Abstract
Subarachnoid hemorrhage is a devastating disease with significant mortality and morbidity, despite advances in treating cerebral aneurysms. There has been recent progress in the intensive care management and monitoring of patients with subarachnoid hemorrhage, but the results remain unsatisfactory. Microglia, the resident immune cells of the brain, are increasingly recognized as playing a significant role in neurological diseases, including subarachnoid hemorrhage. In early brain injury following subarachnoid hemorrhage, microglial activation and neuroinflammation have been implicated in the development of disease complications and recovery. To understand the disease processes following subarachnoid hemorrhage, it is important to focus on the modulators of microglial activation and the pro-inflammatory/anti-inflammatory cytokines and chemokines. In this review, we summarize research on the modulators of microglia-mediated inflammation in subarachnoid hemorrhage, including transcriptome changes and the neuroinflammatory signaling pathways. We also describe the latest developments in single-cell transcriptomics for microglia and summarize advances that have been made in the transcriptome-based classification of microglia and the implications for microglial activation and neuroinflammation.
Keywords: activation, inflammation, microglia, modulator, neuroinflammation, sequencing, signal pathway, single-cell analysis, stroke, subarachnoid hemorrhage, treatment
Introduction
Subarachnoid hemorrhage (SAH) is a significant cause of premature death and leads to a loss of potential life years. It occurs at a rate of 7.2–10.5 per 100,000 population and accounts for approximately 5–10% of strokes each year (Cahill and Zhang, 2009; Wong et al., 2010; Rincon et al., 2013). SAH predominantly affects younger adults, usually between the ages of 40 and 60 years. Approximately 35% of patients with SAH do not survive beyond the first 30 days (Bederson et al., 2009; Schertz et al., 2016). Of those who survive, an estimated 50% have long-term disabilities marked by neuropsychological impairments and a decreased quality of life (Taufique et al., 2016).
The pathophysiological processes that follow SAH have been clinically defined as early brain injury (EBI). The main causes of critical EBI are thought to be a robust neuroinflammatory response, cerebral edema, and microvascular dysfunction. Further brain injury can occur in a delayed phase, resulting from persistent neuroinflammation, delayed cerebral vasospasm, and ischemia. At present, the molecular mechanisms underlying EBI are little understood owing to their complexity (Suzuki, 2015; Suzuki et al., 2017). Concerning treatments, it has been shown that although targeted drugs can significantly reduce vasospasm, the clinical outcomes of SAH patients remain unchanged, as was recently described in a meta-analysis of 14 studies involving 4235 patients (Etminan et al., 2011). However, there are interventions that can improve outcomes, as have been shown in worldwide studies of neuro-intensive care and the multidisciplinary management of SAH (Macdonald and Schweizer, 2017). A promising approach for more effective interventions involves targeting the neuroinflammation that follows SAH. It is therefore of much interest to explore this further, even though the exact nature of EBI following SAH remains incompletely understood.
Microglia are important mediators of neuroinflammation; they are parenchymal macrophages in the brain and spinal cord that make up 10–15% of the glial cell population. They are implicated in various neurological diseases, such as Alzheimer's disease (AD), amyotrophic lateral sclerosis, brain tumor and stroke (Prinz et al., 2011; Colonna and Butovsky, 2017). Microglia are key immune cells that respond to various acute brain injuries, including SAH (Lan et al., 2011; Gris et al., 2019; Zheng et al., 2020). Neuroinflammation-related microglial phenotypes have been classified as “resting’’, ‘‘M1’’ (proinflammatory), and ‘‘M2’’ (anti-inflammatory). Recent evidence suggests that microglia-mediated neuroinflammation plays an important role in SAH injury expansion and brain damage (Zheng et al., 2020). Two recent clinical studies have shown that microglia accumulate and become activated in the human brain parenchyma and cerebrospinal fluid (CSF), following aneurysmal SAH (Schneider et al., 2015; Roa et al., 2020). Some studies have examined the signaling pathways involved in microglial activation and neuroinflammation, and an experimental model of SAH has shown that treatment strategies that modulate these pathways can improve outcomes (Li et al., 2018; Peng et al., 2019; Zheng et al., 2020). These findings imply that research into microglial activation, and function transcription, and cellular interactions is crucial for deciphering the pathophysiology of SAH. At present, two clinical trials are being conducted to test interleukin-1 receptor antagonists as a treatment for SAH (Gaastra et al., 2017; Galea et al., 2018). In addition, the recent development of single-cell analysis technology has the potential to improve the classification of microglia and provide further information concerning their activation (Hammond et al., 2019; Li et al., 2019a). These new areas of research could improve the understanding and treatment of neuroinflammation in SAH.
In this review, we analyze the latest progress in single-cell research concerning the transcriptional heterogeneity of microglia (Figure 1). In addition, we review studies on the modulators of microglia-mediated neuroinflammation following SAH, including both experimental SAH and clinical research (Table 1). Finally, we discuss the limitations of current research and the challenges of translational SAH research.
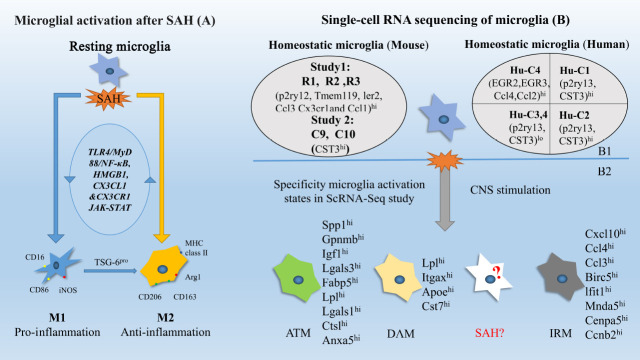
Figure 1.
Microglial activation states.
The left part shows the activation of microglia after subarachnoid hemorrhage (SAH) (A). M1 (Marker CD16, CD86; iNOS) have been observed to have the proinflammatory effect and M2 (Marker CD206, CD163, Arg1 and MHC class II) have an anti-inflammatory effect by interacting with signal pathway TLR4/MyD88/NF-κB, HMGB1, CX3CL1 &CX3CR1 and JAK-STAT. The right part (B) shows the latest results of single-cell RNA sequencing study on transcriptional states of homeostatic microglia (B1) and activated microglia (B2). Two to three clusters of resting microglia in mice were found and with an expression of homeostatic genes. Healthy human microglia were found to have four clusters: Human Cluster 1 (Hu-C1) to human cluster 4 (Hu-C4). Each microglia sub-state has its own up-regulated gene and some clusters have several similar characteristics. The activated microglia have also been analyzed in a single-cell study. DAM was a subcluster of activated microglia found in Alzheimer's disease mouse model. Specialized Axon Tract-Associated Microglia (ATM) were observed in the developmental mouse brain. IRM is another subtype of activated microglia observed in lysolecithin induced animal model. DAM, ATM and IRM all shared a common transcriptional signature of 12 core genes including Spp1, Lpl, and Apoe. There were also differential gene expressions among them. However, microglia transcription heterogeneity leaves unknown in the SAH state.
Table 1.
Modulators of microglia mediated inflammation after SAH in preclinical and clinical study
| Study number | Modulators and function | Study mode |
|---|---|---|
| TLR4/MyD88/NF-κB signal pathway in preclinical study | ||
| Wang et al., 2015 | Baicalein/supressing of TLR4, Myd88 and NF-κB/neuroprotection in 24 and 72 h | SAH rat model (BJ) |
| Zhou et al., 2015 | Fisetin/suppressing TLR4/NF-κB/neuroprotection 24 and 72 h in SAH | SAH rat model (BJ) |
| Lu et al., 2018 | Peroxiredoxin 2 could interact with TLR on microglia in SAH/positively correlated with Hunt-Hess grades in patients/pro-inflammatory at 24 h after SAH | SAH cell model and SAH patients |
| Nishikawa et al., 2018b | MCP (anti-galectin-3) could bind to TLR4 and activate ERK1/2, STAT-3 then have a neuroprotective effect 24 h post SAH | SAH mouse model (EVP) |
| Yin et al., 2018 | Dexmedetomidine could suppress the activation of the TLR4/NF-κB pathway/neuroprotection at 24 h post SAH | SAH rat model (EVP) |
| Gao et al., 2019 | Curcumin/supressing of TLR4, Myd88 and NF-κB at 24 h post SAH/neuroprotection | SAH mouse model (BJ) |
| Zhang et al., 2019 | Astaxanthin could inhibit the TLR4 activation and increase sirtuin 1 levels/reduce pro-inflammatory in EBI | SAH mouse and rat model (BJ) |
| JAK-STAT signal pathway in preclinical study | ||
| Osuka et al., 2006 | SAH produced the cytokine IL-6, activated the JAK-STAT signaling pathway/pro-inflammatory | SAH rat model (BJ) |
| Chen et al., 2009 | rhEPO could activate AK2 and STAT3 in the basilar artery and neuroprotection at 5 d after SAH | SAH rabbit model (BJ) |
| An et al., 2018 | AG490 inhibited JAK2/STAT3 phosphorylation and suppressed the expression and translocation of HMGB1 in EBI | SAH rat model (EVP) |
| Nishikawa et al., 2018b | MCP (anti-galectin-3) could bind to TLR4 and activate ERK1/2, STAT-3 then have a neuroprotection effect 24 h post SAH | SAH mouse model (EVP) |
| Li et al., 2019b | Melatonin attenuatesd EBI via the JAK1/STAT3 signaling pathway/neuroprotection effect at 24 h post SAH | SAH rat model (BJ) |
| HMGB1 signal pathway in preclinical study | ||
| Chang et al., 2014 | Purpurogallin/inhibited HMGB1 and induced vasospasm | SAH rat model (BJ) |
| Lee et al., 2014 | Inflachromene can binding HMGB1& 2 inhibit microglial downregulates proinflammatory | Phenotypic screening |
| Chang et al., 2015 | 4’-O-β-D-glucosyl-5-O-methylvisamminol/inhibited HMGB1 and induced vasospasm | SAH rat model (BJ) |
| Chang et al., 2016 | Rhinacanthin-C/inhibited HMGB1 and induced brain apoptosis | SAH rat model (BJ) |
| Haruma et al., 2016 | mAb could suppress HMGB1 and microglia activation in EBI | SAH rat model (BJ) |
| An et al., 2018 | AG490 inhibited JAK2/STAT3 phosphorylation and suppressed the expression and translocation of HMGB1 in EBI | SAH rat model (EVP) |
| Others mediators in preclinical study | ||
| Huang et al., 2017 | peli1/inhibited cytokines in microglia | SAH mouse model (EVP) |
| Xie et al., 2017 | Albumin/inhibited microglia action | SAH rat model (EVP) |
| Li et al., 2018 | TSG 6/promoted microglial M2 polarization and partially regulated the SOCS3/STAT3 pathway | SAH rat model (EVP) |
| Modulators of neuro-inflammation in clinical study | ||
| Munakata et al., 2009 | Edaravone/free radical scavenger/91 patients (49/42)/1 center | Treatment group outcome increase |
| Vergouwen et al., 2009 | Simvastatin/32 patients (16/16)/1 center | No significant difference between simvastatin group and placebo or low dose simvastatin group |
| Wong et al., 2015 | Simvastatin/255 patients (131/124)/6 centers | |
| Galea et al., 2018 | IL-1Ra/Inhibited IL-1/136 patients (68 drug-treated/68 control)/3 centers | Safe and well tolerated, no significant difference of outcome |
| Nishikawa et al., 2018a | Galectin-3/plasma galectin-3 with a cutoff value of 3.30 or 3.48 ng/mL in EBI predicted poor outcome of SAH patients/83 patients/1 center | SAH patients (non-severe aneurysmal subarachnoid hemorrhage) |
BJ: Blood injection; EBI: early brain injury; EVP: endovascular perforation; HMGB1: high mobility group box 1; IL-1: interleukin-1; IL-6: interleukin-6; IL-1Ra: interleukin-1 receptor antagonist; JAK-STAT: Janus kinase-signal transducer and signal transducer and activator of transcription; M2: phenotype 2 microglia; mAb: anti-HMGB1 monoclonal antibody; MCP: modified citrus pectin; rhEPO: recombinant human erythropoietin; SAH: subarachnoid hemorrhage; SOCS3: suppressor of cytokine signaling-3; TLR4/MyD88/NF-κB: Toll-like receptor 4/MyD88/nuclear factor-kappa B; TSG-6: tumor-specific glycoprotein-6.
Search Strategy and Selection Criteria
Articles were obtained through a literature search in PubMed on 25 April, 2021. These included review articles, original articles, meta-analyses, and clinical trials. For the first search, 47 articles were identified using the following search terms: (microglia [MeSH term]) AND (analysis, single cell [MeSH terms]) AND ((fft [Filter]) AND (2015:2020 [pdat])); we excluded six review articles, four articles on non-parenchymal microglia, and 22 articles that did not fit in with our review. For the second search, 91 articles were identified using the search terms: ((brain inflammation [MeSH term]) OR (microglia [MeSH term])) AND (hemorrhage, intracranial subarachnoid [MeSH terms]) AND ((fft[Filter]) AND (2010:2020[pdat])); we excluded seven review articles and 33 articles that did not fit in with our review. Finally, we selected a further 29 articles related to our review.
Homeostatic and Activated Microglia in Single-Cell Studies
Microglia are derived from the embryonic yolk sac and reside in the brain parenchyma (Ginhoux et al., 2010; Ajami et al., 2011). Morphologically, homeostatic microglia are highly ramified. They are involved in the development of the central nervous system (CNS), and they help to maintain tissue homeostasis by supporting neuronal survival, cell death, and synaptogenesis (Nayak et al., 2014). Activated microglia can adopt polarized phenotypes: either the classically-activated state (M1 phenotype; proinflammatory; specific markers: CD16, CD86, and iNOS), or the alternatively-activated state (M2 phenotype; anti-inflammatory; specific markers: CD206, CD 163, and Arg1) (Zheng and Wong, 2019). Although this is a simplified classification that can only be observed in vitro, it is helpful for understanding the role that microglia play in pathophysiological processes (Tang and Le, 2016; Xiong et al., 2016). It is known that when the M1 phenotype is activated using lipopolysaccharide and pro-inflammatory cytokine interferon-γ, pro-inflammatory cytokines are released, such as tumor necrosis factor-α and interleukin-6 (IL-6); when the alternative M2 phenotype is activated using IL-4 or IL-13, anti-inflammatory factors are expressed, such as transforming growth factor beta and IL-10 (Durafourt et al., 2012; Hu et al., 2015; Orihuela et al., 2016). However, the specific functions of the different M1 and M2 subtypes and their interactions with other cells, such as neurons, have not been fully elucidated. In addition, the transcriptional state of macrophages is complex with at least nine different states (Xue et al., 2014), although some encouraging results are gradually beginning to emerge from research on the microglia transcriptome.
Progress in understanding the transcriptional heterogeneity of microglia in the single-cell era
Recent single-cell research has led to improvements in the classification of microglia on the basis of the transcriptome. In these studies, microglia have been used from the mouse brain at different developmental stages, including the embryonic, adult, and old-age stages (Hammond et al., 2019; Li et al., 2019a). Compared with previous studies, it was found six to nine clusters at all of the different developmental stages, and similar transcriptomes for different adult mouse brains. Using single cell RNA-sequencing, it has been found that homeostatic microglia can be divided into 2–3 clusters (Li et al., 2019a; Masuda et al., 2020). In adult mice, the pool of microglia did not demonstrate region-specific subclasses, which highlighted the homogeneity between the different brain regions, a finding that differed from previous bulk RNA-sequencing studies (Keren-Shaul et al., 2017; Hammond et al., 2019; Li et al., 2019a; Masuda et al., 2020). In addition, recent single-nucleus RNA-sequencing of striatal and cerebellar microglia from adult mice have revealed the epigenetic regulation of the clearance activity of cerebellar microglia (Masuda et al., 2019). The low sensitivity of single-nucleus RNA-sequencing for detecting gene activation means that it is not suitable for detecting microglial activation in human disease (Thrupp et al., 2020). However, these studies provided important new information about the gene transcription of resting microglia in mice.
A small number of RNA-sequencing studies have examined microglia in healthy human brain tissue. In one study, four clusters of microglia were identified, which were named healthy human clusters one to four (HHu-C1 to HHu-C4) (Masuda et al., 2019). Detailed analyses of the differentially-regulated genes revealed some similarities to the gene expression profiles seen in the mouse homeostatic microglia. For instance, CST3 was more highly expressed in HHu-C1 and HHu-C2 than in HHu-C3 and HHu-C4; CST3 was more highly expressed in mouse microglia clusters C9 and C10. Ccl4 mRNA was rarely expressed in the mouse resting microglia but increased in the activated microglia clusters (Masuda et al., 2019, 2020). In addition, P2RY13 mRNA was highly expressed in the HHu-C1 and HHu-C2 clusters; P2RY12 mRNA was highly expressed in the mouse resting microglia (Li et al., 2019a; Masuda et al., 2019). These studies show that human homeostatic microglia have distinct gene expression patterns that partially overlap with those found for adult mouse microglia. There is therefore some degree of homogeneity between human and mouse microglia, thus suggesting the possibility of using a mouse model.
Different microglial transcriptional states have been found in diseases of the CNS. In the case of neurodegenerative diseases, a mouse model simulating multiple sclerosis (MS), with focal demyelination of the subcortical white matter through injection with lysolecithin (Hammond et al., 2019), has enabled the identification of microglia that have suppressed expression of P2ry12 and Cx3cr1. These have been named injury-responsive microglia (IRM), and they have also been observed in other CNS injuries and diseases (Keren-Shaul et al., 2017; Saunders et al., 2018). Single-cell RNA-sequencing has also been carried out for 5 patients with histologically-confirmed early active MS to investigate the classification of the microglia (Masuda et al., 2019). Seven myeloid clusters were found (Hu-C2 to Hu-C8) that express microglial core genes, including three homeostatic microglia clusters and three activated microglia clusters (note that these have the same marker gene with demyelination (C12) and re-myelination (C13) in mice) (Masuda et al., 2019). Recent research has identified several transcriptional states in microglia that relate to different diseases. A subcluster of activated microglia has been found in an AD mouse model, called the disease associated microglia (DAM) (Keren-Shaul et al., 2017) specialized axon tract-associated microglia (ATM) have been observed in the developing mouse brain (Hammond et al., 2019) and IRM have been observed in an animal model induced by lipopolysaccharide which usually used to causes MS lesion (Hammond et al., 2019). DAM, ATM, and IRM all share a common transcriptional signature of 12 core genes, including Spp1, Lpl, and Apoe, but they also have differential gene expression (Hammond et al., 2019). These studies show the transcriptional heterogeneity of microglia from the resting state to the active state, and the diversity of microglial responses to diseases of the CNS. The fact that some of the clusters identified for different CNS diseases expressed some of the same genes indicates that they may be functional genes that are involved in the pathophysiological mechanisms underlying CNS diseases. Further work is needed to explore the exact functions of these genes and the specific clusters found. It remains to be determined how the expression of these genes affects the clinical symptoms, and more work is needed to further unravel the underlying genes and signaling pathways.
The identification of transcriptional signatures can lead to an improved understanding of the role of microglia in the resting and disease states. Because microglia can respond in diverse ways to CNS diseases, the activation of microglia following SAH could lead to different transcriptional characteristics. In some clinical studies, it has been found that APOE gene polymorphism relates to the prognosis of SAH, and a high level of a CCL3 genotype has been observed in aneurysmal SAH (Kay et al., 2003; Aoki et al., 2019). Further studies are needed to clarify the gene expression of microglia in SAH. At present, endovascular treatment is used for most ruptured cerebral aneurysms, which may account for the fact that there are no single-cell data for microglia in the pathophysiological processes following SAH in humans or even in animals. The single-cell analysis of microglia following SAH could provide a key to understanding SAH-related pathologies and could lead to the development of effective SAH treatments in future translational research (Coulibaly and Provencio, 2020).
Studies of Microglial Activation in Subarachnoid Hemorrhage
Microglial polarization is thought to occur in SAH and other CNS diseases. In animal studies of spinal cord injury, activated microglia in the early stages have been found to exhibit mainly the M1 phenotype (Kigerl et al., 2009; Kroner et al., 2014). In studies using experimental models of Traumatic brain injury (TBI) and ischemic stroke, M2-to-M1 phenotype shifts have been observed (Wang et al., 2013; Kumar et al., 2016; Perego et al., 2016). Recent studies of SAH in mice and rats have found dynamic changes in microglial polarization from the M1 to the M2 phenotype, along with morphological transformation from the ramified to the amoeboid shape (Li et al., 2018; Peng et al., 2019; Zheng et al., 2020). In these latter studies, the microglia mainly appeared as ramified (M1 phenotype) on days 1–3 following SAH; in the delayed phase, there were bipolar microglia that expressed both M1 and M2 markers and had a spindle shape; then on days 5–10, the morphology of the microglia transformed to an amoeboid shape, with high expression of CD206 and a M2 phenotype, reflecting an activated status (Zheng et al., 2020). Bipolar-shaped microglia have also been observed in an animal model of epilepsy (Benson et al., 2015). It is relevant to note that these animal experiments are of great importance to SAH research; they represent the most widely used method in SAH neuroinflammation research and are important for understanding the pathophysiological processes involved in SAH microglial activation. Most previous studies have focused on cellular functions in the EBI stage following SAH or the delayed effects on cognition; in this article, we mainly focus on the EBI stage following SAH (Coulibaly and Provencio, 2020).
Previous reports have shown microglial activation in the brain tissue of SAH patients (Lu et al., 2018). Peroxiredoxin 2 (Prx2) is known to activate microglia and has been found in the CSF of patients with SAH; the level of Prx2 in these patients has been found to positively correlate with their neurological status (Lu et al., 2018). Patients with SAH have also been found to have higher microglial heme oxygenase 1 (HO-1) activity in the CSF (Schallner et al., 2015). Following SAH, microglia have been found to exhibit an activated phenotype with a macrophage-like morphology, high levels of CD45 and major histocompatibility complex (MHC) class II, and decreased levels of CX3CL1 and CX3CR1 (Chen et al., 2020). These changes have also been found for microglial activation in the brain tissue of rats (Chen et al., 2020).
Gene co-expression in the microglia of humans and mice represents a promising area for future translational research on SAH. Specifically, clusters that share the same gene expression may lead to new therapeutic developments. However, the differences between human and mouse microglia need to be further evaluated for this work to advance. It would also be of benefit to carry out further immunohistochemical studies using animal models of SAH and to obtain biomarker information relating to microglia-mediated inflammation in SAH patients. In addition, it will be important to further investigate changes in the function and transcriptional heterogeneity of microglia subclusters, as this has the potential to guide the development of modulators of microglial activation, as well as anti-inflammatory drugs for patients with SAH. Finally, because the specific mechanisms and functions of microglial activation are not completely understood, further research is urgently needed to decipher the underlying patterns and pathways.
Modulators of Microglial Activation and Polarization in Subarachnoid Hemorrhage
The microglia-mediated neuroinflammation that follows SAH involves signaling pathways and transcription factors that are not well mapped out. It is important to determine how various factors can affect microglial activation and early brain injury in order to develop effective treatments for SAH. In this section, we summarize the modulators of signaling pathways that are involved in microglial activation (Table 1).
Modulators of the Toll-like receptor signaling pathway
Modulators of the Toll-like receptor (TLR) is a key component of the innate immune system and is widely expressed in the CNS, being found in microglia, neurons, astrocytes, smooth muscle cells of the cerebral arteries, and peripheral blood cells (Buchanan et al., 2010). Within the TLR family, TLR4 is unique because it can signal through both the myeloid differentiation primary response 88 protein (MyD88) and the TRIF pathways. This enables it to coordinate the maximal inflammatory response in SAH (O’Neill and Bowie, 2007). It has been found that TLR4 expression increases in response to SAH in EBI. In addition, the TLR4/nuclear factor-kappaB (NF-κB) signaling pathway has been identified as playing a major role in the microglial activation and neuroinflammation seen in EBI (Liu et al., 2018; Lu et al., 2018). In one study, TLR4 knockout mice with a cisternal blood injection were used as a model of SAH. It was found that during EBI, neuronal apoptosis occurred that was mainly caused by the TLR4-MyD88 and TLR4-TRIF signaling pathways (Hanafy, 2013). In another study, Zhang et al. (2019) also used a rodent model of SAH through prechiasmatic cistern injection. They found that treatment with astaxanthin significantly inhibited the activation of TLR4, increased the expression of sirtuin 1, and inhibited the inflammatory response. Astaxanthin was found to reduce neuronal death, but it did not improve neurological function in the TLR4 knockout mice. In a study on patients with SAH, Lu et al. (2018) found that the levels of peroxiredoxin 2 positively correlated with the Hunt-Hess grades, which might have been due to microglial activation via the TLR4/MyD88/NF-κB signaling pathway. In a rat model of SAH, dexmedetomidine was also found to reduce the neutrophil infiltration, microglial activation, pro-inflammatory factor release, and cell apoptosis (at 24 hours after SAH) by suppressing the TLR4/NF-κB pathway and the NLRP3 inflammasome (Yin et al., 2018). Other potential modulators of neuroinflammation via the TLR4/NF-κB signaling pathway include Fisetin (a natural, neuroprotective flavonoid) (Zhou et al., 2015), Curcumin (a natural phytochemical compound, which is anti-inflammatory and can induce the transformation of microglial phenotypes)(Gao et al., 2019), and Baicalein (a neuroprotective flavonoid) (Wang et al., 2015). However, at present there are no clinical data available relating to their use in SAH.
Modulators of the Janus kinase-signal transducer and signal transducer and activator of transcription
The Janus kinase-signal transducer and signal transducer and activator of transcription (JAK-STAT) is an important signaling pathway. It responds to cytokines and transfers signals from the cell surface to the nucleus. Type I and type II interferons can activate the JAK-STAT signaling pathway and thus regulate cellular proliferation, apoptosis, and inflammation (Kim et al., 2002; Arimoto et al., 2017). The JAK/STAT3 signaling pathway is widely expressed throughout the entire brain. Samraj et al. (2014) found that unphosphorylated STAT3 directly binds to DNA and affects the genes that are involved in the neuroinflammation and delayed cerebral ischemia (DCI) seen in SAH. Using a rat model of SAH, it has been shown that IL-6 is produced in the CSF within two hours following SAH; this activates the JAK-STAT signaling pathway in the basilar artery one to two days following SAH (Osuka et al., 2006). It has also been found that the JAK/STAT3 pathway is activated and upregulated within 24 hours following SAH, leading to increased expression of pro-inflammatory cytokines. Persistent activation of STAT3 leads to the production of suppressor of cytokine signaling-3 (SOCS3), a feedback inhibitor that limits the excessive release of cytokines. An in vitro study using SOCS3-deficient mice found that STAT3 activation and proinflammatory cytokine signaling led to microglial polarization to the M1 phenotype. This was associated with increased expression of iNOS, IL-1β, IL-12 p40, IL-23 p9, IL-6, CC-motif chemokine 2 (CCL2), and CXCL10 (Qin et al., 2012). In another study, it was found that recombinant human erythropoietin activated the JAK2/STAT3 pathway in the basilar artery and decreased the apoptosis index of endothelial cells following SAH (Chen et al., 2009). A study by Li et al. (2019b) also found that melatonin activated the JAK1/STAT3 signaling pathway, improved neurological function, and reduced neuronal apoptosis and brain edema in EBI following SAH. It has been found that AG490 significantly inhibits JAK2/STAT3 phosphorylation; it also suppresses the expression and translocation of High mobility group box 1 (HMGB1) and reduces cortical apoptosis, brain edema, and neurological deficits in SAH animal model (An et al., 2018). It will be important to further investigate the interactions between these modulators and the JAK/STAT proteins in SAH.
It has been shown that the plasma matricellular protein galectin-3 increases after aneurysmal SAH, but the exact function of this protein is still unclear (Jayakumar et al., 2017). In a clinical setting, higher plasma galectin-3 levels at admission have been found to correlate significantly with more severe SAH, as well as poorer outcomes at 6 months (Liu et al., 2016). In these patients with aneurysmal SAH, higher plasma galectin-3 from day 1 to day 3 was seen to relate to DCI but not to vasospasm (Nishikawa et al., 2018a). Galectin-3 is widely expressed in microglia, astrocytes, and oligodendrocytes, and it controls the progression and resolution of CNS inflammation (Pasquini et al., 2011). There is evidence that Galectin-3 is expressed by active microglia interacting with pro-inflammatory stimuli and that it participates in brain immune responses (Yip et al., 2017; Nishikawa and Suzuki, 2018; Venkatraman et al., 2018). Galectin-3 has also been found to participate in post-ischemic repair involving angiogenesis and the migration of microglia; there is evidence that this occurs through integrin-linked kinase signaling pathways (Wesley et al., 2013). Nishikawa et al. (2018b) found that modified citrus pectin, a galectin-3 inhibitor, could prevent disruption to the blood-brain barrier following SAH. This was found to involve modified citrus pectin binding to TLR4 and activating ERK1/2, STAT-3, and MMP-9, and thus provided protection against EBI following SAH. This neuroprotective effect is promising and should be studied further. In another study, Jeon et al. (2010) found that galectin-3 mediates neuroinflammation by activating the JAK/STAT and NF-κB pathways. Such signaling pathways, specifically JAK/STAT and TLR4, are enriched in various brain cells. In the future, knockout animals with an inactivated microglia-specific gene, such as the microglia-specific STAT3 knockout mice, could be used to clarify the connection between microglial signaling pathways and the neuroinflammation that occurs following SAH.
Modulators of HMGB1 and other mediators of inflammation
High mobility group protein B1 (HMG1) is a DNA-binding protein that regulates gene transcription (Kim et al., 2006; Lee et al., 2014). HMG1 also has proinflammatory functions, which can be downregulated using inflachromene. This anti-inflammatory agent has been found to suppress microglia-mediated neuroinflammation, as shown in a study by Lee et al. (2014), which might provide neuroprotection against EBI. It has been found that anti-HMGB1 antibodies can significantly downregulate the expression of TLR4, IL-6, tumor necrosis factor-α, and iNOS, and reverse basilar artery vasospasm (Haruma et al., 2016). Modulators of HMGB1 have also been identified that downregulate M1-related cytokines in an animal model of SAH. These modulators include glycyrrhizin and glycyrrhizin acid, rhinacanthin-C, purpurogallin, and 4′-O-β-D-glucosyl-5-O-methylvisamminol (Sun et al., 2013; Chang and Lin, 2014; Chang et al., 2015, 2016; Li et al., 2017; Ieong et al., 2018). It is possible that the beneficial effects of these modulators may result from the attenuation of microglial activation and the inhibition of TLR4. Mediators of microglial changes have also been studied, such as albumin, which suppresses microglial activation, resulting in reduced Iba-1 and CD68 staining in the cortex one day after SAH (Xie et al., 2017); peli1, an E3 ubiquitin ligase, which mediates the induction of proinflammatory cytokines in microglia via the mitogen-activated protein kinase signaling pathway (Huang et al., 2017) and tumor-specific glycoprotein-6, which transforms the SAH-driven M1 polarization to a skewed M2 polarization (Li et al., 2018).
Modulators of inflammation in clinical studies of SAH
Anti-inflammatory drugs have recently been proposed for treating stroke and neurological diseases. There have already been some encouraging results from clinical trials on patients with ischemic stroke (Zhu et al., 2015; Tian et al., 2018) and intracerebral hemorrhage (Fu et al., 2014). In these studies, fingolimod and alteplase were used, the former having been previously tested for the treatment of MS. In the case of aneurysmal SAH, interleukin-1 receptor antagonist (IL-1Ra) has been tested in a randomized, open-label, single-blinded study conducted by Galea et al. (2018). In this study, patients were administered 100 mg IL-1Ra subcutaneously twice daily, starting within three days following aneurysmal SAH and ending after 21 days or upon discharge. It was found that subcutaneous IL-1Ra was safe and well tolerated by the patients; recovery was assessed at 6 months using the Glasgow Outcome Scale Extended and was found to be better in the experimental group, although this was not statistically significant. A phase III study of IL-1Ra for the treatment of aneurysmal SAH is now planned. In another study, edaravone, a free radical scavenger, was used to treat aneurysmal SAH. The results were promising, showing a trend toward a reduced incidence of delayed ischemic neurological deficit and fewer poor outcomes caused by cerebral vasospasm (Munakata et al., 2009). A further study also assessed the use of simvastatin for the treatment of aneurysmal SAH, although this was not found to be beneficial (Vergouwen et al., 2009; Wong et al., 2015).
It is relevant to note that markers of inflammation have not always been found to relate to the clinical outcomes. For instance, Rasmussen et al. (2019) found that plasma levels of interleukin-6, vascular cell adhesion molecule-1, intercellular adhesion molecule-1, interleukin-8, interleukin-10, interferon gamma, and tumor necrosis factor alpha were not associated with DCI, angiographic vasospasm, or clinical outcome at three months. In another study, Gris et al. (2019) found that IL-6 levels in patients increased following SAH, similar to the SAH mouse model; however, in this study, high levels of Flt-1 and VEGF at admission were associated with poorer outcomes.
It can be seen that studies on the use of anti-inflammatory agents for SAH are limited. In experimental studies, some agents have been found to be effective, but clinical translational studies are needed to assess their therapeutic benefits. An improved understanding of the microglial activation and neuroinflammation in SAH will enable new targets and modulators to be explored. It will also be relevant to assess the use of modulators of TLR, JAK-STAT3, and HMGB1, as well as fingolimod, for treating SAH in patients.
Conclusion
Microglial activation and polarization are now recognized to play an important role in the pathophysiological processes that follow SAH. Recent single-cell analysis studies have indicated that potential interventions could target the microglia-mediated neuroinflammation in SAH. However, it is important to further analyze microglial subclusters in SAH using single-cell technology in order to better understand the evolution and roles of homeostatic and activated microglia in SAH. It will be necessary to carry out further research using animal models of SAH to identify potential pharmacological modulators of microglial activation that are both effective and safe. Subsequently, translational research will be needed to assess the clinical benefits of these modulators in patients with SAH.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Liu WJ, Li JY; T-Editor: Jia Y.
References
- 1.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 2.An JY, Pang HG, Huang TQ, Song JN, Li DD, Zhao YL, Ma XD. AG490 ameliorates early brain injury via inhibition of JAK2/STAT3-mediated regulation of HMGB1 in subarachnoid hemorrhage. Exp Ther Med. 2018;15:1330–1338. doi: 10.3892/etm.2017.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki T, Koseki H, Miyata H, Itoh M, Kawaji H, Takizawa K, Kawashima A, Ujiie H, Higa T, Minamimura K, Kimura T, Kasuya H, Nozaki K, Morita A, Sano H, Narumiya S. RNA sequencing analysis revealed the induction of CCL3 expression in human intracranial aneurysms. Sci Rep. 2019;9:10387. doi: 10.1038/s41598-019-46886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arimoto KI, Löchte S, Stoner SA, Burkart C, Zhang Y, Miyauchi S, Wilmes S, Fan JB, Heinisch JJ, Li Z, Yan M, Pellegrini S, Colland F, Piehler J, Zhang DE. STAT2 is an essential adaptor in USP18-mediated suppression of type I interferon signaling. Nat Struct Mol Biol. 2017;24:279–289. doi: 10.1038/nsmb.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 6.Benson MJ, Manzanero S, Borges K. Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia. 2015;56:895–905. doi: 10.1111/epi.12960. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahill J, Zhang JH. Subarachnoid hemorrhage: is it time for a new direction? Stroke. 2009 doi: 10.1161/STROKEAHA.108.533315. doi: 10.1161/ STROKEAHA.108.533315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CZ, Lin CL, Wu SC, Kwan AL. Purpurogallin, a natural phenol, attenuates high-mobility group box 1 in subarachnoid hemorrhage induced vasospasm in a rat model. Int J Vasc Med 2014. 2014 doi: 10.1155/2014/254270. 254270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CZ, Wu SC, Kwan AL, Lin CL. 4’-O-β-D-glucosyl-5-O-methylvisamminol, an active ingredient of Saposhnikovia divaricata, attenuates high-mobility group box 1 and subarachnoid hemorrhage-induced vasospasm in a rat model. Behav Brain Funct. 2015;11:28. doi: 10.1186/s12993-015-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Chang CZ, Wu SC, Kwan AL, Lin CL. Rhinacanthin-C, a fat-soluble extract from rhinacanthus nasutus, modulates high-mobility group box 1-related neuro-inflammation and subarachnoid hemorrhage-induced brain apoptosis in a rat model. World Neurosurg. 2016;86:349–360. doi: 10.1016/j.wneu.2015.08.071. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Zhang S, Shi J, Ai J, Hang C. Effects of recombinant human erythropoietin (rhEPO) on JAK2/STAT3 pathway and endothelial apoptosis in the rabbit basilar artery after subarachnoid hemorrhage. Cytokine. 2009;45:162–168. doi: 10.1016/j.cyto.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Jiang M, Li H, Wang Y, Shen H, Li X, Zhang Y, Wu J, Yu Z, Chen G. CX3CL1/CX3CR1 axis attenuates early brain injury via promoting the delivery of exosomal microRNA-124 from neuron to microglia after subarachnoid hemorrhage. J Neuroinflammation. 2020;17:209. doi: 10.1186/s12974-020-01882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulibaly AP, Provencio JJ. Aneurysmal subarachnoid hemorrhage: an overview of inflammation-induced cellular changes. Neurotherapeutics. 2020;17:436–445. doi: 10.1007/s13311-019-00829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 17.Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31:1443–1451. doi: 10.1038/jcbfm.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, Yan Y, Huang D, Yu C, Shi FD. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71:1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 19.Gaastra B, Glazier J, Bulters D, Galea I. Haptoglobin genotype and outcome after subarachnoid haemorrhage: new insights from a Meta-Analysis. Oxid Med Cell Longev 2017. 2017 doi: 10.1155/2017/6747940. 6747940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galea J, Ogungbenro K, Hulme S, Patel H, Scarth S, Hoadley M, Illingworth K, McMahon CJ, Tzerakis N, King AT, Vail A, Hopkins SJ, Rothwell N, Tyrrell P. Reduction of inflammation after administration of interleukin-1 receptor antagonist following aneurysmal subarachnoid hemorrhage: results of the Subcutaneous Interleukin-1Ra in SAH (SCIL-SAH) study. J Neurosurg. 2018;128:515–523. doi: 10.3171/2016.9.JNS16615. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Zhuang Z, Lu Y, Tao T, Zhou Y, Liu G, Wang H, Zhang D, Wu L, Dai H, Li W, Hang C. Curcumin mitigates neuro-inflammation by modulating microglia polarization through inhibiting TLR4 axis signaling pathway following experimental subarachnoid hemorrhage. Front Neurosci. 2019;13:1223. doi: 10.3389/fnins.2019.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gris T, Laplante P, Thebault P, Cayrol R, Najjar A, Joannette-Pilon B, Brillant-Marquis F, Magro E, English SW, Lapointe R, Bojanowski M, Francoeur CL, Cailhier JF. Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J Neuroinflammation. 2019;16:253. doi: 10.1186/s12974-019-1629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJM, Piao X, McCarroll SA, Stevens B. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–271. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanafy KA. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation. 2013;10:83. doi: 10.1186/1742-2094-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haruma J, Teshigawara K, Hishikawa T, Wang D, Liu K, Wake H, Mori S, Takahashi HK, Sugiu K, Date I, Nishibori M. Anti-high mobility group box-1 (HMGB1) antibody attenuates delayed cerebral vasospasm and brain injury after subarachnoid hemorrhage in rats. Sci Rep. 2016;6:37755. doi: 10.1038/srep37755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang XP, Peng JH, Pang JW, Tian XC, Li XS, Wu Y, Li Y, Jiang Y, Sun XC. Peli1 contributions in microglial activation, neuroinflammatory responses and neurological deficits following experimental subarachnoid hemorrhage. Front Mol Neurosci. 2017;10:398. doi: 10.3389/fnmol.2017.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ieong C, Sun H, Wang Q, Ma J. Glycyrrhizin suppresses the expressions of HMGB1 and ameliorates inflammative effect after acute subarachnoid hemorrhage in rat model. J Clin Neurosci. 2018;47:278–284. doi: 10.1016/j.jocn.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Jayakumar AR, Apeksha A, Norenberg MD. Role of matricellular proteins in disorders of the central nervous system. 2017;42:858–875. doi: 10.1007/s11064-016-2088-5. [DOI] [PubMed] [Google Scholar]
- 31.Jeon SB, Yoon HJ, Chang CY, Koh HS, Jeon SH, Park EJ. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol. 2010;185:7037–7046. doi: 10.4049/jimmunol.1000154. [DOI] [PubMed] [Google Scholar]
- 32.Kay A, Petzold A, Kerr M, Keir G, Thompson E, Nicoll J. Temporal alterations in cerebrospinal fluid amyloid beta-protein and apolipoprotein E after subarachnoid hemorrhage. Stroke. 2003;34:e240–243. doi: 10.1161/01.STR.0000100157.88508.2F. [DOI] [PubMed] [Google Scholar]
- 33.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169:1276–1290. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim OS, Park EJ, Joe EH, Jou I. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem. 2002;277:40594–40601. doi: 10.1074/jbc.M203885200. [DOI] [PubMed] [Google Scholar]
- 37.Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Alvarez-Croda DM, Stoica BA, Faden AI, Loane DJ. Microglial/Macrophage polarization dynamics following traumatic brain injury. J Neurotrauma. 2016;33:1732–1750. doi: 10.1089/neu.2015.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lan X, Liu R, Sun L, Zhang T, Du G. Methyl salicylate 2-O-β-D-lactoside, a novel salicylic acid analogue, acts as an anti-inflammatory agent on microglia and astrocytes. J Neuroinflammation. 2011;8:98. doi: 10.1186/1742-2094-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Nam Y, Koo JY, Lim D, Park J, Ock J, Kim J, Suk K, Park SB. A small molecule binding HMGB1 and HMGB2 inhibits microglia-mediated neuroinflammation. Nat Chem Biol. 2014;10:1055–1060. doi: 10.1038/nchembio.1669. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Cheng Z, Zhou L, Darmanis S, Neff NF, Okamoto J, Gulati G, Bennett ML, Sun LO, Clarke LE, Marschallinger J, Yu G, Quake SR, Wyss-Coray T, Barres BA. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell rna sequencing. Neuron. 2019a;101:207–223. doi: 10.1016/j.neuron.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li R, Liu W, Yin J, Chen Y, Guo S, Fan H, Li X, Zhang X, He X, Duan C. TSG-6 attenuates inflammation-induced brain injury via modulation of microglial polarization in SAH rats through the SOCS3/STAT3 pathway. J Neuroinflammation. 2018;15:231. doi: 10.1186/s12974-018-1279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Yang S, Sun B, Hang C. Melatonin attenuates early brain injury after subarachnoid hemorrhage by the JAK-STAT signaling pathway. Int J Clin Exp Pathol. 2019b;12:909–915. [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Sun F, Jing Z, Wang X, Hua X, Wan L. Glycyrrhizic acid exerts anti-inflammatory effect to improve cerebral vasospasm secondary to subarachnoid hemorrhage in a rat model. Neurol Res. 2017;39:727–732. doi: 10.1080/01616412.2017.1316903. [DOI] [PubMed] [Google Scholar]
- 45.Liu FY, Cai J, Wang C, Ruan W, Guan GP, Pan HZ, Li JR, Qian C, Chen JS, Wang L, Chen G. Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NF-κB signaling pathway. J Neuroinflammation. 2018;15:347. doi: 10.1186/s12974-018-1388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Liu Y, Zhao J, Liu H, He S. Prognostic value of plasma galectin-3 levels after aneurysmal subarachnoid hemorrhage. Brain Behav. 2016;6:e00543. doi: 10.1002/brb3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y, Zhang X, Zhang Z, Zhou X, Gao Y, Liu G, Wang H, Wu L, Li W, Hang C. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J Neuroinflammation. 2018;15:87. doi: 10.1186/s12974-018-1118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389:655–666. doi: 10.1016/S0140-6736(16)30668-7. [DOI] [PubMed] [Google Scholar]
- 49.Masuda T, Sankowski R, Staszewski O, Prinz M. Microglia heterogeneity in the single-cell era. Cell Rep. 2020;30:1271–1281. doi: 10.1016/j.celrep.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, Scheiwe C, Nessler S, Kunz P, van Loo G, Coenen VA, Reinacher PC, Michel A, Sure U, Gold R, Grün D, Priller J, Stadelmann C, Prinz M. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566:388–392. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 51.Munakata A, Ohkuma H, Nakano T, Shimamura N, Asano K, Naraoka M. Effect of a free radical scavenger, edaravone, in the treatment of patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2009;64:423–428. doi: 10.1227/01.NEU.0000338067.83059.EB. [DOI] [PubMed] [Google Scholar]
- 52.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikawa H, Suzuki H. Possible role of inflammation and galectin-3 in brain injury after subarachnoid hemorrhage. Brain Sci. 2018;8:30. doi: 10.3390/brainsci8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishikawa H, Nakatsuka Y, Shiba M, Kawakita F, Fujimoto M, Suzuki H. Increased plasma galectin-3 preceding the development of delayed cerebral infarction and eventual poor outcome in non-severe aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2018a;9:110–119. doi: 10.1007/s12975-017-0564-0. [DOI] [PubMed] [Google Scholar]
- 55.Nishikawa H, Liu L, Nakano F, Kawakita F, Kanamaru H, Nakatsuka Y, Okada T, Suzuki H. Modified citrus pectin prevents blood-brain barrier disruption in mouse subarachnoid hemorrhage by inhibiting galectin-3. Stroke. 2018b;49:2743–2751. doi: 10.1161/STROKEAHA.118.021757. [DOI] [PubMed] [Google Scholar]
- 56.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 57.Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osuka K, Watanabe Y, Yamauchi K, Nakazawa A, Usuda N, Tokuda M, Yoshida J. Activation of the JAK-STAT signaling pathway in the rat basilar artery after subarachnoid hemorrhage. Brain Res. 2006;1072:1–7. doi: 10.1016/j.brainres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Pasquini LA, Millet V, Hoyos HC, Giannoni JP, Croci DO, Marder M, Liu FT, Rabinovich GA, Pasquini JM. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011;18:1746–1756. doi: 10.1038/cdd.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng J, Pang J, Huang L, Enkhjargal B, Zhang T, Mo J, Wu P, Xu W, Zuo Y, Peng J, Zuo G, Chen L, Tang J, Zhang JH, Jiang Y. LRP1 activation attenuates white matter injury by modulating microglial polarization through Shc1/PI3K/Akt pathway after subarachnoid hemorrhage in rats. Redox Biol. 2019;21:101121. doi: 10.1016/j.redox.2019.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perego C, Fumagalli S, Zanier ER, Carlino E, Panini N, Erba E, De Simoni MG. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiol Dis. 2016;96:284–293. doi: 10.1016/j.nbd.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 62.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 63.Qin H, Yeh WI, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, Reynolds SL, Yanagisawa LL, Fox TH, Park K, Harrington LE, Raman C, Benveniste EN. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci U S A. 2012;109:5004–5009. doi: 10.1073/pnas.1117218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasmussen R, Bache S, Stavngaard T, Møller K. Plasma levels of IL-6, IL-8, IL-10, ICAM-1, VCAM-1, IFNγ, and TNFα are not associated with delayed cerebral ischemia, cerebral vasospasm, or clinical outcome in patients with subarachnoid hemorrhage. World Neurosurg. 2019;128:e1131–e1136. doi: 10.1016/j.wneu.2019.05.102. [DOI] [PubMed] [Google Scholar]
- 65.Rincon F, Rossenwasser RH, Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2013;73:217–222. doi: 10.1227/01.neu.0000430290.93304.33. [DOI] [PubMed] [Google Scholar]
- 66.Roa JA, Sarkar D, Zanaty M, Ishii D, Lu Y, Karandikar NJ, Hasan DM, Ortega SB, Samaniego EA. Preliminary results in the analysis of the immune response after aneurysmal subarachnoid hemorrhage. Sci Rep. 2020;10:11809. doi: 10.1038/s41598-020-68861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samraj AK, Müller AH, Grell AS, Edvinsson L. Role of unphosphorylated transcription factor STAT3 in late cerebral ischemia after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2014;34:759–763. doi: 10.1038/jcbfm.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D, McCarroll SA. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015–1030. doi: 10.1016/j.cell.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schallner N, Pandit R, LeBlanc R, Thomas AJ, Ogilvy CS, Zuckerbraun BS, Gallo D, Otterbein LE, Hanafy KA. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–2625. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schertz M, Mehdaoui H, Hamlat A, Piotin M, Banydeen R, Mejdoubi M. Incidence and mortality of spontaneous subarachnoid hemorrhage in martinique. PloS One. 2016;11:e0155945. doi: 10.1371/journal.pone.0155945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schneider UC, Davids AM, Brandenburg S, Müller A, Elke A, Magrini S, Atangana E, Turkowski K, Finger T, Gutenberg A, Gehlhaar C, Brück W, Heppner FL, Vajkoczy P. Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathol. 2015;130:215–231. doi: 10.1007/s00401-015-1440-1. [DOI] [PubMed] [Google Scholar]
- 72.Sun Q, Wang F, Li W, Li W, Hu YC, Li S, Zhu JH, Zhou M, Hang CH. Glycyrrhizic acid confers neuroprotection after subarachnoid hemorrhage via inhibition of high mobility group box-1 protein: a hypothesis for novel therapy of subarachnoid hemorrhage. Med Hypotheses. 2013;81:681–685. doi: 10.1016/j.mehy.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki H. What is early brain injury? Transl Stroke Res. 2015;6:1–3. doi: 10.1007/s12975-014-0380-8. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki H, Shiba M, Nakatsuka Y, Nakano F, Nishikawa H. Higher cerebrospinal fluid pH may contribute to the development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2017;8:165–173. doi: 10.1007/s12975-016-0500-8. [DOI] [PubMed] [Google Scholar]
- 75.Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 76.Taufique Z, May T, Meyers E, Falo C, Mayer SA, Agarwal S, Park S, Connolly ES, Claassen J, Schmidt JM. Predictors of poor quality of life 1 year after subarachnoid hemorrhage. Neurosurgery. 2016;78:256–264. doi: 10.1227/NEU.0000000000001042. [DOI] [PubMed] [Google Scholar]
- 77.Thrupp N, Sala Frigerio C, Wolfs L, Skene NG, Fattorelli N, Poovathingal S, Fourne Y, Matthews PM, Theys T, Mancuso R, de Strooper B, Fiers M. Single-nucleus RNA-SEQ is not suitable for detection of microglial activation genes in humans. Cell Rep. 2020;32:108189. doi: 10.1016/j.celrep.2020.108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian DC, Shi K, Zhu Z, Yao J, Yang X, Su L, Zhang S, Zhang M, Gonzales RJ, Liu Q, Huang D, Waters MF, Sheth KN, Ducruet AF, Fu Y, Lou M, Shi FD. Fingolimod enhances the efficacy of delayed alteplase administration in acute ischemic stroke by promoting anterograde reperfusion and retrograde collateral flow. Ann Neurol. 2018;84:717–728. doi: 10.1002/ana.25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Venkatraman A, Hardas S, Patel N, Singh Bajaj N, Arora G, Arora P. Galectin-3: an emerging biomarker in stroke and cerebrovascular diseases. Eur J Neurol. 2018;25:238–246. doi: 10.1111/ene.13496. [DOI] [PubMed] [Google Scholar]
- 80.Vergouwen MD, Meijers JC, Geskus RB, Coert BA, Horn J, Stroes ES, van der Poll T, Vermeulen M, Roos YB. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled randomized trial. J Cereb Blood Flow Metab. 2009;29:1444–1453. doi: 10.1038/jcbfm.2009.59. [DOI] [PubMed] [Google Scholar]
- 81.Wang CX, Xie GB, Zhou CH, Zhang XS, Li T, Xu JG, Li N, Ding K, Hang CH, Shi JX, Zhou ML. Baincalein alleviates early brain injury after experimental subarachnoid hemorrhage in rats: possible involvement of TLR4/NF-κB-mediated inflammatory pathway. Brain Res. 2015;1594:245–255. doi: 10.1016/j.brainres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 82.Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wesley UV, Vemuganti R, Ayvaci ER, Dempsey RJ. Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling. Brain Res. 2013;1496:1–9. doi: 10.1016/j.brainres.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong GK, Chan DY, Siu DY, Zee BC, Poon WS, Chan MT, Gin T, Leung M. High-dose simvastatin for aneurysmal subarachnoid hemorrhage: multicenter randomized controlled double-blinded clinical trial. Stroke. 2015;46:382–388. doi: 10.1161/STROKEAHA.114.007006. [DOI] [PubMed] [Google Scholar]
- 85.Wong GK, Wong R, Mok V, Wong A, Poon WS. Natural history and medical treatment of cognitive dysfunction after spontaneous subarachnoid haemorrhage: review of current literature with respect to aneurysm treatment. J Neurol Sci. 2010;299:5–8. doi: 10.1016/j.jns.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 86.Xie Y, Guo H, Wang L, Xu L, Zhang X, Yu L, Liu Q, Li Y, Zhao N, Zhao N, Ye R, Liu X. Human albumin attenuates excessive innate immunity via inhibition of microglial Mincle/Syk signaling in subarachnoid hemorrhage. Brain Behav Immun. 2017;60:346–360. doi: 10.1016/j.bbi.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 87.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin D, Zhou S, Xu X, Gao W, Li F, Ma Y, Sun D, Wu Y, Guo Q, Liu H, Han L, Wang Z, Wang Y, Zhang J. Dexmedetomidine attenuated early brain injury in rats with subarachnoid haemorrhage by suppressing the inflammatory response: The TLR4/NF-κB pathway and the NLRP3 inflammasome may be involved in the mechanism. Brain Res. 2018;1698:1–10. doi: 10.1016/j.brainres.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 90.Yip PK, Carrillo-Jimenez A, King P, Vilalta A, Nomura K, Chau CC, Egerton AM, Liu ZH, Shetty AJ, Tremoleda JL, Davies M, Deierborg T, Priestley JV, Brown GC, Michael-Titus AT, Venero JL, Burguillos MA. Galectin-3 released in response to traumatic brain injury acts as an alarmin orchestrating brain immune response and promoting neurodegeneration. Sci Rep. 2017;7:41689. doi: 10.1038/srep41689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang X, Lu Y, Wu Q, Dai H, Li W, Lv S, Zhou X, Zhang X, Hang C, Wang J. Astaxanthin mitigates subarachnoid hemorrhage injury primarily by increasing sirtuin 1 and inhibiting the Toll-like receptor 4 signaling pathway. FASEB J. 2019;33:722–737. doi: 10.1096/fj.201800642RR. [DOI] [PubMed] [Google Scholar]
- 92.Zheng ZV, Lyu H, Lam SYE, Lam PK, Poon WS, Wong GKC. The dynamics of microglial polarization reveal the resident neuroinflammatory responses after subarachnoid hemorrhage. Transl Stroke Res. 2020;11:433–449. doi: 10.1007/s12975-019-00728-5. [DOI] [PubMed] [Google Scholar]
- 93.Zheng ZV, Wong KCG. Microglial activation and polarization after subarachnoid hemorrhage. Neurol Neuroimmunol Neuroinflamm. 2019;6:1. [Google Scholar]
- 94.Zhou CH, Wang CX, Xie GB, Wu LY, Wei YX, Wang Q, Zhang HS, Hang CH, Zhou ML, Shi JX. Fisetin alleviates early brain injury following experimental subarachnoid hemorrhage in rats possibly by suppressing TLR 4/NF-κB signaling pathway. Brain Res. 2015;1629:250–259. doi: 10.1016/j.brainres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 95.Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, Shi FD. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]