Abstract
Background:
Gallium-68-prostate-specific membrane antigen (68Ga-PSMA) positron emission tomography/computed tomography (PET/CT) has recently been shown to be very high accuracy in biopsy-naïve prostate cancer (PCa) detection and can potentially improve the low specificity noted with diffusion-weighted magnetic resonance imaging (DW-MRI), especially in instances of prostate inflammation. We aimed to compare the diagnostic accuracy of DW-MRI and PSMA PET/CT using apparent diffusion coefficient (ADC) and maximum standardized uptake (SUVmax) values in the diagnosis of PCa.
Patients and Methods:
A retrospective study comparing and analyzing the diagnostic accuracy of prebiopsy DW-MRI and 68Ga-PSMA PET/CTs done in patients with suspected PCa (raised prostate specific antigen [PSA] and/or positive digital rectal examination) from January 2019 to December 2020. The standard of reference was transrectal ultrasound-guided biopsies.
Results:
Sixty-seven patients were included in the study, mean age: 70 years (range 49–84), mean PSA: 23.2 ng/ml (range 2.97–45.6). Biopsy was positive for PCa in 56% (n = 38) and negative in 43% (n = 29). Of the benign results, benign hyperplasia was noted in 75% (n = 22) and prostatitis in 25% (n = 7). Of the PCa, 55% (n = 21) of were high International Society of Urological Pathology (ISUP) grade (4–5) and 45% (n = 17) low/intermediate ISUP grade (1–3). Overall the sensitivity/specificity/Accuracy for prediction of PCa of MRI using prostate imaging and reporting data system version 2 criteria and PSMA PET/CT using PCa molecular imaging standardized evaluation criteria was 92.1%/65.5%/80.5% and 76.3%/96.5%/85.1% respectively. Mean apparent diffusion co-efficient (mean ADC) value of benign lesions and PCa was 1.135 × 10-3 mm2/s and 0.723 × 10-3 mm2/s, respectively (P = 0.00001). Mean SUVmax and ADC of benign and PCa lesions was 4.01 and 16.4 (P = 0.000246). Mean SUVmax/ADC ratio of benign and malignant lesions was 3.8 × 103 versus 25.21 × 103 (P < 0.000026). Inverse correlation was noted between ADC and SUVmax values (R = −0.609), inverse correlation noted between ADC and Gleason's score (R = −0.198), and positive correlation of SUVmax and SUVmax/ADC with Gleason's score (R = 0.438 and R = 0.448). Receiver operating characteristic curve analysis revealed a SUVmax cutoff 6.03 (sensitivity/specificity - 76%/90%, area under the curve (AUC) - 0.935, Youden index (YI) - 0.66), ADC cutoff of 0.817 × 10−3 mm2/s (sensitivity/specificity – 79%/86%, AUC – 0.890, YI - 0.65), and SUVmax/ADC ratio cutoff of 7.43 × 103 (sensitivity/specificity – 87%/98%, AUC - 0.966, YI - 0.85) for PCa diagnosis.
Conclusion:
For diagnosis of biopsy-naïve PCas, the combination of diffusion-weighted MRI and PSMA PET/CT (i.e., SUVmax/ADC ratio) shows better diagnostic accuracy than either used alone and the combination of PET and MRI is especially useful when distinguishing cancer from prostatitis.
Keywords: Apparent diffusion coefficient, cancer, gallium-68, magnetic resonance imaging, positron emission tomography/computed tomography, prostate, prostatitis, prostate specific antigen, prostate-specific membrane antigen, standardized uptake maximum value
Introduction
The results of the magnetic resonance imaging-FIRST (MRI) trial showed that detection of clinically significant prostate cancer (PCa) is better with multiparametric MRI (mpMRI) targeted biopsies than systematic biopsies.[1] Now, as per the EAU guidelines, there is a level 1A/strong recommendation to perform mpMRI in biopsy-naïve patients and combined targeted and systematic biopsies to be performed for only those lesions characterized as prostate imaging and reporting data system (PI-RADS) >3. Although the detection rates of clinically significant PCa increase with increasing PI-RADS score on mpMRI, there is also an increase in the risk of a false-positive diagnosis. A study done by Rourke et al. showed that about 33% of the lesions characterized as PI-RADS 3–4 and up to 19% of lesions characterized as PI-RADS 5 were negative for malignancy, the majority of these false-positive lesions were related to prostate inflammation.[2] Hence there is a need for additional strategies for improved cancer detection to reduce the high number of unwanted biopsies.
In the past 5 years, prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) has emerged as a strong complementary imaging modality to mpMRI for primary PCa detection with comparable/if not better diagnostic accuracy.[3,4,5,6] As an expression of the PSMA is significantly more in cancerous tissue than normal/hyperplastic prostate tissue, the addition of PSMA PET/CT information to mpMRI or use of simultaneous PSMA PET/MRI can improve overall diagnostic accuracy as demonstrated by studies done by Eiber et al. and Scheltema et al.[7,8] Further, in the era of precision oncology with the ever-increasing use of advanced multimodality imaging, a single quantifiable diagnostic parameter for identifying PCa is desirable which may not just be more accurate but also may have better reproducibility compared to visual assessment of lesions alone.
In this study, we aimed to evaluate the diagnostic accuracy of quantifiable variables of primary tumor obtained with biparametric MRI (such as ADC value) and PET/CT (such as maximum standardized uptake value [SUVmax]) for detecting PCa and to assess if the combination of these (i.e., SUVmax/ADC ratio) is better than either used alone.
Patients and Methods
This is a single-center retrospective study analyzing the data of all consecutive patients who underwent prebiopsy PSMA PET/CT and MRI for clinically suspected PCa cases (raised prostate-specific antigen (PSA) and/or positive digital rectal examination) from January 2019 to December 2020. MRI and PET/CT were acquired within a mean duration of 8 days from each other (range: 4–12 days). Patients with prior treatment (hormonal, radiotherapy, or surgery) or without biopsy evidence were excluded from the study. Biopsy was done within 3–12 days from the time of PET/CT and MRI.
Radiopharmaceutical for imaging was gallium-68-labeled PSMA 11 (68Ga PSMA), synthesized using computer run fully automated synthesizer IQS-TS system (ITM Isotopen Technologien München AG, Germany). Quality control of radiopharmaceuticals was done to ensure 95% radiolabeling before injecting to patients. The total synthesis time was about 20 min. About 2–2.2 MBq/kg of synthesized 68Ga-PSMA-11 was injected intravenously (IV) injected in the arm, and scans were acquired after 60 min and another delayed scan of pelvis post 20 mg furosemide IV at 120 min. Imaging was performed on a GE 5 ring PET/CT system Discovery IQ 5 Ring block detectors PET/CT (General Electric, Milwaukee, WI, USA), combining bismuth germanium oxide-based PET crystal and 16-slice CT components. Noncontrast CT and PET data were acquired from the mid-thigh level to the top of the skull with the arms raised. PET emission counts were collected over 2.5 min/table position, acquired in a three-dimensional mode with standard VUE Point HD reconstruction (filter 5.5 mm, subsets 12, 4 iterations, order 4) or Q.clear algorithm (beta value 350). No adverse events were reported in any patient post-PET/CT scans.
PET/CT scans were interpreted by two separate experienced Nuclear Medicine Physicians. PSMA PET/CT was reported as per the PCa molecular imaging standardized evaluation (PROMISE) criteria for quantifying PSMA expression on the prostate (mi-PSMA ES). mi-PSMA ES Score 0-PSMA uptake below mediastinal blood pool, mi-PSMA ES Score 1: PSMA uptake above blood poo but less than liver uptake, mi-PSMA ES Score 2: Uptake more than a liver activity but less than parotid uptake and mi-PSMA ES Score 3: Uptake more than parotid uptake. Score 0 and 1 were considered PET/CT negative for malignancy, and Score 2 and 3 were considered PET/CT positive for malignancy. Along with PROMISE scoring, SUVmax of the index prostate lesion on the 60 min PET image by placing 2 cm circular region of interest (ROI) on the index/dominant prostate lesion. If no visible lesion (defined as the absence of any focal low-grade/high-grade uptake) was noted on PSMA PET/CT, SUVmax of the suspected normal prostate was calculated by placing ROI over the peripheral zone of the body of the prostate. Bi-parametric MRI was acquired on a 3-T MRI. Images were reported as per the PI-RADS v2.0. PI-RADS 4 or 5 were regarded as positive and PI-RADS ≤3 regarded as negative. MRI protocol: 4 sequences were acquired: T1-weighted imaging (repetition time [TR] <600 ms, echo time [TE] =20 ms, field of view [FOV] =20 × 20 cm, matrix 192 × 200), T2-weighted imaging (TR >3000 ms, TE = 120 ms, FOV = 20 cm × 20 cm, matrix = 240 × 230), fat-suppression presaturation-attenuated inversion recovery (TR = 2800 ms, TE = 100 ms, FOV = 25 cm × 40 cm, matrix = 270 × 200), diffusion-weighted imaging (DWI) (TR = 6200 ms, TE = 2000 ms, FOV = 20 cm × 30 cm, matri × 80 × 142, b = 1400 s/m2). No dynamic contrast-enhanced sequences (DCE) were acquired in any patient. ADC map was acquired from DWI and mean ADC value for each patient was calculated by drawing 3 different 2 cm circular ROI over the dominant/index prostate lesion and by deriving the mean of the 3 values. If no visible lesion was noted on MRI (defined as absence of significant abnormality on ADC map), mean ADC of the suspected normal prostate was calculated by placing ROI over peripheral zone of body of prostate.
The gold standard for the evaluation of MRI and PET/CT findings was 12-core trans-rectal ultrasound-guided biopsies. Biopsies were reported as per the International Society of Urologic Pathology (ISUP) guidelines. t-test was used to compare the difference in mean SUVmax, mean ADC, and SUVmax/ADC ratio among benign and malignant groups. In addition, receiver operating characteristic curve (ROC) analysis was performed to evaluate the sensitivity, specificity, area under the curve (AUC), and cutoff value of each parameter. Statistics calculations were done on SPSS software. P < 0.05 was considered statistically significant.
Results
Patient characteristics are summarized in Table 1. As per PI-RADS v. 2, MRI was positive for malignancy in 64% (n = 43) of patients and as per PROMISE criteria, PSMA PET/CT was positive for malignancy in 44.7% (n = 30) patients. Biopsy was positive for PCa in 56% (n = 38) and negative in 43% (n = 29). Of the benign results, benign hyperplasia was noted in 75% (n = 22) and prostatitis in 25% (n = 7). Of the PCa, 55% (n = 21) of were high ISUP grade (4–5) and 45% (n = 17) low/intermediate ISUP grade (1–3).
Table 1.
Patient characteristics
| n=67 | |
|---|---|
| Mean Age (years) | 70.41 (Range 48-84) |
| Mean PSA (ng/ml) | 20.1 (Range 2.97-48) |
| Mean Prostate Volume (cc) | 50.7 (Range 21-163) |
| Mean PSA density (ng/ml2) | 0.44 (Range 0.04-2.19) |
| Digital rectal examination | |
| Abnormal | 25 |
| Normal | 42 |
| Magnetic Resonance Imaging | |
| PI-RADS v. 2 1-3 | 22 |
| PI-RADS v. 2 4-5 | 43 |
| PSMA PET/CT Imaging | |
| PROMISE score 0-1 | 37 |
| PROMISE score 2-3 | 30 |
| Biopsy results | |
| Benign hyperplasia | 22 |
| Prostatitis | 7 |
| Adenocarcinoma | 38 |
| ISUP grade | |
| Grade 1-3 | 16 |
| Grade 4-5 | 22 |
Of the 38 patients positive for malignancy, PSMA PET/CT, regional nodal metastasis was detected in 21% (n = 8/38) patients, 6 of which also showed, in addition, distant metastases to distant nodes and skeleton. Regional nodal metastases were detected in 15.7% (n = 6/38) patients on MRI. In 5% (n = 2/38) patients, there were isolated skeletal metastases without any significant pelvic lymphadenopathy detected on PSMA PET/CT.
Mean ADC value of benign lesions and PCa was 1.135 × 10−3 mm2/s and 0.723 × 10−3 mm2/s, respectively (P = 0.00001). The mean SUVmax and ADC of benign and PCa lesions were 4.01 and 16.4 (P = 0.000246). Mean SUVmax/ADC ratio of benign and malignant lesions was 3.8 × 103 versus 25.21 × 103 (P < 0.000026) [Figures 1-3 and Table 2]. An inverse correlation was noted between ADC and SUVmax values (R = −0.609), an inverse correlation was noted between ADC and Gleason's score (R = −0.198), and a positive correlation of SUVmax and SUVmax/ADC with Gleason's score (R = 0.438 and R = 0.448).
Figure 1.
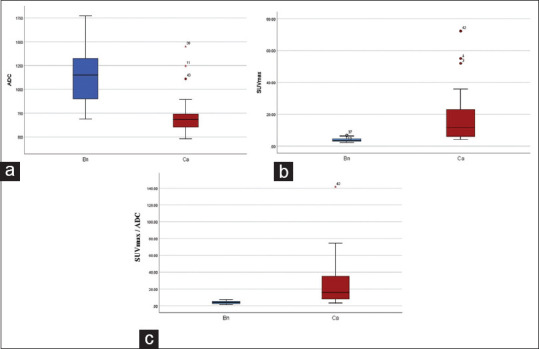
Mean values of ADC (a), SUVmax (b) and SUVmax/ADC ratio (c) between benign (Bn) and cancer (Ca) groups. SUVmax: Maximum standardized uptake value, ADC: Apparent diffusion coefficient
Figure 3.
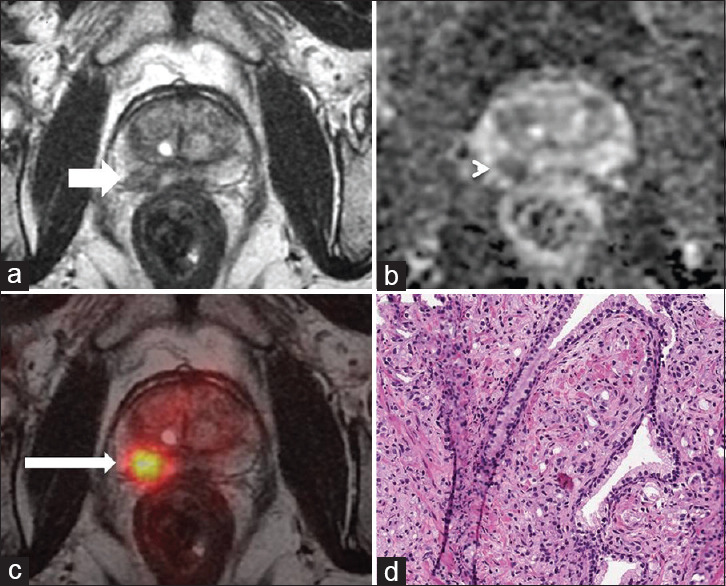
67 years old with PSA - 7.02 ng/ml and nocturia. MRI showed small focal T2 hypo-intense lesion (white arrow (a)) showing restricted diffusion seen as dark signal on ADC map-PIRADS 4 (white arrow head (b), ADC: 0.654 × 10−3 mm2/s). Fused PSMA-PET/MR images shows focal increased uptake in the right peripheral zone (thin white arrow (c), SUVmax - 9.57, SUVmax/ADC 14.6 × 10−3). TRUS guided biopsy from the right lobe suggestive of atypical cells with fused glands - adenocarcinoma, ISUP Grade 4 (d). PSA: Prostate specific antigen, MR: Magnetic resonance, MRI: MR imaging, ADC: Apparent diffusion coefficient, PIRADS 4: Prostate imaging and reporting data system, PSMA: Prostate-specific membrane antigen, PET: Positron emission tomography, SUVmax: Maximum standardized uptake value, TRUS: Transrectal ultrasound guided, ISUP: International Society of Urological Pathology
Table 2.
Independent t-test to compare variables between benign and; malignant groups
| n=67 | Benign (Mean+/-SD) | Malignant (Mean+/-SD) | P |
|---|---|---|---|
| Total PSA (ng/ml) | 17.5 +/-30.4 | 22.2 +/-16.6 | 0.214 |
| Prostate volume (cc) | 62.6 +/- 33.9 | 41.6 +/-19.2 | 0.001 |
| PSA density (ng/ml2) | 0.27 +/-0.27 | 0.57 +/-0.46 | 0.001 |
| Prostate ADC (x10-3 mm2/sec) | 1.135.2 +/-195.9 | 0.723.2+/-277.5 | 0.0001 |
| Prostate SUVmax | 4.02+/-1.3 | 16.4 +/-15.3 | 0.000027 |
| Prostate SUVmax/ADC ratio (x103) | 3.82+/-1.5 | 25.22+/-26.5 | 0.000026 |
Compared to the low-intermediate risk tumors (ISUP grade 1–3), high-risk tumors (ISUP grade 4/5) showed significantly higher mean prostate SUVmax (21.32 vs. 9.7, P = 0.009), significantly lower mean prostate ADC (0.670 × 10−3 mm2/s vs. 0.797 × 10−3 mm2/s, P = 0.02), significantly higher SUVmax/ADC ratio (33.52 vs. 13.79, P = 0.01).
Compared to the patient with no nodal disease (N0) detected on imaging, patients with nodal metastasis had significantly higher primary tumor mean SUVmax (27.99 vs. 13.35, P = 0.007), significantly higher SUVmax/ADC ratio (45.12 vs. 19.91, P = 0.007). There was no significant difference in primary mean ADC values between the N1 and N0 groups (0.694 × 10−3 mm2/s vs. 0.734 × 10−3 mm2/s, P = 0.32).
Compared to patients with nonmetastatic disease (M0), metastatic disease (M1) patients showed a significantly higher mean prostate SUVmax (28 vs. 13.34, P = 0.007), significantly higher SUVmax/ADC ratio (46.85 vs. 19.45, P = 0.003) with no significant difference in mean prostate ADC (0.638 × 10−3 mm2/s vs. 0.746 × 10−3 mm2/s, P = 0.08) between two groups.
Overall, the sensitivity/specificity of the MRI using PI-RADS v.2 criteria for the prediction of PCa was 92.1 and 65.5%, respectively. The sensitivity/specificity of the PSMA PET/CT using PROMISE criteria for the prediction of PCa was 76.3% and 96.5%, respectively [Table 3]. Accuracy of PET/CT was higher than MRI in PSA <20 ng/ml and MRI was better than PET for diagnosing PCa in patient ith PSA > 20 ng/ml [Tables 3 and 4]. There were ten false positives scans on MRI and one false-positive scan on PSMA PET/CT, respectively, and two false negatives on MRI and nine false negatives on PSMA PET/CT. Discordant findings between PSMA PET and MRI for PCa detection were noted in 31% of patients (n = 21), most of these discordances were related to PI-RADS 4/5 lesions (n = 18). Details of these are summarized in Tables 5 and 6.
Table 3.
Comparison of diagnostic accuracy of Bi-parametric MRI and PSMA PET/CT for prediction of biopsy naïve prostate carcinoma
| Sensitivity (%) (95% C.I) | Specificity (%) (95% C.I) | Positive Predictive value (%) (95% C.I) | Negative Predictive value (%) (95% C.I) | Accuracy (%) | |
|---|---|---|---|---|---|
| Bi-parametric MRI-PIRADS v. 2 | 92.1 (77-97) | 65.5 (45-81) | 77.7 (62-88) | 86.3 (64-96) | 80.5 |
| PSMA PET/CT-PROMISE score | 76.3 (59-88) | 96.5 (80-99) | 96.6 (81-99) | 75.6 (58-87) | 85.1 |
Table 4.
Comparison of diagnostic accuracy of bi-parametric MRI and PSMA PET/CT for prediction of biopsy naïve prostate carcinoma based on PSA levels
| Sensitivity | Specificity | Positive Predictive value | Negative Predictive value | Accuracy (%) | |
|---|---|---|---|---|---|
| PSA <10ng/ml (n=25) | |||||
| Bi-parametric MRI | 90% | 60% | 60% | 90% | 72% |
| PSMA PET/CT | 63% | 100% | 100% | 77% | 84% |
| PSA 10-20 ng/ml (n=23) | |||||
| Bi-parametric MRI | 85% | 66% | 80% | 75% | 78% |
| PSMA PET/CT | 85% | 100% | 100% | 81% | 91% |
| PSA >20ng/ml (n=20) | |||||
| Bi-parametric MRI | 100% | 80% | 94% | 100% | 95% |
| PSMA PET/CT | 80% | 100% | 100% | 63% | 85% |
Table 5.
Characteristics of false-positive cases on magnetic resonance imaging as PI-RADS v2
| Age (years) | PSA (ng/ml) | PI-RADS | ADC (mm2/s) x10-3 | PROMISE score | SUVmax | Biopsy |
|---|---|---|---|---|---|---|
| 65 | 6.9 | 5 | 0.707 | 1 | 3.25 | Prostatitis |
| 72 | 8.1 | 4 | 1.109 | 1 | 3.28 | BPH |
| 69 | 5.85 | 4 | 0.852 | 1 | 3.06 | Prostatitis |
| 48 | 8.57 | 4 | 0.741 | 1 | 3.5 | Prostatitis |
| 80 | 12.6 | 4 | 0.900 | 1 | 4.3 | Prostatitis |
| 69 | 11.87 | 5 | 0.840 | 1 | 4.1 | BPH |
| 73 | 8.1 | 4 | 0.1350 | 1 | 3.6 | BPH |
| 49 | 8.57 | 4 | 0.1297 | 1 | 3.28 | Prostatitis |
| 61 | 11.7 | 5 | 0.720 | 1 | 3.7 | Prostatitis |
| 49 | 8.57 | 4 | 0.1297 | 1 | 3.28 | Prostatitis |
Table 6.
Characteristics of false negative cases on prostate-specific membrane antigen positron emission tomography/computed tomography as per PROMISE score
| Age (years) | PSA (ng/ml) | PI-RADS | ADC (mm2/s) x10-3 | PROMISE score | SUVmax | Biopsy | ISUP grade |
|---|---|---|---|---|---|---|---|
| 69 | 7.4 | 5 | 0.825 | 1 | 4.2 | Carcinoma | 2 |
| 66 | 17.5 | 1 | 1.245 | 1 | 4.12 | Carcinoma | 2 |
| 58 | 22.3 | 5 | 0.690 | 1 | 5.47 | Carcinoma | 5 |
| 68 | 15 | 5 | 0.655 | 1 | 5.57 | Carcinoma | 5 |
| 70 | 4.72 | 5 | 0.733 | 1 | 5.9 | Carcinoma | 2 |
| 79 | 34.9 | 5 | 0.648 | 1 | 5.9 | Carcinoma | 1 |
| 67 | 3.8 | 5 | 0.845 | 1 | 6.4 | Carcinoma | 4 |
| 79 | 22.4 | 5 | 0.555 | 1 | 4.13 | 3 | |
| 69 | 8.72 | 5 | 0.670 | 1 | 5.2 | 3 |
ROC curve analysis revealed a SUVmax cutoff 6.03 (sensitivity/specificity - 76%/90%, AUC - 0.935, Youden index [YI] - 0.66), ADC cutoff of 0.817 × 10−3 mm2/s (sensitivity/specificity – 79%/86%, AUC – 0.890, YI – 0.65), and SUVmax/ADC ratio cutoff of 7.43 × 103 (sensitivity/specificity - 87%/98%, AUC - 0.966, YI - 0.85) for PCa diagnosis [Figure 4].
Figure 4.
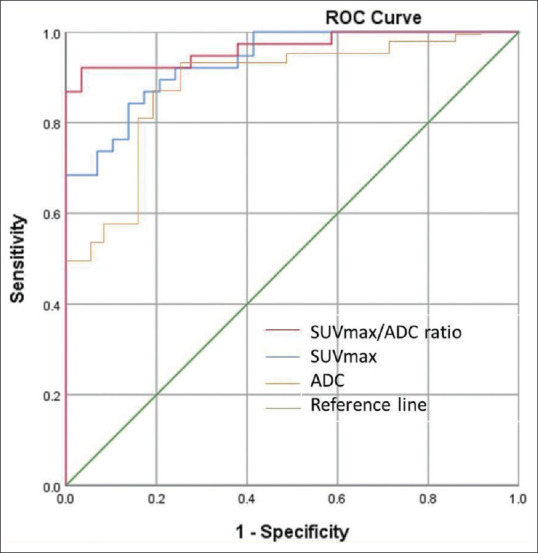
ROC curve analysing ADC, SUVmax and SUVmax/ADC ratio. ADC: Apparent diffusion coefficient, SUVmax: Maximum standardized uptake value, ROC: Receiver operated characteristic
Discussion
Beyond its established utility in the recurrent PCa setting, PSMA PET/CT is now being increasingly used in primary cancer detection and guiding biopsy. A recent systematic review and meta-analysis including seven studies and total of 389 patients showed that PET/CT has excellent sensitivity and negative likelihood ratio for initial diagnosis of PCa.[9] Comparing PSMA PET/CT and MRI in initial PCa diagnosis, Donato et al. showed that PSMA PET/CT has high concordance with mpMRI for the detection of index lesions and is superior to mpMRI in detecting secondary cancer foci and small lesions missed on MRI.[10] In this study, we primarily focused on comparing the diagnostic accuracy of ADC and SUVmax for the detection of index lesions in the prostate. The results of our study show that the combination of primary tumor ADC and SUVmax has better diagnostic accuracy for prebiopsy detection of PCa than using ADC value alone or using PI-RADS 2.0 scoring the standardized system of reporting bi-parametric MRI.
PI-RADS v.2 was designed to promote global standardization and diminish variation in the acquisition, interpretation, and reporting of prostate mpMRI examination based on the best available evidence and expert consensus opinion. Compared to the previous version which gave equal weightage to all sequences, the current version focuses on dominant sequences based on zonal anatomy (DWI for peripheral zone and T2W for transitional zone) and limited the utility DCE only for the transitional zone lesions.[11] A meta-analysis was done by Woo et al. Although pooled sensitivity of PI-RADS v.2 was better than PI-RADS v.1 (95% vs. 88%), the specificity was not significantly different (73% vs. 75%).[12] One of the reasons for the relatively lower specificity of MRI could be due to prostatitis which cannot be reliably distinguished from cancer either using dynamic contrast or diffusion-weighted sequences, as tumors and prostatitis can show similar contrast washout patterns or overlapping ADC values.[13,14,15]
As PSMA expression is significantly higher in cancer compared to prostatitis, adding PSMA PET/CT information before an MRI targeted biopsy will help in reducing the number of false-positive diagnoses and avoid biopsies in a considerable number of patients. In our study, we found that 22.2% (10/45) of patients with PI-RADS 4/5 score were benign on biopsy, of which 50% were related to prostatitis and 50% related to benign hyperplasia. On PET/CT, the mean SUVmax of the prostate of these patients was very low, i.e., 3.34 (range: 2.4–4.3), well below the derived best cutoff point of diagnosis of malignancy in our study (i.e., 6.03) [Figure 2]. Although overall, PSMA PET/CT showed better diagnostic accuracy than biparametric MRI, the accuracy of two diagnostic modalities was dependent on PSA levels. PET/CT scored over MRI in having a better specificity/accuracy with PSA elevation ≤20 ng/ml. However, PET/CT showed lower sensitivity/accuracy than MRI when PSA levels >20 ng/ml [Table 4].
Figure 2.
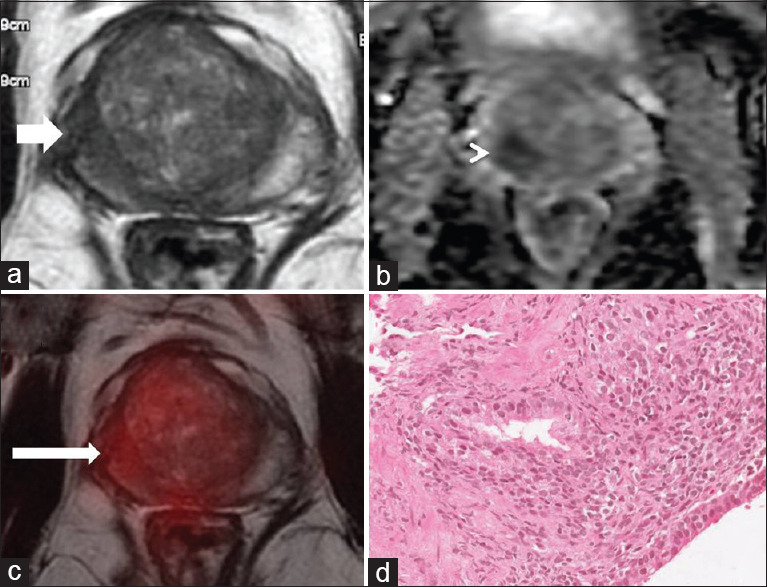
A 67 years old with raised PSA (6.9 ng/ml). MRI suggestive of T2 hypointense area (white arrow (a)) showing restricted diffusion seen as dark signal on ADC map-PIRADS 5 (white arrow head (b), ADC: 0.707 × 10–3 mm2/s). Fused PSMA-PET/MR images shows no increased uptake in the right peripheral zone (thin white arrow (c), SUVmax-3.25, SUVmax/ADC 4.59 × 10−3). TRUS-guided biopsy from the right lobe suggestive of dense lymphocytes around the glands with no atypical cells-suggestive of chronic prostatitis (d). SUVmax: Maximum standardized uptake value, ADC: Apparent diffusion coefficient, PSA: Prostate specific antigen, MR: Magnetic resonance, MRI: MR imaging, PIRADS 5: Prostate imaging and reporting data system, PSMA: Prostate-specific membrane antigen, PET: Positron emission tomography, TRUS: Transrectal ultrasound guided
Similar to the results of our study, the superior diagnostic accuracy of combining ADC and SUVmax was demonstrated in a recent retrospective study by Wang et al. in 63 patients.[16] The study showed sensitivity, specificity, and AUC were 90.6%, 58.1%, and 0.816 for ADC, 67.2%, 97.7%, and 0.905 for SUVmax, and 81.2%, 88.4%, and 0.929 for SUVmax/ADC respectively. The best cutoff values for ADC, SUVmax, and SUVmax/ADC ratio for the prediction of PCa in their study was 1.02 × 10−3, 11.72, and 12.35, compared to ours which was 0.817 × 10−3, 6.03, and 7.43, respectively. Higher SUVmax and SUVmax/ADC ratio values in their study could be attributed slightly higher percentage of cancer cases detected in their study (n = 40/63, 63.4%) compared to ours (n = 25/25, 50%). Another difference was in our study was that we prospectively included only patients with PSA <50 ng/ml (range: 2.97–48 ng/ml), whereas PSA values were slightly higher (range from 4.15 to 1298 ng/ml) in their retrospective study. Positive predictive value for PCa detection is 94.7%–98.5% when PSA increases above 50 ng/ml, 32.7% in PSA range: 10–20 ng/ml, and drops to 20.6% in PSA range: 4–10 ng/ml.[17,18] Hence, the detection of PCa is more uncertain when the PSA is lower and we believe prebiopsy imaging with combined PET and MRI will have more utility at lower PSA levels. At higher PSA levels (i.e., >50 ng/ml), not only the diagnosis of PCa but also the risk of metastatic disease is more likely. The presence of extensive distant metastasis on a “triage” PSMA PET/CT done in patients with very high PSMA levels would alone suffice for disease management obviating the need for additional MRI. However, those patients with very high PSA levels but with localized, locoregional, and also those with an oligometastatic disease on PSMA PET/CT will still benefit from MRI for the management of organ-confined disease (surgery or radiotherapy) owing to its excellent and superior anatomical resolution.
Another similar study done by Uslu-Beşli et al. in 26 patients found an inverse correlation between ADC and SUVmax values of the primary tumor and that SUVmax/ADC ratio predicted the incidence of lymph node metastasis better than either SUVmax of ADC alone.[19] Even in our study, we found that mean SUVmax of the primary tumor or SUVmax/ADC ratio had a significant association with the presence/absence of nodal or distant metastasis but mean ADC values alone were not significantly different in patients with or without loco-regional or distant metastasis. Our study also revealed that higher ISUP grade tumors showed a significantly higher SUVmax/ADC compared to lower ISUP grade tumors. The association of SUVmax/ADC ratio with ISUP grade was better than either using SUVmax or ADC alone. Hence combined PET-MRI may be a better predictor of primary tumor aggressiveness than MRI using ADC alone.
On one hand, where the incidence of false positives is higher on MRI as discussed above, the incidence of false-negative findings appears to be higher on PSMA PET/CT when using PROMISE scoring criteria [Tables 5 and 6]. Using this criterion, seven cancers which were missed on PSMA PET/CT, MRI was positive in six patients. Of these, four were low-grade ISUP (3 patients - Grade 2, 1 patient - Grade 1) and three were high-grade ISUP tumors. Hence, tumor grade may not be the only reason for the false-negative PSMA PET/CT study. A study done by Schmuck et al. showed the value of delayed PET imaging at 80 min postinjection for optimal visualization of PCa.[20] In this study, although we did not statistically evaluate the incremental value of delayed PET imaging over 60 min imaging, our initial experience in certain patients with delayed imaging at 90 min supports its utility in imaging PCas with low Gleason's’ scores (≤7). Larger prospective studies are needed to ascertain the exact reasons for low PSMA expression in certain PCas.
Limitations of our study are small sample size, retrospective design, and it being a single-center study. The data analysis was “per-patient” rather than “per-lesion” focusing on index/prominent lesion/alone. We correlated the imaging findings with biopsy as standard and we do acknowledge that findings of PET/MRI would have been better correlated with postprostatectomy whole mount specimen. One technical limitation in our study was we fixed diffusion weightings known as “b values” of 1400 mm2/s for estimating ADC value and did not evaluate ADC value using b values. Generally, on a 3T MRI, PCa is best-depicted b values of 1500 mm2/s.[21] However, there is a wide variation in b values use in clinical practice and there is no consensus yet on the optimal ideal cutoff of b value for ADC map estimation for PCa detection.[22,23] Another limitation was we did not follow-up or repeat biopsy in patients with negative results to account for any sampling error as the biopsies were not targeted but standard random 12 core biopsies.
Conclusion
PET/CT and MRI are complimentary for biopsy-naïve PCa diagnosis and their combination is a scenario when the whole appears to be better than the sum of its parts. SUVmax/ADC ratio appears to have better diagnostic accuracy than using ADC and SUVmax alone and is especially useful when distinguishing cancer from prostatitis. The utility of SUVmax/ADC ratio as a molecular biomarker of diagnosing clinically significant PCa should be explored in larger prospective studies with the ultimate aim of significantly reducing the number of unwanted prostate biopsies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Special thanks to Mr Ashish Sharma for his contribution towards conceptualization and medical statistics part of this study.
References
- 1.Rouvière O, Puech P, Renard-Penna R, Claudon M, Roy C, Mège-Lechevallier F, et al. MRI-FIRST investigators. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20:100–9. doi: 10.1016/S1470-2045(18)30569-2. [DOI] [PubMed] [Google Scholar]
- 2.Rourke E, Sunnapwar A, Mais D, Kukkar V, DiGiovanni J, Kaushik D, et al. Inflammation appears as high Prostate Imaging-Reporting and Data System scores on prostate magnetic resonance imaging (MRI) leading to false positive MRI fusion biopsy. Investig Clin Urol. 2019;60:388–95. doi: 10.4111/icu.2019.60.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Han D, Wu P, Ren J, Ma S, Zhang J, et al. Comparison of 68Ga-PSMA-617 PET/CT with mpMRI for the detection of PCa in patients with a PSA level of 4-20 ng/ml before the initial biopsy. Sci Rep. 2020;10:10963. doi: 10.1038/s41598-020-67385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M, Zhang Q, Zhang C, Zhao X, Marra G, Gao J, et al. Combination of (68)Ga-PSMA PET/CT and multiparametric MRI improves the detection of clinically significant prostate cancer: A lesion-by-lesion analysis. J Nucl Med. 2019;60:944–9. doi: 10.2967/jnumed.118.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Shao S, Wu P, Liu D, Yang B, Han D, et al. Diagnostic performance of (68)Ga-PSMA PET/CT in the detection of prostate cancer prior to initial biopsy: Comparison with cancer-predicting nomograms. Eur J Nucl Med Mol Imaging. 2019;46:908–20. doi: 10.1007/s00259-018-4255-1. [DOI] [PubMed] [Google Scholar]
- 6.Hicks RM, Simko JP, Westphalen AC, Nguyen HG, Greene KL, Zhang L, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET/MRI compared with multiparametric MRI in the detection of prostate cancer. Radiology. 2018;289:730–7. doi: 10.1148/radiol.2018180788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016;70:829–36. doi: 10.1016/j.eururo.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 8.Scheltema MJ, Chang JI, Stricker PD, van Leeuwen PJ, Nguyen QA, Ho B, et al. Diagnostic accuracy of 68 Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp) MRI to detect intermediate-grade intra-prostatic prostate cancer using whole-mount pathology: Impact of the addition of 68 Ga-PSMA PET to mpMRI. BJU Int. 2019;124(Suppl 1):42–9. doi: 10.1111/bju.14794. [DOI] [PubMed] [Google Scholar]
- 9.Satapathy S, Singh H, Kumar R, Mittal BR. Diagnostic accuracy of 68Ga-PSMA PET/CT for initial detection in patients with suspected prostate cancer: A systematic review and meta-analysis. AJR Am J Roentgenol. 2021;216:599–607. doi: 10.2214/AJR.20.23912. [DOI] [PubMed] [Google Scholar]
- 10.Donato P, Morton A, Yaxley J, Ranasinghe S, Teloken PE, Kyle S, et al. 68Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is 68Ga-PSMA PET/CT guided biopsy the future? Eur J Nucl Med Mol Imaging. 2020;47:1843–51. doi: 10.1007/s00259-019-04620-0. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging – reporting and data system: 2015, Version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo S, Suh CH, Kim SY, Cho JY, Kim SH. diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: A systematic Review and diagnostic meta-analysis. Eur Urol. 2017;72:177–88. doi: 10.1016/j.eururo.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 13.Verma S, Turkbey B, Muradyan N, Rajesh A, Cornud F, Haider MA, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol. 2012;198:1277–88. doi: 10.2214/AJR.12.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagel KN, Schouten MG, Hambrock T, Litjens GJ, Hoeks CM, ten Haken B, et al. Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology. 2013;267:164–72. doi: 10.1148/radiol.12111683. [DOI] [PubMed] [Google Scholar]
- 15.Lee SM, Wolfe K, Acher P, Liyanage SH. Multiparametric MRI appearances of primary granulomatous prostatitis. Br J Radiol. 2019;92:20180075. doi: 10.1259/bjr.20180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Yu F, Yang L, Zang S, Xue H, Yin X, et al. 68Ga-PSMA-11 PET/CT combining ADC value of MRI in the diagnosis of naive prostate cancer: Perspective of radiologist. Medicine (Baltimore) 2020;99:e20755. doi: 10.1097/MD.0000000000020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerstenbluth RE, Seftel AD, Hampel N, Oefelein MG, Resnick MI. The accuracy of the increased prostate specific antigen level (greater than or equal to 20 ng./ml.) in predicting prostate cancer: Is biopsy always required? J Urol. 2002;168:1990–3. doi: 10.1016/S0022-5347(05)64279-6. [DOI] [PubMed] [Google Scholar]
- 18.Vukotic V, Cerovic S, Kozomara M, Lazic M. The predictive value of PSA in diagnosis of prostate cancer in non screened population. Acta Chir Iugosl. 2005;52:81–7. doi: 10.2298/aci0504081v. [DOI] [PubMed] [Google Scholar]
- 19.Uslu-Beşli L, Bakır B, Asa S, Guner E, Demirdag C, Sahin OE, et al. Correlation of SUVmax and apparent diffusion coefficient values detected by Ga-68 PSMA PET/MRI in primary prostate lesions and their significance in lymph node metastasis: Preliminary results of an on-going study. Mol Imaging Radionucl Ther. 2019;28:104–11. doi: 10.4274/mirt.galenos.2019.63825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmuck S, Mamach M, Wilke F, von Klot CA, Henkenberens C, Thackeray JT, et al. Multiple time-point 68Ga-PSMA I and T PET/CT for characterization of primary prostate cancer: Value of early dynamic and delayed imaging. Clin Nucl Med. 2017;42:e286–93. doi: 10.1097/RLU.0000000000001589. [DOI] [PubMed] [Google Scholar]
- 21.Metens T, Miranda D, Absil J, Matos C. What is the optimal b value in diffusion-weighted MR imaging to depict prostate cancer at 3T? Eur Radiol. 2012;22:703–9. doi: 10.1007/s00330-011-2298-9. [DOI] [PubMed] [Google Scholar]
- 22.Thörmer G, Otto J, Reiss-Zimmermann M, Seiwerts M, Moche M, Garnov N, et al. Diagnostic value of ADC in patients with prostate cancer: Influence of the choice of b values. Eur Radiol. 2012;22:1820–8. doi: 10.1007/s00330-012-2432-3. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: Recommendations from a European consensus meeting. Eur Urol. 2011;59:477–94. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]


