Abstract
Background
Caveolin-1 (CAV1) in cancer-associated fibroblasts (CAFs) has pro- or anti-tumourigenic effect depending on the cancer type. However, its effect in intrahepatic carcinoma (ICC) remains unknown. Therefore, this study aimed to investigate the relationship between CAV1 in CAFs and tumour-infiltrating lymphocyte (TIL) numbers or PD-L1 levels in ICC patients.
Methods
Consecutive ICC patients (n = 158) were enrolled in this study. The levels of CAV1 in CAFs, CD8 + TILs, Foxp3+ TILs and PD-L1 in cancer cells were analysed using immunohistochemistry. Their association with the clinicopathological factors and prognosis were evaluated. The correlation between these factors was evaluated.
Results
CAV1 upregulation in CAFs was associated with a poor overall survival (OS) (P < 0.001) and recurrence-free survival (P = 0.008). Clinicopathological factors were associated with high CA19-9 levels (P < 0.001), advanced tumour stage (P = 0.046) and lymph node metastasis (P = 0.004). CAV1 level was positively correlated with Foxp3+ TIL numbers (P = 0.01). There were no significant correlations between CAV1 levels and CD8 + TIL numbers (P = 0.80) and PD-L1 levels (P = 0.97). An increased CD8 + TIL number and decreased Foxp3+ TIL number were associated with an increased OS. In multivariate analysis, positive CAV1 expression in CAFs (P = 0.013) and decreased CD8 + TIL numbers (P = 0.021) were independent poor prognostic factors.
Conclusion
Cellular senescence, represented by CAV1 levels, may be a marker of CAFs and a prognostic indicator of ICC through Foxp3+ TIL regulation. CAV1 expression in CAFs can be a therapeutic target for ICC.
Subject terms: Cancer microenvironment, Biomarkers, Liver cancer
Background
Intrahepatic cholangiocarcinoma (ICC) is the second most common malignant tumour in the liver, with hepatocellular carcinoma being the first. ICC accounts for ~10–15% of all primary liver cancers and around 8–10% of all biliary tract cancers [1–3]. There has been an increased interest in ICC because of its increasing annual incidence and unsatisfactory treatment outcomes. Currently, the only curative treatment options for ICC are hepatectomy and liver transplantation [4, 5], but the indications are too rigorous. Furthermore, the high postoperative recurrence rate and limited efficacy of adjuvant chemotherapy result in a poor prognosis, with a 5-year overall survival (OS) rate of ≤ 30% [6, 7]. These findings suggest that a more effective treatment for ICC is needed. Hence, researchers must focus on new treatment modalities with promising potential.
The tumour microenvironment (TME) plays an important role in regulating cancer cell progression. Recently, it has received increasing attention. An example of a cell associated with the TME is the cancer-associated fibroblasts (CAFs). CAFs are the most prominent stromal components. Mechanistically, CAFs release proteolytic enzymes, growth factors and cytokines that influence the metabolism, transcriptome and signalling pathways of cancer cells [8–10]. The effect of CAFs on the prognosis of various cancer types have been studied [11–13]. However, the role of caveolin-1 (CAV1) expression in CAFs remains poorly understood. Recent studies found that CAV1 expressed in CAFs plays a crucial role in cellular senescence [14, 15]. CAV1 expression results in the release of specific dependent pro-stimulatory factors such as interleukin-6 (IL-6), which stimulate the growth of cancer cells. This mechanism was observed in pancreatic cancer and lung adenocarcinoma [16, 17]. However, in breast cancer, researchers found that Cav-1 expression was downregulated in human breast CAFs compared to that in the paired normal fibroblasts in the same patient [18]. The researchers attributed these results to the fact that the autophagic tumour stroma provided recycled nutrients for tumour growth by the destruction of CAV1 [19]. In this regard, CAV1 may play contrasting roles in tumour progression depending on the cancer types. However, the role of CAV1 in CAFs in ICC remains unknown.
Tumour-infiltrating lymphocytes (TILs) are another important component of the TME. They represent the immune response to tumour progression [20]. CD8 + TILs and forkhead box protein 3-positive (FoxP3 + ) TILs are two specific subsets of TILs that play important but opposite roles [21]. CD8 + TILs are critical immune cells in cancer immunity that kill cancer cells by triggering apoptosis, whereas Foxp3+ TILs are regulatory T cells (Tregs) with an immunosuppressive function of suppressing the proliferation and activation of CD8 + TILs. Several studies found that an increased number of CD8 + TILs was correlated with a good prognosis in colorectal, breast and ovarian carcinoma [22, 23]. Contrastingly, an increased number of Foxp3+ TILs was correlated with a poor prognosis in HCC, melanoma, breast and gastric cancer [24–26]. However, the relationship between TILs and ICC is controversial [27, 28].
Another associated component of the cancer pathway is the programmed death 1 (PD-L1) pathway, which functions as a checkpoint to limit the T-cell-mediated immune response mainly by suppressing the function of CD8 + TILs. Considering this mechanism, a recent study reported that the combination of TILs and PD-L1 expression could be used to classify the TME into four different types [29]. This classification system was validated in various cancer types [30, 31].
Interestingly, different cancer types exhibit different characteristics in this system. It is possible that more component interactions in such a complex system exist in the TME. A recent study found that the CAFs regulated the immunosuppressive TIL distribution in the TME through IL-6, which suggested that the pre-existing tumour immunity and the efficacy of conventional immunotherapies might be improved [32]. Interestingly, IL-6 is a specific pro-stimulatory factor modulated by CAV1 expression. Studies reported that PD-L1 expression in cancer cells was induced by inflammatory cytokines and activation of oncogenic pathways, such as PI3K, STAT3, MEK and Akt-mTOR. Furthermore, CAFs also secrete inflammatory cytokines and growth factors to activate oncogenic pathways in cancer cells. Thus, it is unknown whether the CAV1 expression in CAFs regulates TIL numbers and PD-L1 expression of cancer cells in ICC warrants. This needs further investigation.
To the best of our knowledge, this is the first study to investigate the relationship between cellular senescence of CAFs, which is represented by the expression levels of CAV1, and PD-L1 and TIL numbers, and clinical outcomes of patients with ICC. This is also the first study to explore whether CAV1 expression in CAFs regulates TIL numbers and PD-L1 expression.
Methods
Patients and specimens
A total of 158 ICC patients who underwent macroscopically curative resection at nine high-volume centres for liver surgery at Kumamoto or Fukuoka in Japan between February 2000 and May 2017 were enrolled in this multicenter study. Clinical data of all patients were prospectively collected from the database of each department. Clinical blood tests were performed preoperatively. Tumour-node metastasis (TNM) staging was performed according to the seventh edition of the American Joint Committee on Cancer. Paraffin-embedded sections were evaluated using immunohistochemistry. The pathological diagnosis was based on the Japanese classification of biliary tract cancers.
Follow-up of ICC patients was performed at 3- to 6-month intervals. During follow-ups, clinical examinations and enhanced computed tomography were conducted. Recurrence-free survival (RFS) was defined as the period from surgery to recurrence or death. OS was defined as the period from surgery to death.
Written informed consent was obtained from all patients and the study procedures were approved by the Institutional Review Board of Kumamoto University (No. 1412). We complied with the ethical standards of the relevant local and national committees on human experimentation and with the Declaration of Helsinki, 1964, and its later amendments.
Immunohistochemistry and evaluation for CAV1
Paraffin-embedded sections of ICC were deparaffinised and soaked in distilled water. Sample processing and immunohistochemistry were performed as described below. The endogenous peroxidase activity was blocked with 3% hydrogen peroxide. The sections were incubated with the diluted antibody of CAV1 (1:100 dilution; #3267; Cell Signaling Technology, Denver, MA, USA), and detection was performed using a biotin-free horseradish peroxidase enzyme-labelled polymer from the Envision Plus detection system (Dako, Tokyo, Japan). Positive reactions were visualised using a diaminobenzidine solution, which was followed by counterstaining with Mayer’s haematoxylin. The stromal part of colon cancers and vascular endothelial cells in ICC sections were used as positive controls. All IHC staining results in CAFs were independently scored by two blinded pathologists as follows. Staining intensity was scored as 0, 1, 2 or 3, corresponding to absent, weak, moderate or strong expression, respectively. The percentage of positive cells was scored as 0 for 0%, 1 for 1–25%, 2 for 26–50%, 3 for 51–75% or 4 for 76–100%, respectively. The two scores were multiplied to characterise CAV1 expression as negative (0) or positive (>0).
Immunohistochemistry and evaluation for TIL numbers and PD-L1 expression
Paraffin-embedded sections obtained from ICC were dewaxed in xylene and ethanol and autoclaved for 15 min in an antigen-retrieval solution to retrieve antigen epitopes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. For CD8 and FOXP3 staining, we used rabbit polyclonal anti-CD8 (1:200 dilution; ab4055; Abcam, Cambridge, UK) and anti-FOXP3 (1:200 dilution; ab54501; Abcam, Cambridge, UK) antibodies. For PD-L1 staining, we used the avidin–biotin complex method with a monoclonal rabbit anti- PD-L1 primary antibody (1:200 dilution, clone E1L3 N; Cell Signaling Technology, Tokyo, Japan). Tissue sections were incubated overnight at 4 °C with the primary antibodies. Then, they were incubated with secondary antibody and horseradish peroxidase-labelled polymer (EnVision1kit; Dako, Carpinteria, CA, USA) for 30 min at 25 °C, followed by incubation with 3,30-diaminobenzidine tetrahydrochloride (applied as a 0.02% solution containing 0.005% H2O2 in 0.05 M Tris-HCl; pH 7.6) at 25 °C for 5–15 min, and counterstained with haematoxylin. Stained slides were evaluated using light microscopy at ×200 by two researchers (LC and YK) who were blinded to the clinicopathological data of the patients. For CD8 and FOXP3 staining, serial sections from tumour blocks that contained the largest amount of tumour from each patient were evaluated. Positive cells in each 1-mm-diameter field were counted in three fields that contained a large number of cancer cells. They were expressed as the mean (cells per field) of triplicate counts. Immunohistochemical analysis of PD-L1 expression in cancer cells was performed using a two-way scoring system based on staining intensity and extent. Staining intensity was assigned a score of 0 (negative staining), 1 (weak staining), 2 (moderate staining) or 3 (strong staining). Staining extent was defined as the rate of positively stained cancer cells (0–100%). PD-L1 expression was scored as staining intensity × staining. Score >90 is defined as a positive expression of PD-L1 in cancer cells.
Statistical analyses
Continuous variables with normal distributions are expressed as mean ± standard deviation, whereas those without a normal distribution are expressed as median and range. The differences were assessed for significance using the Student’s t test or the Mann–Whitney U test. Categorical variables were evaluated using the chi-square or the Fisher’s exact tests, whichever was appropriate. The Cox proportional hazard regression analyses were performed to identify the predictors of prognosis. The multivariate analysis was performed for the clinicopathological factors with a P value < 0.05 in the univariate analysis. RFS and OS rates were estimated using the Kaplan–Meier method, and survival curves were compared using the log-rank test. A correlation analysis was assessed using the two-tailed Mann–Whitney U test. Statistical significance was set at P < 0.05. All tests were performed using the JMP software (version 10.0.2; SAS Institute Inc., Cary, NC, USA).
Results
CAV1 expression in CAFs was correlated with a poor prognosis in patients with ICC
There were 83 deaths and 108 recurrences among the 158 patients enrolled in the study. The median follow-up time was 2.9 years. We evaluated the CAV1 expression of CAFs in the cancer cell samples of patients with ICC, and a total of 78 patients showed a positive expression (Fig. 1). Then, we divided the patients into the positive and negative expression groups. The Kaplan–Meier analysis showed that a positive CAV1 expression was significantly correlated with a worse prognosis in terms of both OS (log rank, P < 0.001) and RFS (log rank, P = 0.008), compared to negative CAV1 expression (Fig. 2).
Fig. 1. Representative images of immunohistochemical staining for Caveolin-1 (CAV1), CD8, Foxp3 and PD-L1.
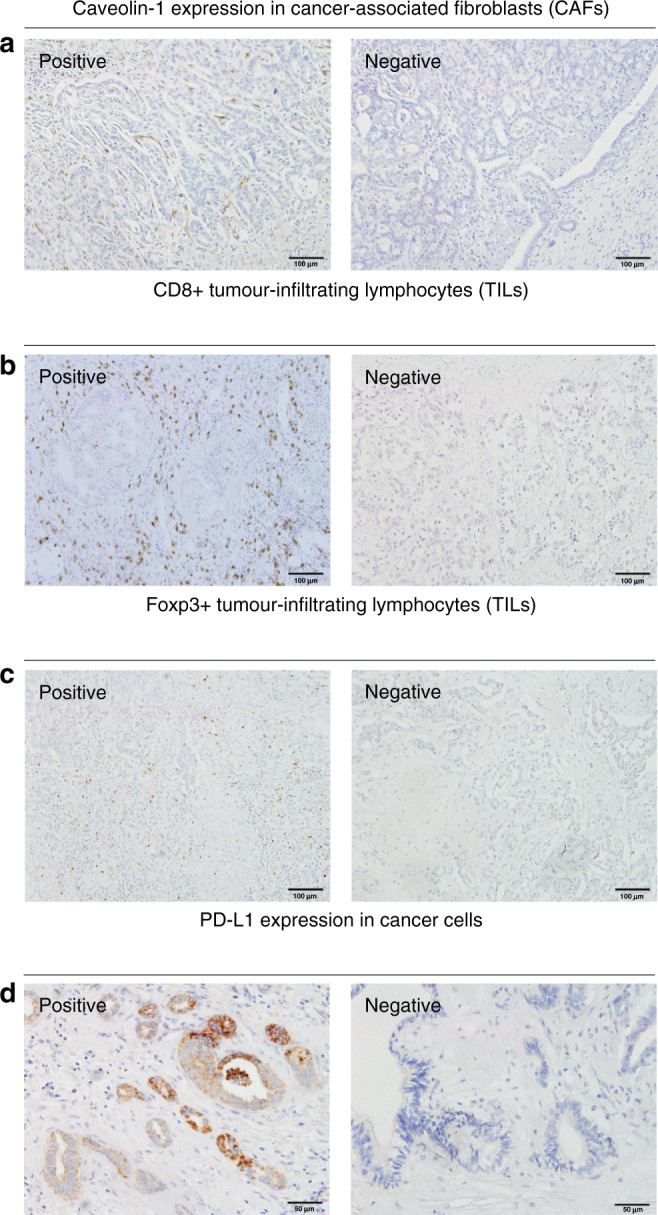
a Expression of CAV1 in cancer-associated fibroblasts (CAFs), b Expression of CD8+ in tumor-infiltrating lymphocytes (TILs), c Expression of Foxp3+ in TILs, d Expression of PD-L1 in cancer cells.
Fig. 2. The Kaplan–Meier curves for survival analysis in patients with intrahepatic cholangiocarcinoma according to CAV1 expression in CAFs.
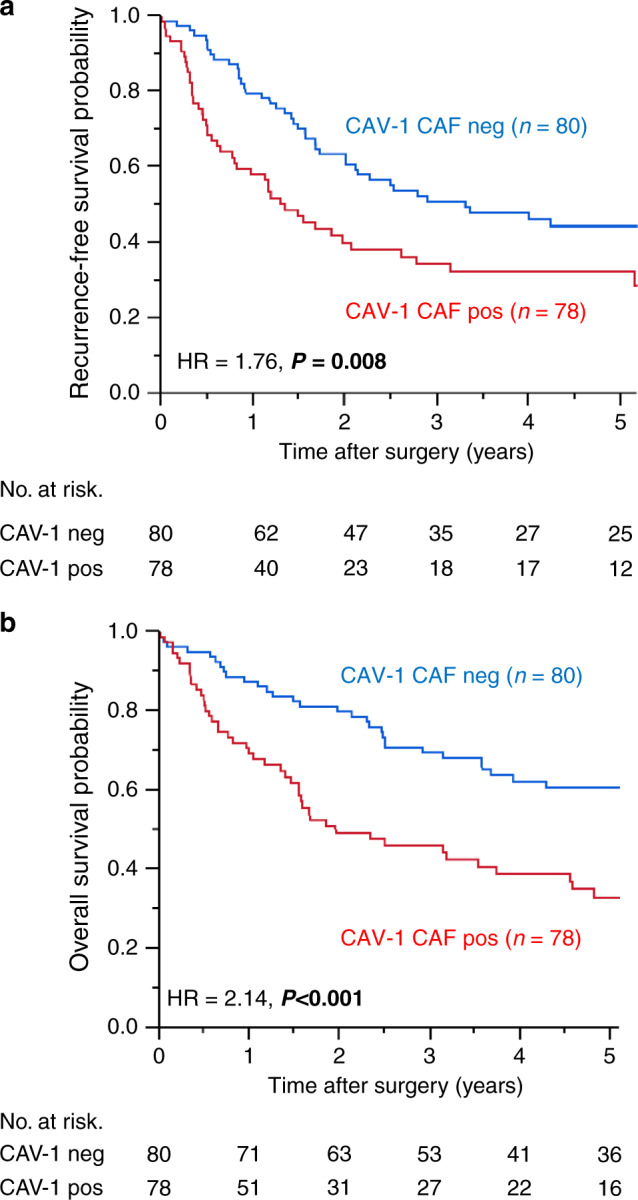
a Recurrence-free survival (RFS) and CAV1 expression, b Overall survival (OS) and CAV1 expression. pos positive, neg negative, HR hazard ratio.
Association between CAV1 expression in CAFs and clinicopathological characteristics and prognosis
Table 1 summarises the clinicopathological characteristics of the CAV1-positive and the CAV1-negative groups. There were no significant differences in age, gender, body mass index, carcinoembryonic antigen, platelet count, total bilirubin level, albumin level, tumour size, operating time, tumour differentiation and postoperative chemotherapy between the CAV1-positive and CAV1-negative groups. However, the CAV1-positive group was significantly associated with high CA19-9 levels (P < 0.001), advanced tumour stage (P = 0.046) and more lymph node metastases (P = 0.004).
Table 1.
Clinicopathological features and CAV1 expression in CAFs.
| Total (n = 158) | CAV1 expression in CAFs | p value | ||
|---|---|---|---|---|
| Negative (n = 80) | Positive (n = 78) | |||
| Age (y) | 67.3 ± 10.3 | 65.8 ± 10.7 | 68.7 ± 9.7 | 0.080 |
| Sex | 0.434 | |||
| Male | 88 (55.7%) | 47 (58.7%) | 41 (52.6%) | |
| female | 70 (44.3%) | 33 (41.3%) | 37 (47.4%) | |
| BMI | 22.8 ± 3.2 | 22.4 ± 3.3 | 23.2 ± 3.1 | 0.117 |
| CEA (ng/mL) | 3.0 (0.6–1692) | 2.9 (0.6–1692) | 3.1 (0.7–150.1) | 0.123 |
| CA19-9 (ng/mL) | 36.0 (0–74079) | 22.8 (0.1–36230) | 54.1 (0–74079) | 0.001 |
| PLT | 19.8 (5.1–296) | 19.6 (5.4–159) | 20.7 (5.1–296) | 0.953 |
| Total bilirubin (mg/dL) | 0.7 (0.1–13.4) | 0.7 (0.1–1.6) | 0.7 (0.2–13.4) | 0.548 |
| Albumin (g/dL) | 4.1 (2.6–5.1) | 4.1 (3.1–5.1) | 4.1 (2.6–4.9) | 0.615 |
| Tumor size (mm) | 39 (1–180) | 35 (1–120) | 40 (2.5–180) | 0.337 |
| pT | 0.046 | |||
| T0-2 | 109 (69.0%) | 61 (76.3%) | 48 (61.5%) | |
| T3,4 | 49 (31.0%) | 19 (23.7%) | 30 (38.5%) | |
| pN | 0.004 | |||
| No | 128 (81.0%) | 72 (90.0%) | 56 (71.8%) | |
| Yes | 30 (19.0%) | 8 (10.0%) | 22 (28.2%) | |
| Operating time (min) | 369.9 ± 131.6 | 385.7 ± 137.6 | 353.7 ± 123.8 | 0.126 |
| Blood loss (ml) | 467.5 (2–5140) | 437.5 (5–2897) | 560 (29–5140) | 0.105 |
| Differentiation | 0.337 | |||
| Well/Mod | 107 (67.7%) | 57 (71.3%) | 50 (64.1%) | |
| Poor | 51 (32.3%) | 23 (28.7%) | 28 (35.9%) | |
| Postoperative chemotherapy | 0.523 | |||
| No | 117 (74.1%) | 61 (76.3%) | 56 (71.8%) | |
| Yes | 41 (25.9%) | 19 (23.7%) | 22 (28.2%) | |
CAV1 caveolin-1, CAFs cancer-associated fibroblasts, BMI body mass index, CEA carcinoembryonic antigen, CA19-9 carbohydrate antigen 19-9, pT pathological tumor stage, pN pathological nodal stage, Mod moderately, Poor; poorly,
Survival analyses of the interaction between CAV1 expression in CAFs and other characteristics
We further explored whether the role of CAV1 expression in CAFs on OS of the patient was affected by the clinicopathological characteristics. The effect of CAV1 expression in CAFs was not significantly affected by sex, CA19-9, tumour size, pT, pN, tumour differentiation and postoperative chemotherapy (Fig. 3). However, we found a notable modifying effect of age, body mass index, and carcinoembryonic antigen levels on the relationship between CAV1 expression in CAFs and OS rate (P for interaction <0.05). Interestingly, upregulation of CAV1 expression in CAFs was not related to the prognosis of patients with carcinoembryonic antigen levels ≥5 ng/mL.
Fig. 3. The relationship between CAV1 expression in CAFs in ICC and the OS.
Loge(HRs) plots of the OS rate in CAV1-positive and CAV1-negative groups are shown.
Association between CAV1 expression in CAFs and TIL numbers and PD-L1 expression in cancer cells
We investigated the association between CAV1 expression in CAFs, TIL numbers and PD-L1 expression in cancer cells. Immunohistochemical staining of CD8 + TILs, Foxp3+ TILs and PD-L1 is shown in Fig. 1. The number of Foxp3+ TILs in the CAV1-positive group was significantly higher than that in the CAV1-negative group (P = 0.01). However, we found no significant correlation between the number of CD8 + TILs and the CAV1 in CAFs (P = 0.80). PD-L1 expression in cancer cells did not correlate with CAV1 expression (P = 0.97) (Fig. 4).
Fig. 4. Correlation analysis between CAV1 expression in CAFs and CD8+ TILs, Foxp3+ TILs, and PD-L1 expression in cancer cells.

a CAV1 expression in CAFs and CD8+ TILs, b CAV1 expression in CAFs and Foxp3+ TILs, c CAV1 expression in CAFs and PD-L1 expression in cancer cells.
TIL numbers were correlated with OS in patients with ICC
We assessed the prognostic impact of TILs in patients with ICC. These patients were divided into the low- and high- number groups of CD8 + and Foxp3+ TILs based on the cut-off value. The Kaplan–Meier analysis showed that the high-number CD8 + TIL group was significantly correlated with a better OS (P = 0.04). In contrast, the high Foxp3+ TILs were significantly correlated with a worse OS (P = 0.02) (Fig. 5).
Fig. 5. The Kaplan–Meier curves for OS analysis in patients with intrahepatic cholangiocarcinoma according to CD8+ and Foxp3+ TILs count.

a OS and CD8+ TILs count, b OS and Foxp3+ TILs count. HR hazard ratio.
Multivariate COX regression analysis of OS in patients with ICC
The univariate analysis showed that female sex, tumour size >5 cm, lymph node metastasis, low number of CD8 + TILs, high number of Foxp3+ TILs and positive expression of CAV1 in CAFs were poor prognostic factors of OS (P < 0.05). Then we conducted a multivariate analysis, which revealed that female sex (HR, 1.693; 95% CI, 1.093–2.620; P = 0.018), tumour size >5 cm (HR, 2.160; 95% CI, 1.369–3.407; P = 0.001), low number of CD8 + TILs (HR, 1.692; 95% CI, 1.081–2.646; P = 0.021) and positive expression of CAV1 in CAFs (HR, 1.791; 95% CI, 1.129–2.841; P = 0.013) were independent poor prognostic factors for OS (Table 2).
Table 2.
Univariate and multivariate analysis for overall survival in patients with ICC.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age ≥70 years | 1.354 | 0.878–2.087 | 0.170 | |||
| Female | 1.560 | 1.012–2.406 | 0.044 | 1.693 | 1.093–2.620 | 0.018 |
| BMI ≥22 | 1.334 | 0.854–2.083 | 0.205 | |||
| CEA ≥5 ng/ml | 1.432 | 0.861–2.381 | 0.167 | |||
| CA19-9 ≥ 37 U/ml | 1.474 | 0.955–2.275 | 0.080 | |||
| Platelet < 10 | 0.738 | 0.321–1.696 | 0.474 | |||
| Total bilirubin >1 mg/dl | 0.870 | 0.449–1.686 | 0.680 | |||
| Albumin <3.5 g/dl | 1.682 | 0.808–3.502 | 0.164 | |||
| Tumour size >50 mm | 2.124 | 1.359–3.320 | 0.001 | 2.160 | 1.369–3.407 | 0.001 |
| pT3,4 | 1.221 | 0.766–1.946 | 0.407 | |||
| pN1 | 1.867 | 1.101–3.168 | 0.021 | |||
| Operating time >500 min | 0.791 | 0.428–1.461 | 0.453 | |||
| Blood loss >400 ml | 1.456 | 0.926–2.288 | 0.103 | |||
| Poorly differentiation | 1.291 | 0.819–2.038 | 0.272 | |||
| Postoperative chemotherapy (−) | 1.371 | 0.855–2.199 | 0.190 | |||
| PD-L1 positive in ICC | 1.208 | 0.735–1.985 | 0.456 | |||
| CD8 + TILs-negative | 1.653 | 1.273–2.545 | 0.023 | 1.692 | 1.081–2.646 | 0.021 |
| Foxp3 + TILs-positive | 1.683 | 1.090–2.598 | 0.017 | |||
| CAV1 positive | 2.142 | 1.379–3.327 | 0.001 | 1.791 | 1.129–2.841 | 0.013 |
ICC intrahepatic cholangiocarcinoma, BMI body mass index, CEA carcinoembryonic antigen, CA19-9 carbohydrate antigen 19-9, pT pathological tumour stage, pN pathological nodal stage, TILs tumour-infiltrating lymphocytes, CAV1 caveolin-1.
Discussion
The role of TME has been increasingly investigated. In the TME, there is reciprocal crosstalk between tumour cells, stromal fibroblasts, inflammatory cells, immune cells and the extracellular matrix existed. CAV1, as a marker of senescent CAFs, plays a two-pronged role in tumour progression depending on the type of tumour. However, its role in ICC remains unknown. Our present study is the first to show that a positive expression of CAV1 in CAFs correlates with a poor OS and RFS in patients with ICC. Patients with a positive CAV1 expression showed elevated CA19-9 levels, advanced tumour stage and increased lymph node metastasis as compared to those seen in patients with a negative CAV1 expression. In addition, we examined the relationship between CAV1 expression and TIL numbers and PD-L1 expression of cancer cells. To the best of our knowledge, this is also the first study to show that CAV1 expression in CAFs is separately associated with Foxp3+ TIL numbers. The combination of CAV1 expression in CAFs and CD8 + TIL numbers accurately predicted the prognosis of patients with ICC. These findings provide a potentially new predictive marker for the OS of ICC and a therapeutic target for patients with ICC.
CAV1, a member of the caveolin family, is a scaffold and structural protein [33] that is widely expressed in epithelial cells, endothelial cells, adipocytes, fibroblasts and CAFs. It is located in the cell membrane and functions through endocytosis, extracellular matrix organisation, cholesterol distribution and signal transduction [34].
In recent years, the association between CAV1 expression in CAFs and tumour progression has been studied in several types of cancer [16, 35, 36]. These studies showed that CAV1 expression in CAFs resulted in two completely opposite outcomes. Numerous cancer studies showed that a negative or downregulation of CAV1 expression in CAFs was correlated with a poor prognosis in ovarian, gastric, breast, lung and prostate cancer [16, 34, 37, 38]. Mechanistically, the loss of CAV1 in CAFs promotes the activation of CAFs through the transforming growth factor-β (TGF-β) pathway, oxidative stress, autophagy and glycolysis, which results in the remodelling of the TME and tumour progression [39]. For instance, a loss of CAV1 expression in CAFs can predict early tumour recurrence, lymph node metastasis and tamoxifen resistance in breast cancer [35, 40]. The possible mechanism involves oxidative stress, which induces autophagy and leads to the lysosomal degradation of CAV1. Moreover, in the destruction of CAV1, the autophagic tumour stroma provides recycled nutrients for tumour growth [19]. Moreover, some studies reported that a high expression of CAV1 in CAFs was associated with a poor prognosis in renal cell carcinoma, colon cancer and metastatic melanoma [41]. Similarly, our previous study found that a high CAV1 expression in CAFs was associated with a poor prognosis in patients with pancreatic cancer [17]. In vitro experiments demonstrated that the downregulation of CAV1 in CAFs by siRNA reduced the invasion of MIAPaCa-2 pancreatic cancer cells in co-culture assays with CAF-conditioned medium. In this study, the results were largely consistent with those of the previous reports. We found that the positive expression of CAV1 in CAFs was significantly correlated with a poor prognosis in patients with ICC. The pro-tumourigenic mechanism in ICC may be induced by CAV1-mediated senescence in CAFs. Krtolica et al. [42] firstly developed the SASP model, in which senescent stromal cells stimulated the growth of pre-neoplastic and neoplastic cells. The specific growth factors and cytokines of SASP were further released by CAV1-regulated senescent fibroblasts, thereby resulting in tumour progression. However, only a few studies observed this phenomenon [16, 17]. For example, a previous study showed that the growth of PC3 prostate cancer cells was stimulated by a conditioned medium derived from the wild-type senescent mouse embryonic fibroblasts but not from the CAV1-null mouse embryonic fibroblasts. After oxidative stress, IL-6 mRNA expression and protein levels were upregulated in senescent wild-type mouse embryonic fibroblasts and in the conditioned medium. CAV1 inhibited Sirl to activate the IL-6 promoter, which induced the upregulation of IL-6 expression [43]. Our previous study had nearly the same results with pancreatic cancer [17].
TILs, which participated in innate and adaptive immunity, are another important component of the TME. Among them, CD8 + T cells and Foxp3 + T cells play a crucial but contradictory role in cancer immunity. CD8 + T cells originate from the bone marrow, differentiate in the thymus medulla, settle in lymph nodes, and migrate to the target tissue to kill cancer cells [44]. In contrast, Foxp3 is a key marker of Tregs, which exert an immunosuppressive function. Previous studies showed that increased CD8 + TIL number was associated with a favourable prognosis in patients with colorectal and ovarian cancers, whereas increased Tregs count was inversely correlated with a poor prognosis in pancreatic cancer [22–26, 29]. Consistently, our previous study found that low CD8 + TILs and high Tregs counts were significantly associated with a poor OS in extrahepatic cholangiocarcinoma [45]. However, the role of CD8 + TILs and Foxp3+ TILs in ICC remains poorly understood. A study by Asahi et al. [28] classified the distribution of TILs centred around the tumour cell into three areas: the outer border, the inner border, and the intra-tumour areas. They found that both CD8 + and Foxp3+ TILs in the inner border and in the intra-tumour areas were not associated with the prognosis of patients with ICC. In addition, Gu et al. obtained nearly the same results [27]. However, both studies only had a small number of patients. In the present study, a high number of CD8 + TILs was significantly correlated with better OS and RFS. Moreover, a high number of Foxp3+ TILs was significantly correlated with worse OS. In addition, we found that CAV1 expression in CAFs was positively correlated with Foxp3+ TILs, which suggested that these could be potential targets for immune therapy. Mechanistically, several recent studies showed that CAFs regulated TILs through the CXCL12 signalling and recruitment of myeloid-derived suppressor cells [46–48]. A previous study by Takuya et al. [32] also showed that CAFs regulated the immunosuppressive TIL population in TME through IL-6. CAFs strongly affected the distribution of CD8 + TILs and Foxp3+ TILs, resulting in a lower number of CD8 + TILs and a higher number of Foxp3+ TILs in the intra-tumoural sites than in the peri-tumoural sites. In vivo and in vitro experiments showed that CAFs regulated TILs in intra-tumoural tissues and promoted tumour progression by secreting high levels of IL-6. Furthermore, IL-6 blockage remodelled TILs in the TME and reversed the effect of CAFs. As discussed, IL-6 was a CAV1 specific pro-tumourigenic factor secreted by senescent CAFs [16]. Hence, we speculated that CAV1 in CAFs might be pro-tumourigenic through the regulation of TILs by IL-6.
Further studies are needed to validate our findings and to investigate the underlying mechanism. Notably, this study indicated that CAV1, a specific marker of CAFs, provided the ability to attenuate the immunosuppression in TME for immune therapies, such as CAR-T-cell and cancer vaccine therapies by regulating the TILs, which could be a potential new therapeutic target.
We acknowledge that this study has some limitations. First, ICC is a very rare disease; therefore, our sample size limits the validity of our results. A larger number of cases and mechanism research is needed to validate our results. Second, the possible mechanism by which CAV1 in CAFs affects TILs is based on our speculation. However, we did not find a significant relationship between CAV1 expression in CAFs and CD8 + TILs. Therefore, other mechanisms may also exist. Third, to the best of our knowledge, this is the first study to report that CAV1 in CAFs is positively correlated with Foxp3+ TILs in ICC. However, our study consisted solely of IHC; therefore, this phenomenon should be confirmed by in vitro and in vivo studies. In addition, whether this phenomenon exists in other cancer types warrant further study.
In conclusion, the expression of CAV1, a marker of CAF senescence, plays an important role in the prognosis of patients with ICC through the regulation of Foxp3+ TIL numbers. CAV1 expression in CAFs and CD8 + TIL numbers can predict the OS of patients with ICC after curative resection. CAV1 can also be a possible new therapeutic target.
Supplementary information
Author contributions
KY and YT contributed to the immunostaining of samples. LC, KY and YY contributed to the evaluation of immunostaining. LC and YY contributed to the statistical analyses. YY, KK, YT, FK, SK, IY, TH, MN, HM and BH contributed to obtaining surgical specimens. LC and YY contributed to writing a paper. BH contributed to revising a paper. All authors approved the final version of the manuscript.
Funding information
Not applicable.
Ethics approval and consent to participate
Ethical approval was provided by the Institutional Review Board of Kumamoto University (number 1412). Consent was obtained from the patients and their families according to Institutional Review Board protocols.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: Due to a table error.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chuan LAN, Yuki Kitano.
Change history
12/20/2021
A Correction to this paper has been published: 10.1038/s41416-021-01615-3
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01569-6.
References
- 1.Patel N, Benipal B. Incidence of cholangiocarcinoma in the USA from 2001 to 2015: a US Cancer statistics analysis of 50 states. Cureus. 2019;11:e3962. doi: 10.7759/cureus.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79.. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:101–4. doi: 10.21037/hbsn.2017.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62.. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldaracena N, Gorgen A, Sapisochin G. Current status of liver transplantation for cholangiocarcinoma. Liver Transplant. 2018;24:294–303. doi: 10.1002/lt.24955. [DOI] [PubMed] [Google Scholar]
- 6.Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–69. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 7.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–74. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 9.Mahale J, Smagurauskaite G, Brown K, Thomas A, Howells LM. The role of stromal fibroblasts in lung carcinogenesis: a target for chemoprevention? Int J Cancer. 2016;138:30–44. doi: 10.1002/ijc.29447. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Ishii G, Goto K, Neri S, Hashimoto H, Yoh K, et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin Cancer Res. 2015;21:642–51. doi: 10.1158/1078-0432.CCR-14-0846. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 12.Harper J, Sainson RC. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin Cancer Biol. 2014;25:69–77. doi: 10.1016/j.semcancer.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–46. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volonte D, Vyas AR, Chen C, Dacic S, Stabile LP, Kurland BF, et al. Caveolin-1 promotes the tumor suppressor properties of oncogene-induced cellular senescence. J Biol Chem. 2018;293:1794–809. doi: 10.1074/jbc.M117.815902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamao T, Yamashita YI, Yamamura K, Nakao Y, Tsukamoto M, Nakagawa S, et al. Cellular senescence, represented by expression of caveolin-1, in cancer-associated fibroblasts promotes tumor invasion in pancreatic cance. Ann Surg Oncol. 2019;26:1552–9. doi: 10.1245/s10434-019-07266-2. [DOI] [PubMed] [Google Scholar]
- 18.Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Minkeu A, et al. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol Ther. 2008;7:1212–25. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capparelli C, Guido C, Whitaker-Menezes D, Bonuccelli G, Balliet R, Pestell TG, et al. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle (Georget, Tex). 2012;11:2285–302. doi: 10.4161/cc.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154–66. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma C, Dong X. Colorectal cancer-derived Foxp3(+) IL-17(+) T cells suppress tumour-specific CD8+ T cells. Scand J Immunol. 2011;74:47–51. doi: 10.1111/j.1365-3083.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 22.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4. [PubMed] [Google Scholar]
- 23.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arigami T, Uenosono Y, Ishigami S, Matsushita D, Hirahara T, Yanagita S, et al. Decreased density of CD3+ tumor-infiltrating lymphocytes during gastric cancer progression. J Gastroenterol Hepatol. 2014;29:1435–41. doi: 10.1111/jgh.12551. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8+ cytotoxic Tcell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–55. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 26.Teng F, Mu D, Meng X, Kong L, Zhu H, Liu S, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res. 2015;5:2064–74. [PMC free article] [PubMed] [Google Scholar]
- 27.Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, et al. Intratumoral IL-17+ cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19:2506–14. doi: 10.1245/s10434-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 28.Asahi Y, Hatanaka KC, Hatanaka Y, Kamiyama T, Orimo T, Shimada S, et al. Prognostic impact of CD8+ T cell distribution and its association with the HLA class I expression in intrahepatic cholangiocarcinoma. Surg Today. 2020;50:931–40. doi: 10.1007/s00595-020-01967-y. [DOI] [PubMed] [Google Scholar]
- 29.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yagi T, Baba Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg. 2019;269:471–8. doi: 10.1097/SLA.0000000000002616. [DOI] [PubMed] [Google Scholar]
- 31.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, et al. Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T cells via IL6 in the tumor microenvironment. Clin Cancer Res. 2018;24:4820–33. doi: 10.1158/1078-0432.CCR-18-0205. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–22.. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 34.Shan-Wei W, Kan-Lun X, Shu-Qin R, Li-Li Z, Li-Rong C. Overexpression of caveolin-1 in cancer-associated fibroblasts predicts good outcome in breast cancer. Breast Care. 2012;7:477–83. doi: 10.1159/000345464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–17.. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiavarina B, Whitaker-Menezes D, Migneco G, Martinez-Outschoorn UE, Pavlides S, Howell A, et al. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: autophagy drives compartment-specific oncogenesis. Cell Cycle. 2010;9:3534–51.. doi: 10.4161/cc.9.17.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen XJ, Zhang H, Tang GS, Wang XD, Zheng R, Wang Y, et al. Caveolin-1 is a modulator of fibroblast activation and a potential biomarker for gastric cancer. Int J Biol Sci. 2015;11:370–9. doi: 10.7150/ijbs.10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8:2420–4. [DOI] [PMC free article] [PubMed]
- 39.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Witkiewicz AK, Kline J, Queenan M, Brody JR, Tsirigos A, Bilal E, et al. Molecular profiling of a lethal tumor microenvironment, as defined by stromal caveolin-1 status in breast cancers. Cell Cycle. 2011;10:1794–809. doi: 10.4161/cc.10.11.15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–63. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krtolica A, Campisi J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int J Biochem Cell Biol. 2002;34:1401–14.. doi: 10.1016/s1357-2725(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 43.Volonte D, Zou H, Bartholomew JN, Liu Z, Morel PA, Galbiati F. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6). J Biol Chem. 2015;290:4202–14. doi: 10.1074/jbc.M114.598268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nolz JC. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell Mol Life Sci. 2015;72:2461–73. doi: 10.1007/s00018-015-1835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018;118:171–80.. doi: 10.1038/bjc.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 48.Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 2016;76:4124–35. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



