Abstract
Objective:
We evaluated the safety of maximal cardiopulmonary exercise testing (CPET) in individuals with sickle cell disease (SCD). Maximal CPET using gas exchange analysis is the gold standard for measuring cardiopulmonary fitness in the laboratory, yet its safety in the SCD population is unclear.
Design:
Systematic review.
Data sources:
Systematic search of Medline (PubMed), EMBASE, Cochrane, ClinicalTrials.gov and professional society websites for all published studies and abstracts through December 2020.
Eligibility criteria for selecting studies:
Two reviewers independently extracted data of interest from studies that assessed safety outcomes of maximal CPET in children and adults with SCD. A modified version of the Newcastle-Ottawa Scale (NOS) was used to assess for risk of bias in studies included.
Results:
In total, 24 studies met inclusion/exclusion criteria. Adverse events were reported separately or as part of study results in 36/939 (3.8%) of participants with SCD undergoing maximal CPET in studies included. Most adverse events were related to transient ischemic changes on electrocardiogram (ECG) monitoring or oxygen desaturation during testing that did not result in arrhythmias or other complications. Only 4/939 (0.43%) participants experienced pain events due to maximal CPET.
Conclusion:
Maximal CPET appears to be a safe testing modality in children and adults with SCD and can be used to better understand the physiologic basis of reduced exercise capacity as well as guide exercise prescription in this population. Some studies did not focus on reporting adverse events related to exercise testing or failed to mention safety monitoring, which contributed to risk of bias.
Keywords: sickle cell anemia, exercise physiology, CPET, stress testing, cardiopulmonary fitness
INTRODUCTION
Sickle cell disease (SCD) affects approximately 100,000 individuals in the United States and millions more worldwide.(1, 2) SCD refers to a group of inherited blood disorders characterized by the production of sickle hemoglobin (HbS). The polymerization of Hb S results in red blood cell sickling, vaso-occlusion and hemolysis. Such pathophysiology leads to complications such as chronic anemia, severe pain episodes and end organ damage like cardiopulmonary disease, which are important causes of increased morbidity and mortality in SCD. In addition to these complications, physical limitations from stroke and avascular necrosis, decreased physical activity, and reduced cardiopulmonary fitness are common findings in individuals with SCD.(3-5) Although the exact etiology of reduced cardiopulmonary fitness in individuals with SCD is not clear, chronic anemia, cardiopulmonary disease and low physical activity levels from a sedentary lifestyle represent contributing factors.(6-8)
Reduced cardiopulmonary fitness represents one of the most important predictors of all-cause mortality in the general population.(9-11) Although the benefits of regular physical activity and exercise in individuals who are healthy or have chronic medical conditions are well-accepted, evidence-based exercise guidelines do not exist for children and adults with SCD.(12) The absence of exercise guidelines in SCD may in part be explained by the paucity of data that establish safety of moderate to high intensity physical activity and exercise in this population. Several pathophysiologic consequences of moderate to vigorous exercise, including the pro-inflammatory response, oxygen desaturation, dehydration and metabolic acidosis, could in theory trigger sickling and cause disease-related complications such as vaso-occlusive pain crises.(13, 14) Such unsubstantiated concerns in SCD, however, may lead to unnecessary restrictions on regular exercise placed by parents, healthcare providers or patients themselves.
Cardiopulmonary exercise testing (CPET) with gas exchange measurements is considered the gold standard for assessing cardiopulmonary fitness and is widely used in both healthcare and non-healthcare settings. Maximal CPET protocols using cycle ergometry or treadmill exercise include incremental increases in exercise workload until volitional exhaustion and as such, expose individuals to exercise at moderate to high intensities. Data derived from exercise testing can also provide useful prognostic information and guide exercise prescription. Although some studies have relied on maximal CPET to evaluate cardiopulmonary fitness in children and adults with SCD, its safety in the SCD population has not been well studied. The purpose of this systematic review is to determine the safety of maximal CPET in individuals with SCD and evaluate the association between maximal CPET and potential CPET-related adverse events.
METHODS
Search Strategy
This systematic review was conducted following standard procedures and exempt from review by the Institutional Review Board. Per PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, the review was registered with PROSPERO [CRD42020157218] and confirmed to be a novel project. A medical librarian developed a search strategy to identify all studies involving maximal intensity exercise testing in individuals of all ages with SCD, including all genotypes. Databases including EMBASE, Cochrane, Medline (PubMed), ProQuest, and ClinicalTrials.gov, as well as relevant conference abstracts and professional society websites, were initially searched for studies written in English and published to date through December 2020 using relevant keywords and Medical Subject Headings (MeSH) (Supplemental Table 1). Given the worldwide distribution of SCD, we also searched the Brazilian database SCIELO, which did not reveal additional relevant studies.
Study Selection
Studies were selected based on predetermined inclusion and exclusion criteria. Two reviewers independently screened the titles and abstracts of the studies and excluded studies from consideration if they were written in a language other than English, included animals or only patients with sickle cell trait (SCT), or used sub-maximal or functional exercise testing to predict aerobic fitness, including the 6-minute walk test. Maximal CPET was defined as exercise testing by either cycle ergometry or treadmill using a ramp protocol (i.e., incremental increase in workload) until either volitional exhaustion occurred or other pre-defined criteria of maximal or peak effort (e.g., using heart rate or respiratory exchange ratio) were met. For studies that met criteria for potential inclusion for analysis, full articles were pulled and reviewed.
Data Analysis
The two reviewers independently collected information on study type, year of publication, sample size, SCD genotype of participants, age of participants, exercise protocol, documentation of adverse event (AE) monitoring, and description of any adverse events (AEs). Any discrepancies in data extraction were resolved by discussion between the reviewers. AEs were defined as any unintended or unfavorable symptoms or events temporarily associated with undergoing maximal CPET, including but not limited to vaso-occlusive pain episodes, acute chest syndrome or major changes in signs/symptoms observed during CPET monitoring (e.g., ECG changes, oxygen saturation, etc.). Serious AEs were defined as AEs that resulted in hospitalization, a persistent incapacitated state or death. We considered AE monitoring to be documented if specified in the study methodology or presumed if authors reported the absence of specific AEs. The two reviewers also independently assessed for risk of bias in the included studies using a modified version of the Newcastle-Ottawa Scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). With this scale, the reviewers assigned a composite star rating to each study based on selection, comparability, and outcome (Table 1). Details of the breakdown of composite scores for each study are also presented elsewhere (Supplemental Table 2).
Table 1.
Description of studies included in systematic review and AEs reported during exercise testing in patients with SCD
| Study | Pub Year |
Study Design | Study Description |
Number Undergoing CPET (% Female) |
Genotype | Children vs. Adults |
Exercise Protocol |
AE Reporting Documented for Maximal CPET |
AEs Reported |
NOS Rating (max 8) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aessopos et al. | 2001 | Observational | Myocardial perfusion during exercise | 30(57%) | Hb S/β0 or Hb S/β+ thalassemia | Adults | Treadmill | No | None | 7 |
| †Ako et al. | 2016 | Observational | MR-augmented CPET to evaluate exercise responses | 14(Not available) | Hb SS only | Adults | Cycle | Yes | None | 7 |
| Alpert et al. | 1984 | Observational | Longitudinal changes in hemodynamic and ECG response during exercise | 74(Not available) | Hb SS only | Children | Cycle | Yes | Ischemic ECG changes observed in 10 subjects during follow-up CPET | 7 |
| Alpert et al. | 1981 | Observational | Hemodynamic and ECG responses during exercise | 47(40%) | Hb SS only | Children | Cycle | Yes | Ischemic ECG changes observed in 7 subjects during CPET | 7 |
| Alsaied et al. | 2018 | Observational | Relationship between diastolic dysfunction and exercise impairment | 20(60%) | Hb SS only | Both | Cycle | Yes | None | 8 |
| Ameringer et al. | 2019 | Observational | Perceptual responses and gas exchange thresholds during exercise | 22(36%) | Hb SS or Hb S/β0 thalassemia | Both | Cycle | Yes | Pain episode in 1 subject within 24 hours of CPET | 7 |
| Anthi et al. | 2007 | Observational | Hemodynamic and functional assessment of pulmonary hypertension | 34(Not available) | Hb SS only | Adults | Cycle | No | None | 6 |
| Badawy et al. | 2018 | Observational | Relationship between exercise capacity and clinical outcomes | 223(64%) | All genotypes | Adults | Treadmill | No | None | 5 |
| Braden et al. | 1996 | Observational | Cardiovascular function during exercise | 27(Not available) | Hb SS only | Adults | Cycle | Yes | None | 7 |
| Callahan et al. | 2002 | Observational | Cardiopulmonary responses to exercise | 17(100%) | Hb SS only | Adults | Cycle | Yes | Mild back pain in 2 subjects after CPET that resolved | 8 |
| †Ceccaldi et al. | 2017 | Observational | Cardiac response and cardiovascular reserve during exercise | 60(60%) | SCA (not specified) | Adults | Not specified | No | None | 5 |
| Chaudry et al. | 2013 | Observational | Impact of pulmonary vascular abnormalities on exercise capacity | 44(55% | Hb SS or Hb S/β0 thalassemia | Children | Cycle | No | None | 5 |
| Covitz et al. | 1983 | Observational | Radionuclide study of cardiac dysfunction during exercise | 22(50% | Hb SS only | Both | Cycle | Yes | Ischemic changes on ECG in 5 subjects | 7 |
| Das et al. | 2008 | Observational | Exercise responses in children on erythrocytapheresis | 16 (56%) | Hb SS only | Children | Treadmill | Yes | None | 7 |
| Faes et al. | 2013 | Observational | Impact of exercise on oxidative stress and endothelial activation | 11(0%) | Hb SS only | Adults | Cycle | ‡No | None | 5 |
| Freund et al. | 1992 | Observational | Lactate exchange and removal during exercise | 7(0%) | Hb SC only | Adults | Cycle | Yes | None | 4 |
| Liem et al. | 2009 | Observational | Relationship between exercise capacity and cardiopulmonary disease | 30(40%) | All genotypes | Both | Cycle | No | §None | 8 |
| Liem et al. | 2015 | Observational | Cardiopulmonary responses to exercise | 60(50%) | Hb SS or Hb S/β0 thalassemia | Children | Cycle | Yes | SpO2 ≤95% during exercise or dropped during exercise in 10 subjects, pain episode requiring hospitalization reported 13 days after CPET in 1 subject | 8 |
| Liem et al. | 2017 | Intervention | Feasibility of aerobic exercise training program | 13(54%) | Hb SS or Hb S/β0 thalassemia | Children | Cycle | Yes | §None | 8 |
| †Ogunsile et al. | 2018 | Observational | Cardiorespiratory fitness and cardiovascular risk factors | 46(74% | All genotypes | Adults | Cycle | Yes | None | 7 |
| Oyono-Enguelle et al. | 2000 | Observational | Cardiorespiratory and metabolic responses to exercise | 11(0%) | Hb SC only | Adults | Cycle | Yes | None | 4 |
| Pianosi et al. | 1991 | Observational | Ventilation and gas exchange parameters during exercise | 34(50%) | Hb SS and Hb SC | Children | Cycle | Yes | None | 8 |
| Van Beers et al. | 2014 | Observational | Relationship between exercise tolerance and cardiopulmonary disease, anemia and cardio-thoracic ratio | 44(73%) | All genotypes | Adults | Cycle | Yes | None | 8 |
| Watson et al. | 2015 | Observational | Longitudinal changes in aerobic capacity | 33(42%) | Hb SS only | Children | Treadmill | No | None | 5 |
AEs, adverse events; NOS rating, Newcastle-Ottawa Scale rating
Conference abstract
Adverse events reported only for submaximal portion of study, not initial maximal CPET
Safety data verified despite absence of reporting in manuscript given studies are by author of this review
RESULTS
Study Characteristics
Initial searches yielded 278,533 results, of which only 195 were potentially applicable to this systematic review. Of these 195 studies, reviewers removed 40 duplicates and then screened titles and abstracts of the remaining 155 studies. An additional 133 studies were removed for the following reasons: animals, participants with sickle cell trait, or a mode of exercise testing other than maximal CPET. With the addition of 2 studies that were found independent of the initial search, a total of 24 studies were included in the final dataset (Figure 1).(4, 5, 8, 15-35) With the exception of 1 interventional study, all of the studies included were observational in design. These studies included a total of 939 unique individuals with SCD who underwent maximal CPET. Half of the studies included only adult patients (N=12), while one-third included children (N=7), and 5 studies included both children and adults. The majority of studies included subjects with either Hb SS or Hb S/β0 thalassemia only (N=14), while the rest included subjects with Hb SC only (N=2), Hb SS or SC only (N=3) or all genotypes (N=4) or did not specify the genotype of participants (N=1). Cycle ergometry was the most commonly used exercise protocol (N=19), while 4 studies used a treadmill and 1 did not specify the type of protocol. NOS ratings, together with key characteristics of included studies, are shown in the Table 1.
Figure 1.
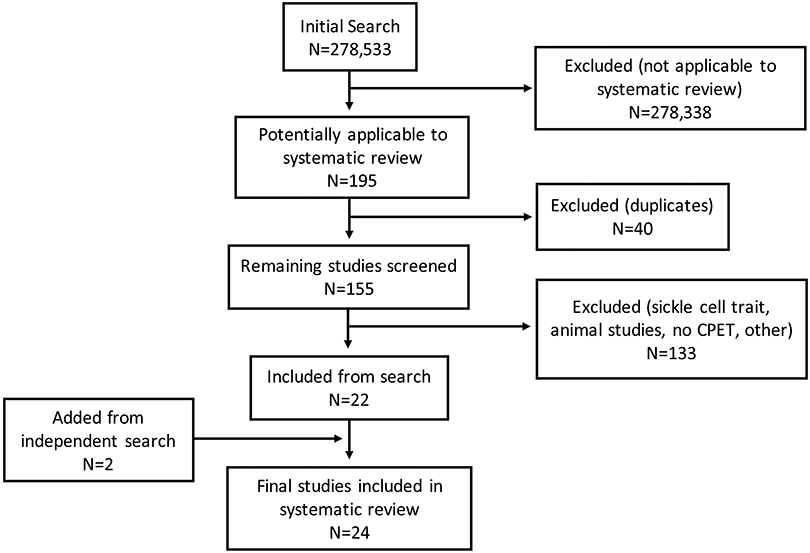
PRISMA diagram of final studies included in this systematic review (CPET, cardiopulmonary exercise test)
Adverse Events
Of the studies included in this review, the majority of the studies (N=16) included documentation of AE monitoring or reported findings that could be classified as AEs.(4, 5, 15-20, 22, 23, 26, 27, 30-32, 34) In 6 of these studies, AEs were described in 36 participants with SCD undergoing maximal CPET and included the following study findings, incidents or events: ischemic changes on ECG (N=22), pain not requiring hospitalization (N=3), pain requiring hospitalization (N=1) or reductions in oxygen saturation during maximal CPET (N=10) (Figure 2).(5, 17, 18, 20, 23, 26) The other 8 studies included neither documentation of AE monitoring nor documentation of AEs as part of study findings.(8, 21, 24, 25, 28, 29, 33, 35) In total, AEs were reported in 36/939 (3.8%) of participants with SCD undergoing maximal CPET in all of the studies included, with only 4/939 (0.43%) participants experiencing pain events.
Figure 2.
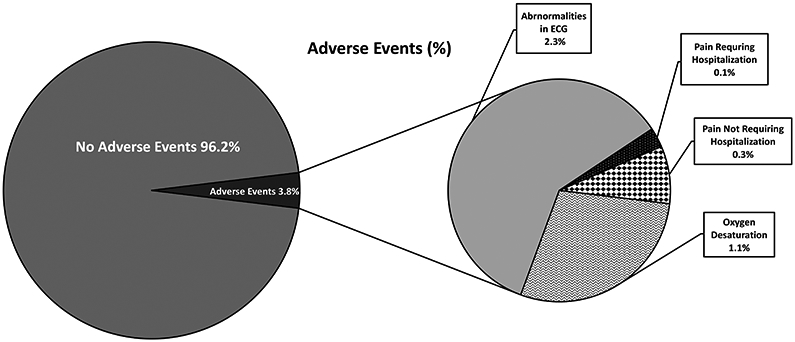
Total percent of adverse events and types of adverse events experienced during maximal cardiopulmonary exercise testing (ECG, electrocardiogram)
DISCUSSION
The gold standard for evaluating cardiopulmonary fitness is graded CPET with indirect calorimetry, which allows for gas exchange analysis and the direct measurement of peak or maximal oxygen consumption. Despite its widespread use in the general population in both clinical and research settings, maximal CPET is underutilized in children and adults with SCD, which in part may be explained by concerns about its safety in the SCD population. This systematic review of the literature supports the safety of maximal CPET in the SCD population. Among 24 studies that utilized maximal CPET in 939 participants with SCD, AEs were reported in less than 5% of tests. The majority of reported AEs were related to ischemic changes on ECG monitoring or oxygen desaturation that did not result in arrhythmias or other cardiopulmonary complications during or after testing. Sickle cell related pain events, however, were reported in less than 1% of tests.
The ability to evaluate individuals with SCD by maximal CPET is important for several reasons. Throughout the lifespan of individuals with SCD, complications like chronic anemia and cardiopulmonary disease may impair physical functioning and result in reduced cardiopulmonary fitness. Routine diagnostic tests such as echocardiography or pulmonary function testing offer static views of the cardiopulmonary system, while the widely used 6-minute walk distance is a submaximal field test that reflects a unidimensional view of exercise capacity. In contrast, CPET with gas exchange analysis is a laboratory-based, reproducible testing modality that allows for a more comprehensive and dynamic assessment of the cardiopulmonary system and exercise capacity.(36, 37) In the clinical setting, the reliance on maximal CPET to directly and accurately measure cardiopulmonary fitness is also useful for the development exercise prescription and physical activity guidelines, which currently do not exist for individuals with SCD. Individualizing and implementing exercise prescription based on data gathered from CPET may translate to short and longterm benefits associated with improved fitness and less sedentary lifestyles for this population. Moreover, adverse events or safety signals observed during testing may help better delineate exercise intensity thresholds below which patients with SCA should stay to maintain safe exercise programming. Finally, maximal CPET is a useful tool by which to measure the benefits of existing and novel treatments on fitness as well as on the individual metabolic and cardiopulmonary factors that lead to improvements in fitness.
Despite the known health benefits of regular exercise in healthy individuals and individuals with chronic medical conditions, concerns about the potential harms of moderate to vigorous physical activity and exercise persist for children and adults with SCD. These unsubstantiated concerns arise because SCD is characterized by a pro-inflammatory state, which could be exacerbated by the potential pro-inflammatory response associated with acute intense exercise and result in SCD-related complications such as pain.(38) In this review, however, the incidence of pain during or after maximal CPET in individuals with SCD was low. Moreover, the inflammatory response to maximal intensity exercise has been previously demonstrated to be comparable in children with or without SCD.(39) Nonetheless, whether or not the low incidence of pain associated with maximal CPET in our systematic review was expected cannot be determined since the incidence of pain during moderate to vigorous intensity exercise or physical activity has not been prospectively studied in the SCD population.
Given the known presence of cardiopulmonary disease among adolescents and adults with SCD, the finding of abnormal cardiopulmonary responses during exercise (e.g., ECG changes or oxygen desaturation) in some of the studies reviewed was not surprising.(5, 17, 18, 26) However, these findings should be considered with caution and do not preclude the use of maximal CPET in individuals with SCD for several reasons. The criteria used to define abnormalities observed during routine monitoring of exercise testing, such as ECG changes that suggest myocardial ischemia or reductions in oxygen saturation (SpO2), may vary among studies. These findings may not be reproducible and do not necessarily suggest more serious consequences, as evidenced by the absence of subsequent complications in subjects who experienced them during exercise testing in the studies. Finally, these findings were considered outcomes that were part of the evaluation or aims of the studies. Although we included them as AEs in this systematic review, others might instead consider these findings a reflection of the utility of CPET to elucidate the physiological impact and significance of cardiopulmonary disease in this population. Similarly, the utility and safety of maximal CPET has been established for other high-risk clinical populations, including adults with high-risk cardiovascular disease, pulmonary hypertension and cystic fibrosis, in whom the incidence of oxygen desaturation or cardiac arrhythmias during exercise testing may be even higher than that reported for patients with SCD in our systematic review.(40-43)
This study is not without its limitations. First, the literature search was limited to articles published in English, although very few studies published in languages other than English were available. A second limitation and contributor to risk of bias is that many of the included studies were not solely focused on evaluating or reporting AEs associated with maximal CPET, with some studies failing to mention safety monitoring at all. With such studies, it was reasonable to assume that subjects in a study had not experienced any AEs related to maximal CPET even if the study did not specifically include a statement that AEs were not observed. A third limitation, which also contributed to risk of bias for study selection, is that many studies did not report if and how maximal effort was determined during exercise testing despite the reported maximal nature of the CPET protocol. A fourth limitation that might have affected sample representativeness is that some studies included participants of a specific genotype only (e.g., hemoglobin SC disease). Moreover, some studies might have excluded participants with known cardiopulmonary disease or other co-morbidities of SCD, so individuals tested in those studies might have been the least likely to have adverse events. For this reason, our findings may not be generalizable to all patients with SCD, especially those with severe disease-related complications. Lastly, the exercise associated with maximal CPET is generally no longer than 8 to 12 minutes in duration and given the ramping nature of the protocol, the highest intensity of exercise occurs only in the last part of the test. Thus, any insight into the safety of maximal CPET gleaned from this review cannot be extrapolated to other forms of exercise or exercise training, including moderate to high intensity exercise sustained at longer durations.
In summary, maximal CPET appears to be a safe modality by which to measure cardiopulmonary fitness in individuals with SCD. More widespread adoption of maximal CPET in children and adults with SCD may allow clinicians and researchers to use CPET as a diagnostic and prognostic tool in this population. As such, establishing the safety of maximal CPET in SCD and leveraging data obtained from testing could also help providers better understand the impact of established and new therapies on physical functioning and develop appropriate exercise prescription programs. Future research to determine the safety and benefits of moderate to vigorous exercise performed for longer durations is warranted.
Supplementary Material
SUMMARY BOX.
What is already known:
Sickle cell disease and its complications have a major impact on cardiopulmonary fitness and lead to exercise limitations in affected individuals.
The safety of high intensity exercise and maximal cardiopulmonary exercise testing in sickle cell disease have not been well studied, which hampers the development of exercise prescription guidelines in this population.
What are the new findings:
Less than 1% of children and adults with sickle cell disease undergoing maximal cardiopulmonary exercise testing experience sickle cell related pain episodes.
Maximal cardiopulmonary exercise testing appears to be a safe modality for evaluating cardiopulmonary fitness and the physiological contributors to exercise limitation in individuals with sickle cell disease.
FUNDING INFO
This work was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (5R01HL136480) and the National Center for Advancing Translational Sciences (5U01TR002004).
Footnotes
ETHICAL APPROVAL INFORMATION
This study did not require research ethics approval because it did not involve human participants or animal subjects.
COMPETING INTERESTS
The authors have no conflicts of interest related to this manuscript to declare.
REFERENCES
- 1.Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85(1):77–8. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omwanghe OA, Kwon S, Muntz DS, Montgomery S, Kemiki O, Hsu LL, et al. Habitual physical activity and exercise patterns in children and adolescents with sickle cell disease. Blood. 2014;124(21). [DOI] [PubMed] [Google Scholar]
- 4.van Beers EJ, van der Plas MN, Nur E, Bogaard HJ, van Steenwijk RP, Biemond BJ, et al. Exercise tolerance, lung function abnormalities, anemia, and cardiothoracic ratio in sickle cell patients. Am J Hematol. 2014;89(8):819–24. [DOI] [PubMed] [Google Scholar]
- 5.Liem RI, Reddy M, Pelligra SA, Savant AP, Fernhall B, Rodeghier M, et al. Reduced fitness and abnormal cardiopulmonary responses to maximal exercise testing in children and young adults with sickle cell anemia. Physiol Rep. 2015;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marinho CL, Maioli MC, Soares AR, Bedirian R, Melo PL, Guimaraes FS, et al. Predictive models of six-minute walking distance in adults with sickle cell anemia: Implications for rehabilitation. J Bodyw Mov Ther. 2016;20(4):824–31. [DOI] [PubMed] [Google Scholar]
- 7.Alsaied T, Niss O, Tretter JT, Powell AW, Chin C, Fleck RJ, et al. Left atrial dysfunction in sickle cell anemia is associated with diffuse myocardial fibrosis, increased right ventricular pressure and reduced exercise capacity. Sci Rep. 2020;10(1):1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthi A, Machado RF, Jison ML, Taveira-Dasilva AM, Rubin LJ, Hunter L, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175(12):1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair SN, Kampert JB, Kohl HW 3rd, Barlow CE, Macera CA, Paffenbarger RS Jr., et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. Jama. 1996;276(3):205–10. [PubMed] [Google Scholar]
- 10.Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, et al. Exercise capacity and mortality in black and white men. Circulation. 2008;117(5):614–22. [DOI] [PubMed] [Google Scholar]
- 11.Carnethon MR, Sternfeld B, Schreiner PJ, Jacobs DR Jr., Lewis CE, Liu K, et al. Association of 20-year changes in cardiorespiratory fitness with incident type 2 diabetes: the coronary artery risk development in young adults (CARDIA) fitness study. Diabetes care. 2009;32(7):1284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exercise immunology review. 2009;15:6–41. [PubMed] [Google Scholar]
- 15.Aessopos A, Tsironi M, Vassiliadis I, Farmakis D, Fountos A, Voskaridou E, et al. Exercise-induced myocardial perfusion abnormalities in sickle beta-thalassemia: Tc-99m tetrofosmin gated SPECT imaging study. Am J Med. 2001;111(5):355–60. [DOI] [PubMed] [Google Scholar]
- 16.Ako EO, Barber NJ, Kowalik GT, Steeden JA, Porter J, Walker JM, et al. MR-augmented cardiopulmonary exercise testing-a proof of concept in Sickle Cell Disease (SCD). Journal of Cardiovascular Magnetic Resonance. 2016;18. [Google Scholar]
- 17.Alpert BS, Dover EV, Strong WB, Covitz W. Longitudinal exercise hemodynamics in children with sickle cell anemia. Am J Dis Child. 1984;138(11):1021–4. [DOI] [PubMed] [Google Scholar]
- 18.Alpert BS, Gilman PA, Strong WB, Ellison MF, Miller MD, McFarlane J, et al. Hemodynamic and ECG responses to exercise in children with sickle cell anemia. Am J Dis Child. 1981;135(4):362–6. [DOI] [PubMed] [Google Scholar]
- 19.Alsaied T, Niss O, Powell AW, Fleck RJ, Cnota JF, Chin C, et al. Diastolic dysfunction is associated with exercise impairment in patients with sickle cell anemia. Pediatric Blood and Cancer. 2018;65(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ameringer S, Elswick RK Jr., Sisler I, Smith W, Lipato T, Acevedo EO. Exercise Testing of Adolescents and Young Adults With Sickle Cell Disease: Perceptual Responses and the Gas Exchange Threshold. J Pediatr Oncol Nurs. 2019;36(5):310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badawy SM, Payne AB, Rodeghier MJ, Liem RI. Exercise capacity and clinical outcomes in adults followed in the Cooperative Study of Sickle Cell Disease (CSSCD). Eur J Haematol. 2018;101(4):532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braden DS, Covitz W, Milner PF. Cardiovascular function during rest and exercise in patients with sickle-cell anemia and coexisting alpha thalassemia-2. Am J Hematol. 1996;52(2):96–102. [DOI] [PubMed] [Google Scholar]
- 23.Callahan LA, Woods KF, Mensah GA, Ramsey LT, Barbeau P, Gutin B. Cardiopulmonary responses to exercise in women with sickle cell anemia. Am J Respir Crit Care Med. 2002;165(9):1309–16. [DOI] [PubMed] [Google Scholar]
- 24.Ceccaldi A, Hatem S, Stankovic-Stojanovic K, Guedeney P, Nicolas-Jilwan F, Haymann JP, et al. Reduced cardiac reserve contributes to exercise tolerance in adult patients with sickle cell anemia. Eur J Heart Fail. 2017;19:489. [Google Scholar]
- 25.Chaudry RA, Bush A, Rosenthal M, Crowley S. The impact of sickle cell disease on exercise capacity in children. Chest. 2013;143(2):478–84. [DOI] [PubMed] [Google Scholar]
- 26.Covitz W, Eubig C, Balfour IC, Jerath R, Alpert BS, Strong WB, et al. Exercise-induced cardiac dysfunction in sickle cell anemia. A radionuclide study. Am J Cardiol. 1983;51(3):570–5. [DOI] [PubMed] [Google Scholar]
- 27.Das BB, Sobczyk W, Bertolone S, Raj A. Cardiopulmonary stress testing in children with sickle cell disease who are on long-term erythrocytapheresis. J Pediatr Hematol Oncol. 2008;30(5):373–7. [DOI] [PubMed] [Google Scholar]
- 28.Faes C, Balayssac-Siransy E, Connes P, Hivert L, Danho C, Bogui P, et al. Moderate endurance exercise in patients with sickle cell anaemia: effects on oxidative stress and endothelial activation. Br J Haematol. 2014;164(1):124–30. [DOI] [PubMed] [Google Scholar]
- 29.Freund H, Lonsdorfer J, Oyono-Enguelle S, Lonsdorfer A, Bogui P. Lactate exchange and removal abilities in sickle cell patients and in untrained and trained healthy humans. J Appl Physiol (1985). 1992;73(6):2580–7. [DOI] [PubMed] [Google Scholar]
- 30.Liem RI, Akinosun M, Muntz DS, Thompson AA. Feasibility and safety of home exercise training in children with sickle cell anemia. Pediatr Blood Cancer. 2017;64(12). [DOI] [PubMed] [Google Scholar]
- 31.Liem RI, Nevin MA, Prestridge A, Young LT, Thompson AA. Functional capacity in children and young adults with sickle cell disease undergoing evaluation for cardiopulmonary disease. Am J Hematol. 2009;84(10):645–9. [DOI] [PubMed] [Google Scholar]
- 32.Ogunsile FJ, Stewart K, Wang H, Lanzkron S. Modifiable cardiovascular risk factors in adults with sickle cell disease. Blood. 2018;132. [Google Scholar]
- 33.Oyono-Enguelle S, Le Gallais D, Lonsdorfer A, Dah C, Freund H, Bogui P, et al. Cardiorespiratory and metabolic responses to exercise in HbSC sickle cell patients. Med Sci Sports Exerc. 2000;32(4):725–31. [DOI] [PubMed] [Google Scholar]
- 34.Pianosi P, D'Souza SJA, Esseltine DW, Charge TD, Coates AL. Ventilation and gas exchange during exercise in sickle cell anemia. Am Rev Respir Dis. 1991;143(2):226–30. [DOI] [PubMed] [Google Scholar]
- 35.Watson AM, Liem RI, Lu Z, Saville B, Acra S, Shankar S, et al. Longitudinal differences in aerobic capacity between children with sickle cell anemia and matched controls. Pediatr Blood Cancer. 2015;62(4):648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keteyian SJ, Brawner CA, Ehrman JK, Ivanhoe R, Boehmer JP, Abraham WT. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise parameters: implications for clinical trials and clinical practice. Chest. 2010;138(4):950–5. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Liu S, Li G, Xiao J. Exercise Regulates the Immune System. Adv Exp Med Biol. 2020;1228:395–408. [DOI] [PubMed] [Google Scholar]
- 39.Liem RI, Onyejekwe K, Olszewski M, Nchekwube C, Zaldivar FP, Radom-Aizik S, et al. The acute phase inflammatory response to maximal exercise testing in children and young adults with sickle cell anaemia. Br J Haematol. 2015;171(5):854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skalski J, Allison TG, Miller TD. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation. 2012;126(21):2465–72. [DOI] [PubMed] [Google Scholar]
- 41.Smith G, Reyes JT, Russell JL, Humpl T. Safety of maximal cardiopulmonary exercise testing in pediatric patients with pulmonary hypertension. Chest. 2009;135(5):1209–14. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen LL, Liang HY, Pinheiro A, Hilser A, Dimaano V, Olsen NT, et al. Safety profile and utility of treadmill exercise in patients with high-gradient hypertrophic cardiomyopathy. Am Heart J. 2017;184:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Causer AJ, Shute JK, Cummings MH, Shepherd AI, Bright V, Connett G, et al. Cardiopulmonary exercise testing with supramaximal verification produces a safe and valid assessment of Vo2max in people with cystic fibrosis: a retrospective analysis. J Appl Physiol (1985). 2018;125(4):1277–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


