Abstract
Nitric oxide is an important mediator of vascular autoregulation and is involved in pathophysiological changes after acute neurological disorders. Nitric oxide is generated by nitric oxide synthases from the amino acid L-arginine. L-arginine can also serve as a substrate for arginases or lead to the generation of dimethylarginines, asymmetric dimethylarginine, and symmetric dimethylarginine, by methylation. Asymmetric dimethylarginine is an endogenous inhibitor of nitric oxide synthase and can lead to endothelial dysfunction. This review discusses the role of L-arginine metabolism in patients suffering from acute and critical neurological disorders often requiring neuro-intensive care treatment. Conditions addressed in this review include intracerebral hemorrhage, aneurysmal subarachnoid hemorrhage, and traumatic brain injury. Recent therapeutic advances in the field are described including current randomized controlled trials for traumatic brain injuries and hemorrhagic stroke.
Keywords: arginine, brain injuries, traumatic, cerebral hemorrhage, dimethylarginine, nitric oxide, stroke, subarachnoid hemorrhage
Introduction
Intracerebral hemorrhage (ICH), aneurysmal subarachnoid hemorrhage (SAH), and traumatic brain injury (TBI) are common neurocritical care diseases with incidences ranging from 6.1/100,000 per year for SAH, between 19.6 and 51.8/100,000 per year for ICH, and up to 73/100,000 for severe traumatic brain injury and are despite the deeper understanding of pathological mechanisms and improved specialized care still associated with high morbidity and mortality (Majdan et al., 2016; An et al., 2017; Iaccarino et al., 2018; Tatlisumak et al., 2018; Busl et al., 2019; Etminan et al., 2019; Maegele et al., 2019). Besides distinct pathophysiological differences between these diseases, a common feature is that after the initial event of injury, secondary cerebral damage can be mediated by various pathological pathways including inflammation, oxidative stress, excitotoxicity, depolarization, decreased cerebral blood flow, blood-brain barrier disruption, intracranial hypertension and cytotoxicity of blood components (Lawton and Vates, 2017; van Lieshout et al., 2018; Wilkinson et al., 2018; Ng and Lee, 2019; Mohme et al., 2020; Weiland et al., 2021).
One pathway of interest is the nitric oxide (NO) metabolism, given the important role of NO as a mediator of vascular autoregulation and the established association between NO disturbances and cerebrovascular disease as well as brain injury (Cherian et al., 2004; Garry et al., 2015). L-arginine serves as a substrate for different enzymatic pathways, which includes the production of NO and L-citrulline catalyzed by NO synthases (NOS). L-arginine metabolism is therefore a central element of the NO pathway (Figure 1). Different types of NOS have been identified, which include the inducible form (iNOS) as well as with the endothelial NOS (eNOS) and neuronal NOS (nNOS) two constitutive forms (Garry et al., 2015).
Figure 1.

The flow chart depicts key pathways of L-arginine metabolism and endogenous mechanisms of interference.
ADMA: Asymmetric dimethylarginine; hCAT-2B: human cationic amino acid transporter 2B; NO: nitric oxide; NOS: nitric oxide synthase; SDMA: symmetric dimethylarginine.
Dimethylarginines, which are products of L-arginine methylation and include asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA), have been identified as risk markers in a range of primarily cardiovascular diseases (Böger, 2006; Tain and Hsu, 2017). Dimethylarginines have multifunctional roles with ADMA acting as a reversible competitive inhibitor of NOS leading to endothelial dysfunction (Böger et al., 1998) as well as oxidative stress (Li et al., 2011) and SDMA competing with L-arginine for the human cationic amino acid transporter 2B (hCAT-2B) (Closs et al., 1997; Böger, 2006). ADMA generation from protein-incorporated L-arginine is catalyzed by protein arginine methyltransferases (Leiper and Vallance, 1999). ADMA is metabolized to citrulline by dimethylarginine dimethylaminohydrolases (DDAH) whereas SDMA is eliminated by renal excretion (Leiper and Vallance, 1999; Bode-Böger et al., 2006).
An alternative enzymatic pathway of L-arginine metabolism is mediated by arginases I and II, which are located in the cytosol and the mitochondria, respectively, and lead to the products L-ornithine and urea (Morris, 2002). Consequently, arginase is a direct competitor with NOS. Another metabolizing enzyme of L-arginine is the Arginine:glycine amidinotransferase (AGAT), which exhibits a promiscuous activity either using glycine or L-lysine as co-substrates to generate L-ornithine and either guanidino acetic acid or L-homoarginine, respectively (Davids et al., 2012).
Disturbances of the NO pathway can lead to different pathophysiological sequels, including most prominent impairment of cerebral blood flow regulation (Cherian et al., 2004). Depletion of the substrate L-arginine can lead to oxidative stress due to uncoupling of NOS with oxygen radical formation potentially leading up to the production of the highly toxic oxidant peroxynitrite (ONOO–) (Xia et al., 1996; Cherian et al., 2004). Uncoupling of NOS can also be mediated by ADMA (Böger et al., 2000; Wells and Holian, 2007). NO holds anti-inflammatory properties by redox regulation of nuclear factor (NF)-κB (Reynaert et al., 2004). Depletion of NO can therefore lead to an enhanced neuroinflammatory reaction. Arginase has also been shown to promote inflammation by decreasing the cellular content of NO and enhancing the NF-κB pathway (Ckless et al., 2007).
This systematic review discusses the role of L-arginine metabolism in patients suffering from acute and critical neurological disorders often requiring neuro-intensive care treatment. Moreover, recent developments of therapeutic advances in this field are described. We are focusing in particular on the pathophysiological mechanisms in ICH, aneurysmal SAH, and TBI. The mechanisms of L-arginine metabolism in ischemic stroke have previously been discussed by Grosse et al. (2020).
Database Search Strategy
The US National Library of Medicine PubMed database (https://pubmed.ncbi.nlm.nih.gov) has been accessed on February 26, 2021, and searched for the following search string: (arginine OR dimethylarginine) AND (‘subarachnoid hemorrhage’ OR ‘traumatic brain injury’ OR ‘intracerebral hemorrhage’ OR ‘hemorrhagic stroke’). This search returned 477 articles. Of these, the title and abstract of articles published within the last 10 years (172 articles) were screened independently by the authors for relevant content. Articles covering vasopressin (also called arginine vasopressin, antidiuretic hormone), its derivates, Agatroban and pituitary hormones were not considered for this review. A total of 33 articles were included in this search strategy. Additional literature identified from these articles or known as relevant to the authors was included as well.
Moreover, ongoing and recently completed clinical trials were identified by searching the US National Library of Medicine ClinicalTrials.gov database (https://www.clinicaltrials.gov/ct2/home) using the search term ‘arginine’ together with either ‘intracerebral hemorrhage’ (4 studies), ‘subarachnoid hemorrhage’ (1 study) or ‘traumatic brain injury’ (4 studies). Trials covering arginine vasopressin, its derivates and pituitary hormones as well as non-interventional studies were excluded, leading to the inclusion of two studies for this review (ClinicalTrials.gov Identifier: NCT02012582 and NCT03711903).
L-arginine Metabolism in Intracerebral Hemorrhage
Whereas detrimental effects of increased NO and overexpression of NOS in the context of ICH have been demonstrated in animal models, clinical data is sparse and controversial (Li et al., 2011). Similarly, studies focusing on the underlying L-arginine metabolism are scarce and mostly focus on the peripheral vascular compartment. A first study measuring L-arginine metabolites in the plasma of 49 patients with primary ICH within three days of onset was conducted by Rashid et al. (2003). The authors observed significant reductions in L-arginine, L-citrulline, and L-ornithine, compared to healthy controls. This was accompanied by reduced levels of NO end products (nitrate and nitrite). Moreover, patients who could be discharged home demonstrated higher levels of NO end products, L-arginine, L-citrulline and serine than patients with in-hospital mortality or discharge to another institution. In a cohort presented by Wanby et al. (2006) plasma of 22 hemorrhagic stroke patients was collected on the first day after admission and analyzed for L-arginine, ADMA, and SDMA. Decreased levels of L-arginine and a reduced L-arginine/ADMA ratio, as an indicator for relative L-arginine deficiency, but no changes in dimethylarginine concentrations were associated with ICH. These findings were partially in contrast to a study of Worthmann et al. (2017), analyzing blood samples ≤ 24 hours and 3 and 7 days after ICH onset in 20 patients. In this cohort, ADMA was significantly elevated in ICH patients compared to controls at all time points. No change in SDMA concentration was detected. L-arginine showed a trend for lower levels at earlier time points but without statistical significance. Moreover, the authors observed elevated levels of SDMA and early ADMA in patients with the unfavorable outcomes and an independent association was confirmed for SDMA at the ≤ 24 hours and 3-day time points.
A recent study examined, besides L-arginine metabolite concentrations in the plasma, also the levels in the cerebrospinal fluid (CSF) of 25 ICH patients over a time course of up to 11 days (Mader et al., 2021). With regard to plasma concentrations compared to control patients, reductions of L-ornithine, L-lysine, and L-citrulline were measured, consistent with the findings of Rashid et al. (2003). Similar to Worthmann et al. (2017), L-arginine showed only a trend to decrease without statistical significance. Intrathecally, an early rise in L-citrulline levels with unchanged L-arginine concentrations was measured, which is suggestive for an early increase in NOS activity. At later timepoints, increasing CSF concentrations of ADMA were measured while accumulating L-arginine and decreasing L-citrulline levels were indicative for a reduction of NOS back to baseline. This observation could be well explained by the known inhibitory effect of ADMA on NOS (Vallance et al., 1992). In contrast to SDMA in the plasma, SDMA levels in the CSF were elevated throughout the observation period, which could also contribute to a later decline in NOS activity by competition with L-arginine for cellular hCAT-2B-mediated uptake leading to intracellular L-arginine depletion (Closs et al., 1997). Moreover, signs for an increase in arginase activity were reported with over time rising levels of L-ornithine and a persistently elevated L-ornithine/L-arginine ratio. Given the direct competition of arginase with NOS for the common substrate L-arginine, this may be another mechanism leading to a reduction of NOS activity back to baseline in the subacute phase. Moreover, rather elevated L-lysine and low L-homoarginine concentrations were possible indicators for reduced AGAT activity. In contrast to the work of Worthmann et al. (2017), no association between dimethylarginines and outcome was detected in this study cohort. Instead, early CSF concentration of L-arginine was found to be an independent predictor of outcome. Patients who were dead or in a persistent vegetative state after six months demonstrated lower CSF L-arginine levels at early timepoints, identifying manipulation of intrathecal L-arginine concentration as a potential therapeutic target. The cationic arginine-rich peptides poly-arginine-18, which is an 18-mer of L-arginine, and its D-enantiomer have recently been evaluated in a collagenase ICH model in rats (Liddle et al., 2019). Intravenous and/or intraperitoneal administration in the acute phase of bleeding has been shown to be safe without exacerbation of hematoma volume. But no reduction of lesion volume or improvement of functional outcome was observed.
L-arginine Metabolism in Aneurysmal Subarachnoid Hemorrhage
A major complication of aneurysmal SAH is cerebral vasospasm (CVS)/delayed cerebral ischemia (DCI), which contributes substantially to the morbidity and mortality of this disease (Lawton and Vates, 2017). An increase in ADMA in the peripheral blood and CSF after SAH was detected in several studies. In a cohort of 20 SAH patients, ADMA concentration of the peripheral blood was not different from control patients in the first 3 days after onset but increased within the first week and remained elevated after 3 months (Rodling-Wahlstrom et al., 2012). In a cohort of 56 SAH patients, plasma levels of ADMA were measured over 10 days and were already significantly elevated within the first 48 hours compared to sex- and age-matched healthy donors (Lindgren et al., 2014). ADMA levels showed then a further increase at later time points (97–240 hours). Interestingly, this increase in ADMA started after proinflammatory markers CRP and IL-6 had peaked, suggesting a potential relationship with systemic inflammation. Patients with higher-grade SAH (defined as Hunt & Hess > 2) demonstrated higher ADMA concentrations and a decreased L-arginine/ADMA ratio. Looking at peak ADMA levels and nadir L-arginine/ADMA ratio, no association with impaired cerebral circulation or functional neurological outcomes was detected. But patients with good functional outcome demonstrated a higher peak L-arginine/ADMA ratio.
Studies in primates revealed that CVS seems to be associated with a reduction in NOS expression (Pluta et al., 1996) as well as reduced CSF levels of nitrite, nitrate, and L-citrulline but increased concentrations of ADMA (Jung et al., 2004). Similar findings were obtained in a cohort of 18 SAH patients (Jung et al., 2007). Patients suffering from SAH demonstrated higher CSF levels of ADMA than control patients. ADMA further increased in patients with CVS and correlated with CVS degree. Measurements of ADMA and vasoconstrictor endothelin-1 in the CSF of 24 patients with SAH showed again that ADMA but not endothelin-1 concentration was correlated with CVS (Jung et al., 2012). But neither correlated with ischemic cerebral lesions. Interestingly, measurement of monomethylated L-arginine, which is also a strong inhibitor of NOS, in serum and CSF showed no correlation with CVS or nitrite but correlated with occurrence and size of ischemic lesions (Jung et al., 2013). Measurement of ADMA concentrations in the CSF of a cohort of 20 SAH patients for 2 weeks revealed an increase on days 3–5, peaking around 7–9 days, and remaining elevated until 12–14 days after SAH (Li et al., 2014). Interestingly, higher ADMA levels at days 7–9 were associated with both the detection of CVS in transcranial Doppler ultrasonography as well as poor outcome (Karnofsky Performance Status scale < 60) after 2 years.
In a cohort of 111 SAH patients, a low plasma L-arginine/ADMA ratio was associated with higher mortality rates and, independently from SAH severity measured by the World Federation of Neurosurgical Societies grade, a higher hazard ratio (Staalso et al., 2013). The L-arginine/ADMA ratio correlated negatively with nitrite/nitrate levels and nitrite/nitrate levels showed an inverse relationship with transcranial Doppler flow velocities. However, neither ADMA itself nor the L-arginine/ADMA ratio demonstrated an association with flow velocities. The same study group evaluated early endothelial function with peripheral arterial tonometry in the fingertip in 48 SAH patients (Bergström et al., 2014). Peripheral endothelial dysfunction was expressed as reactive hyperemia index, which increased in SAH patients after day 2 but remained lower in deceased patients. Similar to previous studies (Rodling-Wahlstrom et al., 2012; Lindgren et al., 2014), ADMA and also L-arginine increased after the acute phase of SAH. A positive correlation was found between the reactive hyperemia index and the L-arginine/ADMA ratio, which was interpreted as impaired endothelial function in the context of L-arginine/ADMA imbalance but reactive hyperemia index showed no association with CVS or DCI.
An evaluation of L-arginine, ADMA, and SDMA in both the peripheral vascular and the intrathecal compartment was performed in a SAH cohort of 34 patients (Appel et al., 2018). Patients with DCI demonstrated a lower baseline plasma L-arginine/ADMA ratio as well as higher CSF concentrations of ADMA and SDMA. A low L-arginine/ADMA plasma ratio at admission was associated with poor functional neurological outcome, at discharge and after 3 months. ADMA in the CSF was confirmed to be independently associated with DCI whereas increased SDMA levels in the CSF were found to be an independent predictor for the poor neurological outcomes. This is in line with a machine learning-driven screen of 138 metabolites in the CSF of 81 SAH patients reporting SDMA, dimethylguanidine valeric acid, and L-ornithine to be associated with poor outcomes at discharge and after 90 days (Koch et al., 2021). Another study including 51 SAH patients further reported higher plasma ADMA and SDMA levels and higher CSF SDMA levels at admission in patients with DCI (Hannemann et al., 2020). Moreover, single nucleotide polymorphisms of the DDAH1 genes were associated with plasma ADMA and carriers of the minor allele rs233112 had a significantly increased relative risk of DCI.
Potential therapeutic manipulations of the L-arginine metabolism have been explored preclinically in several studies for SAH. The effect of L-arginine administration on vasospasm was explored in a rat femoral artery vasospasm model (Akar et al., 2019). The authors described morphometric improvements in animals treated with L-arginine with reduced wall thickness and increased lumen diameter. Reduced pathological changes of the cortical microstructure have been described after intraperitoneal administration of L-arginine aspartate in a rat model of SAH (Netlyukh, 2016). A different study using a prechiasmatic cistern SAH model in rats reported increased levels of ADMA in the CSF over a time course of 14 days peaking around day 5 (Zhao et al., 2015). A positive correlation between ADMA and connexin43 was described. Interestingly, administration of the gap junction inhibitor 18β-glycyrrhetinic acid led to improved basilar artery vessel diameter and neurological score while reducing the CSF level of ADMA. Intraperitoneal administration of L-citrulline, which can serve as a product for new L-arginine synthesis via the argininosuccinate synthase and lyase pathway, was found to improve basilar artery patency and neurological scores in a SAH model in haptoglobin 2-2 transgenic mice (Pradilla et al., 2012).
L-arginine Metabolism in Traumatic Brain Injury
The role of NO in TBI is complex with a time-specific course of brain levels consisting of an initial peak, followed by a potential period of relative deficiency after which a second NO accumulation can be observed (Cherian et al., 2004). Effects of NO pathway disturbances are multifunctional and can even mediate blood-brain barrier disruption as recently demonstrated (Logsdon et al., 2018, 2020). Different studies evaluated the effect of different NOS-inhibitors and administration of L-arginine pre- and post-injury with partially divergent results, as reviewed by Cherian et al. (2004). More recent studies sought to further elucidate the role of underlying L-arginine metabolism including dimethylarginines, further characterize the alterations in humans and evaluate therapeutic effects of polyarginine peptides.
Local ADMA expression was strongly reduced in a controlled cortical impact model in rats (Jung et al., 2014). This was most prominent and lasting within the lesion whereas a more fluctuating time-dependent expression was observed for the perilesional area which interestingly correlated positively with the neurological status. Changes were also detected for the expression of underlying enzymes with a decrease in arginine methyltransferase 1 expression but an increase in the expression of DDAH1 and DDAH2, which negatively correlated with the neuroscore performance. However, measurement of ADMA concentrations in the CSF within 3 days of TBI in a pediatric study population showed an increase compared to healthy controls (Thampatty et al., 2013). Interestingly, this increase was attenuated by therapeutic hypothermia. An increase in the concentration of ADMA in the peripheral blood was described in a severe TBI cohort of 46 patients starting 72 hours after trauma (Wahlström et al., 2014).
Measurement of amino acid concentrations in the rat brain after either mild or severe diffuse traumatic brain injury revealed a reduced L-arginine/L-citrulline ratio already 6 hours after injury and throughout the observation period of 120 hours in severely injured animals but an increased ratio in the mildly injured group (Amorini et al., 2017). The influence of the severity of trauma was also addressed in a clinical study. L-arginine metabolites were measured in the peripheral blood within the first 24 hours in a cohort consisting of mild and severe TBI patients, orthopedically injured patients as well as healthy volunteers (Jeter et al., 2012). Plasma levels of L-arginine, citrulline, ornithine, and hydroxyproline were significantly reduced in the group of severe TBI but no changes were detected in mild TBI patients. Peripheral dimethylarginine plasma levels were unaffected by TBI but an increase in creatine was observed. A metabolomic analysis of brain tissue in rats after a controlled cortical impact revealed that increased ornithine was among four metabolites differentially changed on both days 1 and 3 after TBI (Zheng et al., 2020). Moreover, arginine and proline metabolism were among two pathways identified as significantly affected on day 3 after TBI.
As another relevant component of the L-arginine metabolism, the role of arginase has been further evaluated revealing cell type and subtype-specific implications. Utilizing a genetic approach in a mouse-controlled cortical impact model, the role of overexpression of either arginase I or arginase II in neurons was studied in the context of TBI (Madan et al., 2018). As expected, overexpression of either enzyme subtype led to reduced L-arginine brain levels. Interestingly, animals overexpressing arginase I in neurons demonstrated a significant decrease in lesion size and contusion severity compared to wild-type mice identifying a potential neuroprotective effect of cell-type-specific arginase I expression. In comparison, an induction of systemic endothelial dysfunction after TBI was described to be mediated by arginase I (Villalba et al., 2017). Rats were evaluated for endothelial function in the mesenteric arteries 24 hours after a fluid percussion injury. TBI animals showed diminished vasodilatory and vasoconstrictor responses which were linked to impairment of the NO pathway. Mesenteric arteries of TBI animals demonstrated elevated arginase activity with isoform I being expressed in the endothelium. The vasodilatory function could be rescued by either L-arginine supplementation or arginase inhibition. Increased arginase I levels have also been detected after experimental spinal cord injury (Ahn et al., 2012). Besides constitutive expression in neurons and glial cells also present in control spinal cord tissue, arginase I was additionally detected in macrophages and reactive astrocytes in the core lesion.
The therapeutic potential of cationic arginine-rich peptides for TBI has been evaluated in different recent studies focusing on polyarginine-18 (R-18) (Chiu et al., 2017, 2019, 2020; Batulu et al., 2019). In an in vitro excitotoxicity model, R-18 showed strong neuroprotective abilities and reduced neuronal calcium influx (Chiu et al., 2017). Intravenous administration 30 minutes after experimental TBI in rats lead to reduced axonal injury. However, no significant improvement of hippocampal neuronal loss or functional outcome was detected. In a different rat model of TBI, R-18 reduced neuronal cell apoptosis and brain water content while inhibiting caspases-3/8/9 (Batulu et al., 2019). R-18 treated TBI animals showed lower levels of the pro-apoptotic protein Bax as well as higher levels of the autophagic marker protein LC3 compared to untreated TBI animals. Comparison of R-18 and its D-enantiomer R-18D in a closed-head impact weight drop model in Long-Evans rats revealed that intravenous treatment of R-18D but not R-18 improves sensorimotor and vestibulomotor deficits (Chiu et al., 2019). However, in a subsequent study utilizing a closed-head injury model in Sprague-Dawley rats, no improvement of vestibulomotor functions could be detected and only a trend to improved memory function without statistical significance was observed after R-18D administration (Chiu et al., 2020). Besides L-arginine itself and cationic arginine-rich peptides, other L-arginine-derived compounds have also been identified mediating neuroprotective effects, e.g., agmatine, which is derived from L-arginine by decarboxylation (Kotagale et al., 2019).
Comparison of the Changes in the L-Arginine Metabolism between Different Etiologies
Despite marked differences in the overall pathophysiological processes of ICH, SAH, and TBI, certain similarities can be observed with regard to changes in the L-arginine metabolism. Here we refer to the aforementioned clinical studies and discuss similarities and differences across the different entities (Table 1).
Table 1.
Key findings of selected clinical studies describing alterations of the L-arginine metabolism in patients suffering from ICH, SAH and TBI
| Reference | Entity | Vascular compartment | Intrathecal compartment |
|---|---|---|---|
| Rashid et al., 2003 | ICH | • Early reductions in nitrate/nitrite, L-arginine, L-citrulline and L-ornithine | |
| • L-arginine and L-citrulline associated with feeding status | |||
| Wanby et al., 2006 | ICH | • Early decrease in L-arginine concentration but no change in dimethylarginine concentrations | |
| Worthmann et al., 2017 | ICH | • Elevation in ADMA levels | |
| • Dimethylarginines increased in patients with poor outcome | |||
| Mader et al., 2021 | ICH | • Early reductions in L-citrulline, L-ornithine and L-Lysine | • Persistent elevation of SDMA levels |
| • No increase in dimethylarginine levels | • Delayed increase in ADMA levels | ||
| • Early increase in L-citrulline concentrations | |||
| • Delayed accumulation of L-arginine and L-ornithine | |||
| • Early reduction in L-arginine concentration was an independent risk factor for poor outcome | |||
| Jung et al., 2007 | SAH | • Increased ADMA levels | |
| • ADMA particularly elevated in patients with CVS | |||
| Jung et al., 2012 | SAH | • ADMA but not endothelin-1 concentration was correlated with CVS | |
| Rodling-Wahlstrom et al., 2012 | SAH | • ADMA increased during the first week and remained elevated 3 mon later | |
| Staalso et al., 2013 | SAH | • Low L-arginine/ADMA ratio associated with higher mortality | |
| Bergström et al., 2014 | SAH | • ADMA and L-arginine levels increased after the acute phase | |
| • Correlation of impaired endothelial function with L-arginine/ADMA ratio | |||
| Li et al., 2014 | SAH | • ADMA increase at 3–5 d, peaking around 7–9 d, and remaining elevated until 12–14 d |
|
| • Correlation of higher ADMA levels with CVS and poor outcomes | |||
| Lindgren et al., 2014 | SAH | • Increased ADMA levels | |
| • Higher peak L-arginine/ADMA ratio associated with better outcome | |||
| Appel et al., 2018 | SAH | • DCI and poor outcome associated with lower baseline L-arginine/ADMA ratio | • Association of DCI with higher concentrations of ADMA and SDMA |
| Hannemann et al., 2020 | SAH | • Higher ADMA and SDMA levels associated with DCI | • Higher SDMA levels associated with DCI |
| Koch et al., 2021 | SAH | • SDMA and L-ornithine associated with poor outcomes | |
| Jeter et al., 2012 | TBI | • L-arginine, L-citrulline and L-ornithine concentrations reduced in severe TBI | |
| • Dimethylarginine levels unaffected | |||
| Thampatty et al., 2013 | TBI | • Increased early ADMA concentrations in a pediatric study population | |
| • Increase was attenuated by early hypothermia | |||
| Wahlström et al., 2014 | TBI | • Early increase in ADMA concentrations |
ADMA: Asymmetric dimethylarginine; CVS: cerebral vasospasm; DCI: delayed cerebral ischemia; ICH: intracerebral hemorrhage; SAH: subarachnoid hemorrhage; SDMA: symmetric dimethylarginine; TBI: traumatic brain injury.
Changes in amino acid concentrations
Several studies described systemic reductions in relative (as L-arginine/ADMA ratio) or absolute concentrations of L-arginine and/or related amino acids like L-citrulline and L-ornithine either after disease onset or in association with poor outcome for ICH (Rashid et al., 2003; Wanby et al., 2006; Worthmann et al., 2017; Mader et al., 2021) or TBI (Jeter et al., 2012). Similarly, most clinical data for SAH demonstrated an association between poor outcome or DCI and a relative deficiency in plasma L-arginine (Staalso et al., 2013; Lindgren et al., 2014; Appel et al., 2018) but absolute L-arginine concentrations were reported to not differ from control patients (Jung et al., 2007). Notably, studies have described an increase in systemic L-arginine at later time points after the acute phase, e.g., for ICH (Mader et al., 2021) or SAH (Bergström et al., 2014).
For the intrathecal compartment, only limited data exist for L-arginine concentrations. No changes in CSF L-arginine or L-citrulline concentrations were detected between patients suffering from SAH and controls (Jung et al., 2007). Patients with ICH showed overall a significant increase in CSF L-arginine concentrations after the early phase and an increase in CSF L-ornithine and L-citrulline already early on (Mader et al., 2021). Interestingly, patients with higher early L-arginine concentrations demonstrated a better outcome. Around 10 days after ICH onset, increased L-ornithine was correlated with poor outcomes in the same ICH study. Similarly, elevated L-ornithine was also reported to be associated with poor outcomes in patients with SAH (Koch et al., 2021).
Changes in dimethylarginine concentrations
Increases in ADMA concentrations in the plasma have been described by several studies for ICH (Worthmann et al., 2017), SAH (Rodling-Wahlstrom et al., 2012; Bergström et al., 2014; Lindgren et al., 2014) and TBI (Wahlström et al., 2014). However, other studies did not detect elevations after ICH (Wanby et al., 2006; Mader et al., 2021) or TBI (Jeter et al., 2012) compared to control patients. Intrathecally, increases in ADMA concentrations were reported for ICH (Mader et al., 2021), SAH (Jung et al., 2007; Li et al., 2014), and pediatric TBI (Thampatty et al., 2013). For SAH, ADMA in the CSF was correlated with CVS or DCI in different studies (Jung et al., 2007, 2012; Li et al., 2014; Appel et al., 2018). In ICH, ADMA was found to be increased in patients with poor outcomes and hematoma enlargement in one study (Worthmann et al., 2017).
While SDMA was not elevated in the plasma after ICH (Wanby et al., 2006; Worthmann et al., 2017; Mader et al., 2021), an increase was observed in the CSF (Mader et al., 2021). Plasma SDMA was identified as a predictor for poor outcomes in ICH (Worthmann et al., 2017) but was not confirmed in a different ICH study (Mader et al., 2021). SDMA was also measured in SAH, demonstrating an association between CSF SDMA and DCI (Appel et al., 2018; Hannemann et al., 2020) as well as poor outcomes (Koch et al., 2021).
Pathophysiological considerations
In summary, the heterogeneity in study design and the non-comprehensive nature of data do not allow to point out specific common pathways between different entities with certainty. A common feature reported across different studies is a reduction in L-arginine concentrations, often associated with an unfavorable clinical course (Figure 2). As previously stated, underlying pathophysiological consequences of a depletion of the NOS substrate L-arginine can be impairment of cerebral blood flow regulation (Cherian et al., 2004), oxidative stress via NOS uncoupling (Xia et al., 1996; Cherian et al., 2004) and an increased inflammatory reaction (Reynaert et al., 2004). The potentially detrimental role of elevated CSF L-ornithine described in SAH and ICH can be an indicator for increased arginase activity competing with NOS for L-arginine (Figure 2). Indeed, arginase has been reported to promote inflammation by decreasing the cellular content of NO resulting in an enhancement of the NF-κB pathway (Ckless et al., 2007) and to contributing to endothelial dysfunction in different diseases (Durante et al., 2007). In the context of experimental ischemic stroke, arginase inhibition via L-citrulline or L-ornithine administration demonstrated a neuroprotective effect (Barakat et al., 2018).
Figure 2.
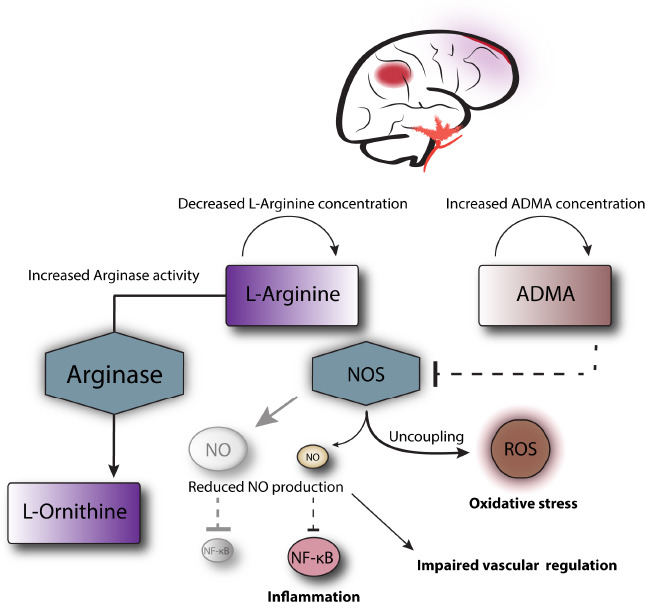
Potential alterations of the L-arginine metabolism in neurocritical care patients and downstream pathophysiological consequences are illustrated.
ADMA: Asymmetric dimethylarginine; NF-κB: nuclear factor κB; NO: nitric oxide; NOS: nitric oxide synthase; ROS: reactive oxygen species.
A detrimental association with intrathecal ADMA concentrations was found predominantly for SAH, where it correlated with CVS and DCI. Presumably, the underlying mechanism is NOS inhibition by ADMA (Figure 2) leading to impairment of endothelial NO production (Böger, 2006) and potentially uncoupling of NOS (Böger et al., 2000; Wells and Holian, 2007). Explanations for the association of SDMA and DCI in SAH and potentially poor outcome in ICH are less well established. Possibly, SDMA could impair intracellular L-arginine concentrations by competition for hCAT-2B (Closs et al., 1997; Böger, 2006). Moreover, it has been demonstrated that SDMA can cause eNOS uncoupling as well (Feliers et al., 2015) and may be involved in proinflammatory pathways by activation of NF-κB and stimulation of reactive oxygen species production (Schepers et al., 2009, 2011).
Alterations in the L-arginine and NO metabolism may also impact the function of endothelial progenitor cells (EPC), as shown in the context of other diseases. In diabetes, uncoupling of eNOS has been shown to impair EPC function and mobilization (Thum et al., 2007). A decrease in EPC in a model of ischemia/reperfusion injury was demonstrated to be rescued by L-arginine administration (Hsieh et al., 2018).
Recent and Ongoing Therapeutic Clinical Trials
A deeper understanding of the role of L-arginine metabolism in acute neurological diseases has built the basis for the development of new therapeutic strategies (Table 2).
Table 2.
Clinical therapeutic phase 2/3 trials investigating study drugs related to L-arginine metabolism (not considering studies investigating AVP derivates and pituitary hormones)
| ClinicalTrials.gov Identifier | Disease | Phase | Study substance |
|---|---|---|---|
| NCT02012582 | TBI | 2a | VAS203 (Ronopterin) |
| NCT02794168 | TBI | 3 | VAS203 (Ronopterin) |
| NCT03168581 | ICH | 2 | CN-105 (Ac-VSRRR- NH2) |
| NCT03711903 | ICH | 2 | CN-105 (Ac-VSRRR- NH2) |
|
| |||
| ClinicalTrials.gov Identifier | Study start | Status (February 2021) | Mechanism of action |
|
| |||
| NCT02012582 | 2009 | Completed 2012 | NOS inhibition |
| NCT02794168 | 2016 | Completed 2020 | NOS inhibition |
| NCT03168581 | 2017 | Completed 2020 | apoE signaling |
| NCT03711903 | 2019 | Recruiting | apoE signaling |
apoE: apolipoprotein E; AVP: arginine vasopression; ICH: intracerebral hemorrhage; NOS: nitric oxide synthase; TBI: traumatic brain injury.
A study substance closely related to the NO/L-arginine pathway is VAS203 (Ronopterin), which is currently evaluated in the NOSTRA-III study (ClinicalTrials.gov Identifier: NCT02794168), a phase 3 clinical trial evaluating the efficacy of VAS203 in patients with moderate and severe TBI (Tegtmeier et al., 2020). VAS203, 4-amino-(6R,S)-5, 8-tetrahydro-L-biopterin, acts as an inhibitor of NOS (Terpolilli et al., 2009). Notably, compared to other NOS inhibitors like L-arginine-methylester, VAS203 does not inhibit NOS at the L-arginine binding site but at the tetrahydrobiopterine cofactor binding site. This leads to a more NOS-specific binding pattern with a potentially improved side effect profile. The preclinical evaluation showed similar vasoconstrictive effects in vitro compared to L-arginine-methyl ester (Terpolilli et al., 2009). Intravenous administration after a controlled cortical impact in mice showed long-lasting prevention of ICP elevation or cerebral blood flow deterioration without affecting the mean arterial blood pressure. A significant improvement of the functional neurological status was observed at 6 days after trauma. VAS203 was shown to partially prevent posttraumatic arteriolar vasodilation suggesting inhibition of excess endothelial NO production as the underlying mechanism of action (Schwarzmaier et al., 2015). Based on these promising preclinical findings, a clinical phase 2a trial, NOSTRA, was conducted (ClinicalTrials.gov Identifier: NCT02012582) (Stover et al., 2014). A total of 32 patients from six European centers were included. VAS203 showed an overall good safety profile except for an association with acute kidney injury in patients treated with the highest dose. No significant effect was observed for intracranial pressure, cerebral perfusion pressure or partial brain oxygen pressure compared to placebo-treated control patients. Nitrate levels were measured via microdialysis but showed high variability and no effect between treatment groups was discernible. Therapy intensity levels increased continuously in the placebo group during the observation period whereas a decrease was observed in the treatment group. Functional neurological outcome after 6 months was improved in VAS203 treated patients compared to the placebo group. The NOSTRA-III study aims to confirm the positive effect on neurological outcomes (Tegtmeier et al., 2020). The actual study completion date was June 30, 2020, and the study has completed multicentric recruitment in Europe with 224 participants.
A second study substance identified from searching ClinicalTrials.gov is CN-105, which is a cationic arginine-rich peptide, a novel class of peptides with potential neuroprotective properties (Edwards et al., 2020). It is currently evaluated for therapeutic use in patients suffering from ICH in two phase 2 clinical trials. The S-CATCH study (ClinicalTrials.gov Identifier: NCT03711903) is currently actively recruiting while the CATCH study (ClinicalTrials.gov Identifier: NCT03168581) has enrolled 38 participants with January 25, 2020, as the actual study completion date. Notably, CN-105 (Ac-VSRRR-amide) is an apolipoprotein E-mimetic peptide assumed to mediate anti-inflammatory and neuroprotective responses via apolipoprotein E signaling (Lei et al., 2016; Guptill et al., 2017; Wang et al., 2021). It is therefore demonstrating a mechanism distinct from the actual L-arginine metabolism primarily addressed in this review. A preclinical study in a murine model of ICH was able to show a decrease in neuroinflammation paired with improved vestibulomotor and neurocognitive performance (Lei et al., 2016). A phase 1 clinical trial confirmed safety in human subjects (Guptill et al., 2017). Preclinical studies in models of TBI and SAH suggested a reduction in neuroinflammation and improved functional outcomes as well (Laskowitz et al., 2017; Liu et al., 2018).
Conclusions
The metabolism of L-arginine is directly related to the production of NO via NOS and metabolic alterations can lead to pathological sequels in acute neurological diseases. The most common critical neurological conditions requiring treatment on a neurocritical care unit are ICH, aneurysmal SAH and TBI. Despite very distinct underlying mechanisms of injury, pathophysiological mechanisms and clinical courses, alterations of the L-arginine metabolism are a common hallmark and appear to be associated with secondary brain injury and/or complications like DCI. Metabolic changes are complex due to specificity for cell type and location, enzyme subtype, timepoint after disease onset, underlying pathology, clinical features and severity of disease, as well as exogenous factors. A common pathological feature between the different diseases observed in several studies is the development of an absolute or relative L-arginine deficiency potentially associated with poor outcomes. Inhibition of NOS by the endogenous metabolite ADMA has been implicated to have detrimental effects particularly in the context of vasospasm after aneurysmal SAH. In contrast, NOS inhibition with the exogenous agent VAS203 showed promising results in a phase 2 clinical trial in TBI patients. Overall, L-arginine and NO metabolism have been identified as relevant pathways in the pathophysiological response in different neurocritical conditions. Future studies may contribute to a more detailed spatiotemporal understanding and help to identify novel therapeutic targets.
Footnotes
Conflicts of interest: Marius M. Mader and Patrick Czorlich have acted as sub investigators for the NOSTRA-III trial (NCT02794168).
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Liu WJ, Wang L; T-Editor: Jia Y.
References
- 1.Ahn M, Lee C, Jung K, Kim H, Moon C, Sim KB, Shin T. Immunohistochemical study of arginase-1 in the spinal cords of rats with clip compression injury. Brain Res. 2012;1445:11–19. doi: 10.1016/j.brainres.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Akar E, Emon ST, Uslu S, Orakdogen M, Somay H. Effect of L-Arginine Therapy on Vasospasm: Experimental Study in Rats. World Neurosurg. 2019;132:e443–446. doi: 10.1016/j.wneu.2019.08.119. [DOI] [PubMed] [Google Scholar]
- 3.Amorini AM, Lazzarino G, Di Pietro V, Signoretti S, Lazzarino G, Belli A, Tavazzi B. Severity of experimental traumatic brain injury modulates changes in concentrations of cerebral free amino acids. J Cell Mol Med. 2017;21:530–542. doi: 10.1111/jcmm.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3–10. doi: 10.5853/jos.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel D, Seeberger M, Schwedhelm E, Czorlich P, Goetz AE, Böger RH, Hannemann J. Asymmetric and symmetric dimethylarginines are markers of delayed cerebral ischemia and neurological outcome in patients with subarachnoid hemorrhage. Neurocrit Care. 2018;29:84–93. doi: 10.1007/s12028-018-0520-1. [DOI] [PubMed] [Google Scholar]
- 6.Barakat W, Fahmy A, Askar M, El-Kannishy S. Effectiveness of arginase inhibitors against experimentally induced stroke. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:603–612. doi: 10.1007/s00210-018-1489-1. [DOI] [PubMed] [Google Scholar]
- 7.Batulu H, Du GJ, Li DZ, Sailike D, Fan YH, Geng D. Effect of poly-arginine R18 on neurocyte cell growth via autophagy in traumatic brain injury. Exp Ther Med. 2019;17:4109–4115. doi: 10.3892/etm.2019.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergström A, Staalsø JM, Romner B, Olsen NV. Impaired endothelial function after aneurysmal subarachnoid haemorrhage correlates with arginine:asymmetric dimethylarginine ratio. Br J Anaesth. 2014;112:311–318. doi: 10.1093/bja/aet331. [DOI] [PubMed] [Google Scholar]
- 9.Bode-Böger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006;17:1128–1134. doi: 10.1681/ASN.2005101119. [DOI] [PubMed] [Google Scholar]
- 10.Böger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med. 2006;38:126–136. doi: 10.1080/07853890500472151. [DOI] [PubMed] [Google Scholar]
- 11.Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 12.Böger RH, Bode-Böger SM, Tsao PS, Lin PS, Chan JR, Cooke JP. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J Am Coll Cardiol. 2000;36:2287–2295. doi: 10.1016/s0735-1097(00)01013-5. [DOI] [PubMed] [Google Scholar]
- 13.Busl KM, Bleck TP, Varelas PN. Neurocritical care outcomes, research, and technology: a review. JAMA Neurol. 2019;76:612–618. doi: 10.1001/jamaneurol.2018.4407. [DOI] [PubMed] [Google Scholar]
- 14.Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu LS, Anderton RS, Cross JL, Clark VW, Edwards AB, Knuckey NW, Meloni BP. Assessment of R18, COG1410, and APP96-110 in excitotoxicity and traumatic brain injury. Transl Neurosci. 2017;8:147–157. doi: 10.1515/tnsci-2017-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu LS, Anderton RS, Cross JL, Clark VW, Knuckey NW, Meloni BP. Poly-arginine peptide R18D reduces neuroinflammation and functional deficits following traumatic brain injury in the long-evans rat. Int J Pept Res Ther. 2019;25:1563–1572. [Google Scholar]
- 17.Chiu LS, Anderton RS, Clark VW, Cross JL, Knuckey NW, Meloni BP. Effect of polyarginine peptide R18D following a traumatic brain injury in Sprague-Dawley rats. Curr Ther Res Clin Exp. 2020;92:100584. doi: 10.1016/j.curtheres.2020.100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ckless K, van der Vliet A, Janssen-Heininger Y. Oxidative-nitrosative stress and post-translational protein modifications: implications to lung structure-function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. Am J Respir Cell Mol Biol. 2007;36:645–653. doi: 10.1165/rcmb.2006-0329SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Closs EI, Basha FZ, Habermeier A, Förstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 20.Davids M, Ndika JD, Salomons GS, Blom HJ, Teerlink T. Promiscuous activity of arginine:glycine amidinotransferase is responsible for the synthesis of the novel cardiovascular risk factor homoarginine. FEBS Lett. 2012;586:3653–3657. doi: 10.1016/j.febslet.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards AB, Mastaglia FL, Knuckey NW, Meloni BP. Neuroprotective cationic arginine-rich peptides (CARPs): an assessment of their clinical safety. Drug Saf. 2020;43:957–969. doi: 10.1007/s40264-020-00962-z. [DOI] [PubMed] [Google Scholar]
- 23.Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and Meta-analysis. JAMA Neurol. 2019;76:588–597. doi: 10.1001/jamaneurol.2019.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feliers D, Lee DY, Gorin Y, Kasinath BS. Symmetric dimethylarginine alters endothelial nitric oxide activity in glomerular endothelial cells. Cell Signal. 2015;27:1–5. doi: 10.1016/j.cellsig.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KT. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Grosse GM, Schwedhelm E, Worthmann H, Choe CU. Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. Int J Mol Sci. 2020;21:1798. doi: 10.3390/ijms21051798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guptill JT, Raja SM, Boakye-Agyeman F, Noveck R, Ramey S, Tu TM, Laskowitz DT. Phase 1 randomized, double-blind, placebo-controlled study to determine the safety, tolerability, and pharmacokinetics of a single escalating dose and repeated doses of CN-105 in healthy adult subjects. J Clin Pharmacol. 2017;57:770–776. doi: 10.1002/jcph.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannemann J, Appel D, Seeberger-Steinmeister M, Bruning T, Zummack J, Böger R. Sequence variation in the DDAH1 gene predisposes for delayed cerebral ischemia in subarachnoidal hemorrhage. J Clin Med. 2020;9:3900. doi: 10.3390/jcm9123900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh KF, Shih JM, Shih YM, Pai MH, Yeh SL. Arginine administration increases circulating endothelial progenitor cells and attenuates tissue injury in a mouse model of hind limb ischemia/reperfusion. Nutrition. 2018;55-56:29–35. doi: 10.1016/j.nut.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Iaccarino C, Carretta A, Nicolosi F, Morselli C. Epidemiology of severe traumatic brain injury. J Neurosurg Sci. 2018;62:535–541. doi: 10.23736/S0390-5616.18.04532-0. [DOI] [PubMed] [Google Scholar]
- 31.Jeter CB, Hergenroeder GW, Ward NH, 3rd, Moore AN, Dash PK. Human traumatic brain injury alters circulating L-arginine and its metabolite levels: possible link to cerebral blood flow, extracellular matrix remodeling, and energy status. J Neurotrauma. 2012;29:119–127. doi: 10.1089/neu.2011.2029. [DOI] [PubMed] [Google Scholar]
- 32.Jung CS, Iuliano BA, Harvey-White J, Espey MG, Oldfield EH, Pluta RM. Association between cerebrospinal fluid levels of asymmetric dimethyl-L-arginine, an endogenous inhibitor of endothelial nitric oxide synthase, and cerebral vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg. 2004;101:836–842. doi: 10.3171/jns.2004.101.5.0836. [DOI] [PubMed] [Google Scholar]
- 33.Jung CS, Oldfield EH, Harvey-White J, Espey MG, Zimmermann M, Seifert V, Pluta RM. Association of an endogenous inhibitor of nitric oxide synthase with cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:945–950. doi: 10.3171/JNS-07/11/0945. [DOI] [PubMed] [Google Scholar]
- 34.Jung CS, Lange B, Zimmermann M, Seifert V. The CSF concentration of ADMA, but not of ET-1, is correlated with the occurrence and severity of cerebral vasospasm after subarachnoid hemorrhage. Neurosci Lett. 2012;524:20–24. doi: 10.1016/j.neulet.2012.06.076. [DOI] [PubMed] [Google Scholar]
- 35.Jung CS, Lange B, Zimmermann M, Seifert V. Role of endogenous monomethylated L-arginine (L-NMMA) after subarachnoid hemorrhage. Neurol Res. 2013;35:709–712. doi: 10.1179/1743132813Y.0000000194. [DOI] [PubMed] [Google Scholar]
- 36.Jung CS, Wispel C, Zweckberger K, Beynon C, Hertle D, Sakowitz OW, Unterberg AW. Endogenous nitric-oxide synthase inhibitor ADMA after acute brain injury. Int J Mol Sci. 2014;15:4088–4103. doi: 10.3390/ijms15034088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch M, Acharjee A, Ament Z, Schleicher R, Bevers M, Stapleton C, Patel A, Kimberly WT. Machine learning-driven metabolomic evaluation of cerebrospinal fluid: insights into poor outcomes after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2021;88:1003–1011. doi: 10.1093/neuros/nyaa557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotagale NR, Taksande BG, Inamdar NN. Neuroprotective offerings by agmatine. Neurotoxicology. 2019;73:228–245. doi: 10.1016/j.neuro.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Laskowitz DT, Wang H, Chen T, Lubkin DT, Cantillana V, Tu TM, Kernagis D, Zhou G, Macy G, Kolls BJ, Dawson HN. Neuroprotective pentapeptide CN-105 is associated with reduced sterile inflammation and improved functional outcomes in a traumatic brain injury murine model. Sci Rep. 2017;7:46461. doi: 10.1038/srep46461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med. 2017;377:257–266. doi: 10.1056/NEJMcp1605827. [DOI] [PubMed] [Google Scholar]
- 41.Lei B, James ML, Liu J, Zhou G, Venkatraman TN, Lascola CD, Acheson SK, Dubois LG, Laskowitz DT, Wang H. Neuroprotective pentapeptide CN-105 improves functional and histological outcomes in a murine model of intracerebral hemorrhage. Sci Rep. 2016;6:34834. doi: 10.1038/srep34834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542–548. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Wu W, Liu M, Zhang X, Zhang QR, Ni L, Hang CH. Increased cerebrospinal fluid concentrations of asymmetric dimethylarginine correlate with adverse clinical outcome in subarachnoid hemorrhage patients. J Clin Neurosci. 2014;21:1404–1408. doi: 10.1016/j.jocn.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 44.Li N, Worthmann H, Deb M, Chen S, Weissenborn K. Nitric oxide (NO) and asymmetric dimethylarginine (ADMA): their pathophysiological role and involvement in intracerebral hemorrhage. Neurol Res. 2011;33:541–548. doi: 10.1179/016164111X13007856084403. [DOI] [PubMed] [Google Scholar]
- 45.Liddle L, Reinders R, South S, Blacker D, Knuckey N, Colbourne F, Meloni B. Poly-arginine-18 peptides do not exacerbate bleeding, or improve functional outcomes following collagenase-induced intracerebral hemorrhage in the rat. PLoS One. 2019;14:e0224870. doi: 10.1371/journal.pone.0224870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindgren C, Hultin M, Koskinen LO, Lindvall P, Borota L, Naredi S. ADMA levels and arginine/ADMA ratios reflect severity of disease and extent of inflammation after subarachnoid hemorrhage. Neurocrit Care. 2014;21:91–101. doi: 10.1007/s12028-013-9945-8. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Zhou G, Kolls BJ, Tan Y, Fang C, Wang H, Laskowitz DT. Apolipoprotein E mimetic peptide CN-105 improves outcome in a murine model of SAH. Stroke Vasc Neurol. 2018;3:222–230. doi: 10.1136/svn-2018-000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Logsdon AF, Meabon JS, Cline MM, Bullock KM, Raskind MA, Peskind ER, Banks WA, Cook DG. Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci Rep. 2018;8:11344. doi: 10.1038/s41598-018-29341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Logsdon AF, Schindler AG, Meabon JS, Yagi M, Herbert MJ, Banks WA, Raskind MA, Marshall DA, Keene CD, Perl DP, Peskind ER, Cook DG. Nitric oxide synthase mediates cerebellar dysfunction in mice exposed to repetitive blast-induced mild traumatic brain injury. Sci Rep. 2020;10:9420. doi: 10.1038/s41598-020-66113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madan S, Kron B, Jin Z, Al Shamy G, Campeau PM, Sun Q, Chen S, Cherian L, Chen Y, Munivez E, Jiang MM, Robertson C, Goodman C, Ratan RR, Lee B. Arginase overexpression in neurons and its effect on traumatic brain injury. Mol Genet Metab. 2018;125:112–117. doi: 10.1016/j.ymgme.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mader MM, Böger R, Appel D, Schwedhelm E, Haddad M, Mohme M, Lamszus K, Westphal M, Czorlich P, Hannemann J. Intrathecal and systemic alterations of L-arginine metabolism in patients after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2021 doi: 10.1177/0271678X20983216. 271678x20983216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maegele M, Lefering R, Sakowitz O, Kopp MA, Schwab JM, Steudel WI, Unterberg A, Hoffmann R, Uhl E, Marzi I. The incidence and management of moderate to severe head injury. Dtsch Arztebl Int. 2019;116:167–173. doi: 10.3238/arztebl.2019.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, Maas A. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health. 2016;1:e76–83. doi: 10.1016/S2468-2667(16)30017-2. [DOI] [PubMed] [Google Scholar]
- 54.Mohme M, Sauvigny T, Mader MM, Schweingruber N, Maire CL, Rünger A, Ricklefs F, Regelsberger J, Schmidt NO, Westphal M, Lamszus K, Tolosa E, Czorlich P. Immune characterization in aneurysmal subarachnoid hemorrhage reveals distinct monocytic activation and chemokine patterns. Transl Stroke Res. 2020;11:1348–1361. doi: 10.1007/s12975-019-00764-1. [DOI] [PubMed] [Google Scholar]
- 55.Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 56.Netlyukh A. [Pathological changes in the microstructure of the sensomotor cortex of white rats with experimental subarachnoid hemorrhage and after experimental influences] Wiad Lek. 2016;69:243–248. [PubMed] [Google Scholar]
- 57.Ng SY, Lee AYW. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528. doi: 10.3389/fncel.2019.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pluta RM, Thompson BG, Dawson TM, Snyder SH, Boock RJ, Oldfield EH. Loss of nitric oxide synthase immunoreactivity in cerebral vasospasm. J Neurosurg. 1996;84:648–654. doi: 10.3171/jns.1996.84.4.0648. [DOI] [PubMed] [Google Scholar]
- 59.Pradilla G, Garzon-Muvdi T, Ruzevick JJ, Bender M, Edwards L, Momin EN, Thompson RC, Tamargo RJ. Systemic L-citrulline prevents cerebral vasospasm in haptoglobin 2-2 transgenic mice after subarachnoid hemorrhage. Neurosurgery. 2012;70:747–756. doi: 10.1227/NEU.0b013e3182363c2f. discussion 756-747. [DOI] [PubMed] [Google Scholar]
- 60.Rashid PA, Whitehurst A, Lawson N, Bath PM. Plasma nitric oxide (nitrate/nitrite) levels in acute stroke and their relationship with severity and outcome. J Stroke Cerebrovasc Dis. 2003;12:82–87. doi: 10.1053/jscd.2003.9. [DOI] [PubMed] [Google Scholar]
- 61.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodling-Wahlstrom M, Olivecrona M, Koskinen LO, Naredi S, Hultin M. Subarachnoid haemorrhage induces an inflammatory response followed by a delayed persisting increase in asymmetric dimethylarginine. Scand J Clin Lab Invest. 2012;72:484–489. doi: 10.3109/00365513.2012.699098. [DOI] [PubMed] [Google Scholar]
- 63.Schepers E, Glorieux G, Dhondt A, Leybaert L, Vanholder R. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol Dial Transplant. 2009;24:1429–1435. doi: 10.1093/ndt/gfn670. [DOI] [PubMed] [Google Scholar]
- 64.Schepers E, Barreto DV, Liabeuf S, Glorieux G, Eloot S, Barreto FC, Massy Z, Vanholder R. Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2374–2383. doi: 10.2215/CJN.01720211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwarzmaier SM, Terpolilli NA, Dienel A, Gallozzi M, Schinzel R, Tegtmeier F, Plesnila N. Endothelial nitric oxide synthase mediates arteriolar vasodilatation after traumatic brain injury in mice. J Neurotrauma. 2015;32:731–738. doi: 10.1089/neu.2014.3650. [DOI] [PubMed] [Google Scholar]
- 66.Staalso JM, Bergström A, Edsen T, Weikop P, Romner B, Olsen NV. Low plasma arginine:asymmetric dimethyl arginine ratios predict mortality after intracranial aneurysm rupture. Stroke. 2013;44:1273–1281. doi: 10.1161/STROKEAHA.111.000605. [DOI] [PubMed] [Google Scholar]
- 67.Stover JF, Belli A, Boret H, Bulters D, Sahuquillo J, Schmutzhard E, Zavala E, Ungerstedt U, Schinzel R, Tegtmeier F. Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: a placebo-controlled randomized Phase IIa trial (NOSTRA) J Neurotrauma. 2014;31:1599–1606. doi: 10.1089/neu.2014.3344. [DOI] [PubMed] [Google Scholar]
- 68.Tain YL, Hsu CN. Toxic dimethylarginines: asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) Toxins (Basel) 2017;9:92. [Google Scholar]
- 69.Tatlisumak T, Cucchiara B, Kuroda S, Kasner SE, Putaala J. Nontraumatic intracerebral haemorrhage in young adults. Nat Rev Neurol. 2018;14:237–250. doi: 10.1038/nrneurol.2018.17. [DOI] [PubMed] [Google Scholar]
- 70.Tegtmeier F, Schinzel R, Beer R, Bulters D, LeFrant JY, Sahuquillo J, Unterberg A, Andrews P, Belli A, Ibanez J, Lagares A, Mokry M, Willschke H, Flüh C, Schmutzhard E. Efficacy of Ronopterin (VAS203) in Patients with Moderate and Severe Traumatic Brain Injury (NOSTRA phase III trial): study protocol of a confirmatory, placebo-controlled, randomised, double blind, multi-centre study. Trials. 2020;21:80. doi: 10.1186/s13063-019-3965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Terpolilli NA, Zweckberger K, Trabold R, Schilling L, Schinzel R, Tegtmeier F, Plesnila N. The novel nitric oxide synthase inhibitor 4-amino-tetrahydro-L-biopterine prevents brain edema formation and intracranial hypertension following traumatic brain injury in mice. J Neurotrauma. 2009;26:1963–1975. doi: 10.1089/neu.2008.0853. [DOI] [PubMed] [Google Scholar]
- 72.Thampatty BP, Klamerus MM, Oberly PJ, Feldman KL, Bell MJ, Tyler-Kabara EC, Adelson PD, Clark RS, Kochanek PM, Poloyac SM. Hypothermia decreases cerebrospinal fluid asymmetric dimethylarginine levels in children with traumatic brain injury. Pediatr Crit Care Med. 2013;14:403–412. doi: 10.1097/PCC.0b013e31827212c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 74.Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. Cardiovasc Pharmacol. 1992;20(Suppl 12):S60–62. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 75.van Lieshout JH, Dibué-Adjei M, Cornelius JF, Slotty PJ, Schneider T, Restin T, Boogaarts HD, Steiger HJ, Petridis AK, Kamp MA. An introduction to the pathophysiology of aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2018;41:917–930. doi: 10.1007/s10143-017-0827-y. [DOI] [PubMed] [Google Scholar]
- 76.Villalba N, Sackheim AM, Nunez IA, Hill-Eubanks DC, Nelson MT, Wellman GC, Freeman K. Traumatic brain injury causes endothelial dysfunction in the systemic microcirculation through arginase-1-dependent uncoupling of endothelial nitric oxide synthase. J Neurotrauma. 2017;34:192–203. doi: 10.1089/neu.2015.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wahlström MR, Olivecrona M, Ahlm C, Bengtsson A, Koskinen LO, Naredi S, Hultin M. Effects of prostacyclin on the early inflammatory response in patients with traumatic brain injury-a randomised clinical study. Springerplus. 2014;3:98. doi: 10.1186/2193-1801-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wanby P, Teerlink T, Brudin L, Brattstrom L, Nilsson I, Palmqvist P, Carlsson M. Asymmetric dimethylarginine (ADMA) as a risk marker for stroke and TIA in a Swedish population. Atherosclerosis. 2006;185:271–277. doi: 10.1016/j.atherosclerosis.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 79.Wang H, Faw TD, Lin Y, Huang S, Venkatraman TN, Cantillana V, Lascola CD, James ML, Laskowitz DT. Neuroprotective pentapeptide, CN-105, improves outcomes in translational models of intracerebral hemorrhage. Neurocrit Care. 2021 doi: 10.1007/s12028-020-01184-y. doi: 10.1007/s12028-020-01184-y. [DOI] [PubMed] [Google Scholar]
- 80.Weiland J, Beez A, Westermaier T, Kunze E, Sirén AL, Lilla N. Neuroprotective strategies in aneurysmal subarachnoid hemorrhage (aSAH) Int J Mol Sci. 2021;22:5442. doi: 10.3390/ijms22115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wells SM, Holian A. Asymmetric dimethylarginine induces oxidative and nitrosative stress in murine lung epithelial cells. Am J Respir Cell Mol Biol. 2007;36:520–528. doi: 10.1165/rcmb.2006-0302SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, Xi G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology. 2018;134:240–248. doi: 10.1016/j.neuropharm.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Worthmann H, Li N, Martens-Lobenhoffer J, Dirks M, Schuppner R, Lichtinghagen R, Kielstein JT, Raab P, Lanfermann H, Bode-Böger SM, Weissenborn K. Dimethylarginines in patients with intracerebral hemorrhage: association with outcome, hematoma enlargement, and edema. J Neuroinflammation. 2017;14:247. doi: 10.1186/s12974-017-1016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao D, Liu Q, Ji Y, Wang G, He X, Tian W, Xu H, Lei T, Wang Y. Effect of 18beta-glycyrrhetinic acid on cerebral vasospasm caused by asymmetric dimethylarginine after experimental subarachnoid hemorrhage in rats. Neurol Res. 2015;37:476–483. doi: 10.1179/1743132814Y.0000000462. [DOI] [PubMed] [Google Scholar]
- 86.Zheng F, Zhou YT, Feng DD, Li PF, Tang T, Luo JK, Wang Y. Metabolomics analysis of the hippocampus in a rat model of traumatic brain injury during the acute phase. Brain Behav. 2020;10:e01520. doi: 10.1002/brb3.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]


