Abstract
Background
Breast cancer in young women is more likely to have higher risk features and be associated with germline BRCA1/BRCA2 mutations. We present the clinicopathologic features of breast cancers in a prospective cohort of young women, and associations between surrogate molecular subtype and BRCA1/BRCA2 mutation status.
Methods
Histopathological features, biomarker status, tumour stage and BRCA status were collected. Invasive tumours were categorised as luminal A-like (ER + and/or PR + , HER2−, grade 1/2), luminal B-like (ER + and/or PR + , HER2 + , or ER + and/or PR + , HER2−, and grade 3), HER2-enriched (ER/PR−, HER2 + ) or triple-negative.
Results
In all, 57.3% (654/1143) of invasive tumours were high grade. In total, 32.9% were luminal A-like, 42.4% luminal B-like, 8.3% HER2-enriched, and 16.4% triple-negative. Among different age groups, there were no differences in molecular phenotype, stage, grade or histopathology. 11% (131) of tumours were from BRCA mutation carriers; 64.1% BRCA1 (63.1% triple-negative), and 35.9% BRCA2 (55.3% luminal B-like).
Discussion
The opportunity to provide comparisons across young age groups, BRCA mutation status, surrogate molecular phenotype, and the identification of more aggressive hormone receptor-positive phenotypes in this population provides direction for future work to further understand and improve disparate outcomes for young women with luminal B-like cancers, particularly BRCA2-associated cancers, with potential implications for tailored prevention and treatment.
Subject terms: Breast cancer, Breast cancer
Background
Breast cancer is the most common cancer type diagnosed among young women (age ≤ 40 years) worldwide and has the highest cancer-related death rate in this age group [1]. Of note, an increased incidence of breast cancer in young women has been seen recently, largely driven by an increase in hormone receptor-positive tumours [2, 3]. Breast cancer arising in this age group is more likely to have more aggressive features than in older women, with a greater proportion of Stage III, high-grade hormone receptor-positive, and hormone receptor-negative tumours in young women [2]. Furthermore, a higher risk of relapse has been shown in women ≤ 40 years when compared to older women, with an inferior relapse-free survival seen among women with oestrogen receptor (ER) positive (+)/human epidermal growth factor 2 receptor (HER2) negative (−) tumours, in particular [4, 5].
Differences in the distribution of the molecular phenotypes by age and race have been demonstrated [6, 7]. Some prior studies have identified a higher proportion of triple-negative and HER2-enriched tumours in young women [4], and among African-American women there is a preponderance of triple-negative breast cancers [8, 9]. In addition, the outcomes by molecular phenotypes vary by age, with a worse prognosis seen in younger women with luminal A-like subtypes and Stage I to III breast cancer compared with older women [5, 10, 11]. Paradoxically, among young women with Stage IV breast cancer, a lower risk of breast cancer-related death has been observed in luminal A-like and luminal B-like subtypes when compared to older women of the same stage [12].
BRCA1 and BRCA2 mutation carriers are well known to have an increased risk of developing breast cancer, [13–15] and in young women, breast cancer is more likely to be associated with germline BRCA1 or BRCA2 mutations [16–19]. Studies evaluating the distribution of molecular phenotypes among BRCA1 or BRCA2 mutation carriers have documented a higher proportion of triple-negative cancers among BRCA1 mutation carriers and a higher proportion of luminal B-like cancers among BRCA2 mutation carriers [20–22]. A low BRCA mutation detection rate (6.4%) among luminal-like early-onset breast cancer patients has been described with a greater proportion being BRCA2 mutation carriers (4.6%), and a lesser proportion being BRCA1 mutation carriers (1.8%) [23]. Moreover, BRCA1 status and ER negativity has been associated with higher pathologic complete response (pCR) after neoadjuvant chemotherapy; and better relapse-free survival and overall survival was observed among those who achieved a pCR [24]. However, the distribution of molecular phenotypes in young women according to BRCA1 or BRCA2 mutation status is not as well-defined.
We previously reported a higher distribution of luminal B-like and HER2-enriched tumours among young women (≤ 40 years) [25]. Here, we present the clinical and pathologic features of invasive disease in the fully assembled prospective cohort of young women with breast cancer, as well as associations between surrogate molecular phenotype and BRCA1 and BRCA2 mutation status.
Patients and methods
Study design and population
The Young Women’s Breast Cancer Study (NCT#01468246) is a multi-institutional prospective cohort study of women newly diagnosed with breast cancer at age 40 years and younger enrolled from 2006 to 2016. As has been described previously [25, 26], women were identified through pathology record review complemented by clinic list review at the Dana–Farber Cancer Institute and Brigham and Women’s Hospital, as well as nine other participating institutions within Massachusetts and three out of state, and were eligible for enrollment provided they were able to respond to questionnaires in English. Participants responding to an invitation by mail, provided written informed consent authorising medical record review that included data abstraction of patient stage, breast tumour biomarker status (ER, progesterone receptor (PR) and HER2) and BRCA mutation status, blood sample and pathology specimen collection, and baseline and follow-up participant questionnaires. Institutional review board (IRB) approval for the study was obtained through the Dana–Farber/Harvard Cancer Centre and other participating centres.
Pathology review
Histopathology slides were reviewed centrally by the study pathologists, including an expert breast pathologist who reviewed all cases (LCC). When available, both the initial core biopsy and subsequent excision or mastectomy specimens were reviewed. Using a standardised case-reporting form, specific histologic features were recorded for each specimen, including histologic grade (Ellston modification of the Bloom–Richardson tumour grade), presence or absence of a central fibrotic focus, zones of geographic necrosis, the pattern of the tumour margins (invasive or pushing/circumscribed) and degree of associated lymphocytic infiltration (none/mild or moderate/marked) [25].
Classification of molecular phenotype
Using the histologic tumour grade from central pathology review and biomarker status (ER, positive or negative; PR, positive or negative; and HER2, positive/amplified or negative/nonamplified) extracted from pathology reports, cases were classified as one of four molecular subtypes. The use of immunohistochemistry as a surrogate for molecular classification by gene expression profiling has been used in a number of large population-based studies [8, 27–30], has been shown to be an acceptable approximate of molecular phenotype [31] and is supported by the St. Gallen International Expert Consensus Statement [32]. As described previously [25], cases that were ER + and/or PR + , HER2-, and either histologic grade 1 or 2 were classified as luminal A-like cancers. Cases that were ER + and/or PR + and HER2 + or ER + and/or PR + and HER2− and that were histologic grade 3 were classified as luminal B-like cancers. Cases that were ER-, PR−, and HER2 + were classified as HER2-enriched. Cases that were negative for ER, PR and HER2 were classified as triple-negative, the clinicopathologic surrogate of basal-like carcinoma [32]. HER2 was considered positive if immunohistochemical stains were reported as 3+ and/or if HER2 fluorescence in situ hybridisation (FISH) showed gene amplification per ASCO/CAP definitions at the time of diagnosis.
BRCA status
BRCA mutation status was retrieved by medical record review or patient survey if not in the medical record. BRCA status was recorded as the deleterious mutation in BRCA1 or BRCA2, no mutation detected (wild-type, including variants of uncertain significance [VUS]), or unknown.
Statistical analyses
Statistical analyses were carried out with SAS 9.4 (SAS + Institute, Cary, NC). Pearson Chi-squared statistics were calculated to assess the difference among three age groups (≤ 30, 31–35 and 36–40 years) of young breast cancer patients’ tumours with respect to the surrogate molecular phenotype of invasive carcinomas, pathological features, and BRCA mutation status.
Results
With the recruitment period finalised, pathology review has been completed on all 1297 eligible participants (Fig. 1) (5 of the 1302 women enrolled were ultimately deemed ineligible). Table 1 summarises the patient characteristics. The mean age at diagnosis was 37 years (range 17–40 years). The majority of the population is white (1101/1297; 84.9%), with a small proportion of Asian, American Indian, Alaskan Native, Black, Haitian, African American and others. Fifty-six women (4.3%) self-identified as Hispanic or Latina. Among participants where family history was available (n = 1114), a family history (first-degree relative) of breast or ovarian cancer was self-reported by 176 (15.8%) of the patients. Germline mutation testing was available for 86.1% (1117/1297) of the patients. One thousand two hundred and seventy-six women (98.4%) presented with unilateral breast cancer. The majority of women presented with early-stage invasive disease (7.6% (98/1297) Stage 0; 31.8% (413/1297) Stage I; 40.5% (525/1297) Stage II, 15.2% (197/1297) Stage III; 4.9% [64/1297] Stage IV) and more than half presented with high-grade tumours (752/1297, 58.0%). Most women had hormone receptor-positive disease. Nine hundred and forty-five (72.9%) of the tumours were ER + , and more than one-fourth, 360 (27.8%), were HER2 + .
Fig. 1. Profile of study population.
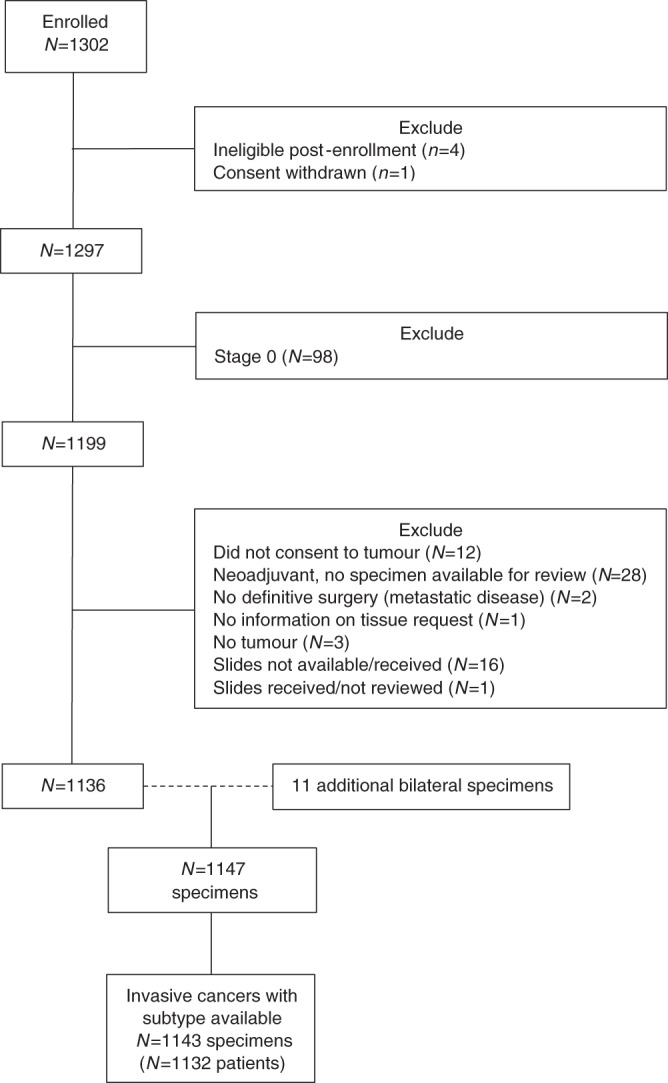
This diagram provides an outline of patients enrolled in the study, those excluded due to ineligibility, those excluded from this analysis due to Stage 0 status, and those with insufficient material for evaluation.
Table 1.
Patient characteristics for entire study population.
| Total patients | N = 1297 |
|---|---|
| Median age at diagnosis (years, range) | 37 (17–40) |
| N (%) | |
| Race* | |
| American Indian or Alaskan native | 6 (0.5) |
| Asian | 88 (6.8) |
| Black, Haitian or African American | 48 (3.7) |
| White | 1101 (84.9) |
| Other/unknown | 38 (2.9) |
| Multiracial | 16 (1.2) |
| First-degree family history breast or ovarian cancer** | |
| Yes | 176 (15.8) |
| No | 936 (84.0) |
| Unsure | 2 (0.2) |
| Missing | 183 |
| Bilateral | |
| Yes | 21 (1.6) |
| No | 1276 (98.4) |
| Stage | |
| 0 | 98 (7.6) |
| I | 413 (31.8) |
| II | 525 (40.5) |
| III | 197 (15.2) |
| IV | 64 (4.9) |
| Tumour grade | |
| Grades 1 and 2 | 534 (41.2) |
| Grade 3 | 752 (58.0) |
| Missing | 11 (0.9) |
| Oestrogen receptor (ER) | |
| Positive | 945 (72.9) |
| Negative | 351 (27.1) |
| Missing | 1 (0.1) |
| Progesterone receptor (PR) | |
| Positive | 848 (65.4) |
| Negative | 441 (34.0) |
| Missing | 8 (0.6) |
| HER2 (any ER status) | |
| Positive | 360 (27.8) |
| Negative | 880 (67.9) |
| Missing/not performed*** | 57 (4.4) |
| Subtype | |
| Luminal A-like (ER and/or PR + , HER2−, grade 1 or 2) | 395 (30.5) |
| Luminal B-like (ER and/or PR + , HER2−, grade 3) | 269 (20.7) |
| Luminal B/HER2 (ER and/or PR + , HER2 + ) | 255 (19.7) |
| HER2-enriched (ER−, PR−, HER2 + ) | 105 (8.1) |
| Triple-negative | 210 (16.2) |
| Missing/unknown subtype | 63 (4.9) |
| Genetic testing | |
| BRCA1 positive | 90 (6.9%) |
| BRCA2 positive | 54 (4.2%) |
| No mutation detected/VUS**** | 973 (75.0%) |
| No testing/Unknown | 180 (13.9%) |
*In total, 56 (4.3%) women self-identified as Hispanic or Latina.
**Mother or sister.
***Missing/unknown subtype includes cases of DCIS for which HER2 was not performed.
****VUS = variant of unknown significance, VUS = 4.2% (54/1297).
The distribution of pathologic features by age group is shown in Table 2. Among invasive tumours with the evaluable surrogate molecular phenotype (n = 1143; Fig. 1), including multiple tumours in the same patient (11 patients with bilateral tumours), the distribution of subtypes was 32.9% (376/1143) luminal A-like, 42.4% (485/1143) luminal B-like, 8.3% (95/1143) HER2-enriched and 16.4% (187/1143) triple-negative. There were no differences in the distribution of surrogate molecular phenotype by age category. Histologic grade 3 was the most common grade in each age category (≤ 30 = 58.6% (85/146), 31–35 = 59.8% (189/318), and 36–40 years = 55.8% (380/683)). Among the different age groups, there were no significant differences in specific histopathological features evaluated, including the presence of tumour necrosis, lymphocytic infiltration and central fibrotic focus. The highest proportion numerically of Stage IV tumours was observed in the youngest age group (7.5%, ≤ 30 years vs. 5.0%, 31–35 years vs 4.4%, in 36–40 years), though stage distribution was not statistically significantly different between age groups (P = 0.11). BRCA mutation status varied significantly by age groups (P = 0.008): the frequency of BRCA1 mutations was 6.0% in the 36–40 years group, 8.8% in the 31–35 years and 10.3% in those ≤ 30 years, and the frequency of BRCA2 mutations was 4.7% in 36–40 years, 2.8% in 31–35 years and 4.1% in ≤ 30 years. The majority of invasive tumours were of ductal histologic subtype.
Table 2.
Distribution of pathologic features by age group.
| Total N = 1147, N (%) | ≤30 years, N = 146, N (%) | 31–35 years, N = 318, N (%) | 36–40 years, N = 683, N (%) | P | |
|---|---|---|---|---|---|
| Subtype | 0.48 | ||||
| Luminal A-like | 376 (32.9) | 48 (33.1) | 98 (30.9) | 230 (33.8) | |
| Luminal B-like | 485 (42.4) | 61 (42.1) | 140 (44.2) | 284 (41.7) | |
| ER and/or PR + , HER2−, grade 3 | 250 (21.9) | 29 (20.0) | 70 (22.1) | 151 (22.2) | |
| ER and/or PR + , HER2 + | 235 (20.6) | 32 (22.1) | 70 (22.1) | 133 (19.5) | |
| HER2-enriched | 95 (8.3) | 12 (8.3) | 35 (11.0) | 48 (7.1) | |
| Triple-negative | 187 (16.4) | 24 (16.6) | 44 (13.9) | 119 (17.5) | |
| Missing | 4 | ||||
| Stage | 0.11 | ||||
| I | 402 (35.1) | 44 (30.1) | 104 (32.7) | 254 (37.2) | |
| II | 502 (43.8) | 70 (48.0) | 133 (41.8) | 299 (43.8) | |
| III | 186 (16.2) | 21 (14.4) | 65 (20.4) | 100 (14.6) | |
| IV | 57 (5.0) | 11 (7.5) | 16 (5.0) | 30 (4.4) | |
| Histologic subtype | |||||
| Ductal | 798 (69.6%) | 107 (73.3%) | 239 (75.2%) | 452 (66.2%) | 0.11 |
| Lobular | 48 (4.2%) | 5 (3.4%) | 9 (2.8%) | 34 (5.0%) | |
| Ductal and lobular features | 114 (9.9%) | 13 (8.9%) | 26 (8.2%) | 75 (11.0%) | |
| Other | 141 (12.3%) | 17 (11.6%) | 37 (11.6%) | 87 (12.7%) | |
| Unknown | 46 (4.0%) | 4 (2.7%) | 7 (2.2%) | 35 (5.1%) | |
| ER | 0.93 | ||||
| Positive | 836 (72.9) | 108 (74.0) | 230 (72.3) | 498 (72.9) | |
| Negative | 311 (27.1) | 38 (26.0) | 88 (27.7) | 185 (27.1) | |
| PR | 0.36 | ||||
| Positive | 759 (66.2) | 92 (63.0) | 204 (64.2) | 463 (67.8) | |
| Negative | 388 (33.8) | 54 (37.0) | 114 (35.9) | 220 (32.2) | |
| HER2 | 0.28 | ||||
| Positive | 330 (28.8) | 44 (30.1) | 105 (33.0) | 181 (26.5) | |
| Negative | 814 (71.0) | 102 (69.9) | 212 (66.7) | 500 (73.2) | |
| Missing | 3 | ||||
| Grade | 0.46 | ||||
| I/II | 488 (42.7) | 60 (41.4) | 127 (40.2) | 301 (44.2) | |
| III | 654 (57.3) | 85 (58.6) | 189 (59.8) | 380 (55.8) | |
| Missing | 5 | ||||
| Tumour necrosis | 0.73 | ||||
| Yes | 178 (15.9) | 23 (16.1) | 53 (16.8) | 102 (15.5) | |
| No | 879 (78.6) | 115 (80.4) | 247 (78.4) | 517 (78.3) | |
| No answer/NA/missing | 90 | ||||
| Lymphocytic infiltration | 0.93 | ||||
| None/Mild | 781 (69.9) | 105 (73.4) | 221 (70.2) | 455 (68.9) | |
| Moderate or marked | 309 (27.6) | 37 (25.9) | 85 (27.0) | 187 (28.3) | |
| No answer/NA/missing | 57 | ||||
| Fibrotic focus | 0.27 | ||||
| Yes | 136 (12.2) | 15 (10.5) | 41 (13.0) | 80 (12.1) | |
| No | 690 (61.7) | 86 (60.1) | 184 (58.4) | 420 (63.6) | |
| No answer/NA/missing | 321 | ||||
| Margins | 0.05 | ||||
| Pushing/circumscribed | 245 (21.9) | 40 (28.0) | 75 (23.8) | 130 (19.7) | |
| Invasive | 642 (57.4) | 73 (51.1) | 166 (52.7) | 403 (61.1) | |
| No answer/NA/missing | 260 | ||||
| Genetic testing | 0.008 | ||||
| BRCA1 positive | 84 (7.3%) | 15 (10.3%) | 28 (8.8%) | 41(6.0%) | |
| BRCA2 positive | 47 (4.1%) | 6 (4.1%) | 9 (2.8%) | 32 (4.7%) | |
| BRCA1/2 not detected/VUS | 972 (84.7%) | 123 (84.3%) | 276 (86.8%) | 573 (83.9%) | |
| Not tested | 44 (3.9%) | 2 (1.4%) | 5 (1.6%) | 37 (5.4%) |
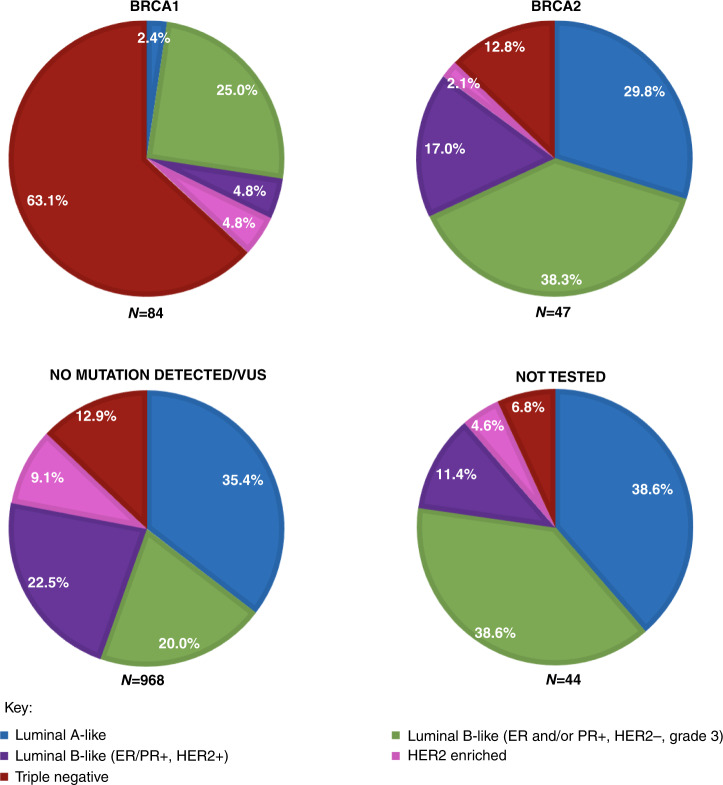
The distribution of surrogate molecular subtype by BRCA mutation status is summarised in Fig. 2. BRCA testing and results were ascertained via medical record review (87.1%, 986/1132) or from survey self-report (9.0%, 102/1132); only 3.9% (44/1132) of women were not tested or had an unknown testing status. Among women who tested positive for a deleterious mutation, 84/131 (64.1%) tumours were in BRCA1 mutation carriers and 47/131 (35.9%) tumours were in BRCA2 mutation carriers. BRCA1 mutation carriers’ tumours were more often triple-negative (63.1%, 53/84), followed by 29.8% (25/84) luminal B-like, 4.8% (4/84) HER2-enriched, and 2.4% (2/84) luminal A-like. BRCA2 mutation carriers’ tumours were most commonly luminal B-like (55.3%, 26/47), followed by 29.8% (14/47) luminal A-like, 12.8% (6/47) triple-negative, and 2.1% (1/47) HER2-enriched. Interestingly, BRCA2 mutation carriers’ tumours were significantly more likely to be luminal B-like/HER2− vs luminal B/HER2 + (38.3% (18/47) vs 17.0% (8/47), P < 0.0001). Among the 968 women with a negative test for BRCA1 and BRCA2 mutation or a non-actionable VUS, the most frequent surrogate molecular subtype identified was luminal B-like (42.5%, n = 412), followed by luminal A-like (35.4% 343), triple-negative (12.9%, 125) and HER2-enriched (9.1%, n = 88). Among the 44 women not tested, the most frequent surrogate molecular subtype identified was luminal B-like (50.0%, n = 22), followed by luminal A-like (38.6%, 17), triple-negative (6.8%, 3) and HER2-enriched (4.6%, n = 2).
Fig. 2. Distribution of molecular phenotypes among BRCA1 + , BRCA2 + , no mutation detected/VUS, and not tested.
Each pie chart illustrates the distribution of molecular phenotypes within BRCA1 (n = 84), BRCA2 (n = 47), no mutation detected/VUS (n = 968) and patients not tested (n = 44).
The distribution of surrogate molecular subtype by age stratified by BRCA1 and BRCA2 mutation status is shown in Supplementary Tables 1 and 2. Though not statistically significant, the largest proportion of triple-negative cancers among BRCA1 mutation carriers was seen in women ≤ 30 years (≤ 30 years; 73.3% vs. 60.7% and 61.0%, in 31–35 years and 36–40 years, respectively). In contrast, all the triple-negative cancers among the BRCA2 mutation carriers were seen in women 36–40 years. Among BRCA mutation carriers, the majority of the tumours were of ductal histologic subtype (106/131; 80.9%). Invasive lobular carcinomas were identified in BRCA2 mutation carriers (3/47; 6.4% of BRCA2 mutation carriers) but not in BRCA1 mutation carriers (Supplementary Table 3).
Discussion
Understanding the distinct clinical and pathologic features of breast cancer in young women is critical to mitigating the disparities in outcomes experienced by this population. Instead of comparing young women to an older population of women for whom it is well-recognised, there are differences in stage at presentation, histologic grade and molecular subtype, we sought to evaluate whether there are clinical and pathological differences among young women with breast cancer within our population of patients, expanded fourfold since our prior publication [25]. We continue to see no differences between age groups (≤ 30, 31–35, vs. 36–40 years) and tumour stage, grade or tumour surrogate molecular phenotype among young women with invasive breast cancer.
Among the large proportion of women in our cohort with known BRCA status, 86.1% had results available for germline mutations, and 11.1% were BRCA mutation carriers (64.1% in BRCA1 mutation carriers and 35.9% in BRCA2 mutation carriers). Copson et al. found similar rates of BRCA mutation in a large cohort of young women with breast cancer, of which 12.4% were BRCA mutation carriers (59.5% in BRCA1 mutation carriers and 40.5% in BRCA2 mutation carriers) [22]. In our cohort, the largest proportion of BRCA mutation carriers was observed in the youngest group (women ≤ 30 years). Interestingly, others have studied women ≤30 years and found a lower proportion of BRCA mutation carriers in the very youngest group (women ≤ 25 years) compared to women in the 26–30 year age group [33]. Among the invasive tumours in BRCA mutation carriers in our cohort, a few lobular subtypes were identified within the BRCA2 mutation carriers. Similarly, Mavaddat et al. found significantly more invasive lobular carcinomas among BRCA2 mutation carriers than among BRCA1 mutation carriers [34].
We have also demonstrated that tumour surrogate molecular phenotype varies by BRCA1 and BRCA2 mutation status among young women (as it does in older women). Larsen et al. studied the molecular phenotype determined by PAM50 within a broad age range (25–74 years) of women and demonstrated that BRCA1 mutation carriers had proportionally more basal-like tumours (20/33, 61%) and BRCA2 mutation carriers had proportionally more luminal B subtype (16/22, 73%) [21]. Although this was a small population and the molecular subtype was determined by PAM50, their findings are consistent with those from our cohort in which BRCA1 mutation carriers’ tumours were proportionately more triple-negative (51/82, 62.2%) and BRCA2 mutation carriers’ tumours were most frequently luminal B-like (25/45, 55.6%). Shah et al. identified 50 cases of BRCA1/2 mutation carriers with ER/PR + and HER2− tumours among all the patients undergoing Oncotype DX testing during an 8-year period, the majority of them were from BRCA2 mutation carriers (31/50, 62%), supporting the finding of more luminal B-like HER2- tumours among BRCA2 mutation carriers when compared to BRCA1 mutation carriers [35]. Similarly, Ha et al. described a higher proportion of triple-negative breast cancers (52/99, 53.3%) among BRCA1 mutation carriers and luminal B-like cancers (41/103, 39.8%) among BRCA2 mutation carriers in a broad age range (23–72 years) of women when using immunohistochemistry as a surrogate for molecular phenotype [20]. Although these studies, which both found a high distribution of luminal B-like tumours among BRCA2 mutation carriers, did not differentiate between luminal B-like HER2 + and luminal B-like HER2- tumours, they did report a high prevalence of HER2− tumours overall among the BRCA2 mutation carriers, which is consistent with our finding that BRCA2 mutation carriers are proportionally more likely to develop luminal B/HER2− breast cancer (18/47, 38.3%), irrespective of young age category. Furthermore, Spurdle et al. found that triple-negative phenotype predicted BRCA1 mutation status, and ER-positive grade 3 phenotype predicted BRCA2 mutation status, particularly in young women (≤50 years) [36].
Mavaddat et al. studied adult women (18 to >70 years) in the Consortium of Investigators of Modifiers of BRCA 1/2 (CIMBA) and found a decrease in the proportion of triple-negative breast cancers with increasing age among BRCA1 mutation carriers, and an increase in triple-negative cancers with age at diagnosis among BRCA2 mutation carriers [34]. In our cohort, the largest proportion of triple-negative cancers among BRCA1 mutation carriers was similarly seen in the youngest group (women ≤ 30 years). In contrast, all the triple-negative cancers among the BRCA2 mutation carriers were present in the oldest age group (women 36–40 years). In addition, and in keeping with the findings of Mavaddat et al., there were more ER-positive tumours in the BRCA2 mutation carriers group than in the BRCA1 mutation carriers in our cohort [34].
The higher risk of developing breast cancer among women with BRCA1 or BRCA2 mutations is well-established, with a lifetime risk described as high as >80% [13]. This has been refined in a recent large prospective cohort of BRCA1 and BRCA2 mutation carriers with reported cumulative breast cancer risks to age 80 years of 72% for BRCA1 mutation carriers and 69% for BRCA2 mutation carriers [37]. The differences in molecular phenotypes among BRCA mutation carriers might have prognostic and predictive implications. For example, in a population-based cohort, Jonasson et al. identified ER-positive status as an adverse prognostic factor in BRCA2 mutation carriers [38]. Understanding the variations in tumour molecular phenotype by BRCA1 or BRCA2 status becomes increasingly important as new breast cancer prevention strategies are developed for asymptomatic BRCA mutation carriers not pursuing bilateral prophylactic mastectomies. Strategies that decrease the risk of hormone receptor-negative tumours, such as the use of receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitors, could be targeted more specifically towards BRCA1 mutation carriers given that tumours occurring in these women are more likely to be triple-negative [39, 40]. In contrast, tumours that develop among BRCA2 mutation carriers are more likely to be high grade, ER + luminal B-like, requiring prevention strategies that decrease the risk of hormone receptor-positive tumours. Further research to confirm the intrinsic, unfavourable biology of ER + BRCA2 breast cancer is clearly warranted. But these differences in molecular phenotypes may explain the relatively recent data from Kotsopoulos et al. which suggest that bilateral oophorectomy prevents early-onset breast cancer among BRCA2 mutation carriers but not among BRCA1 mutation carriers [41]. Although the data is controversial, as a protective effect from bilateral oophorectomy in both BRCA1 and BRCA2 mutation carriers has been observed in some studies [34, 42–44], and more recently no association between risk-reducing salpingo-oophorectomy and breast cancer risk for either BRCA1 or BRCA2 mutation carriers was reported [45]. Furthermore, the distribution of the molecular phenotypes along with the high-risk histopathological features aid in understanding the differences in response to alternative drug targeting for this population, as demonstrated by the beneficial effects of poly ADP ribose polymerase (PARP) inhibitors among BRCA1 and BRCA2 mutation carriers with breast cancer [46].
We have confirmed that young women with breast cancer have a greater likelihood of presenting with high-grade tumours, and have a larger proportion of luminal B-like cancers compared to the general population. Other groups have similarly shown that breast cancers arising in young women are more likely to be of luminal B-like subtype [47–49]. In contrast, some have shown a higher proportion of triple-negative breast cancer among young women [4, 50, 51]. It is important to note that the distribution of the molecular phenotypes differs among different ethnicities, with higher rates of triple-negative and basal-like breast cancer reported among African-American women, interestingly, with a more frequent association with BRCA2 mutations recently reported in this population [8, 52, 53]. Overrepresentation of white women in our cohort may be a potential reason for the differences in the distribution of the molecular subtypes we observed compared with other publications [4, 50, 51].
Central laboratory testing for the hormonal receptors and HER2 status was not performed in this study. Instead, we abstracted this information from pathology reports. Previous work has compared the results obtained by repeating the ER analysis in a central laboratory and the ER results abstracted from pathology reports and shown high concordance [54]. In addition, we used immunohistochemical results instead of molecular testing to classify tumour phenotype as this has been demonstrated to be an adequate surrogate [31].
One additional potential limitation of this study is the lack of uniformity of how BRCA testing was performed. BRCA status was abstracted from the medical record or patient survey and patients were tested in multiple institutions, however, it is important to note that testing during this timeframe would have been performed in a largely consistent manner, through Myriad Genetics. Although the majority of the women had known molecular testing, there are potential variations in the rates of testing during the enrollment period (2006–2016) given the changes in the indications for testing during that timeframe. Other cancer-predisposing gene mutations have been associated with a risk of different subtypes of breast cancer [55]. Given the timing of this cohort, which was mostly accrued pre-panel testing, we do not have available data on the prevalence of other cancer-predisposing gene mutations at this time (e.g., PALB2, TP53, CHEK2, CDH1) and future research is needed in this regard, particularly in young breast cancer patients. Lastly, there may also be an element of referral bias, as the majority of participants were identified at large academic medical centres even with our intentional inclusion of community sites.
Conclusion
Our consideration of BRCA1 and BRCA2 mutation status in a large cohort of young women with breast cancer enhances understanding of the distribution of tumour surrogate molecular subtypes in young women both with and without mutations. The opportunity to provide comparisons across young age groups, and the identification of more aggressive hormone receptor-positive phenotypes in this population also provide direction for future work to further understand and improve the disparate outcomes for young women with luminal B cancers, particularly those with BRCA2-associated breast cancer, with potential implications for tailored prevention and treatment.
Supplementary information
Acknowledgements
Abstract presented at the annual meeting of San Antonio Breast Cancer Symposium 2019 [56].
Author contributions
Study design and methodology: SMR, AHP and LCC. Data collection: YDGA, HV, CS, JDM and LCC. Data analysis and interpretation: YDGA, SMR, GK, AHP and LCC. Drafting manuscript: YDGA, SMR, AHP and LCC. Critical revisions: SMR, JEG, PDP, KJR, RMT, LS, VFR, SEC, EFB, JDM, EW, AHP and LCC. All the authors approved the final version.
Funding information
This work was supported by the Susan G. Komen Foundation and Breast Cancer Research Foundation.
Data availability
Available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Institutional review board (IRB) approval for the study was obtained through the Dana–Farber/Harvard Cancer Centre and other participating centres. The study was performed in accordance with the Declaration of Helsinki and informed written consent was obtained from all the participants.
Consent to publish
Not applicable.
Competing interests
Dr. Rulla Tamimi is an editor of the British Journal of Cancer. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ann H. Partridge, Laura C. Collins.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01597-2.
References
- 1.Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18:1579–89.. doi: 10.1016/S1470-2045(17)30677-0. [DOI] [PubMed] [Google Scholar]
- 2.Thomas A, Rhoads A, Pinkerton E, Schroeder MC, Conway KM, Hundley WG, et al. Incidence and survival among young women with stage I-III breast cancer: SEER 2000-2015. JNCI Cancer Spectr. 2019;3:pkz040. doi: 10.1093/jncics/pkz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. J Am Med Assoc. 2013;309:800–5. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 4.Azim HA, Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18:1341–51. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 5.Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34:3308–14. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 6.Keegan TH, DeRouen MC, Press DJ, Kurian AW, Clarke CA. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14:R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azim HA, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. 2014;16:427. doi: 10.1186/s13058-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. J Am Med Assoc. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 9.Allott EH, Geradts J, Cohen SM, Khoury T, Zirpoli GR, Bshara W, et al. Frequency of breast cancer subtypes among African American women in the AMBER consortium. Breast Cancer Res. 2018;20:12. doi: 10.1186/s13058-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Sahli Z, Wang Y, Wolff AC, Cope LM, Umbricht CB. Young age at diagnosis is associated with worse prognosis in the Luminal A breast cancer subtype: a retrospective institutional cohort study. Breast Cancer Res Treat. 2018;172:689–702. doi: 10.1007/s10549-018-4950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian W, Fu F, Lin Y, Lu M, Chen B, Yang P, et al. The impact of young age for prognosis by subtype in women with early breast cancer. Sci Rep. 2017;7:11625. doi: 10.1038/s41598-017-10414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Xiong XF, Mo YZ, Chen WG, Li M, Liang R, et al. Young age at diagnosis is associated with better prognosis in stage IV breast cancer. Aging. 2019;11:11382–90.. doi: 10.18632/aging.102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King MC, Marks JH, Mandell JB. Group NYBCS. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–6. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 14.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–89. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–71. [PMC free article] [PubMed] [Google Scholar]
- 16.Huzarski T, Byrski T, Gronwald J, Górski B, Domagala P, Cybulski C, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol. 2013;31:3191–6. doi: 10.1200/JCO.2012.45.3571. [DOI] [PubMed] [Google Scholar]
- 17.Young SR, Pilarski RT, Donenberg T, Shapiro C, Hammond LS, Miller J, et al. The prevalence of BRCA1 mutations among young women with triple-negative breast cancer. BMC Cancer. 2009;9:86. doi: 10.1186/1471-2407-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gewefel H, Salhia B. Breast cancer in adolescent and young adult women. Clin Breast Cancer. 2014;14:390–5. doi: 10.1016/j.clbc.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst. 1999;91:943–9. doi: 10.1093/jnci/91.11.943. [DOI] [PubMed] [Google Scholar]
- 20.Ha SM, Chae EY, Cha JH, Kim HH, Shin HJ, Choi WJ. Association of BRCA mutation types, imaging features, and pathologic findings in patients with breast cancer with BRCA1 and BRCA2 mutations. AJR Am J Roentgenol. 2017;209:920–8. doi: 10.2214/AJR.16.16957. [DOI] [PubMed] [Google Scholar]
- 21.Larsen MJ, Kruse TA, Tan Q, Lænkholm AV, Bak M, Lykkesfeldt AE, et al. Classifications within molecular subtypes enables identification of BRCA1/BRCA2 mutation carriers by RNA tumor profiling. PLoS ONE. 2013;8:e64268. doi: 10.1371/journal.pone.0064268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19:169–80. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toss A, Molinaro E, Venturelli M, Domati F, Marcheselli L, Piana S, et al. BRCA detection rate in an Italian Cohort of luminal early-onset and triple-negative breast cancer patients without family history: when biology overcomes genealogy. Cancers. 2020;12:1252. [DOI] [PMC free article] [PubMed]
- 24.Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol. 2011;29:3739–46. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131:1061–6. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 26.Poorvu PD, Gelber SI, Rosenberg SM, Ruddy KJ, Tamimi RM, Collins LC, et al. Prognostic impact of the 21-gene recurrence score assay among young women with node-negative and node-positive ER-positive/HER2-negative breast cancer. J Clin Oncol. 2020;38:725–33.. doi: 10.1200/JCO.19.01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–76. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 28.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomark Prev. 2007;16:439–43. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 32.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans DG, van Veen EM, Byers HJ, Evans SJ, Burghel GJ, Woodward ER, et al. High likelihood of actionable pathogenic variant detection in breast cancer genes in women with very early onset breast cancer. J Med Genet. 2021;1–7. [DOI] [PMC free article] [PubMed]
- 34.Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) Cancer Epidemiol Biomark Prev. 2012;21:134–47. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah PD, Patil S, Dickler MN, Offit K, Hudis CA, Robson ME. Twenty-one-gene recurrence score assay in BRCA-associated versus sporadic breast cancers: differences based on germline mutation status. Cancer. 2016;122:1178–84. doi: 10.1002/cncr.29903. [DOI] [PubMed] [Google Scholar]
- 36.Spurdle AB, Couch FJ, Parsons MT, McGuffog L, Barrowdale D, Bolla MK, et al. Refined histopathological predictors of BRCA1 and BRCA2 mutation status: a large-scale analysis of breast cancer characteristics from the BCAC, CIMBA, and ENIGMA consortia. Breast Cancer Res. 2014;16:3419. doi: 10.1186/s13058-014-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Am Med Assoc. 2017;317:2402–16. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 38.Jonasson JG, Stefansson OA, Johannsson OT, Sigurdsson H, Agnarsson BA, Olafsdottir GH, et al. Oestrogen receptor status, treatment and breast cancer prognosis in Icelandic BRCA2 mutation carriers. Br J Cancer. 2016;115:776–83. doi: 10.1038/bjc.2016.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotsopoulos J, Singer C, Narod SA. Can we prevent BRCA1-associated breast cancer by RANKL inhibition? Breast Cancer Res Treat. 2017;161:11–6. doi: 10.1007/s10549-016-4029-z. [DOI] [PubMed] [Google Scholar]
- 40.Nolan E, Vaillant F, Branstetter D, Pal B, Giner G, Whitehead L, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med. 2016;22:933–9. doi: 10.1038/nm.4118. [DOI] [PubMed] [Google Scholar]
- 41.Kotsopoulos J, Huzarski T, Gronwald J, Singer CF, Moller P, Lynch HT, et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed]
- 42.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. J Am Med Assoc. 2010;304:967–75. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26:1331–7. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rebbeck TR, Friebel T, Wagner T, Lynch HT, Garber JE, Daly MB, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–10. doi: 10.1200/JCO.2004.00.8151. [DOI] [PubMed] [Google Scholar]
- 45.Mavaddat N, Antoniou AC, Mooij TM, Hooning MJ, Heemskerk-Gerritsen BA, Noguès C, et al. Risk-reducing salpingo-oophorectomy, natural menopause, and breast cancer risk: an international prospective cohort of BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2020;22:8. doi: 10.1186/s13058-020-1247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl J Med. 2018;379:753–63. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol. 2010;21:1974–81. doi: 10.1093/annonc/mdq072. [DOI] [PubMed] [Google Scholar]
- 48.Schaffar R, Bouchardy C, Chappuis PO, Bodmer A, Benhamou S, Rapiti E. A population-based cohort of young women diagnosed with breast cancer in Geneva, Switzerland. PLoS ONE. 2019;14:e0222136. doi: 10.1371/journal.pone.0222136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franzoi MA, Rosa DD, Zaffaroni F, Werutsky G, Simon S, Bines J, et al. Advanced stage at diagnosis and worse clinicopathologic features in young women with breast cancer in Brazil: a subanalysis of the AMAZONA III study (GBECAM 0115) J Glob Oncol. 2019;5:1–10. doi: 10.1200/JGO.19.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol. 2011;29:e18–20. doi: 10.1200/JCO.2010.28.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison DH, Rahardja D, King E, Peng Y, Sarode VR. Tumour biomarker expression relative to age and molecular subtypes of invasive breast cancer. Br J Cancer. 2012;107:382–7. doi: 10.1038/bjc.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cherbal F, Gaceb H, Mehemmai C, Saiah I, Bakour R, Rouis AO, et al. Distribution of molecular breast cancer subtypes among Algerian women and correlation with clinical and tumor characteristics: a population-based study. Breast Dis. 2015;35:95–102. doi: 10.3233/BD-150398. [DOI] [PubMed] [Google Scholar]
- 53.Walsh T, Gulsuner S, Lee MK, Troester MA, Olshan AF, Earp HS, et al. Inherited predisposition to breast cancer in the Carolina Breast Cancer Study. NPJ Breast Cancer. 2021;7:6. doi: 10.1038/s41523-020-00214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins LC, Marotti JD, Baer HJ, Tamimi RM. Comparison of estrogen receptor results from pathology reports with results from central laboratory testing. J Natl Cancer Inst. 2008;100:218–21. doi: 10.1093/jnci/djm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu C, Polley EC, Yadav S, Lilyquist J, Shimelis H, Na J, et al. The contribution of germline predisposition gene mutations to clinical subtypes of invasive breast cancer from a clinical genetic testing cohort. J Natl Cancer Inst. 2020;12:1231–41. [DOI] [PMC free article] [PubMed]
- 56.Guzman-Arocho YD, Rosenberg SM, Poorvu P, Ruddy KJ, Kirkner G, Snow C, et al. Clinicopathological features and BRCA 1/2 status in a large prospective cohort of young women with breast cancer. Proceedings of the 2019 San Antonio Breast Cancer Symposium; 2019 Dec 10-14; San Antonio, TC. AACR; Cancer Res. 2020;80 (4 Suppl):Abstract P4-07-02.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available from the corresponding author on reasonable request.