Abstract
Aim:
This study aims to study the clinical-diagnostic relevance of incidental breast uptake (“incidentaloma”) on 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan performed for other indications and to correlate it with radiological imaging and histopathology.
Materials and Methods:
We retrospectively evaluated 3675 FDG-PET scans, identifying 43 patients with breast “incidentaloma.” Thirty of these findings were further investigated with clinical examination, mammography (MMX), UltraSound (US) and/or magnetic resonance (MR). Cases suspected for malignancy underwent US-guided macro-biopsy (USMB) or MR-guided biopsy. Correlations between FDG-PET, radiology findings, age, and histopathology were evaluated.
Results:
patients who performed both US and MMX were 19. Ten consequently underwent USMB, one MR-guided biopsy, the remaining 8 were not further investigated. Nine patients had a diagnosis of malignancy. Among 11 patients who performed only US and consequently, USMB 6 had a diagnosis of malignancy. Histopathology of the 22 patients with both morphological and glucometabolic alterations showed different types of benign or malignant neoplasia, with a cumulative 68.2% incidence of malignancy. Seven lesions showed a SUVmax >2.5, while the remaining 15 a SUVmax <2.5. There was no statistically significant correlation between SUVmax and histology, therefore SUVmax parameter should not be used to discriminate between benign and malignant findings. No significant correlation between patient age and tumor characterization was found.
Conclusions:
incidental mammary uptake during an FDG-PET scan may represent a clue suggesting to investigate PET findings. In this subset of patients, early diagnosis may lead to a change in clinical management with a favorable impact on prognosis and a significant reduction in healthcare costs.
Keywords: Breast cancer, incidental findings, mammography, PET, ultrasonography
Introduction and Aim
An “incidentaloma” is commonly defined as an incidental finding detected in an organ during a scan performed for other clinical indications. In patients with known primary cancer, the frequency of a concomitant second malignancy is not negligible and a quote of these neoplasms might be detected incidentally. Katz and Shaha[1] first coined the term “positron emission tomography (PET)-associated incidental neoplasm” (PAIN) specifically to define the incidental finding of a neoplasm during a PET/computed tomography (CT) scan performed for another indication. The cumulative incidence of incidental findings on 18F-fluoro-2-deoxy-D-glucose (18F-FDG PET/CT) scans ranges between 0.2% and 8.9% and is more frequent in patients over 45 years of age, while the prevalence of a malignant nature “incidentaloma” ranges between 1.2% and 1.7%.[2] Thus, incidental FDG-PET findings require further investigations to clarify their nature.[3]
The most common PAIN localizations are thyroid gland, gastrointestinal tract, and lungs, with a cumulative incidence of 1%–3% of all cancers.[4,5,6] Incidental breast uptakes are quite rare.[6,7] Clinical examination and imaging are essential in the evaluation of breast pathology, but sometimes some lesions could be undetected during screening programs, and casually discovered during some other exams, such as FDG-PET. These findings could be expression of both benignant and malignant lesions, some of which with clinical significance.[8,9,10,11,12] Nevertheless, FDG-PET is not currently recommended for the detection of primary breast cancer, due to the presence of several limitations regarding the evaluation of breast lesions. In particular, FDG-PET lacks sensibility in detecting small lesions, under 1 cm diameter.[13,14] Moreover, different breast cancer histotypes can present a wide range of FDG-avidity, with infiltrating ductal carcinoma (IDC) histotype showing a much higher FDG uptake in comparison to Invasive Lobular Carcinoma.[15,16] As for SUVmax parameter, it is known to be influenced by several conditions, related both to the patient and to the exam protocol conditions. These issues make it a not sufficiently reliable parameter to discriminate between benignant and malignant findings.[17,18,19]
For the reasons above, any FDG breast “incidentaloma” (BI) should be further investigated, as suggested by the “National Comprehensive Cancer Network” Guidelines. However, their management is currently debated, as mammography (MMX) and ultrasound (US) are suggested as first-level exams, while a bioptic approach should be reserved for lesions with a BI-RADS 4 or 5.[20]
The aim of the present study is to define the clinical and diagnostic significance of incidental breast tissue uptakes detected during 18F-FDG scans performed for a different indication. For this purpose, we studied the correlations with traditional radiological imaging and histopathology examination, performed to determine the nature of those findings.
Materials and Methods
Population study
We retrospectively evaluated 3675 FDG-PET scans performed in our Nuclear Medicine Unit during the years 2014–2020 and selected those with an incidental 18F-FDG breast uptake (43 scans) [Figure 1]. Scans of patients with the previous history of biopsy-proven breast cancer were excluded. Consequently, we checked if the BI had been further investigated with clinical exam, MMX, US and/or MR (the latter limited to patients with undetermined results on conventional radiology). Finally, for each patient whose finding was subjected to a biopsy examination (US-guided or MR guided), we reported the histological diagnosis.
Figure 1.

Patient's selection flow-chart
Information on the other imaging modalities and investigations were obtained from the Hospital digital archives, Polaris® and SAP®. The study was performed in accordance with the ethical standards of the local institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all subjects.
Forty-three patients were selected (7 males, 36 females, average age 66.28 ± 14.7 years, min 26 max 90), respectively affected by Lung Cancer (15 patients), non-Hodgkin Lymphoma (10), Hodgkin Lymphoma (2), Melanoma (4), Head and Neck Cancer (3) and 9 other tumors/pathologies.
FDG-PET acquisition protocol and interpretation
All patients were required to fast for 6–8 h and maintain an adequate hydration before the scan. Diabetic patients had blood glucose measured before 18F-FDG delivery. Those with a fasting glucose above 190 mg/dl were postponed until a proper therapy was established. Images were acquired 50–70 min after 18F-FDG injection (1 mCi/10Kg) using a standard technique on a dedicated 3D PET/CT system (Biograph mCT Flow; Siemens Medical Solutions, Malvern, PA, USA). A low-dose CT scan (120 kV and 80 mA/s) was performed for the attenuation correction of the PET emission data acquired from the mid-thigh to the skull vertex.
PET/CT images were all processed and analyzed by a Syngo.via Workstation (Siemens Healthineers). Final PET/CT images were reconstructed along axial, coronal and sagittal planes with a dedicated workstation by an expert nuclear medicine physician. A MIP image has been stored for every patient. Every focal deviation from physiological distribution, background, or blood-pool and liver uptakes was reported, be it hyper or hypo-metabolic. For every finding save screens were registered, and SUVmax was calculated, considering 2.5 value as a cut-off to discriminate between hyper and hypo-metabolic breast incidental uptakes.
Statistical analysis
The nonparametric Median test was applied for independent samples, with the aim to verify the existence of significant differences in two study groups identified respectively as patients with benign and malignant breast neoplasia versus SUVmax trend. The Mann–Whitney U-test was also applied to the same independent samples. To evaluate the diagnostic agreement between the instrumental investigations examined (US and MMX), the concordance index was calculated using the Koen Concordance Test (K). To evaluate the concordance between the patient's age and the finding of malignant or benign neoplasm, the Non-Parametric Median Test for independent samples was applied.
Results
We analyzed 3675 PET/CT scans performed in patients without history of breast cancer. Among those we found 43 BI, with a prevalence of 1.17%. Thirty out of 43 patients underwent diagnostic deepening of the lesions detected with the FDG-PET scan [Table 1]. Among the 13 patients who did not investigate the BI, 4 died early after the FDG-PET scan, before completing the diagnostic process and the remaining 9 were lost during the follow-up or did not perform further investigations because deemed unnecessary. One of these nine patients (female, 81-year-old) had a diagnosis of breast cancer 1 year later, in the site where the 18F-FDG uptake was previously detected.
Table 1.
Patients who underwent diagnostic deepening of the lesions detected with the fluorodeoxyglucose-positron emission tomography scan
| ID (n°) | Sex | Age | Primary disease | Side of BI | SUVmax | US | MMX | Lesion size (mm) | Histology or outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 77 | H-N | Left | 2.2 | Negative | Negative | 21 | IDC G2 |
| 2 | Female | 55 | Melanoma | Left | 3.2 | Negative | Negative | 23 | No biopsy |
| 3 | Female | 89 | Lung tumor | Left | 0.8 | Negative | Negative | 15 | No biopsy |
| 4 | Female | 81 | NHL | Left | 2.5 | Positive | Positive | 10 | Fullicular lymphoma |
| 5 | Female | 52 | NHL | Left | 2.2 | Negative | Negative | 28 | No biopsy |
| 6 | Female | 81 | Melanoma | Left | 0.7 | Positive | Positive | 11 | IDC G2 |
| 7 | Female | 82 | Lung tumor | Right | 2.1 | Positive | Positive | 7 | Adenoidocistic G3 |
| 8 | Female | 56 | Melanoma | Right | 0.8 | Negative | Negative | 12 | No biopsy |
| 9 | Female | 72 | Sarcoma | Left | 1.1 | Positive | Positive | 15 | Fibroadenoma |
| 10 | Female | 26 | HD | Left | 1.3 | Positive | 18 | Phylloid tumor | |
| 11 | Female | 54 | Lung tumor | Left | 1.4 | Positive | 10 | Fibroadenoma | |
| 12 | Female | 69 | Lung tumor | Left | 1.5 | Positive | Positive | 11 | Fibroadenoma |
| 13 | Female | 70 | Lung tumor | Right, left | 1.2 | Negative | Negative | 11 | No Biopsy |
| 14 | Female | 53 | Melanoma | Left | 15.7 | Positive | 12 | Carcinomatous mastitis | |
| 15 | Male | 40 | HD | Right | 0.8 | Positive | 12 | Hodgkin lymphoma | |
| 16 | Female | 90 | ThyrT | Left, right | 1.1 | Positive | 17 | IDC G2 | |
| 17 | Female | 70 | Vasculitis | Right | 3.9 | Positive | Positive | 18 | IDC G2 |
| 18 | Female | 72 | NHL | Left | 1.3 | Positive | Negative | 15 | IDC G2 |
| 19 | Female | 43 | Lung tumor | Right, left | 0.6 | Positive | 9 | Fibroadenoma | |
| 20 | Female | 79 | Lung tumor | Left | 0.9 | Positive | 17 | Fibroadenoma | |
| 21 | Female | 70 | TL | Right | 6.0 | Positive | 17 | T-Lymphoma | |
| 22 | Female | 65 | NHL | Right | 3.8 | Positive | 8 | Mantle cell Lymphoma | |
| 23 | Female | 42 | H-N | Left | 1.3 | Negative | Negative | 18 | No biopsy |
| 24 | Female | 78 | Lung tumor | Left, right | 1.2 | Positive | Positive | 8 | IDCG2 |
| 25 | Female | 67 | OC | Left | 1.1 | Negative | Negative | 17 | No biopsy |
| 26 | Male | 74 | NHL | Left | 2.5 | Positive | 23 | IDC G2 | |
| 27 | Female | 62 | Lung tumor | Left | 1.3 | Positive | Positive | 7 | IDC G2 |
| 28 | Female | 70 | NHL | Right | 1.5 | Positive | Positive | 9 | IDC G2 |
| 29 | Male | 73 | Lung tumor | Right | 1.8 | Negative | 12 | No biopsy | |
| 30 | Female | 67 | Lung tumor | Right, left | 2.5-1.8 | Positive | Positive | 25 | Fibroadenoma |
NHL: Non hodgkin lymphoma, HD: Hodgkin disease, ThyrT: Thyroid tumor, TL: T lymphoma, H-N: Head and neck tumor, OC: Occult cancer, US: Utra sound, MMX: Mammography, IDC: Infiltrating ductal carcinoma, BI: Breast incidentaloma, SUVmax: Standardized uptake value maximum
Nineteen out of 30 patients who performed radiological imaging underwent both US and MMX, while the remaining 11 (25.58%) performed only US. Specifically, in the group that performed both US and MMX, 10 (57.9%) had both US and MMX positive scans and 1 (11.1%) had a positive US scan and a negative MMX; each of these patients consequently performed US-guided macro-biopsy (USMB). The remaining eight patients (26.6%) did not show certain breast morphological abnormalities at US and/or MMX; 3 of them were subsequently subjected to Magnetic resonance (MR) and in 1 (ID n°1) an IDC, G2, was detected. In this case, the biopsy was MR-guided. The diagnostic agreement between the two methods (US and MMX) resulted highly significant (P ≤ 0.001). Ten out of 11 patients who performed only breast US underwent USMB that highlighted a malign finding in 6 cases [Table 2].
Table 2.
Diagnostic deepening performed
| Diagnostic deepening | Total (%) | Positive | Negative |
|---|---|---|---|
| None | 13 (30.2) | - | - |
| US | 11 (25.6) | 10 | 1 |
| MMX + US | 19 (44.2) | 11* | 8 |
| Total | 43 (100) | ||
|
| |||
| Biopsy | Total (%) | Malignant | Nonmalignant |
|
| |||
| USMB-guided | 21 | 14 | 7 |
| MR-guided | 1 | 1 | - |
| Total (%) | 22 (100) | 15 (68.2) | 7 (31.8) |
*10 both US and MMX positive scans, 1 with positive US, but negative MMX. MMX: Mammography, US: Utra sound, USMB: US-guided macro-biopsy, MR: Magnetic resonance
Overall 22 patients performed biopsy examination (21 US-guided and 1 MR-guided). Fifteen out of 22 BI were malignancies, with an overall incidence of 68.2%. Among those, 11 (73.3%) were primary breast cancers (9 IDC G2, 2 Adenoidocystic Cancer and 1 Carcinomatous Mastitis) and 4 (26.7%) were atypical Lymphoma localizations (1 Hodgkin's Lymphoma, 2 non Hodgkin's Lymphoma, and 1 cutaneous T cell Lymphoma) [Figure 2]. The remaining 7 BI were benign lesions (6 Fibroadenoma and 1 Phyllodes Tumor) [Figure 3 and Table 1].
Figure 2.

Female, 65-year-old, undergoing fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography to evaluate a vasculitis. A focal fluoro-2-deoxy-D-glucose uptake (SUVmax 3,9) was identified in the right breast. Histology: infiltrating ductal carcinoma
Figure 3.
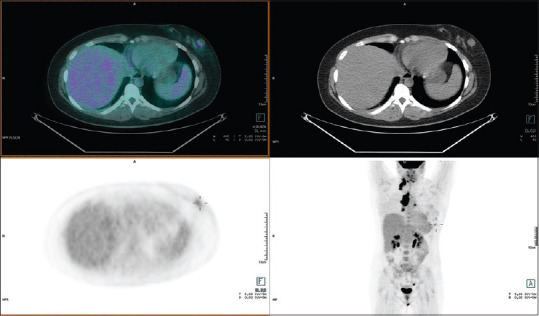
Female, 69-year-old, undergoing fluoro-2-deoxy-D-glucose positron emission tomography for restaging sarcoma. 18F- fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography: Mild fluoro-2-deoxy-D-glucose uptake (SUVmax 1,5) was identified in the left breast. Histology: fibroadenoma
Within the cohort of patients who performed histopathological correlation, median SUVmax value was 2.02 ± 2.38 (minimum value 0.60; maximum value 15.7). Seven patients (31,8%) had a SUVmax >2.5 while the remaining 15 (68,2%) had a SUVmax <2.5. In 2 cases, ID n° 14 and 21, breast lesion's SUVmax were respectively 15.7 and 6, while all the other ranged between the 1st percentile and 3rd percentile. The malignant lesion with the lowest SUVmax value (0.7) was histopathologically diagnosed as IDC, G2, while the benignant lesion with the higher SUVmax value (2.5) was a fibroadenoma. Among BI with confirmed diagnostic/histological findings, no statistically significant correlation was found between lesional SUVmax and histology (benign vs. malignant).
Discussion
When evaluating an FDG-PET scan, the attention is usually focused on the primary disease and not on the possible incidental coexistence of another primary malignant lesion. Nevertheless, the prevalence of a second incidental neoplasm is far from negligible. A nonspecific 18F-FDG spot can be detected in various conditions and it should be reminded that it could be neoplastic until proven otherwise.[21,22]
During a FDG-PET scan the identification of an abnormal breast uptake can occur, even if it is quite rare and it can be a false positive image in a nonnegligible percentage of cases. Several literature evidences report that a BI detected during a FDG-PET scan performed for other reasons can identify a breast cancer.[23,24] Anyhow, the reported frequency of malignancy is highly variable, ranging from 29.7% to 71.5%.[18,25] Our results are consistent with Bertagna et al. In fact, we found that in 22 cases (73.3%) the detection of a FDG-PET BI was confirmed by a radiological exam. Of these findings, a relevant number of cases (68.2%) was diagnosed as malignant at the following histopathological examination. The well-timed identification of these malignancies has a huge impact on patient's diagnostic and therapeutic management, as the early diagnosis may potentially lead to a consistent benefit in terms of prognosis and costs reduction for the Healthcare System. Thus, our experience strengthens the importance of further investigate every abnormal breast uptake observed during a FDG-PET scan.
It is well known that different breast cancers histotypes have a different affinity and tropism for 18F-FDG. Buck et al.[10] and Avril et al.[26] reported that 18F-FDG uptake is significantly higher in IDC than in Lobular Carcinoma and Yoon et al.[27] also described that medium SUVmax values are significantly superior in IDC in comparison to Lobular Carcinoma. Bertagna et al.[18] reported that the 68% of malignant BI was infiltrating or in situ ductal carcinoma at the histopathological exam. Our case series is consistent with this previous literature data. In fact, among the malignant BI, every primary breast cancer was histopathologically proved to be IDC. Among benign BI the prevalent histology was fibroadenoma, in keeping with other literature reports.[18]
When evaluating a BI, some articles report a significant difference in terms of SUVmax values between benign and malignant tumors.[17] On the contrary, some other authors do not consider SUVmax as a sufficiently reliable parameter to discriminate BI nature.[10] SUVmax parameter is known to be influenced by various factors both related to individual patient biologic aspects and to several procedural aspects (such as different tomographs performance, scanning protocols, and injected 18F-FDG activity). In our study we found no statistically significant correlation between the SUVmax values of the BI and the corresponding histological findings (P = 0.361), even if, in the case of malignancy, a modestly higher SUVmax trend was identified. This means that a medium-high SUVmax value does not necessarily correspond to a malignant BI and vice versa. Remarkably, patient ID n. 8 had a lesion with a very low SUVmax value (0.7), which is considerable hypometabolic, that resulted to be a ICD, G2. Thus, we believe that this finding suggests that nuclear medicine physicians should always report any abnormal breast uptake, even if hypometabolic, because it can be malignant until proven otherwise. Anyhow, SUVmax parameter alone is not sufficiently reliable to discriminate between benign and malignant BI. Therefore, our results highlight that a clinical examination is mandatory to evaluate the characteristics of any BI detected. In case of medium-high probability of malignancy, it is essential to proceed with further radiological-histological investigations. Conversely, cases of low probability of malignancy should be addressed to clinical follow-up, postponing further investigations until evidence of lesions’ variation (e.g. size increase).
Dedicated breast imaging is surely the most reliable tool for both screening and diagnosis of breast lesions, whether benign or malignant. Similarly, the gold standard for the histological confirmation is through a biopsy examination (be it US-guided, stereotaxic, or MR-guided).[28] In the present case series, radiological breast dedicated exams (US, MMX) showed a highly statistically significant diagnostic concordance in the evaluation of breast findings (P < 0.001). Literature data confirms reliability and compliance of breast-related radiological methods in both screening and diagnosis of breast neoformations.[20] In our case series, only one BI resulted positive at breast US scan but negative of MMX, probably in relation with the small size of the finding (Ø 5 mm). The final histology was IDC, G2. Only in one case US and MMX were both negative and the subsequent MR scan showed a breast area of pathological “contrast enhancement,” that was found to be ICD at histological examination. Recent evidences demonstrate that in patients with extremely dense breast tissue and normal MMX a supplementary MR imaging is useful.[29]
Finally, 8 FDG-PET breast findings were not radiologically confirmed. This may be explained by the possible existence of mammary artefacts, both from movement during PET scan acquisition or for the breast para-physiological distribution of the radioactive tracer (in patients of childbearing age it is necessary to consider the phase of the menstrual cycle, keeping in mind that in the postmenstrual phase the breast tissue physiologically captures 18F-FDG). Moreover, several benign and inflammatory breast conditions can potentially mimic malignancies.[18,30,31,32,33]
A limit of the present study might be the relative low number of cases under examination, in a time window of 6 years (2014–2020). This can be correlated to various factors: (a) BI on FDG-PET scan performed for other reasons is quite rare to find; other types of “incidentalomas” are more frequent, as confirmed by literature data (1,2,4,5) (b) we considered a uniform case series, in which every PET/CT scan was performed with the same tomograph, excluding the exams performed with a second resident tomograph which was dismissed in 2015.
Conclusions
Although the case series in question is small and implementation is necessary, our data suggest that the identification of an incidental breast uptake during a FDG-PET scan performed for another reason can represent an important “alarm bell.” An FDG-PET BI radiologically confirmed has a medium-high probability (68.2% in the present case series) to be a malignant breast neoformation, regardless of lesional SUVmax value. For these reasons, a clinical evaluation is always mandatory. Moreover, in suspicious cases, a “second look” exam (US, MMX and/or MR scan) and if appropriate, even a biopsy examination should always be performed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Katz SC, Shaha A. PET-associated incidental neoplasms of the thyroid. J Am Coll Surg. 2008;207:259–64. doi: 10.1016/j.jamcollsurg.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Treglia G, Calcagni ML, Rufini V, Leccisotti L, Meduri GM, Spitilli MG, et al. Clinical significance of incidental focal colorectal 18F-fluorodeoxyglucose uptake: Our experience and a review of the literature. Color Dis. 2012;14:174–80. doi: 10.1111/j.1463-1318.2011.02588.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertagna F, Treglia G, Orlando E, Dognini L, Giovanella L, Sadeghi R, et al. Prevalence and clinical significance of incidental F18-FDG breast uptake: A systematic review and meta-analysis. Jpn J Radiol. 2014;32:59–68. doi: 10.1007/s11604-013-0270-0. [DOI] [PubMed] [Google Scholar]
- 4.Bertagna F, Treglia G, Piccardo A, Giubbini R. Diagnostic and clinical significance of F-18-FDG-PET/CT thyroid incidentalomas. J Clin Endocrinol Metab. 2012;97:3866–75. doi: 10.1210/jc.2012-2390. [DOI] [PubMed] [Google Scholar]
- 5.Stone WZ, Wymer DC, Canales BK. Fluorodeoxyglucose-positron-emission tomography/computed tomography imaging for adrenal masses in patients with lung cancer: Review and diagnostic algorithm. J Endourol. 2014;28:104–11. doi: 10.1089/end.2013.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernsdorf M, Graff J. Clinical application of 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in breast cancer. Clin Physiol Funct Imaging. 2014;34:426–33. doi: 10.1111/cpf.12106. [DOI] [PubMed] [Google Scholar]
- 7.Kang BJ, Lee JH, Yoo IeR, Kim SH, Choi JJ, Jeong SH, et al. Clinical significance of incidental finding of focal activity in the breast at 18F-FDG PET/CT. AJR Am J Roentgenol. 2011;197:341–7. doi: 10.2214/AJR.10.6126. [DOI] [PubMed] [Google Scholar]
- 8.Benveniste AP, Yang W, Benveniste MF, Mawlawi OR, Marom EM. Benign breast lesions detected by positron emission tomography-computed tomography. Eur J Radiol. 2014;83:919–29. doi: 10.1016/j.ejrad.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Beatty JS, Williams HT, Gucwa AL, Hughes MP, Vasudeva VS, Aldridge BA, et al. The predictive value of incidental PET/CT findings suspicious for breast cancer in women with non-breast malignancies. Am J Surg. 2009;198:495–9. doi: 10.1016/j.amjsurg.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Buck A, Schirrmeister H, Kühn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29:1317–23. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 11.Chierichetti F, Pizzolato G. 18F-FDG-PET/CT. Q J Nucl Med Mol Imaging. 2012;56:138–50. [PubMed] [Google Scholar]
- 12.Litmanovich D, Gourevich K, Israel O, Gallimidi Z. Unexpected foci of 18F-FDG uptake in the breast detected by PET/CT: Incidence and clinical significance. Eur J Nucl Med Mol Imaging. 2009;36:1558–64. doi: 10.1007/s00259-009-1147-4. [DOI] [PubMed] [Google Scholar]
- 13.Paydary K, Seraj SM, Zadeh MZ, Emamzadehfard S, Shamchi SP, Gholami S, et al. Molecular Imaging and Biology. Vol. 21. New York: Springer LLC; 2019. The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty D, Basu S, Ulaner GA, Alavi A, Kumar R. Diagnostic role of fluorodeoxyglucose PET in breast cancer: A history to current application. PET Clin. 2018;13:355–61. doi: 10.1016/j.cpet.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Groheux D, Majdoub M, Sanna A, de Cremoux P, Hindié E, Giacchetti S, et al. Early metabolic response to neoadjuvant treatment: FDG PET/CT criteria according to breast cancer subtype. Radiology. 2015;277:358–71. doi: 10.1148/radiol.2015141638. [DOI] [PubMed] [Google Scholar]
- 16.Kitajima K, Fukushima K, Miyoshi Y, Nishimukai A, Hirota S, Igarashi Y, et al. Association between 18F-FDG uptake and molecular subtype of breast cancer. Eur J Nucl Med Mol Imaging. 2015;42:1371–7. doi: 10.1007/s00259-015-3070-1. [DOI] [PubMed] [Google Scholar]
- 17.Lim S, Lee EH, Park JM, Chang YW, Kim HH, Jeong SH. Role of combined BI-RADS assessment using mammography and sonography for evaluation of incidental hypermetabolic lesions in the breast on 18F-FDG PET-CT. Acta Radiol. 2013;54:1117–24. doi: 10.1177/0284185113492453. [DOI] [PubMed] [Google Scholar]
- 18.Bertagna F, Evangelista L, Piccardo A, Bertoli M, Bosio G, Giubbini R, et al. Multicentric study on 18F-FDG-PET/CT breast incidental uptake in patients studied for non-breast malignant purposes. Rev Esp Med Nucl Imagen Mol. 2015;34:24–9. doi: 10.1016/j.remn.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Mohamadien NR, Sayed MH. Correlation between semiquantitative and volumetric 18F-FDG PET/computed tomography parameters and Ki-67 expression in breast cancer. Nucl Med Commun. 2021;42:656–64. doi: 10.1097/MNM.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 20.Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362–89. doi: 10.6004/jnccn.2018.0083. [DOI] [PubMed] [Google Scholar]
- 21.Dong C, Hemminki K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958-1996: A search for common mechanisms. Br J Cancer. 2001;85:997–1005. doi: 10.1054/bjoc.2001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: An experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003;8:162–7. doi: 10.1007/s10147-003-0322-z. [DOI] [PubMed] [Google Scholar]
- 23.Eubank WB, Mankoff DA. Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2005;35:84–99. doi: 10.1053/j.semnuclmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Korn RL, Yost AM, May CC, Kovalsky ER, Orth KM, Layton TA, et al. Unexpected focal hypermetabolic activity in the breast: Significance in patients undergoing 18F-FDG PET/CT. AJR Am J Roentgenol. 2006;187:81–5. doi: 10.2214/AJR.05.0548. [DOI] [PubMed] [Google Scholar]
- 25.Shin KM, Kim HJ, Jung SJ, Lim HS, Lee SW, Cho SH, et al. Incidental breast lesions identified by 18F-FDG PET/CT: Which clinical variables differentiate between benign and malignant breast lesions? J Breast Cancer. 2015;18:73–9. doi: 10.4048/jbc.2015.18.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avril N, Adler LP. F-18 Fluorodeoxyglucose-Positron Emission Tomography Imaging for Primary Breast Cancer and Loco-Regional Staging. Radiol Clin North Am; 2007;45:645–57. doi: 10.1016/j.rcl.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Yoon HJ, Kang KW, Chun IK, Cho N, Im SA, Jeong S, et al. Correlation of breast cancer subtypes, based on estrogen receptor, progesterone receptor, and HER2, with functional imaging parameters from 68Ga-RGD PET/CT and 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2014;41:1534–43. doi: 10.1007/s00259-014-2744-4. [DOI] [PubMed] [Google Scholar]
- 28.Siu AL. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2016;164:279–96. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 29.Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PH, Monninkhof EM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091–102. doi: 10.1056/NEJMoa1903986. [DOI] [PubMed] [Google Scholar]
- 30.Metser U, Miller E, Lerman H, Even-Sapir E. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: Characterization and incidence. AJR Am J Roentgenol. 2007;189:1203–10. doi: 10.2214/AJR.07.2083. [DOI] [PubMed] [Google Scholar]
- 31.Akkas BE, Ucmak Vural G. Fat necrosis may mimic local recurrence of breast cancer in FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 2013;32:105–6. doi: 10.1016/j.remn.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Ceyrat Q, Ziade C, Tlili G, Fernandez P, Meyer M. Galactocele, pitfall for the evaluation by 18F-FDG PET/CT. Clin Nucl Med. 2018;43:e237–8. doi: 10.1097/RLU.0000000000002119. [DOI] [PubMed] [Google Scholar]
- 33.Levin D, Lantsberg S, Giladi MR, Kazap DE, Hod N. Post contusion breast hematoma mimicking malignancy on FDG PET/CT. Clin Nucl Med. 2020;45:552–4. doi: 10.1097/RLU.0000000000003050. [DOI] [PubMed] [Google Scholar]


