Optogenetics is a powerful technology that employs light and genetics to manipulate physiology and behavior with unprecedented precision. The high acuity of light stimulation permits fine control both in space (e.g., to target just one tissue in an animal) and in time (e.g., to interfere with a specific disease stage), whilst genetic targeting restricts manipulation to a functionally-relevant cell population (Figure 1A). These unique capabilities have laid the ground for answering previously unresolvable questions in neuroscience and for new treatment avenues. Already shortly after its inception, optogenetics was harnessed to understand neural circuit function in animal models of neurological and neurodegenerative disorders, including spinal cord injury, stroke, and Parkinson's disease (PD). Notably, in some of these models, optically-evoked neuronal activity was sufficient to elicit a functional improvement, e.g. through the formation of new microcircuitries or release of neurotrophic factors (Ordaz et al., 2017). These initial discoveries were recently followed by targeted neuroregeneration strategies. These generally aim at either replacement of degenerated sensory functions by optogenetic actuators or site-specific optical delivery of pro-survival signals to counter neurodegeneration (Kleinlogel et al., 2020; Ingles-Prieto et al., 2021). It is these two optogenetic neuroregeneration strategies that we discuss here, from the origins of the field of optogenetics to the recent pioneering clinical application (Sahel et al., 2021).
Figure 1.
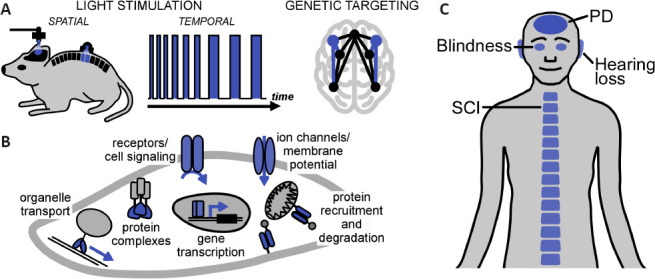
Optogenetics and neuroregeneration.
(A) Optogenetics combines spatial (micrometers to centimeters) and temporal (milliseconds to days) light stimulation with genetic targeting to (in)activate selected cells or tissues at selected stages of development or disease. (B) Cellular processes accessible to optogenetics. Membrane proteins, such as opsins, and cytosolic clustering photoreceptors permit light control of a wide range of processes. The light-sensing protein or light-controlled process is highlighted in blue. (C) Targets for optogenetic neuroregeneration in animal models and patients. PD: Parkinson's disease; SCI: spinal cord injury.
Light signals regulate physiological and behavioral processes across all domains of life, ranging from phototaxis in microbes to plant development and animal vision. Conceptually, there are two major reasons for the abundance of optical cues in Nature. First, diverse classes of naturally-occurring molecules are capable of absorbing visible photons, such as poly-isoprenoid retinals or ring system flavins. These biochemical molecules are then incorporated in photoreceptor proteins that translate light absorption into cell state alterations. For instance, retinals linked covalently to opsins catalyze transmembrane ion flow or intracellular cell signaling, whilst flavins bind in small microbial or plant photoreceptor proteins that control transcriptional or enzyme activities. Second, light is exceptionally well suited to encode spatial information (e.g., the site of irradiance on a growing plant shoot) as well as temporal information (e.g., rapid changes of objects in a field of view). This high acuity is made possible by the strategic genetic placement of photoreceptive cells in light-sensing tissues and by, in many cases rapid, desensitization of photoreceptors and downstream signals. These two remarkable features of light sensing, diverse biochemistry, and spatio-temporal precision, have not only been studied extensively by photobiologists but also by researchers in the fields of bioengineering, synthetic biology, and biomedicine. Indeed, over the past three decades, new research has emerged towards artificial light control of living systems.
Engineered light control of neurons looks back on a vivid history. Since the late 1980s, synthetic molecules are being developed that permit neuronal photocontrol. Prominent examples are “caged neurotransmitters”, derivatives of neurotransmitters (e.g., glutamate or acetylcholine) that carry photocleavable moieties. These “cages” are then cleaved off irreversibly optically allowing the neurotransmitter to act rapidly and locally. Whilst powerful, these and other synthetic approaches are not optimal to study multicellular circuits, such as those driving brain function. For instance, the uncaged neurotransmitter acts indiscriminately on the species of encountered neuron or receptor subtype. Ideally, spatio-temporal light stimulation would need to be married to genetic targeting that limits activation to a specific neuronal population; this is achieved in optogenetics.
Optogenetics refers to the genetic targeting of photoreceptor proteins, e.g. using cell-type-specific promoters or cell-type-specific gene recombination, to achieve light-induced responses only in a subset of functionally-relevant cells. In 2002, a seminal study demonstrated that expression of an exogenous Drosophila opsin can render cultured hippocampal neurons light-sensitive (Zemelman et al., 2002). Specifically, light stimulation resulted in action potential firing only in the transfected neurons of the otherwise homogenous preparation. One limitation of the employed system was that three genes, the opsin, a G-protein α-subunit, and an arrestin, were required for functional light responses, somewhat complicating potential in vivo applications. The discovery of channelrhodopsins (ChRs), in particular channelrhodopsin-2 from the green alga Chlamydomonas rheinhardtii (Nagel et al., 2003), was a key step towards the broad uptake of optogenetics. Chlamydomonas rheinhardtii naturally exhibits rapid photocurrents and ChRs encode relatively small (~300 amino acids for truncated variants) “stand alone” light-activated ion channels. Since this discovery, ChRs have become instrumental in optogenetics and answered long-standing questions in neuroscience and beyond. The ChR breakthrough was followed by several major waves of development. First, new microbial opsins were discovered and existing opsins were reengineered towards neuronal silencing, increased light sensitivity, and multi-color experiments. Second, further microbial, plant, and even metazoan photoreceptor classes were employed for control of a wider range of cellular processes, including enzyme activity, intracellular signaling, and gene regulation (Figure 1B). In prominent examples, this was mainly made possible using flavin-binding photoreceptors, which respond to light with clustering or unfolding reactions, or G-protein coupled receptor opsins, which trigger essential signaling pathways. After two decades of method development, optogenetics has now matured to a stage where diverse biological problems, ranging from the metabolic engineering of microbes to curing human disorders, are within reach.
The ability to spatio-temporally control neurons has been harnessed for the restoration of lost neurosensory functions, including vision and hearing (Figure 1C) (Kleinlogel et al., 2020). The accessibility of the eye to light and the lack of effective treatments for blindness spurred translational approaches to restore vision in animal models (Busskamp et al., 2010; Cehajic-Kapetanovic et al., 2015; van Wyk et al., 2015; Berry et al., 2019). The retina became the first clinical target for optogenetic therapies with currently three clinical trials underway (NCT03326336, NCT02556736, and NCT04278131), all of which employ ChRs delivered through a gene therapy with non-pathogenic adeno-associated virus vectors. Notably, a recent first application of optogenetics in humans demonstrated successful vision restoration in one patient with advanced retinitis pigmentosa, a hereditary photoreceptor degenerative disease (Sahel et al., 2021). In this study, the adeno-associated virus vector was injected intravitreally (i.e., into the eyeball) and the ChR was predominantly expressed in retinal ganglion cells, the “output” neurons of the retina. Due to the very high light intensities required for activation of the employed ChR (“ChrimsonR”), the patient was equipped with biomimetic goggles amplifying and spectrally-adapting ambient light. After a training period of ~7 months, some ability to locate and count objects both under laboratory and real-life conditions was regained. Although restored vision remained elementary, the study presents a milestone for clinical optogenetics notably without reported major adverse effects (e.g., inflammation; however, also see below). Multiple approaches are under development to improve the quality of restored vision. The retina executes remarkable pre-analysis of the visual scene leading to about 35 parallel channels of visual information transmitted to the brain by ganglion cells. This computational power is lost when turning ganglion cells into “replacement photoreceptors” using optogenetics. Therefore, current endeavors focus on integrating signal processing into the biomimetic googles or targeting hierarchically higher inner retinal neurons to restore retinal signaling. To this end, we and others have put major effort into the development of synthetic promoters that reliably steer expression to specific neuron subtypes, in particular to bipolar cells that are the first-order interneurons of the retina (Kleinlogel et al., 2020). The field also explored G-protein coupled receptor opsins to “hijack” native signaling in bipolar cells and ultimately restore more natural retinal function (Cehajic-Kapetanovic et al., 2015; van Wyk et al., 2015; Berry et al., 2019). Conceptually similar approaches were followed for optogenetic restoration of hearing. Also in this case a ChR was employed and first-order interneurons of the cochlea (the spiral ganglion cells) were targeted. However, as opposed to the retina, the cochlea performs little computation and other main challenges exist. These are the speed of optogenetic activation, because the auditory system relies on high temporal fidelity, as well as light delivery, because the cochlea is not naturally accessible to light. To address these challenges, fast ChRs with sub-millisecond kinetics and flexible cochlear implants consisting of light-emitting diodes have been developed (Kleinlogel et al., 2020). A further challenge for cochlear optogenetics is efficient targeting of the spiral ganglion cells both in non-human primates and humans.
Neurosensory optogenetic regeneration ultimately resulted in induced bioelectrical signals (i.e., ion flow across the cell membrane), either directly through a ChR or through ion channels downstream of G-protein coupled receptors. In a complementary strategy, we have recently demonstrated the direct optogenetic delivery of pro-survival signals in a model of neurodegeneration (Ingles-Prieto et al., 2021). This was made possible not using opsins but rather dimerizing flavin-binding photoreceptors. These photoreceptors were incorporated in receptor tyrosine kinases (Grusch et al., 2014), a large type I membrane protein family that includes the receptors for many neurotrophic factors. A receptor tyrosine kinase of particular relevance is REarranged during Transfection (RET), the cognate receptor for glial cell line-derived neurotrophic factor ligands (GFLs). GFLs have been explored as a disease-modifying treatment of PD, but limited outcomes were observed in clinical trials and attributed to difficulties in GFL delivery and advanced loss of midbrain neurons (Tenenbaum and Humbert-Claude, 2017). In addition, for GFLs and other (neuro)trophic factors continuous exposure may lead to complex protein-receptor interactions, compensation or toxicity, even if injury-free transfer past the blood-brain barrier can be achieved. Collectively, these long-standing challenges have led to calls for reversible and local trophic factor delivery strategies (Tenenbaum and Humbert-Claude, 2017). We have realized this using the optogenetic RET variant “Opto-RET” in two genetic models of PD (Drosophila and human cells) (Ingles-Prieto et al., 2021). In both models, loss of the PINK1 gene, which is linked to autosomal recessive PD, resulted in functional and structural deficiencies of mitochondria, in line with the human pathology. Strikingly, the emerging tissue degeneration and behavioral fitness phenotypes could be completely and specifically reversed by light activation of Opto-dRET in situ. In addition, the tractable human cell model allowed identifying the requirement for activity in the PI3K and NF-κB signaling pathways, which were known to act as an important node of crosstalk downstream of receptor tyrosine kinases, to suppress PINK1 loss-of-function (Ingles-Prieto et al., 2021). This work demonstrates the utility of optogenetics as a cell-type-specific and remote-controlled method to exert beneficial trophic effects in a neurodegeneration context. The next step is exploring the efficacy of Opto-dRET and other light-activated kinases in mammalian in vivo models for which past successes in the optical control of neuronal pathways in rodent and non-human primate brains provide an encouraging methodological basis. This first demonstration of optogenetic trophic factor delivery nicely complements studies in which increased neurotrophic factor levels were observed as an outcome of electrical optogenetic stimulation. Through this mechanism, even some functional improvement and recovery were achieved in models of spinal cord injury and stroke (Ordaz et al., 2017).
Successful restoration of sensory functions and protection from degeneration highlight the neuroregeneration potential of optogenetics. In both applications, spatio-temporal and cell type-specific regulation have permitted overcoming long-standing challenges. The future will likely see even finer control of a wider range of cellular pathways in cell types of choice. As potential limitations of optogenetics, phototoxicity and protein overexpression generally require testing in every biological context. Furthermore, whilst transgenics and genetic targeting are developed in rodents, this is not the case for larger animal models that are intermediates towards clinical applications. At the clinical end of the spectrum, optogenetics is to date limited to the retina; however, developments towards treatments of hearing loss and cardiac arrhythmias are ongoing. The main challenges are safety, gene delivery, and light delivery. The safety of adeno-associated virus-based gene therapy has been demonstrated. The optogenetic retinal gene delivery in one patient reported no major adverse effects. It is noteworthy that the targeted tissue was immune-privileged and subject to advanced degeneration. How microbial ChRs are tolerated in other tissues, including those in the periphery that are subject to sensitive homeostasis, is an integral part of future studies. Wirelessly-powered electronics or transcranial energy delivery may provide safe deep tissue light delivery. Collectively, these developments will not only enable wider applications in animal models of increasing complexity and potentially humans, but also benefit studies of disorders of the peripheral nervous system and even non-neuronal tissues.
This study was funded by grants of the Australian Research Council (DP200102093, to HJ), the National Health and Medical Research Council (APP1187638, to HJ), the Swiss National Science Foundation (31003A_176065 and 310030E 188991, to SK) and the Bertarelli foundation (BCL7O2, to SK). The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government. The EMBL Australia Partnership Laboratory (EMBL Australia) is supported by the National Collaborative Research Infrastructure Strategy (NCRIS) of the Australian Government.
The authors declare no conflict of interest. SK is co-founder and shareholder of Arctos Medical AG, which is developing optogenetic gene therapies to restore vision.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y.
References
- 1.Berry MH, Holt A, Salari A, Veit J, Visel M, Levitz J, Aghi K, Gaub BM, Sivyer B, Flannery JG, Isacoff EY. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat Commun. 2019;10:1221. doi: 10.1038/s41467-019-09124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, Biel M, Humphries P, Paques M, Mohand-Said S, Trono D, Deisseroth K, Sahel JA, Picaud S, Roska B. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- 3.Cehajic-Kapetanovic J, Eleftheriou C, Allen AE, Milosavljevic N, Pienaar A, Bedford R, Davis KE, Bishop PN, Lucas RJ. Restoration of vision with ectopic expression of human rod opsin. Curr Biol. 2015;25:2111–2122. doi: 10.1016/j.cub.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grusch M, Schelch K, Riedler R, Reichhart E, Differ C, Berger W, Ingles-Prieto A, Janovjak H. Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J. 2014;33:1713–1726. doi: 10.15252/embj.201387695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingles-Prieto A, Furthmann N, Crossman SH, Tichy AM, Hoyer N, Petersen M, Zheden V, Biebl J, Reichhart E, Gyoergy A, Siekhaus DE, Soba P, Winklhofer KF, Janovjak H. Optogenetic delivery of trophic signals in a genetic model of Parkinson's disease. PLoS Genet. 2021;17:e1009479. doi: 10.1371/journal.pgen.1009479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinlogel S, Vogl C, Jeschke M, Neef J, Moser T. Emerging approaches for restoration of hearing and vision. Physiol Rev. 2020;100:1467–1525. doi: 10.1152/physrev.00035.2019. [DOI] [PubMed] [Google Scholar]
- 7.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ordaz JD, Wu W, Xu XM. Optogenetics and its application in neural degeneration and regeneration. Neural Regen Res. 2017;12:1197–1209. doi: 10.4103/1673-5374.213532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahel JA, Boulanger-Scemama E, Pagot C, Arleo A, Galluppi F, Martel JN, Esposti SD, Delaux A, de Saint Aubert JB, de Montleau C, Gutman E, Audo I, Duebel J, Picaud S, Dalkara D, Blouin L, Taiel M, Roska B. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med. 2021;27:1223–1229. doi: 10.1038/s41591-021-01351-4. [DOI] [PubMed] [Google Scholar]
- 10.Tenenbaum L, Humbert-Claude M. Glial cell line-derived neurotrophic factor gene delivery in Parkinson's disease: A delicate balance between neuroprotection, trophic effects, and unwanted compensatory mechanisms. Front Neuroanat. 2017;11:29. doi: 10.3389/fnana.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Wyk M, Pielecka-Fortuna J, Lowel S, Kleinlogel S. Restoring the ON switch in blind retinas: Opto-mGluR6, a next-generation, cell-tailored optogenetic tool. PLoS Biol. 2015;13:e1002143. doi: 10.1371/journal.pbio.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]


