Abstract
Immune suppressive myeloid cells play a major role in cancer by negatively regulating immune responses, promoting tumor progression, and limiting the efficacy of cancer immunotherapy. Immune suppression is mediated by various mechanisms dependent upon the type of myeloid cell involved. In recent years, a more universal mechanism of immune suppressive activity of myeloid cells has emerged: generation of oxidized lipids. Oxidized lipids accumulate in all types of myeloid cells and are often transferred between cells. In this review, we discuss mechanisms involved in the generation and biological role of myeloid cell-derived oxidized lipids in cancer.
Myeloid cells have long been recognized as one of the major regulators of tumor progression and metastasis. They are one of the major hurdles in generating a productive anti-tumor immune response and contribute to the limitation of cancer immunotherapy. Therefore, effectively targeting myeloid cells has become a key strategy in improving current therapies. The biology and role of myeloid cells in cancer have been discussed in many recent reviews (1-5). The myeloid lineage is a complex, closely connected network of cells with diverse functions. They utilize a large variety of molecules and mechanisms to regulate the immune system in cancer. These mechanisms depend on the specific type and state of activation of different myeloid cells. However, in recent years one common mechanism that myeloid cells exploit in cancer has emerged, utilizing oxidized lipids. In this review, we will discuss the potential role of oxidized lipids in the function of different myeloid cells.
Brief overview of myeloid cells in cancer
The myeloid compartment is comprised of several major groups of cells: granulocytic represented by polymorphonuclear neutrophils (PMN), eosinophils, and basophils; monocytic represented by monocytes (MON) and macrophages (MΦ), dendritic cells (DC), and megakaryocytes. Of these, the most well-studied in the context of cancer, and thus the focus of this review, are PMN, MON, MΦ, and DC. Populations of PMN and MON consist of classically activated cells (evolved as major protectors of organisms from pathogens) and pathologically activated myeloid-derived suppressor cells (MDSC). Although MDSC share many features with classical PMN and MON (origin, phenotype, morphology), they also have distinct transcriptomics, biochemical and functional traits with the most distinguishing feature of MDSC being the immune suppressive activity (3). Based on the differentiation pathway (granulocytic and monocytic), MDSC are defined as either PMN-MDSC or M-MDSC. PMN-MDSC are the most abundant, representing >90% of all MDSC. To suppress the function of T, B, and NK cells, M-MDSCs secret immunosuppressive cytokines (IL-10, IL-6, TGFβ) and nitric oxide (NO) as well as express checkpoint inhibitors, while PMN-MDSC utilize reactive oxygen species (ROS), peroxynitrite (PNT), arginase I, and prostaglandin E2 (PGE2) (3). The prevalence of MDSCs has been closely associated with poor patient prognosis and response to therapy in a variety of tumor types (6).
MΦ have a broad role in host defense and maintenance of tissue homeostasis (7). Based on their origin, MΦ can be classified into two major groups: tissue resident macrophages derived from embryonic progenitors or bone marrow derived MΦ differentiated from MON. Additionally, MΦ can be polarized in vitro to a classically activated M1 phenotype when incubated with interferon γ or lipopolysaccharide or alternatively activated M2 phenotype when incubated with IL-4 and IL-13 (8,9). In cancer, M1/M2 polarization of tumor associated macrophages (TAM) is difficult to capture, reflecting the dynamic nature of TAM polarization and complexity of signals from the tumor microenvironment. Nonetheless, TAM can be polarized to have either pro- or anti-tumor functions (reviewed in (2)). In recent years, evidence has emerged that MΦ in cancer can be distinguished as classical (non-suppressive) and pathologically activated (suppressive) (10), similar to what was observed for MDSC. These suppressive MΦ may include multiple subsets and utilize mechanisms that may be shared by M1 and M2 MΦ. For instance, both arginase I and NO, a distinct feature of M2 and M1 MΦ, respectively, were directly implicated in the immune suppressive activity of TAM. To identify the multiple subsets of TAM, single cell RNA sequencing and spectral cytometry have been used, but the functional characterization of each population is still largely lacking (11). Corresponding to the divergent polarization of TAM, the presence of TAM has been correlated to both shorter relapse-free survival and overall survival (12) as well as better outcomes in the same types of cancer (13).
DC differentiate from specialized progenitors and function as professional antigen presenting cells that endocytose, process, and present antigens to T cells to generate cytotoxic antigen specific responses. These processes are critical for the induction of an anti-tumor immune response and success of cancer immunotherapy (14,15). DC can be broadly classified into classical DC (cDC) of which two subsets cDC1 and cDC2 are defined, plasmacytoid DC, and monocyte-derived DC (inflammatory DC) (16). cDC1 are considered the major cross-presenting cells promoting antitumor responses, whereas monocyte-derived DC are implicated in inhibition of immune responses (16).
Outline of the metabolism of oxidized lipids
Lipids are small amphiphilic molecules containing a non-polar hydrocarbon moiety and a polar head group. If the length of the hydrocarbon chain is 10 or more carbons, lipids can assemble into monolayers (at the air-water interface), micelles in solution, and vesicles having bilayer membranes. Lipids are indispensable in forming biomembranes (17). Lipids also self-organize into supramolecular structures allowing for the creation of microenvironments that can contain proteins, nucleic acids and/or small molecules (18).
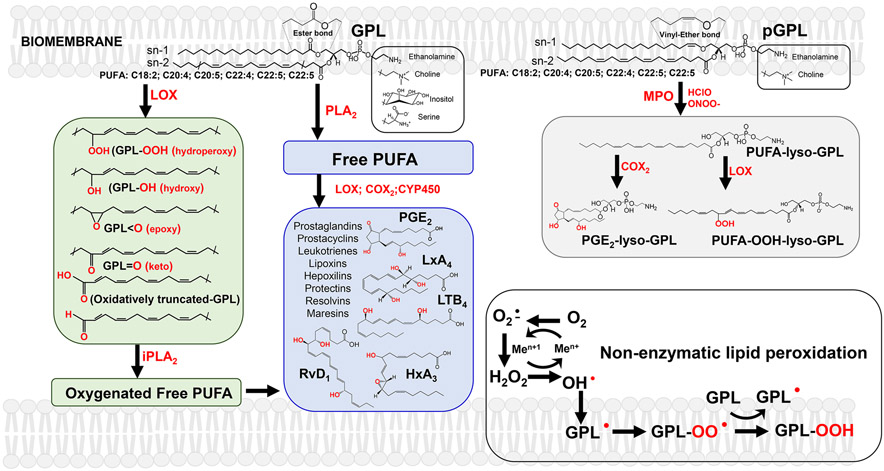
The requirement of context-specific properties of biomembranes, maintenance of their parameters within a relatively narrow range of fluidity/rigidity and optimized regulation of the membrane-embedded protein machinery, defined the selection of several major classes of lipids. Among them, the most important and common in membranes are phospholipids (PL) which are composed of fatty acid(s) (FA), an alcohol, a phosphate and a polar group. Glycerophospholipids (GPL) are the major class of PL in which one or two FA are attached at the sn-1, sn-2 positions of the glycerol backbone through an ester or ether bond and a polar group occupies the sn-3 position via a phosphodiester bond (Figure 1). GPLs are further classified based on the nature of their polar groups: choline, ethanolamine, serine, inositol, etc. (19).
Figure 1. The major metabolic pathways leading to the formation of signaling molecules from PUFA-GPL and pGPL.
Right upper panel (grey): Cleavage of vinyl ether bond of pGLP by oxidants (eg, hypochlorous acid (HClO) generated by myeloperoxidase (MPO) or peroxynitrite (ONOO-) result in the production of unusual lyso-GPLs with the retained sn-2 PUFA oxidizable residues. These types of lyso-GPLs can be readily be used as substrates of cyclooxygenases (COX) and lipoxygenases (LOX) to yield respective hydroperoxy-lyso-GPLs and their secondary oxygenated derivatives (including PGE2-lyso-GPLs) with multiple signaling functions.
Middle panel (blue) : Canonical PLA2 catalyzed release of free PUFA from the sn-2 position of GPLs and subsequent oxygenation of liberated free PUFA by COX, LOX and isoforms of cytochrome P450 (CYP450). Among these oxygenated molecules formed are prostaglandins, prostacyclins, leukotrienes, lipoxins, hepoxilins, protectins, resolvins, maresins.
Left panel (green) : LOX-catalyzed oxidation of GPL yields hydroperoxy-, hydroxy-, keto-, epoxy- and oxidatively- truncated GPL species with signaling functions. Hydrolysis of these peroxidized GPL by Ca2+-independent PLA2 (iPLA2) represents a non-canonical pathway leading to the formation of free oxygenated PUFA acting as lipid mediators.
Right lower panel (white): non-enzymatic lipid peroxidation
FA are classified as saturated FA (SUFA), monounsaturated FA (MUFA) or polyunsaturated FA (PUFA) with the latter two containing one or more double bonds, respectively. SUFA- and MUFA-residues are usually localized at both sn-1 and sn-2 positions, whereas PUFA preferably occupy the sn-2 position. In eukaryotic cells, long-chain PUFA are usually synthesized from saturated FA by two major classes of enzymes, elongases that add an ethylene group and desaturases that insert a double bond in the FA. In mammalian cells, these enzymes can “build” the myriads of individual molecular species by differing the number and position of up-to 6 double bonds. There are two major families of PUFA in which the double bonds are localized starting from the 3rd (ω-3) or the 6th (ω-6) carbon atom, counting from the PUFA terminal methyl group. The KEGG’s pathway for the biosynthesis of unsaturated FA lists a total of 30 PUFA (20). Combined with ~15 other possible SUFA and MUFA, each class of GPLs can encompass >2x103 individual species. In some types of GLP (eg, mitochondrial cardiolipins), this number may be much greater reaching in excess of 4x106 different species.
It has become clear that the large number of different GLP species including not only intact GLP but also metabolites, act as signaling molecules. Two metabolic mechanisms – i) hydrolysis by different phospholipases, ii) oxygenation reactions - and their combination represent the major pathways leading to the production of lipid signals.
Lipid mediators are the products of sequential hydrolysis of PUFA-GLP by type 2 phospholipases which catalyze the release of free PUFA from the sn-2 position thus making them available for the oxygenation by cyclooxygenases (COX), lipoxygenases (LOX) and isoforms of cytochrome P450 (CYP450) (21). These reactions represent the canonical mechanisms for the formation of several classes of well-known lipid mediators with multiple regulatory functions. Based on their carbon chain length, they are represented by octadecanoids, eicosanoids, docosanoids and docosahexanoids, the oxygenated derivatives of PUFA with 18, 20, and 22 carbons (22). Among these oxygenated molecules are prostaglandins, prostacyclins, leukotrienes, lipoxins, hepoxilins, protectins, resolvins, maresins – lipid signals that function to recruit myeloid cells and have pro- and anti-inflammatory effects.
Lately, it has become apparent that an alternative mechanism may generate the same lipid mediators. This process may be executed enzymatically, whereby Fe-containing proteins, LOX and peroxidases, directly attack GLP PUFA residues and generate their peroxidation products. These peroxidized GLPs include thousands of oxidatively modified molecules with oxygen-containing functional groups such as hydroperoxy-, hydroxy-, epoxy- and keto- that are positioned on the GLP FA residues (Figure 1). Similar to free PUFA-based lipid mediators, the peroxidized GLP may act through specific receptors or signal via covalent modification of protein targets. The latter mechanism is based on the formation of oxidatively-truncated highly electrophilic peroxidation products capable of attacking the nucleophilic sites in target proteins(23,24).
One additional way to metabolically generate lipid signals utilizes a GLP subclass called plasmalogens. Plasmalogens (pGLP) can contain two different types of bonds: a vinyl ether linkage at the sn-1 position of the glycero-phospholipid backbone and an ester-bond at the sn-2 position (similar to diacyl-GLPs (25)) (Figure 1). In the context of signaling, the important property of pGLP is the vulnerability of their vinyl ether bond to cleavage by hypochlorous acid (HClO) generated by myeloperoxidase (MPO) and peroxynitrite (ONOO-) massively produced by MDSC and MΦ (21) (Figure 2). In pro-inflammatory conditions, elevated levels of HClO or ONOO- can lead to the production of unusual lyso-GPLs with the retained sn-2 PUFA oxidizable residues. These special lyso-GPLs can be readily used as substrates of COX and LOX to yield respective hydroperoxy-GPLs with potentially multiple signaling functions (21). As an example, massive production of sn-2-PUFA-lyso-phosphatidylethanolamines occurs during PMN MPO attack causing awakening of dormant cancer cells, as discussed below (26).
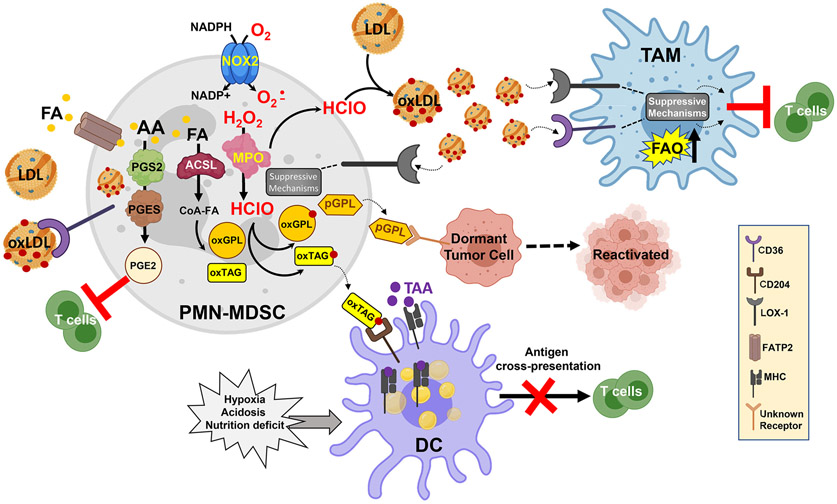
Figure 2. Effect of oxidized lipids of function of myeloid cells in cancer.
Oxidative machinery of PMN-MDSC include MPO and superoxide produced by NADPH oxidase (NOX2) support the generation of a large variety of oxidized lipids. Oxidized fatty acids (oxFA) undergo esterification to triglycerides (TAG) and various phospholipids (PL). oxTAG can be transferred to DC where they undergo degradation and oxidatively truncated FA are then incorporated into LD. Electrophilic LD bind HSP70 and prevent the transfer to the cell surface of peptide-MHC class I complexes formed in lysosomes and endosomes during vacuolar pathway of cross-presentation; thus inhibiting cross-present tumor antigens by DC. Formation of LD containing oxFA can also be mediated by activation of ER stress and spliced XBP1. In PMN-MDSC, accumulation of oxidized lipids result in increased biosynthesis of PGE2 and plasmalogens leading to enhanced suppressive activity of these cells. Plasmalogens promote reactivation of dormant tumor cells. Oxidized LDL signal in PMN-MDSC and TAM via several receptors to potentiate suppressive activity of these cells, which include up-regulation of arginase 1, NO production, secretion of immune suppressive cytokines.
In addition to membrane GLPs, there are several classes of non-amphiphilic lipid molecules, neutral lipids. Their formation is frequently associated with the aberrant metabolism of FAs. Dis-coordinated FA metabolism in hypoxia or in suppressed beta-oxidation, leads to the accumulation of free FA and severe lipotoxicity. As a response, cells attempt to insulate free FA by integrating them into esterified forms, di-acylglycerols (DAGs) and tri-acylglycerols (TAGs) as well as in cholesterol esters (CE). These covalent derivatives of FA are highly hydrophobic and usually excluded from biomembranes to produce separate intracellular organizations, lipid droplets (LD). (Figure 1). Recent studies demonstrated that LD represent highly-specialized organelles with active metabolism and signaling functions (27). This new understanding of the functional LD importance has been developed in parallel to the appreciation of the presence of readily “peroxidizable” PUFA-residues in DAGs and TAGs. Indeed, PUFA de-esterification/re-esterification into these neutral lipids can serve as interesting sources of signaling lipid mediators. Peroxidation of PUFA-containing DAG/TAG molecules and formation of their oxidatively-truncated electrophilic derivatives forming covalent adducts with proteins may be also utilized for intracellular insulation and hiding of the proteins in the hydrophobic core of LDs (28).
Lipids can also be used as a source of energy via fatty acid oxidation (FAO). FAO begins with the activation of the FA through enzymatic conjugation with acyl-CoA. Then the FA must translocate into the mitochondria with short-chain FA less than six carbons diffusing passively and longer FA needing to be first conjugated to carnitine via carnitine palmitoyl transferase 1 (CPT1). The reaction is a rate limiting step in FAO and can be inhibited with the drug etomoxir which will be discussed below. Once shuttled into the mitochondria, the carnitine is removed by carnitine palmitoyl transferase 2 (CPT2). FA are then broken down by beta-oxidation to yield large amounts of acetyl-CoA, NADH and FADH2 that is shuttled into the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) to generate ATP (29).
Role of oxidized lipids in myeloid cell dysfunction in cancer
MDSC.
Lipid metabolism has been directly implicated in MDSC suppressive activity. Tumor infiltrated MDSC upregulated FA uptake and FAO relative to peripheral MDSC. This altered metabolism promoted the suppressive activity of MDSC since blockade of FAO decreased immunosuppression (30). A subsequent study explored the mechanism by which tumor infiltrating MDSC increased lipid uptake. They demonstrated that the tumor-derived G-CSF and GM-CSF induced upregulation of a variety of lipid transport receptors including CD36, macrophage scavenger receptor 1 (MSR1)/CD204 and fatty acid transporters 1 and 6 (FATP1 and 6) (31). The lipids transported from the microenvironment promoted immune suppression by MDSC, which was reversed by CD36 deletion (31).
Additional receptors/transporters have been reported to be involved in lipid uptake by MDSC. For instance, PMN-MDSC overexpressed oxidized low-density lipoprotein (LDL) receptor 1 (OLR1/LOX-1) (32). While the impact of LOX-1 expression on lipid metabolism and suppressive activity of PMN-MDSC is not yet fully explored, LOX-1 was shown to be selectively expressed on human PMN-MDSC as opposed to classical PMN and associated with an immune suppressive gene signature in PMN-MDSC. ER stress was shown to be the major inducer of LOX-1 expression in PMN-MDSC (32,33). A number of studies correlated LOX-1 positive PMN-MDSC and negative clinical outcomes in cancer and proposed that LOX-1 is a potential biomarker of PMN-MDSC (34-37).
Along the same lines, another study established that in contrast to classical PMN, PMN-MDSC expressed FATP2, a transporter for long chain fatty acids. Experiments with targeted deletion in neutrophils of Slc27a2 gene that encodes FATP2 abrogated the suppressive activity of PMN-MDSC (38). Increased uptake of arachidonic acid (AA) and production of PGE2 were implicated in FATP2 mediated suppression by PMN-MDSC (38). (Figure 2). Inhibition of FATP2 with the small molecule lipofermata reduced suppressive activity of MDSC and demonstrated potent antitumor activity (38,39). In another study, lipofermata reduced melanoma tumor growth in aged mice which was not mediated by its direct effect on tumor cells (40). PGE2 is a product of lipid oxygenation initiated when AA is metabolized by cyclooxygenase 2 (COX2). Generation of PGE2 has been shown to not only promote MDSC development, but also mediate their suppressive activity (41,42). PGE2 was directly implicated in MDSC immune suppressive activity not only in cancer but also in newborns (43-46). Overall, these studies provide a link between lipid metabolism and lipid uptake by MDSC to the immune suppressive activity.
Macrophages.
It has long been appreciated that metabolism is involved in MΦ polarization. M1-like MΦ preferentially use glycolysis, have a broken TCA cycle, and generate lipids via fatty acid synthesis. Conversely, M2-like MΦ have an intact TCA cycle and rely on FAO to fuel OXPHOS for energy production (29,47,48). In fact, the polarization of MΦ can be altered by modifying the metabolic pathways being utilized. A recent study found that unsaturated FA or LD polarized bone-marrow derived cells into immune suppressive M2-like MΦ. This polarization could be reversed with etomoxir, the FAO inhibitor, or inhibition of diacylglycerol-O-acyltransferase (DGAT) the enzyme responsible for the import of FFA into LD (49).
Similar studies demonstrated that MΦ from tumor bearing mice or cultured with tumor cells had increased lipid accumulation and utilized scavenger receptors CD36 and LOX-1 to transport FA from the lipid rich TME (50-52). FA undergo lipolysis mediated by lysosomal acid lipase to support OXPHOS and M2 activation which is reversed by inhibition of these lipases (51). Additionally, inhibition of FAO with etomoxir or CD36 deletion in TAMs decreased tumor growth and M2 polarization (52). Together, these studies demonstrate that lipid metabolism and uptake can alter the polarization and enhance protumor functions of TAM.
Ferroptosis is a redox-driven cell death program (53), which includes iron-dependent generation of (phospho)lipid hydroperoxides and failure of their reduction to alcohols by glutathione peroxidase 4 (GPX4). The decay of hydroperoxy-lipids to oxidatively truncated reactive electrophilic species affects yet to be identified, critical protein targets leading to the cell death (54). Recent studies implicated ferroptosis in regulation of MΦ biology. Inducible nitric oxide synthase (iNOS)/NO•, a characteristic of M1 MΦ, conferred MΦ resistance to ferroptosis by inhibiting pro-ferroptotic lipid peroxidation (55). This study and another showed that exposure of M2 MΦ to NO• protected them from ferroptosis and that GPX4 expression was not required for survival of M2 (alternatively activated) MΦ if sufficient amounts of NO• were generated (55,56). These studies suggest that induction of ferroptosis can alter the MΦ polarization. Along the same lines, induction of ferroptosis in TAM resulted in a metabolic switch from mitochondrial OXPHOS to glycolysis, which induced pro-inflammatory signaling pathways, enabling MΦ activation with potent tumoricidal activities (57).
Dendritic cells.
Early studies showed that PUFA reduced expression of DC co-stimulatory molecules and pro-inflammatory cytokine production in response to different stimuli (58,59). Two subsequent studies identified that PPARγ was involved in the PUFA, docosahexaenoic acid (DHA), mediated suppression of DC activation (60,61). Along the same lines, accumulation of lipids was found in DC of tumor-bearing mice and cancer patients and was associated with defective cross-presentation by these cells (62). The scavenger receptor, Msr1/CD204, was implicated in lipid uptake by DC and blockade of CD204 abrogated the accumulation of lipids from tumor explant supernatant (62). A later study demonstrated that the nature of lipids rather than the amount is critical for negative regulation DC function. Accumulation of oxidatively truncated lipids was directly implicated in blockade of cross-presentation by DC. DC cross-presentation utilizes two major pathways: cytosolic and vacuolar (63). The latter, includes processing peptides and formation of peptide-MHC Class I complexes in lysosomes/late endosomes. LD containing oxidatively truncated lipids were implicated in blocking the vacuolar pathway of cross-presentation. The mechanism of this phenomenon involved LD containing oxidatively truncated lipids binding to chaperon heat shock protein 70 (HSP70) preventing it from transporting peptide-MHC Class I complexes to the cell surface (64,65) (Figure 2).
Although an important role of lipid accumulation in DC dysfunction is established (66-69), the exact mechanism by which oxidized lipids can be accumulated by DC is not clear. DC lack the potent machinery of lipid oxidation, but it was proposed that the tumor derived changes to DC allowed for them to generate oxidized lipids (70). Tumor-associated DC (tDC) have significantly higher levels of the ER stress mediator XBP1, which was correlated with increased expression of genes involved in triglyceride biosynthesis suggesting an increased ability to produce lipids. The increased lipid accumulation and up-regulation of ROS allowed for lipid peroxidation to occur in tDC. Consistent with previous reports, this led to a reduction in antigen presentation via MHC complexes (70). However, this mechanism operated only in tumor site, whereas defective DC function was observed in peripheral lymphoid organs as well. A recent study demonstrated another mechanism. PMN-MDSC accumulate oxidized lipids after generating them by utilizing MPO and superoxide produced by NADPH oxidase. PMN-MDSC can then transfer the oxidized lipids to DC, impairing the ability of DC to cross-present (71) (Figure 2).
Although cross-presenting activity of MΦ was long considered to be inferior to that of DC, recent studies indicated that MΦ, including TAM, were able to cross-present antigens. Interestingly, it appears that vacuolar pathway is predominant mechanism of cross-presentation in these cells (72). Therefore, similar to what was described above for DC, oxidatively truncated lipids also may substantially inhibit cross-presentation by TAM.
Lipid metabolism has also been implicated in promoting DC dysfunction. Examining the metabolic difference between immunogenic and tolerogenic DC revealed that tolerogenic DC, amongst other changes, had significantly higher OXPHOS and FAO. Additionally, inhibition of FAO with etomoxir prevented the function of tolerogenic DC and partially restored T cell stimulatory capacity (73). Similarly, resveratrol and vitamin D3 were also shown to promote OXPHOS and tolerogenic DCs (74,75). Overall, these studies demonstrate that increased lipid metabolism and accumulation of oxidized lipids have profound ability to inhibit the function of DC.
Targeting oxidized lipids from myeloid cells.
Studies described above implicated lipid metabolism in regulation of myeloid cell function in cancer; thus, targeting it could provide therapeutic benefit for patients. The challenge is in the selection of targets. A summary of the preclinical and clinical attempts at targeting lipids from myeloid cells is shown in Table 1. As we discussed above, several studies implicated FAO in dysfunction of myeloid cells in cancer. While inhibition of the FAO with etomoxir was shown to inhibit immune suppression in preclinical models (30,52), this drug is not specific to myeloid cells creating the potential for toxic side effects. In fact, a clinical trial with etomoxir for the treatment of congestive heart failure was terminated due to toxicity (76). Due to the fact that the enzymes in FAO are required for normal metabolism, careful drug design and dosing as well as targeted delivery is probably needed to safely target this pathway.
Table 1:
Targeting lipids in myeloid cells
| Target Type | Target | Drug | Preclinical or Clinical |
Effects | Potential Pitfall |
|---|---|---|---|---|---|
| FAO rate limiting enzyme | CPT1 | Small molecule Etomoxir | Preclinical | Not specific to metabolism of myeloid cells | |
| Clinical |
|
Terminated due to toxicity | |||
| Enzyme that transports FFA into LD | DGAT1 DGAT2 |
Small molecule A922500 & PF-06424439 | Preclinical |
|
|
| Lipolysis inhibition | Lipase | orlistat | Preclinical |
|
|
| Enzyme involved in PGE2 generation | COX-2 | Small molecule Celecoxib | Preclinical |
|
|
| Clinical | Potential cardiovascular effects | ||||
| Lipid Receptors and Transporters | CD36 | Antibody blockade | Preclinical | High redundancy in these receptors may represent therapeutic challenge | |
| Thrombospondin mimetic peptides | Clinical |
|
|||
| CD204 | Antibody blockade | Preclinical |
|
||
| LOX-1 | MEDI6570 | Clinical |
|
||
| FATP2 | Small molecule Lipofermata | Preclinical |
COX-2, the enzyme involved in generating PGE2, has also been examined as a potential target for cancer therapy. Preclinically, the COX-2 inhibitor celecoxib reduced the number and function of MDSC and potentiated DC based immunotherapy (77). An analysis of 11 clinical trials found that the COX-2 inhibitor celecoxib was beneficial for the treatment of advanced cancer, but patients had an increased risk of cardiovascular events which is common for COX-2 inhibitors (78,79). Once again highlighting that a more precise approach to target lipid metabolism of myeloid cells is needed. Among them could be targeting of specific receptors involved in myeloid cell lipid uptake such as the scavenger receptors CD36, LOX-1 or CD204. Preclinically, antibody blockade of CD36 reduced tumor growth alone or in combination with anti-PD-1, although the involvement of MDSC was not examined in these studies (80,81). Of note, there is high redundancy of these receptors, which may represent a therapeutic challenge. For instance, attempts at targeting CD36 indirectly with thrombospondin mimetic peptides have been unsuccessful (82). One possible direction is to focus on molecules that are selectively up-regulated on immune suppressive myeloid cells, but studies of this nature are currently ongoing.
Myeloid cell derived oxidized lipids in regulation of dormant tumor cells
Tumor cells that have entered a reversible cell cycle arrest are considered dormant (83). Tumor cell dormancy can occur for a variety of reasons including an adaptation to environmental insults, the effect of oncogenes, and as a consequence of cancer treatment (83). Tumor dormancy has a positive and negative impact on tumor progression. On one hand, dormant tumor cells don’t proliferate limiting tumor expansion and supporting tumor remission. On the other hand, dormant tumors cells are more resistant to chemo- or radiation therapies. Furthermore, these cells are capable of evading immune surveillance making them resistant to immunotherapies (84). If reactivated, dormant tumor cells quickly progress resulting in tumor recurrence which can happen many years after the start of the remission. One of the biggest challenges is understanding the mechanism of reactivation of dormant tumor cells.
Due to the rarity of dormant tumor cells and the challenges identifying them in patients, few studies have delved into the altered metabolism of these cells. One study found that dormant cells relied on mitochondrial respiration rather than glycolysis for cellular energetics (85). Another study revealed that dormant tumor cells had increased expression of genes related to lipid metabolism (86). A recent study utilized a reporter construct that allowed for the identification of non-cycling or cycling persister cells that remained after chemotherapy. It found that although both, non-cycling and cycling persister cells, shifted their metabolism to FAO relative to untreated cells, cycling cells had higher FA metabolism than non-cycling cells (87). While the authors called these cells persister cells, a non-cycling tumor cell could probably be considered dormant. Thus, these studies suggest that FAO plays a role in the dormancy and reactivation of tumor cells.
Studies have indicated that lipid moieties can cause dormant tumor cell reactivation. A decade ago, one study showed that the lipid mediator, epoxyeicosatrienoic acid, caused escape from tumor dormancy in several tumor models (88). Similarly, in a 3D bone-like microenvironment, PGE2 induced dormant breast cancer reactivation (89). Neither of these studies investigated the impact of immune cells on the reactivation with lipid moieties. Recently we demonstrated that PMN-MDSC were able to reactivate proliferation of dormant tumor cells (26) (Figure 2). Importantly, neutrophils from tumor-free mice or healthy donors as well as other myeloid cells were not able to reactivate dormant tumor cells. Only after exposure to stress in vivo or stress hormones in vitro, neutrophils acquired the ability to induce exit of tumor cells from dormancy (26). This effect was mediated by release of pro-inflammatory S100A8/A9 proteins leading to elevated MPO activity and accumulation of oxidized lipids, plasmalogens, in neutrophils. Direct experiments demonstrated that oxidatively modified by MPO phosphatidylethanolamine plasmalogens were able to activate proliferation of dormant tumor cells via up-regulation of fibroblast growth factor pathway (26). These findings provide one possible mechanism for tumor recurrence. Patients in remission, who have undetectable dormant tumor cells, are encountering stress throughout their daily lives. If neutrophils in the vicinity of dormant tumor cells are exposed to stress hormones, they can release oxidized lipids that in turn induce exit of tumor cells from the state of dormancy.
Conclusion
The importance of lipid metabolism in tumorigenesis has been shown to be crucial for tumor growth and survival. The impact of lipid metabolism and oxidatively modified lipids from myeloid cells on immune suppression and tumor cell dormancy is an emerging field of study. The vast number of lipid signaling moieties as well as the different receptors and signaling pathways involved complicates understanding of these processes. Yet, this also provides a well of potential drug targets to improve response to chemotherapy or immunotherapy. The challenge is in identifying the nature of oxidized lipid species and particular receptors to target that are uniquely upregulated in the tumor and that do not have crucial roles in normal lipid metabolism.
Acknowledgment
This work was partially supported by NIH grants CA165065, CA243142 to VEK.
Footnotes
Conflict of interests statement
Authors declare no conflict of interest
References
- 1.Pathria P, Louis TL, Varner JA. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol 2019;40:310–27 [DOI] [PubMed] [Google Scholar]
- 2.Pinto ML, Rios E, Duraes C, Ribeiro R, Machado JC, Mantovani A, et al. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Frontiers in immunology 2019;10:1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 2021;21:485–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin Immunol 2018;35:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014;1319:47–65 [DOI] [PubMed] [Google Scholar]
- 7.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593–604 [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–86 [DOI] [PubMed] [Google Scholar]
- 9.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000;164:6166–73 [DOI] [PubMed] [Google Scholar]
- 10.Kwak T, Wang F, Deng H, Condamine T, Kumar V, Perego M, et al. Distinct Populations of Immune-Suppressive Macrophages Differentiate from Monocytic Myeloid-Derived Suppressor Cells in Cancer. Cell reports 2020;33:108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A, et al. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell 2019;177:1330–45 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 2005;14:425–31 [PubMed] [Google Scholar]
- 13.Yahaya MAF, Lila MAM, Ismail S, Zainol M, Afizan N. Tumour-Associated Macrophages (TAMs) in Colon Cancer and How to Reeducate Them. J Immunol Res 2019;2019:2368249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottcher JP, Reis e Sousa C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018;4:784–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner A, Ruffell B. Dendritic Cells and Cancer Immunity. Trends Immunol 2016;37:855–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol 2017;45:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deamer D The Role of Lipid Membranes in Life's Origin. Life (Basel) 2017;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segre D, Ben-Eli D, Deamer DW, Lancet D. The lipid world. Orig Life Evol Biosph 2001;31:119–45 [DOI] [PubMed] [Google Scholar]
- 19.Tyurina YY, Tyurin VA, Anthonymuthu T, Amoscato AA, Sparvero LJ, Nesterova AM, et al. "Redox lipidomics technology: Looking for a needle in a haystack". Chem Phys Lipids 2019;221:93–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000;28:27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyurina YY, St Croix CM, Watkins SC, Watson AM, Epperly MW, Anthonymuthu TS, et al. Redox (phospho)lipidomics of signaling in inflammation and programmed cell death. J Leukoc Biol 2019;106:57–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol 2015;15:511–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spickett CM. Formation of Oxidatively Modified Lipids as the Basis for a Cellular Epilipidome. Front Endocrinol (Lausanne) 2020;11:602771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni Z, Goracci L, Cruciani G, Fedorova M. Computational solutions in redox lipidomics - Current strategies and future perspectives. Free Radic Biol Med 2019;144:110–23 [DOI] [PubMed] [Google Scholar]
- 25.Broniec A, Klosinski R, Pawlak A, Wrona-Krol M, Thompson D, Sarna T. Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic Biol Med 2011;50:892–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Science translational medicine 2020;12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacci M, Lorito N, Smiriglia A, Morandi A. Fat and Furious: Lipid Metabolism in Antitumoral Therapy Response and Resistance. Trends in cancer 2021;7:198–213 [DOI] [PubMed] [Google Scholar]
- 28.Mohammadyani D, Tyurin VA, O'Brien M, Sadovsky Y, Gabrilovich DI, Klein-Seetharaman J, et al. Molecular speciation and dynamics of oxidized triacylglycerols in lipid droplets: Mass spectrometry and coarse-grained simulations. Free Radic Biol Med 2014;76:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016;16:553–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res 2015;3:1236–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, et al. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology 2017;6:e1344804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol 2016;1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai E, Zhang L, Li C. LOX-1+ PMN-MDSC enhances immune suppression which promotes glioblastoma multiforme progression. Cancer Manag Res 2019;11:7307–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HR, Park SM, Seo SU, Jung I, Yoon HI, Gabrilovich DI, et al. The Ratio of Peripheral Regulatory T Cells to Lox-1(+) Polymorphonuclear Myeloid-derived Suppressor Cells Predicts the Early Response to Anti-PD-1 Therapy in Patients with Non-Small Cell Lung Cancer. American journal of respiratory and critical care medicine 2019;199:243–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Si Y, Merz SF, Jansen P, Wang B, Bruderek K, Altenhoff P, et al. Multidimensional imaging provides evidence for down-regulation of T cell effector function by MDSC in human cancer tissue. Sci Immunol 2019;4 [DOI] [PubMed] [Google Scholar]
- 36.Cassetta L, Bruderek K, Skrzeczynska-Moncznik J, Osiecka O, Hu X, Rundgren IM, et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J Immunother Cancer 2020;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominguez GA, Polo AT, Roop J, Campisi AJ, Somer RA, Perzin AD, et al. Detecting Prostate Cancer Using Pattern Recognition Neural Networks With Flow Cytometry-Based Immunophenotyping in At-Risk Men. Biomark Insights 2020;15:1177271920913320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019;569:73–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adeshakin AO, Liu W, Adeshakin FO, Afolabi LO, Zhang M, Zhang G, et al. Regulation of ROS in myeloid-derived suppressor cells through targeting fatty acid transport protein 2 enhanced anti-PD-L1 tumor immunotherapy. Cell Immunol 2021;362:104286. [DOI] [PubMed] [Google Scholar]
- 40.Alicea GM, Rebecca VW, Goldman AR, Fane ME, Douglass SM, Behera R, et al. Changes in Aged Fibroblast Lipid Metabolism Induce Age-Dependent Melanoma Cell Resistance to Targeted Therapy via the Fatty Acid Transporter FATP2. Cancer Discov 2020;10:1282–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res 2007;67:4507–13 [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 2005;202:931–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He YM, Li X, Perego M, Nefedova Y, Kossenkov AV, Jensen EA, et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat Med 2018;24:224–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Perego M, Xiao Q, He Y, Fu S, He J, et al. Lactoferrin-induced myeloid-derived suppressor cell therapy attenuates pathologic inflammatory conditions in newborn mice. J Clin Invest 2019;129:4261–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagaraj S, Nelson A, Youn JI, Cheng P, Quiceno D, Gabrilovich DI. Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Research 2012;72:928–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A 2017;114:1117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015;42:419–30 [DOI] [PubMed] [Google Scholar]
- 48.Remmerie A, Scott CL. Macrophages and lipid metabolism. Cell Immunol 2018;330:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H, Han Y, Rodriguez Sillke Y, Deng H, Siddiqui S, Treese C, et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol Med 2019;11:e10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kume N, Moriwaki H, Kataoka H, Minami M, Murase T, Sawamura T, et al. Inducible expression of LOX-1, a novel receptor for oxidized LDL, in macrophages and vascular smooth muscle cells. Ann N Y Acad Sci 2000;902:323–7 [DOI] [PubMed] [Google Scholar]
- 51.Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 2014;15:846–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, et al. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res 2020;80:1438–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017;171:273–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol 2013;4:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nature chemical biology 2020;16:278–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piattini F, Matsushita M, Muri J, Bretscher P, Feng X, Freigang S, et al. Differential sensitivity of inflammatory macrophages and alternatively activated macrophages to ferroptosis. Eur J Immunol 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gu Z, Liu T, Liu C, Yang Y, Tang J, Song H, et al. Ferroptosis-Strengthened Metabolic and Inflammatory Regulation of Tumor-Associated Macrophages Provokes Potent Tumoricidal Activities. Nano Lett 2021;21:6471–9 [DOI] [PubMed] [Google Scholar]
- 58.Zeyda M, Saemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, et al. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J Biol Chem 2005;280:14293–301 [DOI] [PubMed] [Google Scholar]
- 59.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol 2005;174:5390–7 [DOI] [PubMed] [Google Scholar]
- 60.Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, Diaz-Delfin J, et al. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: comparison with other polyunsaturated fatty acids. J Leukoc Biol 2008;84:1172–82 [DOI] [PubMed] [Google Scholar]
- 61.Kong W, Yen JH, Vassiliou E, Adhikary S, Toscano MG, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis 2010;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 2010;16:880–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol 2012;12:557–69 [DOI] [PubMed] [Google Scholar]
- 64.Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nature communications 2017;8:2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol 2014;192:2920–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perrin-Cocon L, Diaz O, Andre P, Lotteau V. Modified lipoproteins provide lipids that modulate dendritic cell immune function. Biochimie 2012 [DOI] [PubMed] [Google Scholar]
- 67.Gardner JK, Mamotte CD, McGonigle T, Dye DE, Jackaman C, Nelson DJ. Lipid-laden partially-activated plasmacytoid and CD4(−)CD8alpha(+) dendritic cells accumulate in tissues in elderly mice. Immunity & ageing : I & A 2014;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gardner JK, Mamotte CD, Patel P, Yeoh TL, Jackaman C, Nelson DJ. Mesothelioma tumor cells modulate dendritic cell lipid content, phenotype and function. PloS one 2015;10:e0123563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng X, He Y, Huang J, Tao Y, Liu S. Metabolism of Dendritic Cells in Tumor Microenvironment: For Immunotherapy. Frontiers in immunology 2021;12:613492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015;161:1527–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ugolini A, Tyurin VA, Tyurina YY, Tcyganov EN, Donthireddy L, Kagan VE, et al. Polymorphonuclear myeloid-derived suppressor cells limit antigen cross-presentation by dendritic cells in cancer. JCI insight 2020;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muntjewerff EM, Meesters LD, van den Bogaart G. Antigen Cross-Presentation by Macrophages. Frontiers in immunology 2020;11:1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malinarich F, Duan K, Hamid RA, Bijin A, Lin WX, Poidinger M, et al. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. J Immunol 2015;194:5174–86 [DOI] [PubMed] [Google Scholar]
- 74.Ferreira GB, Vanherwegen AS, Eelen G, Gutierrez ACF, Van Lommel L, Marchal K, et al. Vitamin D3 Induces Tolerance in Human Dendritic Cells by Activation of Intracellular Metabolic Pathways. Cell Rep 2015;10:711–25 [DOI] [PubMed] [Google Scholar]
- 75.Svajger U, Obermajer N, Jeras M. Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology 2010;129:525–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ceccarelli SM, Chomienne O, Gubler M, Arduini A. Carnitine palmitoyltransferase (CPT) modulators: a medicinal chemistry perspective on 35 years of research. J Med Chem 2011;54:3109–52 [DOI] [PubMed] [Google Scholar]
- 77.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC cancer 2010;10:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Shen P, Zhang XC, Zhao MD, Zhang XG, Yang L. Efficacy and safety profile of celecoxib for treating advanced cancers: a meta-analysis of 11 randomized clinical trials. Clin Ther 2014;36:1253–63 [DOI] [PubMed] [Google Scholar]
- 79.Guo Q, Li Q, Wang J, Liu M, Wang Y, Chen Z, et al. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: A preliminary, three-center, clinical trial study. Medicine (Baltimore) 2019;98:e16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol 2020;21:298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu S, Chaudhary O, Rodriguez-Morales P, Sun X, Chen D, Zappasodi R, et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity 2021;54:1561–77 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeanne A, Schneider C, Martiny L, Dedieu S. Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front Pharmacol 2015;6:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Recasens A, Munoz L. Targeting Cancer Cell Dormancy. Trends Pharmacol Sci 2019;40:128–41 [DOI] [PubMed] [Google Scholar]
- 84.Quesnel B Tumor dormancy and immunoescape. APMIS 2008;116:685–94 [DOI] [PubMed] [Google Scholar]
- 85.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014;514:628–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakayama T, Sano T, Oshimo Y, Kawada C, Kasai M, Yamamoto S, et al. Enhanced lipid metabolism induces the sensitivity of dormant cancer cells to 5-aminolevulinic acid-based photodynamic therapy. Sci Rep 2021;11:7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oren Y, Tsabar M, Cuoco MS, Amir-Zilberstein L, Cabanos HF, Hutter JC, et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest 2012;122:178–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sosnoski DM, Norgard RJ, Grove CD, Foster SJ, Mastro AM. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin Exp Metastasis 2015;32:335–44 [DOI] [PubMed] [Google Scholar]