Abstract
Since the discovery of three scene-selective regions in the human brain, a central assumption has been that all three regions directly support navigation. We propose instead that cortical scene processing regions support three distinct computational goals: (i) the parahippocampal place area supports scene categorization, which involves recognizing the kind of place we are in; (ii) the occipital place area supports visually-guided navigation, which involves finding our way through the immediately visible environment, avoiding boundaries and obstacles; and (iii) the retrosplenial complex supports map-based navigation, which involves finding our way from a specific place to some distant, out-of-sight place. We further hypothesize that these systems develop along different timelines, with both navigation systems developing slower than the scene categorization system.
Keywords: scene categorization, visually-guided navigation, map-based navigation, parahippocampal place area (PPA), occipital place area (OPA), retrosplenial complex (RSC)
Carving up human cortical scene processing
The human ability to recognize a place (or scene) forms the bedrock for many of our essential, everyday behaviors. In a brief glance, we extract a wealth of information from scenes, such as the category of the scene (e.g., “a kitchen”), its identity (e.g., “my kitchen”), and other critical properties like whether it is safe or what behavior is appropriate for the current context. At the same time, we extract information that is vital for navigation, allowing us to effortlessly find our way through the immediately visible environment without running into the kitchen walls or banging into the kitchen table, for example. What’s more, we are able to situate the local visual environment within a broader spatial map, allowing us, for instance, to know where our favorite restaurant is relative to our house. But how do we accomplish these remarkable feats?
One promising strategy to understand human visual scene processing is to characterize the neural systems that accomplish it. Over the past three decades, cognitive neuroscience has revealed a set of three cortical regions that together make up the visual scene processing system in humans: the parahippocampal place area (PPA) [1], the occipital place area (OPA) [2], and the retrosplenial complex (RSC) [3]. However, beyond establishing the general involvement of these regions in human visual scene processing – i.e., responding about 2–4 times more to images of scenes than to images of objects and faces in human functional magnetic resonance imaging (fMRI) studies – two fundamental questions remain unanswered: i) What precise role does each region play within the broad domain of adult human cortical scene processing? and ii) How does this functional organization develop from infancy to adulthood?
We review adult fMRI and neuropsychological studies, and developmental fMRI studies on human cortical scene processing, and offer two hypotheses. First, we hypothesize that human cortical scene processing is composed of three distinct systems: one we call the “scene categorization” system, including PPA, which is involved in recognizing a scene as a kind of place (e.g., a kitchen), but not as a specific place (e.g., my kitchen); another we call the “visually-guided navigation” system, including OPA, which is involved in finding our way through the immediately visible environment, avoiding boundaries and obstacles; and a third we call the “map-based navigation” system, including RSC, which is involved in making our way from a specific place to some distant, out-of-sight place (Figure 1, Key Figure). This three-scene-systems hypothesis challenges the pervasive theory that human cortical scene processing is entirely in the service of navigation [1, 4–18]. Second, we hypothesize that these systems develop along different timelines – more specifically, that both the visually-guided navigation and map-based navigation systems are slower to develop than the scene categorization system (Figure 1).
Figure 1, Key Figure. The three scene systems (a la Marr’s levels of analysis) and their development.
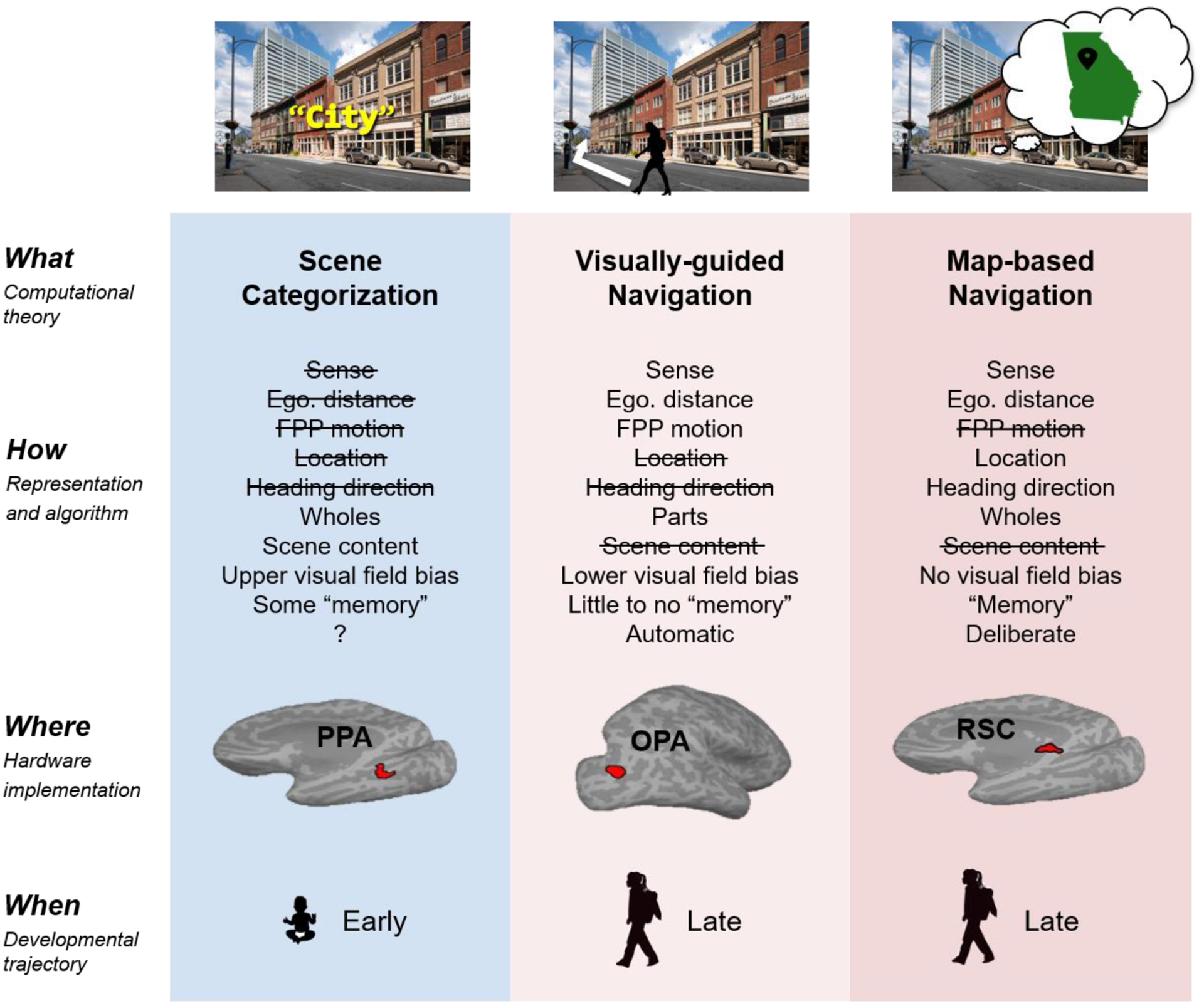
For example, the “scene categorization” system (including PPA) supports our ability to recognize the kind of place we are in (e.g., a city versus a beach), not a specific place, and is not involved in navigation. Accordingly, it does not represent information critical for navigation: sense (left/right), egocentric distance (near/far), first-person perspective (FPP) motion, location, or heading direction. Instead, it represents two kinds of information critical for categorizing scenes: i) spatial layout or “wholes” (i.e., the spatial arrangement of the major surfaces that make up the overall shape of a scene, and ii) scene content (e.g., the particular objects and textures that fill that space). By contrast, the “visually-guided” and “map-based” navigation systems (including OPA and RSC, respectively) do represent information essential for navigation. However, they do so differently, with the visually-guided navigation system representing information critical to guiding navigation through the immediately visible environment (i.e., sense, egocentric distance, FPP motion, and the local “parts” of a scene that constitute boundaries or obstacles), and the map-based navigation system representing information critical to guiding navigation from a specific place to some distant, out-of-sight place (i.e., sense, egocentric distance, location, heading direction, and the overall shape of a scene). Each system is further dissociated based on visual field bias, memory requirements, and the automaticity of the process they support. Finally, consistent with the distinct computational goals, representations, and cortical regions across the three scene systems, each is further hypothesized to develop along a different timeline. In particular, the scene categorization system is hypothesized to develop earlier than both the visually-guided navigation and map-based navigation systems.
PPA is involved in scene categorization, not navigation
A central assumption since the discovery of the three scene-selective regions has been that all three directly support navigation. For example, in the seminal PPA paper, Epstein and Kanwisher [1] found that PPA responded significantly more to images of empty rooms than to the same rooms in which the walls, floors, and ceilings had been fractured and rearranged, thus disrupting spatial layout. The authors then argued that the representation of spatial layout in PPA signals its role in navigation, since spatial layout information is critical for navigation and reorientation [19–23]. Other more recent fMRI studies found that PPA represents certain aspects of the spatial layout of scenes (e.g., whether a scene is “open” or “closed”) [6, 7, 17], and similarly argued that such encoding of spatial layout information reflects the role of PPA in navigation. But spatial layout information need not be used for navigation only, and could also easily facilitate scene categorization (e.g., a beach is “open”; a kitchen is “closed”). Indeed, several behavioral and computer vision studies have found that spatial layout information (including open/closed information) can be used to categorize scenes, and at times even necessary to do so [24–28]. Thus, we argue that none of the studies claiming that PPA is involved in navigation provide direct evidence for this claim, and that all of the findings are, in fact, open to the alternative interpretation that spatial layout sensitivity reflects the role of PPA in scene categorization.
To directly test the role that PPA plays within human cortical scene processing, we conducted an fMRI experiment [29] in which participants were asked to perform either a scene categorization task or a visually-guided navigation task on the exact same stimuli. In the scene categorization task, participants imagined standing in a room and indicated whether the scene was a kitchen, living room, or bedroom. In the visually-guided navigation task, participants imagined walking along a path on the floor that led only to one of three doors and indicated whether they could leave through the door on the left, center, or right wall. We found that PPA responded significantly more during the scene categorization task than during the visually-guided navigation task. Crucially, we further found that PPA did not respond any more during the visually-guided navigation task than during a baseline task (i.e., a one-back task, where participants indicated whether the presented image was the exact same or different from the previous one), suggesting a complete lack of task-based modulation in PPA during the visually-guided navigation task. By contrast, OPA showed the exact opposite pattern, responding significantly more during the visually-guided navigation task than during both the scene categorization and baseline tasks. These results reveal a double dissociation between the responses in PPA and OPA, and thus strongly suggest distinct neural systems selectively involved in scene categorization and visually-guided navigation, respectively. Meanwhile, RSC responded similarly during all three tasks, suggesting the lack of RSC involvement in either scene categorization or visually-guided navigation, consistent with our hypothesis that RSC and OPA play different roles even within navigation– a topic we explore in the next section. Additional evidence against the role of PPA in visually-guided navigation comes from several other fMRI studies showing that PPA – unlike OPA – does not represent information necessary for visually-guided navigation. Specifically, PPA does not represent: i) “sense” (left-right) information [30], ii) egocentric distance (near-far) information [31], iii) first-person perspective motion information through scenes (i.e., actually mimicking the visual experience of navigating through immediately visible environments) [32, 33], or iv) possible routes through a local scene [34].
Intriguingly, our finding of dissociable neural systems involved in recognizing places (including PPA, in the temporo-occipital cortex) and navigating through them (including OPA, in the parieto-occipital cortex), suggests that the human cortical scene processing system is similar to the well characterized ventral/dorsal distinction for human object processing, with one system responsible for recognition (a “what” system) and another for visually-guided action (a “how” system), respectively [35, 36]. Note, however, that our finding of two distinct systems for scene categorization (including PPA) and visually-guided navigation (including OPA) does not mean that the two systems cannot and do not interact, as is widely recognized in the case of the dorsal and ventral streams for object processing [35, 37]. Indeed, two studies [38, 39] found resting-state functional correlations between PPA and OPA, suggesting that these regions are connected (at least functionally, maybe anatomically), thereby enabling crosstalk between them. However, the nature of any such interaction remains mysterious, and, in any case, this interaction need not be essential to the operations performed in either system. Just as in object processing, where one need not recognize the category of an object (e.g., “cup”) to successfully act on it (and vice versa), in scene processing, one need not recognize the category of a scene (e.g., “kitchen”) to successfully navigate through it (and vice versa). Importantly, causal evidence for independent scene categorization and visually-guided navigation systems is still lacking. Future studies of patients with focal lesions to either PPA or OPA are thus required to provide a definitive answer to this question.
However, perhaps the biggest challenge to our hypothesis that PPA is not involved in navigation are several studies reporting that PPA is involved in a key component of map-based navigation, known as landmark recognition [8–15]. In other words, while PPA may not be involved in visually-guided navigation – as described above – it may be involved in map-based navigation. However, these otherwise elegantly designed studies have significant methodological limitations (i.e., not correcting for multiple comparisons and/or “double dipping” [40]; not comparing across regions to show neural specificity; and/or not matching on behavioral performance to rule out task difficulty), and hence cannot be taken to show the claimed result. Instead, a recent fMRI multivoxel pattern analysis (MVPA) study [41] – which did not suffer from the above methodological problems – found that PPA does not represent the location of particular places (e.g., the restaurant on 10th Street versus the restaurant on Peachtree Street) or the heading direction of the navigator (e.g., the navigator is facing the restaurant in the north part of town versus the south part of town) – but instead represents only the scene category (e.g., a restaurant). RSC showed the exact opposite pattern of results, representing location and heading direction information, but not category information.
Such a double dissociation strongly suggests distinct neural systems involved in scene categorization (including PPA) and map-based navigation (including RSC). Unlike RSC or PPA, OPA represented neither location, heading direction, nor category information, again, supporting our hypotheses that RSC and OPA play different roles within navigation, and that PPA and OPA play different roles within scene processing more broadly. Additional evidence arguing against the role of PPA in map-based navigation comes from two other fMRI studies also showing that PPA – unlike RSC – does not represent landmark information (i.e., location and/or direction information) [42, 43]. Finally, another fMRI study found that, during a landmark recognition task, responses in parahippocampal cortex (perhaps overlapping with the PPA; although the PPA was never functionally defined) – unlike RSC – did not vary as a function of one’s ability to navigate [44, 45].
Interestingly, support for the idea that PPA is involved in landmark recognition also comes from several neuropsychological studies, reporting that patients with damage to parahippocampal and lingual gyri (areas encompassing and surrounding the PPA) commonly present with landmark agnosia – an inability to recognize landmarks [46–50]. However, in all of these studies, there is either i) diffuse damage, including not only parahippocampal cortex, but also other navigationally-relevant structures including the hippocampus and retrosplenial cortex, ii) not enough detail about the lesion to determine whether the parahippocampal cortex is even implicated at all, or iii) a lack of the proper control conditions to rule out alternative hypotheses, especially regarding the role of PPA in scene categorization – making it impossible then to draw any clear conclusions about the function of PPA from these studies. Future work is therefore required to test patients with damage to PPA. Indeed, another more recent study on two stroke patients with focal damage to PPA found significant impairment in the encoding of novel scenes, yet sparing of famous landmarks [51], suggesting a role for PPA in recognizing scene category, but not identity.
So, if PPA is not involved in navigation (visually-guided or map-based), then what role does it play within human cortical scene processing? We propose that PPA plays a role in scene categorization for four reasons. First, as previously described [29], PPA responded more during a scene categorization task than both a visually-guided navigation and a baseline task. Second, as previously described [41, 43], PPA represents basic-level category information about places (e.g., a restaurant), but not landmark information (e.g., the restaurant on 10th Street). Third, two fMRI MVPA studies found that scene category (e.g., beach versus forest, across multiple exemplars per category) – from both color photographs and simple line drawings – is more accurately decoded from the activation patterns in PPA than RSC, and that the activation patterns in PPA, but not RSC, were correlated to behavioral performance on a scene categorization task [52, 53]. OPA was not tested in either of these studies. Fourth, PPA represents scene content information – information undoubtedly used for scene categorization. For example, PPA responds to objects that are i) good exemplars of specific scenes (e.g., a bed is found in a bedroom, while a refrigerator is found in a kitchen) [17, 54–56], ii) strongly associated with a given context (e.g., a toothbrush is only found in a bathroom) versus low “contextual” objects (e.g., an apple is found in many places) [57, 58], and iii) large and not portable (e.g., a couch versus a small fan) [59–61]. Similarly, several other studies found that PPA represents the summary statistics of object ensembles (e.g., the leaves on a tree), and texture information (e.g., brick) [62–65].
Finally, there is some limited evidence that the PPA is not a unified region, but instead can be broken up into posterior and anterior portions [38, 39, 66], or even by hemisphere [67]. However, despite such suggestions, there is no evidence that these proposed subregions actually support functionally distinct processing; in fact, the best available evidence suggests that these subregions support similar functions, albeit in a graded or hierarchical fashion from posterior to anterior (i.e., with stronger responses or information processing in the anterior portion than the posterior one) [56, 68, 69]. Critically, even if functionally distinct subregions exist, it is unlikely such a result would challenge the fundamental claim here that at least some portion of PPA is dedicated to categorization, not navigation (indeed, this must be true, given the evidence reviewed here that when treated as a unitary region – i.e., even averaging over subregions that might support other processes, if they exist – PPA still shows functional responses consistent with scene categorization, not navigation).
OPA and RSC are involved in different kinds of navigation
Unlike PPA, there is clear and consistent evidence for the roles of OPA and RSC in navigation. Moreover, there is a growing consensus that these two regions play distinct roles even within navigation, with OPA supporting visually-guided navigation and RSC supporting map-based navigation.
Beginning with OPA, as previously discussed [29], OPA responded significantly more during a visually-guided navigation task than either a scene categorization task or baseline task, and similarly during the latter two tasks, strongly suggesting its complete lack of involvement in scene categorization. Furthermore, several other fMRI studies found that OPA represents at least five kinds of information relevant for visually-guided navigation: i) sense information [30], ii) egocentric distance information [31], iii) first-person perspective motion information through scenes [32, 33], iv) local scene elements (“parts”), including boundaries (e.g., walls) and/or individual obstacles (e.g., furniture) that constrain how one can navigate the immediately visible space [54, 65, 70–72], and v) possible routes through a local scene [34]. Additional support for OPA’s involvement in visually-guided navigation comes from two fMRI studies showing a lower visual field bias in OPA – where paths tend to be [73, 74]. Finally, causal evidence in support of OPA’s role in visually-guided navigation comes from a transcranial magnetic stimulation (TMS) study showing that TMS to the OPA impairs accuracy of navigation to locations in a virtual arena, and that this impairment is specific to locations defined by distance to a bounding wall [75].
But is OPA only involved in visually-guided navigation, and not map-based navigation? The answer appears to be yes. Indeed, several fMRI studies found that OPA – unlike RSC – does not represent landmark information (i.e., location and heading direction) [41, 43]. Furthermore, OPA shows little to no memory effects [69] – consistent with its hypothesized role in visually-guided navigation, and not map-based navigation, since visually-guided navigation, by definition, must operate in the here-and-now, requiring little to no memory, while map-based navigation requires a “linking” of the currently visible scene to representations of the broader environment stored in memory.
Next, RSC does not respond during either a visually-guided navigation or scene categorization task [29], strongly suggesting a lack of involvement in either of these processes. Instead, studies of information processing in RSC point to a role for this region in map-based navigation. Like OPA, RSC represents sense and egocentric distance information [30, 31] – information critical for any navigation system. However, unlike OPA, RSC does not represent information critical for visually-guided navigation (e.g., first-person perspective motion information) [32], but rather represents information necessary for map-based navigation more specifically. For example, many fMRI studies have found that RSC represents landmark information, including both location information and heading direction [41, 43, 44, 61, 76–80]. Moreover, several fMRI studies found that RSC shows strong memory effects – responding two times more strongly to familiar than unfamiliar scenes [18, 69, 81], and significantly more to personally familiar scenes than famous scenes [82] – as required for a system that must link the currently visible scene to representations of the broader environment stored in memory. Finally, consistent with the hypothesis that map-based navigation involves deliberate processing (i.e., it depends on conscious, intentional control), a recent fMRI study [83] found greater RSC responses during active versus “passive” navigation through complex (versus simple) virtual mazes. By contrast, OPA responded strongly to both active and passive navigation, in both complex and simple mazes, suggesting that visually-guided navigation instead operates automatically.
A final, tentative piece of evidence for the dissociation between visually-guided versus map-based navigation systems comes from the neuropsychological literature on patients with topographical disorientation. For example, Aguirre and colleagues [46] proposed a taxonomy of spatial navigation deficits in which they argued for a distinction between cases of egocentric disorientation, where patients show a profound deficit in localizing objects in space relative to the body [84], and cases of heading disorientation, where participants are unable to derive directional information from the currently visible scene [85]. Egocentric disorientation is related to posterior parietal damage, whereas heading disorientation is related to retrosplenial and/or posterior cingulate damage, sites that may encompass OPA and RSC, respectively. However, while the similarity between this neurological dissociation and our proposed division of labor between OPA and RSC is striking, future experiments are needed to rigorously test such patients in terms of our “two scene navigation systems” hypothesis and clarify the precise contributions of OPA and RSC to these deficits.
Differential development across the three cortical scene processing systems?
If the three regions indeed develop along different timelines – as hypothesized here – then this finding would provide strong support for our three-scene-systems hypothesis. However, no studies to date have tested this hypothesis for three reasons. First, most developmental fMRI studies only investigated a single scene-selective region (i.e., PPA), precluding the critical comparisons across regions [86–89]. Second, the few studies that have compared more than one region have failed to find conclusive evidence. For example, one study investigating OPA, RSC, and PPA found that RSC is adultlike in scene selectivity (i.e., responding 2–4 times more to images of scenes than to images of objects and faces) by 7–8 years old, while OPA and PPA are not – consistent with our differential development hypothesis [90]. However, this study did not test for the critical region by age group interaction necessary to support this claim. To that end, a second study investigating the three scene-selective regions in children 5 and 8 years old did test for the critical region by age group interaction and found no differences in scene selectivity in any of the three regions between the two age groups [91]. Thus, although these studies detected scene-selective regions in children as early as 5 years (Box 1), they did not establish whether these regions are developing at different rates – at least in the age ranges tested – as hypothesized here. Third, and perhaps most importantly then, given the hypothesis that the three scene-selective regions support distinct functions, focusing on the development of scene selectivity alone may be insufficient. Instead, we propose that investigating the information processing necessary for each specific function is needed to understand the developmental trajectory of each of these regions. For example, one recent fMRI study investigated the development of visually-guided navigation by asking when in development OPA represents first-person perspective motion information – information shown to be represented in OPA in adulthood [32] – in children 5 and 8 years old [91]. The study found that, although OPA already exhibited scene selectivity by age 5 years, and not different from children age 8 (as previously discussed), responses to first-person perspective motion were not yet detectable at this same age, and did not emerge until age 8 years. This protracted development was specific to OPA, and was not found in either PPA or RSC, as expected since prior work in adults found that these regions do not represent first-person perspective motion information [32] – consistent with our three-scene-systems hypothesis.
Box 1. The origins of scene-selective cortex.
The finding that PPA, RSC, and OPA are all scene selective by age 5 years, albeit perhaps with subsequent development, raises the question: When does human cortical scene processing first come online? Does it take 5 years (as suggested by the current data), or is it present much earlier in life, within the first few months (or days even) of life? Addressing this question, a recent study [118] found that the overall scene preferences (i.e., significantly greater responses to images of scenes than faces) can already be detected in and around the parahippocampal gyrus (consistent with the location of PPA) and the lateral occipital cortex (consistent with the location of OPA) by just 4 to 6 months, but scene selectivity (i.e., greater responses to scenes than objects – what the above studies find as early as 5 years old) is not yet detectable in these regions at this age. RSC was not discussed in this study. Moreover, a functional near-infrared spectroscopy (fNIRS) study – using an independently defined ROI approach, similarly used in fMRI – found that infants between 3 and 12 months of age exhibit scene-preferring regions (i.e., responding significantly more to scenes than faces), like that of adults [119], most likely reflecting OPA, given fNIRS is only able to detect signals from lateral regions of the brain (and not the more medial and deep regions, like PPA and RSC). Finally, another recent resting-state fMRI study found that the functional connectivity underlying the scene-selective cortical system is already intact in as little as 27 days of age – with PPA showing biased connectivity with RSC, as well as with peripheral early visual cortex – suggesting that the connectivity underlying these regions develops prior to, and potentially scaffolds, the development of scene selectivity [120]. Connectivity to OPA was not discussed. Thus, while the foundations of the scene processing system (in both “proto-function” and connectivity) may be present early in infancy, it is still not clear when the more focal, adultlike PPA, OPA, and RSC emerge.
The finding that visually-guided navigation undergoes protracted development dovetails with a number of behavioral findings. Most directly, a recent study [92] – using a task known to depend on OPA in adulthood [75] – found that the ability to navigate to locations (defined by distance to a bounding wall) in a virtual arena was still maturing late into childhood, not reaching adult-like status until sometime after 8 years old. Likewise, several other behavioral studies have found that other visually-guided navigation abilities including obstacle avoidance [93, 94] and locomotion through a local environment [95, 96] also continue to mature well into childhood, not reaching adult-like status until after 7–8 years old. At the same time, however, the idea of a late developing visually-guided navigation system might seem surprising, given that humans begin navigating early in life (e.g., crawling around 9 months) and show remarkably sophisticated navigational ability within the first few years. For example, young children can use boundaries to recover their orientation after becoming disoriented [97], and infants understand whether it is safe to locomote over a “visual cliff” [98]. How then can we reconcile these observations with the hypothesis that visually-guided navigation undergoes protracted development? One possibility is that these tasks rely on qualitatively different systems. For example, the reorientation task may involve spatial memory systems in the hippocampus, while the visual cliff task may depend on basic depth perception. A second possibility is that these tasks do rely on the same visually-guided navigation system, and that despite development extending well into childhood (as discussed above), the foundations of this system are nevertheless intact early and sufficient to support these early navigational behaviors.
Whatever the case – whether early-emerging representations present in OPA are sufficient to support early visually-guided navigational abilities, and followed by subsequent development, or such early navigational abilities depend on qualitatively different systems – all of the above findings suggest that visually-guided navigation is indeed late developing. But, what about the development of the scene categorization and map-based navigation systems then? Here we suggest that these systems develop along different timelines and offer a specific developmental hypothesis – described next.
Visually-guided and map-based navigation develop later than scene categorization?
Reflecting the basic fact that typically developing infants view scenes from the day they are born, but only begin to actively navigate through them much later in life, we hypothesize that the visually-guided navigation system (including OPA) is slower to develop than the scene categorization system (including PPA). While no pediatric fMRI nor behavioral studies to date have directly tested this hypothesis (given the reasons outlined in the previous section), perhaps the work investigating the development of human object processing can provide some clues. For example, building on the two systems for adult human object processing, as described above, several behavioral studies have found that the “vision-for-action” (dorsal) system is slower to develop than the “vision-for-perception” (ventral) system [99–106], raising the tantalizing possibility that separable systems for recognition and action in scene processing, like object processing, also follow the same developmental trajectories. As such, we hypothesize that the visually-guided navigation system (including OPA, in the dorsal stream) is slower to develop than the scene categorization system (including PPA, in the ventral stream). Indeed, behavioral work in infants shows that before infants ever independently navigate their surroundings (e.g., by crawling), they can discriminate the deep versus shallow side of a visual cliff [107]. By contrast, when older infants are encouraged to actually navigate the same environment (e.g., by crawling or even walking), they fail to do so accurately, navigating right over the edge of the cliff, for example [108].
More speculative still, we further hypothesize that the map-based navigation system (including the RSC) develops later than the scene categorization system. Again, no pediatric fMRI nor behavioral studies to date have tested this hypothesis (i.e., by directly comparing map-based navigation and scene categorization abilities across childhood). However, partial support for this possibility comes from a large literature showing that map-based navigation ability – especially that relying on allocentric representations of the broader spatial environment – undergoes protracted development late into childhood [92, 109–117].
Concluding remarks and future perspectives
Here we provide a fundamental shift in our current understanding of adult human cortical scene processing and highlight the need for research on its development by offering two novel hypotheses for future investigation. First, we propose that human cortical scene processing is composed of three distinct systems: i) scene categorization (including PPA), ii) visually-guided navigation (including OPA), and iii) map-based navigation (including RSC) (but see Outstanding Questions). Second, we propose that these systems develop along different timelines, and, more specifically, that the visually-guided navigation system and the map-based navigation system are slower to develop than the scene categorization system.
OUTSTANDING QUESTIONS.
How exactly are scene categories represented in PPA? For example, like objects, are scenes first categorized at the basic level (e.g., “kitchen” versus “room” or “galley kitchen”) in PPA?
Do nonhuman primates (or even other animals) also possess three systems for scene processing? While three visual scene-selective regions have been identified in adult macaques, with each region in a similar anatomical location to the human PPA, OPA, and RSC, it is not clear whether there is a homologous functional organization for scene-selective regions in the cortex of human and nonhuman primates.
Can the three-scene-systems hypothesis guide development of better computational models? Training models to perform specific scene processing tasks (i.e., scene categorization, visually-guided navigation, or map-based navigation) may yield improved fits between models and the brain, and therefore may be an avenue for gaining insight into the computational mechanisms underlying each system, possibly beyond that obtainable from fMRI alone.
HIGHLIGHTS:
It is widely believed that cortical scene processing in adult humans – composed of the parahippocampal place area (PPA), the occipital place area (OPA), and the retrosplenial complex (RSC) – is wholly devoted to navigation.
Challenging this pervasive theory, recent research suggests that PPA is not involved in navigation, but instead involved in “scene categorization.”
By contrast, OPA and RSC are involved in navigation, albeit different kinds (i.e., “visually-guided navigation” and “map-based navigation”, respectively).
Thus, we propose that adult human cortical scene processing comprises three dissociable systems.
Furthermore, we propose that PPA, OPA, and RSC develop along different timelines, and offer the specific hypothesis that both the visually-guided navigation and map-based navigation systems are slower to develop than the scene categorization system.
ACKNOWLEDGMENTS
We thank the Facility for Education and Research in Neuroscience (FERN) Imaging Center in the Department of Psychology, Emory University, Atlanta, Georgia. We also thank Rebecca Saxe, Nancy Kanwisher, Michael McCloskey, Annie Cheng, and Christopher Jones for helpful comments on the manuscript. This work was supported by grants from the National Eye Institute (R01 EY29724 to D.D.D. and T32 EY007092 to F.S.K.), the National Science Foundation (DGE-1444932 to A.S.P.), and a grant from the Simons Foundation to Simons Center for the Social Brain at MIT (F.S.K).
GLOSSARY
- Landmark recognition
The ability to recognize the locations of particular places or objects in the local environment so they can be used as landmarks to orient ourselves while navigating the broader environment.
- Occipital place area (OPA)
A scene-selective region located in the parieto-occipital cortex, around the transverse occipital sulcus.
- Parahippocampal place area (PPA)
The first scene-selective cortical region reliably identified in adult humans using fMRI, PPA is located medially in the inferior temporo-occipital cortex, between the posterior parahippocampal gyrus and the anterior lingual gyrus, and sometimes extending to the adjacent fusiform gyrus and collateral sulcus.
- Reorientation
An organism’s ability to find its bearings after being disoriented, using both spatial layout and egocentric information (i.e., sense and egocentric distance).
- Retrosplenial complex (RSC)
A scene-selective region – more recently called the Medial Place Area (MPA) [74] – located in the medial parietal cortex, immediately behind the splenium, the most caudal part of the corpus callosum, often including the precuneus and posterior cingulate cortex.
- Scene
A large-scale space or environment that a person can recognize as a particular kind of place, navigate through, or situate within a broader environment.
- Scene content
The internal features of a scene encompassing objects, textures, colors, and materials.
- Scene-selective regions
Brain regions, including PPA, RSC, and OPA, that respond more strongly in fMRI when people view images of scenes (e.g., landscapes, cityscapes, rooms) than when they view other visual stimuli, such as objects and faces.
- Spatial layout
The geometry of a space, defined by the spatial arrangement of the large, extended surfaces/planes composing the space.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel D. Dilks, Department of Psychology, Emory University, 36 Eagle Row, Atlanta, GA 30322, USA
Frederik S. Kamps, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA
Andrew S. Persichetti, Section on Cognitive Neuropsychology, Laboratory of Brain and Cognition, NIMH/NIH, 10 Center Drive, Bethesda, MD 20892, USA
REFERENCES
- 1.Epstein and Kanwisher (1998) A cortical representation of the local visual environment. Nature 392 (6676), 598–601. [DOI] [PubMed] [Google Scholar]
- 2.Dilks DD et al. (2013) The occipital place area is causally and selectively involved in scene perception. J Neurosci 33 (4), 1331–6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire EA (2001) The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol 42 (3), 225–38. [DOI] [PubMed] [Google Scholar]
- 4.Epstein RA and Baker CI (2019) Scene Perception in the Human Brain. Annu Rev Vis Sci 5, 373–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein RA et al. (2017) The cognitive map in humans: spatial navigation and beyond. Nat Neurosci 20 (11), 1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S et al. (2011) Disentangling Scene Content from Spatial Boundary: Complementary Roles for the Parahippocampal Place Area and Lateral Occipital Complex in Representing Real-World Scenes. Journal of Neuroscience 31 (4), 1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kravitz DJ et al. (2011) Real-world scene representations in high-level visual cortex: it’s the spaces more than the places. J Neurosci 31 (20), 7322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janzen G and van Turennout M (2004) Selective neural representation of objects relevant for navigation. Nat Neurosci 7 (6), 673–7. [DOI] [PubMed] [Google Scholar]
- 9.Janzen G and Jansen C (2010) A neural wayfinding mechanism adjusts for ambiguous landmark information. Neuroimage 52 (1), 364–70. [DOI] [PubMed] [Google Scholar]
- 10.Janzen G et al. (2008) Memory consolidation of landmarks in good navigators. Hippocampus 18 (1), 40–7. [DOI] [PubMed] [Google Scholar]
- 11.Janzen G and Weststeijn CG (2007) Neural representation of object location and route direction: an event-related fMRI study. Brain Res 1165, 116–25. [DOI] [PubMed] [Google Scholar]
- 12.Wegman J and Janzen G (2011) Neural encoding of objects relevant for navigation and resting state correlations with navigational ability. J Cogn Neurosci 23 (12), 3841–54. [DOI] [PubMed] [Google Scholar]
- 13.Schinazi VR and Epstein RA (2010) Neural correlates of real-world route learning. Neuroimage 53 (2), 725–35. [DOI] [PubMed] [Google Scholar]
- 14.Sun L et al. (2021) The parahippocampal place area and hippocampus encode the spatial significance of landmark objects. Neuroimage 236, 118081. [DOI] [PubMed] [Google Scholar]
- 15.Marchette SA et al. (2015) Outside Looking In: Landmark Generalization in the Human Navigational System. J Neurosci 35 (44), 14896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein RA and Higgins JS (2007) Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cereb Cortex 17 (7), 1680–93. [DOI] [PubMed] [Google Scholar]
- 17.Harel A et al. (2013) Deconstructing Visual Scenes in Cortex: Gradients of Object and Spatial Layout Information. Cerebral Cortex 23 (4), 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein RA et al. (2007) Visual scene processing in familiar and unfamiliar environments. J Neurophysiol 97 (5), 3670–83. [DOI] [PubMed] [Google Scholar]
- 19.Ghaem O et al. (1997) Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport 8 (3), 739–44. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum RS et al. (2004) “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus 14 (7), 826–35. [DOI] [PubMed] [Google Scholar]
- 21.Cheng K and Newcombe NS (2005) Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon Bull Rev 12 (1), 1–23. [DOI] [PubMed] [Google Scholar]
- 22.Rauchs G et al. (2008) Partially segregated neural networks for spatial and contextual memory in virtual navigation. Hippocampus 18 (5), 503–18. [DOI] [PubMed] [Google Scholar]
- 23.Spelke E et al. (2010) Beyond core knowledge: Natural geometry. Cogn Sci 34 (5), 863–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliva A and Schyns PG (1997) Coarse blobs or fine edges? Evidence that information diagnosticity changes the perception of complex visual stimuli. Cognitive Psychology 34 (1), 72–107. [DOI] [PubMed] [Google Scholar]
- 25.Oliva A and Torralba A (2001) Modeling the shape of a scene: a holistic representation of the spatial envelope. International journal in computer vision 42, 145–175. [Google Scholar]
- 26.Greene MR and Oliva A (2009) Recognition of natural scenes from global properties: Seeing the forest without representing the trees. Cognitive Psychology 58 (2), 137–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walther DB and Shen D (2014) Nonaccidental properties underlie human categorization of complex natural scenes. Psychol Sci 25 (4), 851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene MR and Oliva A (2010) High-Level Aftereffects to Global Scene Properties. Journal of Experimental Psychology-Human Perception and Performance 36 (6), 1430–1442. [DOI] [PubMed] [Google Scholar]
- 29.Persichetti AS and Dilks DD (2018) Dissociable Neural Systems for Recognizing Places and Navigating through Them. J Neurosci 38 (48), 10295–10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dilks DD et al. (2011) Mirror-image sensitivity and invariance in object and scene processing pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience 31 (31), 11305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persichetti AS and Dilks DD (2016) Perceived egocentric distance sensitivity and invariance across scene-selective cortex. Cortex 77, 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamps FS et al. (2016) The occipital place area represents first-person perspective motion information through scenes. Cortex 83, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitcher D et al. (2019) A functional dissociation of face-, body- and scene-selective brain areas based on their response to moving and static stimuli. Sci Rep 9 (1), 8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonner MF and Epstein RA (2017) Coding of navigational affordances in the human visual system. Proc Natl Acad Sci U S A 114 (18), 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodale MA and Milner AD (1992) Separate visual pathways for perception and action. Trends in Neuroscience 15, 20–25. [DOI] [PubMed] [Google Scholar]
- 36.Kravitz DJ et al. (2011) A new neural framework for visuospatial processing. Nature reviews. Neuroscience 12 (4), 217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milner AD (2017) How do the two visual streams interact with each other? Experimental Brain Research 235 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldassano C et al. (2013) Differential connectivity within the Parahippocampal Place Area. Neuroimage 75, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasr S et al. (2013) Spatial encoding and underlying circuitry in scene-selective cortex. Neuroimage 83, 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vul E and Kanwisher N (2010) Begging the question: The non-independence error in fMRI data analysis. In Foundations and Philosophy for Neuroimaging (Hanson S and Bunzl M eds), pp. 71–91. [Google Scholar]
- 41.Persichetti AS and Dilks DD (2019) Distinct representations of spatial and categorical relationships across human scene-selective cortex. Proc Natl Acad Sci U S A 116 (42), 21312–21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vass LK and Epstein RA (2017) Common Neural Representations for Visually Guided Reorientation and Spatial Imagery. Cereb Cortex 27 (2), 1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchette SA et al. (2014) Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nat Neurosci 17 (11), 1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auger SD et al. (2012) Retrosplenial cortex codes for permanent landmarks. PLoS One 7 (8), e43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auger SD and Maguire EA (2013) Assessing the mechanism of response in the retrosplenial cortex of good and poor navigators. Cortex 49 (10), 2904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguirre GK and D’Esposito M (1999) Topographical disorientation: a synthesis and taxonomy. Brain 122 (Pt 9), 1613–28. [DOI] [PubMed] [Google Scholar]
- 47.Whiteley AM and Warrington EK (1978) Selective Impairment of Topographical Memory - Single Case-Study. Journal of Neurology Neurosurgery and Psychiatry 41 (6), 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hecaen H et al. (1980) Loss of Topographic Memory with Learning-Deficits. Cortex 16 (4), 525–542. [DOI] [PubMed] [Google Scholar]
- 49.Pallis CA (1955) Impaired Identification of Faces and Places with Agnosia for Colours - Report of a Case Due to Cerebral Embolism. Journal of Neurology Neurosurgery and Psychiatry 18 (3), 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Derenzi E et al. (1977) Topographical Amnesia. Journal of Neurology Neurosurgery and Psychiatry 40 (5), 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epstein R et al. (2001) Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cognitive neuropsychology 18 (6), 481–508. [DOI] [PubMed] [Google Scholar]
- 52.Walther DB et al. (2011) Simple line drawings suffice for functional MRI decoding of natural scene categories. Proceedings of the National Academy of Sciences of the United States of America 108 (23), 9661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walther DB et al. (2009) Natural scene categories revealed in distributed patterns of activity in the human brain. J Neurosci 29 (34), 10573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamps FS et al. (2016) The occipital place area represents the local elements of scenes. Neuroimage 132, 417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacEvoy SP and Epstein RA (2009) Decoding the Representation of Multiple Simultaneous Objects in Human Occipitotemporal Cortex. Current Biology 19 (11), 943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonner MF and Epstein RA (2021) Object representations in the human brain reflect the co-occurrence statistics of vision and language. Nat Commun 12 (1), 4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bar M and Aminoff E (2003) Cortical analysis of visual context. Neuron 38 (2), 347–358. [DOI] [PubMed] [Google Scholar]
- 58.Bar M et al. (2008) Scenes unseen: The parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. Journal of Neuroscience 28 (34), 8539–8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mullally SL and Maguire EA (2011) A New Role for the Parahippocampal Cortex in Representing Space. Journal of Neuroscience 31 (20), 7441–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konkle T and Oliva A (2012) A Real-World Size Organization of Object Responses in Occipitotemporal Cortex. Neuron 74 (6), 1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Troiani V et al. (2014) Multiple object properties drive scene-selective regions. Cereb Cortex 24 (4), 883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cant JS and Goodale MA (2011) Scratching beneath the surface: new insights into the functional properties of the lateral occipital area and parahippocampal place area. J Neurosci 31 (22), 8248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cant JS and Xu Y (2012) Object ensemble processing in human anterior-medial ventral visual cortex. J Neurosci 32 (22), 7685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park J and Park S (2017) Conjoint representation of texture ensemble and location in the parahippocampal place area. J Neurophysiol 117 (4), 1595–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henriksson L et al. (2019) Rapid Invariant Encoding of Scene Layout in Human OPA. Neuron 103 (1), 161–171 e3. [DOI] [PubMed] [Google Scholar]
- 66.Aminoff E et al. (2007) The parahippocampal cortex mediates spatial and nonspatial associations. Cerebral Cortex 17 (7), 1493–1503. [DOI] [PubMed] [Google Scholar]
- 67.Stevens WD et al. (2012) Hemispheric Asymmetry of Visual Scene Processing in the Human Brain: Evidence from Repetition Priming and Intrinsic Activity. Cerebral Cortex 22 (8), 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park S et al. (2015) Parametric Coding of the Size and Clutter of Natural Scenes in the Human Brain. Cereb Cortex 25 (7), 1792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steel A et al. (2021) A network linking scene perception and spatial memory systems in posterior cerebral cortex. Nat Commun 12 (1), 2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dillon MR et al. (2018) Places in the Brain: Bridging Layout and Object Geometry in Scene-Selective Cortex. Cereb Cortex 28 (7), 2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park J and Park S (2020) Coding of Navigational Distance and Functional Constraint of Boundaries in the Human Scene-Selective Cortex. J Neurosci 40 (18), 3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng A et al. (2021) Concavity as a diagnostic feature of visual scenes. Neuroimage 232, 117920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silson EH et al. (2015) A Retinotopic Basis for the Division of High-Level Scene Processing between Lateral and Ventral Human Occipitotemporal Cortex. J Neurosci 35 (34), 11921–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silson EH et al. (2016) Scene-Selectivity and Retinotopy in Medial Parietal Cortex. Front Hum Neurosci 10, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julian JB et al. (2016) The Occipital Place Area Is Causally Involved in Representing Environmental Boundaries during Navigation. Curr Biol 26 (8), 1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Auger SD et al. (2015) A central role for the retrosplenial cortex in de novo environmental learning. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolbers T and Buchel C (2005) Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci 25 (13), 3333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sherrill KR et al. (2013) Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J Neurosci 33 (49), 19304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morgan LK et al. (2011) Distances between Real-World Locations Are Represented in the Human Hippocampus. Journal of Neuroscience 31 (4), 1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iaria G et al. (2007) Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci 25 (3), 890–9. [DOI] [PubMed] [Google Scholar]
- 81.Sugiura M et al. (2005) Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. J Cogn Neurosci 17 (2), 183–98. [DOI] [PubMed] [Google Scholar]
- 82.Silson EH et al. (2019) Distinct subdivisions of human medial parietal cortex support recollection of people and places. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suzuki S et al. (2021) Two scene navigation systems dissociated by deliberate versus automatic processing. Cortex 140, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stark M et al. (1996) Impairment of an egocentric map of locations: Implications for perception and action. Cognitive Neuropsychology 13 (4), 481–523. [Google Scholar]
- 85.Takahashi N et al. (1997) Pure topographic disorientation due to right retrosplenial lesion. Neurology 49 (2), 464–9. [DOI] [PubMed] [Google Scholar]
- 86.Golarai G et al. (2007) Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci 10 (4), 512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scherf KS et al. (2007) Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev Sci 10 (4), F15–30. [DOI] [PubMed] [Google Scholar]
- 88.Scherf KS et al. (2011) “What” precedes “which”: developmental neural tuning in face- and place-related cortex. Cereb Cortex 21 (9), 1963–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Golarai G et al. (2010) Differential development of the ventral visual cortex extends through adolescence. Front Hum Neurosci 3, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meissner TW et al. (2019) Prolonged functional development of the parahippocampal place area and occipital place area. Neuroimage 191, 104–115. [DOI] [PubMed] [Google Scholar]
- 91.Kamps FS et al. (2020) Late Development of Navigationally Relevant Motion Processing in the Occipital Place Area. Curr Biol 30 (3), 544–550 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Julian JB et al. (2019) Dissociable spatial memory systems revealed by typical and atypical human development. Dev Sci 22 (2), e12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berard JR and Vallis LA (2006) Characteristics of single and double obstacle avoidance strategies: a comparison between adults and children. Exp Brain Res 175 (1), 21–31. [DOI] [PubMed] [Google Scholar]
- 94.Pryde KM et al. (1997) Age-related trends in locomotor ability and obstacle avoidance. Human Movement Science 16 (4), 507–516. [Google Scholar]
- 95.Franchak JM and Adolph KE (2010) Visually guided navigation: Head-mounted eye-tracking of natural locomotion in children and adults. Vision Research 50 (24), 2766–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Franchak JM et al. (2011) Head-Mounted Eye Tracking: A New Method to Describe Infant Looking. Child Development 82 (6), 1738–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hermer L and Spelke ES (1994) A geometric process for spatial reorientation in young children. Nature 370 (6484), 57–9. [DOI] [PubMed] [Google Scholar]
- 98.Gibson EJ and Walk RD (1960) The “visual cliff”. Sci Am 202, 64–71. [PubMed] [Google Scholar]
- 99.Diamond A and Goldman-Rakic PS (1989) Comparison of human infants and rhesus monkeys on Piaget’s AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res 74 (1), 24–40. [DOI] [PubMed] [Google Scholar]
- 100.Diamond A et al. (1989) Successful performance by monkeys with lesions of the hippocampal formation on AB and object retrieval, two tasks that mark developmental changes in human infants. Behav Neurosci 103 (3), 526–37. [DOI] [PubMed] [Google Scholar]
- 101.Bertenthal BI (1996) Origins and early development of perception, action, and representation. Annu. Rev. Psychol 47, 431–459. [DOI] [PubMed] [Google Scholar]
- 102.Gilmore RO and Johnson MH (1997) Egocentric action in early infancy: spatial frames of reference for saccades. Psychological Science 8, 224–230. [Google Scholar]
- 103.Gilmore RO and Johnson MH (1997) Body-centered representations for visually-guided action emerge during early infancy. Cognition 65, B1–B9. [DOI] [PubMed] [Google Scholar]
- 104.Csibra G et al. (1998) Neural correlates of saccade planning in infants: a high-density ERP study. Int J Psychophysiol 29 (2), 201–15. [DOI] [PubMed] [Google Scholar]
- 105.Atkinson J et al. (2003) Neurobiological models of visuospatial cognition in children with Williams syndrome: measures of dorsal-stream and frontal function. Dev Neuropsychol 23 (1–2), 139–72. [DOI] [PubMed] [Google Scholar]
- 106.Dilks DD et al. (2008) Vision for perception and vision for action: normal and unusual development. Dev Sci 11 (4), 474–86. [DOI] [PubMed] [Google Scholar]
- 107.Campos JJ, Langer A, Krowitz A (1970) Cardiac Responses on the Visual Cliff in Prelocomotor Human Infants. Science 170 (3954), 196–197. [DOI] [PubMed] [Google Scholar]
- 108.Adolph K (2000) Specificity in learning: Why infants fall over a veritable cliff. Psychological Science 11 (4), 290–295. [DOI] [PubMed] [Google Scholar]
- 109.Acredolo LP (1977) Developmental-Changes in Ability to Coordinate Perspectives of a Large-Scale Space. Developmental Psychology 13 (1), 1–8. [Google Scholar]
- 110.Overman WH et al. (1996) Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behavioral Neuroscience 110 (6), 1205–1228. [DOI] [PubMed] [Google Scholar]
- 111.Lehnung M et al. (1998) Spatial memory and orientation in healthy and brain-injured children. European Journal of Neuroscience 10, 143–143. [Google Scholar]
- 112.Leplow B et al. (2003) Navigational place learning in children and young adults as assessed with a standardized locomotor search task. British Journal of Psychology 94, 299–317. [DOI] [PubMed] [Google Scholar]
- 113.Akers KG and Hamilton DA (2007) Comparison of developmental trajectories for place and cued navigation in the Morris water task. Developmental Psychobiology 49 (6), 553–564. [DOI] [PubMed] [Google Scholar]
- 114.Nazareth A et al. (2018) Charting the development of cognitive mapping. Journal of Experimental Child Psychology 170, 86–106. [DOI] [PubMed] [Google Scholar]
- 115.Newcombe NS (2019) Navigation and the developing brain. Journal of Experimental Biology 222. [DOI] [PubMed] [Google Scholar]
- 116.Allen GL et al. (1979) Developmental issues in cognitive mapping: the selection and utilization of environmental landmarks. Child Dev 50 (4), 1062–70. [PubMed] [Google Scholar]
- 117.Cousins JH et al. (1983) Way finding and cognitive mapping in large-scale environments: a test of a developmental model. J Exp Child Psychol 35 (1), 1–20. [DOI] [PubMed] [Google Scholar]
- 118.Deen B et al. (2017) Organization of high-level visual cortex in human infants. Nat Commun 8, 13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Powell LJ et al. (2018) Using individual functional channels of interest to study cortical development with fNIRS. Dev Sci 21 (4), e12595. [DOI] [PubMed] [Google Scholar]
- 120.Kamps FS et al. (2020) Connectivity at the origins of domain specificity in the cortical face and place networks. Proc Natl Acad Sci U S A 117 (11), 6163–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]


