Abstract
Glaucoma is one of the world's most frequent visual impairment causes and leads to selective damage to retinal ganglion cells and their axons. Despite glaucoma's most accepted risk factor is increased intraocular pressure (IOP), the mechanisms behind the disease have not been fully elucidated. To date, IOP lowering remains the gold standard; however, glaucoma patients may still lose vision regardless of effective IOP management. Therefore, the exclusive IOP control apparently is not enough to stop the disease progression, and developing new resources to protect the retina and optic nerve against glaucoma is a goal of vast clinical importance. Besides pharmacological treatments, environmental conditions have been shown to prevent neurodegeneration in the central nervous system. In this review, we discuss current concepts on key pathogenic mechanisms involved in glaucoma, the effect of enriched environment on these mechanisms in different experimental models, as well as recent evidence supporting the preventive and therapeutic effect of enriched environment exposure against experimental glaucomatous damage. Finally, we postulate that stimulating vision may become a non-invasive and rehabilitative therapy that could be eventually translated to the human disease, preventing glaucoma-induced terrible sequelae resulting in permanent visual disability.
Keywords: brain-derived neurotrophic factor, chondroitin sulfate, enriched environment, glaucoma, optic nerve axons, optic nerve glia, retinal ganglion cells, visual stimulation
Introduction
Glaucoma is a multifactorial, progressive, and neurodegenerative disorder, characterized by the death of retinal ganglion cells (RGCs), and optic nerve (ON) axon loss (Nucci et al., 2016). With more than 70 million people, and approximately 10% of them suffering from bilateral blindness, glaucoma is recognized as a leading cause of irreversible blindness worldwide (Weinreb et al., 2014; Nucci et al., 2016). In fact, glaucoma is predicted to strike more than 110 million people by 2040, with strong Public Health implications (Tham et al., 2014). Due to the lack of symptoms during the disease early stages, half of those affected by glaucoma are estimated to not be aware of their condition; by the time vision loss has developed, significant and irreversible damage has occurred already. Glaucomatous visual field defects usually start in the periphery, and progress to central vision, with devastating consequences to the patient´s quality of life (Cesareo et al., 2015). Increased intraocular pressure (IOP) is the main risk factor for the onset and progression of neuronal damage within glaucoma (Nucci et al., 2016). Thus, glaucoma therapy is currently based on IOP reduction by medical, surgical or parasurgical treatments. A considerable percentage of patients experiences disease progression despite a satisfactory IOP control (Nucci et al., 2016). In fact, it is estimated that one in eight patients will eventually become blind in at least one eye as a result of glaucoma progression, even in populations with access to the best available treatments (Khatib and Martin, 2017). Therefore, although the current management of glaucoma is mainly focused on IOP control, a therapy that prevents RGC death and ON axon loss should be the main goal of glaucoma treatment. Unfortunately, the mechanisms that lead to RGC loss in glaucoma are still unclear. Since glaucoma is a complex and multifactorial disease, several molecular pathways are likely to converge, inducing structural and functional alterations. Signals that promote RGC death in glaucoma might be exacerbated by risks factors, tilting the neuron's fate towards dysfunction and demise. In recent years, there has been a considerable progress in our understanding of multiple pathways that lead to RGC degeneration and ON injury. In this vein, several factors such as a reduced antioxidant defense system activity (Kimura et al., 2017), glutamate excitotoxicity (Moreno et al., 2005b), and sustained glial reactivity (Weinreb et al., 2016; Baudouin et al., 2020), among others, have been suggested as possible additional causes for early and/or advanced stages of glaucomatous damage. The contribution of different players to the pathogenesis of glaucoma supports the necessity of developing therapeutic strategies that ideally may act on several molecular and cellular targets, with the sustainability of treatments over time and their degree of invasiveness, being important points to consider. Here, we will discuss advances in key factors contributing to glaucomatous neurodegeneration, with a particular focus on the advantages of the enriched environment as a non-invasive therapeutic strategy against glaucoma.
Database Search Strategy
We performed search on both PubMed and Google Scholar for articles with keywords of “glaucoma, retina, and enriched environment” from 1995 to the present.
Experimental Models of Glaucoma
The development of clinically relevant models for glaucoma research has greatly improved our understanding of the pathobiological mechanisms of the disease, as well as the possibility of searching for neuroprotective agents. In recent years, rat and mouse glaucoma models have become key tools due to many advantages, such as their potential for experimental manipulation, low cost, and relatively human-like ocular anatomy and biology (Pang and Clark, 2020). Ocular pressure-dependent models generally address the most important risk factor, although strategies for inducing ocular hypertension vary considerably. Several groups have managed to increase IOP in rodents by impeding the outflow of aqueous humor through laser photocoagulation, episcleral veins cauterization or injection of hypertonic saline, as well as by intracameral injections of microbeads, or viscoelastics (Biswas and Wan, 2019). Furthermore, mouse genetic models, in particular, the DBA2J mouse strain that develops pigment dispersal iris disease by spontaneous mutations leading to elevated IOP (Fernandes et al., 2015) are frequently used. All these models have both advantages and disadvantages. We have developed a model of glaucoma through weekly injections of chondroitin sulfate (CS) in the eye anterior chamber of Wistar rats. Weekly intracameral injections of CS significantly increase IOP as compared with vehicle-injected contralateral eye. Although sustained ocular hypertension requires multiple intracameral injections, we have shown that the maneuver itself remains IOP, retinal function, or histology unaffected. Several advantages support this model: 1) highly consistent and moderate ocular hypertension, 2) reasonably long course, 3) low cost, and 4) easy to perform. The use of viscoelastics (hyaluronic acid or CS) to induce ocular hypertension has been validated by several groups (Foureaux et al., 2015; Mayordomo-Febrer et al., 2015; Pirhan et al., 2016; Chan et al., 2019; Yu et al., 2020). Chondroitin sulfate chronic administration significantly decreases the scotopic electroretinographic response and induces a significant RGCs and ON fibers loss (Moreno et al., 2005a; Belforte et al., 2010). Moreover, ocular hypertension induced by CS-injections decreases visual evoked potentials (VEPs), retinal anterograde transport from RGCs to main central targets, retinal and proximal ON glial reactivity, and even induces alterations in non-image-forming visual functions (i.e., pupil light reflex, and locomotor activity daily rhythms) (Belforte et al., 2010; De Zavalía et al., 2011; Bordone et al., 2017; González Fleitas et al., 2020b). Taken together, these results support intracameral injections of CS in the anterior chamber of the rat eye reproduce the hallmarks of primary open-angle glaucoma, which is the most common form of the disease. Therefore, this model may be a useful tool to understand the cellular and molecular mechanisms involved in glaucomatous damage and to develop new therapeutic strategies.
Enriched Environment
The concept of the enriched environment (EE) emerged as an experimental approach to studying the effects of experience in the brain. EE was first defined as a complex combination of social and unanimated stimulation that was able to induce profound effects on brain morphology, chemistry, and physiology (Rosenzweig et al., 1960). In EE, animals are reared in wide cages, in the presence of a variety of objects with different shapes, colors, and textures regularly changed, and running wheels, which achieve a boost in exploratory, sensory, and cognitive activity, social interaction, and voluntary physical exercise. The exposure to EE triggers cellular and molecular plasticity across different brain areas, as compared to animals housed in standard laboratory conditions (standard environment (SE)) (Kempermann, 2019). EE beneficial effects were found across the lifespan in different species, as well as in the context of experimental models of brain damage (Nithianantharajah and Hannan, 2006; Hui et al., 2011; Sale, 2018). At the cellular level, EE potentiates dendritic length and spine density in several neuronal populations and promotes syn-aptogenesis and neurogenesis in the hippocampus (Kondo, 2017). At the molecular level, these effects are supported by alterations in gene expression, histone acetylation, and chromatin remodeling (Griñán-Ferré et al., 2018; Wang et al., 2020). Moreover, EE housing increases neurotrophins levels (particularly brain-derived neurotrophic factor (BDNF), and nerve growth factor), which play essential roles in neuronal trophic signaling (Nithianantharajah and Hannan, 2006; Kondo, 2017). Age is not a limitation for EE consequences. In aging, EE positively impacts neuronal physiology, for example, by modulating the dynamics of several neurotransmitter sys-tems. In fact, it has been shown that EE has similar effects in the early post-natal stage and adulthood (Sale et al., 2014). Macroscopically, there is evidence that EE modifies behavior (Sale et al., 2014), increasing learning and memory, and reducing age-related cognitive decay (Kondo, 2017; Mandolesi et al., 2017).
Extensive research has explored the neuroprotective effects of EE in the context of central nervous system diseases. In this line, it has been shown that EE has protective effects in different animal models of neurodegenerative disorders, and different types of brain damage in adults (Nithianantharajah and Hannan, 2006), such as stroke, Parkinson's disease, Alzheimer's disease, epilepsy, and traumatic brain injury (Sale et al., 2014; Kline et al., 2016). Additionally, EE mediates axonal regeneration (Hutson et al., 2019), functional white matter recovery (Forbes et al., 2020), and neuroinflammation inhibition (Zhang et al., 2020). As already mentioned, BDNF plays a major role in EE-mediated neuroprotection (Kondo, 2017). EE housing increases the levels of BDNF and its receptor TrkB in the superior colliculus (SC), the visual cortex (Franklin et al., 2006), and the hippocampus (Sale et al., 2014). In addition, TrkB expression knockdown impairs EE-mediated neuroprotection and spatial memory during hypobaric hypoxia (Jain et al., 2013).
The early-life visual system is significantly influenced by the environment. Postnatal EE housing not only accelerates retinal acuity maturation, but also counteracts the effects of dark rearing on RGC dendritic stratification, through a BDNF-dependent mechanism (Sale et al., 2014). Prenatal exposure to EE accelerates neural progenitor migration, and spontaneous apoptosis in retinal neurons (Sale, 2018). The impact of EE in visual brain areas has been also analyzed in experimental amblyopia (Consorti et al., 2019), a permanent loss of visual acuity in one deprived eye, due to various causes, which provokes a binocular functional misbalance that results in abnormal early visual experience. This neurodevelopmental, purely cortical, visual deficit is mainly characterized by a loss of visual acuity, and low contrast sensitivity. Although it is widely accepted that normal visual function can only be recovered by the reestablishment of a “correct” visual experience within a narrow period (i.e., the critical period) during visual development, it has been demonstrated that exposure to EE for 2–3 weeks promotes full visual acuity, and ocular dominance recovery, both at the electrophysiological and behavioral level in adult rats with experimental amblyopia (Baroncelli et al., 2012). The adult retina has long been considered less plastic than the brain cortex or the hippocampus, the canonical sites of experience-dependent plasticity; however, in recent years, the neuroprotective role of EE has gained relevance in rodent adult retina and ON (Dorfman et al., 2013, 2014, 2015; Aranda et al., 2019; González Fleitas et al., 2019, 2020b), due to its non-invasiveness and sustainability over time. Table 1 summarizes EE protocols successfully used in different retinal diseases in rodents. A description of EE-induced neuroprotection against key pathophysiological mechanisms involved in glaucoma will be provided in the next sections.
Table 1.
Summary of protocols of EE used in experimental models of retinal diseases
| Experimental model | EE protocol | EE´s exposure period | EE preserves | Reference |
|---|---|---|---|---|
| Dark rearing in Thy-1-mGFP single mice | Cage dimensions: 44 × 62 × 28 cm3. EE cages contained several food hoppers, a running wheel and differently shaped objects (tunnels, shelters, stairs) that were completely substituted once a week. | Pregnant females exposed 7 d before delivery and pups exposed from birth up to P30 | -RGCs dendritic maturation (dendritic stratification in the a and b sublaminae of the IPL) -BDNF immunoreactivity in GCL |
Landi et al., 2007 |
| Glutamate excitotoxicity in Wistar rats | Cage dimensions: 88 × 50 × 44 cm3. EE cages contained different toys, objects, running tunnels and rotating rods. Half of the objects were changed daily, while the other half was left unchanged. | From birth up to 5 wk | -Retinal structure -Number of cells in GCL |
Szabadfi et al., 2009 |
| Retinitis pigmentosa in rd10 mutants and wild-type mice (C57BL/6J) | Cage dimensions: 60 × 38 × 20 cm3. Running wheels were repositioned once a week and tunnels and objects were replaced with the same frequency. 6–8 pups per cage. | Whole pregnancy occurred in EE, and pups remained in EE from birth up to 210 d | -Photoreceptor survival and morphology -Morphology of inner retinal cells -Photopic ERG response -Visual acuity and contrast sensitivity -CNTF and mTOR mRNA levels |
Barone et al., 2012 |
| Retinal ischemia in Wistar rats | Cage dimensions: 46.5 × 78 × 95 cm3. Food hoppers, water bottles, running wheels, tubes, ramps, and differently shaped objects (balls, ropes, stones) were repositioned once a day and fully substituted once a week. | 3 wk after ischemia | -Retinal function (ERG) -Retinal structure -TUNEL+ cell number in GCL -RGCs number -Retinal GFAP reactivity -Anterograde transport |
Dorfman et al., 2013 |
| Six animals per cage. | 3 d after ischemia | -Retinal glutamate uptake -Retinal glutamine syntase activity |
||
| Retinal ischemia in Wistar rats | Cage dimensions: 85 × 50 × 44 cm3. EE cages contained different toys, objects, running tunnels and rotating rods. Half of the objects were changed daily, while the other half was left unchanged. | 2 wk after ischemia | -Retinal structure -Number of cells in ONL -Number of cells in GCL |
Kiss et al., 2013 |
| Retinal ischemia in Wistar rats | Cage dimensions: 46.5 × 78 × 95 cm3. Food hoppers, water bottles, running wheels, tubes, ramps, and differently shaped objects (balls, ropes, stones) were repositioned once a day and fully substituted once a week. | 3 wk before ischemia | -Retinal function (ERG) -Retinal structure -Retinal synaptophysin-immunoreactivity -Retinal microglia/macrophages reactivity -RGCs number -Anterograde transport |
Gonzalez Fleitas et al., 2019 |
| Six animals per cage. | ||||
| Diabetic retinopathy in Wistar rats | Cage dimensions: 46.5 × 78 × 95 cm3. Food hoppers, water bottles, running wheels, tubes, ramps, and differently shaped objects (balls, ropes, stones) were repositioned once a day and fully substituted once a week. | 6 wk after diabetes induction | -Retinal function (ERG) -Retinal synaptophysinimmunoreactivity -B-R barrier -Retinal VEGF levels-Retinal TNFα and TBARS levels -Retinal BDNF immunoreactivity |
Dorfman et al., 2014 |
| Six animals per cage. | 10 wk after diabetes induction | -Retinal function (ERG) -Retinal VEGF levels |
||
| Optic neuritis in Wistar rat | Cage dimensions: 46.5 × 78 × 95 cm3. Food hoppers, water bottles, running wheels, tubes, ramps, and differently shaped objects (balls, ropes, stones) were repositioned once a day and fully substituted once a week. | 3 wk after optic nerve inflammation | -Visual function (PLR/VEPs) -RGCs number -Anterograde transport -ON axons number -ON microglia/macrophages and astrocytes reactivity -ON myelination |
Aranda et al., 2019 |
| Six animals per cage. | 4 d after optic nerve inflammation | -ON NOS-2 and TBARS levels -ON COX-2, IL1β mRNA and TNFα mRNA levels -ON BDNF levels |
||
| Glaucoma in Wistar rats | Cage dimensions: 46.5 × 78 × 95 cm3. Food hoppers, water bottles, running wheels, tubes, ramps, and differently shaped objects (balls, ropes, stones) were repositioned once a day and fully substituted once a week. | 10 wk after first CS-intracameral injection | -Visual function (VEPs) -Anterograde transport -RGCs number -Retinal microglia/macrophages reactivity -ON axons number -ON microglia/macrophages and astrocytes reactivity -ON myelination |
Gonzalez Fleitas et al., 2020 |
| Six animals per cage. | 6 wk after first CS-intracameral injection | -Retinal BDNF levels |
BDNF: Brain-derived neurotrophic factor; B-R: blood-retina; CNTF: ciliary neurotrophic factor; COX-2: cyclooxygenase-2; CS: chondroitin sulfate; EE: entriched environment; ERG, electroretinogram; GCL: ganglion cell layer; GFAP: glial fibrillary acidic protein; IL-1β: interleukin-1β; IPL: inner plexiform layer; mTOR: mammalian target of rapamycin; NOS-2: inducible nitric oxide synthase; ON: optic nerve; ONL: outer nuclear layer; PLR: pupil light reflex; RGCs: retinal ganglion cells; TBARS: thiobarbituric acid reactive substances; TNFα: tumor necrosis factor; VEGF: vascular endothelial growth factor; VEPs: visual evoked potentials.
Oxidative Stress in Glaucoma and the Effect of Enriched Environment
Notwithstanding the local antioxidant high levels, the retina is particularly sensitive to oxidative stress due to several factors, such as high oxygen consumption, light exposure, oxidization of polyunsaturated fatty acids, and photoreceptor phagocytosis (Nishimura et al., 2017). Oxidative stress critically impacts the RGC apoptotic pathway and disrupts the antioxidant capacity of surrounding glial cells (Rohowetz et al., 2018). Growing evidence obtained from clinical and experimental studies over the past decade highlights the involvement of oxidative stress in glaucomatous neurodegeneration (Moreno et al., 2004; Pinazo-Durán et al., 2015). Oxidative stress markers and lipid peroxidation products increase in the aqueous humor and the trabecular meshwork from glaucomatous eyes (Nucci et al., 2013; Goyal et al., 2014; Tang et al., 2019). In agreement, the ocular antioxidant defense system decreases in the anterior chamber of patients with advanced glaucoma (Goyal et al., 2014; Liu et al., 2021). We have demonstrated that retinal superoxide dismutase and catalase activities significantly decrease in eyes with chronic ocular hypertension, consistently with a significant increase in retinal lipid peroxidation (Moreno et al., 2004). In addition, experimental glaucoma induced by photocoagulation of the trabecular meshwork, increases superoxide radical levels in the retina and ON head with alterations in antioxidant enzymes (Chidlow et al., 2017). Based on these results, it seems possible that a decrease in antioxidant enzyme activities may surpass RGC resistance against oxidative damage, supporting the involvement of oxidative stress in glaucomatous damage. In this sense, we have demonstrated that exposure to EE prevents lipid peroxidation in experimental optic neuritis (Aranda et al., 2019), and diabetic retinopathy (Dorfman et al., 2014). These results suggest that the exposure to EE is beneficial against oxidative damage to the retina and ON and could potentially prevent oxidative stress in glaucoma.
Excitotoxicity in Glaucoma and the Effect of Enriched Environment
Glutamate is the main excitatory neurotransmitter in the retina. After its release from presynaptic terminals, glutamate binds to postsynaptic receptors, inducing the influx of sodium and calcium and, consequently, membrane depolarization. As a result of several insults, like oxidative stress, inappropriate electrical activity or energy supply, ischemia/reperfusion injury, or decreased neurotrophins levels, glutamate can accumulate and provoke toxicity to retinal neurons (Baudouin et al., 2020). Retinal glutamatergic synapse is surrounded by glial cells, mainly astrocytes and Müller glia that removes extracellular glutamate through specific transporters and metabolizes it by glutamine synthetase to the nontoxic amino acid glutamine (Ishikawa, 2013). It has been shown that excitotoxicity plays a key role in glaucoma-induced RGC degeneration, through the overactivation of both non-NMDA and NMDA receptors (Nucci et al., 2016). Many subtypes of RGCs are highly susceptible to glutamatergic excitotoxicity due to the abundance of ionotropic glutamate receptors (Opere et al., 2018). An elevation of glutamate concentration in the vitreous humor was reported in several species with glaucoma, whereas other authors show no significant increase of this parameter in samples from human or experimental glaucoma (Ishikawa, 2013). Glutamate concentrations in vitreous samples could not be necessarily correlated with the local concentration of glutamate at RGC membrane receptors. For experimental measurement, vitreous humor must be removed using a method that will disturb its state, possibly altering the measured amount of glutamate. Glutamate levels are likely to increase eventually in localized areas of the retina during glaucomatous neurodegeneration (Ishikawa, 2013), although there are no available tools to assess this parameter in vivo. Notwithstanding, the synaptic concentrations of glutamate can be estimated by analyzing the mechanisms regulating retinal glutamate clearance and recycling. It has been suggested that Müller cells may increase RGC susceptibility to stress signals in response to elevated IOP, reducing retinal ability to regulate glutamate homeostasis (Bringmann et al., 2009). In rats subjected to elevated IOP, we have demonstrated that the retinal glutamate/glutamine cycle activity is significantly altered (Moreno et al., 2005b). Due to the lack of extracellular enzymes that degrade glutamate, the responsibility for the maintenance of low glutamate synaptic concentrations is of glutamate transporters. Retinal glutamate uptake significantly decreases in hypertensive eyes (Moreno et al., 2005b), consistently with excitatory amino acid transporters dysfunction (Nucci et al., 2016). In fact, a downregulation of excitatory amino acid transporter type 1 transporter was observed in glaucoma patient Müller cells (Naskar et al., 2000). Müller cells quickly convert glutamate to glutamine, which favors glutamate uptake by these cells versus retinal neurons. Then, glutamine is released from Müller cells as a precursor for glutamate synthesis by neuronal glutaminase. We have shown that ocular hypertension decreases glutamine synthetase activity and increases glutaminase activity, contributing to glutamate production and synaptic glutamate accumulation (Moreno et al., 2005b). Moreover, increased glutamine uptake in hypertensive eyes could potentiate substratum availability for glutamate synthesis, also contributing to glaucomatous RGC loss (Moreno et al., 2005b). These results disclose the relevance of glutamatergic excitotoxicity for retinal alterations in glaucoma. We have analyzed the effect of EE against retinal glutamatergic excitotoxicity in adult rats (Dorfman et al., 2013). EE housing prevents the decrease in retinal glutamate uptake and glutamine synthetase activity induced by ischemia/reperfusion (Dorfman et al., 2013). Moreover, when supraphysiological concentrations of glutamate were intravitreally injected, the exposure to EE protects not only the retinal function and histology, but also reduces the loss of RGCs induced by glutamate (Dorfman et al., 2013). Therefore, the exposure to EE could be an effective strategy against glutamatergic excitotoxicity in different scenarios, eventually including glaucoma.
Glial Response in Glaucoma and the Effect of Enriched Environment
Glial cells are currently considered fundamental players in the progression of glaucomatous neurodegeneration. Microglia and macroglia, comprising astrocytes, Müller cells, and oligodendroglial lineage cells, adopt specific configurations in the visual system, that make each compartment unique and distinctively affected by glaucoma (Williams et al., 2017). Microglial cells take part in neuroinflammatory processes, showing signs of increased reactivity in the retina and ON of human and experimental glaucoma (Rolle et al., 2021). In fact, microglial activation is considered one of the first events in glaucomatous neural damage occurring prior to RGC loss (Bordone et al., 2017; Wei et al., 2019). In experimental glaucoma, increased levels of cytokines and chemokines, such as complement factors, tumor necrosis factor-alpha (TNF-α), and interleukin-6 that amplify the response and promote morphological changes in microglial cells, has been shown (Russo et al., 2016; Rolle et al., 2021). Under pathological conditions, microglia communicates via signaling proteins to Müller cells and astrocytes, which also undergo a pronounced transformation termed “reactive gliosis” (Liddelow et al., 2017). It has been recently reported that blocking the reactive astrocyte phenotype preserves the electrical activity and number of RGCs in a microbead occlusion model of glaucoma, and that an injury is a necessary condition for RGCs to become susceptible to astrocyte-mediated toxicity (Guttenplan et al., 2020). Reactive Müller cells, detected by the upregulation of intermediate filament glial fibrillary acidic protein (GFAP) and extracellular signal-regulated kinase, were identified in glaucoma patients and experimental models (Russo et al., 2016; Bordone et al., 2017). Moreover, subtle and severe re-activity of astrocytes from the ON head is observed during glaucoma, opening the question whether these changes occur before microglial reactivity (Williams et al., 2017). In addition, ON oligodendrocytes, which are essential for axonal maintenance and correct transmission of visual information from the retina to brain targets, are also affected in experimentally induced glaucoma (Son et al., 2010; Bordone et al., 2017).
Consistent data obtained by our group supports EE housing positive effect against glial alterations in the retina and ON in experimental models of optic neuritis and diabetic retinopathy (Dorfman et al., 2014; Aranda et al., 2019). The exposure to EE prevents Müller cells’ reactivity against retinal ischemic damage (Dorfman et al., 2013). Furthermore, EE housing significantly reduces microglial/macrophages reactivity, astrogliosis, and myelin alterations, and avoided the increase in TNFα and interleukin-1β mRNA levels in the proximal region of the ON induced by experimental optic neuritis (Aranda et al., 2019), supporting EE's impact in reducing neuroin-flammation, and glial alterations (Aranda et al., 2019).
Environmental Enrichment and Glaucoma
As already mentioned, oxidative damage, excitotoxicity, decreased neurotrophins, and glial alterations play key roles in glaucoma pathogenesis. Based on the evidence supporting the antioxidant, antiinflammatory, and pro-BDNF properties of EE housing, together with its effect against glutamate toxicity, we have studied the therapeutic effect of exposure to EE in an experimental model of glaucoma (González Fleitas et al., 2020b). For this purpose, male Wistar rats in which one eye received weekly intracameral injections of CS were housed in SE or EE for 10 weeks, as shown in Figure 1. Flash VEPs are clinically used to analyze different ophthalmologic diseases, as an index of the visual function from the retina to the visual cortex. Concomitantly with data from human and experimentally induced glaucoma (Belforte et al., 2010; Hines-Beard et al., 2016; Jha et al., 2017), CS-induced ocular hypertension provokes a decrease in VEPs, which is prevented by EE housing (Figure 1). Evidence supports the axonal compartment as a preference location of glaucomatous alterations, and a misconnection between the retina and its primary central targets has been shown in different glaucoma models (Crish et al., 2010; Bordone et al., 2017), and histological changes and metabolic adaptations add to glaucomatous damage susceptibility (Yang et al., 2018; Cooper et al., 2020).
Figure 1.
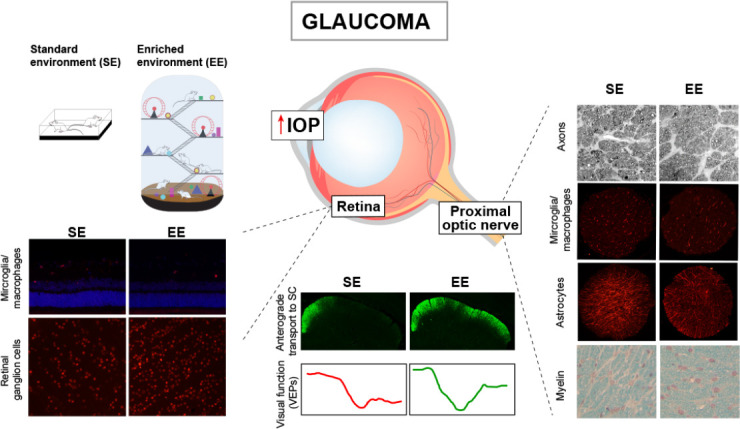
Effect of the enriched environment (EE) on experimental glaucoma.
Wistar rats were submitted to weekly intracameral injections of CS in one eye and vehicle in the contralateral eye, and housed in the standard environment (SE) or EE for 10 weeks. The exposure to EE prevents the alterations induced by chronic ocular hypertension on visual function (assessed by VEP recording), retinal anterograde transport (intravitreal injection of cholera toxin β-subunit), axon number (toluidine blue staining), microglial/macrophages reactivity (ionized calcium-binding adaptor molecule 1-immunoreactivity), astrocytosis (GFAP-immunoreactivity), as well as myelin alterations (Luxol fast blue staining), in the proximal portion of the optic nerve. EE housing also prevents retinal reactive microglia/macrophages and RGCs loss (Brn3a-immunoreactivity) induced by ocular hypertension. CS: Chondroitin sulfate; GFAP: glial fibrillary acidic protein; IOP: intraocular pressure; RGCs: retinal ganglion cells; SC: superior colliculus; VEP: visual evoked potentials.
EE housing avoids the deficit induced by ocular hypertension in the anterograde transport from the retina to its main central targets (i.e., the SC, and the lateral geniculate nucleus), as shown in Figure 1. Based on several reports showing axonal alterations in different regions of the ON in different glaucoma models (i.e., prelaminar, laminar, and proximal post-laminar) (Son et al., 2010; Bordone et al., 2017), the levels of phosphorylated neurofilament heavy chain (PNFH), involved in neuronal morphology, axonal transport, and plasticity, were assessed. The exposure to EE prevents the decrease in PNFH immunoreactivity and the number of axons in hypertensive eyes, which is compatible with the preservation of axonal transport, and VEP amplitude (González Fleitas et al., 2020b).
EE neuroprotection is not exclusively restricted to the axonal compartment in glaucoma, as it significantly impacts on the retina and ON glial reactivity. Ocular hypertension triggers microglial/ macrophage and astrocyte reactivity in the proximal ON that is also prevented by EE (Figure 1). Moreover, EE housing prevents myelination alterations in this region of the ON induced by experimental glaucoma (Bordone et al., 2017; González Fleitas et al., 2020b). At the retinal level, EE lessens microglial/macrophages in the inner retina and avoided central and peripheral RGC loss in hypertensive eyes (Figure 1). These data suggest that the preservation of RGCs could be due to the protection of axons and the decreased microglial reactivity induced by EE both in the ON and the retina. In order to analyze whether EE housing, apart from preventing glaucoma development, is able to reduce glaucomatous damage progression, SE-housed animals with 6 weeks of ocular hypertension (a time-point at which histological and functional alterations are already evident), were transferred to EE (delayed EE). Delayed EE notably reduces functional and structural alterations in the retina and the ON of glaucomatous eyes (González Fleitas et al., 2020b), supporting a therapeutic effect of EE on ongoing glaucomatous damage.
Emerging strategies of neuroprotection in the context of glaucoma focus on neurotrophin-dependent mechanisms on the basis of their well-documented roles in neuronal survival (Kimura et al., 2016). Treatments based on BDNF have gained relevance over the last years because of its powerful neuroprotective function, especially for RGCs (Galindo-Romero et al., 2013). BDNF shows retinal and ON beneficial effects in different injury models, such as light-induced photoreceptor degeneration, optic neuritis, and axotomy, among others (Cerri et al., 2015; Brandli et al., 2016; Aranda et al., 2019), supporting that BDNF could constitute a common protective mechanism against different visual deleterious conditions. BDNF levels and its signaling through TrkB receptors are significantly reduced in the ON head of glaucoma patients (Gupta et al., 2014). Moreover, decreased BDNF levels in the aqueous humor from early human glaucoma have also been reported (Shpak et al., 2018), although another study did not find changes in this parameter (Zhang et al., 2017). In animal models of glaucoma, several studies have shown that BDNF supplementation is successful at slowing the progression of RGC degeneration (Mysona et al., 2017). Nevertheless, the therapeutic use of BDNF in glaucoma is greatly limited by the fact that BDNF does not cross the blood-brain barrier (Kimura et al., 2016). Gene therapies that increase retinal BDNF or its signaling by delivering adenoviruses in the vitreous humor demonstrate promising results against glaucomatous damage (Chitranshi et al., 2019; Wójcik-Gryciuk et al., 2020). However, many of these strategies present translational limitations to human glaucoma due to their grade of invasiveness, as repeated injections are required for sustained effects, and the difficulty in setting doses that do not downregulate TrkB receptor expression. In this line, a better approach would be to locally enhance endogenous BDNF production and signaling. We have demonstrated that a long-term exposure to EE significantly increases BDNF protein content in glaucomatous retinas (González Fleitas et al., 2020b). In addition, EE housing increases ON BDNF levels, and the administration of an antagonist of TrkB receptor abolishes the protective effect of EE in an experimental model of optic neuritis (Aranda et al., 2019), supporting a key role of the BDNF/TrkB receptor pathway in neuroprotection induced by EE. In conclusion, our results support that EE housing preserves visual functions, provides neuroprotection to RGCs and their axons, and avoids glial cell alterations following the exposure to chronic ocular hypertension, probably through a BDNF-dependent mechanism. Notwithstanding, the involvement of the antioxidant, and antiinflammatory effects involved in the EE-induced neuroprotection cannot be ruled out.
Apart from hypertensive glaucoma, there is a condition that shows glaucomatous optic neuropathy without elevation of IOP, termed normal tension glaucoma (NTG). It has been postulated that IOP-independent risk factors, such as vascular dysregulation, higher translaminar pressure gradient due to impaired cerebrospinal fluid dynamics, immune-related disorders, and myopia-related biomechanical factors, play important roles in the development of NTG (Kim and Park, 2016; Trivli et al., 2019; Zhang et al., 2019). In addition, recent studies have shown the possible involvement of oxidative stress, and glutamate neurotoxicity in the pathogenesis of NTG (Trivli et al., 2019; Harada et al., 2020), supporting that there is a close pathogenic link between primary open-angle glaucoma and NTG (Bulut et al., 2016; Zhang et al., 2019). Since EE behaves as antioxidant, and anti-excitotoxic therapy, and is beneficial against a disruption in ocular blood flow (as shown in retinal ischemia/reperfusion), it may be expectable that EE could have a positive impact also against NTG.
New Perspectives: Visual Stimulation for Recovering Vision in Glaucoma?
In contrast to the original concept indicating that the adult retina is less susceptible than cortical areas to experience-dependent plasticity, the already mentioned data highlights the possibility of inducing retinal plasticity even in the adult stage. As already mentioned, EE preserves retinal function and structure not only against experimental glaucoma, but also against retinal ischemia, diabetic retinopathy, and optic neuritis (Dorfman et al., 2013, 2014, 2015; Aranda et al., 2019; González Fleitas et al., 2020b). Furthermore, the previous exposure to EE increases retinal resilience against acute ischemic damage (González Fleitas et al., 2019).
A natural question that emerges is about the translational feasibility of the EE paradigm application to human glaucoma. Although evidence suggests that at least for the brain, the greatest benefits are obtained from the sum or synergic effects of the full repertoire of EE (Faherty et al., 2003; Sozda et al., 2010), one step forward to EE paradigm clinical application is identifying the role of independent EE components (e.g., social, sensory, motor) in eliciting the beneficial effects evoked by the full enriched experience, followed by the design of therapeutic strategies based on the most promising and effective variables (Baroncelli et al., 2012; Sale et al., 2014). In that context, we isolated the components (cognitive, social, and visual) of EE condition, and analyzed their particular effect on the retinal protection against acute ischemic damage in adult rats. For this purpose, animals submitted to unilateral retinal ischemia were exposed to modified environments in order that several components of the usual EE could be separately studied (González Fleitas et al., 2020a). The contribution of the social component was evaluated by housing five animals at a time in standard laboratory cages, and the outcome was compared with animals housed in standard cages containing two animals per cage. The exposure to social environment does not reproduce the neuroprotective effects of EE on retinal function and structure, suggesting that isolated social stimulation does not contribute to the retinal protection against acute retinal ischemia. The cognitive component of EE was tested by using SE cages containing objects that were daily changed for novelty maintenance purposes (novelty environment). It has been demonstrated that the interaction with complex objects apart from social interaction successfully improves functional recovery after brain lesion in rodents (Will and Kelche, 1992). However, in animals housed in a novelty environment no significant retinal protection was observed, suggesting that different mechanisms from those acting in retinal protection could act in brain plasticity. As EE is visually complex compared with standard laboratory housing, we analyzed the contribution of the visual input to EE neuroprotection in animals submitted to bilateral or unilateral retinal ischemia. In animals with bilateral ischemia, EE housing did not induce retinal protection against acute ischemia. As it has been suggested that the neutroprotection achieved by EE can exclusively rely on the physical component of environmental enrichment (Kobilo et al., 2011), we analyzed if bilaterally induced ischemia could affect locomotor activity thus the altered physical activity be the cause of loss of EE-induced retinal neuroprotection. Nevertheless, locomotor activity shows no differences among intact animals, and after unilateral or bilateral ischemia, which demonstrates that the abolishment of EE protective effect in animals bilaterally submitted to ischemia cannot be explained by altered locomotor activity, and that at least visual input in one eye may be a necessary condition for protection induced by EE in the contralateral (ischemic) eye. Thus, we hypothesized that visual stimulation could protect retinal alterations induced by ischemia. In order to analyze the effect of visual stimulation, animals were housed as follows: (i) standard visual environment: cages similar to SE (two animals/cage) surrounded by four PC monitors, which projected a 50% gray image during the total light phase, and (ii) visual environment (VE): cages equal to SE (two animals/cage) surrounded by four PC monitors, which projected a 100% contrast black/white pattern for 6 seconds, before a 50% gray image for 12 seconds, also during the entire light phase. VE achieves a significant retinal functional and structural protection, and preserves RGC number in ischemic retinas, indicating that visual stimulation (but not social interaction or cognitive stimulation) reproduces EE protective effect. How visual stimulation is able to avoid damage against retinal ischemia is still a matter of debate. In agreement with BDNF role in the visual pathway protection induced by EE, it is possible that a BDNF/TrkB-dependent mechanism is involved in VE, since VE increases retinal BDNF levels in ischemic eyes, and administrating a TrkB receptor antagonist prevents its protective effect against ischemia. Taken together, our results support visual stimulation as a plausible potent stimulus achieving retinal protection in adult rats, possibly through a mechanism related to BDNF/TrkB. In line with our results, high-contrast visual stimulation induces axonal regeneration after ON crush due to increased RGCs activity (Lim et al., 2016). Although a similar visual stimulation protocol was used in the report by Lim and collaborators, the authors show an enhanced axonal regeneration with biased visual stimulation to the lesioned nerve through the occlusion of the intact eye, combined with the induction of the mammalian target of rapamycin pathway. Differences between the effect of visual stimulation to the non-lesioned or lesioned pathway to induce retinal or ON neuroprotection could be explained by the lesion site and the pathogenic mechanisms involved in each experimental model.
Since acute retinal ischemic damage shares some of the pathogenic mechanisms involved in chronic glaucoma, it is tempting to speculate that visual stimulation could also protect the retina and ON against glaucomatous injury, a hypothesis that is currently under investigation. There is evidence in humans showing that experimental paradigms analogous to EE, such as playing video games or being trained in perceptual learning tasks, can be quite successful in eliciting amblyopia recovery in adult subjects (Foss, 2017). Although amblyopia involves an alteration that mainly impacts on the visual cortex, whereas glaucoma primarily affects retinal and ON cells, it seems that visual stimulation could be a rehabilitative therapy for retinal disorders in adulthood that eventually may translate to the human condition, averting glaucoma-induced dreaded sequelae that result in permanent visual disability.
One of the many complications of glaucoma is its chronicity, which implies that treatments (of any kind) should be considered in the long-term. For mimicking enriched environments (as described for rodents), glaucomatous patients could be advised to change several aspects of their lifestyle, such as increasing social interactions, physical exercise, and cognitive challenges. In the case of visual overstimulation, a very frequent application (e.g., playing video games once or twice a day) would be useful. In this way, preservation of the visual functions in glaucomatous patients through an intensified use of the sensory and motor systems, and/or social interactions could be a novel, harmless, and non-invasive strategy to promote visual recovery.
Footnotes
Conflicts of interest: None.
Financial support: This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica, Nos. PICT 1563 and PICT 2731 (to RER); The University of Buenos Aires, No. 20020100100678 (to RER); Consejo Nacional de Investigaciones Científicas y Técnicas, No. PIP 0707 (to RER), Argentina.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica, Nos. PICT 1563 and PICT 2731 (to RER); The University of Buenos Aires, No. 20020100100678 (to RER); Consejo Nacional de Investigaciones Científicas y Técnicas, No. PIP 0707 (to RER), Argentina.
C-Editors: Zhao M, Wang L; T-Editor: Jia Y.
References
- 1.Aranda ML, González Fleitas MF, Dieguez HH, Milne GA, Devouassoux JD, Keller Sarmiento MI, Chianelli M, Sande PH, Dorfman D, Rosenstein RE. Therapeutic benefit of environmental enrichment on optic neuritis. Neuropharmacology. 2019;145:87–98. doi: 10.1016/j.neuropharm.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Baroncelli L, Bonaccorsi J, Milanese M, Bonifacino T, Giribaldi F, Manno I, Cenni MC, Berardi N, Bonanno G, Maffei L, Sale A. Enriched experience and recovery from amblyopia in adult rats: Impact of motor, social and sensory components. Neuropharmacology. 2012;62:2388–2397. doi: 10.1016/j.neuropharm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Barone I, Novelli E, Piano I, Gargini C, Strettoi E. Environmental enrichment extends photoreceptor survival and visual function in a mouse model of retinitis pigmentosa. PLoS One. 2012;7:e50726. doi: 10.1371/journal.pone.0050726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog Retin Eye. 2020;Res:100916. doi: 10.1016/j.preteyeres.2020.100916. [DOI] [PubMed] [Google Scholar]
- 5.Belforte N, Sande P, de Zavalía N, Knepper PA, Rosenstein RE. Effect of chondroitin sulfate on intraocular pressure in rats. Investig Ophthalmol Vis Sci. 2010;51:5768–5775. doi: 10.1167/iovs.10-5660. [DOI] [PubMed] [Google Scholar]
- 6.Biswas S, Wan KH. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019;97:e331–340. doi: 10.1111/aos.13983. [DOI] [PubMed] [Google Scholar]
- 7.Bordone MP, González Fleitas MF, Pasquini LA, Bosco A, Sande PH, Rosenstein RE, Dorfman D. Involvement of microglia in early axoglial alterations of the optic nerve induced by experimental glaucoma. J Neurochem. 2017;142:323–337. doi: 10.1111/jnc.14070. [DOI] [PubMed] [Google Scholar]
- 8.Brandli A, Johnstone DM, Stone J. Remote ischemic preconditioning protects retinal photoreceptors: Evidence from a rat model of light-induced photoreceptor degeneration. Investig Ophthalmol Vis Sci. 2016;57:5302–5313. doi: 10.1167/iovs.16-19361. [DOI] [PubMed] [Google Scholar]
- 9.Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Müller cell gliosis: Neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Bulut M, Yaman A, Erol MK, Kurtuluş F, Toslak D, Coban DT, Başar EK. Cognitive performance of primary open-angle glaucoma and normal-tension glaucoma patients. Arq Bras Oftalmol. 2016;79:100–104. doi: 10.5935/0004-2749.20160030. [DOI] [PubMed] [Google Scholar]
- 11.Cerri E, Origlia N, Falsini B, Barloscio D, Fabiani C, Sansò M, Ottino S, Giovannini L, Domenici L. Conjunctivally applied BDNF protects photoreceptors from light-induced damage. Transl Vis Sci Technol. 2015;4:1. doi: 10.1167/tvst.4.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesareo M, Ciuffoletti E, Ricci F, Missiroli F, Giuliano MA, Mancino R, Nucci C. Visual disability and quality of life in glaucoma patients. Prog Brain Res. 2015;221:359–374. doi: 10.1016/bs.pbr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Chan KC, Yu Y, Ng SH, Mak HK, Yip YWY, van der Merwe Y, Ren T, Yung JSY, Biswas S, Cao X, Chau Y, Leung CKS. Intracameral injection of a chemically cross-linked hydrogel to study chronic neurodegeneration in glaucoma. Acta Biomater. 2019;94:219–231. doi: 10.1016/j.actbio.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chidlow G, Wood JPM, Casson RJ. Investigations into hypoxia and oxidative stress at the optic nerve head in a rat model of glaucoma. Front Neurosci. 2017;11:478. doi: 10.3389/fnins.2017.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chitranshi N, Dheer Y, Mirzaei M, Wu Y, Salekdeh GH, Abbasi M, Gupta V, Vander Wall R, You Y, Graham SL, Gupta V. Loss of Shp2 rescues BDNF/TrkB signaling and contributes to improved retinal ganglion cell neuroprotection. Mol Ther. 2019;27:424–441. doi: 10.1016/j.ymthe.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consorti A, Sansevero G, Torelli C, Berardi N, Sale A. From basic visual science to neurodevelopmental disorders: The voyage of environmental enrichment-like stimulation. Neural Plast. 2019;2019:5653180. doi: 10.1155/2019/5653180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper ML, Pasini S, Lambert WS, D’Alessandro KB, Yao V, Risner ML, Calkins DJ. Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress. Proc Natl Acad Sci U S A. 2020;117:18810–18821. doi: 10.1073/pnas.2009425117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010;107:5196–5201. doi: 10.1073/pnas.0913141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Zavalía N, Plano SA, Fernandez DC, Lanzani MF, Salido E, Belforte N, Sarmiento MIK, Golombek DA, Rosenstein RE. Effect of experimental glaucoma on the non-image forming visual system. J Neurochem. 2011;117:904–914. doi: 10.1111/j.1471-4159.2011.07260.x. [DOI] [PubMed] [Google Scholar]
- 20.Dorfman D, Aranda ML, González Fleitas MF, Chianelli MS, Fernandez DC, Sande PH, Rosenstein RE. Environmental enrichment protects the retina from early diabetic damage in adult rats. PLoS One. 2014;9:e101829. doi: 10.1371/journal.pone.0101829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorfman D, Aranda ML, Rosenstein RE. Enriched environment protects the optic nerve from early diabetes-induced damage in adult rats. PLoS One. 2015;10:e0136637. doi: 10.1371/journal.pone.0136637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorfman D, Fernandez DC, Chianelli M, Miranda M, Aranda ML, Rosenstein RE. Post-ischemic environmental enrichment protects the retina from ischemic damage in adult rats. Exp Neurol. 2013;240:146–156. doi: 10.1016/j.expneurol.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain Res Dev Brain Res. 2003;141:55–61. doi: 10.1016/s0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes KA, Harder JM, Williams PA, Rausch RL, Kiernan AE, Nair KS, Anderson MG, John SWM, Howell GR, Libby RT. Using genetic mouse models to gain insight into glaucoma: Past results and future possibilities. Exp Eye Res. 2015;141:42–56. doi: 10.1016/j.exer.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes TA, Goldstein EZ, Dupree JL, Jablonska B, Scafidi J, Adams KL, Imamura Y, Hashimoto-Torii K, Gallo V. Environmental enrichment ameliorates perinatal brain injury and promotes functional white matter recovery. Nat Commun. 2020;11:964. doi: 10.1038/s41467-020-14762-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foss AJE. Use of video games for the treatment of amblyopia. Curr Opin Ophthalmol. 2017;28:276–281. doi: 10.1097/ICU.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 27.Foureaux G, Franca JR, Nogueira JC, De Oliveira Fulgêncio G, Ribeiro TG, Castilho RO, Yoshida MI, Fuscaldi LL, Fernandes SOA, Cardoso VN, Cronemberger S, Faraco AAG, Ferreira AJ. Ocular inserts for sustained release of the angiotensin-converting enzyme 2 activator, diminazene aceturate, to treat glaucoma in rats. PLoS One. 2015;10:e0133149. doi: 10.1371/journal.pone.0133149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin TB, Murphy JA, Myers TL, Clarke DB, Currie RW. Enriched environment during adolescence changes brain-derived neurotrophic factor and TrkB levels in the rat visual system but does not offer neuroprotection to retinal ganglion cells following axotomy. Brain Res. 2006;1095:1–11. doi: 10.1016/j.brainres.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Galindo-Romero C, Valiente-Soriano FJ, Jiménez-López M, García-Ayuso D, Villegas-Pérez MP, Vidal-Sanz M, Agudo-Barriuso M. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Investig Ophthalmol Vis Sci. 2013;54:974–985. doi: 10.1167/iovs.12-11207. [DOI] [PubMed] [Google Scholar]
- 30.González Fleitas MF, Aranda ML, Dieguez HH, Devouassoux JD, Chianelli MS, Dorfman D, Rosenstein RE. Pre-ischemic enriched environment increases retinal resilience to acute ischemic damage in adult rats. Exp Eye Res. 2019;178:198–211. doi: 10.1016/j.exer.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 31.González Fleitas MF, Aranda ML, Diéguez HH, Milne G, Langellotti L, Miranda M, Altschuler F, Dorfman D, Rosenstein RE. The “Use It or Lose It” dogma in the retina: visual stimulation promotes protection against retinal ischemia. Mol Neurobiol. 2020a;57:435–449. doi: 10.1007/s12035-019-01715-5. [DOI] [PubMed] [Google Scholar]
- 32.González Fleitas MF, Devouassoux JD, Aranda ML, Calanni JS, Chianelli MS, Dorfman D, Rosenstein RE. Enriched environment provides neuroprotection against experimental glaucoma. J Neurochem. 2020b;152:103–121. doi: 10.1111/jnc.14885. [DOI] [PubMed] [Google Scholar]
- 33.Goyal A, Srivastava A, Sihota R, Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr Eye Res. 2014;39:823–829. doi: 10.3109/02713683.2011.556299. [DOI] [PubMed] [Google Scholar]
- 34.Griñán-Ferré C, Izquierdo V, Otero E, Puigoriol-Illamola D, Corpas R, Sanfeliu C, Ortuño-Sahagún D, Pallàs M. Environmental enrichment improves cognitive deficits, AD hallmarks and epigenetic alterations presented in 5xFAD mouse model. Front Cell Neurosci. 2018;12:224. doi: 10.3389/fncel.2018.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta V, You Y, Li J, Gupta V, Golzan M, Klistorner A, van den Buuse M, Graham S. BDNF impairment is associated with age-related changes in the inner retina and exacerbates experimental glaucoma. Biochim Biophys Acta - Mol Basis Dis. 2014;1842:1567–1578. doi: 10.1016/j.bbadis.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Guttenplan KA, Stafford BK, El-Danaf RN, Adler DI, Münch AE, Weigel MK, Huberman AD, Liddelow SA. Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Rep. 2020;31:107776. doi: 10.1016/j.celrep.2020.107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada C, Noro T, Kimura A, Guo X, Namekata K, Nakano T, Harada T. Suppression of oxidative stress as potential therapeutic approach for normal tension glaucoma. Antioxidants. 2020;9:874. doi: 10.3390/antiox9090874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hines-Beard J, Bond WS, Backstrom JR, Rex TS. Virus-mediated EpoR76E gene therapy preserves vision in a glaucoma model by modulating neuroinflammation and decreasing oxidative stress. J Neuroinflammation. 2016;13:39. doi: 10.1186/s12974-016-0499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui J jie, Zhang Z jun, Liu S shan, Xi G jun, Zhang X rong, Teng GJ, Chan KC, Wu EX, Nie B bin, Shan B ci, Li L jiang, Reynolds GP. Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: A magnetic resonance study. Behav Brain Res. 2011;217:122–127. doi: 10.1016/j.bbr.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Hutson TH, Kathe C, Palmisano I, Bartholdi K, Hervera A, De Virgiliis F, McLachlan E, Zhou L, Kong G, Barraud Q, Danzi MC, Medrano-Fernandez A, Lopez-Atalaya JP, Boutillier AL, Sinha SH, Singh AK, Chaturbedy P, Moon LDF, Kundu TK, Bixby JL, Lemmon VP, Barco A, Courtine G, Di Giovanni S, et al. Cbp-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aaw2064. eaaw2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa M. Abnormalities in glutamate metabolism and excitotoxicity in the retinal diseases. Scientifica (Cairo) 2013;2013:528940. doi: 10.1155/2013/528940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain V, Baitharu I, Prasad D, Ilavazhagan G. Enriched environment prevents hypobaric hypoxia induced memory impairment and neurodegeneration: role of BDNF/PI3K/GSK3β pathway coupled with CREB activation. PLoS One. 2013;8:e62235. doi: 10.1371/journal.pone.0062235. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Jha MK, Thakur D, Limbu N, Badhu BP, Paudel BH. Visual evoked potentials in primary open angle glaucoma. J Neurodegener Dis. 2017;2017:9540609. doi: 10.1155/2017/9540609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kempermann G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat Rev Neurosci. 2019;20:235–245. doi: 10.1038/s41583-019-0120-x. [DOI] [PubMed] [Google Scholar]
- 45.Khatib TZ, Martin KR. Protecting retinal ganglion cells. Eye (Lond) 2017;31:218–224. doi: 10.1038/eye.2016.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KE, Park KH. Update on the prevalence, etiology, diagnosis, and monitoring of normal-tension glaucoma. Asia Pac J Ophthalmol (Phila) 2016;5:23–31. doi: 10.1097/APO.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 47.Kimura A, Namekata K, Guo X, Harada C, Harada T. Neuroprotection, growth factors and BDNF-TRKB signalling in retinal degeneration. Int J Mol Sci. 2016;17:1584. doi: 10.3390/ijms17091584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura A, Namekata K, Guo X, Noro T, Harada C, Harada T. Targeting oxidative stress for treatment of glaucoma and optic neuritis. Oxid Med Cell Longev. 2017;2017:2817252. doi: 10.1155/2017/2817252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiss P, Szabadfi K, Horvath G, Tamas A, Farkas J, Gabriel R, Reglodi D. Gender-dependent effects of enriched environment and social isolation in ischemic retinal lesion in adult rats. Int J Mol Sci. 2013;14:16111–16123. doi: 10.3390/ijms140816111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Prog Neurobiol. 2016;142:45–67. doi: 10.1016/j.pneurobio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kondo M. Molecular mechanisms of experience-dependent structural and functional plasticity in the brain. Anat Sci Int. 2017;92:1–17. doi: 10.1007/s12565-016-0358-6. [DOI] [PubMed] [Google Scholar]
- 53.Landi S, Cenni MC, Maffei L, Berardi N. Environmental enrichment effects on development of retinal ganglion cell dendritic stratification require retinal BDNF. PLoS One. 2007;2:e346. doi: 10.1371/journal.pone.0000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD. Neural activity promotes long-distance, target-specific regeneration ofadult retinal axons. Nat Neurosci. 2016;19:1073–1084. doi: 10.1038/nn.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu A, Wang L, Feng Q, Zhang D, Chen K, Yiming GH, Wang Q, Hong Y, Whelchel A, Zhang X, Li X, Dong L. Low expression of GSTP1 in the aqueous humour of patients with primary open-angle glaucoma. J Cell Mol Med. 2021;25:3063–3079. doi: 10.1111/jcmm.16361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mandolesi L, Gelfo F, Serra L, Montuori S, Polverino A, Curcio G, Sorrentino G. Environmental factors promoting neural plasticity: Insights from animal and human studies. Neural Plast. 2017;2017:7219461. doi: 10.1155/2017/7219461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayordomo-Febrer A, López-Murcia M, Morales-Tatay JM, Monleón-Salvado D, Pinazo-Durán MD. Metabolomics of the aqueous humor in the rat glaucoma model induced by a series of intracamerular sodium hyaluronate injection. Exp Eye Res. 2015;131:84–92. doi: 10.1016/j.exer.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Moreno MC, Aldana Marcos HJ, Croxatto JO, Sande PH, Campanelli J, Jaliffa CO, Benozzi J, Rosenstein RE. A new experimental model of glaucoma in rats through intracameral injections of hyaluronic acid. Exp Eye Res. 2005a;81:71–80. doi: 10.1016/j.exer.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Moreno MC, Campanelli J, Sande P, Sáenz DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Moreno MC, Sande P, Marcos HA, De Zavalía N, Sarmiento MIK, Rosenstein RE. Effect of glaucoma on the retinal glutamate/glutamine cycle activity. FASEB J. 2005b;19:1161–1162. doi: 10.1096/fj.04-3313fje. [DOI] [PubMed] [Google Scholar]
- 62.Mysona BA, Zhao J, Bollinger KE. Role of BDNF/TrkB pathway in the visual system: therapeutic implications for glaucoma. Expert Rev Ophthalmol. 2017;12:69–81. doi: 10.1080/17469899.2017.1259566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naskar R, Vorwerk CK, Dreyer EB. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Investig Ophthalmol Vis Sci. 2000;41:1940–1944. [PubMed] [Google Scholar]
- 64.Nishimura Y, Hara H, Kondo M, Hong S, Matsugi T. Oxidative stress in retinal diseases. Oxid Med Cell Longev. 2017;2017:4076518. doi: 10.1155/2017/4076518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 66.Nucci C, Di Pierro D, Varesi C, Ciuffoletti E, Russo R, Gentile R, Cedrone C, Duran MDP, Coletta M, Mancino R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol Vis. 2013;19:1841–1846. [PMC free article] [PubMed] [Google Scholar]
- 67.Nucci C, Russo R, Martucci A, Giannini C, Garaci F, Floris R, Bagetta G, Morrone LA. New strategies for neuroprotection in glaucoma, a disease that affects the central nervous system. Eur J Pharmacol. 2016;787:119–126. doi: 10.1016/j.ejphar.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 68.Opere CA, Heruye S, Njie-Mbye YF, Ohia SE, Sharif NA. Regulation of excitatory amino acid transmission in the retina: Studies on neuroprotection. J Ocul Pharmacol Ther. 2018;34:107–118. doi: 10.1089/jop.2017.0085. [DOI] [PubMed] [Google Scholar]
- 69.Pang IH, Clark AF. Inducible rodent models of glaucoma. Prog Retin Eye Res. 2020;75:100799. doi: 10.1016/j.preteyeres.2019.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinazo-Durán MD, Zanón-Moreno V, Gallego-Pinazo R, García-Medina JJ. Oxidative stress and mitochondrial failure in the pathogenesis of glaucoma neurodegeneration. Prog Brain Res. 2015;220:127–153. doi: 10.1016/bs.pbr.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Pirhan D, Yüksel N, Emre E, Cengiz A, Kürşat Yildiz D. Riluzole- and resveratrol-induced delay of retinal ganglion cell death in an experimental model of glaucoma. Curr Eye Res. 2016;41:59–69. doi: 10.3109/02713683.2015.1004719. [DOI] [PubMed] [Google Scholar]
- 72.Rohowetz LJ, Kraus JG, Koulen P. Reactive oxygen species-mediated damage of retinal neurons: Drug development targets for therapies of chronic neurodegeneration of the retina. Int J Mol Sci. 2018;19:3362. doi: 10.3390/ijms19113362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rolle T, Ponzetto A, Malinverni L. The role of neuroinflammation in glaucoma: an update on molecular mechanisms and new therapeutic options. Front Neurol. 2021;11:612422. doi: 10.3389/fneur.2020.612422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenzweig MR, Krech D, Bennett EL. A search for relations between brain chemistry and behavior. Psychol Bull. 1960;57:476–492. doi: 10.1037/h0044689. [DOI] [PubMed] [Google Scholar]
- 75.Russo R, Varano GP, Adornetto A, Nucci C, Corasaniti MT, Bagetta G, Morrone LA. Retinal ganglion cell death in glaucoma: exploring the role of neuroinflammation. Eur J Pharmacol. 2016;787:134–142. doi: 10.1016/j.ejphar.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 76.Sale A. A Systematic Look at Environmental Modulation and Its Impact in Brain Development. Trends Neurosci. 2018;41:4–17. doi: 10.1016/j.tins.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Sale A, Berardi N, Maffei L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol Rev. 2014;94:189–234. doi: 10.1152/physrev.00036.2012. [DOI] [PubMed] [Google Scholar]
- 78.Shpak AA, Guekht AB, Druzhkova TA, Kozlova KI, Gulyaeva NV. Brain-derived neurotrophic factor in patients with primary open-angle glaucoma and age-related cataract. Curr Eye Res. 2018;43:224–231. doi: 10.1080/02713683.2017.1396617. [DOI] [PubMed] [Google Scholar]
- 79.Son JL, Soto I, Oglesby E, Lopez-Roca T, Pease ME, Quigley HA, Marsh-Armstrong N. Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 2010;58:780–789. doi: 10.1002/glia.20962. [DOI] [PubMed] [Google Scholar]
- 80.Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szabadfi K, Atlasz T, Horváth G, Kiss P, Hamza L, Farkas J, Tamás A, Lubics A, Gábriel R, Reglodi D. Early postnatal enriched environment decreases retinal degeneration induced by monosodium glutamate treatment in rats. Brain Res. 2009;1259:107–112. doi: 10.1016/j.brainres.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Tang B, Li S, Cao W, Sun X. The association of oxidative stress status with open-angle glaucoma and exfoliation glaucoma: a systematic review and meta-analysis. J Ophthalmol. 2019;2019:1803619. doi: 10.1155/2019/1803619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Trivli A, Koliarakis I, Terzidou C, Goulielmos GN, Siganos CS, Spandidos DA, Dalianis G, Detorakis ET. Normal-tension glaucoma: Pathogenesis and genetics. Exp Ther Med. 2019;17:563–574. doi: 10.3892/etm.2018.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Meng ZX, Chen YZ, Li YP, Zhou HY, Yang M, Zhao TT, Gong YL, Wu Y, Liu T. Enriched environment enhances histone acetylation of NMDA receptor in the hippocampus and improves cognitive dysfunction in aged mice. Neural Regen Res. 2020;15:2327–2334. doi: 10.4103/1673-5374.285005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei X, Cho KS, Thee EF, Jager MJ, Chen DF. Neuroinflammation and microglia in glaucoma: time for a paradigm shift. J Neurosci Res. 2019;97:70–76. doi: 10.1002/jnr.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weinreb RN, Leung CK, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, Martin KR. Primary open-angle glaucoma. Nat Rev Dis Primers. 2016;2:16067. doi: 10.1038/nrdp.2016.67. [DOI] [PubMed] [Google Scholar]
- 89.Will B, Kelche C. Environmental approaches to recovery of function from brain damage: A review of animal studies (1981 to 1991) Adv Exp Med Biol. 1992;325:79–103. doi: 10.1007/978-1-4615-3420-4_5. [DOI] [PubMed] [Google Scholar]
- 90.Williams PA, Marsh-Armstrong N, Howell GR, Bosco A, Danias J, Simon J, Di Polo A, Kuehn MH, Przedborski S, Raff M, Trounce I. Neuroinflammation in glaucoma: A new opportunity. Exp Eye Res. 2017;157:20–27. doi: 10.1016/j.exer.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wójcik-Gryciuk A, Gajewska-Woźniak O, Kordecka K, Boguszewski PM, Waleszczyk W, Skup M. Neuroprotection of retinal ganglion cells with aav2-bdnf pretreatment restoring normal trkb receptor protein levels in glaucoma. Int J Mol Sci. 2020;21:6262. doi: 10.3390/ijms21176262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang XL, van der Merwe Y, Sims J, Parra C, Ho LC, Schuman JS, Wollstein G, Lathrop KL, Chan KC. Age-related changes in eye, brain and visuomotor behavior in the DBA/2J mouse model of chronic glaucoma. Sci Rep. 2018;8:4643. doi: 10.1038/s41598-018-22850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu H, Zhong H, Chen J, Sun J, Huang P, Xu X, Huang S, Zhong Y. Efficacy, drug sensitivity, and safety of a chronic ocular hypertension rat model established using a single intracameral injection of hydrogel into the anterior chamber. Med Sci Monit. 2020;26:e925852. doi: 10.12659/MSM.925852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang HJ, Mi XS, So KF. Normal tension glaucoma: From the brain to the eye or the inverse? Neural Regen Res. 2019;14:1845–1850. doi: 10.4103/1673-5374.259600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Yuan M, Yang S, Chen X, Wu J, Wen M, Yan K, Bi X. Enriched environment improves post-stroke cognitive impairment and inhibits neuroinflammation and oxidative stress by activating Nrf2-ARE pathway. Int J Neurosci. 2020;2020:1–9. doi: 10.1080/00207454.2020.1797722. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Yang Q, Guo F, Chen X, Xie L. Link between neurodegeneration and trabecular meshwork injury in glaucomatous patients. BMC Ophthalmol. 2017;17:223. doi: 10.1186/s12886-017-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


