Abstract
Spinal cord injury represents a devastating central nervous system injury that could impair the mobility and sensory function of afflicted patients. The hallmarks of spinal cord injury include neuroinflammation, axonal degeneration, neuronal loss, and reactive gliosis. Furthermore, the formation of a glial scar at the injury site elicits an inhibitory environment for potential neuroregeneration. Besides axonal regeneration, a significant challenge in treating spinal cord injury is to replenish the neurons lost during the pathological process. However, despite decades of research efforts, current strategies including stem cell transplantation have not resulted in a successful clinical therapy. Furthermore, stem cell transplantation faces serious hurdles such as immunorejection of the transplanted cells and ethical issues. In vivo neuronal reprogramming is a recently developed technology and leading a major breakthrough in regenerative medicine. This innovative technology converts endogenous glial cells into functional neurons for injury repair in the central nervous system. The feasibility of in vivo neuronal reprogramming has been demonstrated successfully in models of different neurological disorders including spinal cord injury by numerous laboratories. Several reprogramming factors, mainly the pro-neural transcription factors, have been utilized to reprogram endogenous glial cells into functional neurons with distinct phenotypes. So far, the literature on in vivo neuronal reprogramming in the model of spinal cord injury is still small. In this review, we summarize a limited number of such reports and discuss several questions that we think are important for applying in vivo neuronal reprogramming in the research field of spinal cord injury as well as other central nervous system disorders.
Keywords: astrocyte, microRNA, NeuroD1, neuronal relay, neuronal reprogramming, NG2 glia, pericyte, reactive gliosis, Sox2, spinal cord injury
Introduction
Spinal cord injury (SCI) is a devastating neurological disorder that often impairs the daily function of patients for their entire life (McDonald and Sadowsky, 2002; Gupta et al., 2010; Boakye et al., 2012). While rodent SCI models have offered substantial understanding about the molecular and cellular mechanisms, there is still no effective therapy yielding significant functional recovery in SCI patients (Nori et al., 2017). Thus far, the only Food and Drug Administration-approved treatment for SCI patients in the United States is to give a high dose of methylprednisolone within 8 hours after the injury. By this acute treatment, about 20% of motor function can be preserved (Bracken et al., 1990). However, the potential of methylprednisolone for increased complications makes its application very controversial (Hurlbert, 2000; Ahuja et al., 2017). SCI induces a cascade of pathological changes that eventually lead to axonal degeneration, loss of neurons, and reactive gliosis; the glial scar that is formed primarily by reactive astrocytes often persists long after SCI is inflicted (Norenberg et al., 2004). Furthermore, the inhibitory environment of the glial scar at the injury site impedes axonal regeneration (Burda and Sofroniew, 2014; Cregg et al., 2014; O’Shea et al., 2017).
Besides axonal regeneration, one major challenge facing the SCI field is how to regenerate new functional neurons in the injury site in order to restore the lost functions. While stem cell therapy offers great promise for regenerating new neurons upon grafting into the injury site, immunosuppression is required to ensure the survival of engrafted stem cells including those of human origin (Lu et al., 2017; Kumamaru et al., 2019). The induced pluripotent stem cells (iPSCs) appear to have lower ethical and immunogenic concerns than other stem cells, because they can be derived from patient's own cells to overcome immunorejection when used for transplantation (Marchetto et al., 2010; Brennand et al., 2011; Okita et al., 2011; Zhao et al., 2011; Araki et al., 2013; Okano et al., 2013). In fact, human iPSCs have been experimentally transplanted, and shown to differentiate into neuronal cell types and promote functional recovery after SCI in mice (Nori et al., 2011; Lu et al., 2014). The iPSCs resemble the embryonic stem cells and can be propagated in culture prior to differentiation into different neuronal cell types in a controlled manner (Marei et al., 2017). However, the iPSCs still suffer from potential problems such as tumorigenesis after transplantation (Lee et al., 2013). Besides stem cells, researchers have also transplanted other cell types such as Schwann cells, radial glia, and olfactory ensheathing cells (Chen et al., 1996; Ramon-Cueto et al., 1998; Hasegawa et al., 2005). However, like stem cell transplantation, most of the cell replacement therapies that appear to improve functional recovery in rodents have not shown a significant effect in clinical trials (Feron et al., 2005).
In vivo neuronal reprogramming has recently emerged as a novel technology to regenerate new neurons from endogenous glial cells by overexpression of neurogenic transcription factors in the central nervous system (CNS) (Li and Chen, 2016). This approach completely eliminates the critical problem of immunorejection that the cell transplantation therapy is facing. Thus far, in vivo neuronal reprogramming has been successfully demonstrated in different laboratories with different disease models (Tai et al., 2020). In the injured spinal cord, the transcription factor Sox2 has been shown to reprogram astrocytes (Su et al., 2014; Wang et al., 2016) and NG2 glia (Tai et al., 2021) into proliferating neuroblasts, which can further differentiate into mature neurons with additional treatments. In addition, the combination of growth factor treatment and forced expression of the transcription factor Ngn2 is also able to stimulate neurogenesis from neural progenitors in the injured spinal cord (Ohori et al., 2006). Recently, our research has indicated that NeuroD1 can convert reactive astrocytes into functional neurons in the spinal cord dorsal horn with high efficiency (Puls et al., 2020).
Given the unique anatomical structure of the spinal cord, a major focus in SCI research has been on axonal regeneration, i.e. to regrow the severed axons through the injury site and reconnect with downstream targets. Reprogrammed neurons can potentially form a “neuronal relay” passing information over the injury site. Furthermore, in vivo reprogramming can replenish propriospinal neurons that are lost during the injury to facilitate the rebuild of local neuronal circuits, which is required to achieve functional recovery after SCI (Laliberte et al., 2019). Lastly, in vivo reprogramming may alleviate the glial scar formation by reducing the number of reactive glial cells and improving the inhibitory environment at the injury site for better endogenous regeneration (Tai et al., 2021). Below, we review a limited number of reports on in vivo neuronal reprogramming in the spinal cord (Table 1) and discuss several strategic questions on applying this technology to SCI research.
Table 1.
In vivo neuronal reprogramming in the spinal cord
| Cell source | Gene delivery method (timing) | Reprogramming factor | Efficiency (NeuN+) | Neuronal subtype | SCI model | Functional recovery | References |
|---|---|---|---|---|---|---|---|
| Neural progenitors | Retrovirus (0 dpi) | Ngn2 | 3%; 21.1% (+GFs); 28.2% (+GFs+BDNF) | GABAergic (major) | Complete transection (T10) | N/A | Ohori et al., 2006 |
| Astrocytes | Lentivirus (0 dpi) | Sox2 | 3%; 6% (+VPA) | Glutamatergic, GABAergic | Lateral hemisection (T7–T9) | N/A | Su et al., 2014 |
| Astrocytes | Lentivirus (0 dpi) | Sox2 + shRNA-p53 + BDNF-NOG | 20000* | Glutamatergic (major) | N/A | N/A | Wang et al., 2016 |
| Astrocytes | Lentivirus (0 dpi) | Sox2 + shRNA-p53 + BDNF-NOG | 6000* | N/A | Contusive injury (T7–T9) | N/A | Wang et al., 2016 |
| Astrocytes | Retrovirus (4 dpi), AAV (0 dpi) | NeuroD1 | 93.5–95% | Glutamatergic (major), GABAergic↑ by NeuroD1+Dlx2 | Stab injury (T11–T12) | N/A | Puls et al., 2020 |
| Astrocytes | AAV (10 dpi, 16 wpi) | NeuroD1 | > 95% | Glutamatergic | Contusive injury (T11–T12) | N/A | Puls et al., 2020 |
| NG2 glia | Lentivirus (0 dpi) | Sox2 | 5000; 44000 (+ BDNF-NOG); 84000 (+P75-2)* | Glutamatergic, GABAergic, glycinergic | N/A | N/A | Tai et al., 2021 |
| NG2 glia | Lentivirus (1 wpi) | SOX2 + p75-2 | 45.80% | N/A | Dorsal hemisection (C5) | Forelimb motor skill | Tai et al., 2021 |
AAV: Adeno-associated virus; BDNF: brain-derived neurotrophic factor; dpi: day(s) post injury; GFs: growth factors; NOG: noggin; N/A: not available; p75-2: a mutant form of the neurotrophic factor NT3; SCI: spinal cord injury; VPA: valproic acid; wpi: week(s) post injury; *: the number of neurons per injection.
Search Strategy
A PubMed search was conducted between March and May 2021 by using keywords including neuronal reprogramming, spinal cord injury, stem cell transplantation, axon regeneration, NeuroD1, Sox2, Ngn2, microRNAs, pro-neural transcription factors, astrocytes, oligodendrocyte progenitors, NG2 glia, microglia, pericytes.
Neuronal Reprogramming Factors
Sox2 is a transcription factor for maintaining neural progenitor and stem cell identity. Therefore, persistent expression of Sox2 actually inhibits neuronal differentiation from stem cells. In the injured spinal cord, Sox2 has been shown to reprogram astrocytes into proliferating neuroblasts, which can further differentiate into mature neurons with additional treatments (Su et al., 2014; Wang et al., 2016). In this model, Sox2 is expressed under the control of human GFAP (hGFAP) promoter, which produces a high level of Sox2 expression in astrocytes but reduced level in reprogrammed neuroblasts allowing further neuronal differentiation. This dynamic Sox2 expression during the reprogramming process is important since persistent Sox2 expression hinders neuronal reprogramming if it happens at all (Su et al., 2014). However, even with a reduced level of Sox2 expression achieved by low hGFAP promoter activity in reprogrammed neuroblasts, additional factors such as brain-derived neurotrophic factor and valproic acid are needed for neuronal differentiation and maturation (Su et al., 2014; Wang et al., 2016). With that being said, cautions need to be taken for potential tumorigenicity of reprogrammed cells with an even residual level of Sox2 expression in the long term. On the other hand, one major advantage of Sox2-mediated neuronal reprogramming is the production of clonal neuronal progeny (appeared as clusters) from the proliferation of Sox2-induced neuroblasts (Wang et al., 2016) and thus higher number of reprogrammed neurons than astrocyte-to-neuron direct reprogramming.
NeuroD1 is widely expressed and critical to neuronal differentiation in the developing CNS. As a terminal differentiation factor, unlike Sox2, NeuroD1 inhibits cell proliferation and exerts glia-to-neuron direct reprogramming without going through a proliferating neuroblast stage (Guo et al., 2014). NeuroD1 is also a survival factor in granule neurons of the cerebellum and hippocampus, where it is highly expressed throughout adulthood in the mouse (Miyata et al., 1999). Furthermore, NeuroD1 is a basic helix-loop-helix transcription factor that can bind to the E-box DNA enhancer elements (Massari and Murre, 2000; Seo et al., 2007). Transcription factors that can bind the nucleosome DNA sequences and open up the chromatin for other factors to bind, are referred to as “pioneer” factors (Iwafuchi-Doi and Zaret, 2014). As a pioneer factor, NeuroD1 reprograms the chromatin landscape to elicit neuronal programming in embryonic stem cells (Pataskar et al., 2016) and microglia (Matsuda et al., 2019) when overexpressed. Ectopic expression of NeuroD1 can induce expression of endogenous NeuroD1 as well as other downstream target genes such as Hes6 and NeuroD4 (Pataskar et al., 2016), which may be important effectors for NeuroD1-mediated neuronal conversion.
Cell death is common during neuronal reprogramming since the converting cells undergo dramatic metabolic changes, during which cells either overcome the “checkpoint” and survive, or undergo apoptosis (Gascon et al., 2016). When combined with Bcl2, an anti-apoptotic gene, Ngn2-mediated neuronal conversion acquires a much higher efficiency, although the authors claim that Bcl2 plays an additional role that is independent of apoptotic pathways (Gascon et al., 2016). An earlier report also showed that Ngn2-expressing retrovirus is able to promote neurogenesis in the injured spinal cord, but the number of newly generated neurons greatly decreases over time even when combined with neurotrophic factor treatment (Ohori et al., 2006). In sharp contrast, we rarely observe apoptotic cells during and after NeuroD1-mediated conversion as determined by the TUNEL assay (Puls et al., 2020). This difference in cell survival in neurons converted by different transcription factors may be explained by the fact that NeuroD1 is not only a reprogramming factor but also a survival factor. During development, NeuroD1 is required for survival of a variety of neuron types in the developing and adult CNS (Miyata et al., 1999; Morrow et al., 1999; Gao et al., 2009). This dual role of NeuroD1 during neuronal conversion may explain its higher conversion efficiency over other reprogramming factors.
MicroRNAs (miRNAs) are endogenously derived, short non-coding RNAs that regulate gene expression post-transcriptionally (Bartel, 2004). MiRNAs are crucial to differentiation of neural cell types during CNS development (Rajman and Schratt, 2017), as well as pathological processes after neural injury (Ambros, 2004; Alvarez-Garcia and Miska, 2005; Christensen and Schratt, 2009) including SCI (Liu et al., 2009; Yan et al., 2012; Nieto-Diaz et al., 2014). MiRNAs are small and chemically modifiable, which make them ideal candidates for therapy. Modifications such as cholesterol linkage at the 3′ end, 2′-O-methylation, and locked-nucleic-acid significantly increase their penetration, stability, and efficacy (Yan et al., 2012). A variety of techniques have been developed over the years to target the CNS with high efficiency and cellular specificity (van Rooij and Kauppinen, 2014; Wen, 2016). In fact, some modified miRNAs are already in clinical trials (Hydbring and Badalian-Very, 2013; Simonson and Das, 2015). The idea of using miRNAs to perform neuronal reprogramming has been developed. For example, miR-124 and miR-9 together are able to convert fibroblasts into neurons in culture (Yoo et al., 2011). Whether miRNAs can reprogram glial cells into neurons in the injured spinal cord needs to be tested in the future. NeuroD1-converted neurons are mostly glutamatergic subtype in the brain and spinal cord (Guo et al., 2014; Puls et al., 2020). Although the underlying mechanism is unclear, NeuroD1 as a basic helix-loop-helix transcription factor could play an instructive role by turning on neuronal genes (Figure 1A) (Boutin et al., 2010). However, miRNAs possess a different mechanism of action by mainly inhibiting translation of the target genes (Lim et al., 2005; Conaco et al., 2006). Therefore, forced expression of miRNAs may play a permissive, rather than instructive, role during astrocyte-to-neuron conversion, and that the fate of neuronal subtype of miRNA-converted neurons may more likely be determined by the local environment (Figure 1B). For example, the region-specific environment may affect subtype determination of newly converted neurons in the spinal cord (i.e., dorsal horn, enriched in GABAergic interneurons; and ventral horn, enriched in cholinergic motor neurons) (Figure 1B). If this is true, miRNA-mediated neuronal conversion may have the advantage of generating different neuronal subtypes in different spinal cord regions, which is important for functional repair after SCI.
Figure 1.
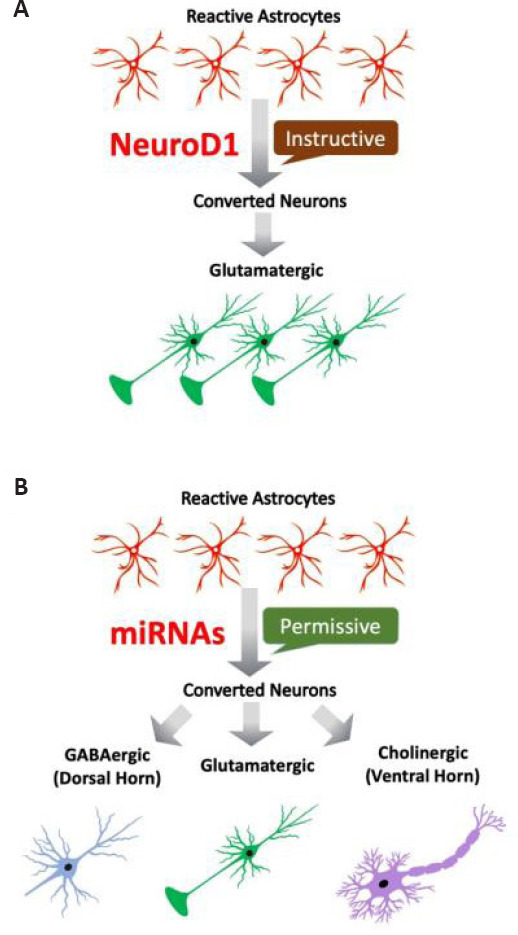
Predicted difference in neuronal subtypes of reprogrammed neurons from reactive astrocytes by NeuroD1 and miRNAs in the injured spinal cord.
NeuroD1 is a glutamatergic lineage transcription factor. Persistent NeuroD1 expression elicits an instructive role in pushing reprogrammed neurons into glutamatergic neuronal subtype (A). However, miRNAs play a permissive role by inhibiting non-neuronal gene expression, and therefore are predicted to allow generation of distinct neuronal subtypes possibly influenced by regional cues (B).
Cell Source for In Vivo Neuronal Reprogramming
Resident astrocytes react to injury-induced cytokine release and drastically upregulate expression of the astrocytic marker GFAP and the neural progenitor markers Nestin and Vimentin (Sofroniew, 2015). These reactive astrocytes also become proliferative and hypertrophic in cell morphology, and are a major contributor to the formation of glial scar, a dense tissue structure that is inhibitory to axonal regeneration (Silver and Miller, 2004). We have successfully converted GFAP+ reactive astrocytes into functional neurons with high efficiency in the injured spinal cord (Puls et al., 2020). We target reactive astrocytes for neuronal conversion for two reasons: 1) we are unlikely to exhaust the supply of these cells by conversion since they are proliferative; 2) reducing the number of reactive astrocytes by conversion may inhibit glial scar formation and create a more permissive environment for axonal regeneration. NG2 glia have also been reprogrammed into functional spinal neurons that contribute to the functional recovery of the mouse after SCI (Tai et al., 2021). NG2 glia represent largely the oligodendrocyte progenitor cells (OPCs) that also express Olig2 and PDGFRa. OPCs proliferate under the physiological condition and repopulate myelinating oligodendrocytes in the adult spinal cord. Upon injury, OPCs migrate to the injury site and contribute to glial scar formation (Barnabe-Heider et al., 2010). OPCs and astrocytes are not mutually exclusive under the injury condition. In fact, it is hard to distinguish the two cell types by gene expression upon injury since both ones express GFAP, Olig2, and NG2 (Barnabe-Heider et al., 2010). Pericytes help maintain vasculature integrity and have been shown as a major cellular component of glial scar in the injured spinal cord (Goritz et al., 2011). In addition, pericytes can be directly reprogrammed into functional neurons in culture (Karow et al., 2018). Therefore, pericytes could be another candidate cell source for neuronal reprogramming for SCI repair. Interestingly, pericytes also express high level of NG2 and might already have been reprogrammed to neurons in the injured spinal cord in the recent report, which targets NG2 glia for SCI repair (Tai et al., 2021). Microglia, an endogenous immune cell type, in the spinal cord, has been shown to convert into dopaminergic neurons in the striatum (Matsuda et al., 2019). It will be exciting to see if neuronal reprogramming can occur in microglia in the injured spinal cord. In sum, multiple cell types can be potentially utilized for reprogramming purposes in the injured spinal cord, and yet the most important consideration may be to achieve sufficient neuronal reprogramming for repair without overly disrupting the physiological function of these cells.
Neuronal Reprogramming in Gray versus White Matters
The concept of “neuronal relay” for SCI repair has been discussed for many years (Bonner and Steward, 2015); newly converted neurons could form relays connecting ascending and descending axons to their projection targets. Indeed, Sox2-converted neuroblasts were found to generate neuronal clusters in the white matter of the injured spinal cord where they have the potential to form synapses with the sprouting axonal terminals (Su et al., 2014; Wang et al., 2016). However, the lack of neurotrophic support in the white matter could hinder the survival of these neurons, hence the need for exogenous BDNF to assure the maturation and survival of the Sox2-converted neurons after SCI (Wang et al., 2016). We have rarely observed neuronal conversion by NeuroD1 in the white matter of the spinal cord in part due to the fact that we routinely target our viral injection to the gray matter by a stereotaxic apparatus. In a severe injury, the boundary between gray and white matters in the spinal cord may be obscured especially around the injury site. In addition, the injury-induced inflammation further masks this regional difference. Nevertheless, the newly reprogrammed neurons could benefit more in the gray matter from neighboring neurons by secreted neurotrophic factors and synaptic connections, which are critical to neuronal survival. On the other hand, glial cells in gray vs white matter may possess distinct intrinsic properties that affect neuronal reprogramming. In consistent with this notion, white-matter astrocytes in the corpus callosum are resistant to NeuroD1-mediated reprogramming compared with the ones in the cortex and striatum. Furthermore, the few reprogrammed neurons from astrocytes in the corpus callosum show immature neuronal phenotype including lack of excitability and neuronal subtype marker expression (Liu et al., 2020).
Timing for Neuronal Reprogramming
In our recent report on NeuroD1-mediated neuronal reprogramming in the injured spinal cord, we tested both retrovirus and adeno-associated virus (AAV) expression systems for gene delivery (Puls et al., 2020). Retrovirus only infects proliferating cells, the number of which is small under the physiological condition. To collect an adequate number of infected cells for our analysis, we adopted a 4-day-delayed injection paradigm, i.e., injecting NeuroD1-expressing retrovirus at 4 days post injury (Puls et al., 2020). This delayed injection was based on the finding that injury-induced proliferation of reactive glial cells dramatically increases 2–4 days post injury (Chen et al., 2008; Hong et al., 2014). For AAV that infects both proliferating and resting cells, we tested different time points post injury for injection. With different AAV injection timings in both stab injury and contusive injury models, we were able to demonstrate the high efficiency of neuronal reprogramming from reactive astrocytes by NeuroD1 in the spinal cord (Puls et al., 2020). In order to achieve functional recovery after SCI, more factors will have to be taken into consideration. We suggest injecting AAVs to convert reactive astrocytes to neurons at 2 weeks after SCI. The reasons for this delayed injection are: 1) Reactive astrocytes play a beneficial role at the acute phase of SCI in restricting injury spread (Okada et al., 2006; Herrmann et al., 2008); reducing these cells by conversion at this period may actually be detrimental; 2) Converted neurons may survive better after toxic factors and neuroinflammation induced by SCI have subsided; 3) Tissue environment and motor behavior of the SCI animals have stabilized by 2 weeks after SCI (Okada et al., 2006). Surely, the timing scheme of AAV injection will need to be optimized in order to maximize functional recovery after SCI. Determining factors will also include cell source for reprogramming, behavioral assays engaged, SCI model and severity. In consistent with the notion of delayed AAV injection, Tai et al. (2021) demonstrated that neuronal reprogramming by injecting Sox2/p75-2-expressing lentivirus at one week after a dorsal hemisection SCI significantly improved forelimb functional recovery in the grid walking paradigm, which examines basic and skilled locomotion.
Neurotransmitter Subtypes of Reprogrammed Neurons
To rebuild the optimal neuronal circuitry for functional repair after SCI, both excitatory and inhibitory neurons would be needed to keep the excitatory and inhibitory balance. Recently, our research has indicated that NeuroD1 can convert reactive astrocytes into functional neurons in the spinal cord with high efficiency (~90%) (Puls et al., 2020). However, the neurons converted by highly and continuously expressed NeuroD1 are mostly glutamatergic (i.e., excitatory) subtype (Puls et al., 2020), which is consistent with the fact that NeuroD1 is a glutamatergic neuron-lineage determination factor during development (Hevner et al., 2006; Roybon et al., 2015). Although transcription factors such as Ascl1/Mash1 and Dlx2 are able to convert astrocytes into GABAergic neurons, their neuronal conversion efficiency is much lower than that of NeuroD1 (Heinrich et al., 2010; Liu et al., 2015). Interestingly, NeuroD1 has been shown to compete with Mash1/Ascl1 in the determination of neuronal subtype during forebrain development (Roybon et al., 2010), suggesting that relative gene expression levels between these transcription factors may be crucial for neuronal subtype output. We have also shown that NeuroD1 + Dlx2 can increase the proportion of GABAergic phenotype in the converted neurons in the spinal cord compared with NeuroD1 alone (Puls et al., 2020). Therefore, we believe that we can convert astrocytes into inhibitory neurons by combining NeuroD1 with other antagonizing transcription factors, or by simply modulating NeuroD1 expression level during the neuronal conversion process. Sox2, a stem cell transcription factor, also converts astrocytes into mainly glutamatergic neurons (Wang et al., 2016), suggesting a possibly default neuronal subtype of astrocyte-converted neurons. Interestingly, Sox2-reprogrammed neurons from NG2 glia showed an increased proportion of GABAergic neuronal subtype in the injured spinal cord (Tai et al., 2021), indicating that cell source also plays an important role in determining neuronal subtypes from in vivo reprogramming. Consistently, a similar increase in the number of GABAergic neurons was observed in NeuroD1-mediated neuronal reprogramming from NG2 glia when compared with astrocytes (Guo et al., 2014).
Conclusion and Future Perspectives
In vivo reprogramming has brought regenerative medicine into a new exciting era. It is like gene therapy in terms of technique (i.e., both involve manipulation of gene expression). However, unlike gene therapy that only corrects certain gene functions in diseased tissues, in vivo reprogramming literally changes the cellular identity of the target cells from one cell type to another by overexpressing unique reprogramming factors.
One line of future research direction is to dissect molecular mechanisms underlying the reprogramming process with the goal of optimizing reprogramming outcomes. Neuronal reprogramming is not a natural developmental process and may engage distinct molecular mechanisms. Overexpression of a transcription factor that only appears during development may trigger “unusual” signaling pathways of the molecular machinery in the adult cells. Although injury-induced reactive astrocytes resemble neural progenitors in terms of their gene expression profiles (Sirko et al., 2013; Gotz et al., 2015), unique molecular interactions may occur during neuronal conversion and produce distinct phenotypes. For example, NeuroD1 plays a role in GABAergic neuron differentiation in the spinal cord dorsal horn during embryonic development (Brohl et al., 2008), but it converts reactive astrocytes into glutamatergic neurons in the adult spinal cord (Puls et al., 2020).
Another demanding research direction in the field of in vivo neuronal reprogramming is to fine-tune this technology to achieve functional benefit in models of various neurological disorders. In the injured spinal cord, while in vivo neuronal reprogramming has been repeatedly demonstrated as a feasible approach for injury repair, reports with functional recovery data at the behavioral level are rare (Tai et al., 2021). To facilitate translation towards the clinic, future focus in this research field should be to maximize functional benefits of neuronal reprogramming after SCI. This could be challenging because there are many parameters to optimize in order to achieve functional benefits. These parameters may include site(s) and timing for viral injection, reprogramming factor(s) to use, models of SCI, and types of behavioral assays to perform. For example, multiple injection sites may be required to reprogram a substantial number of new neurons in order to have sufficient impact to achieve functional recovery (Tai et al., 2021). Nonetheless, we strongly believe that neuronal reprogramming will become an effective treatment for SCI in the foreseeable future.
Acknowledgments
The authors would like to thank members of the Li lab for their insightful discussion.
Footnotes
Author contributions: Literature search, manuscript writing and table and figure preparation: XC and HL. Both authors approved the final manuscript.
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by startup funds from Medical College of Georgia at Augusta University (to HL) and by National Institutes of Health R01NS117918, R21NS104394, and R21NS119732 (to HL).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by startup funds from Medical College of Georgia at Augusta University (to HL) and by National Institutes of Health R01NS117918, R21NS104394, and R21NS119732 (to HL).
C-Editors: Zhao M, Zhao LJ, Li JY; T-Editor: Jia Y.
References
- 1.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 4.Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, Abe M. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 5.Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Boakye M, Leigh BC, Skelly AC. Quality of life in persons with spinal cord injury: comparisons with other populations. J Neurosurg Spine. 2012;17:29–37. doi: 10.3171/2012.6.AOSPINE1252. [DOI] [PubMed] [Google Scholar]
- 8.Bonner JF, Steward O. Repair of spinal cord injury with neuronal relays: From fetal grafts to neural stem cells. Brain Res. 2015;1619:115–123. doi: 10.1016/j.brainres.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutin C, Hardt O, de Chevigny A, Core N, Goebbels S, Seidenfaden R, Bosio A, Cremer H. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc Natl Acad Sci U S A. 2010;107:1201–1206. doi: 10.1073/pnas.0909015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second national acute spinal cord injury study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 11.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brohl D, Strehle M, Wende H, Hori K, Bormuth I, Nave KA, Muller T, Birchmeier C. A transcriptional network coordinately determines transmitter and peptidergic fate in the dorsal spinal cord. Dev Biol. 2008;322:381–393. doi: 10.1016/j.ydbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen A, Xu XM, Kleitman N, Bunge MB. Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp Neurol. 1996;138:261–276. doi: 10.1006/exnr.1996.0065. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Miles DK, Hoang T, Shi J, Hurlock E, Kernie SG, Lu QR. The basic helix-loop-helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. J Neurosci. 2008;28:10983–10989. doi: 10.1523/JNEUROSCI.3545-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen M, Schratt GM. microRNA involvement in developmental and functional aspects of the nervous system and in neurological diseases. Neurosci Lett. 2009;466:55–62. doi: 10.1016/j.neulet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 17.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neuroly. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gascon S, Murenu E, Masserdotti G, Ortega F, Russo GL, Petrik D, Deshpande A, Heinrich C, Karow M, Robertson SP, Schroeder T, Beckers J, Irmler M, Berndt C, Angeli JP, Conrad M, Berninger B, Gotz M. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell. 2016;18:396–409. doi: 10.1016/j.stem.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 23.Gotz M, Sirko S, Beckers J, Irmler M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia. 2015;63:1452–1468. doi: 10.1002/glia.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta R, Bathen ME, Smith JS, Levi AD, Bhatia NN, Steward O. Advances in the management of spinal cord injury. J Am Acad Orthop Surg. 2010;18:210–222. doi: 10.5435/00124635-201004000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa K, Chang YW, Li H, Berlin Y, Ikeda O, Kane-Goldsmith N, Grumet M. Embryonic radial glia bridge spinal cord lesions and promote functional recovery following spinal cord injury. Exp Neurol. 2005;193:394–410. doi: 10.1016/j.expneurol.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Heinrich C, Blum R, Gascon S, Masserdotti G, Tripathi P, Sanchez R, Tiedt S, Schroeder T, Gotz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Hong P, Jiang M, Li H. Functional requirement of dicer1 and miR-17-5p in reactive astrocyte proliferation after spinal cord injury in the mouse. Glia. 2014;62:2044–2060. doi: 10.1002/glia.22725. [DOI] [PubMed] [Google Scholar]
- 31.Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 32.Hydbring P, Badalian-Very G. Clinical applications of microRNAs. F1000Res. 2013;2:136. doi: 10.12688/f1000research.2-136.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwafuchi-Doi M, Zaret KS. Pioneer transcription factors in cell reprogramming. Genes Dev. 2014;28:2679–2692. doi: 10.1101/gad.253443.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karow M, Camp JG, Falk S, Gerber T, Pataskar A, Gac-Santel M, Kageyama J, Brazovskaja A, Garding A, Fan W, Riedemann T, Casamassa A, Smiyakin A, Schichor C, Gotz M, Tiwari VK, Treutlein B, Berninger B. Direct pericyte-to-neuron reprogramming via unfolding of a neural stem cell-like program. Nat Neurosci. 2018;21:932–940. doi: 10.1038/s41593-018-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumamaru H, Lu P, Rosenzweig ES, Kadoya K, Tuszynski MH. Regenerating corticospinal axons innervate phenotypically appropriate neurons within neural stem cell grafts. Cell Rep. 2019;26:2329–2339. doi: 10.1016/j.celrep.2019.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laliberte AM, Goltash S, Lalonde NR, Bui TV. Propriospinal neurons: essential elements of locomotor control in the intact and possibly the injured spinal cord. Front Cell Neurosci. 2019;13:512. doi: 10.3389/fncel.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Chen G. In vivo reprogramming for CNS repair: regenerating neurons from endogenous glial cells. Neuron. 2016;91:728–738. doi: 10.1016/j.neuron.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 40.Liu MH, Li W, Zheng JJ, Xu YG, He Q, Chen G. Differential neuronal reprogramming induced by NeuroD1 from astrocytes in grey matter versus white matter. Neural Regen Res. 2020;15:342–351. doi: 10.4103/1673-5374.265185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–429. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Miao Q, Yuan J, Han S, Zhang P, Li S, Rao Z, Zhao W, Ye Q, Geng J, Zhang X, Cheng L. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J Neurosci. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu P, Ceto S, Wang Y, Graham L, Wu D, Kumamaru H, Staufenberg E, Tuszynski MH. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J Clin Invest. 2017;127:3287–3299. doi: 10.1172/JCI92955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LS, Tuszynski MH. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marei HE, Althani A, Lashen S, Cenciarelli C, Hasan A. Genetically unmatched human iPSC and ESC exhibit equivalent gene expression and neuronal differentiation potential. Sci Rep. 2017;7:17504. doi: 10.1038/s41598-017-17882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuda T, Irie T, Katsurabayashi S, Hayashi Y, Nagai T, Hamazaki N, Adefuin AMD, Miura F, Ito T, Kimura H, Shirahige K, Takeda T, Iwasaki K, Imamura T, Nakashima K. Pioneer factor neurod1 rearranges transcriptional and epigenetic profiles to execute microglia-neuron conversion. Neuron. 2019;101:472–485. doi: 10.1016/j.neuron.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 49.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 50.Miyata T, Maeda T, Lee JE. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 1999;13:1647–1652. doi: 10.1101/gad.13.13.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- 52.Nieto-Diaz M, Esteban FJ, Reigada D, Munoz-Galdeano T, Yunta M, Caballero-Lopez M, Navarro-Ruiz R, Del Aguila A, Maza RM. MicroRNA dysregulation in spinal cord injury: causes, consequences and therapeutics. Front Cell Neurosci. 2014;8:53. doi: 10.3389/fncel.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 54.Nori S, Ahuja CS, Fehlings MG. Translational advances in the management of acute spinal cord injury: What is new? What is hot? Neurosurgery. 2017;64:119–128. doi: 10.1093/neuros/nyx217. [DOI] [PubMed] [Google Scholar]
- 55.Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127:3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohori Y, Yamamoto S, Nagao M, Sugimori M, Yamamoto N, Nakamura K, Nakafuku M. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26:11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 59.Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, Ikeda E, Yamanaka S, Miura K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 60.Okita K, Nagata N, Yamanaka S. Immunogenicity of induced pluripotent stem cells. Circ Res. 2011;109:720–721. doi: 10.1161/RES.0b013e318232e187. [DOI] [PubMed] [Google Scholar]
- 61.Pataskar A, Jung J, Smialowski P, Noack F, Calegari F, Straub T, Tiwari VK. NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. EMBO J. 2016;35:24–45. doi: 10.15252/embj.201591206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puls B, Ding Y, Zhang F, Pan M, Lei Z, Pei Z, Jiang M, Bai Y, Forsyth C, Metzger M, Rana T, Zhang L, Ding X, Keefe M, Cai A, Redilla A, Lai M, He K, Li H, Chen G. Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-mediated astrocyte-to-neuron conversion. Front Cell Dev Biol. 2020;8:591883. doi: 10.3389/fcell.2020.591883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajman M, Schratt G. MicroRNAs in neural development: from master regulators to fine-tuners. Development. 2017;144:2310–2322. doi: 10.1242/dev.144337. [DOI] [PubMed] [Google Scholar]
- 64.Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roybon L, Mastracci TL, Li J, Stott SR, Leiter AB, Sussel L, Brundin P, Li JY. The origin, development and molecular diversity of rodent olfactory bulb glutamatergic neurons distinguished by expression of transcription factor NeuroD1. PLoS One. 2015;10:e0128035. doi: 10.1371/journal.pone.0128035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roybon L, Mastracci TL, Ribeiro D, Sussel L, Brundin P, Li JY. GABAergic differentiation induced by Mash1 is compromised by the bHLH proteins Neurogenin2, NeuroD1, and NeuroD2. Cereb Cortex. 2010;20:1234–1244. doi: 10.1093/cercor/bhp187. [DOI] [PubMed] [Google Scholar]
- 67.Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 69.Simonson B, Das S. MicroRNA therapeutics: the next magic bullet? Mini Rev Med Chem. 2015;15:467–474. doi: 10.2174/1389557515666150324123208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, Fischer IR, Plesnila N, Staufenbiel M, Haass C, Snapyan M, Saghatelyan A, Tsai LH, Fischer A, Grobe K, Dimou L, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell. 2013;12:426–439. doi: 10.1016/j.stem.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 71.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tai W, Wu W, Wang LL, Ni H, Chen C, Yang J, Zang T, Zou Y, Xu XM, Zhang CL. In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell. 2021;28:923–937. doi: 10.1016/j.stem.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tai W, Xu X-M, Zhang C-L. Regeneration through in vivo cell fate reprogramming for neural repair. Front Cell Neurosci. 2020;14:10714. doi: 10.3389/fncel.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6:851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang LL, Su Z, Tai W, Zou Y, Xu XM, Zhang CL. The p53 pathway controls SOX2-Mediated reprogramming in the adult mouse spinal cord. Cell Rep. 2016;17:891–903. doi: 10.1016/j.celrep.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen MM. Getting miRNA Therapeutics into the target cells for neurodegenerative diseases: a mini-review. Front Mol Neurosci. 2016;9:129. doi: 10.3389/fnmol.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan H, Hong P, Jiang M, Li H. MicroRNAs as potential therapeutics for treating spinal cord injury. Neural Regen Res. 2012;7:1352–1359. doi: 10.3969/j.issn.1673-5374.2012.17.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]


