Keywords: amplitude of low-frequency fluctuation, clinical study, coronavirus disease 2019, follow-up, functional magnetic resonance imaging, long-term physical consequences, neuropsychiatric sequelae, resting-state function
Abstract
Although some short-term follow-up studies have found that individuals recovering from coronavirus disease 2019 (COVID-19) exhibit anxiety, depression, and altered brain microstructure, their long-term physical problems, neuropsychiatric sequelae, and changes in brain function remain unknown. This observational cohort study collected 1-year follow-up data from 22 patients who had been hospitalized with COVID-19 (8 males and 11 females, aged 54.2 ± 8.7 years). Fatigue and myalgia were persistent symptoms at the 1-year follow-up. The resting state functional magnetic resonance imaging revealed that compared with 29 healthy controls (7 males and 18 females, aged 50.5 ± 11.6 years), COVID-19 survivors had greatly increased amplitude of low-frequency fluctuation (ALFF) values in the left precentral gyrus, middle frontal gyrus, inferior frontal gyrus of operculum, inferior frontal gyrus of triangle, insula, hippocampus, parahippocampal gyrus, fusiform gyrus, postcentral gyrus, inferior parietal angular gyrus, supramarginal gyrus, angular gyrus, thalamus, middle temporal gyrus, inferior temporal gyrus, caudate, and putamen. ALFF values in the left caudate of the COVID-19 survivors were positively correlated with their Athens Insomnia Scale scores, and those in the left precentral gyrus were positively correlated with neutrophil count during hospitalization. The long-term follow-up results suggest that the ALFF in brain regions related to mood and sleep regulation were altered in COVID-19 survivors. This can help us understand the neurobiological mechanisms of COVID-19-related neuropsychiatric sequelae. This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (approval No. 2020S004) on March 19, 2020.
Chinese Library Classification No. R445.2; R741.02; R563.1
Introduction
Coronavirus disease 2019 (COVID-19) is a highly infectious illness caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). In 2020, it rapidly developed into a global pandemic and poses a serious threat to health by attacking multiple organs. People who recover from COVID-19 can present a diverse spectrum of sequelae, including post-inflammatory pulmonary fibrosis (Zhan et al., 2020) and myocardial injury (Chen et al., 2020), which substantially affect daily life. Therefore, long-term follow-up research on persistent symptoms and physical and neurological sequelae is urgently needed in survivors of COVID-19 (Yelin et al., 2020). To date, several effects on the central nervous system (CNS) have been recognized in the early stages of SARS-CoV-2 infection (Mao et al., 2020; Pezzini and Padovani, 2020), such as acute ischemic and hemorrhagic cerebrovascular illness (Hernández-Fernández et al., 2020; Li et al., 2020a; Mao et al., 2020), acute disseminated encephalomyelitis (Parsons et al., 2020), and Guillain-Barré syndrome (Uncini et al., 2020; Zhao et al., 2020).
In COVID-19 infection, spike glycoproteins on the surface of SARS-CoV-2 have a high affinity for the angiotensin-conversion enzyme 2 (ACE2) receptor, which presents widely throughout the body (Bougakov et al., 2021). However, the precise mechanism through which CNS infection occurs has remained controversial. Bougakov et al. (2021) reported that the mechanism underlying multiple neurological symptoms might be related to direct or indirect viral infection. Direct invasion of the CNS by SARS-CoV-2 has been proposed to occur via two routes: the hematogenous route and axonal transport in the olfactory nerve (De Santis, 2020; Politi et al., 2020). The indirect mechanism involves hypoxia due to respiratory failure or an aberrant immune response (Gandhi et al., 2020; Li et al., 2020b). Regardless of how SARS-CoV-2 infects the CNS, it induces psychopathological sequelae (Wu et al., 2020). Reviewing what has been examined thus far, psychopathological sequelae can occur during the acute or subacute infection period in patients with COVID-19 and have been reported to be significantly related to inflammatory biomarkers collected during hospitalization (Mazza et al., 2020, 2021). Additionally, 3-month follow-up studies have found changes in cerebral microstructure and cerebral blood flow in individuals who have recovered from COVID-19 (Lu et al., 2020; Qin et al., 2021). However, no observational studies have investigated the long-term (over 6 months) health consequences of COVID-19. Functional magnetic resonance imaging (fMRI) is a noninvasive method for evaluating hemodynamic changes resulting from neuronal activity (Logothetis, 2008) and the amplitude of low-frequency fluctuations (ALFF), which can be obtained by computing the regional intensity, reflects spontaneous neuronal activity (Yang et al., 2007; Huang et al., 2020; Xing et al., 2021).
Therefore, the present study investigated the long-term sequelae in people 1 year after they had been hospitalized with COVID-19. To provide insight into the impact of SARS-CoV-2 on the brain, we also searched for inflammatory biomarkers that could predict the neuropsychiatric sequelae.
Participants and Methods
Study design
This retrospective observational cohort study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (Hunan, China) (approval No. 2020S004) on March 19, 2020 (Additional file 1 (290.4KB, pdf) ). The study was performed in accordance with the Declaration of Helsinki, and all participants provided written informed consent before entering the study. The writing and editing of the article were performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement (Additional file 2).
STROBE Statement—Checklist of items that should be included in reports of cohort studies
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract (b) Provide in the abstract an informative and balanced summary of what was done and what was found |
1-4 |
|
| |||
| Introduction | |||
|
| |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 4-5 |
|
| |||
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 5 |
|
| |||
| Methods | |||
|
| |||
| Study design | 4 | Present key elements of study design early in the paper | 6-7 |
|
| |||
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 6-7 |
|
| |||
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up (b) For matched studies, give matching criteria and number of exposed and unexposed |
6-7 |
|
| |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 6-7 |
|
| |||
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 7-9 |
|
| |||
| Bias | 9 | Describe any efforts to address potential sources of bias | 9 |
|
| |||
| Study size | 10 | Explain how the study size was arrived at | |
|
| |||
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 9-10 |
|
| |||
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding (b) Describe any methods used to examine subgroups and interactions (c) Explain how missing data were addressed (d) If applicable, explain how loss to follow-up was addressed (e) Describe any sensitivity analyses |
9-10 |
|
| |||
| Results | |||
|
| |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed (b) Give reasons for non-participation at each stage (c) Consider use of a flow diagram |
6-7,11 |
|
| |||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders (b) Indicate number of participants with missing data for each variable of interest (c) Summarise follow-up time (eg, average and total amount) |
9-10 |
|
| |||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time | 10-11 |
|
| |||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included (b) Report category boundaries when continuous variables were categorized (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period |
9-11 |
|
| |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | 11 |
|
| |||
| Discussion | |||
|
| |||
| Key results | 18 | Summarise key results with reference to study objectives | 11-12 |
|
| |||
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias |
16 |
|
| |||
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 11-16 |
|
| |||
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | |
|
| |||
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 2 |
*Give information separately for exposed and unexposed groups. Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.
Participants
Our study recruited 22 unpaid individuals who had been hospitalized with, and subsequently recovered from, COVID-19 and 29 healthy volunteers between January 28 and February 8, 2021. The 22 COVID-19 survivors who volunteered to participate in our study were recruited from among 237 patients who had been admitted with COVID-19 and who had been discharged from the First Hospital of Changsha about 1 year earlier. Clinical characteristics including the type of COVID-19, symptoms during hospitalization, and inflammatory markers such as lymphocyte count (Lymph#), neutrophil count (Neu#), and C-reactive protein (CRP) were collected from the hospital records. Healthy volunteers who had never tested positive or been hospitalized with COVID-19, and who matched the COVID-19 survivors in age, sex, and education level, were recruited through social media. All participants underwent fMRI scanning and completed several questionnaires that included information on age, sex, education level, smoking history, handedness, the Athens Insomnia Scale (AIS) (Soldatos et al., 2000), and the Hospital Anxiety and Depression Scale (HADS) (Michopoulos et al., 2008). The HADS consists of two subscales, the HADS-A and HADS-D, designed to detect anxious and depressive states, respectively. The inclusion criteria were as follows: (1) normal visual acuity, hearing, and right-handedness; (2) no history of brain structural abnormalities, epilepsy, traumatic brain injury, or mental or psychiatric illness; (3) no contraindications to MRI; and (4) fMRI data showing < 2.0 mm of displacement and/or < 2.0° rotation in any of the axes. The exclusion criteria were as follows: (1) patients with the following devices installed and carried in the body: cardiac pacemakers, cardiac stents, artificial heart valves, metal foreign bodies in the eyeball, and large blood vessels; (2) patients with severe hyperthermia; and (3) patients with claustrophobia. Finally, the recovered from COVID-19 (RecCOVID) group comprised 19 individuals and the healthy control (HC) group comprised 25 individuals (Figure 1).
Figure 1.
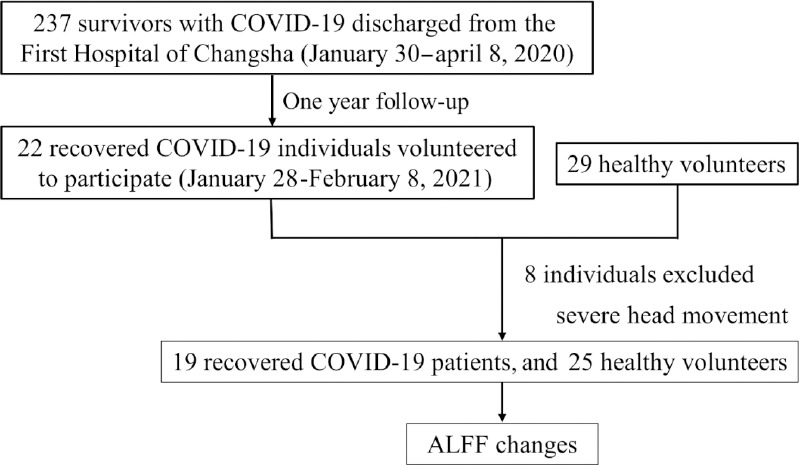
Experimental design flowchart.
ALFF: Amplitude of low-frequency fluctuation; COVID-19: coronavirus disease 2019.
MRI acquisition
We collected all MRI data with a 3T Siemens Skyra MRI scanner (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. Throughout the scanning, the participants were instructed to keep their bodies still, close their eyes, and not to think about anything in particular. Additionally, participants maintained a supine position, wore earplugs, and foam pads were placed between the head and the coil to minimize motion artifacts caused by head movements. The MRI scanning sequences included T1-weighted imaging, T2-weighted imaging, three-dimensional magnetization-prepared rapid acquisition gradient echo (3D-MPRAGE). Resting-state fMRI sessions measure the blood oxygenation level dependent (BOLD) signal. The 3D-MPRAGE scanning parameters were as follows: 176 sagittal slices, repetition time = 2000 ms, echo time = 2.26 ms, flip angle = 8°, voxel size = 1 mm × 1 mm × 1 mm, slice thickness = 1 mm, field of view = 256 mm × 256 mm. The BOLD parameters were: 36 axial slices, repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, voxel size = 4 mm × 4 mm × 4 mm, slice thickness = 4 mm, field of view = 240 mm × 240 mm (Sun et al., 2021).
MRI processing
Data Processing Assistant for Resting-State fMRI (DPABI, 4.3, Advanced edition) software (http://rfmri.org/dpabi) (Yan et al., 2016) based on MATLAB 2016b was used for MRI data preprocessing. The specific process was as follows: (1) data format conversion: digital imaging and communications in medicine (DICOM) images were converted into neuroimaging informatics technology initiative (NIfTI) images; (2) removal of the first time point: discard the initial 10 scanning volumes to allow for steady-state magnetization; (3) slice timing and realignment: excluded data from participants with head movements > 2 mm or head rotation > 2°; (4) after registering the resting-state image with the T1-structural image for each participant, all images were manually reoriented to the anterior commissure-posterior commissure axis; (5) nuisance covariates were regressed out, including the 24 Friston parameters for head motion, white matter signals, and cerebrospinal fluid signals. This reduces confounds from non-neuronal signals; (6) normalization: spatially normalized resting-state and T1-structural images were converted to Montreal Neurological Institute (MNI) space through the exponential Lie algebra registration method (Ashburner, 2007); (7) smoothing: the resampled image was spatially smoothed using a 6-mm full-width half-maximum Gaussian kernel to reduce spatial noise; (8) each voxel of the filtered time series was transformed into the frequency domain by a fast Fourier transform to calculate the power spectrum; and (9) the square root of each voxel's frequency (0.01–0.08 Hz) signal was calculated and subtracted from the average value. This quantity was divided by the whole-brain voxel deviation to obtain a standardized whole-brain ALFF map with m-distribution of ALFF values. For the detailed calculation method, please refer to Zang et al. (2007).
Statistical analysis
Paired-sample t-tests were used to compare clinical symptoms of the RecCOVID group at the initial time of illness and at the 1-year follow-up. Two-sample t-tests and chi-square tests were used to compare demographic characteristics between the RecCOVID and HC groups. Analyses were performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA) with P < 0.05 being the threshold for significance. Differences in ALFF values were examined in DPABI software with multiple comparisons using the 5000 permutation test with threshold-free cluster enhancement (Smith and Nichols, 2009; Chen et al., 2018) at the whole-brain level among the RecCOVID and HC groups, with a single voxel-level threshold of P < 0.05. The age, sex, years of education, and head movement parameters of the two groups were used as covariates.
Pearson correlation analysis was applied to explore associations between the ALFF values in several brain regions (derived from the anatomical automatic labeling atlas) and inflammatory markers, AIS scores, HADS-A scores, and HADS-D scores in the COVID-19 group. Significance levels were set at P < 0.05.
Results
Demographic characteristics of COVID-19 survivors
Age, sex, and education level were well matched with no significant differences between the two groups. As shown in Table 1, the history of smoking history, head motion parameters, and HADS scores presented no significant differences. In contrast, AIS scores were higher in the RecCOVID group than in the HC group, and sleep disruptions were more severe in the RecCOVID group. Details are summarized in Table 1.
Table 1.
Demographic characteristics in the RecCOVID and HC groups
| RecCOVID group (n=19) | Healthy control group (n=25) | t/c2 value | P-value | |
|---|---|---|---|---|
| Age (yr)a | 54.210±8.696 | 50.480±11.576 | 1.221 | 0.229 |
| Sex (male/female)b | 8/11 | 7/18 | 0.956 | 0.328 |
| Education level (yr)a | 13.420±3.610 | 12.720±4.088 | 0.602 | 0.550 |
| Smoking history (yes/ no)b | 2/17 | 4/21 | 0.275 | 0.600 |
| Handedness (right/left) | 19/0 | 25/0 | – | – |
| Head motion (mm)a | 0.085±0.075 | 0.087±0.055 | –0.076 | 0.940 |
| Athens Insomnia Scale scorea | 8.320±4.534 | 5.560±3.001 | 2.295 | 0.029 |
| Hospital Anxiety and Depression Scale-Aa | 3.210±4.131 | 5.040±3.458 | –1.559 | 0.128 |
| Hospital Anxiety and Depression Scale-Da | 3.740±4.954 | 4.160±3.520 | –0.317 | 0.754 |
aData are expressed as expressed as mean ± SD and were analyzed by two-sample t-test. bData are expressed as number, and were analyzed by chi-square test. COVID-19: Coronavirus disease 2019.
Clinical data for the COVID-19 survivors
The RecCOVID group included 10 mild cases and 9 severe cases, according to WHO guidelines (World Health Organization). Clinical data are shown in Table 2. During hospitalization, the common clinical manifestations in the RecCOVID group of this study were fever (79%), cough (74%), dyspnea (42%), olfactory loss (42%), taste loss (37%), fatigue (37%) and myalgia (21%). After 1 year of follow-up, the fever (11%), cough (37%), olfactory loss (5%) and taste loss (5%) had significantly improved (P < 0.05). However, compared with the time when they had the SARS-CoV-2 infection, at the 1-year follow-up, the RecCOVID group presented with greater chest tightness (32%, P = 0.010) and headache (36%, P = 0.056). Dyspnea (32%), fatigue (21%), and myalgia (36%) were persistent symptoms at the 1-year follow-up after discharge (Table 2).
Table 2.
Clinical information for the COVID-19 survivors at the time of illness and at the 1-year follow-up
| Hospitalization | Follow-up | t-value | P-value | |
|---|---|---|---|---|
| Clinical type (mild/severe) | 10/9 | – | – | – |
| Hospital day (d) | 19.160±10.383 | – | – | - |
| Follow-up time (d) | – | 345.790±15.796 | – | – |
| Inflammatory markers | ||||
| Lymphocyte count (×109/L) | 1.132±0.473 | – | – | – |
| Neutrophil count (×109/L) | 3.131±1.060 | – | – | – |
| C-reactive protein (mg/L) | 31.497±28.363 | – | – | – |
| Symptoms [number (percentage)] | ||||
| Fever | 15(79) | 2(11) | –6.245 | 0 |
| Cough | 14(74) | 7(37) | –2.348 | 0.031 |
| Expectoration | 0 | 3(16) | 1.837 | 0.083 |
| Dyspnea | 8(42) | 6(32) | –1.143 | 0.268 |
| Headache | 1/18 (5) | 5(36) | 2.041 | 0.056 |
| Fatigue | 7(37) | 4(21) | –1 | 0.331 |
| Myalgia | 4(21) | 5(36) | 0.369 | 0.716 |
| Decreased appetite | 0 | 1/18 (5) | 1 | 0.331 |
| Nausea | 1/18 (5) | 1/18 (5) | 0 | 1 |
| Vomiting | 1/18 (5) | 0 | –1 | 0.331 |
| Diarrhea | 2(11) | 1/18 (5) | –1 | 0.331 |
| Chest tightness | 0 | 6(32) | 2.882 | 0.010 |
| Chest pain | 1/18 (5) | 5(36) | 1.714 | 0.104 |
| Olfactory loss | 8(42) | 1/18 (5) | –3.24 | 0.005 |
| Taste loss | 7(37) | 1/18 (5) | –2.882 | 0.010 |
Data for clinical type are expressed as expressed as number, and data for hospital day, follow-up time, and inflammatory markers are expressed as the mean ± SD. Data for symptoms are expressed as number (percentage) and were analyzed by paired sample t-test. COVID-19: Coronavirus disease 2019.
fMRI results for the COVID-19 survivors
Compared with the HC group, the RecCOVID group demonstrated significantly increased ALFF values in the left precentral gyrus (PreCG), middle frontal gyrus, inferior frontal gyrus of operculum, inferior frontal gyrus of triangle, insula, hippocampus (HIP), parahippocampal gyrus, fusiform gyrus, postcentral gyrus, inferior parietal angular gyrus, supramarginal gyrus, angular gyrus, thalamus, middle temporal gyrus, inferior temporal gyrus, caudate (CAU) and putamen (PUT) (Figure 2A). The left HIP was the peak among the clusters (Figure 2B; cluster size: 78 mm3, peak MNI coordinates: –30, –33, –3, peak t value: 5.4507). More details are provided in Table 3.
Figure 2.
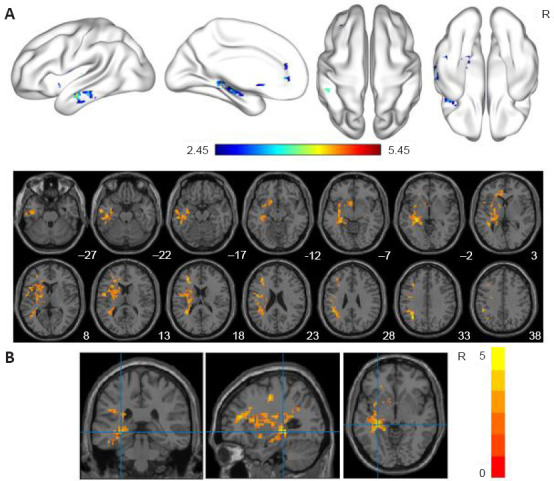
ALFF analyses in the individuals who had recovered from COVID-19.
(A) Significantly increased ALFF in the RecCOVID group. The 5000 permutations test was performed with threshold-free cluster enhancement (single voxel-level threshold of P < 0.05, cluster size > 10 voxels). (B) Peak in the cluster (cluster size: 78 mm3, peak MNI coordinates: −30, −33, −3, peak t value: 5.4507). The dark red (A) and yellow (B) on the color bar indicate brain regions in which the ALFF values differed significantly between the RecCOVID and HC groups. ALFF: Amplitude of low-frequency fluctuation; COVID-19: coronavirus disease 2019; HC: healthy control; MNI: Montreal Neurological Institute; R: right.
Table 3.
Significant ALFF differences between the RecCOVID and HC groups
| Brain regions | Side (R/L) | AAL | Cluster size | MNI coordinate | Peak intensity | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| X | Y | Z | |||||
| PreCG/MFG/ | L | 1/7/11/13 | 1190 | –30 | –33 | –3 | 5.4507 |
| IFGoperc/ | /29/37/39 | ||||||
| IFGtriang/INS/HIP/ | /55/57/61 | ||||||
| PHG/FFG/PoCG/ | /63/65/77 | ||||||
| IPL/SMG/ANG/ | /85/89 | ||||||
| THA/MTG/ITG | |||||||
| CAU/PUT | L | 71/73 | 53 | –6 | 12 | –6 | 3.8569 |
AAL: Anatomical automatic labeling atlas; ALFF: amplitude of low-frequency fluctuation; ANG: angular gyrus; CAU: caudate; COVID-19: coronavirus disease 2019; FFG: fusiform gyrus; HIP: hippocampus; IFGoperc: inferior frontal gyrus of operculum; IFGtriang: inferior frontal gyrus of triangle; INS: insula; IPL: inferior parietal angular gyrus; ITG: inferior temporal gyrus; L: left; MNI: Montreal Neurological Institute; MFG: middle frontal gyrus; MTG: middle temporal gyrus; PHG: parahippocampal gyrus; PoCG: postcentral gyrus; PreCG: precentral gyrus; PUT: putamen; R: right; SMG: supramarginal gyrus; THA: thalamus.
Correlation results for COVID-19 survivors
Before multiple comparison correction, the ALFF values in the left CAU were positively correlated with the AIS (r = 0.466, P = 0.044; Figure 3A) and those in the left PreCG were positively correlated with the Neu# (r = 0.761, P = 0.000; Figure 3B) in the RecCOVID group. However, after correcting for multiple comparisons (FDR-corrected P < 0.05), only the latter correlation survived (PFDR = 0.019).
Figure 3.
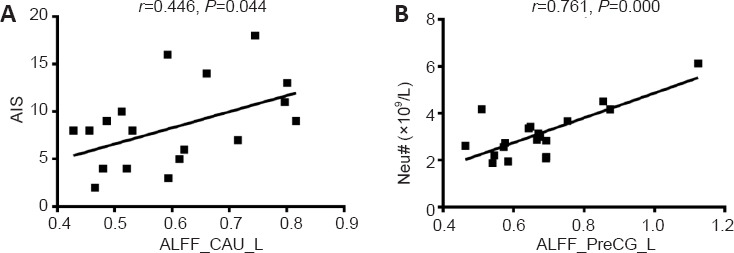
Correlations between left CAU and PreCG ALFF values and AIS score and Neu#.
(A) ALFF values in the left CAU showed a positive correlation with AIS score. (B) ALFF values in the left PreCG showed a positive correlation with Neu#. AIS: Athens Insomnia Scale; ALFF: amplitude of low-frequency fluctuation; CAU: caudate; COVID-19: coronavirus disease 2019; L: left; Neu#: neutrophil count; PreCG: precentral gyrus.
Discussion
This study explored differences in clinical symptoms between the acute phase and a 1-year follow-up period in cases of COVID-19. The 1-year follow-up data was also used to explore differences in brain function between healthy controls and those who had recovered from COVID-19. The main findings were as follows: (a) dyspnea, fatigue, myalgia, and insomnia were the most common symptoms at follow-up; (b) compared to the HC group, the COVID-19 survivors exhibited increased brain activity in the left PreCG, middle frontal gyrus, inferior frontal gyrus of operculum, inferior frontal gyrus of triangle, insula, HIP, parahippocampal gyrus, fusiform gyrus, postcentral gyrus, inferior parietal angular gyrus, supramarginal gyrus, angular gyrus, thalamus, middle temporal gyrus, inferior temporal gyrus, CAU, and PUT; (c) there was a significant correlation between ALFF values in the left CAU and AIS scores, and the increase in ALFF values in the left PreCG was highly correlated with the levels of Neu#.
We found that dyspnea, fatigue, and myalgia were common even at the 1-year follow-up after SARS-CoV-2 infection. While anxiety and depression scale scores were within the normal range, insomnia became obvious over time. This is consistent with the results of other studies. A previous SARS study showed that 40% of survivors continued to suffer from chronic fatigue problems after an average of 41.3 months post infection (Lam et al., 2009). Several studies have also suggested that fatigue or muscle weakness is more common in women recoverees and patients who had severe COVID-19 (Huang et al., 2021; Xiong et al., 2021).
Moreover, Tansey et al. (2007) found that 33% of survivors had a significant decrease in mental health 1 year after infection, even though most patients recovered well from the SARS infection. Compared with the 1-month data, a 3-month follow-up survey of 226 survivors showed that symptoms of depression persisted, while symptoms of post-traumatic stress disorder, anxiety, and insomnia had significantly improved (Mazza et al., 2021). The latest Chinese cohort study observed that sleep difficulties, anxiety, and depression were common symptoms at a 6-month follow-up (Huang et al., 2021), which is similar to our results. We speculate that compared with anxiety and depression, symptoms of insomnia become more obvious with longer follow-up times. The mechanism underlying the physical and neuropsychological sequelae of COVID-19 may be a combination of multiple factors, including direct viral infection, immune stress, hormone therapy, intensive care unit treatment history, and social isolation (Huang et al., 2021).
In the current study, the group that had recovered from COVID-19 had significantly increased ALFF values in multiple brain regions compared with the HC group, with the greatest difference in the left HIP. The main function of the HIP is memory formation and spatial navigation, and it plays an important role in supporting flexible cognition and behavior (Bellmund et al., 2018). Kim et al. (2015) suggested that uncontrollable stress can affect the shape and function of the HIP. However, the COVID-19 outbreak has caused a certain degree of psychological stress not only for patients, but also for healthy people who are not sick. Thus, we suspect that the increased regional intensity in the HIP could result partially from stress and partially from inflammation or other reasons. In addition to the HIP, other brain areas with increased ALFF values included the insula, parahippocampal gyrus, and thalamus, which are all important parts of the limbic system. The limbic system processes sensory input from external and internal environments, and uses memory and motivation to determine the emotional, autonomous, motor, and cognitive responses that are essential for self-protection and survival (McLachlan, 2009). Supporting these reported changes in limbic system function, abnormal cortical thickness in the left limbic system was reported in a study of patients with COVID-19 6 months after discharge (Qin et al., 2021). Taken together, these data suggest that the limbic system may be vulnerable to COVID-19 infection, but how this happens needs further study.
ALFF values in the CAU and PUT were greater in the RecCOVID group than in the HC group. The human striatum is composed of the CAU and PUT, and it has been reported that ACE2 is expressed in the striatum (Baig et al., 2020). Therefore, elevations in ALFF may be related to the richness of ACE2 in the striatum. Higher ALFF values in the CAU was associated with greater insomnia in the COVID-19 survivors. The CAU is involved in many associative, executive, motivational, and emotional processes (Kas et al., 2021). Increased spontaneous neuronal activity in the CAU might trigger insomnia, thus accounting for the higher rates in the RecCOVID group at the 1-year follow-up. However, a longitudinal fluorine-18 fluorodeoxyglucose positron emission tomography study examining the consequences of COVID-19 after 6 months showed a hypometabolic pattern in the bilateral CAU (Kas et al., 2021). The discrepancy in results might be related to the different follow-up times and the smaller sample size (seven) used in Kas et al. (2021).
In our study, the RecCOVID group also had higher ALFF values in the left frontal lobe (PreCG, middle frontal gyrus, inferior frontal gyrus of operculum, and inferior frontal gyrus of triangle), parietal lobe (postcentral gyrus, inferior parietal angular gyrus, supramarginal gyrus, and angular gyrus), and temporal lobe (fusiform gyrus, middle temporal gyrus, and inferior temporal gyrus) than did the HC group. This might indicate that COVID-19 damages the function of multiple brain lobes to varying degrees. Furthermore, ALFF values in the left PreCG of the RecCOVID group were positively associated with Neu#. This implies that the more severe the inflammation was when the patients were hospitalized, the faster the recovery was after 1 year of follow-up in the PreCG. SARS-CoV-2 was recently detected in frontal lobe sections from postmortem examination, confirming the presence of the virus in brain tissue (Paniz-Mondolfi et al., 2020). Thus, we think this may be related to direct infection of the virus, which causes a fast inflammatory response that recovers quickly.
We wish to highlight two interesting findings. First, brain regions at follow-up only showed higher ALFF values than controls; no regions exhibited lower ALFF values. Second, ALFF values were elevated in the left hemisphere, but not in the right hemisphere. As the study was conducted 1 year after discharge, we speculate that the cause of the first finding may be related to the compensatory repair of brain tissue following hypoxia or inflammation, which manifests as increased spontaneous activity of neurons and increased ALFF. The second finding could be related to asymmetrical development of the brain (Duboc et al., 2015), resulting in different degrees of viral damage to the left and right cerebral hemispheres. However, the exact mechanism underlying these two findings remains unclear and further research is needed.
This study has several limitations. First, the small sample size was small. Subsequent follow-up studies should focus on increasing the sample size. Second, to avoid cross-infection of patients, head MRI was not performed in the acute phase.
In this study, dyspnea, fatigue, and myalgia were common even at the 1-year follow-up after SARS-CoV-2 infection, and insomnia became obvious over time. We observed significant differences in ALFF values in brain regions related to mood and sleep regulation in individuals who had recovered from COVID-19, which may provide imaging evidence for neuropsychiatric sequelae in the long-term recovery of COVID-19. Inflammatory markers collected during hospitalization were related to the ALFF, and this finding may help shed some light on the neurobiological mechanisms underlying COVID-19-associated neuropsychiatric sequelae.
Additional files:
Additional file 1 (290.4KB, pdf) : Hospital ethics approval (Chinese).
Additional file 2: STROBE checklist.
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
Editor note: JL is an Editorial Board member of Neural Regeneration Research. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer review handled independently of this Editorial Board member and his research group.
Financial support: This study was supported by Key Emergency Project of Pneumonia Epidemic of Novel Coronavirus Infection of China, No. 2020SK3006 (to JL); Clinical Research Center for Medical Imaging in Hunan Province of China, No. 2020SK4001 (to JL); and the Innovative Major Emergency Project Funding against the New Coronavirus Pneumonia in Hunan Province of China, No. 2020SK3014 (to JYL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (Hunan, China) (approval No. 2020S004) on March 19, 2020.
Declaration of participant consent: The authors certify that they have obtained all appropriate participant consent forms from the participants. In the forms, the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: The writing and editing of the article were performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of School of Mathematics and Statistics, Central South University.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: After publication, the data will be made available to other researchers on reasonable request to the corresponding author.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by Key Emergency Project of Pneumonia Epidemic of Novel Coronavirus Infection of China, No. 2020SK3006 (to JL); Clinical Research Center for Medical Imaging in Hunan Province of China, No. 2020SK4001 (to JL); and the Innovative Major Emergency Project Funding against the New Coronavirus Pneumonia in Hunan Province of China, No. 2020SK3014 (to JYL).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y.
References
- 1.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 3.Bellmund JLS, Gärdenfors P, Moser EI, Doeller CF. Navigating cognition: Spatial codes for human thinking. Science. 2018;362:eaat6766. doi: 10.1126/science.aat6766. [DOI] [PubMed] [Google Scholar]
- 4.Bougakov D, Podell K, Goldberg E. Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol. 2021;58:564–575. doi: 10.1007/s12035-020-02152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Lu B, Yan CG. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum Brain Mapp. 2018;39:300–318. doi: 10.1002/hbm.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Wang KJ, Luo YC, Wang BZ, Zhang MM, Xu YQ, Yang YN, Ma YT. Predictive value of neutrophil/lymphocyte ratio on myocardial injury in severe COVID-19 patients. Zhonghua xin xue guan bing za zhi. 2020;48:572–579. doi: 10.3760/cma.j.cn112148-20200422-00336. [DOI] [PubMed] [Google Scholar]
- 7.De Santis G. SARS-CoV-2: A new virus but a familiar inflammation brain pattern. Brain Behav Immun. 2020;87:95–96. doi: 10.1016/j.bbi.2020.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duboc V, Dufourcq P, Blader P, Roussigné M. Asymmetry of the brain: development and implications. Annu Rev Genet. 2015;49:647–672. doi: 10.1146/annurev-genet-112414-055322. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi S, Srivastava AK, Ray U, Tripathi PP. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem Neurosci. 2020;11:1379–1381. doi: 10.1021/acschemneuro.0c00217. [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martín Ó, Barrena C, Molina-Nuevo JD, García-García J, Lozano-Setién E, Alcahut-Rodriguez C, Martínez-Martín Á, Sánchez-López A, Segura T. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143:3089–3103. doi: 10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Xu L, Kuang L, Wang W, Cao J, Xiao MN. Abnormal brain activity in adolescents with Internet addiction who attempt suicide: an assessment using functional magnetic resonance imaging. Neural Regen Res. 2020;15:1554–1559. doi: 10.4103/1673-5374.274346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kas A, Soret M, Pyatigoskaya N, Habert MO, Hesters A, Le Guennec L, Paccoud O, Bombois S, Delorme C. The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging. 2021;48:2543–2557. doi: 10.1007/s00259-020-05178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem. 2015;22:411–416. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam MH, Wing YK, Yu MW, Leung CM, Ma RC, Kong AP, So WY, Fong SY, Lam SP. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020a;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020b;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, Jia T, Zhao Y, Wang D, Xiao A, Yin B. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazza MG, Palladini M, De Lorenzo R, Magnaghi C, Poletti S, Furlan R, Ciceri F COVID-19 BioB Outpatient Clinic Study group, Rovere-Querini P, Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P COVID-19 BioB Outpatient Clinic Study group, Benedetti F. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLachlan RS. A brief review of the anatomy and physiology of the limbic system. Can J Neurol Sci. 2009;36(Suppl 2):S84–87. [PubMed] [Google Scholar]
- 24.Michopoulos I, Douzenis A, Kalkavoura C, Christodoulou C, Michalopoulou P, Kalemi G, Fineti K, Patapis P, Protopapas K, Lykouras L. Hospital Anxiety and Depression Scale (HADS): validation in a Greek general hospital sample. Ann Gen Psychiatry. 2008;7:4. doi: 10.1186/1744-859X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020;267:2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020;77:1028–1029. doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 29.Qin Y, Wu J, Chen T, Li J, Zhang G, Wu D, Zhou Y, Zheng N, Cai A, Ning Q, Manyande A, Xu F, Wang J, Zhu W. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131:e147329. doi: 10.1172/JCI147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 31.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 32.Sun JJ, Pan XQ, Yang R, Jin ZS, Li YH, Liu J, Wu DX. Changes in sensorimotor regions of the cerebral cortex in congenital amusia: a case-control study. Neural Regen Res. 2021;16:531–536. doi: 10.4103/1673-5374.293154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tansey CM, Louie M, Loeb M, Gold WL, Muller MP, de Jager J, Cameron JI, Tomlinson G, Mazzulli T, Walmsley SL, Rachlis AR, Mederski BD, Silverman M, Shainhouse Z, Ephtimios IE, Avendano M, Downey J, Styra R, Yamamura D, Gerson M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- 34.Uncini A, Vallat JM, Jacobs BC. Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry. 2020;91:1105–1110. doi: 10.1136/jnnp-2020-324491. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance. [Accessed January 12, 2020]. https://www.who.int/docs/default-source/coronaviruse/clinicalmanagement-of-novel-cov.pdf.
- 36.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing XX, Zheng MX, Hua XY, Ma SJ, Ma ZZ, Xu JG. Brain plasticity after peripheral nerve injury treatment with massage therapy based on resting-state functional magnetic resonance imaging. Neural Regen Res. 2021;16:388–393. doi: 10.4103/1673-5374.290912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 40.Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 41.Yelin D, Wirtheim E, Vetter P, Kalil AC, Bruchfeld J, Runold M, Guaraldi G, Mussini C, Gudiol C, Pujol M, Bandera A, Scudeller L, Paul M, Kaiser L, Leibovici L. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20:1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Zhan X, Liu B, Tong ZH. Postinflammatroy pulmonary fibrosis of COVID-19: the current status and perspective. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:728–732. doi: 10.3760/cma.j.cn112147-20200317-00359. [DOI] [PubMed] [Google Scholar]
- 44.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


