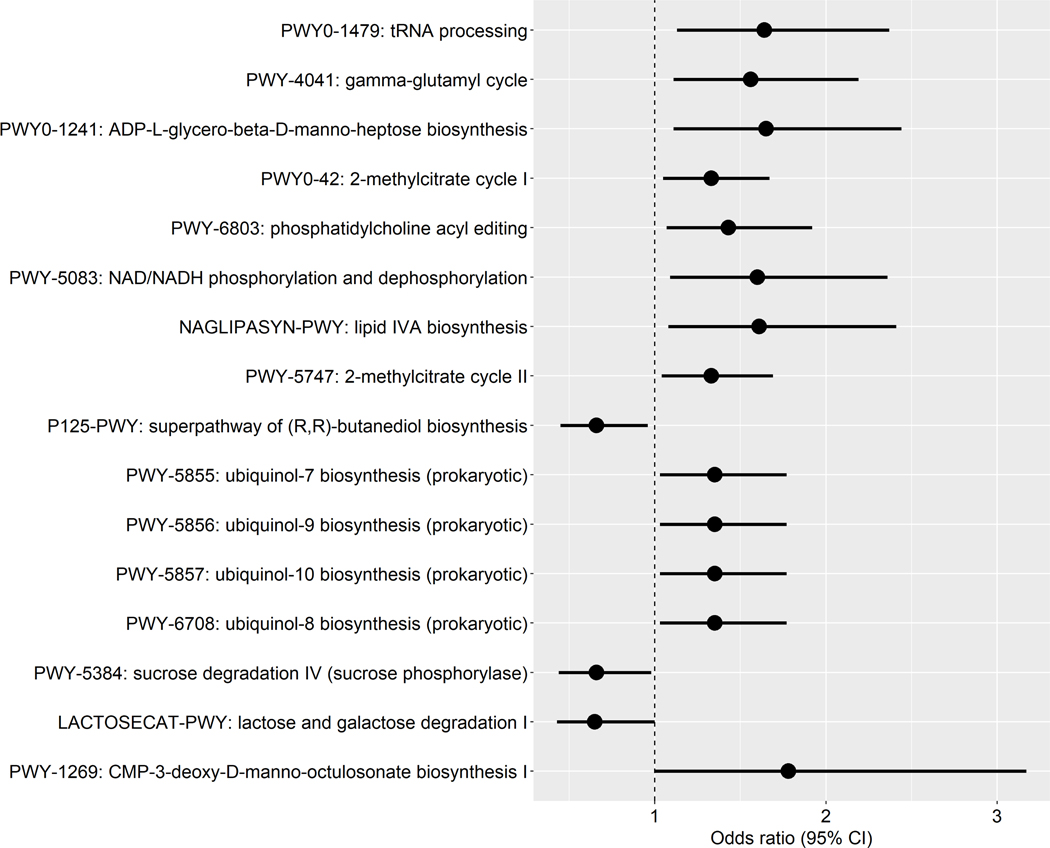
We identified 16 pathways in the oral microbiome that were associated with gastric IM at the nominal level (P = 0.002–0.05) (Figure 2A). Some of the IM-enriched pathways were involved in lipopolysaccharide (LPS) biosynthesis (PWY0–1241: ADP-L-glycero-β-D-manno-heptose biosynthesis, NAGLIPASYN-PWY: lipid IVA biosynthesis) and ubiquinol biosynthesis (PWY-5855/5856/5857/6708: ubiquinol-7/9/10/8 biosynthesis (prokaryotic)). Those under-represented pathways in IM were mainly involved in sugar degradation (PWY-5384: sucrose degradation IV (sucrose phosphorylase), LACTOSECAT-PWY: lactose and galactose degradation I).
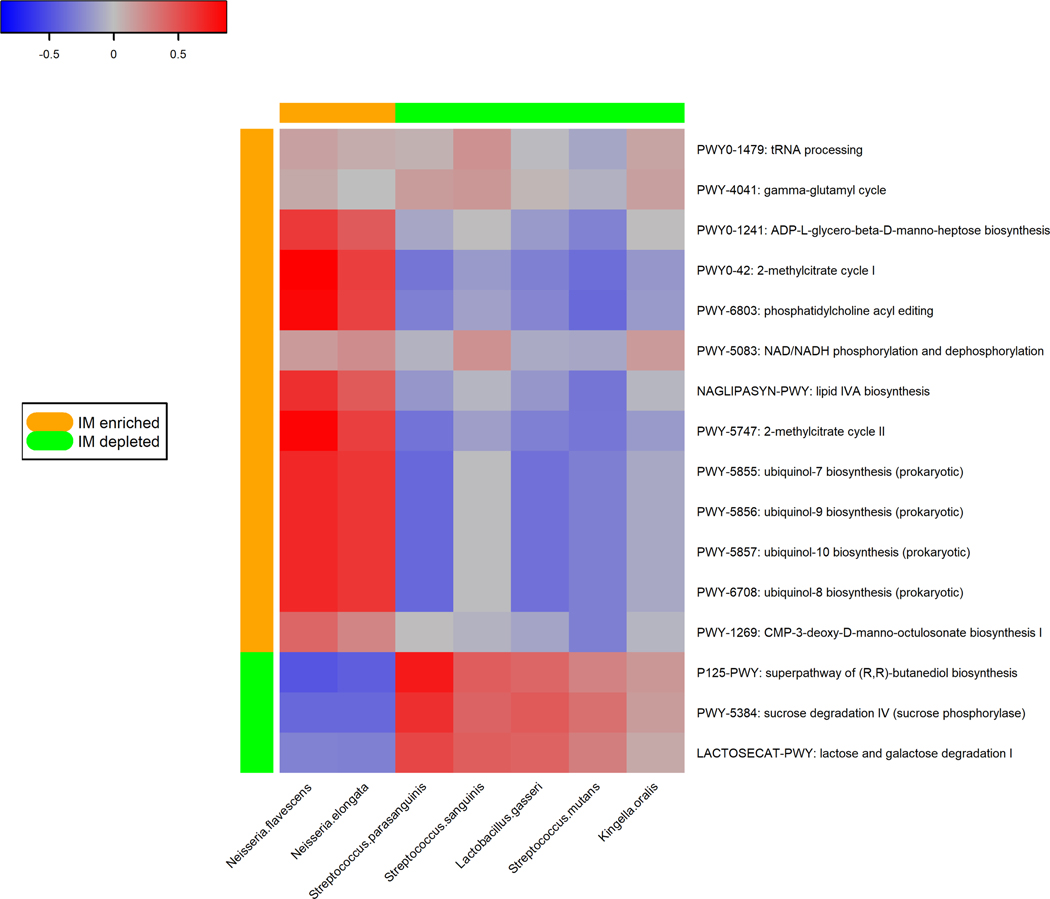
Figure 2.
Pathways in the oral microbiome associated with gastric intestinal metaplasia. (A) Forest plot of odds ratios (ORs) and 95% confidence intervals (95% CI) for associations between clr-transformed pathway relative abundance and gastric IM in standard conditional logistic regression models. (B) Correlations between IM-associated oral species and functional pathways. Spearman correlation coefficient values were estimated for each pairwise comparison of clr-transformed species and pathway relative abundance. Here we show only species in Table 2 and Figure 1 with known contribution to each pathway according to the species-specific pathway data.
We estimated pair-wise correlations of the relative abundance between the selected species (Table 2) and the IM-associated pathways that they contributed to (Figure 2B). IM-enriched pathways tended to be positively correlated with IM-enriched species (N. elongata and N. flavescens). Pathways under-represented in IM were positively correlated with protective species (L. gasseri, S. mutans, S. sanguinis, and S. parasanguinis). Average contributions by pathway-correlated oral species to overall pathway abundances were shown in Supplementary Figure S2.
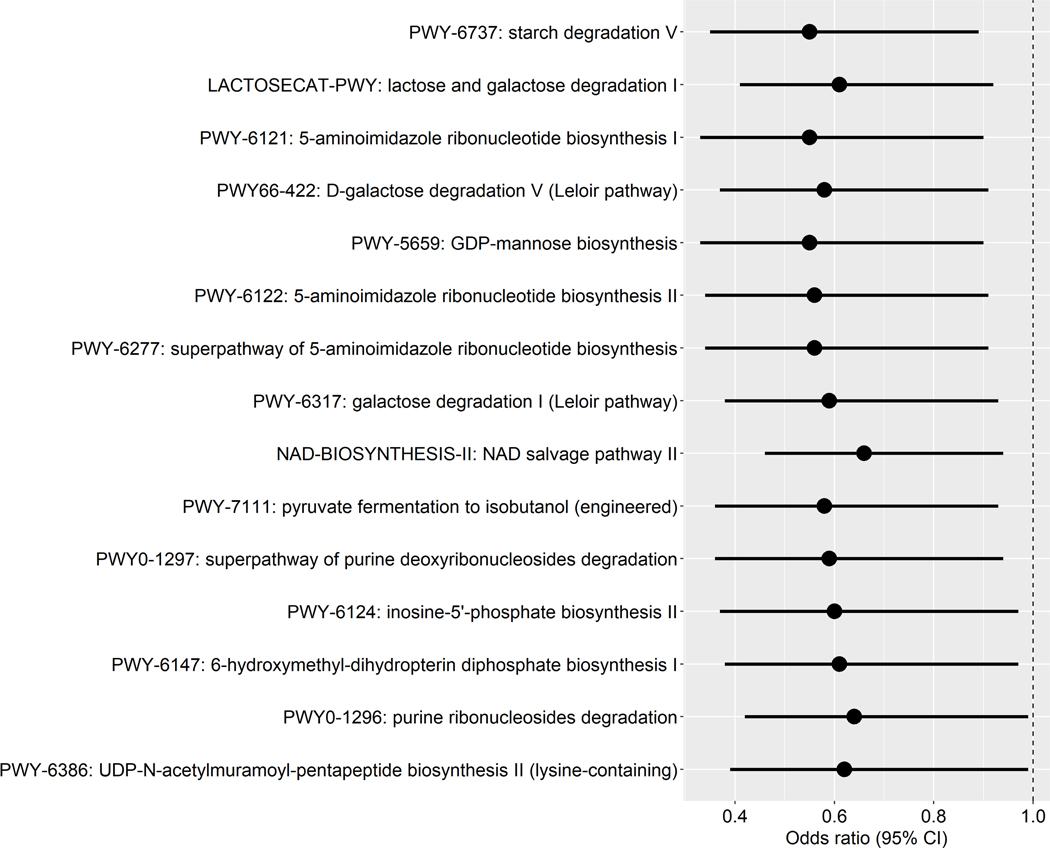
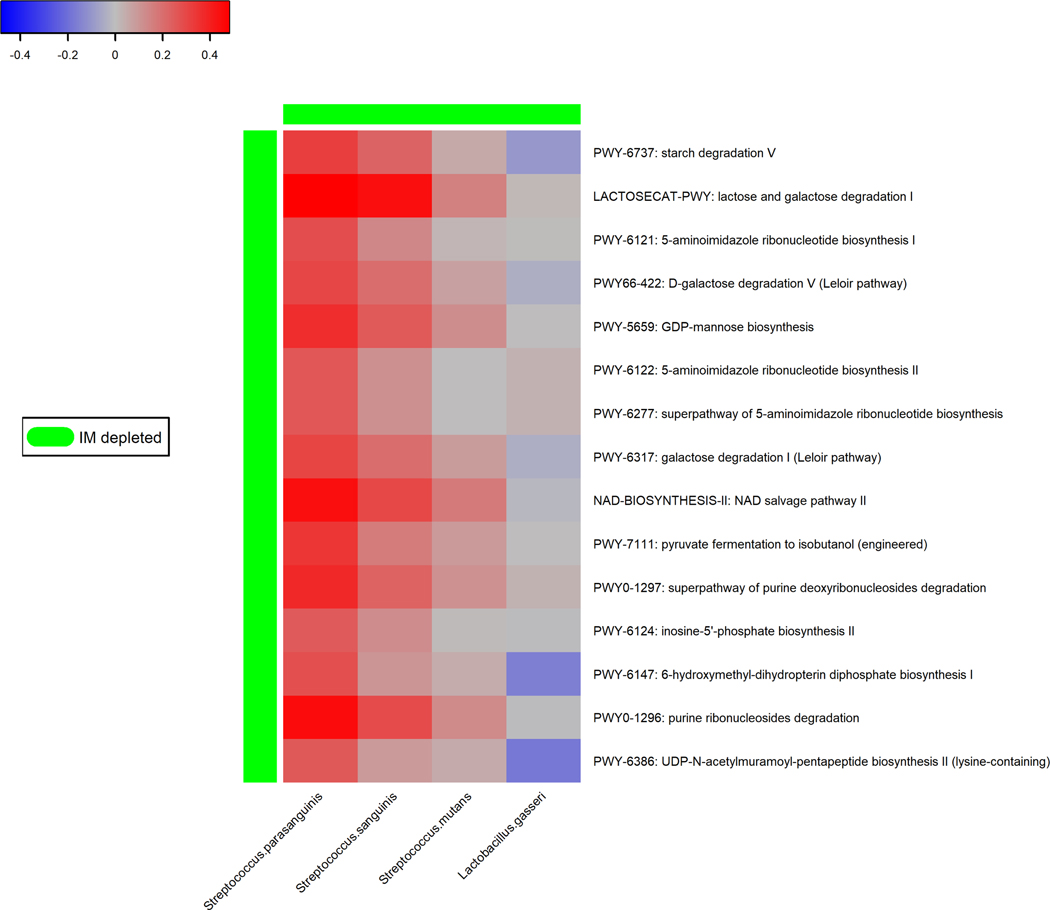
We identified 15 pathways in the gastric microbiome that were associated with gastric IM at the nominal level (P = 0.003–0.05) (Figure 3A). Several of these pathways were related to sugar degradation (LACTOSECAT-PWY: lactose and galactose degradation I, PWY66–422: D-galactose degradation V (Leloir pathway), PWY-6317: galactose degradation I (Leloir pathway)). Most of the under-represented pathways in IM were positively associated with under-represented species (S. parasanguinis and S. sanguinis) (Figure 3B). Average contributions by pathway-correlated gastric species to overall pathway abundances were shown in Supplementary Figure S3.
Figure 3.
Pathways in the gastric microbiome associated with gastric intestinal metaplasia. (A) Forest plot of odds ratios (ORs) and 95% confidence intervals (95% CI) for associations between clr-transformed pathway relative abundance and gastric IM in standard conditional logistic regression models. (B) Correlations between IM-associated gastric species and functional pathways. Spearman correlation coefficient values were estimated for each pairwise comparison of clr-transformed species and pathway relative abundance. Here we show only species in Table 3 and Figure 1 with known contribution to each pathway according to the species-specific pathway data.
Associations between oral and gastric GO categories and gastric IM (Supplementary Tables S9 and S10) largely corresponded to the associations between functional pathways and IM (details in the Supplementary Materials). Correlations between IM-associated oral and gastric species and GO categories are shown in Supplementary Figure S4 and S5.
Discussion
In this study of oral and gastric microbiome and gastric premalignant lesions (IM), we identified species related to periodontal disease (P. stomatis, J. ignava, F. alocis)29,30 and opportunistic pathogens (N. elongata, N. flavescens) that were enriched in gastric IM, as well as probiotic species (L. gasseri) and commensals (S. mutans, S. parasanguinis, S. sanguinis) that were under-represented in gastric IM. Several species (J. ignava, L. gasseri, S. mutans, S. parasanguinis, S. sanguinis) in both the oral and gastric microbiota were consistently associated with gastric IM. Further, we identified metagenomic functions as potential mechanism by which these bacteria influence disease risk, including via LPS and ubiquinol biosynthesis and sugar degradation.
Previous prospective studies have reported positive associations of tooth loss and periodontal disease with gastric cancer risk31. However, studies investigating specific periodontal pathogens are limited8,32. In our previous study with 37 cases of gastric precancerous lesions, we observed that DNA levels of periodontal pathogen Aggregatibacter actinomycetemcomitans was related to a non-significant elevated OR of gastric precancerous lesions (OR: 1.36, P = 0.17)8. Using metagenomic sequencing in the present study, we also observed a positive albeit non-significant association between A. actinomycetemcomitans and gastric IM (OR: 1.22; P = 0.088) which was stronger in those carrying H. pylori antibodies (OR: 2.69; P = 0.002). In addition, we observed positive associations of several newly appreciated species related to periodontal disease (P. stomatis, J. ignava, and F. alocis)29,30 with gastric IM. A previous case-control study with 16S rRNA gene analysis identified increased abundance of P. stomatis in gastric biopsies of gastric cancer patients compared with individuals with superficial gastritis12, and another study found the enrichment of Peptostreptococcus in biopsies of gastric atrophy and IM33. P. stomatis may contribute to the acidic and hypoxic tumor microenvironment, which promotes bacterial colonization34. F. alocis can induce the secretion of proinflammatory cytokines from gingival epithelial cells35. Taken together, the data suggest a role of these highly host-interactive organisms in gastric cancer and warrant further investigations.
Several Neisseria species were enriched in gastric IM, such as N. elongata and N. flavescens. Neisseria species are oral cavity commensals and have been recognized as opportunistic pathogens. A recent small metagenomics study revealed enrichment of Neisseriaceae-Neisseria-N. sicca in gastric wash samples of gastric cancer patients compared with individuals with superficial gastritis36. In our collaborative prospective study of oral microbiome and gastric cancer that was also based on metagenomics data, the order Neisseriales, family Neisseriaceae, and genus Neisseria were enriched in oral wash samples collected before cancer occurrence, compared with controls37 (Supplementary Table S11). The relative abundance of N. elongata was positively related to an increased risk of gastric cancer in one of the cohorts. However, a 16S rRNA-based study observed significant depletion of Neisseria in gastric cancer13,38,39. This discrepancy could reflect that 16S rRNA gene sequencing typically provides only family- or genus-level taxonomy. Neisseria species were correlated with pathways and GO categories for LPS and ubiquinol biosynthesis (Figure 2B and Supplementary Figure S4). LPS is a gram-negative bacterial antigen that increases inflammation in the tumor microenvironment and drives tumorigenesis40. LPS-related pathways were enriched in gastric cancer36. Most Gram-negative bacteria produce ubiquinone, which can form a microbial environment characteristic of inflammation41. Additional research is warranted to investigate these potential mechanisms by which Neisseria species may influence gastric cancer risk.
In our study, several commensals in the oral cavity and digestive tract, including L. gasseri, S. mutans, S. parasanguinis, and S. sanguinis, were associated with lower odds of gastric IM, with consistent associations across the oral and gastric microbiota. S. mutans is a major pathogen causing human dental caries42. S. parasanguinis is an early colonizer of dental surfaces and is related to a healthy microbiota43. S. sanguinis, a member of the oral biofilm community, is considered benign, or even beneficial, with regard to dental caries44. Dental caries-associated bacteria such as Streptococcus species elicit potent Th1 immune responses and promote CD8+ T-cell responses45 that may decrease cancer development46. The abundances of Lactobacillus and Streptococcus species were correlated with pathways for sugar degradation that were under-represented in gastric IM, suggesting a role of fermentation of sugars and production of lactic acid in gastric cancer. Lactic acid produced by Lactobacillus can lower the gastrointestinal tract pH, thus creating a hostile environment for resident pathogenic bacteria and eliciting antibacterial effects47.Probiotics can induce the coccoid conversion of H. pylori and suppress H. pylori colonization and multiplication48,49. Supporting our results, significant reduction in the abundance of Streptococcus was observed in gastric microbiota of gastric cancer compared with chronic gastritis13. However, several studies using 16S rRNA gene sequencing reported significantly higher abundance of Lactobacillus and Streptococcus in gastric carcinoma relative to chronic gastritis13,14. Again, differences in sequencing methods and study design may explain the discrepancy.
Although seropositivity of H. pylori or CagA was positively associated with gastric IM (Table 1), the relative abundance of H. pylori in gastric microbiome was not (data not shown). This observation is consistent with the observation that H. pylori is absent in gastric tissues in the large majority of patients with advanced atrophy, IM or gastric cancer5 even when serology is positive, suggesting the disappearance of active H. pylori infection during the later stages of gastric cancer development6. The loss of H. pylori and impairment of acid secretion in these lesions may facilitate the colonization of other bacteria in the stomach that may play a role in gastric cancer development. Many of the associations between the non-H. pylori bacteria and gastric IM we found were stronger among individuals carrying H. pylori antibodies, suggesting their additive effects on the H. pylori-initiated gastric cancer development. Some experimental studies suggested that H. pylori can act synergistically with a community of bacteria to promote gastric neoplasia, and the gastric cancer risk may depend on the microbiota following H. pylori infection10,11. Future larger studies should be conducted to investigate interaction between H. pylori and specific taxa in gastric cancer risk.
Strengths and limitations
Strengths of our study included the matched design, comprehensive shotgun metagenomic sequencing, inclusion of both oral and gastric microbiome profiling, and adjustment for gastric cancer risk factors throughout analysis. Several case-control studies of gastric premalignant lesions, predominantly gastric IM, have identified shared risk factors and molecular alterations for gastric cancer17 under the premise that a risk factor’s association with the precancerous lesion parallels its association with cancer. Thus, IM can be used to identify risk factors for gastric cancer and elucidate the underlying carcinogenesis. However, although our study is the largest of its kind, case sample sizes (n=89 oral and n=55 gastric) remained small, limiting statistical power and our ability to investigate race-specific associations. Although we only study the compositions at a single time, the abundance of core members of the oral and gut microbiota are stable over time at the genus level50.
Conclusion
We found evidence that individuals with gastric IM exhibited different microbial composition and functions compared with healthy individuals. Future studies are needed to confirm our findings and investigate the underlying mechanisms. Given that bacterial profiles may be modified, identification of bacterial risk factors of malignancy might enable interventions and more cost-effective cancer screening by risk stratification.
Supplementary Material
Novelty and impact:
The colonization of bacteria other than H. pylori in precancerous and cancerous lesions of the stomach may play a role in the development of gastric cancer, but the evidence is not well established. This study identified species related to periodontal disease (P. stomatis, J. ignava, F. alocis) and opportunistic pathogens (N. elongata, N. flavescens) that were enriched in gastric IM, as well as probiotic species (L. gasseri) and commensals (S. mutans, S. parasanguinis, S. sanguinis) that were under-represented in gastric IM. Several species (J. ignava, L. gasseri, S. mutans, S. parasanguinis, S. sanguinis) in both the oral and gastric microbiota were consistently associated with gastric IM. Further, we identified metagenomic functions as potential mechanism by which these bacteria influence disease risk, including via LPS and ubiquinol biosynthesis and sugar degradation. The findings on potential roles of certain oral and gastric microbiota in the development of gastric precancerous lesions, if confirmed by future studies, may be considered in interventions and more cost-effective cancer screening.
Acknowledgments
Funding
This work was supported by the National Institutes of Health grants [R01 CA204113, R01 DK110014, P42 ES010349, P30 ES000260, and P30 ES009089], and by the C & D and Sergei Zlinkoff funds.
Abbreviations:
- CagA
cytotoxin-associated gene A
- CI
confidence interval
- clr
centered log-ratio
- FDR
false discovery rate
- GC
gastric cancer
- GO
Gene Ontology
- H. pylori
Helicobacter pylori
- IM
intestinal metaplasia
- JSD
Jensen-Shannon Divergence
- LPS
lipopolysaccharide
- OR
odds ratio
- PCoA
principal coordinate analysis
- PERMANOVA
permutational multivariate analysis of variance
- SCCS
Southern Community Cohort Study
- SMHS
Shanghai Men’s Health Study
- SWHS
Shanghai Women’s Health Study
Footnotes
Data Availability Statement
Metagenomic sequencing data generated in this study is available in the database of Genotypes and Phenotypes (dbGaP) with accession code phs002566.v1.p1. Investigators who would like to access individual-level study data should submit an application to the NIH Data Access Committee (DAC) to request the datasets. Further information is available from the corresponding author upon request.
Ethics Statement
This study was approved by the NYU institutional review board and all participants provided written informed consent.
Conflict of interest: The authors disclose no conflicts.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- 3.Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev 2015;20:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology 2006;130:188–206; quiz 12–3. [DOI] [PubMed] [Google Scholar]
- 5.Kwak HW, Choi IJ, Cho SJ, et al. Characteristics of gastric cancer according to Helicobacter pylori infection status. J Gastroenterol Hepatol 2014;29:1671–7. [DOI] [PubMed] [Google Scholar]
- 6.Karnes WE Jr., Samloff IM, Siurala M, et al. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology 1991;101:167–74. [DOI] [PubMed] [Google Scholar]
- 7.Salazar CR, Francois F, Li Y, et al. Association between oral health and gastric precancerous lesions. Carcinogenesis 2012;33:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salazar CR, Sun J, Li Y, et al. Association between selected oral pathogens and gastric precancerous lesions. PloS one 2013;8:e51604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen I, Yamazaki K. Can oral bacteria affect the microbiome of the gut? J Oral Microbiol 2019;11:1586422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lofgren JL, Whary MT, Ge Z, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 2011;140:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CW, Rickman B, Rogers AB, Ge Z, Wang TC, Fox JG. Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res 2008;68:3540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coker OO, Dai Z, Nie Y, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018;67:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018;67:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eun CS, Kim BK, Han DS, et al. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter 2014;19:407–16. [DOI] [PubMed] [Google Scholar]
- 15.Knight R, Vrbanac A, Taylor BC, et al. Best practices for analysing microbiomes. Nat Rev Microbiol 2018;16:410–22. [DOI] [PubMed] [Google Scholar]
- 16.Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Corrigendum: Shotgun metagenomics, from sampling to analysis. Nat Biotechnol 2017;35:1211. [DOI] [PubMed] [Google Scholar]
- 17.Farinati F, Cardin R, Libera GD, et al. Determinants for the development of chronic atrophic gastritis and intestinal metaplasia in the stomach. Eur J Cancer Prev 1995;4:181–6. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Perez GI, Dworkin BM, Chodos JE, Blaser MJ. Campylobacter pylori antibodies in humans. Ann Intern Med 1988;109:11–7. [DOI] [PubMed] [Google Scholar]
- 19.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 1995;55:2111–5. [PubMed] [Google Scholar]
- 20.Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol 2019;20:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abubucker S, Segata N, Goll J, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput Biol 2012;8:e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzek BE, Wang Y, Huang H, McGarvey PB, Wu CH, UniProt C. UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics 2015;31:926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes AD, Reid JN, Macklaim JM, McMurrough TA, Edgell DR, Gloor GB. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 25.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol 2005;67:301–20. [Google Scholar]
- 26.Reid S, Tibshirani R. Regularization Paths for Conditional Logistic Regression: The clogitL1 Package. Journal of Statistical Software 2014;58:1–21. [PMC free article] [PubMed] [Google Scholar]
- 27.Peters BA, Wilson M, Moran U, et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med 2019;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schirmer M, Franzosa EA, Lloyd-Price J, et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol 2018;3:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willems A, Collins MD. Evidence for the placement of the gram-negative Catonella morbi (Moore and Moore) and Johnsonella ignava (Moore and Moore) within the Clostridium subphylum of the gram-positive bacteria on the basis of 16S rRNA sequences. Int J Syst Bacteriol 1995;45:855–7. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Chaparro PJ, Goncalves C, Figueiredo LC, et al. Newly identified pathogens associated with periodontitis: a systematic review. J Dent Res 2014;93:846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi C, Gudino CV, Gibson FC 3rd, Genco CA Review: Pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol 2010;25:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun JH, Li XL, Yin J, Li YH, Hou BX, Zhang Z. A screening method for gastric cancer by oral microbiome detection. Oncol Rep 2018;39:2217–24. [DOI] [PubMed] [Google Scholar]
- 33.Sung JJY, Coker OO, Chu E, et al. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020;69:1572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ternes D, Karta J, Tsenkova M, Wilmes P, Haan S, Letellier E. Microbiome in Colorectal Cancer: How to Get from Meta-omics to Mechanism? Trends Microbiol 2020;28:401–23. [DOI] [PubMed] [Google Scholar]
- 35.Moffatt CE, Whitmore SE, Griffen AL, Leys EJ, Lamont RJ. Filifactor alocis interactions with gingival epithelial cells. Mol Oral Microbiol 2011;26:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu YL, Pang W, Huang Y, Zhang Y, Zhang CJ. The Gastric Microbiome Is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Front Cell Infect Microbiol 2018;8:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Long J, Wang C, et al. Prospective Study of Oral Microbiome and Gastric Cancer Risk among Low-income Asian, African American and European American Populations (submitted). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrell JJ, Zhang L, Zhou H, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012;61:582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters BA, Wu J, Pei Z, et al. Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers. Cancer Res 2017;77:6777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagliani N, Hu B, Huber S, Elinav E, Flavell RA. The fire within: microbes inflame tumors. Cell 2014;157:776–83. [DOI] [PubMed] [Google Scholar]
- 41.Vich Vila A, Imhann F, Collij V, et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci Transl Med 2018;10. [DOI] [PubMed] [Google Scholar]
- 42.van Houte J. Role of micro-organisms in caries etiology. J Dent Res 1994;73:672–81. [DOI] [PubMed] [Google Scholar]
- 43.Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ 2001;65:1028–37. [PubMed] [Google Scholar]
- 44.Becker MR, Paster BJ, Leys EJ, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 2002;40:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miettinen M, Matikainen S, Vuopio-Varkila J, et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun 1998;66:6058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. [DOI] [PubMed] [Google Scholar]
- 47.Vieco-Saiz N, Belguesmia Y, Raspoet R, et al. Benefits and Inputs From Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters During Food-Animal Production. Front Microbiol 2019;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujimura S, Watanabe A, Kimura K, Kaji M. Probiotic mechanism of Lactobacillus gasseri OLL2716 strain against Helicobacter pylori. J Clin Microbiol 2012;50:1134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am J Gastroenterol 1998;93:2097–101. [DOI] [PubMed] [Google Scholar]
- 50.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009;326:1694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.