Abstract
Background:
Optimal peptide concentration in treatment with 177Lu-DOTATOC/DOTATATE is a matter of debate. Most of the studies with peptide receptor radionuclide therapy mention peptide dose ranging between 100 and 250 μg. The aim of this is to identify possible differences in radiation-absorbed doses (D/Gy) to tumor and kidney as a function of the peptide mass dose in order to identify the most suitable peptide dose for treatment. The therapeutic index (Dtumor/Dkidneys) was assessed as a key parameter for the treatment response.
Materials and Methods:
Five patients with metastasized Grade 1 to Grade 2 neuroendocrine tumor were analyzed in this study. Patients (n = 4) received two cycles of treatment with intravenously injected 177Lu-DOTATOC containing peptide mass doses of 200 μg and 90 μg, alternatively; one patient was treated with 90 μg peptide mass in both the therapy cycles. Whole-body (head to mid-thigh) three-dimensional single-photon emission computerized tomography (3D SPECT)/CT images were acquired at 1, 4, 24, 48, and 72 h following the injection of 177Lu-DOTATOC. Attenuation correction for 3D SPECT images was performed using CT data acquired and fused with the SPECT data (SPECT/CT).
Results:
Overall, 28 target lesions (liver n = 17, lung n = 4, lymph nodes n = 1, and bone n = 2) were analyzed after 1st and 2nd therapy cycles. Tumor normalized absorbed doses varied by a factor of 74 between 0.35 and 26 mGy/MBq. Averaged over all patients, a higher normalized mean tumor dose (10.51 mGy/MBq) was achieved for a peptide dose of 200 μg compared to 90 μg (4.58 mGy/MBq). Kidneys doses varied by a factor of up to 4 between patients (0.25–1.0 mGy/MBq) (independent of dose cycle and peptide dose) and by a factor of up to 2 between dose cycles. The mean kidney dose was 13.7% higher for the 90 μg peptide dose compared to 200 μg. Given the higher tumor dose, the mean therapeutic index of a 200 μg mass dose was considerably higher (16.95), compared to a 90 μg mass dose (9.63). This coincided with the observation, that lesion volume reduction was more pronounced after an initial treatment with a 200 μg mass dose. Biologically effective dose was only 5. 1%–19.3% higher than the absorbed dose for individual dose cycles.
Conclusions:
Higher peptide dose of 200 μg appears to be more suitable than 90 μg in terms of tumor dose, kidney dose, and therapeutic index for treatment with 177Lu-DOTATOC.
Keywords: 177Lu-DOTATOC, dosimetry, peptide mass, peptide receptor radionuclide therapy
Introduction
Peptide receptor radionuclide therapy (PRRT) is an effective treatment option for Grade 1/Grade 2 gastroenteropancreatic neuroendocrine tumor (NET).[1] The recently concluded NETTER-1 study showed treatment benefit for midgut NET treated with 4 cycles of 7.4 GBq 177Lu-DOTATATE at 8-week interval in combination with octreotide 30 mg in comparison to octreotide 60 mg.[2]
Although a consensus exists on the number of cycles for PRRT, the amount of peptide used for radiolabeling has not been standardized.[3] Standard of care for NET recommends stopping octreotide or lanreotide at least 4–6 weeks before PRRT to prevent saturation of somatostatin receptors.[3] In general, the peptide dose recommended for labeling by the guidelines ranges between 100 and 250 μg. There is a general opinion that there remains a significant proportion of free non-radiolabeled peptides in the end product of 177Lu-DOTATATE or 177Lu-DOTATOC, which could theoretically block somatostatin receptors on target lesions. Sabet et al. have analyzed exactly this issue.[4] In their study, they reported about five patients where treatment with peptide doses ranging between 180 and 300 μg did not lead to significant saturation of the target lesions in comparison to normal liver and spleen. For their study, the authors used various standardized uptake values (SUVs) on 68Ga-DOTATOC PET performed immediately before and after PRRT. Although SUV is a useful parameter, it is dependent on several other factors (Prasad et al., 2008).[5] In addition, there is no study which has directly looked at dosimetry data of 177Lu-DOTATATE or 177Lu-DOTATOC to compare the peptide mass effect on the tumor and kidneys dose. With this background, we undertook this pilot study to investigate the possible differences in radiation-absorbed doses (D/Gy) to tumor and kidneys as a function of the peptide mass dose in order to identify the most suitable peptide dose for treatment. The therapeutic index (Dtumor/Dkidneys) was assessed as a key parameter for the treatment response.
Study objectives
The purpose of this study was to investigate the optimum peptide dose of 177Lu-DOTATOC with respect to efficacy and radiation safety for the treatment of patients with metastatic lesions from Grade 1 and Grade 2 NETs.
The detailed objectives were:
To evaluate the kidney dosimetry after IV injection of 177Lu-DOTATOC (radiation safety dosimetry)
To evaluate the individual tumor dosimetry
To investigate the relationship between absorbed dose and biologically effective dose (BED) for the kidneys
To examine the peptide dose for treatment with 177Lu-DOTATOC
To evaluate the preliminary efficacy of 177Lu-DOTATOC in reducing tumor size and uptake.
Materials and Methods
Study population
The study was performed after taking the institutional review board clearance (EA2 176/17). All the patients gave written informed consent for undergoing treatment with PRRT. Five patients (3 females and 2 males; age range 62–70 years) with metastasized NETs treated with two cycles of 177Lu-DOTATOC containing different peptide doses at Charité Berlin were evaluated. An overview of the study population is shown in Table 1. The locations of the primary tumors were as follows: three patients had ileum NETs, 1 patient had rectum NET, and one patient had pancreas NET. Two patients received 200 μg peptide dose in the first cycle and 90 μg of peptide dose in the second cycle. Two patients received first 90 μg peptide dose and second 200 μg peptide dose. One patient received 90 μg peptide dose in both therapy cycles. The second treatment was given between 3 and 6 months after the first treatment. The administered radionuclide dose ranged between 4.9 and 7.66 GBq. This large variation in dose took place because of the clinical need of dose adjustment due to poor renal function or pretreatment with chemotherapy. Patients were instructed not to go to toilet before the 1st imaging time point.
Table 1.
Overview of analysed study population
| Patient | Number analysed tumours | Tumour Ki67 index [%] | Dose Cycle D1 | Dose Cycle D2 | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Peptide Dose [µg] | A0 [MBq] | Missing imaging time points | Time post D1 [days] | Peptide Dose [µg] | A0 [MBq] | Missing imaging time points | |||
| A | 5 | 15 | 200 | 5200 | - | 90 | 7360 | - | |
| B | 6 | 15 | 200 | 5100 | 1h | 90 | 5100 | 72h | |
| C | 6 | 5 | 90 | 5300 | 72h | 200 | 4900 | - | |
| D | 7 | 10 | 90 | 7000 | - | 200 | 5100 | - | |
| E | 8 | 1 | 90 | 7660 | - | 90 | 7440 | - | |
Imaging acquisition and processing
Semiquantitative single-photon emission computerized tomography (SPECT)/CT imaging was performed at Charité Berlin:
Whole-body (head to knees) three-dimensional (3D) SPECT/CT images were acquired at approximately 1, 4, 24, 48, and 72 h following injection of 177Lu-DOTATOC
Attenuation correction for 3D SPECT images was performed using CT data acquired and fused with the SPECT data (SPECT/CT)
SPECT images were reconstructed using a 3D ordered subset expectation maximization algorithm with 8 iterations and 16 subsets, applying scatter, attenuation, and uniformity corrections. A Gaussian postreconstruction filter of 9 mm full width at half maximum was also applied.
The SPECT images had been fused with the corresponding low-dose CT image. The reader had checked and assessed the quality of the coregistration of the fused images.
The analysis was carried out using MIRADA, MATLAB, and OLINDA software. The segmented images were jointly reviewed by an imaging physicist and a nuclear medicine physician.
Absolute activity calibration was performed using the assumption that all counts in the SPECT image at time point 1 (1 h post injection) corresponded to the injected activity (corrected for decay). The calibration factor (CF) was calculated using a volume of interest (VOI) around the whole body according to:

where A0 is the injected activity, TP1 is the first imaging time point, Tinj is the time of injection, and τphys is the physical half-life of 177Lu. Given that the SPECT image does not contain the arms and parts of the legs, it is expected that the CF and therefore the measured activities in volumes of interest could be overestimated by 10%–30%. This effect was not corrected.
Quantitative assessment of tumor uptake
The tumor uptake was measured for lesions in the 177Lu-DOTATOC SPECT images, which had been identified and selected for analysis by the nuclear medicine physician. The analysis was performed using MIRADA XD3 software according to the following steps:
An elliptical VOI was drawn around the lesion. This VOI was intended to include all activity from the lesion, but it also contained some activity from the background. The activity concentration ACVOI + and the volume VVOI + of this VOI were recorded on the image analysis worksheet.
The tumor volume was estimated by setting the threshold for an automated segmentation to 40% of the maximum value in the VOI on the 24 h time point scan.
A circular VOI ideally of 30 mm diameter was placed in a disease-free area in the liver, and the activity concentration ACBkg was recorded on the image analysis worksheet. The VOI should cover as much disease-free area as possible to get a reliable mean background value.
The background activity was subtracted from the activity of the VOI surrounding the lesion to determine the activity of the tumor ATU:
ATU = ACVOI+ × VVOI+ – (VVOI+ – VTU) × ACBkg (2)
This analysis was performed for all scan time points to generate time activity curves (TACs) for all lesions. For dosimetry, the TACs were normalized to the injected activity A0.
Activity concentrations ACTU were calculated as:

and to account for different injected activities, they were normalized to injected activity A0:
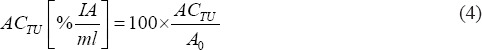
Furthermore, from the injected amount of peptide dose, the peptide dose accumulated in a tumor per milliliter tumor was calculated according to:
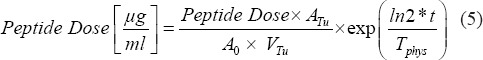
Since the peptide dose is a biological parameter, it was corrected for physical decay (second term in the equation) where t is the time of the imaging post-injection and Tphys the physical half-life of 177Lu.
In addition, to evaluate potential uptake differences between dose cycles and peptide doses, the mean values over all lesions per patient and dose cycle were calculated.
Quantitative assessment of organ uptake
The organ uptake was measured for both kidneys. The evaluation for the kidneys was performed according to the following steps:
An elliptic VOI was drawn around each kidney and the threshold for automated segmentation was adapted manually for each time point to match the uptake in the kidney and not to overlap with the segmented tumor volume in the liver. The activity concentration ACKidneyVOI in this VOI was recorded on the image analysis worksheet.
Based on the ACKidneyVOI and the volume of the kidney VOI VKidneyVOI, the total activity in each kidney was calculated as:
AKidney = ACKidneyVOI × VKidneyVOI (6)
Total activity in the kidneys was calculated as the sum of the activity in the two kidneys.
AKidneys = AKidneys1 + VKidney2 (7)
Total body activity was calculated using a VOI drawn around the whole body. Since it is needed for safety dosimetry calculations, the remainder body (RB) activity was calculated subtracting the kidney activity from the whole-body activity.
This analysis was also performed for all scan time points to generate TACs for the kidney and RB. The TACs were then normalized to the injected activity A0.
Calculation of cumulated activities for organs and tumors for dosimetry
The total number of disintegrations or cumulated activity à was determined for kidney, RB, and all evaluated lesions by calculating the area under the curve (AUC) from the TAC. The normalized cumulated activity or “residence time” τ (MBq × h/MBq) was computed as the cumulated activity à divided by the administered activity A0:

First, the normalized TACs were fitted with a biexponential function using MATLAB code developed based on the curve-fitting toolbox in MATLAB R2013a:
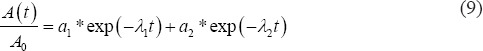
with the fit parameters a1 and a2 and λ1 and λ2 where 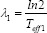 and
and 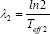 with Teff being the effective half-life of 177Lu-DOTATOC.
with Teff being the effective half-life of 177Lu-DOTATOC.
The à calculation was divided into three distinct time segments:
The AUC from time 0 to the first measured time point TPfirst: AUC0:TPfirst was calculated assuming a constant activity ATPfirst from time 0 to TPfirst
The AUC from the first measured time point TPfirst to the last measured time point TPlast: AUCTPfirst: TPlast was integrated numerically using trapezoidal approximation
The AUC from TPlast to infinity: AUCTPlast: Inf was integrated using the fitted biexponential function.
The total à for organs and tumors was calculated as the sum of the AUCs of the three segments:
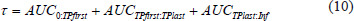
The à of the RB was calculated by subtracting the kidneys à from the total body Ã.
Safety dosimetry
The safety dosimetry calculations for the kidneys were performed with OLINDA/EXM 1.1.[6] Kidneys and RB were used as source organs for dose calculations for this study, for which residence times were provided as input. Furthermore, the adult (hermaphrodite) male model was selected for the dose calculations using the original phantom masses.
Absorbed dose per unit injected activity (mGy/MBq) and absorbed dose D (Gy) were extracted for the kidneys as a target organ, since the kidneys are considered an organ at risk for radiation damage.
DKidneys = ÃKidneys * SKidneys→ + ÃRB →Kidneys (11)
where S is the dose conversion factor from a source to a target organ for a specific isotope and phantom geometry.
In general, a dose limit of 23 Gy is applied to the kidneys in external-beam radiation therapy and can also be used for radionuclide therapy although higher values of 27 Gy or even 29 Gy due to the lower dose rates in radionuclide therapy have also been suggested.[7,8,9]
The expected absorbed dose for a therapeutic activity of 30 GBq was estimated by multiplying the absorbed dose per unit injected activity with this activity.
For further evaluation of the influence of peptide dose and 177Lu-DOTATOC dose cycles on kidney dose, the ratio of absorbed dose per unit injected activity between dose cycles D1 and D2 was also calculated.
Tumor dosimetry
The tumor dosimetry was also performed with OLINDA/EXM 1.1. The absorbed dose D for the lesions was calculated using the sphere model.
DTU = ÃTU * Ssphere→sphere (12)
The sphere model allows the input of a residence time for a tumor and calculates the absorbed doses for a number of predefined tumor masses for a specific isotope.
The resulting tumor dose was then determined by interpolating these values using a power function to the previously determined tumor volume/mass of the lesion assuming a density of 1 g/cm3.
For further evaluation of optimum peptide dose and efficacy of 177Lu-DOTATOC dose cycles, the following parameters were also calculated:
Mean absorbed dose over all lesions per patient and dose cycle (lesion TACs that could not be fitted properly were excluded)
Ratio of absorbed dose per unit injected activity between dose cycles D1 and D2
Therapeutic index: tumor to kidney ratio of absorbed dose per unit injected activity
Lesion volume reduction between dose cycles D1 and D2
Mean absorbed dose and therapeutic index over all dose cycles D1, all dose cycles D2, all 90 μg peptide dose, all 200 μg peptide dose, all D1 with 90 μg peptide dose, and all D1 with 200 μg peptide dose.
Biologically effective dose
The biologically effective dose (BED) is expected to more accurately predict the dose effect, for example, renal toxicity for kidneys, than absorbed dose by taking into account: (1) time-dependent dose rate, (2) repair time of sub lethal damage, and (3) organ radio sensitivity. It was originally established for external-beam radiation therapy, but has recently also been applied to radionuclide therapy, in particular for kidney dosimetry.[8,10,11,12]
The standard BED is defined as the product of absorbed dose and relative effectiveness per unit dose (RE).[8]
BED = D × RE (13)
where D is the absorbed dose. For radionuclide therapy, RE is calculated as:

where G is the Lea-Catcheside factor, which expresses the reduction in cell kill due to sublethal damage repair during continuous irradiation applying to type B events (double-hit cell kill). This factor G is specific to radionuclide therapy and would be equal to 1 for external-beam radiation therapy. The α/β ratio is related to radiosensitivity (α) and potential-sparing capacity (β).[10] This ratio is tissue specific and was set to 2.5 Gy for the kidneys according to Guerriero et al. and Wessels et al.[11,12]
For fractionated radionuclide therapy with i cycles of different doses, BED can be defined as:[8]

The Lea-Catcheside factor G for one source region and biexponential clearance (G1,2) can be calculated according to:[8]
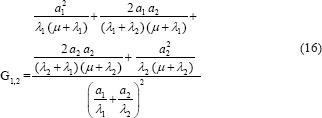
where a1, a2, λ1, and λ2 are the fit parameters from the biexponential fit to the normalized TACs as described in section 3.5, and μ is a repair constant that was set to μ = 0.24 h−1 according to Guerriero et al. and Wessels et al.[11,12]
In this study, only the kidneys were included as a source organ. This simplified calculation of G was chosen over more complicated formulas that include more than one source region. This was justified by the expectation that the contribution to the kidneys dose from the RB is low.
Results
Quantitative assessment of tumor uptake
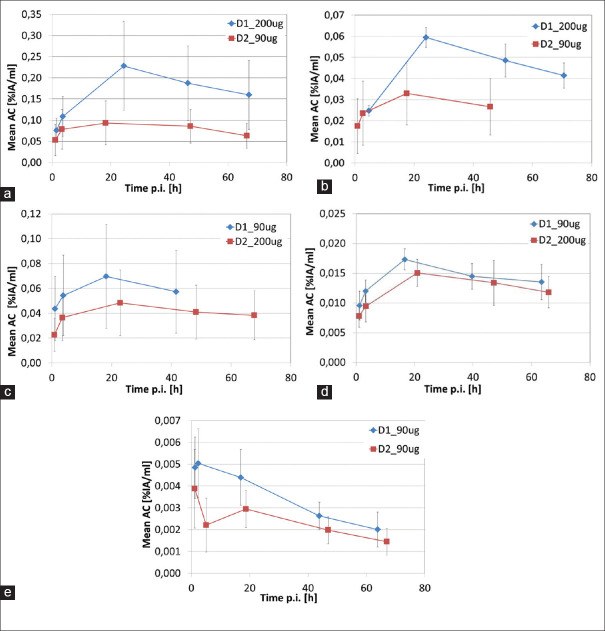
Figure 1 displays the mean activity concentration in the tumors (averaged over all lesions per patient and dose cycle) normalized to injected activity. The activity concentrations at all-time points were higher for the first dose cycle D1 for all patients independent on which peptide dose was used in which cycle. Three out of five patients showed an activity accumulation phase until the 24 h time point with a clear exponential decrease of activity concentration afterward. One patient (E) did not have this clear accumulation phase. Another patient (D) did not show a clear decrease after 24 h, which made it difficult to fit the TACs with a biexponential function for individual lesions. These lesions were excluded from further analysis.
Figure 1.
TAC for mean activity concentrations normalised to the injected activity [%IA/ml] averaged over all lesions per patient and dose cycle: (a) patient A, (b) patient B, (c) patient C, (d) patient D, (e) patient E.
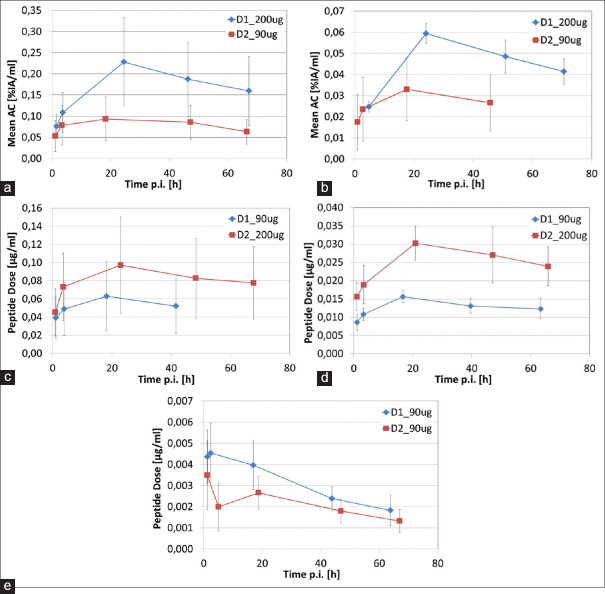
The peptide dose accumulated pro milliliter tumor was higher for all patients for an injected peptide dose of 200 μg compared to 90 μg independent of whether the higher peptide dose was used in the first or second dose cycle [Figure 2]. For the patient that received the lower peptide dose of 90 μg in both dose cycles, the higher peptide dose in the tumor was accumulated in the first dose cycle D1.
Figure 2.
Peptide doses accumulated in the lesions: (a) – (e) patients A - E.
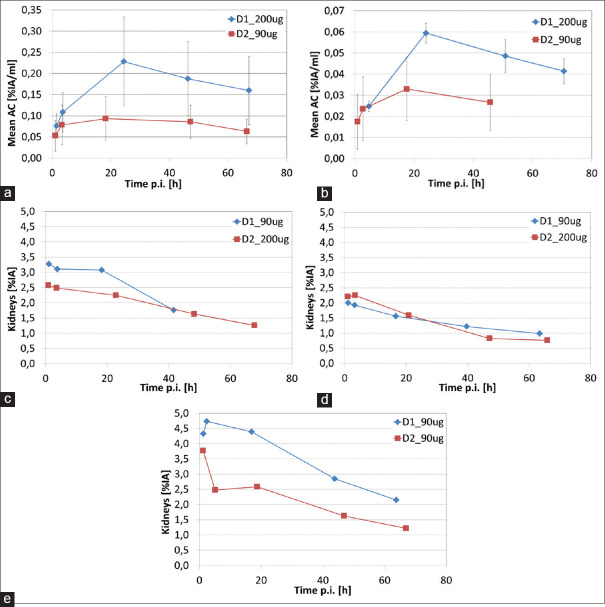
Quantitative assessment of kidney uptake
The TACs for the kidneys are displayed in Figure 3. Two kidney TACs (patient E D1 and D D1) showed a short accumulation phase reaching maximum activity at the second time point with clearance after. For two other kidney TACs (patient A D1 and E D2), the activity for the second time point TP2 was lower than for TP1 and TP3, and hence TP2 was excluded from the biexponential curve fitting. In addition, TP2 also had to be excluded from the curve fitting for another TAC (patient A D2) since including it leads to very unrealistic fitting parameters and residence times for BED calculation.
Figure 3.
Kidneys TACs normalized to injected activity: (a) – (e) patients A – E.
Safety dosimetry
The results for kidney safety dosimetry and tumor dosimetry are displayed in Tables 2 and 3. Kidney-absorbed doses per unit injected activity varied by a factor of up to 4 between patients from 0.25 to 1.0 mGy/MBq (independent of dose cycle and peptide dose) and by a factor of up to 2 between dose cycles. Averaged over patients and either peptide dose, dose cycle, or both, the mean kidney dose was 13.7% higher for the 90 μg peptide dose compared to 200 μg, 32.6% higher for dose cycle D1 compared to D2, and combining peptide dose and dose cycle D1 the kidney dose was 18.8% higher for D1 with 90 μg peptide dose compared to D1 with 200 μg peptide dose.
Table 2.
Absorbed doses and dose ratios for tumours and kidneys, therapeutic index and lesion volume reduction averaged over all lesions for each patient and dose cycle
| Patient | Dose Cycle | Peptide Dose [µg] | Injected activity [MBq] | No. Of tumours | Σ Tumour Volumes [ml] | t [MBq*h/MBq] | Tumours Absorbed Dose [Gy] Mean STD | Normalized Absorbed Dose [mGy/MBq] | Ratio D1/D2 - Normalized Dose | t [MBq*h/MBq] | Absorbed Dose [Gy] | Kidneys Normalized Absorbed Dose [mGy/MBq] | Extrapolated Dose [Gy/30GBq] | Ratio D1/D2 -Normalized Dose | Therapeutic index (T/K) | Tumour volume reduction D1 --> D2 [%] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | D1 | 200 | 5200 | 5 | 8,9 | 0,25 | 133,55 | 98,19 | 25,68 | 2,09 | 2,40 | 3,62 | 0,70 | 20,88 | 1,21 | 36,89 | 47,8% |
| D2 | 90 | 7360 | 5 | 4,6 | 0,09 | 90,43 | 42,71 | 12,29 | 1,98 | 4,24 | 0,58 | 17,28 | 21,33 | ||||
| B | D1 | 200 | 5100 | 6 | 263,1 | 3,15 | 32,50 | 7,52 | 6,37 | 1,64 | 1,73 | 2,58 | 0,51 | 15,18 | 2,05 | 12,60 | 52,4% |
| D2 | 90 | 5100 | 6 | 125,3 | 0,79 | 19,86 | 16,48 | 3,89 | 0,85 | 1,26 | 0,25 | 7,41 | 15,76 | ||||
| C | D1 | 90 | 5300 | 6 | 163,4 | 2,26 | 37,85 | 15,86 | 7,14 | 1,00 | 1,71 | 2,66 | 0,50 | 15,03 | 0,82 | 14,25 | 0,8% |
| D2 | 200 | 4900 | 6 | 162,1 | 2,36 | 34,96 | 14,22 | 7,14 | 2,10 | 2,99 | 0,61 | 18,33 | 11,68 | ||||
| D | D1 | 90 | 7000 | 3 | 316,6 | 2,05 | 22,85 | 16,21 | 3,26 | 1,96 | 2,05 | 4,20 | 0,60 | 18,00 | 1,69 | 5,44 | -4,6% |
| D2 | 200 | 5100 | 3 | 331,2 | 0,85 | 8,49 | 0,75 | 1,66 | 1,20 | 1,81 | 0,35 | 10,65 | 4,69 | ||||
| E | D1 | 90 | 7660 | 8 | 366,0 | 0,38 | 4,11 | 4,84 | 0,54 | 1,53 | 3,60 | 7,97 | 1,04 | 31,21 | 1,42 | 0,52 | 12,7% |
| D2 | 90 | 7440 | 8 | 319,6 | 0,19 | 2,61 | 1,76 | 0,35 | 2,53 | 5,45 | 0,73 | 21,98 | 0,48 | ||||
Table 3.
Absorbed doses for kidneys and tumours, therapeutic index and percent differences for all parameters between peptide doses, dose cycles and combined dose cycle D1 and peptide dose averaged over all patients
| Mean Kidneys Dose [mGy/MBq] | Percent difference [%] | Mean Tumour Dose [mGy/MBq] | Percent difference [%] | Therapeutic index (T/K) | Percent difference [%] | |
|---|---|---|---|---|---|---|
| all 90 µg | 0,62 | 13,7 | 4,58 | -56,4 | 9,63 | -43,2 |
| all 200 µg | 0,54 | 10,51 | 16,95 | |||
| all D1 | 0,67 | 32,6 | 8,60 | 62,0 | 13,94 | 24,6 |
| all D2 | 0,50 | 5,31 | 11,18 | |||
| all D1/90 µg | 0,71 | 18,8 | 3,65 | -77,2 | 6,74 | -72,8 |
| all D1/200 µg | 0,60 | 16,02 | 24,73 |
For an expected therapeutic activity of 30 GBq, the dose limit of 23 Gy would have been exceeded with 31 Gy for one patient (E) based on extrapolation from the absorbed dose calculated for dose cycle D1 and would have been close to the dose limit with 21 Gy for the same patient based on extrapolation dose cycle 2. For another patient (A), the kidney dose would also have been close to the dose limit with 21 Gy and 17 Gy based on extrapolation from dose cycles D1 and D2, respectively.
Tumor dosimetry
Tumor doses varied by a factor of 74 between 0.35 and 26 mGy/MBq between patients (averaged over all lesions per patient). As shown in Table 3, the mean tumor dose (averaged over all patients) for dose cycle D1 was approximately 62% higher than for dose cycle D2, which can be partly explained by the lesion volume reduction observed for dose cycle D2 for three of the five patients. The mean tumor dose was 56% lower for the lower peptide dose of 90 μg compared with 200 μg. Similarly, only looking at dose cycle D1, the mean tumor dose was 77% lower for 90 μg peptide dose compared to 200 μg. The same general trends were observed for the therapeutic index.
Biologically effective dose
BED for kidneys for all patients and dose cycles are presented in Table 4. The Lea-Catcheside factor G1,2 for all patients and dose cycles was lower or equal to 0.11 with a variation between 0.035 and 0.112 depending on the kinetics of the kidneys. These low G1,2 values indicate high sublethal damage repair during a therapy cycle. The relative effectiveness RE was relatively low between 1.051 and 1.193, meaning that the BED was between 5.1% and 19.3% higher than the absorbed dose.
Table 4.
Kidneys BED for all patients and dose cycles. 1The absorbed doses shown here only include the kidneys as source organ. 2For these patients and dose cycles time point 2 had to be excluded from the bi-exponential fit and hence was also excluded for dose calculations
| Patient ID | Dose Index | Peptide Dose [µg] | Injected Activity [MBq] | Absorbed Dose1 [Gy] | Fit parameters (bi-exponential fit) | G1,2 | RE | BED [Gy] | BED [mGy/MBq] | Expected BED [Gy/30GBq] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a1 | λ1 | a2 | λ2 | ||||||||||
| A | D1 | 200 | 5200 | 2.572 | -1,0066 | 0,0305 | 1,0481 | 0,0300 | 0,089 | 1,091 | 2,80 | 0,539 | 16,18 |
| D2 | 90 | 7360 | 4.272 | 0,0247 | 0,1252 | 0,0279 | 0,0148 | 0,066 | 1,112 | 4,75 | 0,645 | 19,36 | |
| B | D1 | 200 | 5100 | 2,55 | 0,0420 | 0,0792 | 0,0141 | 0,0123 | 0,080 | 1,082 | 2,76 | 0,541 | 16,23 |
| D2 | 90 | 5100 | 1,25 | 0,0837 | 1,6098 | 0,0207 | 0,0262 | 0,103 | 1,051 | 1,31 | 0,258 | 7,73 | |
| C | D1 | 90 | 5300 | 2,61 | 509,343 | 0,0444 | -509,312 | 0,0444 | 0,101 | 1,105 | 2,89 | 0,544 | 16,33 |
| D2 | 200 | 4900 | 2,97 | 0,0440 | 0,0171 | -0,0185 | 0,0395 | 0,058 | 1,069 | 3,17 | 0,648 | 19,43 | |
| D | D1 | 90 | 7000 | 4,14 | 0,0038 | 0,0746 | 0,0167 | 0,0084 | 0,035 | 1,058 | 4,38 | 0,626 | 18,77 |
| D2 | 200 | 5100 | 1,77 | 0,0240 | 0,0199 | -1,3740 | 6,7847 | 0,112 | 1,079 | 1,91 | 0,375 | 11,24 | |
| E | D1 | 90 | 7660 | 7,98 | 0,0569 | 0,0155 | -0,0228 | 0,4688 | 0,060 | 1,193 | 9,52 | 1,242 | 37,27 |
| D2 | 90 | 7440 | 4.902 | 0,0089 | 0,0656 | 0,0300 | 0,0136 | 0,057 | 1,112 | 5,45 | 0,732 | 21,97 | |
Discussion
The therapeutic index in the form of ratio between radiation dose delivered to tumor and radiation dose to the kidney plays a significant role in the management of NETs. Several previous studies have looked into the possible risk factors for deterioration of kidneys function under PRRT. The study by Cremonesi et al. has shown that there are clinical factors such as diabetes, age >70 years, and prior chemotherapy, which influence the probability of induction of higher-grade renal toxicity.[13] In addition, their group mentioned for the first time the use of biological effective dose as more suitable for renal dosimetry.[13] The Uppsala group performed intratherapeutic dosimetry in 200 patients to show that patients might develop high-grade renal toxicity with as low as two therapy cycles whereas some patients could tolerate up to 10 doses without significant nephrotoxicity.[13] Analog to Cremonesi et al. we also found that BED was higher than the absorbed dose.[13] Not surprisingly, for an expected therapeutic activity of 30 GBq, the dose limit of 23 Gy would have been exceeded in one patient and would have reached the dose limit of 23 Gy in another patient. Interestingly, the one patient where the kidney dose would have received 31 Gy (E) based on extrapolation of dose cycle D1 received 6 therapy cycles with total cumulative dose of 40 GBq without any evidence of adverse effect on kidneys function. The patient had no clinical risk factors. This indicates (1) that the absorbed dose to the kidneys was not the same for the following dose cycles and (2) underscores the need to take into account the biological radiation sensitivity in defining the upper limit for dosimetry planning. Other than the differential response to radiation, other factors such as nonradiolabeled peptide in 177Lu-DOTATATE or 177Lu-DOTATOC might also have an influence on the pharmacokinetic and pharmacodynamics in the tumor and its microenvironment.
The dosimetry data of five patients studied at our center clearly show the value of nonradiolabeled peptide in defining the therapeutic index. Although the tumor dose was found to be highly variable, averaged over all patients, lesions treated with a peptide dose of 200 μg showed a higher 177Lu-DOTATOC mean tumor dose in comparison to 90 μg.
Higher peptide dose turned out to be more beneficial for kidneys too. Although the absorbed dose to the kidneys varied by a factor of up to 4 between patients (independent of dose cycle and peptide dose) and by a factor of up to 2 between dose cycles, the mean kidneys dose was 13.7% higher for the 90 μg peptide dose compared to 200 μg. This meant that the mean therapeutic index of a 200 μg mass dose was considerably higher as compared to a 90 μg mass dose. This coincided with the observation, that lesion volume reduction was more pronounced after an initial treatment with a 200 μg mass dose. Of course, it is hard to prove that other biological factors such as Ki67, radiation sensitivity, and tumor microenvironment also played some role in significant volume reduction with μg mass dose.
A major limitation of this study is that only five patients with a total of 10 treatment cycles have been analyzed with two patients per dosing strategy (200 μg - >90 μg and 90 μg - >200 μg) and one patient receiving a repeated dose of 90 μg which reduced the robustness of any statistics calculated. From 2 patients (3 treatment cycles), only 4 time points were available, and for some lesions from other patients, time points had to be excluded from curve fitting and dose calculation to avoid unrealistic fit parameters, absorbed dose, and BED values.
The BED for the kidneys was between 5.1% and 19.3% higher than the absorbed dose for the kidneys. These differences between patients and dose cycles are related to (1) differences in the kinetics of the kidneys TACs between patients and dose cycles and (2) differences in absorbed dose. The high sublethal damage repair and low RE are caused by the slow clearance of 177Lu-DOTATOC, i.e., the long irradiation times, which are favorable for kidneys protection.
Interestingly, Kletting et al. have also tried to establish the optimal peptide dose for use in PRRT with 90Y DOTATATE.[14] Based on a whole-body physiologically based pharmacokinetic (PBPK) model, the authors found that the optimal peptide concentration is 87 ± 50 nmol (135 ± 78 μg) and 5.1 ± 2.8 GBq for NET patients. More patients are needed to correlate the results of our study based on individualized 3D dosimetry with the PBPK model.[14]
Conclusions
The results indicate that a peptide dose of 200 μg would be favorable to increase the therapeutic index. It is, furthermore, possible that the therapeutic activity might have to be reduced in order not to exceed a tolerable dose of 23 Gy for the kidneys. The preliminary results of this study have to be confirmed by a larger patient dataset.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasia: Peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology. 2017;105:295–309. doi: 10.1159/000475526. [DOI] [PubMed] [Google Scholar]
- 2.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O’Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–16. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabet A, Nagarajah J, Dogan AS, Biersack HJ, Sabet A, Guhlke S, et al. Does PRRT with standard activities of 177Lu-octreotate really achieve relevant somatostatin receptor saturation in target tumor lesions? Insights from intra-therapeutic receptor imaging in patients with metastatic gastroenteropancreatic neuroendocrine tumors. EJNMMI Res. 2013;3:82. doi: 10.1186/2191-219X-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad V, Ambrosini V, Alavi A, Fanti S, Baum RP. PET/CT in neuroendocrine tumors: Evaluation of receptor status and metabolism. PET Clin. 2008;3:355–79. doi: 10.1016/j.cpet.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: The second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7. [PubMed] [Google Scholar]
- 7.IAEA Guidelines. Practical Guidance on Peptide receptor Radionuclide Therapy (PRRNT) for Neuroendocrine Tumors, IAEA Human Health Series. Vol. 20. Vienna: IAEA; 2013. [Google Scholar]
- 8.Baechler S, Hobbs RF, Prideaux AR, Wahl RL, Sgouros G. Extension of the biological effective dose to the MIRD schema and possible implications in radionuclide therapy dosimetry. Med Phys. 2008;35:1123–34. doi: 10.1118/1.2836421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konijnenberg M, Melis M, Valkema R, Krenning E, de Jong M. Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J Nucl Med. 2007;48:134–42. [PubMed] [Google Scholar]
- 10.Cremonesi M, Ferrari M, Bodei L, Tosi G, Paganelli G. Dosimetry in Peptide radionuclide receptor therapy: A review. J Nucl Med. 2006;47:1467–75. [PubMed] [Google Scholar]
- 11.Wessels BW, Konijnenberg MW, Dale RG, Breitz HB, Cremonesi M, Meredith RF, et al. MIRD pamphlet No.20: The effect of model assumptions on kidney dosimetry and response – Implications for radionuclide therapy. J Nucl Med. 2008;49:1884–99. doi: 10.2967/jnumed.108.053173. [DOI] [PubMed] [Google Scholar]
- 12.Guerriero F, Ferrari ME, Botta F, Fioroni F, Grassi E, Versari A, et al. Kidney dosimetry in 177Lu and 90Y peptide receptor radionuclide therapy: Influence of image timing, time-activity integration method, and risk factors. Biomed Res Int 2013. 2013 doi: 10.1155/2013/935351. 935351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cremonesi M, Ferrari M, Di Dia A, Botta F, De Cicco C, Bodei L, et al. Recent issues on dosimetry and radiobiology for peptide receptor radionuclide therapy. Q J Nucl Med Mol Imaging. 2011;55:155–67. [PubMed] [Google Scholar]
- 14.Kletting P, Kull T, Maaß C, Malik N, Luster M, Beer AJ, Glatting G. Optimized peptide amount and activity for 90Y-labeled DOTATATE therapy. J Nucl Med. 2016;57:503–8. doi: 10.2967/jnumed.115.164699. [DOI] [PubMed] [Google Scholar]