Abstract
Encrustation of ureteral stents can represent a complex challenge. Patients can require multiple intervention types as well as several operative sessions. Our aim was to establish a practical guide for managing such cases as well as an accompanying treatment algorithm. Nearly all cases can now be successfully managed with minimally invasive methods such as ureteroscopy and/or percutaneous nephrolithotomy. Use of a validated tool for grading burden of encrustation is recommended. Careful patient counselling as well as operative planning are of paramount importance. Identifying high risk patient groups such as pregnancy and implementing prevention strategies are also crucial.
Keywords: ureteroscopy, stents, risk factors, encrustation, indwelling time
INTRODUCTION
Ureteral stents hold an indispensable status in urologic practice. Primarily serving to drain the upper urinary tract, their usage is indicated in a wide range of both emergency and elective scenarios. Engineering and design of this versatile tool have undergone continued modifications since the introduction of the Gibbons indwelling ureteral stent catheter in 1972 [1]. This includes changes in material composition, structure and coatings among other advancements [2]. As the global burden of urolithiasis rises, this is mirrored in the rising volume of endourological procedures being performed worldwide [3, 4]. A recent worldwide survey of flexible ureteroscopy (URS) practice, found that over 80% of urologists place a ureteral stent in more than half of all cases they perform [5]. This alone highlights the central role ureteral stents serve as well as their frequency of usage. However, while their importance is never in question, they are associated with adverse sequelae, not least, a negative effect on quality of life (QoL) [6, 7]. Among these shortcomings is the liability for encrustation. This is most often due to crystallization associated with presence of urease producing organisms in urine. A principal contributor to this is where the stent has been in situ for a long time and even ‘forgotten’ as an unintended consequence of stent insertion. The latter is reported to occur in between 4–13% of cases [8]. Stent encrustation (SE) is a difficult challenge for the clinician and in the modern era, minimally invasive endourological approaches dominate in its management [9, 10]. However, despite advancements in this field, it can be challenging to navigate and plan, thus affecting patient’s care. Sancaktutar et al. determined that the financial burden associated with treating such cases is on average 6.9 times (range: 1.8–21 fold) higher than a standard stent removal [11]. Moreover, recommended treatment strategies are not currently addressed in international guidelines [12, 13]. Our aim therefore, was to provide an evidence-based guide for this demanding problem as well as formulate an accompanying treatment algorithm to aid clinicians.
Risk factors
Among several risk factors for SE, prolonged indwelling time stands as the major one [14]. Kawahara et al. reported there to be a degree of SE to exist at a rate of 56.8% at 6–12 weeks and 75.9% after 12 weeks [14]. These findings echo an earlier study by el-Faqih et al., who found the rate of SE to be 76.6% after 12 weeks [15].
There are a multitude of factors that contribute to why stents become ‘forgotten’, but educational level, socio-economic background, healthcare system and insurance status appear to play a large role in this. Indeed, in a retrospective series of SE reported by Weedin et al., only 1/52 patients had medical insurance (Medicare) [16], which serves as a reminder of the holistic approach required. In another recent series, 15.5% of patients with SE were unaware that a stent had ever been inserted [17]. The latter highlights the importance of careful patient counselling in all endourological procedures. Novel aids such as smartphone applications as well as other non-digital alternatives can help reduce risk of forgotten stents [18]. The highest rate of SE, particularly those cases which require additional endourological intervention, occurs in urolithiasis patients, especially cystine and uric acid stone formers [19]. Other known risk factors to remember are pregnancy, where the associated physiological changes increase encrustation rate. This accelerated process is also known to occur in patients with immunosuppression, malignancy and those with an underlying malabsorptive state. As well as smaller calibre stents being more susceptible to SE, composition material is also an important factor [14]. Numerous studies have found that silicone stents for example, are less prone to SE than polyurethane alternatives and might even offer a better QoL [2]. Bacterial colonisation of the stent can lead to development of a microbial biofilm causing recurrent symptomatic infection and stent encrustation. Modifying a stent’s coating and architecture can aid to reduce encrustation rate, decreasing the bacterial biofilm and the crystal formation accordingly [20].
Presentation and assessment
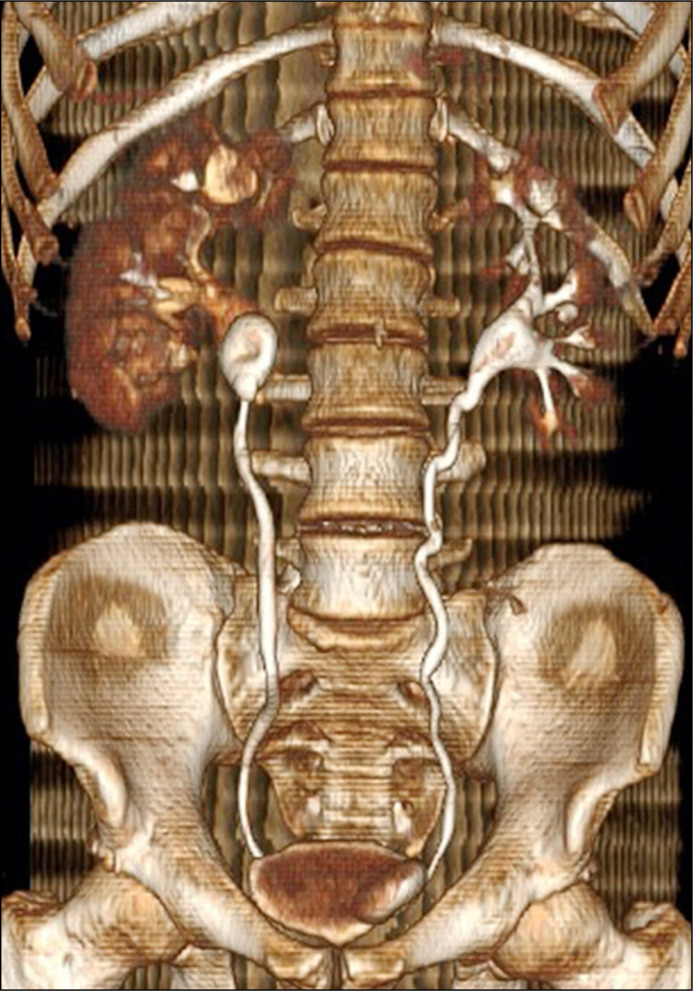
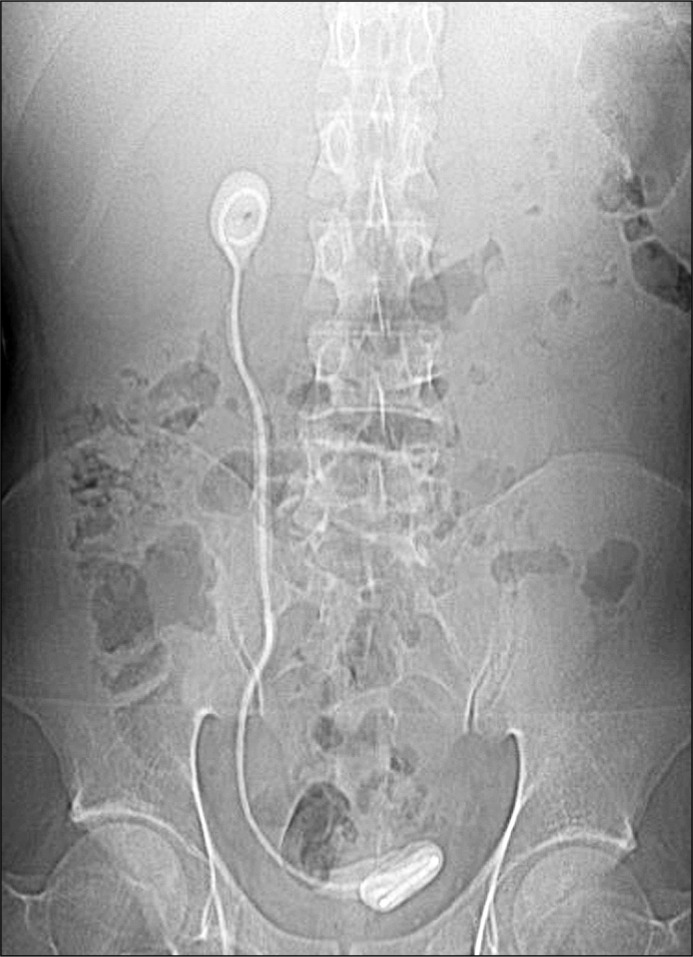
Presenting symptoms are usually made up of a constellation of flank pain, haematuria, storage lower urinary tract symptoms (LUTS) and infection. The latter is a common sequelae and urinary sepsis can develop as a result. Positive urine cultures have been reported in up to 75.2% of SE cases [16]. A specimen should therefore be sent for culture and sensitivity testing and antibiotic selection should be made accordingly. Note that fungal infection is also possible, particularly in patients with comorbidities such as cirrhosis, diabetes and malnutrition [21]. In patients with concomitant hydronephrosis and an infected obstructed system, decompression with percutaneous nephrostomy (PCN) should be performed before definitive management is attempted. Many patients are often asymptomatic, and the initial diagnosis may come at the time of cystoscopic removal in the outpatient setting. This may be immediately obvious at point of entry to bladder in cases of SE to the lower coil. However, in cases where SE is sparing the lower coil and only affecting a higher segment, resistance to removal may be the first indication. In such cases, no force should be applied. Imaging should be obtained of the patient and while plain KUB (kidney, ureter, bladder) X-ray might be the easiest to arrange, non-contrast computed tomography (NCCT) is the modality of choice (Figures 1 & 2). This will allow the diagnosis to be confirmed, the location(s) of encrustation to be mapped and grading of severity to be made. It also allows for assessment of the contralateral renal unit. Plain radiographs alone often fail to identify and map the site of calcification accurately [22].
Figure 1.
Reconstructed computed tomography displaying stent encrustation.
Figure 2.
Plain X-Ray showing stent encrustation.
In severe cases, SE can render the renal unit to be non-functioning. Accordingly, nuclear renal imaging is recommended to determine its status. In extreme cases, decision may be made to proceed to simple nephrectomy where the kidney is atrophic. Discussion at a dedicated stone meeting (MDT) with radiologists can aid planning and decision making.
In clinical practice, there are three main groups that are commonly encountered. Firstly, those at high-risk for encrustation with long-term stents where early replacement needs to be scheduled. Secondly, those with forgotten stents where careful operative planning and additional endourological measures are warranted. Finally, those cases where some degree of crystallization on the stent is visible upon inspection of the stent after unremarkable extraction.
Grading
A number of classification systems now exist in endourology, which includes validated tools for SE [23]. This includes the Forgotten Encrusted CALcified (FECal) system, which categorises SE cases into 5 levels from minimal encrustation of a coil (Level 1) to encrustation of the entire stent length (Level 5) [24]. It is the original and most used tool of this kind, which can aid operative planning including the need for a multimodal treatment strategy. A similar tool is the Kidney, Ureter, Bladder (KUB) system, which also gives indication of patients requiring prolonged operative time [25]. Based on radiographic imaging, encrustation is assessed in three segments: proximal coil (K), body of ureter (U) and lower coil in bladder (B). Each of these three segments is graded 1–3 and a final score is calculated (3–15). A final score ≥9 is associated with significantly higher risk of needing multiple surgeries, multiple intervention methods and operation time over 3 hours. The Visual Grading for Ureteral Encrusted Stent (V-GUES) tool has been recently developed to aid the clinician at determining likelihood of treatment success of both removing the stent and accompanying stone, wherein the severity is graded from A to D [26].
Operative planning
Patients should be carefully counselled regarding endourological management of SE. It should be highlighted that removal of the stent and any associated stone may necessitate more than one operative session. A growing number of studies report success at clearance within a single session [27]. However, most series report that between 1 and 3 operative sessions are required [28]. It may well also be necessary to insert a new stent at the end of the procedure. Optimisation of patients should occur pre-operatively including confirmation of negative urine culture. While it may not be possible to completely clear the infection pre-operatively, an antibiotic course of sufficient duration should be scheduled and guidance from a microbiologist sought accordingly. Rates of antibiotic resistance are higher in this patient group. One study reported E. Coli resistance to quinolones in this group to exceed 90% [16]. Intravenous antibiotic prophylaxis is recommended at the time of anaesthetic induction. SE patients represent a high-risk group and will likely have many surgical criteria, which place them at a high-risk of post-operative complications such as positive urine culture, multiple comorbidities and large stone burden as well as the presence of encrusted potentially infected stent. The importance therefore of pre-operative optimisation cannot therefore be underestimated. Longer operating time is also a known risk factor for post-operative sepsis in stone surgery and therefore close attention should be paid to this and whether surgery should be staged and continued on another occasion where needed [29].
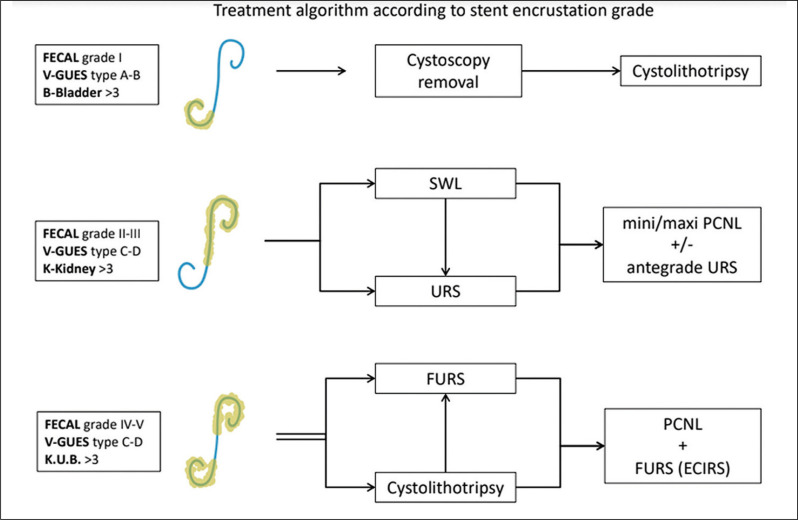
The clinical team should ensure availability of additional equipment in case a multimodal approach e.g., endoscopic combined intrarenal surgery (ECIRS). Alnadhari et al. reported that on average, 4.2 (1–10) intervention methods were required to render their 40-patient sample, stone and stent free [30]. A proposed algorithm is provided in Figure 3.
Figure 3.
Management algorithm.
FECAL – Forgotten Encrusted CALci- fied; V-GUES – Visual Grading for Ureteral Encrusted Stent; K.U.B. – kidney, ureter, bladder; SWL – shock wave lithotripsy; URS – ureteroscopy; PCNL – percutaneous nephrolithotomy; FURS (ECIRS) – flexible ureteroscopy (endoscopic combined intrarenal surgery)
Cystolitholapaxy
After performing systematic cystoscopic inspection, in cases of lower coil encrustation, it is not recommended to use biopsy forceps to try and fragment the encrustations. This usually has negligible effect and only serves to blunt the forceps. Depending on availability of equipment, options include cystolitholapaxy with a stone punch or pneumatic lithotripsy. Care is required with the former not to cause inadvertent damage to the bladder mucosa, as the resultant effect can be bleeding and poor vision even before higher segments of the stent are arrived at. Use of laser cystolithotripsy is now recognised as a safe and effective alternative. This is usually done via a larger laser fiber (500–1000 μm) with a low pulse energy and a frequency that is adjusted based on the power of the laser system used. Although holmium laser (Ho:YAG) has been the gold standard for this, there are increasing reports of using thulium fiber laser (TFL). A ureteric catheter can aid to stabilize the laser fiber and thereby facilitate control and minimise vibration when the laser is activated. Use of a resectoscope can be considered, which has the advantage of enabling continuous irrigation. The latter keeps the vision clear, adds a cooling effect and higher power laser settings can be used more safely in the bladder compared to the upper urinary tract without the risk of associated heat damage. In contrast to ureteroscopic lithotripsy where an empty bladder is recommended, a well filled bladder aids vision for cystolithotripsy. Review of literature reveals that most centres adopt a strategy of treating encrustation of the distal stent segment before proceeding to the proximal part.
Ureteroscopy
Once the bladder coil is released, insertion of a semi-rigid ureteroscope can be placed alongside the encrusted stent and the laser (Ho:YAG or TFL) employed to start clearing the encrustations. We recommend starting with settings such as (0.4–0.6 J, 5–10 Hz) and paying attention to use of a lower range of power settings in the ureter compared to the kidney. In cases where it is constricted, consider use of a smaller ureteroscope (4.5–6 Fr). Once removed, the distal portion of the stent can be carefully fixed to the patient’s thigh or secured with forceps to secure gentle traction. In cases of severe SE, such as where the whole body of the ureter is affected, space can be created via the technique of piecemeal removal in order to enable advancement higher up the ureter [31]. Accordingly, transection of the stent can be achieved with cutting settings (1–1.5 J, 5–10 Hz). The incised stent segments can be removed using grasping forceps or basket (e.g., N Gage or Dakota). This process may need to be repeated several times, but care must be taken as to avoid thermal or mechanical damage to ureter. When only the encrustation of the proximal coil remains, one can continue with flexile URS. Thomas et al reported success in their prospective series of employing URS alone to successfully treat 98% of cases. The authors implemented use of transecting the stent in 71% [27]. Given the stress placed on the instrument in such difficult cases, re-usable scopes are vulnerable to damage. Therefore, single use ureteroscopes are an alternative to consider if available [32]. The latter are now available in smaller sizes (e.g., Pusen 7.5 Fr) [33]. Survey results suggest that over 70% of urologists now use ureteral access sheath (UAS) routinely [5]. However, it is generally not recommended in SE scenarios due to potential damage to an already traumatized ureter. Once the stent has been fully retrieved, a mutual decision should be made with the anaesthetic team regarding the safety of proceeding to clear any additional stone(s) if present, or to defer to another session. Insertion of a new stent is usually required unless the case has been truly uncomplicated. This is usually if a staged procedure is required or for a short period afterwards due to ureteric oedema. Using a stent on string has been proposed as a helpful reminder for patients not to forget they have a stent or to minimise the stent dwell time. The advent of TFL offers several potential advantages in complex stone cases. Its ability to treat larger stones and maintain good visibility can be transferred to SE cases. It also causes reduced bleeding to surrounding tissue and achieves shorter operative times [34, 35]. It is also possible that a percutaneous nephrostomy (PCN) tube becomes encrusted, which can be approached using the same rationale as for a ureteral stent.
Note that in cases where there is known encrustation entire body of the stent as well as the lower coil, it is possible to use one laser fiber for both encrustations in the bladder and sections further up. In this case, a smaller fiber e.g., 150–270 μm can be employed. Again, low settings should be selected when starting and power increased as needed, as high power is associated with more bleeding and reduced visibility.
Shockwave lithotripsy
While it is rarely an option to achieve monotherapy with shockwave lithotripsy (SWL) and although its usage is now increasingly shadowed by URS, it can still play an important role. El-Tatawy et al. reported outcomes in patients undergoing SWL monotherapy before trial removal with cystoscopy [17]. This was successful in 70.7% of cases but up to 4 sessions were required to achieve this. Its application lends itself better to cases of milder encrustation. The decision for its use it will be largely affected by local expertise and whether there is a fixed on-site lithotripter. SWL can be employed pre-surgery or as an additional adjunctive treatment in between operative sessions.
Percutaneous nephrolithotomy
Larger SE burdens may require treatment with percutaneous nephrolithotomy (PCNL) in order to treat proximal coil encrustations. This can also enable antegrade ureteroscopy as required. In order to reduce morbidity, a miniaturised approach can be considered [35]. Grading systems are particularly helpful in such scenarios as with the V-GUES system, type C or D usually indicates that percutaneous access will also be required especially if aiming to achieve clearance in a single session.
Guideline positions
Currently, the only reference made to SE in the latest European Association of Urology (EAU) and American Urological Association (AUA) guidelines on urolithiasis, is in reference to the increased risk of encrustation in pregnancy [12, 13]. Beyond this, there are also no recommendations regarding forgotten stents or relevant prevention strategies.
Medical management
The role of non-surgical treatment for SE is limited to small reports of chemolysis [21]. This can be administered via a PCN. This may serve to soften the encrustations in advance of a definitive procedure. In our experience, it serves a very limited role but is a consideration for special scenarios such as high encrustation burden, uric acid stone and severely comorbid patients where any strategy to minimise or shorten a general anaesthetic are a priority.
Prevention strategies
Prevention of SE represents a clinical priority and steps must be taken to minimize stent placement in the first instance. This also includes education of both patients and clinicians, wherein the latter must adhere to clear counselling of patients. In a review of malpractice claims associated with endourology in the USA, 16% were because of retained stents [36]. We recommend that the operator who has placed the stent takes responsibility for organising its removal rather than delegating this task. Identifying high-risk patient groups is also key to this. In cases where SE has been treated yet the patient requires stent replacement as a long-term stent, for perhaps a lifelong period e.g., extrinsic compression due to malignancy, these should be scheduled for early exchange and their SE disposition highlighted in medical notes. Stent registries have evolved in recent years to include alerts to update clinician of stent dwell time [37]. A more recent approach is the development of smartphone applications, which also offer two-way communication whereby the patient can log symptoms for review by the clinical team [38]. However, it is important to consider that a method is selected, which is tailored to the individual patient and their circumstances as not all members of society will choose to or be able to engage in digital platforms. Other strategies, which are currently under research include development of next generation biodegradable stents as well as novel coatings. Summary of recommendations are provided in Table 1.
Table 1.
Summary of recommendations
| Summary point | Recommendation |
|---|---|
| High-risk patients for stent encrustation | Identify high-risk patient groups such as stents placed in pregnancy |
| Grading | Use a validated tool for grading severity such as FECal or KUB |
| Patient counselling | Inform patients of possible need for multiple operative sessions to clear stent/stone burden |
| Obtain pre-operative urine culture and treat infection if present | Gaining control over any infection and identifying multi resistant organisms is key to helping to minimise operative complications. |
| Operative planning | Assemble and plan for possible need for multimodal intervention such as combined ECIRS |
| Endourological methods | Nearly all cases of severe encrustation can be successfully managed with endourological methods alone without the need for open surgery |
| SWL | Can be used as an accessory treatment but monotherapy rarely successful if moderate to severe encrustation is present |
| Laser settings | Use low energy settings, especially in the ureter to reduce risk of heat injury. |
| Risk factors for post-operative sepsis | Maintain awareness of risk factors such as prolonged operative time, urinary tract infection and comorbidities such as diabetes |
| Surgeon responsibility | The operator takes ownership of organising stent removal or relevant other follow-up. |
| Prevention strategies | Educate patients and consider use of novel strategies such as digital reminders but remain awareness of patient’s likelihood of being able to use this aid e.g., elderly |
FECal – forgotten encrusted calcified; KUB – kidney, ureter, bladder; ECIRS – endoscopic combined intrarenal surgery; SWL – shockwave lithotripsy
CONCLUSIONS
Encrustation of ureteral stents can be a difficult problem to treat. Grading with a classification system is recommended as well as close attention to risk factors for complications such as post-operative sepsis. A stepwise approach is required to safely render the patient stent free. Endourological interventions now represent the mainstay of management and can achieve good outcomes, however a multimodal approach may be required, along with a staged procedure where necessary.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Danoff DS. The Gibbons indwelling silicone ureteral stent catheter. J Urol. 1977;117:33. doi: 10.1016/s0022-5347(17)58326-3. [DOI] [PubMed] [Google Scholar]
- 2.Mosayyebi A, Vijayakumar A, Yue QY, et al. Engineering solutions to ureteral stents: material, coating and design. Cent European J Urol. 2017;70:270–274. doi: 10.5173/ceju.2017.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z, Prosperi M, Bird VY. Prevalence of kidney stones in the USA: The National Health and Nutrition Evaluation Survey. J Clin Urol. 2019;12:296–302. [Google Scholar]
- 4.Geraghty RM, Jones P, Somani BK. Worldwide Trends of Urinary Stone Disease Treatment Over the Last Two Decades: A Systematic Review. J Endourol. 2017;31:547–556. doi: 10.1089/end.2016.0895. [DOI] [PubMed] [Google Scholar]
- 5.Pietropaolo A, Bres-Niewada E, Skolarikos A, et al. Worldwide survey of flexible ureteroscopy practice: a survey from European Association of Urology sections of young academic urologists and uro-technology groups. Cent European J Urol. 2019;72:393–397. doi: 10.5173/ceju.2019.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi HB, Stainthorpe A, MacDonagh RP, Keeley FX, Jr, Timoney AG, Barry MJ. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169:1065–1069. doi: 10.1097/01.ju.0000048980.33855.90. [DOI] [PubMed] [Google Scholar]
- 7.Galal E, Abdelhamid MH, Fath El-Bab T, Abdelhamid A. The role of mirabegron in relieving double-J stent-related discomfort: a randomized controlled clinical trial. Cent European J Urol. 2021;74:76–80. doi: 10.5173/ceju.2021.0273.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small AC, Thorogood SL, Shah O, Healy KA. Emerging Mobile Platforms to Aid in Stone Management. Urol Clin North Am. 2019;46:287–301. doi: 10.1016/j.ucl.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Whitehurst L, Jones P, Somani BK. Mortality from kidney stone disease (KSD) as reported in the literature over the last two decades: a systematic review. World J Urol. 2019;37:759–776. doi: 10.1007/s00345-018-2424-2. [DOI] [PubMed] [Google Scholar]
- 10.Adanur S, Ozkaya F. Challenges in treatment and diagnosis of forgotten/encrusted double-J ureteral stents: the largest single-center experience. Ren Fail. 2016;38:920–926. doi: 10.3109/0886022X.2016.1172928. [DOI] [PubMed] [Google Scholar]
- 11.Sancaktutar AA, Söylemez H, Bozkurt Y, Penbegül N, Atar M. Treatment of forgotten ureteral stents: how much does it really cost? A cost-effectiveness study in 27 patients. Urol Res. 2012;40:317–325. doi: 10.1007/s00240-011-0409-3. [DOI] [PubMed] [Google Scholar]
- 12.Türk C, NeIsius A, Petrik A, et al. EAU guidelines on urolithiasis. EAU Guidelines. presented at the EAU Annual Congress Amsterdam; 2020; . https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urolithiasis-2020.pdf . [Google Scholar]
- 13.Assimos D, Krambeck A, Miller NL, et al. Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART I. J Urol. 2016;196:1153–1160. doi: 10.1016/j.juro.2016.05.090. [DOI] [PubMed] [Google Scholar]
- 14.Kawahara T, Ito H, Terao H, Yoshida M, Matsuzaki J. Ureteral stent encrustation, incrustation, and coloring: morbidity related to indwelling times. J Endourol. 2012;26:178–182. doi: 10.1089/end.2011.0385. [DOI] [PubMed] [Google Scholar]
- 15.el-Faqih SR, Shamsuddin AB, Chakrabarti A, Atassi R, Kardar AH, Osman MK, Husain I. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487–1491. doi: 10.1016/s0022-5347(17)38146-6. [DOI] [PubMed] [Google Scholar]
- 16.Weedin JW, Coburn M, Link RE. The impact of proximal stone burden on the management of encrusted and retained ureteral stents. J Urol. 2011;185:542–547. doi: 10.1016/j.juro.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 17.El-Tatawy H, El-Abd AS, Gameel TA, et al. Management of 'forgotten' encrusted JJ stents using extracorporeal shockwave lithotripsy: A single-centre experience. Arab J Urol. 2019;17:132–137. doi: 10.1080/2090598X.2019.1595485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hameed BZ, Shah M, Naik N, Reddy SJ, Somani BK. Use of ureteric stent related mobile phone application (UROSTENTZ App) in COVID-19 for improving patient communication and safety: a prospective pilot study from a university hospital. Cent European J Urol. 2021;74:51–56. doi: 10.5173/ceju.2021.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legrand F, Saussez T, Ruffion A, et al. Double Loop Ureteral Stent Encrustation According to Indwelling Time: Results of a European Multicentric Study. J Endourol. 2021;35:84–90. doi: 10.1089/end.2020.0254. [DOI] [PubMed] [Google Scholar]
- 20.Rebl H, Renner J, Kram W, et al. Prevention of Encrustation on Ureteral Stents: Which Surface Parameters Provide Guidance for the Development of Novel Stent Materials? Polymers (Basel) 2020;12:558. doi: 10.3390/polym12030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchholz N, Hakenberg O, Masood J. Handbook of urinary stents: basic science and clinical applications. JP Medical Ltd; 2016. ISBN-13: 978-1907816659. [Google Scholar]
- 22.Adanur S, Ozkaya F. Challenges in treatment and diagnosis of forgotten/encrusted double-J ureteral stents: the largest single-center experience. Ren Fail. 2016;38:920–926. doi: 10.3109/0886022X.2016.1172928. [DOI] [PubMed] [Google Scholar]
- 23.Jones P, Pietropaolo A, Chew BH, Somani BK. Atlas of scoring systems, grading tools and nomograms in Endourology: A comprehensive overview from The TOWER Endourological Society research group. J Endourol. 2021 Apr 20; doi: 10.1089/end.2021.0124. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Acosta-Miranda AM, Milner J, Turk TM. The FECal Double-J: a simplified approach in the management of encrusted and retained ureteral stents. J Endourol. 2009;23:409–415. doi: 10.1089/end.2008.0214. [DOI] [PubMed] [Google Scholar]
- 25.Arenas JL, Shen JK, Keheila M, et al. Kidney, Ureter, and Bladder (KUB): A Novel Grading System for Encrusted Ureteral Stents. Urology. 2016;97:51–55. doi: 10.1016/j.urology.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 26.Manzo BO, Alarcon P, Lozada E, et al. A Novel Visual Grading for Ureteral Encrusted Stent Classification to Help Decide the Endourologic Treatment. J Endourol. 2021;35:1314–1319. doi: 10.1089/end.2020.1225. [DOI] [PubMed] [Google Scholar]
- 27.Thomas A, Cloutier J, Villa L, Letendre J, Ploumidis A, Traxer O. Prospective Analysis of a Complete Retrograde Ureteroscopic Technique with Holmium Laser Stent Cutting for Management of Encrusted Ureteral Stents. J Endourol. 2017;31:476–481. doi: 10.1089/end.2016.0816. [DOI] [PubMed] [Google Scholar]
- 28.Kartal IG, Baylan B, Gok A, et al. The Association of Encrustation and Ureteral Stent Indwelling Time in Urolithiasis and KUB Grading System. Urol J. 2018;15:323–328. doi: 10.22037/uj.v0i0.4592. [DOI] [PubMed] [Google Scholar]
- 29.Bhojani N, Miller LE, Bhattacharyya S, et al. Risk Factors for Urosepsis After Ureteroscopy for Stone Disease: A Systematic Review with Meta-Analysis. J Endourol. 2021;35:991–1000. doi: 10.1089/end.2020.1133. [DOI] [PubMed] [Google Scholar]
- 30.Alnadhari I, Alwan MA, Salah MA, et al. Treatment of retained encrusted ureteral Double-J stent. Arch Ital Urol Androl. 2019;90:265–269. doi: 10.4081/aiua.2018.4.265. [DOI] [PubMed] [Google Scholar]
- 31.Pietropaolo A, Whitehurst L, Somani BK. Piecemeal Retrograde Removal of Encrusted and Encased Stuck Ureteral Stent: Video Tips and Tricks. Videourology. 2020;34 doi: 10.1089/vid.2019.0057. [DOI] [Google Scholar]
- 32.Johnston TJ, Baard J, de la Rosette J, et al. A clinical evaluation of the new digital single-use flexible ureteroscope (UscopePU3022): an international prospective multicentered study. Cent European J Urol. 2018;71:453–461. doi: 10.5173/ceju.2018.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emiliani E, Mercadé A, Millan F, Sánchez-Martín F, Konstantinidis CA, Angerri O. First clinical evaluation of the new single-use flexible and semirigid Pusen ureteroscopes. Cent European J Urol. 2018;71:208–213. doi: 10.5173/ceju.2018.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones P, Beisland C, Ulvik Ø. Current status of thulium fibre laser lithotripsy: an up-to-date review. BJU Int. 2021;128:531–538. doi: 10.1111/bju.15551. [DOI] [PubMed] [Google Scholar]
- 35.Ulvik Ø. Ureteroscopy and thulium fiber laser lithotripsy: early clinical experience. Videourology. 2021;35 doi: 10.1089/vid.2020.0084. [DOI] [Google Scholar]
- 36.Duty B, Okhunov Z, Okeke Z, Smith A. Medical malpractice in endourology: analysis of closed cases from the State of New York. J Urol. 2012;187:528–532. doi: 10.1016/j.juro.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 37.Lynch MF, Ghani KR, Frost I, Anson KM. Preventing the forgotten ureteral stent: implementation of a web-based stent registry with automatic recall application. Urology. 2007;70:423–426. doi: 10.1016/j.urology.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Duty B, Okhunov Z, Okeke Z, Smith A. Medical malpractice in endourology: analysis of closed cases from the State of New York. J Urol. 2012;187:528–532. doi: 10.1016/j.juro.2011.10.045. [DOI] [PubMed] [Google Scholar]