Abstract
Countries continue to debate the need for decontamination of cold-chain food packaging to reduce possible severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) fomite transmission among frontline workers. While laboratory-based studies demonstrate persistence of SARS-CoV-2 on surfaces, the likelihood of fomite-mediated transmission under real-life conditions is uncertain. Using a quantitative microbial risk assessment model of a frozen food packaging facility, we simulated 1) SARS-CoV-2 fomite-mediated infection risks following worker exposure to contaminated plastic packaging; and 2) reductions in these risks from masking, handwashing, and vaccination. In a frozen food facility without interventions, SARS-CoV-2 infection risk to a susceptible worker from contact with contaminated packaging was 1.5 × 10−3 per 1h-period (5th – 95th percentile: 9.2 × 10−6, 1.2 × 10−2). Standard food industry infection control interventions, handwashing and masking, reduced risk (99.4%) to 8.5 × 10−6 risk per 1h-period (5th – 95th percentile: 2.8 × 10−8, 6.6 × 10−5). Vaccination of the susceptible worker (two doses Pfizer/Moderna, vaccine effectiveness: 86–99%) with handwashing and masking reduced risk to 5.2 × 10−7 risk per 1h-period (5th – 95th percentile: 1.8 × 10−9, 5.4 × 10−6). Simulating increased transmissibility of current and future variants (Delta, Omicron), (2-, 10-fold viral shedding) among a fully vaccinated workforce, handwashing and masking continued to mitigate risk (1.4 × 10−6 - 8.8 × 10−6 risk per 1h-period). Additional decontamination of frozen food plastic packaging reduced infection risks to 1.2 × 10−8 risk per 1h-period (5th – 95th percentile: 1.9 × 10−11, 9.5 × 10−8). Given that standard infection control interventions reduced risks well below 1 × 10−4 (World Health Organization water quality risk thresholds), additional packaging decontamination suggest no marginal benefit in risk reduction. Consequences of this decontamination may include increased chemical exposures to workers, food quality and hazard risks to consumers, and unnecessary added costs to governments and the global food industry.
Keywords: COVID-19, Quantitative microbial risk assessment, Cold-chain fomite-mediated transmission, Plastic packaging
1. Introduction
According to the World Health Organization (WHO, 2020a) and the United States (U.S.) Centers for Disease Control and Prevention (CDC, 2021), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) fomite-mediated transmission is rare (Lewis, 2021; Mondelli et al., 2021), compared to the predominant aerosol and droplet transmission modes (Meyerowitz et al., 2021). Fomites (e.g., surfaces) can become contaminated from an infected individual by: 1) shedding onto hands which then touch a surface; or 2) expelled respiratory particles (from coughing, speaking) (Bourouiba, 2020; Morawska et al., 2009) which then fall to a surface (Fernstrom & Goldblatt, 2013). An individual may then transfer infectious particles from a contaminated surface to their facial mucosa (Bueckert et al., 2020). However, definitive epidemiological evidence of fomite transmission is lacking. Few case reports implicate fomites as a possible SAR-CoV-2 source (Cai et al., 2020; Xie et al., 2020) of which, asymptomatic aerosol transmission could not be eliminated as an alternative transmission mode.
Despite these sparse data, a report of the isolation of infectious SARS-CoV-2 from imported frozen cod packaging in Qingdao, China (Liu et al., 2020) has raised alarm for fomites to serve as vectors for seeding SARS-CoV-2 into areas with controlled transmission (Ji et al., 2021). Further, laboratory studies suggest prolonged SARS-CoV-2 infectivity (days to weeks) (Riddell et al., 2020) on surfaces (Pastorino et al., 2020; van Doremalen et al., 2020) and low temperatures and humidity (common in cold-chain conditions) were associated with virus stability (months or longer) (Aboubakr et al., 2021). SARS-CoV-2 viral RNA has been detected on surfaces in playgrounds, retail stores (Harvey et al., 2021; Singh et al., 2021), and healthcare settings (Jiang et al., 2020; Ong et al., 2020). However, the relationship between detectable viral RNA and infectious virus is tenuous (estimated 4:1 ratio viral RNA copies to infectious virus) (Sender et al., 2021). Of 63 studies testing for SARS-CoV-2 RNA on surfaces, only 13 attempted to isolate infectious virus. Of these, viable SARS-CoV-2 virus was identified in only four instances: frozen cod packaging (Liu et al., 2020), a nightstand of an infected case (Marcenac et al., 2021), an isolation room of patients undergoing mechanical ventilation (Ahn et al., 2020), and on a windowsill of a patient's quarantine unit (Santarpia et al., 2020). In a cold-chain food setting, evidence is lacking on the frequency of SARS-CoV-2 contamination on packaging and the infection risks to workers.
To prevent potential SARS-CoV-2 outbreaks associated with imported food products, China implemented testing and disinfection (e.g., wet wiping of plastic packaging (Ji et al., 2021; Malenovska, 2020)) on all imported cold-chain (temperature-controlled transport and storage) products and packaging. However, there is no definitive evidence of SARS-CoV-2 fomite transmission from contact with contaminated food or packaging (Goldman, 2021), suggesting that these decontamination measures may be extreme (Goldman, 2020; Lewis, 2021) and may lead to unintended chemical exposures for workers and consumers (Dewey et al., 2021). Thus, using a quantitative microbial risk assessment (QMRA) model, our goals were to simulate in a frozen food packaging facility: 1) SARS-CoV-2 fomite-mediated infection risks following worker exposure to contaminated plastic packaging; and 2) reductions in these risks from masking, handwashing, vaccination, and additional packaging decontamination.
2. Materials and methods
2.1. Model overview
We applied the validated QMRA model of Sobolik et al. (2022) to simulate contamination of plastic packaging (cartons, plastic-wrapped palletized cartons) with SARS-CoV-2 respiratory particles from two coughing, infected workers. We simulated the SARS-CoV-2 exposure doses and infection risks to a susceptible worker in a receiving warehouse resulting exclusively from fomite transmission.
2.2. Model structure
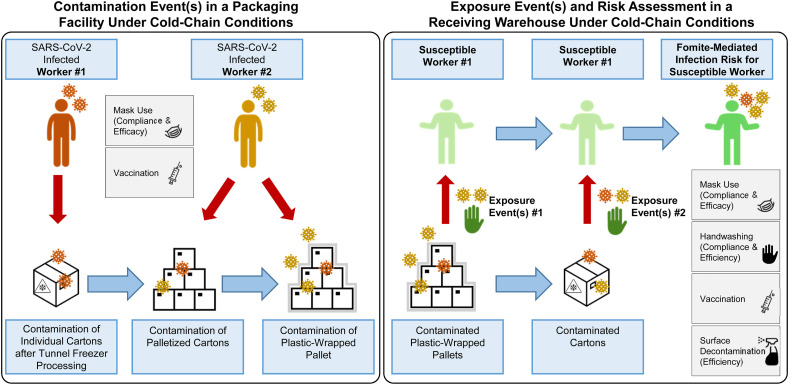
The model initiates with two infected workers in a representative frozen food manufacturing facility (Fig. 1 ). In this facility, products (e.g., potatoes, blueberries, peas etc.) are placed within a blast tunnel quick freezer (−18 °C), where they are frozen along its conveyor belt. Once frozen, products exit the tunnel freezer and fall into individual plastic-lined cartons. We assumed the first worker was within ≤3 feet of the cartons, with an estimated 144–216 cartons (dimensions: [0.38m × 0.28m x 0.15m] or [0.38m × 0.30m x 0.23m]) processed per hour. The second infected worker transferred these cartons onto a wooden pallet (36–54 cartons/pallet), either manually or by automation, and then plastic-wrapped the pallet (four pallets processed/hour). We assumed infected workers coughed SARS-CoV-2-laden aerosol (<50 μm) and droplet (50–750 μm) respiratory particles when in close proximity to the cartons, during palletization, and plastic-wrap processing. Plastic-wrapped, palletized cartons were stored and transported under cold-chain conditions (−20 °C) to a receiving warehouse where a susceptible worker was exposed to the virus exclusively via direct contact with contaminated plastic wrap and/or surface-contaminated cartons during manual unpacking of the pallets. Ambient air temperature outside of the tunnel freezer in the frozen food facility and in the receiving warehouse was assumed to be 4 °C. Workers were assumed to wear gloves continuously (glove changes not simulated) following current Good Manufacturing Practices (FDA, 2016).
Fig. 1.
Conceptual framework for fomite-mediated SARS-CoV-2 transmission involving exposure of a susceptible worker to individual plastic cartons, palletized cartons, and plastic wrap in a receiving warehouse under cold-chain conditions. This schematic depicts a representative frozen food manufacturing facility, initiating with two infected workers (left panel). Up to 10 contamination events per infected worker (0–10 coughs) can occur at three stages in the packaging pipeline (See Materials and methods): 1) contamination of the top-face of individual plastic cartons (144–216 individual cartons processed per hour) via respiratory droplet and aerosol fallout from the first infected worker (orange in schematic) while closing cartons filled with frozen product at the end of the tunnel freezer; 2) contamination of cartons via respiratory particle spray (droplets and aerosols) as cartons are placed (manually or via automation) on a pallet by the second infected worker (yellow in schematic); and 3) contamination of the plastic-wrapped palletized cartons by respiratory particle spray (droplet and aerosol) from the second infected worker (yellow in schematic). Four pallets, each containing approximately 36–54 individual plastic cartons, are processed per hour. All workers were assumed to be continuously wearing gloves (glove changes were not simulated) and the model assumed there was no indirect transfer of virus from the infected workers' hands to the plastic fomites along the packaging pipeline. Given current estimates of limited to no SARS-CoV-2 viral decay at and below 4 °C, the model assumed no loss in viral infectivity during the duration of cold-chain storage and shipment (−20 °C) of individual and plastic-wrapped palletized cartons prior to their handling and during unloading by a susceptible worker in a receiving warehouse. Infection risks resulting exclusively from fomite transmission were simulated as contacts between the susceptible worker's fingers and palms (of both hands) and the fomite surface (accounting for the surface area of the hand relative to the fomite surface); virus transfer from fomite to hands; and virus transfer from fingertips to facial mucous membranes (accounting for the surface area of the fingers relative to the combined surface area of the eyes, nose, and mouth). Gray boxes indicate infection control interventions implemented for the infected (masking, vaccination) and susceptible (handwashing, masking, vaccination) workers. In the scenarios with additional plastic surface decontamination, this was simulated prior to the susceptible worker contacting the fomites.
The two model outcomes included: 1) the SARS-CoV-2 infection risks from fomite-mediated exposures to the cartons and plastic-wrapped pallets following a 1-hour period; and 2) the relative reduction in SARS-CoV-2 infection risk from masking, handwashing, vaccination, and package surface decontamination. The model was developed in R (v.4.0.3; R Development Core Team; Vienna, Austria) using the “mc2d” package (Pouillot & Delignette-Muller, 2010). We conducted 10,000 Monte Carlo iterations for each scenario and reported the median infection risk with 5th and 95th percentiles. Additional details on model assumptions, vetting, and stability, and variability/uncertainty analyses are in Appendix A Supplementary data.
2.3. Data sources
Model parameters were derived from the peer-reviewed literature (Table 1 ) and included: (i) viral shedding through cough events; (ii) fomite-mediated transmission parameters; (iii) dose-response parameters for SARS-CoV-2 infection risk; and (iv) risk mitigation interventions.
Table 1.
Model parameter inputs and distributions.
| Parameter | Units | Description | Distribution | Input Values | Type of Variability/Uncertainty | Citations |
|---|---|---|---|---|---|---|
|
Viral Shedding |
||||||
| Log10(Cvirus) | PFU/mL | Concentration of virus in saliva | Triangular | 6.8 (6.1, 7.4) | Variability and parameter uncertainty | (To et al., 2020; Wolfel et al., 2020) |
| 100X increased viral shedding | 8.8 (8.1, 9.4) | |||||
| 10X increased viral shedding | 7.8 (7.1, 8.4) | |||||
| 2X increased viral shedding | 7.1 (6.4, 7.7) | |||||
| VF,c | mL/Cough | Fraction of volume associated with aerosols (2–45 μm) | Triangular | 2.3 × 10−6 (1.4 × 10−6, 2.6 × 10−6) | Variability and parameter uncertainty | Chao et al. (2009) |
| VF,c | mL/Cough | Fraction of volume associated with droplets (50–60 μm) | Triangular | 6.0 × 10−6 (3.5 × 10−6, 6.7 × 10−6) | Variability and parameter uncertainty | Chao et al. (2009) |
| VF,c | mL/Cough | Fraction of volume associated with droplets (60–100 μm) | Triangular | 4.9 × 10−6 (1.1 × 10−6, 8.4 × 10−6) | Variability and parameter uncertainty | Chao et al. (2009) |
| VF,c | mL/Cough | Fraction of volume associated with droplets (100–750 μm) | Triangular | 6.8 × 10−3 (4.0 × 10−3, 7.6 × 10−3) | Variability and parameter uncertainty | Chao et al. (2009) |
| FC | Cough/h | Number of coughs per hour | Empirical | 1/11 equal probability (0, 10) | Variability and parameter uncertainty | (Adhikari et al., 2019; Loudon & Roberts, 1967) |
| λvirus | Hour | Aerosol viral decay of SARS-CoV-2 at 40% relative humidity, 38 °F | Point value | 0 | Fixed parameter | (DHS, 2021a) |
| pp | Probability | Probability respiratory particles will remain in the air as respiratory spray between 0 and 1m distancing | Uniform | 50–60 μm: | Assumption uncertainty | Bourouiba et al. (2014) |
| 1m: 0.82; | ||||||
| 60–100 μm: | ||||||
| 1m: 0.44; | ||||||
| >100 μm: | ||||||
| 1m: 0.04 | ||||||
| ppdroplets | Probability | Probability respiratory particles (>100 μm) will remain in the air as respiratory spray between 0 and 1m distancing | Uniform | (0.01, 0.22) | Assumption uncertainty | Bourouiba et al. (2014) |
| ppfalldroplets |
Probability |
Probability respiratory particles (>100 μm) will settle to the fomite surfaces between 0 and 1m distancing |
Uniform |
(0.07, 0.78) |
Assumption uncertainty |
Bourouiba et al. (2014) |
|
Risk Mitigation Interventions1 |
||||||
| Smask | Log reduction | Source protection surgical mask efficacy | Uniform | (0.39, 0.57) | Variability | (Lindsley et al., 2021; Maurer et al., 2021; Ueki et al., 2020) |
| RSmask | Percent reduction | Recipient surgical mask efficacy | Uniform | (0.37, 0.998) | Variability | (Lindsley et al., 2021; Maurer et al., 2021; Ueki et al., 2020) |
| SDeff | Log reduction | Plastic fomite surface decontamination efficiency | Point value | 3 Log10 virus | Fixed parameter | (EPA, 2020; Malenovska, 2020) |
| HWeff | Log reduction | Handwashing efficiency | Point value | 2 Log10 virus | Fixed parameter | (Grove et al., 2006; Liu et al., 2010) |
| HWfreq | Handwashing/h | Frequency of handwashing per hour | Point value | 1.0 | Fixed parameter | Expert elicitation |
| Rair | Air changes/h | Frequency of room air changes per hour (ACH) | Point value | 2.0 | Fixed parameter | Expert elicitation |
| VEoptimal | Percent reduction | Vaccine effectiveness (VE) | Uniform | (0.86, 0.99) | Variability | (Andrejko et al., 2021; Pawlowski et al., 2021; Swift et al., 2021) |
| VEreduced | Percent reduction | Vaccine effectiveness (VE) | Uniform | (0.64, 0.80) | Variability | (Khan & Mahmud, 2021; Moustsen-Helms et al., 2021) |
| VET |
Percent reduction |
Vaccine effectiveness against transmission (VET) |
Triangular |
0.89 (0.82, 0.95) |
Variability |
Prunas et al. (2021) |
|
Fomite-Mediated Transmission |
||||||
| SAcarton.top | m2 | Surface area of top of individual plastic carton | Uniform | (0.106, 0.116) | Variability and parameter uncertainty | Assumed |
| SAcarton | m2 | Surface area of a single individual plastic carton | Uniform | (0.41, 0.54) | Variability and parameter uncertainty | Assumed |
| Cartons | Cartons/h | Number of individual plastic cartons processed per h | Uniform | (144, 216) | Variability and parameter uncertainty | Assumed |
| Pallets | Pallets/h | Number of pallets processed per h | Point value | 4.0 | Fixed parameter | Assumed |
| SAplasticwrap.side | m2 | Surface area of a single side of plastic wrapped pallet | Uniform | (4.20, 6.97) | Variability and parameter uncertainty | Assumed |
| SAplasticwrap | m2 | Surface area of entire plastic wrapped pallet | Uniform | (25.2, 41.8) | Variability and parameter uncertainty | Assumed |
| Fingerssa | m2 | Surface area of three finger tips touching the surface | Point value | 0.00042 | Fixed parameter | Bouwknegt et al. (2015) |
| Hsa | m2 | Area of two hands (palms only) | Point value | 0.049 | Fixed parameter | Bouwknegt et al. (2015) |
| decay.time | Days | Transport time between the frozen food manufacturing facility and the receiving warehouse (days) | Uniform | (30, 90) | Variability | Assumed |
| Fdecay | Days−1 | Viral decay rate (PFU per day) | Uniform | (0.14, 0.22) | Variability | Kwon et al. (2021) |
| TEfh | PFU | Viral transfer fraction from fomite to hand with relative humidity (15–32%); acrylic surface 2 | Triangular | 0.217 (0.067, 0.367) | Variability and parameter uncertainty | Lopez et al. (2013) |
| TEhf | PFU | Viral transfer fraction from hand to fomite surface | Point value | 0.025 | Fixed parameter | (Bean et al., 1982; Greene et al., 2015; Nicas & Jones, 2009; Rusin et al., 2002) |
| TEhm | PFU | Viral transfer fraction from hand to face | Triangular | 0.200 (0.137, 0.263) | Variability and parameter uncertainty | Lopez et al. (2013) |
| freq.hs | Contacts/min | Frequency of contacts from hand to individual plastic cartons | Point value | Cartons/60 | Fixed parameter | Assumed |
| freq.hs.pw | Contacts/min | Frequency of contacts from hand to plastic wrap | Uniform | (4/60, 20/60) | Variability and parameter uncertainty | Assumed |
| freq.hf | Contacts/min | Frequency of contacts from hand to face | Point value | 0.80 | Variability and parameter uncertainty | Nicas and Best (2008) |
| Handdecay | Minutes | Viral decay rate on hands (PFU/min) | Uniform | (0.92, 1.47) | Variability | Nicas and Jones (2009) |
| eyes.sa | m2 | Surface area of mucous membranes—eyes | Uniform | (1 × 10−5, 2 × 10−4) | Variability | Wilson et al. (2018) |
| nose.sa | m2 | Surface area of mucous membranes—nose | Uniform | (1 × 10−5, 1 × 10−3) | Variability | Wilson et al. (2018) |
| mouth.sa |
m2 |
Surface area of mucous membranes—mouth |
Uniform |
(1 × 10−4, 4.1 × 10−3) |
Variability |
Wilson et al. (2018) |
|
SARS-CoV-2 Dose and Risk Characterization |
||||||
| Ratioinfectious | No units | Infectious to non-infectious ratio | Point value | 1:100 | Fixed parameter | Pitol and Julian (2021) |
| krisk | PFU−1 | Dose-response parameter | Point value | 0.00680 | Fixed parameter | Pitol and Julian (2021) |
Note.1All interventions were assumed to be implemented with 100% compliance. 2Fomite-to-hand transfer rate derived from laboratory studies with bacteriophage MS2.
2.4. Fomite-mediated transmission modeling
SARS-CoV-2 contamination of the plastic cartons was calculated using the combined aerosol and droplet particle fallout, Fallt,a (infectious virus) and Fallt (infectious virus/m3) by the first infected, coughing worker as described (Sobolik et al., 2022). Contamination of the palletized cartons and plastic-wrapped pallets was calculated using the combined aerosol and droplet particle spray, Ct,aerosol (plaque-forming unit [PFU]/m3) and Ct,droplet (PFU/m3), expelled from coughs by the second infected worker as described (Sobolik et al., 2022) with the resulting fomite surface viral concentration:
Cartons, Fomitecartons, (PFU/m2):
Plastic-wrapped pallets, Fomiteplasticwrap, (PFU/m2):
where fV was the facility air volume (m3), Hsa was the surface area of the susceptible worker's hand touching the fomite surface (m2), SAcontamcompcart was the cross-sectional area of the composite contaminated individual cartons (m2), SAcompcart was the cross-sectional area of the composite individual cartons (m2), SAcontamcomppw was the cross-sectional area of the contaminated plastic wrap (m2), and SAcomppw was the cross-sectional area of the composite total plastic wrap (m2). When calculating contamination of the packaging, aerosol particles (<50 μm) were assumed to be homogenously mixed throughout the facility, consistent with the QMRA modeling of Azimi et al. (2021); Nicas et al. (2005); Zhang et al. (2021). The droplet proportion (50–750 μm) capable of reaching the cartons (droplet fallout) or plastic wrap (droplet spray) within 0–3 feet distancing was derived from previous models (Bourouiba et al., 2014).
The SARS-CoV-2 concentration transferred to a hand, Chand,carton (PFU/h), following contact with the cartons, Fomitecartons (PFU/m2), was calculated as per (Nicas & Best, 2008).
Hsurface,carton was the contact frequency between the hand and the cartons (contacts/min), Fomitecartons was the viral concentration on the cartons (PFU/m2) at time t, TEfh was the proportion of virus transferred from fomite to hand, and λv,hand was the SARS-CoV-2 viral decay on the hand. The same approach was taken for calculating the SARS-CoV-2 concentration transferred to a hand, Chand,pw (PFU/h), following contact with the plastic wrap (Appendix A Supplementary data).
2.5. Risk assessment
The fomite-mediated dose (Dfomite,i) to the susceptible worker following contact while unloading the palletized cartons was calculated from the viral contamination on the hand (Chand,i) at time t, where i = carton or plastic wrap, the frequency of hand-to-face contacts (Hface), the surface area of the hands (Hsa), the surface area ratio of fingers (Fsa) to face (Facesa), the fraction of pathogens transferred from hand-to-face (TEhm), and the exposure duration (t).
The total viral dose, Dfomite,total (PFU), at time t, was:
The probability of SARS-CoV-2 infection to the susceptible worker was calculated using Dfomite,total (Appendix A Supplementary data).
2.6. Evaluating infection control interventions
Standard infection control interventions were selected based on current industry (FAO, 2012; FDA, 2015) and coronavirus disease 2019 (COVID-19) prevention practices (FDA, 2020; WHO, 2020b). These interventions included masking (surgical), hourly handwashing of ungloved hands (2 log10 virus removal) (Grove et al., 2015), and vaccination (two doses of Pfizer/Moderna) of: 1) only the susceptible worker in the receiving warehouse; and 2) all workers, and assuming breakthrough infections among vaccinated workers (Appendix A Supplementary data). To handwashing and masking, we simulated the added effect of surface decontamination (3 log10 virus removal) (EPA, 2020; Malenovska, 2020) applied directly to plastic packaging (cartons, plastic wrap) as described (Ji et al., 2021). As there are no infection risk targets for food manufacturing workers, we applied the targets of 1.0 × 10−4 and 1.0 × 10−6 used by Ryan et al. (2014); Wilson et al. (2021).
2.7. Data availability
Model code developed and used in this study is available to readers through GitHub at the following DOI: https://doi.org/10.5281/zenodo.5904275.
3. Results and discussion
3.1. SARS-CoV-2 fomite-mediated infection risks to unvaccinated workers
Assuming no SARS-CoV-2 immunity from vaccination or prior infection, the risk of fomite-mediated transmission without standard infection control interventions was 1.5 × 10−3 per 1h-period (5th – 95th percentile: 9.2 × 10−6, 1.2 × 10−2) (Fig. 2 A). This is consistent with (Wilson et al., 2021), who simulated an infection risk of approximately 1.0 × 10−3 resulting from a single contact with a high SARS-CoV-2 bioburden (1 to 10,000 genome copies/cm2)-contaminated fomite with no disinfection. Similarly, comparable fomite-mediated risks on the order of 1 in 10,000 were reported by Pitol & Julian (2021) and Harvey et al. (2021) associated with contacting community fomites (playgrounds, crosswalk buttons etc.). In contrast, higher relative risks associated with SARS-CoV-2 fomite transmission (range: 2 × 10−2 – 3.2 × 10−1 infection risks) were reported in modeling studies of child daycare centers (Kraay et al., 2021) and hospitals (Jones, 2020; Mizukoshi et al., 2021). SARS-CoV-2 bioburden on environment-specific fomites (Wilson et al., 2021) and fomite-specific contact frequencies likely explain these differences in risk estimates. These data confirm that even in the absence of interventions, exposure to packaging under cold-chain conditions resulted in very low (under 2.0 × 10−3) fomite-mediated risks.
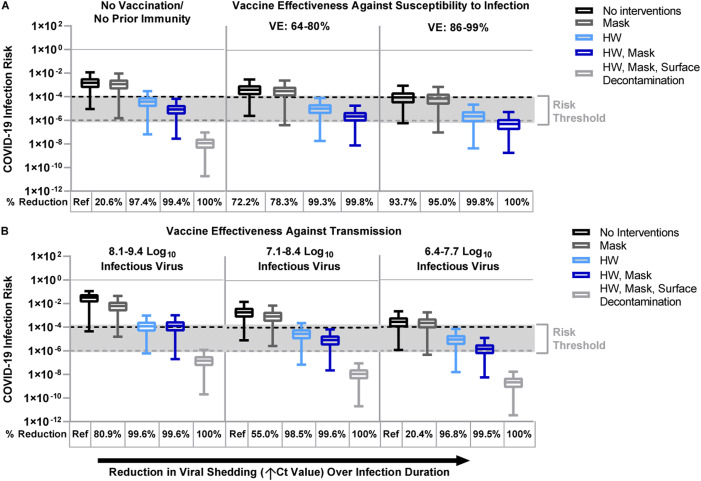
Fig. 2.
Fomite-mediated SARS-CoV-2 infection risks associated with individual and combined standard infection control interventions (hourly handwashing of ungloved hands [2 log10 virus removal efficiency] (Grove et al., 2015), surgical masking). Vaccination was incorporated into the model representing two doses of mRNA vaccine (Moderna/Pfizer) and was applied with and without the standard infection control interventions. Additional decontamination of plastic packaging [3 log10 virus removal efficiency] (EPA, 2020) was applied in combination with the standard infection control interventions. Ventilation (two air changes per hour [ACH]) was applied to all simulations. An infectious to non-infectious particle ratio of 1:100 (Pitol & Julian, 2021) was applied to all viral shedding concentrations. Reductions in SARS-CoV-2 infection risk (%) to the susceptible worker relative to no interventions are reported below each panel. Panel A represents the impact of standard infection control interventions with and without vaccination on fomite-mediated SARS-CoV-2 risk. For the first vaccination scenario, we assumed only the susceptible worker was vaccinated with two doses of mRNA vaccine (Moderna/Pfizer) and vaccine effectiveness (VE) against susceptibility to infection was simulated across three vaccination states. These included: 1) no vaccination/no prior immunity; 2) lower VE ranging from 64% (Moustsen-Helms et al., 2021) - 80% (Khan & Mahmud, 2021) representative of reduced protection (variants of concern, waning immunity, immunocompromised and elderly or at-risk populations); and 3) optimal VE ranging from 86% (Andrejko et al., 2021; Pawlowski et al., 2021) – 99% (Swift et al., 2021) among healthy adults 14 days or more after second mRNA dose. Panel B: the second vaccine scenario represented vaccine effectiveness against transmission, where all workers are assumed to be vaccinated with two doses of mRNA vaccines and hence the model simulated breakthrough infections. Vaccine effectiveness against transmission (VET) was modeled by applying the combined effect of the reduction in risk of infection to the susceptible worker and the risk of transmissibility given a breakthrough infection among the vaccinated workers. We used the VET estimate (88.5% [95% CI: 82.3%, 94.8%]) derived from Prunas et al. (2021). VET was modeled across a range of three peak infectious viral shedding concentrations representative of possible increased transmissibility and/or infectiousness of variants of concern: 1) 8.1–9.4 log10 viral particles; 2) 7.1–8.4 log10 viral particles; and 3) 6.4–7.7 log10 viral particles. These viral shedding levels represent 100-, 10-, and 2-times, respectively, the increased viral shedding concentration simulated in the base model analysis. Dashed lines represent 1:10,000 (black) and 1:1,000,000 (gray) infection risk targets. Results are presented as the median risk values with 5th and 95th percentile bars.
We then evaluated the risk reductions from standard infection control interventions. Masking reduced risk by 20.6% (1.2 × 10−3 risk per 1h-period, 5th – 95th percentile: 1.5 × 10−6, 9.4 × 10−3), handwashing by 97.4% (3.9 × 10−5 risk per 1h-period, 5th – 95th percentile: 6.6 × 10−8, 2.9 × 10−4), and handwashing with masking by 99.4% (8.5 × 10−6 risk per 1h-period, 5th – 95th percentile: 2.8 × 10−8, 6.6 × 10−5), relative to no interventions. Similarly, Pitol and Julian (2021) demonstrated that hand hygiene could substantially reduce the risk of SARS-CoV-2 transmission from contaminated surfaces. In an 8-hour shift, cumulative fomite risks remained very low (handwashing and masking: 7.6 × 10−5 [5th – 95th percentile: 1.7 × 10−7, 5.8 × 10−4]). To contextualize these risks, handwashing and masking effectively reduce risk across varying exposure durations and to well below WHO risk guidelines for drinking water (Cryptosporidium [9.5 × 10−4], Campylobacter [7.3 × 10−4], and rotavirus [2.4 × 10−3]) (WHO, 2017).
The addition of plastic surface decontamination to these standard infection control interventions reduced risks by 100% (1.2 × 10−8 risk per 1h-period, 5th – 95th percentile: 1.9 × 10−11, 9.5 × 10−8), relative to no interventions. Because risk reductions from masking and handwashing (99.4%, 8.5 × 10−6 risk per 1h-period) already fell well below risk targets of 1 × 10−4 (Ryan et al., 2014; Wilson et al., 2021), additional decontamination of frozen food packaging suggested minimal added benefit in risk reduction.
3.2. Impact of vaccination on SARS-CoV-2 fomite-mediated infection risks to workers
Vaccination of the susceptible worker with two doses of mRNA vaccine, without additional infection control interventions, reduced infection risk by 93.7% (optimal vaccine efficacy [VE] 86–99%: 9.6 × 10−5 risk per 1h-period, 5th – 95th percentile: 6.2 × 10−7, 9.6 × 10−4), relative to no vaccination (Fig. 2A). Optimal VE (86–99%) combined with infection control interventions further enhanced the risk reduction by 95.0% (masking: 7.5 × 10−5 risk per 1h-period, 5th – 95th percentile: 9.9 × 10−8, 7.4 × 10−4), 99.8% (hourly handwashing: 2.4 × 10−6 risk per 1h-period, 5th – 95th percentile: 4.4 × 10−9, 2.3 × 10−5), and 100% (hourly handwashing and masking: 5.2 × 10−7 risk per 1h-period, 5th – 95th percentile: 1.8 × 10−9, 5.4 × 10−6), relative to no vaccination (Fig. 2A). Across all vaccination states (no vaccination/no partial immunity, reduced VE 64–80%, and optimal VE 86–99%), combined handwashing and masking ensured SARS-CoV-2 fomite-mediated infection risks ranged between 8.5 × 10−6 (no vaccination) to 5.2 × 10−7 (optimal VE).
Importantly, these VE ranges encompass uncertainties in vaccine effectiveness with waning immunity (Wang et al., 2021) and emerging SARS-CoV-2 variants (Liu et al., 2021), heterogeneity in vaccine effectiveness, and variable vaccine protection among higher-risk populations (Monin et al., 2021). Based on these results, fomite-mediated risks will continue to decrease with increased vaccination rates among food workers.
3.3. Impact of infection control interventions on SARS-CoV-2 fomite-mediated risk to workers from new variants of concern (VOC)
To account for variations in the infectiousness of new VOC, we simulated increased viral shedding concentrations (2-, 10-, and 100-fold) resulting from breakthrough infections among a fully vaccinated workforce (two doses of Pfizer/Moderna; vaccine effectiveness against transmission (VET) 88.5% [5th – 95th percentile: 82.3%, 94.8%]) (Prunas et al., 2021). Ten-fold increased viral shedding (7.1–8.4 log10 infectious virus) resulted in an infection risk of 2.0 × 10−3 per 1h-period (5th – 95th percentile: 8.4 × 10−6, 1.6 × 10−2) (Fig. 2B). Handwashing and masking substantially reduced this risk by 99.6% (8.8 × 10−6 risk per 1h-period, 5th – 95th percentile: 2.3 × 10−8, 7.0 × 10−5), relative to no interventions. Similar trends were observed when using a 2-fold increased viral shedding. In the rare event of a VOC inducing a 100-fold increased viral shedding (8.1–9.4 log10 infectious virus), handwashing and masking still led to small fomite-mediated risks of 1.3 × 10−4 risk per 1h-period (5th – 95th percentile: 2.0 × 10−7, 1.0 × 10−3). Although new data on SARS-CoV-2 B.1.617.2 (Delta) and other VOCs continue to emerge (B.1.1.529 [Omicron]), the analysis presented here captures the increased viral shedding of Delta (median 7.83 log10 copies/mL [range: 6.3–8.83 log10 copies/mL]), which is estimated to be ten times higher and 40–60% more transmissible than historical variants (Callaway, 2021; Teyssou et al., 2021).
Risks from this study are conservative estimates of fomite-mediated transmission. Because the fraction of SARS-CoV-2 that enters mucous membranes via fomite-mediated transmission is likely smaller than through intranasal administration (basis of dose-response model), our model may overestimate fomite-mediated risks. Moreover, viral decay during product transport to the receiving warehouse was not included in the primary risk analysis given sparse data on viral stability on surfaces at 4 °C and below (DHS, 2021b; Riddell et al., 2020). When incorporating viral persistence data from laboratory studies conducted at 5 °C on surfaces (Kwon et al., 2021), extended transport duration (30–90 days) reduced risk by 2.2–6.9 log10, relative to the baseline scenario (Appendix A Supplementary data). Further, while analyses in this study were conducted with a 1:100 infectious to non-infectious particle ratio (Pitol & Julian, 2021), fomite-mediated transmission will be even less likely with ratios of 1:1,000–1:1,000,000, as studies suggest (McCormick & Mermel, 2021).
4. Conclusion
Susceptible workers (unvaccinated, no precautions) in frozen food facilities are at low risk of SARS-CoV-2 fomite-mediated transmission under cold-chain conditions. Standard infection control interventions (masking and handwashing) reduced risk (8.5 × 10−6) below the target of 1 × 10−4 (Ryan et al., 2014; Wilson et al., 2021). Thus, handwashing and masking mitigate the likelihood of transmitting SARS-CoV-2 via fomites into non-SARS-CoV-2 circulating areas (Ji et al., 2021; Liu et al., 2020). Across all vaccination states of the worker, handwashing with masking maintained low SARS-CoV-2 infection risks: 10−6 (no vaccination) to 10−7 (optimal VE). Therefore, worker vaccination should continue to be prioritized with standard infection control interventions (Hagen, 2021). Lastly, we found that the added benefit of decontaminating packaging (1.2 × 10−8 risk) was nominal and might be excessively conservative.
Surface decontamination of products meant for human consumption increases risks to workers and consumers. Continuous exposure to disinfectants was associated with respiratory diseases, including worsening asthma control (Dumas et al., 2017) and increased risk of chronic obstructive pulmonary disease (Dumas et al., 2019). Risks to consumers of ingested disinfectants through damaged packaging could range from irritation (sinuses, skin, eyes) to liver damage, depending on the disinfectant type and quantity (Kuehn, 2020; Li et al., 2020). Increasing disinfectant use since the start of the COVID-19 pandemic has resulted in a 16.4% increase in exposure calls as reported by the U.S National Poison Data System, CDC (January–March 2019) (Chang et al., 2020).
Furthermore, testing imported frozen foods for SARS-CoV-2 and disinfecting packaging (Liu et al., 2020) potentially introduces delays in product distribution, which could jeopardize product integrity, contribute to food spoilage, and lead to shortages or instability in the global food supply (Cable et al., 2021). Increased use of disinfectants are costly, with global sales of surface disinfectant in 2020 increasing by more than 30%, compared to 2019 (totaling US$4.5 billion) (Lewis, 2021). Thus, additional surface decontamination of cold-chain food packaging could be viewed as excessive and is more likely to increase chemical risks to workers, food hazard risks to consumers, and unnecessary added costs to the global food industry. These results support the continued use of global (WHO, 2020b; Cockburn, 2020) and U.S. federal (FDA, 2020) SARS CoV-2 risk mitigation strategies (handwashing, masking, vaccination) to advance the safety of essential food workers, maintain global food supply chains, and ensure consumer food security (Cable et al., 2021), even with future higher transmissible variants.
Funding
This work was partially supported by the National Institutes of Health (NIH) T32 grant (J.S.S., grant 2T32ES012870-16), the U.S. Department of Agriculture (USDA) (J.S.L. 2019-67017-29642; J.S.S., grant 2020-67034-31728), the National Institute General Medical Sciences (B.A.LR01 GM124280; B.A.L R01 GM124280-03S1), and the NIH (E.T.S., T32AI138952) and Emory University's Infectious Disease Across Scales Training Program (E.T.S). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, or the U.S. Department of Agriculture.
CRediT authorship contribution statement
Julia S. Sobolik: Conceptualization, Methodology, Formal analysis, Visualization, Writing – original draft, Funding acquisition. Elizabeth T. Sajewski: Conceptualization, Methodology, Writing – review & editing. Lee-Ann Jaykus: Conceptualization, Methodology, Visualization, Writing – review & editing. D. Kane Cooper: Methodology, Validation, Writing – review & editing. Ben A. Lopman: Conceptualization, Methodology, Writing – review & editing, Funding acquisition. Alicia N.M. Kraay: Conceptualization, Methodology, Writing – review & editing. P. Barry Ryan: Conceptualization, Methodology, Writing – review & editing. Jodie L. Guest: Conceptualization, Methodology, Writing – review & editing. Amy Webb-Girard: Conceptualization, Methodology, Writing – review & editing. Juan S. Leon: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare no competing interest.
Acknowledgments
The authors would like to thank Drs. Sanjay Gummalla and Lory Reveil (American Frozen Food Institute) for their valuable time and input as frozen food experts and for conducting surveys of facilities. The authors also thank members of the American Frozen Food Institute for participating in surveys to help provide context to the model framework.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.foodcont.2022.108845.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transboundary and Emerging Diseases. 2021;68(2):296–312. doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari U., Chabrelie A., Weir M., Boehnke K., McKenzie E., Ikner L., Wang M., Wang Q., Young K., Haas C.N., Rose J., Mitchell J. A case study evaluating the risk of infection from middle eastern respiratory syndrome coronavirus (MERS-CoV) in a hospital setting through bioaerosols. Risk Analysis. 2019;39(12):2608–2624. doi: 10.1111/risa.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J.Y., An S., Sohn Y., Cho Y., Hyun J.H., Baek Y.J., Kim M.H., Jeong S.J., Kim J.H., Ku N.S., Yeom J.S., Smith D.M., Lee H., Yong D., Lee Y.J., Kim J.W., Kim H.R., Hwang J., Choi J.Y. Environmental contamination in the isolation rooms of COVID-19 patients with severe pneumonia requiring mechanical ventilation or high-flow oxygen therapy. Journal of Hospital Infection. 2020;106(3):570–576. doi: 10.1016/j.jhin.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejko K.L., Pry J., Myers J.F., Jewell N.P., Openshaw J., Watt J., Jain S., Lewnard J.A., Team C.C.-C.-C.S. Prevention of coronavirus disease 2019 (COVID-19) by mRNA-based vaccines within the general population of California. Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi P., Keshavarz Z., Cedeno Laurent J.G., Stephens B., Allen J.G. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(8):1–8. doi: 10.1073/pnas.2015482118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B., Moore B.M., Sterner B., Peterson L.R., Gerding D.N., Balfour H.H., Jr. Survival of influenza viruses on environmental surfaces. Journal of Infectious Diseases. 1982;146(1):47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Bourouiba L., Dehandschoewercker E., Bush J.W.M. Violent expiratory events: On coughing and sneezing. Journal of Fluid Mechanics. 2014;745:537–563. doi: 10.1017/jfm.2014.88. [DOI] [Google Scholar]
- Bouwknegt M., Verhaelen K., Rzetutka A., Kozyra I., Maunula L., von Bonsdorff C.H., Vantarakis A., Kokkinos P., Petrovic T., Lazic S., Pavlik I., Vasickova P., Willems K.A., Havelaar A.H., Rutjes S.A., Husman A.M.D. Quantitative farm-to-fork risk assessment model for norovirus and hepatitis A virus in European leafy green vegetable and berry fruit supply chains. International Journal of Food Microbiology. 2015;198:50–58. doi: 10.1016/j.ijfoodmicro.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Bueckert M., Gupta R., Gupta A., Garg M., Mazumder A. Infectivity of SARS-CoV-2 and other coronaviruses on dry surfaces: Potential for indirect transmission. Materials. 2020;13(22):1–16. doi: 10.3390/ma13225211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cable J., Jaykus L.A., Hoelzer K., Newton J., Torero M. The impact of COVID-19 on food systems, safety, and security-a symposium report. Annals of the New York Academy of Sciences. 2021;1484(1):3–8. doi: 10.1111/nyas.14482. [DOI] [PubMed] [Google Scholar]
- Cai J., Sun W., Huang J., Gamber M., Wu J., He G. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerging Infectious Diseases. 2020;26(6):1343–1345. doi: 10.3201/eid2606.200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. Delta coronavirus variant: Scientists brace for impact. Nature. 2021;585:17–18. doi: 10.1038/d41586-021-01696-3. [DOI] [PubMed] [Google Scholar]
- Chang A., Schnall A.H., Law R., Bronstein A.C., Marraffa J.M., Spiller H.A., Hays H.L., Funk A.R., Mercurio-Zappala M., Calello D.P., Aleguas A., Borys D.J., Boehmer T., Svendsen E. Cleaning and disinfectant chemical exposures and temporal associations with COVID-19 - National Poison data system, United States, January 1, 2020-March 31, 2020. Morbidity and Mortality Weekly Report. 2020;69(16):496–498. doi: 10.15585/mmwr.mm6916e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.Y.H., Wan M.P., Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Li Y., Xie X., Katoshevski D. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. Journal of Aerosol Science. 2009;40(2):122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W. 2020. COVID-19: Back to the workplace, adapting workplaces and protecting workers.https://osha.europa.eu/en/themes/covid-19-resources-workplace from. [Google Scholar]
- Dewey H.M., Jones J.M., Keating M.R., Budhathoki-Uprety J. ACS Chemical Health & Safety; 2021. Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on Health and safety. [DOI] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas O., Varraso R., Boggs K.M., Quinot C., Zock J.P., Henneberger P.K., Speizer F.E., Le Moual N., Camargo C.A., Jr. Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Network Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas O., Wiley A.S., Quinot C., Varraso R., Zock J.P., Henneberger P.K., Speizer F.E., Le Moual N., Camargo C.A., Jr. Occupational exposure to disinfectants and asthma control in US nurses. European Respiratory Journal. 2017;50(4):1–18. doi: 10.1183/13993003.00237-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom A., Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. Journal of Pathogens. 2013 doi: 10.1155/2013/493960. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization . 2012. Guidelines on the application of general principles of food hygiene to the control of viruses in food. 1-13.http://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/en/ from. [Google Scholar]
- Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. The Lancet Infectious Diseases. 2020;20(8):892–893. doi: 10.1016/S1473-3099(20)30561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman E. SARS wars: The fomites strike back. Applied and Environmental Microbiology. 2021;87(13) doi: 10.1128/AEM.00653-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C., Vadlamudi G., Eisenberg M., Foxman B., Koopman J., Xi C. Fomite-fingerpad transfer efficiency (pick-up and deposit) of Acinetobacter baumannii—with and without a latex glove. American Journal of Infection Control. 2015;43(9):928–934. doi: 10.1016/j.ajic.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove S.F., Lee A., Lewis T., Stewart C.M., Chen H., Hoover D.G. Inactivation of foodborne viruses of significance by high pressure and other processes. Journal of Food Protection. 2006;69(4):957–968. doi: 10.4315/0362-028x-69.4.957. [DOI] [PubMed] [Google Scholar]
- Grove S.F., Suriyanarayanan A., Puli B., Zhao H., Li M., Li D., Schaffner D.W., Lee A. Norovirus cross-contamination during preparation of fresh produce. International Journal of Food Microbiology. 2015;198:43–49. doi: 10.1016/j.ijfoodmicro.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Hagen A. American Society for Microbiology; 2021. How dangerous is the Delta variant (B.1.617.2)?https://asm.org/Articles/2021/July/How-Dangerous-is-the-Delta-Variant-B-1-617-2 from. [Google Scholar]
- Harvey A.P., Fuhrmeister E.R., Cantrell M.E., Pitol A.K., Swarthout J.M., Powers J.E., Nadimpalli M.L., Julian T.R., Pickering A.J. Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. Environmental Science and Technology Letters. 2021;8(2):168–175. doi: 10.1021/acs.estlett.0c00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F.C., Jiang X.L., Wang Z.G., Meng Z.H., Shao S.F., Anderson B.D., Ma M.J. Detection of severe acute respiratory syndrome coronavirus 2 RNA on surfaces in quarantine rooms. Emerging Infectious Diseases. 2020;26(9):2162–2164. doi: 10.3201/eid2609.201435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Li X., Chen S., Ren L. Transmission of SARS-CoV-2 via fomite, especially cold chain, should not be ignored. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(11) doi: 10.1073/pnas.2026093118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M. Relative contributions of transmission routes for COVID-19 among healthcare personnel providing patient care. Journal of Occupational and Environmental Hygiene. 2020;17(9):408–415. doi: 10.1080/15459624.2020.1784427. [DOI] [PubMed] [Google Scholar]
- Khan N., Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021;161(3):827–836. doi: 10.1053/j.gastro.2021.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraay A.N.M., Hayashi M.A.L., Berendes D.M., Sobolik J.S., Leon J.S., Lopman B.A. Risk for fomite-mediated transmission of SARS-CoV-2 in child daycares, schools, nursing homes, and offices. Emerging Infectious Diseases. 2021;27(4):1229–1231. doi: 10.3201/eid2704.203631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn B.M. More than 1 in 3 US adults use disinfectants unsafely. JAMA. 2020;324(4):328. doi: 10.1001/jama.2020.12600. [DOI] [PubMed] [Google Scholar]
- Kwon T., Gaudreault N.N., Richt J.A. Environmental stability of SARS-CoV-2 on different types of surfaces under indoor and seasonal climate conditions. Pathogens. 2021;10(2) doi: 10.3390/pathogens10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. COVID-19 rarely spreads through surfaces. So why are we still deep cleaning? Nature. 2021;590(7844):26–28. doi: 10.1038/d41586-021-00251-4. [DOI] [PubMed] [Google Scholar]
- Lindsley W.G., Blachere F.M., Law B.F., Beezhold D.H., Noti J.D. Efficacy of face masks, neck gaiters and face shields for reducing the expulsion of simulated cough-generated aerosols. Aerosol Science and Technology. 2021;55(4):449–457. doi: 10.1080/02786826.2020.1862409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Sangion A., Li L. Evaluating consumer exposure to disinfecting chemicals against coronavirus disease 2019 (COVID-19) and associated health risks. Environment International. 2020;145 doi: 10.1016/j.envint.2020.106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liu Y., Xia H., Zou J., Weaver S.C., Swanson K.A., Cai H., Cutler M., Cooper D., Muik A., Jansen K.U., Sahin U., Xie X., Dormitzer P.R., Shi P.-Y. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596(7871):273–275. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- Liu P., Yang M., Zhao X., Guo Y., Wang L., Zhang J., Lei W., Han W., Jiang F., Liu W.J., Gao G.F., Wu G. Cold-chain transportation in the frozen food industry may have caused a recurrence of COVID-19 cases in destination: Successful isolation of SARS-CoV-2 virus from the imported frozen cod package surface. Biosafety and Health. 2020;2(4):199–201. doi: 10.1016/j.bsheal.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Yuen Y., Hsiao H.M., Jaykus L.A., Moe C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Applied and Environmental Microbiology. 2010;76(2):394–399. doi: 10.1128/AEM.01729-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez G.U., Gerba C.P., Tamimi A.H., Kitajima M., Maxwell S.L., Rose J.B. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Applied and Environmental Microbiology. 2013;79(18):5728–5734. doi: 10.1128/AEM.01030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon R.G., Roberts R.M. Droplet expulsion from the respiratory tract. American Review of Respiratory Disease. 1967;95(3):435–442. doi: 10.1164/arrd.1967.95.3.435. [DOI] [PubMed] [Google Scholar]
- Malenovska H. Coronavirus persistence on a plastic carrier under refrigeration conditions and its reduction using wet wiping technique, with respect to food safety. Food and Environmental Virology. 2020;12(4):361–366. doi: 10.1007/s12560-020-09447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcenac P., Park G.W., Duca L.M., Lewis N.M., Dietrich E.A., Barclay L., Tamin A., Harcourt J.L., Thornburg N.J., Rispens J., Matanock A., Kiphibane T., Christensen K., Pawloski L.C., Fry A.M., Hall A.J., Tate J.E., Vinjé J., Kirking H.L., Pevzner E. Detection of SARS-CoV-2 on surfaces in households of persons with COVID-19. International Journal of Environmental Research and Public Health. 2021;18(15):8184. doi: 10.3390/ijerph18158184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer L., Peris D., Kerl J., Guenther F., Koehler D., Dellweg D. Community masks during the SARS-CoV-2 pandemic: Filtration efficacy and air resistance. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2021;34(1):11–19. doi: 10.1089/jamp.2020.1635. [DOI] [PubMed] [Google Scholar]
- McCormick W., Mermel L.A. The basic reproductive number and particle-to-plaque ratio: Comparison of these two parameters of viral infectivity. Virology Journal. 2021;18(1):92. doi: 10.1186/s12985-021-01566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E.A., Richterman A., Gandhi R.T., Sax P.E. Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Annals of Internal Medicine. 2021;174(1):69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi A., Nakama C., Okumura J., Azuma K. Assessing the risk of COVID-19 from multiple pathways of exposure to SARS-CoV-2: Modeling in health-care settings and effectiveness of nonpharmaceutical interventions. Environment International. 2021;147 doi: 10.1016/j.envint.2020.106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli M.U., Colaneri M., Seminari E.M., Baldanti F., Bruno R. Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. The Lancet Infectious Diseases. 2021;21(5):e112. doi: 10.1016/S1473-3099(20)30678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin L., Laing A.G., Munoz-Ruiz M., McKenzie D.R., Del Molino Del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., Abdul-Jawad S., Kamdar S., Harvey-Jones E., Graham R., Cooper J., Khan M., Vidler J., Kakkassery H., Sinha S.…Irshad S. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. The Lancet Oncology. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. Journal of Aerosol Science. 2009;40(3):256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustsen-Helms I.R., Emborg H.-D., Nielsen J., Nielsen K.F., Krause T.G., Mølbak K., Møller K.L., Berthelsen A.-S.N., Valentiner-Branth P. 2021. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 Vaccine in long-term care facility residents and healthcare workers – a Danish cohort study medRxiv, 2021.2003.2008.21252200. [DOI] [Google Scholar]
- Nicas M., Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. Journal of Occupational and Environmental Hygiene. 2008;5(6):347–352. doi: 10.1080/15459620802003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas M., Jones R.M. Relative contributions of four exposure pathways to influenza infection risk. Risk Analysis. 2009;29(9):1292–1303. doi: 10.1111/j.1539-6924.2009.01253.x. [DOI] [PubMed] [Google Scholar]
- Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: Emission of respirable pathogens. Journal of Occupational and Environmental Hygiene. 2005;2(3):143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Prolonged infectivity of SARS-CoV-2 in fomites. Emerging Infectious Diseases. 2020;26(9):2256–2257. doi: 10.3201/eid2609.201788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski C., Lenehan P., Puranik A., Agarwal V., Venkatakrishnan A.J., Niesen M.J.M., O'Horo J.C., Virk A., Swift M.D., Badley A.D., Halamka J., Soundararajan V. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (New York, N.Y.) 2021;2(8):979–992. doi: 10.1016/j.medj.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitol A.K., Julian T.R. Community transmission of SARS-CoV-2 by surfaces: Risks and risk reduction strategies. Environmental Science and Technology Letters. 2021;8(3):263–269. doi: 10.1021/acs.estlett.0c00966. [DOI] [PubMed] [Google Scholar]
- Pouillot R., Delignette-Muller M.L. Evaluating variability and uncertainty separately in microbial quantitative risk assessment using two R packages. International Journal of Food Microbiology. 2010;142(3):330–340. doi: 10.1016/j.ijfoodmicro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Prunas O., Warren J.L., Crawford F.W., Gazit S., Patalon T., Weinberger D.M., Pitzer V.E. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. medRxiv. 2021 doi: 10.1101/2021.07.13.21260393. 2007.2013.21260393. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virology Journal. 2020;17(1):145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin P., Maxwell S., Gerba C. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. Journal of Applied Microbiology. 2002;93(4):585–592. doi: 10.1046/j.1365-2672.2002.01734.x. [DOI] [PubMed] [Google Scholar]
- Ryan M.O., Haas C.N., Gurian P.L., Gerba C.P., Panzl B.M., Rose J.B. Application of quantitative microbial risk assessment for selection of microbial reduction targets for hard surface disinfectants. American Journal of Infection Control. 2014;42(11):1165–1172. doi: 10.1016/j.ajic.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., Crown K.K., Brett-Major D.M., Schnaubelt E.R., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Scientific Reports. 2020;10(1):12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R., Bar-On Y.M., Gleizer S., Bernshtein B., Flamholz A., Phillips R., Milo R. The total number and mass of SARS-CoV-2 virions. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(25) doi: 10.1073/pnas.2024815118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Sadat A., Abdi R., Colaruotolo L.A., Francavilla A., Petker K., Nasr P., Moraveji M., Cruz G., Huang Y., Arora A., Chao A., Walker S., Wang X., Rathnayake S., Ragupathy S., Newmaster S.G., Hanner R.H., Goodridge L.D., Corradini M.G. Detection of SAR-CoV-2 on surfaces in food retailers in Ontario. Current Research in Food Science. 2021;4:598–602. doi: 10.1016/j.crfs.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolik J.S., Sajewski E.T., Jaykus L.-A., Cooper D.K., Lopman B.A., Kraay A.N.M., Ryan P.B., Leon J.S. Controlling risk of SARS-CoV-2 infection in essential workers of enclosed food manufacturing facilities. Food Control. 2022;133 doi: 10.1016/j.foodcont.2021.108632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift M.D., Breeher L.E., Tande A.J., Tommaso C.P., Hainy C.M., Chu H., Murad M.H., Berbari E.F., Virk A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciab361. e1376-e1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssou E., Delagreverie H., Visseaux B., Lambert-Niclot S., Brichler S., Ferre V., Marot S., Jary A., Todesco E., Schnuriger A., Ghidaoui E., Abdi B., Akhavan S., Houhou-Fidouh N., Charpentier C., Morand-Joubert L., Boutolleau D., Descamps D., Calvez V., Soulie C. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. Journal of Infection. 2021;83(4):e1–e3. doi: 10.1016/j.jinf.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K., Tsang O.T., Yip C.C., Chan K.H., Wu T.C., Chan J.M., Leung W.S., Chik T.S., Choi C.Y., Kandamby D.H., Lung D.C., Tam A.R., Poon R.W., Fung A.Y., Hung I.F., Cheng V.C., Chan J.F., Yuen K.Y. Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki H., Furusawa Y., Iwatsuki-Horimoto K., Imai M., Kabata H., Nishimura H., Kawaoka Y. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. mSphere. 2020;5(5) doi: 10.1128/mSphere.00637-20. e00637-00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention . 2021, Apr.5, 2021. Science brief: SARS-CoV-2 and surfaces (fomite) transmission for indoor community environments.https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html Retrieved Jun 1 from. [PubMed] [Google Scholar]
- U.S. Department of Homeland Security . 2021. Estimated Airborne Decay of SARS-CoV-2 (virus that causes COVID-19) under a range of temperatures, relative humidity, and UV index.https://www.dhs.gov/science-and-technology/sars-airborne-calculator from. [Google Scholar]
- U.S. Department of Homeland Security . 2021. Estimated Surface Decay of SARS-CoV-2 (virus that causes COVID-19) on surfaces under a range of temperatures, relative humidity, and UV Index.https://www.dhs.gov/science-and-technology/sars-calculator from. [Google Scholar]
- U.S. Environmental Protection Agency List N: Disinfectants for coronavirus (COVID-19) 2020. https://cfpub.epa.gov/wizards/disinfectants/ from.
- U.S. Food and Drug Administration . 21 CFR Parts 11, 16 and 112, 2015-28159, 1-216. 2015. FSMA final rule on produce safety standards for the growing, harvesting, packing, and holding of produce for human consumption.https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-produce-safety from. [Google Scholar]
- U.S. Food and Drug Administration 21 CFR Part 110 current Good manufacturing practice in manufacturing, packing, or holding human food. 2016. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=110&showFR=1 from.
- U.S. Food and Drug Administration . 2020. Employee Health and food safety checklist for human and animal food operations during the COVID-19 pandemic.https://www.fda.gov/food/food-safety-during-emergencies/employee-health-and-food-safety-checklist-human-and-animal-food-operations-during-covid-19-pandemic from. [Google Scholar]
- Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.H., Barnes C.O., Cipolla M., Ramos V., Oliveira T.Y., Cho A., Schmidt F., da Silva J., Bednarski E., Aguado L., Yee J., Daga M., Turroja M., Nussenzweig M.C. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.M., Reynolds K.A., Sexton J.D., Canales R.A. Modeling surface disinfection needs to meet microbial risk reduction targets. Applied and Environmental Microbiology. 2018;84(18) doi: 10.1128/AEM.00709-18. e00709-00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.M., Weir M.H., Bloomfield S.F., Scott E.A., Reynolds K.A. Modeling COVID-19 infection risks for a single hand-to-fomite scenario and potential risk reductions offered by surface disinfection. American Journal of Infection Control. 2021;49(6):846–848. doi: 10.1016/j.ajic.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brunink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2017. Guidelines for drinking-water quality. Microbial aspects(978-92-4-154995-0)https://www.who.int/water_sanitation_health/dwq/gdwq0506_7.pdf from. [Google Scholar]
- World Health Organization . 2020. Transmission of SARS-CoV-2: Implications for infection prevention precautions [scientific brief]. 1-10.https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations from. [Google Scholar]
- World Health Organization COVID-19 and food safety: Guidance for food businesses: Interim guidance. 2020. https://www.who.int/publications/i/item/covid-19-and-food-safety-guidance-for-food-businesses from. 1–6.
- Xie C., Zhao H., Li K., Zhang Z., Lu X., Peng H., Wang D., Chen J., Zhang X., Wu D., Gu Y., Yuan J., Zhang L., Lu J. The evidence of indirect transmission of SARS-CoV-2 reported in Guangzhou, China. BMC Public Health. 2020;20(1):1202. doi: 10.1186/s12889-020-09296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Ji Z., Yue Y., Liu H., Wang J. Infection risk assessment of COVID-19 through aerosol transmission: A case study of south China seafood market. Environmental Science and Technology. 2021;55(7):4123–4133. doi: 10.1021/acs.est.0c02895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Model code developed and used in this study is available to readers through GitHub at the following DOI: https://doi.org/10.5281/zenodo.5904275.