Abstract
Bioplastics — typically plastics manufactured from bio-based polymers — stand to contribute to more sustainable commercial plastic life cycles as part of a circular economy, in which virgin polymers are made from renewable or recycled raw materials. Carbon-neutral energy is used for production and products are reused or recycled at their end of life (EOL). In this Review, we assess the advantages and challenges of bioplastics in transitioning towards a circular economy. Compared with fossil-based plastics, bio-based plastics can have a lower carbon footprint and exhibit advantageous materials properties; moreover, they can be compatible with existing recycling streams and some offer biodegradation as an EOL scenario if performed in controlled or predictable environments. However, these benefits can have trade-offs, including negative agricultural impacts, competition with food production, unclear EOL management and higher costs. Emerging chemical and biological methods can enable the ‘upcycling’ of increasing volumes of heterogeneous plastic and bioplastic waste into higher-quality materials. To guide converters and consumers in their purchasing choices, existing (bio)plastic identification standards and life cycle assessment guidelines need revision and homogenization. Furthermore, clear regulation and financial incentives remain essential to scale from niche polymers to large-scale bioplastic market applications with truly sustainable impact.
Subject terms: Polymer chemistry, Polymers, Sustainability
Plastics support modern life but are also associated with environmental pollution. This Review discusses technologies for the production and recycling of bioplastics as part of a more sustainable and circular economy.
Introduction
Polymers exhibit diverse material properties — ranging, for example, from flexible to stiff, from permeable to impermeable and from hydrophilic to hydrophobic. These properties are determined by the structure of the repeating building blocks of the polymers: the monomers. Once the polymeric material has been processed and formed into its final and commercially relevant shape, typically using heat, they are called plastics1. Most plastics are thermoplastics composed of linear polymer chains that allow thermal reshaping, such as those used in bottles and textiles, whereas some polymers are crosslinked during processing to form thermosets, which are tougher than thermoplastics and their shape is largely unaffected by temperature, such as those used in car tyres and epoxies. In this Review, we refer to these materials as ‘polymers’ when discussing their physicochemical properties and their synthesis before processing into their final shape, and use ‘plastics’ to describe the more commercially relevant forms of polymers processed into products. Many of these materials have an integral role in modern life and are especially important in the transportation, food, health-care and energy industries. Plastics are also essential to many aspects of sustainability: lightweight plastic materials improve the fuel efficiency of cars and aeroplanes, plastic insulators can increase energy savings and plastic food packaging increases shelf life, which can reduce food waste2. Annual plastic production is >380 million tonnes and increasing at an annual rate of 4%; consequently, 6,300 million tonnes of plastic waste have been generated since 1950 (refs3,4). Increasing concern regarding the environmental impact of plastic waste and the plastic-related emission of greenhouse gases (GHGs) motivates the transition towards a ‘circular plastic economy’. In a circular economy, the use of non-renewable resources and waste production is minimized, while reuse and recycling dominate the life cycles of materials.
Although most commercial plastics are made from fossil resources, these materials can also be made from renewable resources and are commonly referred to as bioplastics. In this case, the monomers are extracted or synthesized from biomass compounds (such as sugars in plants) and then polymerized to either make a direct replacement for an existing plastic, such as polyethylene (PE), or novel polymers, such as polyhydroxyalkanoates (PHAs). Biomass extraction can also yield non-synthetic natural polymers, such as starch, natural rubber and proteins. Note that, although the term ‘bioplastic’ is frequently used, it remains misunderstood, owing to the ambiguity of the definition (Box 1). Bioplastics are plastics that are either made from renewable resources (‘bio-based’), are biodegradable, are made through biological processes or a combination of these. Some biodegradable but fossil-based plastics are also referred to as bioplastics2,5; however, the use of this terminology is advised against, as it is misleading6,7.
Bioplastics that are 100% bio-based are currently produced at a scale of ~2 million tonnes per year and are considered a part of future circular economies to help achieve some of the United Nations’ (UN) Sustainable Development Goals, such as by diverting from fossil resources, introducing new recycling or degradation pathways and using less toxic reagents and solvents in production processes5,8–11. Depending on type, bioplastics can offer improved circularity by using renewable (non-fossil) resources, a lower carbon footprint, biodegradation as an alternative end-of-life (EOL) option and improved material properties. These benefits, however, are highly dependent on several factors, including the chemical structure, the manufacturing process and the most likely EOL scenario. All these factors have to be evaluated across the life cycle, along metrics such as climate impact, ecotoxicity and recyclability, using tools such as a life cycle assessment (LCA) to elucidate the environmental benefit over alternatives6,12,13. Similar to traditional plastics, bioplastics also raise concerns relating to the leaching of monomers, oligomers and additives, and, therefore, require the same scrutiny in product design and formulation14. Given the trade-offs, the implementation of bioplastics faces several challenges (Box 1).
In this Review, we discuss the benefits and risks of technologies for the production and recycling of bioplastics towards informing circular economy principles. We start by briefly reviewing the environmental issues relating to plastic production and disposal, before outlining the principles of a circular economy. The remainder of the Review is organized according to the stages along the supply chain of bioplastics. We address technological advances in bioplastic feedstocks and manufacturing, consider the EOL options and culminate in an appraisal of commercial and regulatory aspects. This Review covers scientific literature, governmental and non-governmental organization reports and industry trends up to the end of 2021.
Box 1 Disambiguation of ‘bioplastics’ and challenges for their implementation.
Bioplastic definitions
The prefix ‘bio’ in bioplastics can mean several things: the monomers were derived from renewable resources (biomass) and then polymerized through chemical mechanisms; the polymer was extracted from biomass; the polymer or the plastic is biodegradable (note that the processing of a polymer into its plastic product can affect the original biodegradability); the material is produced through biological processes; or a combination of these261 (see the figure). The use of ‘bioplastics’ for fossil-derived degradable plastics is discouraged6,7.
Using more descriptive terminologies can be helpful: for example, bio-based durable polyethylene (bioPE) is made from biomass derivatives but is not readily biodegradable, polybutylene succinate (PBS) is typically fossil-based yet biodegradable (that is, easily hydrolysable), and polyhydroxyalkanoates (PHAs) are biodegradable and bio-based, at least when the synthesizing microorganisms are grown on biomass. Note that biodegradation refers to the depolymerization of polymers by biological organisms, whereas composting is a form of biodegradation that yields CO2, H2O, heat and humus; therefore, the compostability of a polymer depends on the microbial and chemical environment.
Bio-based plastics are not, by default, more sustainable than fossil-based plastics. Although use of renewable resources can reduce carbon emissions, other factors along the life cycle can offset these benefits. Sustainability benefits and trade-offs must be elucidated from life cycle assessments that scrutinize all steps along the fossil-based and bio-based plastic life cycles, from feedstock harvesting, through various processing steps to end-of-life scenarios6,13,172.
Challenges: the ‘5Es’
We have identified five main challenges that hinder the implementation of bioplastics.
Economics
Most bioplastics are currently more expensive to produce than fossil-based plastics, mostly owing to economies of scale and the price competitiveness of crude oil.
Efficiency
Bioplastic manufacturing processes can be less energy efficient than fossil-based plastic processes and come with other environmental burdens associated with agricultural farming.
End of life
For most bioplastics, recycling streams have yet to be established to make them truly ‘circular’. Consumers remain uncertain of how to deal with bioplastics after use. Compostable bioplastics are often rejected by composters.
Ethics
Using first-generation biomass, which is often edible, remains controversial owing to potential competition with food production. Processes to efficiently use second-generation biowastes need to be established.
Education
Consumers and plastic converters are confused about the usefulness of bioplastics, owing to inconsistent labelling, contradicting life cycle assessments and ‘greenwashing’. Improved information distribution and consistent global standards need to be established.
PBAT, polybutylene adipate-co-terephthalate; PC, polycarbonate; PE, polyethylene; PEF, polyethylene furanoate; PET, polyethylene terephthalate; PLA, polylactic acid; PP, polypropylene; PS, polystyrene; PU, polyurethane; PVA, polyvinyl alcohol; PVC, polyvinylchloride. 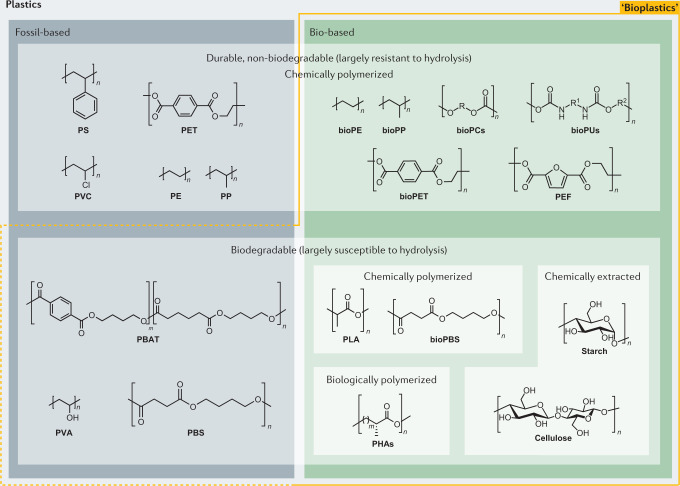
Environmental impact of plastic
The ‘plastic problem’
Environmental plastic pollution has become a priority of major global entities, including the UN15,16, the World Economic Forum (WEF)17, the World Health Organization18 and the European Union (EU)19. The plastics industry has traditionally implemented mostly linear processes focused on extracting raw materials and converting them into useful products, rather than recycling or reusing products2,20. The overall production of non-fibre plastics since 1950 has been dominated by PE (36.4%), polypropylene (PP; 21%) and polyvinylchloride (PVC; 12%), while the fibres market largely comprises polyethylene terephthalate (PET; 70%). The largest global plastic volumes in commercial sectors in 2015 were in packaging (35.9%), construction (16.0%), textiles (14.5%) and consumer goods (10.3%)3. The automotive, electronics and agricultural industries also use considerable amounts of plastics (for example, 10.1%, 6.2% and 3.4%, respectively, in the EU in 2016)2,19,21. Packaging is considered the greatest source of waste globally, with 146 million tonnes produced in 2015, of which 141 million tonnes went unrecycled (96.6%). Packaging also tends to have the shortest working life out of all industrial plastic sectors3. For single-use plastics, the working life, from use to disposal, can be as short as a few minutes.
Owing to poor waste management, ~1–5% of all plastic ends up as waste in terrestrial and, predominantly, oceanic environments. Approximately 80% of oceanic plastic debris comes from land, typically from mismanaged landfills and kerbsides that are plundered by sea tides and wind16,22. Around two million tonnes of plastic debris leach into rivers each year, occurring in both developing countries, which lack adequate collection and waste treatment infrastructures, and industrialized nations, such as China and the USA22–24. This issue is amplified by ‘external dumping’ — that is, plastic shipped from wealthier nations to those with inferior waste management infrastructures and regulations15,16,25,26. This problem is now being addressed by UN member states through the 2019 Basel Convention’s Plastic Waste Amendments, which aim to regulate global plastic waste trade27.
Within oceanic environments, submerged plastic pieces can choke marine life. Moreover, microplastic particles, which are <1–5 mm in size and are typically created by abrasion and ultraviolet (UV) light degradation, can ascend within the food chain; today, microplastic particles can be found in tap water, air, fish and salt18,28,29. These particles are potentially harmful because of their particulate nature and because they can absorb and carry contaminants, such as additives and hydrophobic organic chemicals30. The majority (98%) of oceanic microplastic particles comes from land-based sources, mainly from washing synthetic clothes (35% of total marine microplastics, coming mainly from Asia) and abrasion of car tyres (especially from North America)31–33. Although current levels of microplastic particles in freshwater are considered too low to be harmful18,30,34, they can have deleterious effects at higher levels. Worms, amphipods, oysters and crabs exhibited impaired growth, inflammation and reduced cognitive function upon exposure to higher levels of microplastic particles35–37. Gravitational sinking and seabed currents lead to localized and concentrated deposits of microplastics in some sea-floor ecosystems38. Plastic pollution is also costly: in the Asia-Pacific region alone, the economic damage to the tourism, fishing and shipping industries is estimated to be US$1.3 billion per year15, and the WEF estimates the annual cost of global marine litter to be $40 billion39.
The COVID-19 pandemic has exacerbated the ‘plastic problem’, creating urgent demand for single-use plastic personal protective equipment such as masks, gloves and face shields. Several bans on single-use plastic were temporarily reverted, reusable shopping bags were banned and, in some places, traditionally recyclable plastic food containers were considered hazardous, owing to potential pathogenic contamination40. Consequently, the amount of medical plastic waste has increased by 3–10-fold beyond local waste treatment capacities in certain places, such as China and Jordan, and the UN expects short-lived items, such as hundreds of millions of masks, to end up mainly in landfills or the ocean40,41. Indeed, the pandemic has gravely accentuated the inability of existing waste management systems to cope with surging amounts of potentially hazardous plastic waste42. Yet, as the UN Secretary-General António Guterres has stated, “pandemic recovery is our chance to change course,” through policies and investments in sustainable technologies43.
Climate change
Human activity has driven warming of the planet and increased extreme weather conditions, mainly rooted in the emission of GHGs through exploitation of fossil resources, such as CO2 and CH4 (ref.44). Plastic production consumes ~5–7% of the global oil supply and released >850 million tonnes of CO2 into the atmosphere in 2019, representing 2% of the global CO2 output45,46. The majority of plastic-related CO2 emissions are incurred by raw material extraction (61%) and polymer production (30%), while only 9% is associated with the EOL stage, mainly incineration45,47. Undocumented open burning of plastic waste might add >1 Gt of CO2 equivalent emissions to these numbers48. Simulations suggest that complete replacement of fossil feedstocks with sugarcane would reduce GHG emissions by ~25%; recycling all existing plastics could have a similar effect. As the largest carbon footprint of plastics is associated with production, switching existing processes to a renewable energy supply would cut plastic-related emissions by 62%. These measures, along with halved consumption, would lower current levels by 93%47, suggesting that these four systematic changes need to act in concert to effectively mitigate emissions. Conversely, petrochemical plastic capacities are growing, with more than 300 new industrial projects (with a value of more than US$200 billion) announced since 2010 in the USA alone, of which about half are in the construction or completion stages. This growth could lead to plastic production and incineration generating 2.8 Gt of CO2 emissions by 2050. Such levels would account for 10–15% of the estimated global carbon budget of CO2 output to keep global warming below 2 °C, which is the goal stated in the UN Paris Agreement of 2015 (refs2,45,47,49,50).
Besides plastic-related carbon emissions, microplastic waste has the potential to affect the capacity of oceanic organisms to capture CO2. Phytoplankton capture ~31% of global anthropogenic CO2 emissions and are, thus, a crucial global CO2 sink; however, their capabilities to sequester and sediment CO2 into deep ocean soil are believed to be impaired by microplastic45,50–52. This issue could become an autocatalytic process, because warming itself can negatively affect phytoplankton activity53.
End-of-life and circular economy principles
In an ideal circular economy, plastics would be made from renewable or recycled resources (Fig. 1). However, the traditional life of most plastic materials is linear (Fig. 1): 79% of all plastic produced has been dumped into landfill sites or reached the environment, while the remainder has been incinerated (12%) or recycled (9%). Although recycling has increased since the 1980s, the recycling of non-fibre plastic remains stagnated at 18% and almost no textile fibres are recycled3. The lack of progress is largely due to the limitations of the dominant form of recycling — mechanical recycling — which converts waste plastics into new shapes through mechanical force and heat. Product quality is, therefore, highly dependent on input quality and, thus, mechanical recycling ideally requires well-sorted and contamination-free plastic waste, which is often scarce. By contrast, different forms of chemical recycling offer a more resilient and flexible way to recycle mixed and contaminated plastic waste, as well as popular multilayer materials2,54. Chemical recycling first depolymerizes the polymer to recover the monomers, which, after appropriate separation, can undergo repolymerization into materials of defined quality55–58 (discussed further below). However, these processes are more complex and, thus, more expensive, particularly during the implementation phase, and, therefore, require financial incentives. Biodegradation is an EOL option largely for easily hydrolysable polymers, such as aliphatic esters like polylactic acid (PLA), but should be performed only in controlled industrial settings to ensure complete digestion without uncontrolled side effects, such as leakage of contaminants or microplastic formation46,59.
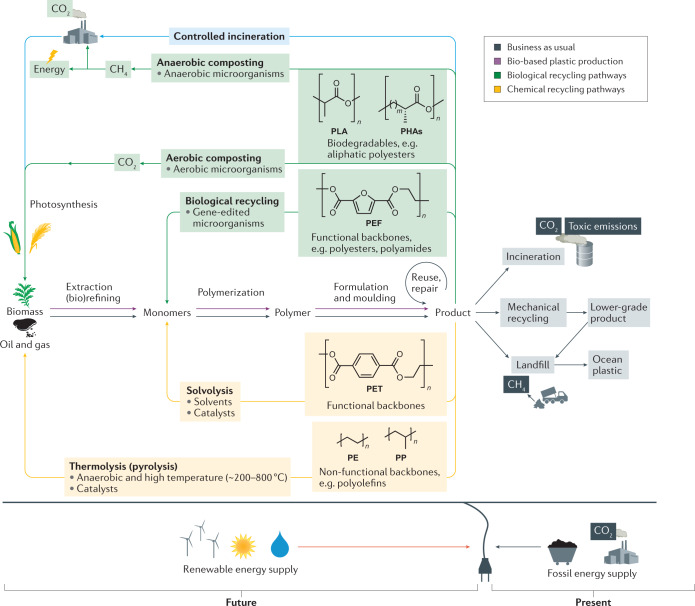
Fig. 1. The circular plastic economy.
The plastics industry has traditionally been based on linear life cycles (grey arrows): crude oil is cracked and refined into monomers and polymer products using fossil energy, which, at their end of life, are either disposed of (~80%) with potential environmental leakage, incinerated (~10%) or, in the minority of cases (10% globally), mechanically recycled into lower-grade products, which also end up landfilled2,3,19,23,24. In a ‘circular plastic economy’ (green arrows), plastic waste becomes raw material for a recycling process at its end of life, and all production and recycling processes are supplied with renewable energy21,47,62. Renewable resources (lignocellulosic biomass and pyrolysis oils) are the starting materials for polymer products, which all have a defined circular end-of-life scenario. CO2 generated through bioplastic incineration (blue arrow), aerobic composting or incineration of CH4 from anaerobic composting229 is a net-zero addition to the carbon cycle, as it is captured by photosynthesis into new biomass. Advanced recycling routes enable upcycling of plastic waste: polymers with functional backbones (such as polyesters or polyamides) can be depolymerized biologically or chemically, and the subsequent monomers are polymerized into tailored high-quality or virgin-quality products55,151,181,210. Polymers with non-functional backbones such as polyolefins (including polyethylene (PE), bio-based PE, polypropylene (PP) and polystyrene) are better suited for cracking into hydrocarbon oil and gas by thermolysis and can then follow a similar upcycling path58,199,200,260. PEF, polyethylene furanoate; PET, polyethylene terephthalate; PHA, polyhydroxyalkanoate; PLA, polylactic acid.
Innovating ‘beginning-of-life’ options to minimize environmental impact are equally important as EOL considerations. Educating consumers and companies towards ‘life cycle thinking’ will encourage a holistic view on plastic products beyond their obvious impacts associated with the use and disposal of plastics. Less tangible but potentially more detrimental environmental impacts are associated with feedstock harvesting, processing energies and transportation12. To this end, the sustainable harvesting and catalytic conversion of local, non-food, renewable resources and biological wastes into bio-based plastics can provide greater sustainability than established fossil fuel extraction and refining practices47,48,60.
Circular economy principles and technologies, therefore, need to be enacted at every step along the plastic supply chain to minimize environmental impact and plastic waste. Useful measures towards future circular economies include a drastic reduction in plastic consumption, the design of products that can be reused and recycled in their markets, improved process energy efficiency in plastic and bioplastic manufacturing combined with the use of renewable power, increased collection rates and market penetration of robust and circular recycling and ‘upcycling’ methods16,61,62 (Fig. 1). Owing to the cost-competitiveness of established plastic industry practices and linear business models, the implementation of these measures requires not only technological progress but also economic investment and financial incentives created by legislators16,21. The financial investments would be well justified, as chemical recycling is estimated to achieve global profits of $55 billion per year57.
Bio-based raw materials
Similar to the traditional concept of an oil refinery, ‘biorefineries’ convert renewable bio-based feedstocks into useful chemicals63–66. Biomass is a relatively quickly renewing resource and is typically divided into first-generation and second-generation feedstocks. The former typically corresponds to readily fermentable sugars from edible polysaccharide sources, such as corn and sugarcane, and edible vegetable oils. Although some studies suggest that it is possible to sustainably co-produce biomass for both food and fuel (and, thus, also for bio-based materials)67,68, first-generation biomass remains controversial, owing to ethical concerns about the potential competition with food resources, especially in local settings46. Currently, 0.02% of global agricultural land use is devoted to producing precursors for bioplastics8. A total replacement of fossil resources for plastics with biomass, however, is unlikely, highlighting the need for reduced consumption and improved recycling. A complete switch of the 170 million tonnes of global packaging plastics produced per year to bioplastics has been estimated to require 54% of the current corn production and 60% more than Europe’s annual freshwater withdrawal69.
Second-generation biomass describes various non-edible biowastes that offer a more ethically viable and widely available, albeit more complex, feedstock70. For example, more than 1 billion tonnes of agricultural and food waste are produced globally each year, and ~20% of domestic waste is food waste71–73. Research towards future biorefineries aims to establish processes to convert lignocellulosic biomass, such as wheat straw and sugarcane bagasse. These agricultural wastes are typically inexpensive but require additional pretreatment steps to liberate fermentable cellulose and hemicellulose sugars from the protective, phenolic, crosslinked lignin polymer network74–77.
Another source of polysaccharides is seaweed78,79, which includes brown and red algae. The most abundant polysaccharides in brown algae are alginates, which comprise up to 40% of their dry weight. Alginates can react and gel with divalent or trivalent cations and can be blended with starches to make biodegradable plastic films with low gas permeability and other desirable mechanical properties.
Vegetable and plant oils also provide access to various monomers. Similar to sugars, edible oils raise concerns over food competition and deforestation80,81, while non-edible oils or waste oils are more ethically and ecologically viable80. Vegetable oils9,82 contain triglycerides with unsaturated fatty acids, which can be epoxidized to make epoxy resins. Polyols that are naturally found in some fruits and vegetables are used for the synthesis of bio-based polyurethanes (bioPUs). Terpenes9, which contain isoprene units, are compounds found in plant oils. Polyisoprene is produced on a multimillion-tonne scale and is widely used as natural rubber. Limonene, a terpene obtained from lemon peel, can be used to make bio-based polycarbonates (bioPCs) that are free of bisphenol A by reacting limonene epoxide with CO2 (refs83,84).
CO2 is an abundant yet low-energy feedstock that can be used directly for polymer synthesis with effective catalysts85,86. This approach allows CO2 from air to be sequestered in a polymer, until its ultimate release through incineration or composting. Exhaust gases from NH3 factories and coal power plants contain ~97% and 15% CO2 (sold at <$70 per tonne), respectively. These emission-intensive industries can, thus, provide CO2 as a building block87,88 for the synthesis of polyurethanes, polycarbonates and various other chemicals86,89,90.
Crude oil is the raw material for most traditional durable polymers but it is also used to produce many biodegradable polymers (discussed below). Owing to the environmental pollution associated with harvesting and incinerating fossil products, crude oil is considered a non-sustainable feedstock.
Each bioplastic feedstock has its own set of challenges. It is crucial to increase the conversion efficiency of renewable resources into useful chemicals in biorefineries91. Examples include enhanced pretreatment methodologies and more robust microorganisms for fermentation70,92.
Bioplastics
Today, almost every monomer required for the production of drop-in polymers — that is, chemically equivalent replacements for fossil-derived polymers — can be obtained from biomass. Additionally, biomass can support the synthesis of novel polymers that are not easily derived from fossil resources. The methods for processing biomass to obtain vinyl monomers93, carboxylic acids94–96, alcohols90, amides93 and rubbers65,93 have been extensively reviewed. In this section, we provide an overview of the most commercially relevant polymers for bioplastic manufacturing. Figure 2 shows examples of bio-based, fossil-based, durable and degradable plastics and their synthesis routes from various feedstocks. The environmental and economic parameters of the most well-known synthetic bioplastics on the market are compared with existing fossil-based plastics in Table 1. The corresponding materials properties as well as the commercial volumes of bioplastic production are summarized in Supplementary Tables 1 and 2, respectively.
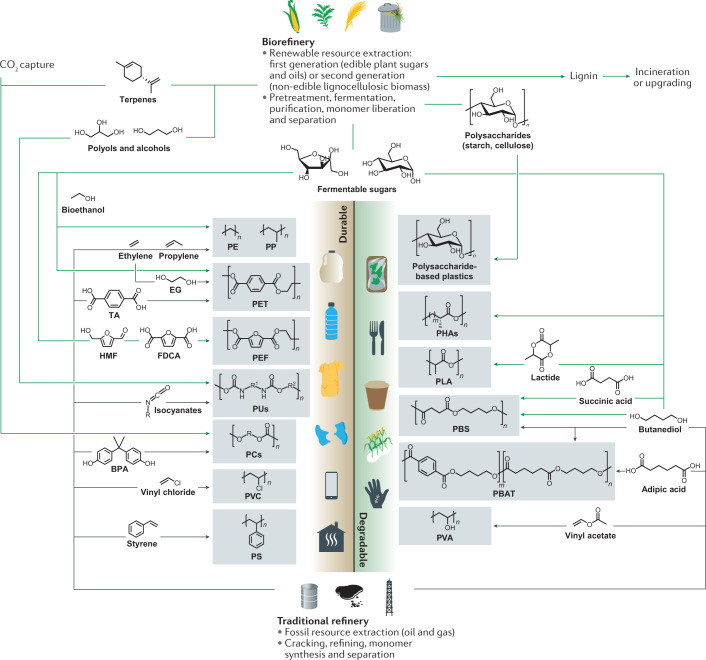
Fig. 2. Routes for synthesizing polymers from fossil-based and bio-based resources.
Petrochemical feedstocks (bottom) are the traditional resources for most commercial monomers and polymers for durable and single-use applications (such as polyethylene (PE), polypropylene (PP), polyvinylchloride (PVC) and polyethylene terephthalate (PET)), as well as for several fossil-based biodegradable polymers (such as polybutylene adipate-co-terephthalate (PBAT) and polyvinyl alcohol (PVA))142,180. Several plastic families, such as polyamides, are not included here for reasons of space and complexity. Using renewable raw materials (top), biorefineries upgrade first-generation and second-generation biomass (that is, edible plant products and non-edible biowastes, respectively) into the same building blocks as those derived from petroleum, as well as others64,70,93. These monomers can be polymerized into several durable drop-in polymers (such as bioPE and bioPET), new durable polymers (such as polyethylene furanoate (PEF))6,92,127,128,144, as well as biodegradable ones (such as polylactic acid (PLA) and bio-polybutylene succinate (bioPBS))105,152. Polyhydroxyalkanoates (PHAs) are biosynthesized in microorganisms from various feedstocks106,110,121,235. Advanced catalysis unlocks captured CO2, which, together with plant-oil-derived terpenes and epoxides, can be used to synthesize polycarbonates (PCs)85,86. Bio-based non-isocyanate polyurethanes (PUs) can be made from plant-oil-based polyols93. Separated lignin is often incinerated for energy recovery but its phenolic network can also be converted into useful chemicals143,164. Polysaccharides can be extracted from plant biomass and converted chemically into plasticized starch and cellulose-based products13,163,165. BPA, bisphenol A; EG, ethylene glycol; FDCA, 2,5-furandicarboxylic acid; HMF, 5-hydroxymethylfurfural; PS, polystyrene; TA, terephthalic acid.
Table 1.
Comparison of environmental properties and typical prices of some commercially relevant synthetic fossil-based and bio-based polymers
| Polymer | Biodegradation (industrial) | Biodegradation (ocean) | GWP cradle-to-gate (tonne CO2eq per tonne polymer) | AP cradle-to-gate (kg SO2eq per tonne polymer) | Price (US$ per kg)5,254 | Refs |
|---|---|---|---|---|---|---|
| Fossil-based and durable | ||||||
| HDPE | NA | NA | 1.8–2.6 | 6–22 | 1.4–1.6 | 111 |
| LDPE | NA | NA | 1.9–3.1 | 27 | 1.36 | 111 |
| PP | NA | NA | 1.5–3.6 | 49 | 1.1 | 151,225 |
| PS | NA | NA | 3.2 | NA | 0.7–1.5 | 180 |
| PET | NA | NA | 2.4–5 | 10–18 | 1.2–1.4 | 111 |
| PVC | NA | NA | 1.5–2.2 | 3 | 1.9 | 180 |
| Fossil-based and degradable | ||||||
| PBAT | 2–3 months | >1 year | NA | NA | 4.1 | 111,167,255 |
| PBS | 2–5 months | >1 year | NA | NA | 4.5 | 68,167,256 |
| PVA | 1–2 weeks | 4 months | NA | NA | 2 | 147 |
| PCL | 4–6 weeks | 6 weeks | NA | NA | NA | 149,150,167 |
| Bio-based and durable | ||||||
| PEF | 9 months | NA | 2.1 | NA | NA | 128,130,257 |
| bioPET | NA | NA | 2–5.5 | 13–75 | NA | 151 |
| bioPE | NA | NA | 0.68 | 30 | 1.8–2.4 | 258 |
| Bio-based and degradable | ||||||
| bioPBS | >3 months | >1 year | 2.2 | 75 | NA | 167,169,256 |
| PLA | 6–9 weeks | >1.5 years | 0.5–2.9 | 7–21 | 2–3 | 111,167,206 |
| PGA | 2–3 months | 1–2 months | NA | NA | NA | 151,152 |
| P3HB | 1–4 months | 1–6 months | −2.3–4 | 14–25 | 3–8 | 167,225,235 |
| P4HB | 4–6 weeks | 1–6 months | NA | NA | 3–8 | 151,167,259 |
Degradable polymers are those that contain readily hydrolysable aliphatic ester bonds in their backbone and polyvinyl alcohol (PVA), whose degradation follows a diketone pathway. Durable polymers are those with a backbone that is typically more resistant to enzymatic and non-enzymatic hydrolysis, such as aromatic esters, amides and those with C–C bonds. Note that non-zero degradation may occur in any polymer. Biodegradation refers to industrial compostability under EN 13432 or ASTM D6400 conditions or degradation in ocean water according to the references. The values or ranges, where available, for global warming potential (GWP) and acidification potential (AP) are taken from a 2020 review of cradle-to-gate life cycle assessments6 or from other references, where indicated. HDPE, high-density polyethylene; LDPE, low-density polyethylene; NA, not available; P3HB, poly(3-hydroxybutyrate); P4HB, poly(4-hydroxybutyrate); PBAT, polybutylene adipate-co-terephthalate; PBS, polybutylene succinate; PCL, polycaprolactone; PE, polyethylene; PEF, polyethylene furanoate; PET, polyethylene terephthalate; PGA, polyglycolic acid; PLA, polylactic acid; PP, polypropylene; PS, polystyrene; PVA, polyvinyl alcohol; PVC, polyvinylchloride.
Bio-based aliphatic (degradable) polyesters
Aliphatic (or non-aromatic) polyesters are degradable because the ester groups in their backbones can be easily cleaved by hydrolysis or enzymatic activity. The most common bio-based aliphatic polyesters are PLA, polybutylene succinate (PBS) and PHAs.
PLA is an aliphatic homopolymer and is the most price-competitive synthetic bioplastic, with a production capacity of >250,000 tonnes per year97–99. PLA is typically made through the polycondensation of lactic acid97, which can be derived from the fermentation of sugars, or through the ring-opening polymerization of lactide, the cyclic dimer of lactic acid100–102. PLA can be optically clear and has found use as a replacement for polyolefin films or polystyrene foams, including incorporation into single-use items. Indeed, Total Corbion PLA has recently announced that PLA-coated paper cups are repulpable and, thus, recyclable, whereas traditionally PE-coated paper cups are not103. However, the relatively short repeat unit and the methyl side group make PLA brittle and slow to crystallize. Thus, PLA typically needs to be modified and blended (such as with other biopolymers or nucleation agents) before being processed8,104.
PBS is an aliphatic copolyester with longer hydrocarbon repeat units and, therefore, a more flexible molecular structure than that of PLA. As such, the material properties of PBS are more similar to those of polyolefins, namely, a low glass transition temperature and a high elongation at break (>500%). PBS is typically synthesized from non-renewable feedstocks, but its monomers, succinic acid and butanediol, can be obtained from renewable sources. Methods to produce succinic acid by fermenting lignocellulosic sugars are under development, and butanediol can be produced by hydrocracking starches and sugars90,105.
PHAs are an emerging family of biodegradable aliphatic polyesters with a commercial market that is expected to reach annual volumes of >100,000 tonnes in the coming years106. Instead of chemical synthesis, PHAs can be produced by various bacteria, including Pseudomonas and Ralstonia strains, as well as algae107–109. These microorganisms store PHA intracellularly at levels of up to 80% of their cell volume110,111. Various carbon-rich feedstocks can be used for cultivation, including inexpensive food residues and liquefied plastic wastes, highlighting the usefulness of the biological PHA production process in enabling circularity112–114. The material properties of PHAs can be tuned by varying the repeat unit chain length, the side chain functionalities and co-monomer composition, resulting in (co)polymers such as rigid and brittle poly(3-hydroxybutyrate) (P3HB) or the softer and more flexible poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH)110,111,115–117. Their desirable mechanical properties and good O2 and CO2 barrier properties indicate that PHAs would be suitable replacements for bulk packaging materials such as PE and PP115,118,119. Similar to PLA, PHA linings for paper cups could potentially alleviate the non-recyclability of PE-lined paper cups120. Most PHAs degrade faster than PLA, which makes them attractive for applications in which biodegradation is desired. Gene-editing technologies such as CRISPR–Cas9 can potentially be used to guide the type and properties of PHA that is produced; these tools can also tune metabolic pathways to increase cell size, increase PHA yields and increase resilience to stress to improve production efficiencies, which can consequently decrease production cost and enable the application of PHAs in a wider range of commercial products121,122. Furthermore, extracellular PHA production has been observed in certain bacterial mutants, which could substantially reduce the production cost by simplifying or obviating the need for cell lysis and extraction steps123. PHAs are often extracted from cells using halogenated organic solvents; however, more environmentally benign and solvent-free cell disruption methodologies are being developed124,125.
Bio-based aromatic (durable) polyesters
Polyethylene furanoate (PEF) is expected to enter markets in the coming years and is considered a promising high-performance plastic similar to PET. Its slightly altered semi-aromatic structure gives rise to a higher gas diffusion barrier, higher tensile strength and higher glass transition temperature, which may be useful for long-shelf-life packaging126. However, PEF is also more thermally sensitive and, thus, requires more careful processing127–129. PEF biodegrades faster than PET under certain industrial composting conditions (within 9 months) but, otherwise, is considered a similarly durable material without notable biodegradation in the environment126,130. PEF can be produced similarly to PET through polycondensation of the bio-derived monomers monoethylene glycol and 2,5-furandicarboxylic acid, as pursued by Avantium (Netherlands)127,131. Alternatively, PEF can be synthesized by ring-opening polymerization of cyclic PEF oligomers, as pursued by Sulzer (Switzerland), which can decrease reaction times and improve molecular weight control128,132,133. Various molecular modifications and copolymerizations are being explored to tune the mechanical strength, glass transition temperature and degradability of PEF134,135. But cost-effective production of the monomer 2,5-furandicarboxylic acid remains a challenge94.
bioPET is the bio-derived drop-in variant of PET. Its identical properties make it suitable for direct application in the beverage (one-third) and textile (two-thirds) markets56, as well as PET established recycling streams. Terephthalic acid, which is esterified with ethylene glycol to make PET, can be derived synthetically or microbially from biomass through intermediates such as para-xylene and 2,5-furandicarboxylic acid136,137.
Bio-based polyurethanes
Polyurethanes are commercially applied on a scale beyond 18 million tonnes, mainly as flexible and rigid foams. The use of toxic phosgene and potentially carcinogenic isocyanate monomers have raised health concerns during the life cycle of traditional polyurethanes138. Instead, non-isocyanate bioPUs can be made from cyclic carbonates and diamines derived from vegetable oils93,139–141. Cyclic carbonates can also be produced by cycloaddition of epoxides with CO2 (ref.90).
Bio-based polyolefins
Polyolefins, such as PE and PP, constitute >50% of global plastics production and >90% of packaging materials142. Their widespread use is due to their excellent chemical stability and tailorable mechanical properties. Progress in catalysis since the 1950s, such as the development of Ziegler–Natta catalysts, has made controlled-quality, large-scale petrochemical processing efficient and cost-effective. bioPE is chemically equivalent to PE and, therefore, equally processable and recyclable on existing infrastructure, including future recycling methodologies such as thermolysis142. Ethylene can be derived by dehydration of ethanol from sugarcane, by steam cracking of biomass or through methanol-to-olefin routes93,143. Bio-based PE-like materials with a low density of ester and carbonate bonds in the polymer backbone that serve as breaking points have been fabricated through the polycondensation of bio-based difunctional oligoethylene monomers. This approach enables the use of renewable resources and improved recycling by solvolysis, which is usually not applicable to polyolefins144. The development of bioPP is less established, but several synthetic avenues are possible, including metathesis from bio-ethylene and butylenes143.
Fossil-derived biodegradable polymers
In this Review, we largely focus on bioplastics made from polymers derived from renewable resources. The term bioplastic has also been used for fossil-based plastics that are biodegradable5, but its use is controversial, owing to the potential confusion with bio-based materials from renewable (biomass) feedstocks6,7. In the following, we briefly review fossil-based polymers that are frequently associated with bioplastics because of their biodegradability and that have the potential to be produced from biomass in the future93.
Polyvinyl alcohol (PVA) is a widely used (1.2 Mt per year) water-soluble polymer. Most PVA is produced using ethylene, which is typically made from fossil fuels but could be obtained from bioethanol. PVA is the only vinyl polymer that is readily biodegradable, which is believed to occur by enzymatic oxidation of the hydroxyl groups into diketones, which are then hydrolysed and cleaved145–147.
Polybutylene adipate-co-terephthalate (PBAT) is a biodegradable aromatic–aliphatic copolyester sold as Ecoflex by BASF (Germany) and under other brand names by several suppliers in Asia. It is used in agricultural mulch films, which can degrade in soil over a period of >9 months111,148.
Several degradable fossil-derived polymers have found applications in the biomedical sector. Polycaprolactone is a popular biocompatible and biodegradable material used in applications such as sutures and implantable drug delivery devices145,146. Polycaprolactone hydrolyses non-enzymatically in humans within years and is biodegraded by fungi and bacteria in seawater within weeks149,150. Polyglycolic acid is the simplest aliphatic ester. Its fast industrial and marine degradation rates (Table 1) and its high gas barrier make it an interesting candidate for plastic packaging. Despite its large financial market share in the biomedical sector, the production volumes of polyglycolic acid are negligible from a commodity standpoint151,152. A copolymer of polyglycolic acid and PLA, polylactide-co-glycolide (PLGA), is also commonly used in biomedical applications, owing to its biocompatibility and faster biodegradation than polycaprolactone. PLGA can be synthesized through polycondensation or ring-opening polymerization with various molecular weights and monomer ratios, enabling the degradation rate and mechanical stiffness to be tuned152,153.
Polyanhydrides are a class of polymers whose highly hydrolytically labile anhydride bond can be exploited in materials for drug and protein release154–158. Degradability can be tuned by varying the monomers between the anhydride linkages in the backbone. Aliphatic polyanhydrides (such as poly(sebacic anhydride)) degrade over days, whereas aromatic polyanhydrides degrade over months to years. Producing polyanhydrides that have material properties similar to those of the more durable ‘engineering plastics’ was suggested by Bucher and Slade in 1909 (ref.159) but has not been commercially explored, except for certain medical applications160–162.
Other non-synthetic bioplastics
Although this Review focuses largely on synthetic bioplastics, the extraction of polymers directly from biomass is a simple and often cost-effective method. Starches, which constitute a considerable portion of food waste, are the main material used for non-synthetic starch-based bioplastics, produced by direct processing of the starch into films163. Lignin separated from biowastes in second-generation biorefineries is mostly (98%) incinerated for power generation. However, its complex phenolic structure has sparked interest in upgrading lignin into polymer additives, polymer grafts or monomers for specialty plastics143,164. Cellulose, the most abundant natural polymer, can be extracted from plant biomass or specific cellulose-producing bacteria and processed into food-packaging materials or used as a nano-filler additive with other bioplastics to improve barrier properties and the tensile strength of food packaging165,166. Cellulose is biodegradable167, yet regenerated cellulose, which is popular as ‘viscose’ or ‘rayon’ textile fibres, constitutes 60% of sea-bed microplastics31. A related material, cellulose acetate, is used to make cigarette filters, which are a notable litter issue. Cellulose acetate degrades extremely slowly owing to its acetylation, which renders the material hydrophobic168. Composite blends of different polymers with other natural additives, such as starch, lignin or silica, can also afford materials with a range of properties169,170.
Life cycle assessment of bioplastics
LCAs can be performed with various boundaries that result in different outcomes. Scenarios can be set up from cradle-to-gate (resource extraction to plastic factory outlet), cradle-to-grave (resource extraction, plastic manufacturing and disposal process) and cradle-to-cradle (entire process including recycling or biodegradation processes at end of first life)171. There are international standards in place to guide the structure, conduct, limitations and assumptions made of general LCAs, such as ISO 14040, and specific guidelines for bioplastics, such as EN 16760. However, heterogeneity in LCA approaches and assumptions persist6,172,173. For example, manufacturer’s processing parameters are mostly confidential and, therefore, assumptions are typically made. Many LCAs focus on carbon emissions in cradle-to-gate scenarios, often for lack of reliable data on EOL scenarios. Therefore, some exemplary sustainability indicators presented in Table 1 exhibit quite a broad range of values, owing to the heterogeneity of scenarios and assumptions made6. Broader boundaries (such as cradle-to-cradle) could derive more inclusive and informative LCAs, as post-consumer polymer waste contributes considerably to plastic pollution, and recycling scenarios have a notable impact on the LCA performance of plastics2,6,98. Other means to improve LCA meaningfulness are more detailed reporting of inventories (such as the use of additives and local energy mixes), the extension of scope to multiple plastic use cycles and the inclusion of the effects of plastic pollution, such as leakage into oceans174,175. As such, current LCAs are not always able to inform decision-makers in choosing the ‘right’ and most sustainable polymer for a given application. Developing a systematic framework for bioplastic LCAs in which key parameters and assumptions are consistent would increase their usefulness.
Bio-based plastics are not, by definition, more sustainable than fossil-derived plastics. In many LCAs, bioplastics yield a considerable reduction in global warming potential through the use of renewable resources. However, these benefits are often counterbalanced by side effects of feedstock farming, such as acidification potential and eutrophication due to increased fertilizer and pesticide use6,167,172. Improvements in the supply chain of raw material extraction, such as measures that eliminate extensive pesticide use and forest burning practices, could substantially reduce these negative impacts of bioplastics175. LCA outcomes depend heavily on assumptions made for energy use and processing efficiencies in all production stages and, thus, some LCAs have associated bioplastics, such as PLA, with higher energy and water use6,98,172. Bioplastics often score negatively in landfill scenarios, whereas the situation looks more promising once plastic recycling is taken into account (see next section)98. Social and economic sustainability should also be included in LCAs to evaluate whether bioplastics contribute not only to a circular economy but a truly sustainable circular economy176.
End-of-life treatment scenarios
Leakage of plastic into the environment is a central issue of inappropriate EOL management3,22. Recycling of bioplastics is widely regarded as the most environmentally friendly EOL option and better than simple composting. However, bioplastics recycling streams are less established than those for traditional plastics98,99. Sorting of mixed plastic waste becomes even more demanding with novel (non-drop-in) bioplastics by increasing its heterogeneity, which raises concerns of higher rejection rates177,178. Spectroscopic techniques such as near-infrared scanners can be used to selectively identify bioplastics; for example, PLA can be identified with 98% accuracy179. Advanced sorting technologies include X-ray and UV spectroscopy, inert detectable markers in materials for ‘barcoding’ and using artificial-intelligence-based robotic sorting19,178.
Plastic and bioplastic recycling is generally complicated by the presence of additives in almost every finished plastic product3. For example, typical PVC flooring can be composed of up to 80% fillers, plasticizers and pigments180. An ‘ingredients table’ (such as those found on food packaging or shampoo bottles) could detail the composition of a plastic product and, therefore, inform of its suitability for local recycling options. Furthermore, the complex and multimaterial design of plastic products typically prohibits recycling, which is why accounting for recyclability and simplicity in product design can greatly increase recycling rates. For example, achieving the necessary barrier properties for packaging through high-barrier monomaterials could improve recyclability by replacing non-recyclable multilayers2,128. Physical methods such as biaxial orientation can increase plastic film strength, clarity and barrier properties without the need for chemical additives180. Progressive extended producer responsibility (EPR) schemes, such as charging producers higher fees for less recyclable plastics, would help incentivize the design of easy-to-recycle products.
In this section, we discuss the EOL options for bioplastics, considering current and future recycling scenarios (Fig. 1).
Mechanical recycling
Mechanical recycling is the simplest, cheapest and most common form of recycling181,182, and typically involves sorting the plastic waste by polymer type, removing labels, washing, mechanical shredding, melting and remoulding into new shapes. Mechanical recycling of bioplastics is generally not yet commercially available, but re-extrusion has been performed in the literature. The mechanical recycling of PLA and PHA is associated with the usual reduction in quality, such as loss of tensile strength and molecular weight125,151. Given the inability of mechanical recycling to effectively remove contaminants and additives from polymer waste, combined with the inherent thermal and mechanical stress, the products are generally ‘downcycled’ into goods of lower quality. Coloured or low-density materials (films, foams), as well as medical contaminants, are further complications and can render products non-recyclable21,59,181. Food-grade recycled materials are, therefore, hard to obtain183,184. Virgin polymers are often mixed with the recyclates to improve the quality of the recycled ones180,181. Nevertheless, mechanical recycling is often described as the most desirable EOL option, owing to its divergence from virgin resources. The environmental impact of mechanically recycled plastic is typically lower than that of virgin plastic. For example, the environmental impact (GHG emissions from transport and process energy use) of recycled PET (rPET) is two times lower than that of virgin PET, increasing to three times for recycled PE and PP (rPE and rPP, respectively) relative to their virgin materials185,186. The overall capacity of this form of recycling is, however, very limited: globally, ~10% of PET and high-density PE is recycled, whereas for polystyrene and PP, the numbers are closer to zero. Textiles and fibre products are also rarely recycled3.
Deposit-refund systems and EPR schemes can increase return and collection rates for post-consumer plastics and increase the quality of the plastic collected187. The plastic that is most commonly mechanically recycled is PET from beverage bottles. As a polycondensation polymer, its quality can be upgraded within existing recycling streams, wherein solid-state post-polymerization (effectively, heating of recycled flakes under vacuum to remove volatile polymerization by-products) increases the molecular weight of recyclates for commercial applications. Examples of countries with high recycling rates are Norway (97%, 2018)188, where an effective deposit system exists; Japan (83%, 2019), which has several EPR laws and fees in place189; and India (~90%, 2018)190, where informal collectors can make a living from returned bottles that recyclers pay for. In Germany, 99% of PET bottles under deposit schemes are recycled but only 65% of non-deposit bottles191. Recollection rates were roughly 30% in the USA in 2018 (ref.192). Globally, PET bottle-to-bottle recycling was at only 7% before 2016 (refs2,193); the rest was downcycled into PET fibres (72%), sheets (10%) and tape (5%), which are generally non-recyclable19,194.
Chemical recycling
In contrast to mechanical recycling, chemical recycling offers the potential for making high-quality polymers from waste — termed ‘upcycling’. Plastic products are depolymerized into their monomeric subunits, which can then be repolymerized through controlled polymerization mechanisms into polymers of desired quality (such as with controlled molecular weight). For example, low-molecular-weight fibre polyesters can be depolymerized into monomers, which can then be polymerized into longer-chain polyesters that are required for bottles56,195. Impurities and colour can also be removed. Chemical recycling is performed mainly through solvolysis or thermolysis.
In solvolysis, polymers with cleavable groups along their backbone, such as ester bonds in PET, PEF and PLA, can be subjected to solvent-based depolymerization processes such as hydrolysis, glycolysis or methanolysis56,181,196,197. Aliphatic polyesters, such as PLA, PBS or PHAs, are more hydrolysable than aromatic ones. For example, PLA can be hydrolysed to 95% lactic acid without a catalyst at 160–180 °C for 2 h with an energy demand four times lower than that of virgin lactic acid production151 or depolymerized back into ~90% cyclic lactide monomers after 6 h using Zn transesterification catalysts198. The resulting monomers present a useful feedstock for the production of high-quality plastics. However, the need for chemicals and more complex separation units make chemical recycling more expensive and, therefore, currently less economically competitive than mechanical recycling. Chemical recycling accounts for <1% of all recycled plastics. Several large chemical companies are developing processes to make ‘chemcycled’ products cost-competitive with virgin polymers57. As this approach provides monomers suitable for repolymerization into high-quality condensation polymers, such as polyesters and polyamides, the design and use of chemically recyclable polymers in plastic applications can solve persisting EOL issues and support a circular materials economy55,181.
In thermolysis, typically polyolefins, which do not possess hydrolysable functional groups, are pyrolysed at temperatures of ~200–800 °C (depending on the polymer and catalyst used) in the total or partial absence of O2. Under these conditions, the C–C bonds break, converting the polymer back into feedstock in the form of hydrocarbon oil or gas, or directly into olefin monomers. This feedstock can then be fed into traditional refineries and polymerization factories58,142,199. Thermolysis is most suitable for hydrocarbon polyolefin materials such as (bio)PE, (bio)PP and polystyrene. Thermolysis of polystyrene can recover >90% of liquid hydrocarbon oil58. One issue is the production of potentially toxic gases, as a result of the (often unknown) additives, that require appropriate capturing. Polyesters and other O-bearing, N-bearing and S-bearing polymers emit GHGs, such as CO, CO2, NOx and SOx, whereas halogenated polymers, such as PVC, produce HCl gas and chlorobenzene. The olefin monomer yield, selectivity and energy efficiency of thermolysis can be improved by incorporating advanced techniques, such as microwave pyrolysis, catalytic cracking, pressure and temperature profiling, and by adjusting the reactor configuration for surface maximization58,180,200.
Biodegradation and composting
Biodegradation and composting describe the microbial digestion and metabolic conversion of polymeric material into CO2, H2O and other inorganic compounds by various known species111. This process is typically aided by physical processes, especially those that help with fragmentation and the reduction of particle size. For example, amorphization of crystalline structures in typically semi-crystalline plastics through micronization or extrusion can make them more susceptible to enzymatic degradation201,202. Hydrolysis cleaves susceptible bonds in accessible amorphous regions of a polymer, typically aliphatic esters, and microbial enzymes and acids or bases can enhance hydrolysis. Photodegradation using UV light breaks tertiary and aromatic C–C bonds, typically leaving a brittle and discoloured material. This process can be enhanced by embedding metallic catalysts in the polymer203. Similarly, oxo-degradation (that is, decomposition by oxidation) can be triggered by metals; however, this can lead to fragmentation into microplastics and insufficient digestion. Thus, oxo-degradation has been restricted in the EU and Switzerland19,204.
Despite earlier hopes, biodegradation is non-trivial, as the rate of biodegradation is highly dependent on a polymer’s chemical structure, stabilizing additives, the surrounding conditions (such as the presence of H2O and O2) and any microorganisms used205. These conditions are often not met in home compost, open water or even in industrial composting facilities. Composters often reject biodegradable plastics, such as PLA shopping bags and utensils, as required decomposition times exceed typical composting process times of 6–8 weeks8,206. Typical biodegradation times for selected fossil-derived and bio-based polymers under industrial conditions and in ocean water are reported in Table 1.
Numerous certifications and labels are used to identify biodegradable materials (Box 2), typically related to industrial standards such as EN 13432 or ASTM D6400. However, revision and global harmonization of these guidelines are required, as the conditions mentioned in these standards may not necessarily be met in local disposal settings and, thus, may confuse consumers and converters39,179,207.
Box 2 Labelling bioplastics.
Plastic products are often labelled to indicate their chemical composition, whether they can be recycled, are bio-based and/or can be biodegraded and under which conditions. Consumers and converters are currently faced with various labels for bioplastics based on different industrial testing standards, some of which are referenced by major legislators, including the United Nations, the European Union (EU) or the US government. Some of these standards, particularly those certifying biodegradation, which were established around 2000, are currently under investigation, with the aim of revision and harmonization. It is important to understand the basis for these certifications and also who the agencies behind them are.
Identification labels
The most commonly observed labels on plastic products are the plastic resin identification codes (examples from ASTM D7611/D7611M-20 in panel a of the figure), which identify the polymer but provide no information on the recyclability. The older version of these labels — the ‘chasing arrows’ — still appears on products, and many consumers still falsely believe that products with these labels are recyclable, which may cause ‘wishcycling’ and lead to consumers placing non-recyclable items in recycling bins262. In the USA, only products labelled ‘1’ (polyethylene terephthalate (PETE)) or ‘2’ (high-density polyethylene) have a viable market and are, therefore, recycled262,263. Environmental organizations such as Greenpeace as well as some US states, such as California and New York, favour laws to prevent companies from using recycling symbols for non-recyclable products, and instead aim to use extended producer responsibility (EPR) laws to foster the design of recyclable materials262,264. Bioplastics such as polylactic acid are currently labelled as ‘7’ (other) and are typically not recycled.
Recycling-oriented labels
The ‘green dot’ symbol (panel b of the figure) used in the EU indicates that the producer has paid an EPR fee that is intended to fund collection and recycling programmes, but not that the product can be recycled. The on-pack recycling label (‘OPRL’) used in the UK (panel c of the figure) recommends whether consumers should place individual plastic packaging components into trash or recycling bins, based on the nationwide probability that the component is successfully collected, sorted and reprocessed into a new product with a viable market. The German certification body DIN CERTCO has established new labels to certify the recyclability of a plastic product based on the polymer and existing infrastructure to recycle the latter (panel d of the figure). Similarly, new labels to certify the recycled content are being proposed. The US-based How2Recycle label aims to provide more information on the recyclability of individual packaging parts.
Bio-based content labels
The labels shown in panels e–g of the figure certify the bio-based carbon content in plastic products. The DIN biobased (panel e of the figure) and OK biobased (panel f of the figure) labels are granted by DIN CERTCO and the Austrian technical service company TÜV Austria, respectively. The US Department of Agriculture’s BioPreferred program issues a label based on third-party analysis (panel g of the figure) and, in Japan, labels are issued by the Japan BioPlastics Association (JBPA). All these labels follow standards such as EN 16640 (Europe), ISO 16620 (international) and ASTM D6866 (USA).
Industrial compostability labels
The ‘OK compost’ (panel h of the figure) and ‘seedling’ (panel i of the figure) labels used in the EU and the ‘BPI compostable’ (panel j of the figure) label used in the USA have become more prevalent in recent years, yet, consumers have to understand the need for industrial capacity to biodegrade. The ‘industrial’ sub-label is based on four tests specified in the standards EN 13432 and ASTM D6400: biodegradation (90% of material is converted into CO2 in inoculum derived from compost at 58 °C after 6 months), disintegration (90% of material is smaller than 2 mm after 3 months at 40–70 °C, depending on the standard), ecotoxicity (90% of regular plant growth in soil with plastic present) and the heavy metal content must not exceed a certain threshold265.
‘Custom’ compostability/biodegradability labels
The ‘home’ compost label (panel k of the figure) has seen increased use but is not based on a legislative standard. This label was proposed by TÜV Austria as a modification of EN 13432, with tests performed at 20–30 °C over time frames that are twice as long as those in the original tests. Similarly, TÜV Austria has developed further labels and certification procedures for different environments in which plastics may end up (panels l–n of the figure). New bioplastic testing standards are under review, such as prEN 17427 (2020) by the European Committee for Standardization (CEN), which focuses on tests aimed to inform home compostability specifically for plastic bags.
Panel a reprinted, with permission, from ASTM D7611/D7611M-20 Standard Practice for Coding Plastic Manufactured Articles for Resin Identification, copyright ASTM International, 100 Barr Harbour Drive, West Conshohocken, PA 19428, USA. A copy of the complete standard may be obtained from ASTM International, www.astm.org. Panel b copyright Der Grüne Punkt – Duales System Deutschland GmbH. Panel c copyright OPRL Ltd. Panels d and e reprinted with permission from DIN CERTCO, www.dincertco.de. Panels f, h and k–n copyright TÜV AUSTRIA Group. Panel g copyright Department of Agriculture’s BioPreferred program based on third-party analysis. Panel i copyright European Bioplastics e.V. Panel j courtesy of the Biodegradable Products Institute.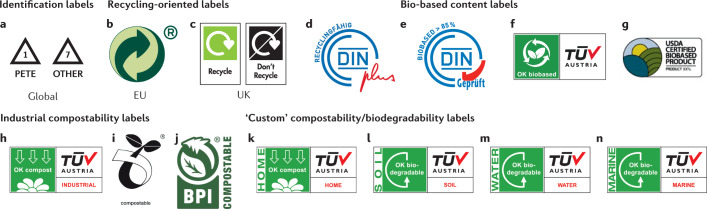
Biological recycling
Instead of complete biodegradation (composting), microorganisms and their hydrolysing enzymes can be used to depolymerize condensation polymers into monomers, instead of CO2, similar to chemical recycling208. Such biological processes are still underexplored but hold promise as they could be cleaner than the chemical approach209. Aliphatic esters can be readily hydrolysed, but aromatic polyesters are typically resistant to enzymatic hydrolysis. However, Ideonella sakaiensis 201-F6, a bacterium that was discovered in a Japanese recycling site, can depolymerize PET at ambient temperatures within 40 days201. Interestingly, its PETase enzyme is specific to aromatic polyester degradation and ineffective for aliphatic polyesters202. Leaf compost cutinase can be genetically modified to increase substrate specificity and thermal stability. The optimized enzyme can depolymerize 90% of micronized, amorphous PET into its monomers over 10 h at temperatures close to the glass transition of PET (~75 °C)210. Near this temperature, the amorphous chain mobility increases, which increases the susceptibility to microbial degradation. The derived terephthalic acid monomer can be reused to synthesize bottle-grade PET210,211. This technology has also been used to depolymerize PEF212,213.
Compared with polyesters, polyurethanes are much less biodegradable, owing to the strength of the urethane bonds. However, fungi and various soil bacteria can help hydrolyse the ester groups within polyester-containing polyurethane214,215. Better understanding of enzymatic activity and gene editing to increase the specificity of microorganisms could potentially enhance the biorecycling of polyurethanes.
Biodegradation of polyolefin materials is even more challenging, as they lack cleavable functional groups along their backbones, are highly hydrophobic, have a high molecular weight and contain stabilizing additives216,217. Small fragments, <5,000 Da, are believed to be metabolized by some organisms; however, the molecular weight of most polyolefin plastics is millions of daltons. Partial biodegradation (5–20%) of PE films by waxworm bacteria as well as Pseudomonas strains has been observed, occurring over 1–2 months218–221.
Non-degradable polymers, such as PEF, can be made more degradable by copolymerization with more hydrolysable, more hydrophilic and less crystalline copolymers222,223. However, copolymerization can negatively affect the properties of the material. Polyolefins can also be blended with biodegradable polymers, such as starch, protein or natural fibre, to increase the material’s susceptibility to biodegradation224. However, it remains unclear whether such compounds decompose into sufficiently small particles or whether they are merely fragmented to form microplastic.
Incineration
In the USA, ~20% of EOL plastic waste is incinerated (2014)3; in Europe, it is ~40% (2017)182. If only C/H/O-containing renewable material is combusted, CO2 emissions are net-zero and some of the resulting thermal energy can be recovered for energy production. However, combustion of N-containing, S-containing and Cl-containing polymers produces toxic NOx, SOx and HCl. Similarly, additives in polymers may release various toxic substances upon burning that require potentially costly capture and treatment interventions180,225. Furthermore, there are concerns of a ‘locking-in’ effect, whereby the high investment cost for incineration plants and the need for constant waste influx may jeopardize the adoption of recycling technologies2.
Landfill
In many countries, landfills are still the dominant waste disposal option: in the USA, 58% of waste ends up in landfills (2014)3, and in Europe, it is 27.3% (2017)182. Mismanaged and leaky landfills are considered a major source of environmental pollution. Biodegradable polymers should also be kept out of landfills as they can compost anaerobically to CH4, which has a GHG impact that is >20 times higher than that of CO2 (refs98,207). In the USA, the decomposition of organic material (such as paper and food scraps) in the ~1,500–2,000 operational landfills is the third largest CH4 emitter behind enteric fermentation (in farm animals) and natural gas systems226. Only 10% of CH4 produced in landfills was estimated to be captured globally in 2006, which is an approach that offers potential for energy recovery while benefitting the climate and public health227,228. The UN has mentioned that landfilling fees could make recycling more cost-competitive16.
Anaerobic digestion
Controlled anaerobic digestion (which occurs in the absence of O2) in a methanization ‘biogas’ facility produces CH4 from biodegradable polymer waste. The CH4 can then be captured and burned, which produces CO2 and H2O, and the heat and energy can be recovered for use. This process yields a net-zero carbon balance for the bioplastic waste while also producing energy229,230. The efficiency of anaerobic digestion can be increased by including elements such as a ‘bioreactor landfill’, in which H2O is circulated to enhance microbial activities for CH4 production227. Anaerobic digestion is feasible for several types of polymers, including thermoplastic starch, polycaprolactones and PHAs, as well as for PLA at elevated temperatures167.
Industrial applications and bioplastic market
Worldwide, annual production of 100% bio-based polymers is currently ~2 million tonnes2,8, with biodegradable plastic accounting for two-thirds of that amount207. When partially bio-based PUs and polyamide copolymers are included, worldwide production was ~7.5 million tonnes in 2018 and is expected to reach 9.1 million tonnes in 2023, with market capitalizations of $1.1 billion and $1.7 billion, respectively4. In comparison, total fossil-based plastic production is currently >380 million tonnes per year6. Owing to similar expected fossil-based growth, the global market share of bioplastics is expected to remain low at 2%, with a compound annual growth rate of 4%. The growth rate in Europe is 10%, mainly driven by upcoming market regulations and increased consumer demand for sustainable products. Global growth could reach 10–20% if bioplastics were subsidized and politically supported similarly to biofuels4,231. The compound annual growth rate in global bioplastic packaging is 18%232. The largest growth in demand and capacity can be expected for drop-in polymers that can be processed on standard equipment (such as bioPE) and cost-competitive ones with existing large-scale facilities (such as PLA blends and cellulose). The companies producing these materials have announced scale-up plans on the order of 60 kt and 75 kt in the coming years, respectively233,234. However, PLA currently lacks adequate recycling options. Increased demand and recycling-oriented regulations might create incentives for improved PLA recycling to come online, such as chemical recycling and improved biodegradation facilities, but also pave the way for new bioplastics, such as PHAs, that are more hydrolysable and, therefore, more compatible with existing composters235 (see, for example, information on Danimer Scientific’s PHA). Key industrial stakeholders for the most prominent bioplastics on the market are reported in Supplementary Table 2.
Food packaging and fast-moving consumer goods are the largest markets for short-lived to medium-lived plastics and, therefore, also for bioplastics. Companies are faced with balancing established material properties known to the company and consumers with various sustainability-related factors along the supply chain of a new, potentially more sustainable material (Fig. 3). The WEF highlights that investor interest in environmental, social and governance assets is growing, with 86% believing that these will be better long-term investments. $30 trillion are now spent on sustainable assets globally (one-third of total investments), with plastic and climate change topping the list of sustainable investor interests236. Several major organizations and companies have made commitments to develop and produce more sustainable plastics, which are set to increase future bioplastic demand. The Alliance to End Plastic Waste, which comprises major chemical companies, brand owners and smaller sustainability-focused entities, has promised to spend a total of $1.5 billion for projects related to sustainable plastic. Nestlé has committed up to $2 billion to develop food-grade recycled plastics and sustainable plastic technologies, including 100% bioPET. Carmaker Peugeot Citroën SA has pledged to make 20% of its plastics renewables-based. Toyota has committed to buying 25% of the bioPE output from Braskem’s Brazilian plant, which comes at a 30–50% price markup compared with fossil-based PE, thus showing Toyota’s willingness to pay premiums for sustainable polymers8.
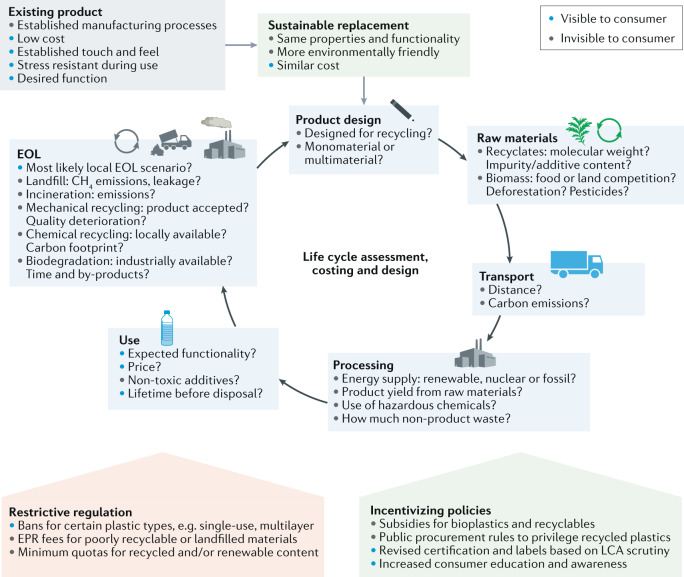
Fig. 3. Implementation framework for companies switching to sustainable materials.
Existing products in company portfolios have a set of properties that enable their business case. When switching to a more sustainable plastic replacement in the same application, companies are faced with a balancing act between conserving the same functionality, ideally at similar cost, and the requirements of a life cycle assessment to prove that the alternative is more environmentally friendly than the incumbent material6,19. Some aspects are visible to the consumer, but most aspects that affect the sustainability of a product remain invisible12. Yet, all aspects must be addressed to comply with upcoming regulations, extended producer responsibility (EPR) schemes and certification rules16,187. EOL, end of life.
Investment and scaling of bioplastic technologies, however, remains a high-risk business, with the central problem of uncertain demand owing to high prices and undefined EOL treatment, although larger scales could reduce prices and create demand and incentives for recycling infrastructure. In 2010, Metabolix and Archer Daniels Midland opened a plant for the production of 55 kt of PHA per year in Iowa (USA)237. But, 2 years later, forecasted sales projections were not fulfilled and profits could not cover operational costs. Archer Daniels Midland wrote off its $339 million investment and Metabolix sold the technology to CheilJedang (South Korea) and rebranded into Yield10 Bioscience to shift its focus to crop research. Today, the commercial production of PHA has seen a revival, with companies such as Danimer Scientific (USA) and RWDC Industries (USA and Singapore) scaling up in light of confirmed contracts in the packaging and fast-moving consumer goods sectors106. Tepha (USA) is focusing on medical PHA applications, for which the profit margins are much higher, which may provide a stepping stone towards bulk plastic production110. NatureWorks (USA) scaled rapidly and, in 2002, opened a plant to produce 70 kt of PLA per year; necessary optimization of a process step at a large scale manifested in billion-dollar losses over several years before breaking even68,238.
The threat of rising oil prices owing to a supply shortage, once advertised as the main driver for renewable-resource-based materials, has not materialized. Technological advances in horizontal deep drilling and fracking continue to enable the harvesting of increasingly remote oil reservoirs, and oil prices are expected to remain competitive for decades to come63. The prices of bioplastics and fossil-based plastics8,66,118 are compared in Table 1, showing that current bioplastic premiums can be ~50% (bioPE) but also 3–4 times more expensive (PHAs) than established fossil plastics. Besides higher production costs, there is an increase in demand over supply for popular bioplastics such as PLA and PHAs106. Note that most prices are taken from the literature before the COVID-19 crisis, which temporarily reduced oil and petrochemical prices, although these have now returned to pre-pandemic values239.
There is now a bioplastic replacement for almost every application of fossil-derived polymers; however, most replacements are more expensive and currently end up landfilled or incinerated. There are several examples of bioplastics that have penetrated the fossil-based plastic market (Fig. 2). For single-use disposable items, bioplastics are growing in popularity. In food packaging, their typically insufficient barrier properties are often enhanced with a slim halogenated polymer or metal layer. The European Commission has ranked the usefulness of biodegradable plastic in applications from beneficial (for example, bags for biowaste and teabags) to detrimental (such as single-use cups and bottles)207. For applications in which durability is crucial, the use of bio-based polymers is underexplored.
Policy and regulations
Governments and international bodies are increasingly prioritizing circular economy principles. The UN named plastic pollution a priority during its 73rd Session (2018–2019). In 2019, 187 UN member nations amended the directives of the 1989 Basel Convention on global hazardous materials shipping and trade to include plastic waste, adding new transparency and regulatory requirements27. The UN Industrial Development Organization and G20 nations, as well as the Plastic Waste Partnership, are collaborating on circular economy measures. Activities span the plastic life cycle, from selective plastic bans and easily understandable labelling to helping consumers participate in waste management and financial incentives for renewable resources and chemical recycling16,39. Regarding bioplastics, the Convention’s Open-ended Working Group recommends that nations clearly define and standardize the identification of bio(degradable) plastics, improve bioplastic production processes to become economically and ecologically competitive with fossil-based plastics and develop universal techno-economic analysis methodologies to quantify the environmental benefit of bioplastics179.
The WEF, together with the Ellen MacArthur Foundation and McKinsey & Company, is promoting science-based policy initiatives for a circular plastics economy2. Recommendations include adopting EPR schemes and clearer labelling standards for bioplastic materials. A recent report offers strategies to curb plastic leakage into oceans by 80% by 2040 (ref.48). These proposals include reducing waste exports into countries with high leakage rates by 90%, doubling global mechanical recycling capacity, improving design-for-recycling to expand global recyclable plastic from 21% to 54% and implementing known solutions to eliminate major microplastic sources48.
The EU has announced several plastics policies under the framework of the European Green Deal and its Circular Economy Action Plan. One goal is a recycling target of 50% for plastic packaging by 2030. As of January 2021, several single-use plastic items (such as straws, cutlery, food and beverage containers made from polystyrene and cotton bud sticks) and all oxo-degradable plastics have been banned for sale in the EU240. Following the Basel agreements, low-grade plastic waste exports outside EU borders will be restricted as of 2021 (ref.241). A tax on non-recycled plastic of €800 per tonne was implemented in 2021 to motivate manufacturing industries to adopt alternative recyclable, reusable or compostable materials242. EPR schemes implemented in many EU countries have proved useful in shifting EOL costs from local governments to producers but are generally limited to packaging materials. Their scope needs to be expanded to other plastic-intensive industries (including agriculture, textiles, medical and construction) and their implementation requires improvement, especially by harmonizing definitions of what is considered ‘recyclable’ from a local and chemical perspective187. Regarding bioplastics, the EU is developing a regulatory framework to determine which applications are appropriate for them, with the goal of preventing false sustainability claims (that is, greenwashing) by companies. As part of the regulatory framework, the EU plans to revise and harmonize existing standards (such as EN 13432) to account for more realistic biodegradation testing207,243.
China, the world’s largest producer of single-use plastics, recently announced that it would ban non-recyclables other than degradable bioplastics by 2025 (ref.244). As a result, Chinese manufacturers plan to dramatically increase PLA production to 700,000 tonnes per year and combined PBAT and PBS output to 1.24 million tonnes per year by 2023 (ref.245). These capacities may affect global market prices for these polymers, and plans for controlled disposal of these increased volumes remain unclear. Several other countries, including Japan, Malaysia, Singapore and South Korea, have created financial subsidies for bioplastics246.
In the USA, the current administration has committed to more environment-focused and climate-focused policies. Their goals include catalysing private sector investment into domestic clean energy technologies and materials and addressing ocean plastics247,248. The Break Free from Plastic Pollution Act was introduced in the House of Representatives in February 2020. This bill aims to limit single-use items and non-recyclables in markets after 2022 by establishing a tax on carry-out bags and making plastic producers fiscally responsible for collecting and recycling their products following EPR principles. The bill also prevents the export of plastic waste to non-OECD (Organisation for Economic Co-operation and Development) countries249. These efforts, however, are at odds with policies that have promoted fossil-derived plastic production. Financial incentives and lack of regulations have enabled fracking and shale gas in the USA, making plastic based on these fossil feedstocks cheap and competitive with renewable materials250,251. The International Monetary Fund estimates that 85% of global subsidies benefit fossil fuels, despite estimates that incentives for sustainable technologies could save 28% of carbon emissions, curb air-pollution-related deaths by 46% and increase governmental revenues by 3.8% of the gross domestic product252,253.
Conclusions and future perspectives
Use of renewable resources alone does not imply sustainability. Sustainability is highly dependent on how a material is made, where it is used and how it can be recycled, and less so on the building blocks of a material. Nevertheless, with technological advances, bioplastics have the potential to move several plastic-intensive industries towards a circular economy. Bio-based replacements are available for almost every fossil-based application; however, these are mostly in small and costly quantities, and do not always have substantial environmental benefits. Besides first-generation biomass, lignocellulosic agricultural and other biowastes present a renewable, abundant and more ethically viable feedstock. However, biorefinery processes have to increase in efficiency and adhere to green chemistry principles (such as using non-toxic chemicals and reducing the energy demand) to supply polymer building blocks in a cost-competitive and sustainable manner. Gene editing is a promising tool to increase microorganism efficiencies in biomass utilization, bioplastic polymerization (especially for PHAs) and biological depolymerization for recycling. Although many modern bioplastics are degradable polymers, bio-based and thermolysis-oil-based versions of durable polyolefins (for example, bioPE and chemically recycled PE) and polyesters (such as PEF and bioPET) can also be sustainable and recyclable by exploiting established and highly efficient, solvent-free and water-free catalytic processes.
The ability to evaluate, scrutinize and compare sustainability and the environmental impact of fossil-based and bio-based materials remains essential, yet non-trivial. LCA is the main tool but requires homogenization of methodology standards to make LCAs more transparent, consistent and comparable. Existing bioplastic labels need to be revised for application on both global and local scales — they must convey widely recognized appropriate standards and still identify the EOL of plastics in the local market. Together, reliable LCAs and clearer product labelling will help avoid ‘greenwashing’, help to identify ‘sustainability bottlenecks’ along supply chains, improve public education on bioplastics and help guide investment in promising sustainable technologies.
The COVID-19 pandemic has highlighted that there is an urgent requirement for methods that can recycle increasing amounts of mixed waste streams of potentially poor quality, containing contaminants from the medical, food and other sectors. Innovation and financial incentivization in advanced recycling technologies, such as chemical and biological recycling, would further unlock (bio)plastic circularity. With improvements in efficiency and cost-competitiveness, these techniques can enable the upcycling of plastic waste. For any recycling technology, including existing mechanical recycling capacities, plastic products must be designed to be recyclable, such as through the use of monomaterials rather than non-recyclable multilayers. The superior properties of some bioplastics could aid this process. EPR schemes can incentivize design-for-recycling but require clearer definitions regarding local and chemical recyclability. Robust sorting technology that separates bioplastics from existing plastics is another key to circularity. Deposit-refund systems have proved effective in EU countries to increase recollection rates and afford pure recycling streams.
Biodegradation is no ‘silver bullet’ to curb plastic pollution and typically ranks as the least desired fate of bioplastics, especially in anaerobic landfill scenarios without gas capture. Industrial anaerobic digestion offers a potential route for CH4 and energy recovery. True and fast biodegradation without the release of toxic chemicals may prove useful in settings in which there are no other forms of recycling, but more research on the impact of microplastics as intermediates is required. Besides recycling, behavioural changes towards using less plastic, and the strict usage of renewable energy for polymer and plastic production, remain essential strategies to mitigate plastic waste and carbon emissions.
Environmental sustainability has yet to be met with financial sustainability. Low oil prices, narrow profit margins and existing fossil-fuel subsidies reduce the cost-competitiveness of bio-based manufacturers, which represent a fragmented market of small entities and subdivisions of petrochemical companies. Drop-in bioplastics processed on standard equipment (such as bioPE) and cost-competitive ones (such as PLA blends and cellulose) will likely see the lowest barriers to adoption in existing markets. Some firms rely on selling products with higher margins, as in the medical or nutrition areas, to yield the profits needed to scale-up bioplastic production. For some popular bioplastics based on PLA and PHA, however, demand currently exceeds the supply as an increasing number of stakeholders in the food industry seek to use these materials in their packaging, albeit often without clear recycling options in mind. Upcoming regulatory incentives, including taxation of non-bio-based materials, will further drive the demand for existing and new bioplastics (such as PEF). Circularity-oriented policies will encourage companies to scrutinize all steps along their product’s expected life cycle and help consumers choose more sustainable plastics.
Supplementary information
Acknowledgements
The authors thank R. Mülhaupt, E. Olivetti, G. Storti, G. W. Liu, S. Srinivasan, A. Kirtane and A. Lopes for discussions related to this body of work. The authors have been supported in part by the following funding sources: Bill & Melinda Gates Foundation (INV-002177, INV-009529), Karl Van Tassel (1925) Career Development Chair and the Department of Mechanical Engineering, MIT.
Author contributions
All authors contributed to the discussion of content, writing and editing of the manuscript prior to submission.
Competing interests
All authors are co-inventors on multiple patents or patent applications describing bio-based or biodegradable materials. Complete details for R.L. can be found at the following link: https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0. Complete details of all relationships for profit and not for profit for G.T. can be found at the following link. https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0.
Footnotes
Peer review information
Nature Reviews Materials thanks Michael Bank, Bernd Rehm and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BPI Certification Mark: https://bpiworld.org/Value-Of-Certification
Danimer Scientific’s PHA: https://danimerscientific.com/about-us/faqs/
European Bioplastics Seedling label: https://www.european-bioplastics.org/bioplastics/standards/labels/
How2Recycle: https://how2recycle.info/
Japan BioPlastics Association: http://www.jbpaweb.net
OPRL: https://www.oprl.org.uk/get-involved/what-is-the-scheme/
prEN 17427: https://standards.globalspec.com/std/14260972/pren-17427
The Green Dot Trademark: https://www.pro-e.org/the-green-dot-trademark
TÜV Austria bioplastic certificates: https://www.tuv-at.be/green-marks/
USDA BioPreferred program: http://www.biopreferred.gov
Contributor Information
Robert Langer, Email: rlanger@mit.edu.
Giovanni Traverso, Email: cgt20@mit.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41578-021-00407-8.
References
- 1.Elias, H.-G. & Mülhaupt, R. in Ullmann’s Encyclopedia of Industrial Chemistry 1–70 (Wiley, 2015).
- 2.World Economic Forum, Ellen MacArthur Foundation & McKinsey & Company. The new plastics economy: rethinking the future of plastics (2016). Report on the envisioned shift from linear to circular plastic economies, addressing strategies such as bioplastics, advanced recycling and extended producer responsibility.
- 3.Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3:25–29. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinthapalli R, et al. Biobased building blocks and polymers — global capacities, production and trends, 2018–2023. Ind. Biotechnol. 2019;15:237–241. doi: 10.1089/ind.2019.29179.rch. [DOI] [Google Scholar]
- 5.Karan H, Funk C, Grabert M, Oey M, Hankamer B. Green bioplastics as part of a circular bioeconomy. Trends Plant Sci. 2019;24:237–249. doi: 10.1016/j.tplants.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Walker S, Rothman R. Life cycle assessment of bio-based and fossil-based plastic: a review. J. Clean. Prod. 2020;261:121158. doi: 10.1016/j.jclepro.2020.121158. [DOI] [Google Scholar]
- 7.Pellis A, Malinconico M, Guarneri A, Gardossi L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021;60:146–158. doi: 10.1016/j.nbt.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J. Global Markets and Technologies for Bioplastics. BCC Research Report Code: PLS050E 1–231 (BCC Research, 2019).
- 9.Zhu Y, Romain C, Williams CK. Sustainable polymers from renewable resources. Nature. 2016;540:354–364. doi: 10.1038/nature21001. [DOI] [PubMed] [Google Scholar]
- 10.Gandini A, Lacerda TM, Carvalho AJF, Trovatti E. Progress of polymers from renewable resources: furans, vegetable oils, and polysaccharides. Chem. Rev. 2016;116:1637–1669. doi: 10.1021/acs.chemrev.5b00264. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, H. N. & Gross, R. A. in ACS Symposium Series Vol. 1372 Ch. 1 (ACS, 2020).
- 12.Miller SA. Five misperceptions surrounding the environmental impacts of single-use plastic. Environ. Sci. Technol. 2020;54:14143–14151. doi: 10.1021/acs.est.0c05295. [DOI] [PubMed] [Google Scholar]
- 13.Hottle TA, Bilec MM, Landis AE. Sustainability assessments of bio-based polymers. Polym. Degrad. Stab. 2013;98:1898–1907. doi: 10.1016/j.polymdegradstab.2013.06.016. [DOI] [Google Scholar]
- 14.Zimmermann L, Dombrowski A, Völker C, Wagner M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020;145:106066. doi: 10.1016/j.envint.2020.106066. [DOI] [PubMed] [Google Scholar]
- 15.Independent Group of Scientists appointed by the Secretary-General. Global Sustainable Development Report 2019: the future is now – science for achieving sustainable development. United Nationshttps://sustainabledevelopment.un.org/content/documents/24797GSDR_report_2019.pdf (2019).
- 16.United Nations Industrial Development Organization (UNIDO). Addressing the challenge of marine plastic litter using circular economy methods: relevant considerations. UNIDOhttps://www.unido.org/sites/default/files/files/2019-06/UNIDO_Addressing_the_challenge_of_Marine_Plastic_Litter_Using_Circular_Economy.pdf (2019). Comprehensive report on the plastic problem and potential global actions to combat it.
- 17.World Economic Forum (WEF). Top 10 Emerging Technologies 4–15 (WEF, 2019).
- 18.World Health Organization (WHO). Microplastics in drinking-water. WHOhttps://apps.who.int/iris/bitstream/handle/10665/326499/9789241516198-eng.pdf?ua=1 (2019).
- 19.European Commission. A European strategy for plastics in a circular economy. European Commissionwww.europarc.org/wp-content/uploads/2018/01/Eu-plastics-strategy-brochure.pdf (2018).
- 20.MacArthur DE. Beyond plastic waste. Science. 2017;358:843. doi: 10.1126/science.aao6749. [DOI] [PubMed] [Google Scholar]
- 21.Bucknall DG. Plastics as a materials system in a circular economy. Philos. Trans. R. Soc. A. 2020;378:20190268. doi: 10.1098/rsta.2019.0268. [DOI] [PubMed] [Google Scholar]
- 22.Jambeck J, et al. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt C, Krauth T, Wagner S. Export of plastic debris by rivers into the sea. Environ. Sci. Technol. 2017;51:12246–12253. doi: 10.1021/acs.est.7b02368. [DOI] [PubMed] [Google Scholar]
- 24.Lebreton LCM, et al. River plastic emissions to the world’s oceans. Nat. Commun. 2017;8:15611. doi: 10.1038/ncomms15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitt JR. Waste exports to the developing world: A global response. Geo. Int. Environ. Law Rev. 1995;7:485–514. [Google Scholar]
- 26.Brooks AL, Wang S, Jambeck JR. The Chinese import ban and its impact on global plastic waste trade. Sci. Adv. 2018;4:eaat0131. doi: 10.1126/sciadv.aat0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United Nations. Report of the Conference of the Parties to the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal on the Work of its Fourteenth Meeting. Fourteenth Meeting of the Conference of the Parties to the Basel Convention Meeting Documents 1–123 (United Nations, 2019).
- 28.Kosuth M, Mason SA, Wattenberg EV. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE. 2018;13:e0194970. doi: 10.1371/journal.pone.0194970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catarino AI, Macchia V, Sanderson WG, Thompson RC, Henry TB. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018;237:675–684. doi: 10.1016/j.envpol.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 30.Ziccardi LM, Edgington A, Hentz K, Kulacki KJ, Driscoll SK. Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: a state-of-the-science review. Environ. Toxicol. Chem. 2016;35:1667–1676. doi: 10.1002/etc.3461. [DOI] [PubMed] [Google Scholar]
- 31.Henry B, Laitala K, Klepp IG. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019;652:483–494. doi: 10.1016/j.scitotenv.2018.10.166. [DOI] [PubMed] [Google Scholar]
- 32.Boucher, J. & Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sourceshttps://portals.iucn.org/library/sites/library/files/documents/2017-002-En.pdf (International Union for Conservation of Nature and Natural Resources, 2017).
- 33.Jan Kole P, Löhr AJ, Van Belleghem FGAJ, Ragas AMJ. Wear and tear of tyres: a stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health. 2017;14:1265. doi: 10.3390/ijerph14101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Science Advice for Policy by European Academies (SAPEA). A Scientific perspective on microplastics in nature and society. Evidence review report no. 4. SAPEAhttps://www.sapea.info/topics/microplastics/ (2019).
- 35.Straub S, Hirsch PE, Burkhardt-Holm P. Biodegradable and petroleum-based microplastics do not differ in their ingestion and excretion but in their biological effects in a freshwater invertebrate Gammarus fossarum. Int. J. Environ. Res. Public Health. 2017;14:774. doi: 10.3390/ijerph14070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Von Moos N, Burkhardt-Holm P, Köhler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012;46:11327–11335. doi: 10.1021/es302332w. [DOI] [PubMed] [Google Scholar]
- 37.Crump A, et al. Microplastics disrupt hermit crab shell selection. Biol. Lett. 2020;16:20200030. doi: 10.1098/rsbl.2020.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane IA, et al. Seafloor microplastic hotspots controlled by deep-sea circulation. Science. 2020;368:1140–1145. doi: 10.1126/science.aba5899. [DOI] [PubMed] [Google Scholar]
- 39.ten Brink, P. et al. Circular Economy Measures to Keep Plastics and Their Value in the Economy, Avoid Waste and Reduce Marine Litter. Economics Discussion Papers No. 2018-3 (Kiel Institute for the World Economy, 2018).
- 40.Patrício Silva AL, et al. Increased plastic pollution due to COVID-19 pandemic: challenges and recommendations. Chem. Eng. J. 2021;405:1266831. doi: 10.1016/j.cej.2020.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanapalli KR, et al. Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Sci. Total Environ. 2021;750:141514. doi: 10.1016/j.scitotenv.2020.141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klemeš JJ, Fan Y, Van, Tan RR, Jiang P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sustain. Energy Rev. 2020;127:109883. doi: 10.1016/j.rser.2020.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.United Nations. COVID-19 recovery offers ‘chance to change course’, Guterres tells One Planet Summit. United Nationshttps://news.un.org/en/story/2021/01/1081772 (2021).
- 44.IPCC. Sixth assessment report. IPCChttps://www.ipcc.ch/report/ar6/wg1/ (2021).
- 45.Center for International Environmental Law (CIEL). Plastic & climate. The hidden costs of a plastic planet. CIELhttps://www.ciel.org/wp-content/uploads/2019/05/Plastic-and-Climate-FINAL-2019.pdf (2018).
- 46.Mülhaupt R. Green polymer chemistry and bio-based plastics: dreams and reality. Macromol. Chem. Phys. 2013;214:159–174. doi: 10.1002/macp.201200439. [DOI] [Google Scholar]
- 47.Zheng J, Suh S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Change. 2019;9:374–378. doi: 10.1038/s41558-019-0459-z. [DOI] [Google Scholar]
- 48.The Pew Charitable Trusts and SYSTEMIQ. Breaking the plastic wave - a comprehensive assessment of pathways towards stopping ocean plastic pollution. The Pew Charitable Trusts and SYSTEMIQhttps://www.pewtrusts.org/-/media/assets/2020/07/breakingtheplasticwave_report.pdf (2020).
- 49.United Nations Framework Convention on Climate Change (UNFCCC). Report of the Conference of the Parties on its Twenty-First Session, Held in Paris from 30 November to 13 December 2015 (UNFCCC, 2016).
- 50.Shen M, et al. (Micro)plastic crisis: un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020;254:120138. doi: 10.1016/j.jclepro.2020.120138. [DOI] [Google Scholar]
- 51.Wieczorek AM, Croot PL, Lombard F, Sheahan JN, Doyle TK. Microplastic ingestion by gelatinous zooplankton may lower efficiency of the biological pump. Environ. Sci. Technol. 2019;53:5387–5395. doi: 10.1021/acs.est.8b07174. [DOI] [PubMed] [Google Scholar]
- 52.Gruber N, et al. The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science. 2019;363:1193–1199. doi: 10.1126/science.aau5153. [DOI] [PubMed] [Google Scholar]
- 53.Behrenfeld MJ, et al. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444:752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 54.Rahimi A, García J. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017;1:0046. doi: 10.1038/s41570-017-0046. [DOI] [Google Scholar]
- 55.Coates GW, Getzler YDYL. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020;5:501–516. doi: 10.1038/s41578-020-0190-4. [DOI] [Google Scholar]
- 56.Pudack C, Stepanski M, Fässler P. PET recycling – Contributions of crystallization to sustainability. Chem. Ing. Tech. 2020;92:452–458. doi: 10.1002/cite.201900085. [DOI] [Google Scholar]
- 57.Hundertmark, T., McNally, C., Simons, T. J. & Vanthournout, H. No Time to Waste: What Plastics Recycling Could Offer. McKinsey on Chemicals (McKinsey & Company, 2018).
- 58.Anuar Sharuddin SD, Abnisa F, Wan Daud WMA, Aroua MK. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016;115:308–326. doi: 10.1016/j.enconman.2016.02.037. [DOI] [Google Scholar]
- 59.Organisation for Economic Co-operation and Development (OECD). Improving Plastics Management: Trends, Policy Responses, and the Role of International Co-operation and Trade. OECD Environmental Policy Paper No. 12 (OECD, 2018).
- 60.Sheldon RA. Chemicals from renewable biomass: a renaissance in carbohydrate chemistry. Curr. Opin. Green Sustain. Chem. 2018;14:89–95. doi: 10.1016/j.cogsc.2018.08.003. [DOI] [Google Scholar]
- 61.Lau WWY, et al. Evaluating scenarios toward zero plastic pollution. Science. 2020;369:1455–1461. doi: 10.1126/science.aba9475. [DOI] [PubMed] [Google Scholar]
- 62.Fadeeva Z, Berkel RVan. Unlocking circular economy for prevention of marine plastic pollution: an exploration of G20 policy and initiatives. J. Environ. Manag. 2021;277:111457. doi: 10.1016/j.jenvman.2020.111457. [DOI] [PubMed] [Google Scholar]
- 63.Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature. 2012;488:294–303. doi: 10.1038/nature11475. [DOI] [PubMed] [Google Scholar]
- 64.Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates — the US Department of Energy’s “Top 10” revisited. Green Chem. 2010;12:539–554. doi: 10.1039/b922014c. [DOI] [Google Scholar]
- 65.Erickson B, Nelson, Winters P. Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnol. J. 2012;7:176–185. doi: 10.1002/biot.201100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ioannidou SM, et al. Sustainable production of bio-based chemicals and polymers via integrated biomass refining and bioprocessing in a circular bioeconomy context. Bioresour. Technol. 2020;307:123093. doi: 10.1016/j.biortech.2020.123093. [DOI] [PubMed] [Google Scholar]
- 67.Kline KL, et al. Reconciling food security and bioenergy: priorities for action. Glob. Change Biol. Bioenergy. 2017;9:557–576. doi: 10.1111/gcbb.12366. [DOI] [Google Scholar]
- 68.van den Oever, M., Molenveld, K., van der Zee, M. & Bos, H. Bio-based and biodegradable plastics – facts and figures. Focus on food packaging in the Netherlands. Report 1722. Wageningen Food & Biobased Researchhttps://edepot.wur.nl/408350 (2017).
- 69.Brizga J, Hubacek K, Feng K. The unintended side effects of bioplastics: carbon, land, and water footprints. One Earth. 2020;3:45–53. doi: 10.1016/j.oneear.2020.06.016. [DOI] [Google Scholar]
- 70.Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB. Biomass pretreatment: fundamentals toward application. Biotechnol. Adv. 2011;29:675–685. doi: 10.1016/j.biotechadv.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Lin CSK, et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013;6:426–464. doi: 10.1039/c2ee23440h. [DOI] [Google Scholar]
- 72.Pleissner D, et al. Valorization of organic residues for the production of added value chemicals: a contribution to the bio-based economy. Biochem. Eng. J. 2016;116:3–16. doi: 10.1016/j.bej.2015.12.016. [DOI] [Google Scholar]
- 73.Food and Agriculture Organization of the United Nations (FAO). Food wastage footprint - impacts on natural resources. FAOhttp://www.fao.org/3/i3347e/i3347e.pdf (2013).
- 74.Questell-Santiago YM, Galkin MV, Barta K, Luterbacher JS. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 2020;4:311–330. doi: 10.1038/s41570-020-0187-y. [DOI] [PubMed] [Google Scholar]
- 75.Taherzadeh MJ, Karimi K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 2008;9:1621–1651. doi: 10.3390/ijms9091621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Usmani Z, et al. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020;304:123003. doi: 10.1016/j.biortech.2020.123003. [DOI] [PubMed] [Google Scholar]
- 77.Zhu H, Ma Q, Sheng J, Yang R. Freeze–thaw repetition as an auxiliary method to promote efficient separation of hemicellulose from poplar. Green Chem. 2020;22:942–949. doi: 10.1039/C9GC03792F. [DOI] [Google Scholar]
- 78.Abdul Khalil HPS, et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: a review. Renew. Sustain. Energy Rev. 2017;77:353–362. doi: 10.1016/j.rser.2017.04.025. [DOI] [Google Scholar]
- 79.Kaplan, D. L. (ed.) Biopolymers from Renewable Resources Vol. 53 (Springer, 1998).
- 80.Vijay V, Pimm SL, Jenkins CN, Smith SJ. The impacts of oil palm on recent deforestation and biodiversity loss. PLoS ONE. 2016;11:e01596681. doi: 10.1371/journal.pone.0159668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carlson KM, et al. Effect of oil palm sustainability certification on deforestation and fire in Indonesia. Proc. Natl Acad. Sci. USA. 2018;115:121–126. doi: 10.1073/pnas.1704728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meier MAR, Metzger JO, Schubert US. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007;36:1788–1802. doi: 10.1039/b703294c. [DOI] [PubMed] [Google Scholar]
- 83.Hauenstein O, Reiter M, Agarwal S, Rieger B, Greiner A. Bio-based polycarbonate from limonene oxide and CO2 with high molecular weight, excellent thermal resistance, hardness and transparency. Green Chem. 2016;18:760–770. doi: 10.1039/C5GC01694K. [DOI] [Google Scholar]
- 84.Abts, G., Eckel, T. & Wehrmann, R. in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2014).
- 85.Grignard B, Gennen S, Jérôme C, Kleij AW, Detrembleur C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019;48:4466–4514. doi: 10.1039/C9CS00047J. [DOI] [PubMed] [Google Scholar]
- 86.Chapman AM, Keyworth C, Kember MR, Lennox AJJ, Williams CK. Adding value to power station captured CO2: tolerant Zn and Mg homogeneous catalysts for polycarbonate polyol production. ACS Catal. 2015;5:1581–1588. doi: 10.1021/cs501798s. [DOI] [Google Scholar]
- 87.Jiang L, et al. PEF plastic synthesized from industrial carbon dioxide and biowaste. Nat. Sustain. 2020;3:761–767. doi: 10.1038/s41893-020-0549-y. [DOI] [Google Scholar]
- 88.Venkata Mohan S, Modestra JA, Amulya K, Butti SK, Velvizhi G. A circular bioeconomy with biobased products from CO2 sequestration. Trends Biotechnol. 2016;34:506–519. doi: 10.1016/j.tibtech.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 89.Kember MR, Williams CK. Efficient magnesium catalysts for the copolymerization of epoxides and CO2; using water to synthesize polycarbonate polyols. J. Am. Chem. Soc. 2012;134:15676–15679. doi: 10.1021/ja307096m. [DOI] [PubMed] [Google Scholar]
- 90.Dechent S-E, Kleij AW, Luinstra GA. Fully bio-derived CO2 polymers for non-isocyanate based polyurethane synthesis. Green Chem. 2020;22:969–978. doi: 10.1039/C9GC03488A. [DOI] [Google Scholar]
- 91.Hillmyer MA. The promise of plastics from plants. Science. 2017;358:868–870. doi: 10.1126/science.aao6711. [DOI] [PubMed] [Google Scholar]
- 92.Sheldon RA. Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem. 2014;16:950–963. doi: 10.1039/C3GC41935E. [DOI] [Google Scholar]
- 93.Harmsen PFH, Hackmann MM, Bos HL. Green building blocks for bio-based plastics. Biofuel. Bioprod. Biorefin. 2014;8:306–324. doi: 10.1002/bbb.1468. [DOI] [Google Scholar]
- 94.Sajid M, Zhao X, Liu D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): recent progress focusing on the chemical-catalytic routes. Green Chem. 2018;20:5427–5453. doi: 10.1039/C8GC02680G. [DOI] [Google Scholar]
- 95.Kucherov FA, Romashov LV, Galkin KI, Ananikov VP. Chemical transformations of biomass-derived C6-furanic platform chemicals for sustainable energy research, materials science, and synthetic building blocks. ACS Sustain. Chem. Eng. 2018;6:8064–8092. doi: 10.1021/acssuschemeng.8b00971. [DOI] [Google Scholar]
- 96.Van Putten RJ, et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013;113:1499–1597. doi: 10.1021/cr300182k. [DOI] [PubMed] [Google Scholar]
- 97.Chafran LS, et al. Preparation of PLA blends by polycondensation of d,l-lactic acid using supported 12-tungstophosphoric acid as a heterogeneous catalyst. Heliyon. 2019;5:e01810. doi: 10.1016/j.heliyon.2019.e01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hottle TA, Bilec MM, Landis AE. Biopolymer production and end of life comparisons using life cycle assessment. Resour. Conserv. Recycl. 2017;122:295–306. doi: 10.1016/j.resconrec.2017.03.002. [DOI] [Google Scholar]
- 99.Morão A, Bie FD. Life cycle impact assessment of polylactic acid (PLA) produced from sugarcane in Thailand. J. Polym. Environ. 2019;27:2523–2539. doi: 10.1007/s10924-019-01525-9. [DOI] [Google Scholar]
- 100.Yu Y, Storti G, Morbidelli M. Ring-opening polymerization of l,l-lactide: kinetic and modeling study. Macromolecules. 2009;42:8187–8197. doi: 10.1021/ma901359x. [DOI] [Google Scholar]
- 101.Penczek S, Szymanski R, Duda A, Baran J. Living polymerization of cyclic esters–a route to (bio)degradable polymers. Influence of chain transfer to polymer on livingness. Macromol. Symp. 2003;201:261–269. doi: 10.1002/masy.200351129. [DOI] [Google Scholar]
- 102.Dusselier M, Wouwe PV, Dewaele A. Shape-selective zeolite catalysis for bioplastics production. Green Chem. 2015;349:78–80. doi: 10.1126/science.aaa7169. [DOI] [PubMed] [Google Scholar]
- 103.Total Corbion PLA. Closing the loop for paper-coated packaging and serviceware: now compostable and recyclable with Luminy® from Total Corbion PLA. Total Corbion PLAhttps://www.total-corbion.com/news/closing-the-loop-for-paper-coated-packaging-and-serviceware-now-compostable-and-recyclable-with-luminy-from-total-corbion-pla/ (2021).
- 104.Kannan, G., Grieshaber, S. E. & Zhao, W. in Handbook of Thermoplastics 2nd edn (eds Olabisi, O. & Adewale, K.) 319–346 (CRC Press, 2015).
- 105.Xu J, Guo B. Poly(butylene succinate) and its copolymers: research, development and industrialization. Biotechnol. J. 2010;5:1149–1163. doi: 10.1002/biot.201000136. [DOI] [PubMed] [Google Scholar]
- 106.Tullo, A. H. Will the Biodegradable Plastic PHA Finally Deliver?https://cen.acs.org/business/biobased-chemicals/biodegradable-plastic-PHA-finally-deliver/99/i22 (Chemical & Engineering News, 2021).
- 107.Singh AK, Sharma L, Mallick N, Mala J. Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J. Appl. Phycol. 2017;29:1213–1232. doi: 10.1007/s10811-016-1006-1. [DOI] [Google Scholar]
- 108.Koller M, et al. Characteristics and potential of micro algal cultivation strategies: a review. J. Clean. Prod. 2012;37:377–388. doi: 10.1016/j.jclepro.2012.07.044. [DOI] [Google Scholar]
- 109.Mendhulkar VD, Shetye LA. Synthesis of biodegradable polymer polyhydroxyalkanoate (PHA) in cyanobacteria Synechococcus elongates under mixotrophic nitrogen- and phosphate-mediated stress conditions. Ind. Biotechnol. 2017;13:85–93. doi: 10.1089/ind.2016.0021. [DOI] [Google Scholar]
- 110.Koller M. Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules. 2018;23:362. doi: 10.3390/molecules23020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Künkel, A. et al. in Ullmann’s Encyclopedia of Industrial Chemistry 1–29 (Wiley, 2016).
- 112.Nikodinovic-Runic J, et al. Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Adv. Appl. Microbiol. 2013;84:139–200. doi: 10.1016/B978-0-12-407673-0.00004-7. [DOI] [PubMed] [Google Scholar]
- 113.Medeiros Garcia Alcântara J, et al. Current trends in the production of biodegradable bioplastics: the case of polyhydroxyalkanoates. Biotechnol. Adv. 2020;42:1075821. doi: 10.1016/j.biotechadv.2020.107582. [DOI] [PubMed] [Google Scholar]
- 114.Ward PG, Goff M, Donner M, Kaminsky W, O’Connor KE. A two step chemo-biotechnological conversion of polystyrene to a biodegradable thermoplastic. Environ. Sci. Technol. 2006;40:2433–2437. doi: 10.1021/es0517668. [DOI] [PubMed] [Google Scholar]
- 115.Plackett, D. & Siró, I. in Multifunctional and Nanoreinforced Polymers for Food Packaging 498–526 (Elsevier, 2011).
- 116.Utsunomia C, Ren Q, Zinn M. Poly(4-hydroxybutyrate): current state and perspectives. Front. Bioeng. Biotechnol. 2020;8:257. doi: 10.3389/fbioe.2020.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen, G.-Q. in Plastics from Bacteria: Natural Functions and Applications 121–132 (Springer, 2010).
- 118.Hann S, Scholes R, Lee T, Ettlinger S, Jørgensen H. Biobased and biodegradable plastics in Denmark. Ind. Biotechnol. 2020;16:164–175. doi: 10.1089/ind.2020.29213.sha. [DOI] [Google Scholar]
- 119.Varghese SA, Pulikkalparambil H, Rangappa SM, Siengchin S, Parameswaranpillai J. Novel biodegradable polymer films based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Ceiba pentandra natural fibers for packaging applications. Food Packag. Shelf Life. 2020;25:100538. doi: 10.1016/j.fpsl.2020.100538. [DOI] [Google Scholar]
- 120.Lingle, R. Bioplastics boost packaging film and paper barrier and performance. Packaging Digesthttps://www.packagingdigest.com/sustainability/bioplastics-boost-packaging-film-and-paper-barrier-and-performance (2015).
- 121.Chen GQ, Jiang XR. Engineering microorganisms for improving polyhydroxyalkanoate biosynthesis. Curr. Opin. Biotechnol. 2018;53:20–25. doi: 10.1016/j.copbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 122.Li D, Lv L, Chen J, Chen G. Controlling microbial PHB synthesis via CRISPRi. Appl. Microbiol. Biotechnol. 2017;101:5861–5867. doi: 10.1007/s00253-017-8374-6. [DOI] [PubMed] [Google Scholar]
- 123.Sabirova JS, et al. Mutation in a “tesB-like” hydroxyacyl-coenzyme A-specific thioesterase gene causes hyperproduction of extracellular polyhydroxyalkanoates by Alcanivorax borkumensis SK2. J. Bacteriol. 2006;188:8452–8459. doi: 10.1128/JB.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kunasundari B, Sudesh K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011;5:620–634. doi: 10.3144/expresspolymlett.2011.60. [DOI] [Google Scholar]
- 125.Vu DH, Åkesson D, Taherzadeh MJ, Ferreira JA. Recycling strategies for polyhydroxyalkanoate-based waste materials: an overview. Bioresour. Technol. 2020;298:122393. doi: 10.1016/j.biortech.2019.122393. [DOI] [PubMed] [Google Scholar]
- 126.Loos K, et al. A perspective on PEF synthesis, properties, and end-life. Front. Chem. 2020;8:585. doi: 10.3389/fchem.2020.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Jong, E., Dam, M. A., Sipos, L. & Gruter, G. J. M. in ACS Symposium Series (ed. Smith, P.) Vol. 1105 1–13 (ACS, 2012).
- 128.Rosenboom J-G, Hohl DK, Fleckenstein P, Storti G, Morbidelli M. Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers. Nat. Commun. 2018;9:2701. doi: 10.1038/s41467-018-05147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burgess SK, et al. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate) Macromolecules. 2014;47:1383–1391. doi: 10.1021/ma5000199. [DOI] [Google Scholar]
- 130.Gruter, G.-J. Technology and markets day path to the future. Avantiumhttps://www.avantium.com/wp-content/uploads/2019/06/20190606-Technology-Day_CTO_Gert-Jan_Gruter_breakout_final_.pdf (2019).
- 131.Knoop RJI, Vogelzang W, van Haveren J, Van Es DS. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. A Polym. Chem. 2013;51:4191–4199. doi: 10.1002/pola.26833. [DOI] [Google Scholar]
- 132.Fleckenstein P, Rosenboom J-G, Storti G, Morbidelli M. Synthesis of cyclic (ethylene furanoate) oligomers via cyclodepolymerization. Macromol. React. Eng. 2018;12:1800018. doi: 10.1002/mren.201800018. [DOI] [Google Scholar]
- 133.Morales-Huerta JC, Martínez De Ilarduya A, Muñoz-Guerra S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer. 2016;87:148–158. doi: 10.1016/j.polymer.2016.02.003. [DOI] [Google Scholar]
- 134.Terzopoulou Z, et al. Effect of catalyst type on recyclability and decomposition mechanism of poly(ethylene furanoate) biobased polyester. J. Anal. Appl. Pyrolysis. 2017;126:357–370. doi: 10.1016/j.jaap.2017.05.010. [DOI] [Google Scholar]
- 135.Righetti MC, et al. Polymorphism and multiple melting behavior of bio-based poly(propylene 2,5-furandicarboxylate) Biomacromolecules. 2020;21:2622–2634. doi: 10.1021/acs.biomac.0c00039. [DOI] [PubMed] [Google Scholar]
- 136.Saraci E, Wang L, Theopold KH, Lobo RF. Bioderived muconates by cross-metathesis and their conversion into terephthalates. ChemSusChem. 2018;11:773–780. doi: 10.1002/cssc.201701874. [DOI] [PubMed] [Google Scholar]
- 137.Packaging Europe. Suntory to introduce 100% plant-based PET bottle prototypes. Packaging Europehttps://packagingeurope.com/suntory-to-introduce-100-plant-based-pet-bottle-prototypes/ (2021).
- 138.Cornille A, Auvergne R, Figovsky O, Boutevin B, Caillol S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017;87:535–552. doi: 10.1016/j.eurpolymj.2016.11.027. [DOI] [Google Scholar]
- 139.Blattmann H, Lauth M, Mülhaupt R. Flexible and bio-based nonisocyanate polyurethane (NIPU) foams. Macromol. Mater. Eng. 2016;301:944–952. doi: 10.1002/mame.201600141. [DOI] [Google Scholar]
- 140.Figovsky O, Shapovalov L, Leykin A, Birukova O, Potashnikova R. Recent advances in the development of non-isocyanate polyurethanes based on cyclic carbonates. PU Mag. 2013;10:2–9. [Google Scholar]
- 141.Rokicki G, Parzuchowski PG, Mazurek M. Non-isocyanate polyurethanes: synthesis, properties, and applications. Polym. Adv. Technol. 2015;26:707–761. doi: 10.1002/pat.3522. [DOI] [Google Scholar]
- 142.Hees T, Zhong F, Stürzel M, Mülhaupt R. Tailoring hydrocarbon polymers and all-hydrocarbon composites for circular economy. Macromol. Rapid Commun. 2019;40:1800608. doi: 10.1002/marc.201800608. [DOI] [PubMed] [Google Scholar]
- 143.Wang Z, Ganewatta MS, Tang C. Sustainable polymers from biomass: bridging chemistry with materials and processing. Prog. Polym. Sci. 2020;101:101197. doi: 10.1016/j.progpolymsci.2019.101197. [DOI] [Google Scholar]
- 144.Häußler M, Eck M, Rothauer D, Mecking S. Closed-loop recycling of polyethylene-like materials. Nature. 2021;590:423–427. doi: 10.1038/s41586-020-03149-9. [DOI] [PubMed] [Google Scholar]
- 145.Kawai F, Hu X. Biochemistry of microbial polyvinyl alcohol degradation. Appl. Microbiol. Biotechnol. 2009;84:227–237. doi: 10.1007/s00253-009-2113-6. [DOI] [PubMed] [Google Scholar]
- 146.Matsumura S, Kurita H, Shimokobe H. Anaerobic biodegradability of polyvinyl alcohol. Biotechnol. Lett. 1993;15:749–754. doi: 10.1007/BF01080150. [DOI] [Google Scholar]
- 147.Ben Halima N. Poly(vinyl alcohol): review of its promising applications and insights into biodegradation. RSC Adv. 2016;6:39823–39832. doi: 10.1039/C6RA05742J. [DOI] [Google Scholar]
- 148.Zumstein MT, et al. Biodegradation of synthetic polymers in soils: tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018;4:eaas90241. doi: 10.1126/sciadv.aas9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Labet M, Thielemans W. Synthesis of polycaprolactone: a review. Chem. Soc. Rev. 2009;38:3484–3504. doi: 10.1039/b820162p. [DOI] [PubMed] [Google Scholar]
- 150.Woodruff MA, Hutmacher DW. The return of a forgotten polymer — polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256. doi: 10.1016/j.progpolymsci.2010.04.002. [DOI] [Google Scholar]
- 151.Lamberti FM, Román-Ramírez LA, Wood J. Recycling of bioplastics: routes and benefits. J. Polym. Environ. 2020;28:2551–2571. doi: 10.1007/s10924-020-01795-8. [DOI] [Google Scholar]
- 152.Jem KJ, Tan B. The development and challenges of poly(lactic acid) and poly(glycolic acid) Adv. Ind. Eng. Polym. Res. 2020;3:60–70. [Google Scholar]
- 153.Sun X, Xu C, Wu G, Ye Q, Wang C. Poly(lactic-co-glycolic acid): applications and future prospects for periodontal tissue regeneration. Polymers (Basel). 2017;9:189. doi: 10.3390/polym9060189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kumar N, Langer RS, Domb AJ. Polyanhydrides: an overview. Adv. Drug Deliv. Rev. 2002;54:889–910. doi: 10.1016/S0169-409X(02)00050-9. [DOI] [PubMed] [Google Scholar]
- 155.Park JH, Ye M, Park K. Biodegradable polymers for microencapsulation of drugs. Molecules. 2005;10:146–161. doi: 10.3390/10010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Domb AJ, Langer R. Polyanhydrides. I. Preparation of high molecular weight polyanhydrides. J. Polym. Sci. A Polym. Chem. 1987;25:3373–3386. doi: 10.1002/pola.1987.080251217. [DOI] [Google Scholar]
- 157.Tabata Y, Gutta S, Langer R. Controlled delivery systems for proteins using polyanhydride microspheres. Pharm. Res. 1993;10:487–496. doi: 10.1023/A:1018929531410. [DOI] [PubMed] [Google Scholar]
- 158.Vogel BM, Mallapragada SK. Synthesis of novel biodegradable polyanhydrides containing aromatic and glycol functionality for tailoring of hydrophilicity in controlled drug delivery devices. Biomaterials. 2005;26:721–728. doi: 10.1016/j.biomaterials.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 159.Bucher JE, Slade WC. Anhydrides of isophthalic acid and terephthalic acid. J. Am. Chem. Soc. 1909;31:1319–1321. doi: 10.1021/ja01942a009. [DOI] [Google Scholar]
- 160.Kricheldorf HR, Lubbers D. Polyanhydrides. 2. Thermotropic poly(ester anhydride)s derived from terephthalic acid, substituted hydroquinones, and 4-hydroxybenzoic acids. Macromolecules. 1992;25:1377–1381. doi: 10.1021/ma00031a002. [DOI] [Google Scholar]
- 161.Conix A. Aromatic polyanhydrides, a new class of high melting fiber-forming polymers. J. Polym. Sci. 1958;29:343–353. doi: 10.1002/pol.1958.1202912002. [DOI] [Google Scholar]
- 162.Dang W, et al. Effects of GLIADEL® wafer initial molecular weight on the erosion of wafer and release of BCNU. J. Control. Rel. 1996;42:83–92. doi: 10.1016/0168-3659(96)01371-5. [DOI] [Google Scholar]
- 163.Zhang Y, Rempel C, Liu Q. Thermoplastic starch processing and characteristics — a review. Crit. Rev. Food Sci. Nutr. 2014;54:1353–1370. doi: 10.1080/10408398.2011.636156. [DOI] [PubMed] [Google Scholar]
- 164.Schutyser W, et al. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018;47:852–908. doi: 10.1039/C7CS00566K. [DOI] [PubMed] [Google Scholar]
- 165.Vilarinho F, Sanches Silva A, Vaz MF, Farinha JP. Nanocellulose in green food packaging. Crit. Rev. Food Sci. Nutr. 2018;58:1526–1537. doi: 10.1080/10408398.2016.1270254. [DOI] [PubMed] [Google Scholar]
- 166.Azeredo HMC, Barud H, Farinas CS, Vasconcellos VM, Claro AM. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019;3:7. doi: 10.3389/fsufs.2019.00007. [DOI] [Google Scholar]
- 167.Burgstaller, M. et al. Gutachten zur Behandlung biologisch abbaubarer Kunststoffe. TEXTE 57/2018 of the Umwelt Bundesamt (German Federal Environmental Office). Umwelt Bundesamthttps://www.umweltbundesamt.de/publikationen/gutachten-zur-behandlung-biologisch-abbaubarer (2018). Extensive review of biodegradation of most relevant bioplastics, including their biodegradation times in various environments (in German).
- 168.Robertson RM, Thomas WC, Suthar N, Brown DM. Accelerated degradation of cellulose acetate cigarette filters using controlled-release acid catalysis. Green Chem. 2012;14:2266–2272. doi: 10.1039/c2gc16635f. [DOI] [Google Scholar]
- 169.Huang Z, et al. Biodegradability studies of poly(butylene succinate) composites filled with sugarcane rind fiber. Polym. Test. 2018;66:319–326. doi: 10.1016/j.polymertesting.2018.02.003. [DOI] [Google Scholar]
- 170.Ferrero B, Fombuena V, Fenollar O, Boronat T, Balart R. Development of natural fiber-reinforced plastics (NFRP) based on biobased polyethylene and waste fibers from Posidonia oceanica seaweed. Polym. Compos. 2015;36:1378–1385. doi: 10.1002/pc.23042. [DOI] [Google Scholar]
- 171.Pawelzik P, et al. Critical aspects in the life cycle assessment (LCA) of bio-based materials–reviewing methodologies and deriving recommendations. Resour. Conserv. Recycl. 2013;73:211–228. doi: 10.1016/j.resconrec.2013.02.006. [DOI] [Google Scholar]
- 172.Yates MR, Barlow CY. Life cycle assessments of biodegradable, commercial biopolymers — a critical review. Resour. Conserv. Recycl. 2013;78:54–66. doi: 10.1016/j.resconrec.2013.06.010. [DOI] [Google Scholar]
- 173.Spierling S, et al. Bio-based plastics - A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018;185:476–491. doi: 10.1016/j.jclepro.2018.03.014. [DOI] [Google Scholar]
- 174.Bishop George, Styles David, Lens Piet N.L. Environmental performance comparison of bioplastics and petrochemical plastics: a review of life cycle assessment (LCA) methodological decisions. Resour. Conserv. Recycl. 2021;168:105451. doi: 10.1016/j.resconrec.2021.105451. [DOI] [Google Scholar]
- 175.Tsiropoulos I, et al. Life cycle impact assessment of bio-based plastics from sugarcane ethanol. J. Clean. Prod. 2015;90:114–127. doi: 10.1016/j.jclepro.2014.11.071. [DOI] [Google Scholar]
- 176.Blum NU, Haupt M, Bening CR. Why “circular” doesn’t always mean “sustainable”. Resour. Conserv. Recycl. 2020;162:105042. doi: 10.1016/j.resconrec.2020.105042. [DOI] [Google Scholar]
- 177.Wahab DA, Hussain A, Scavino E, Mustafa MM, Basri H. Development of a prototype automated sorting system for plastic recycling. Am. J. Appl. Sci. 2006;3:1924–1928. doi: 10.3844/ajassp.2006.1924.1928. [DOI] [Google Scholar]
- 178.Gundupalli SP, Hait S, Thakur A. A review on automated sorting of source-separated municipal solid waste for recycling. Waste Manag. 2017;60:56–74. doi: 10.1016/j.wasman.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 179.Open-ended Working Group of the Basel Convention. Technical guidelines for the identification and environmentally sound management of plastic wastes and for their disposal. Matters Related to Work Program 1–94 (2020).
- 180.Chanda, M. & Roy, S. K. Plastics Technology Handbook (Taylor & Francis, 2006).
- 181.Hong M, Chen EYX. Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 2017;19:3692–3706. doi: 10.1039/C7GC01496A. [DOI] [Google Scholar]
- 182.PlasticsEurope. Plastics - the facts 2017. Plastics Europehttps://www.plasticseurope.org/application/files/5715/1717/4180/Plastics_the_facts_2017_FINAL_for_website_one_page.pdf (2017).
- 183.Briassoulis D, Hiskakis M, Babou E. Technical specifications for mechanical recycling of agricultural plastic waste. Waste Manag. 2013;33:1516–1530. doi: 10.1016/j.wasman.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 184.Knappe, F. et al. Technische Potenzialanalyse zur Steigerung des Kunststoffrecyclings und des Rezyklateinsatzes [German]. (Wuppertal Institut, 2021).
- 185.Gu F, Guo J, Zhang W, Summers PA, Hall P. From waste plastics to industrial raw materials: a life cycle assessment of mechanical plastic recycling practice based on a real-world case study. Sci. Total Environ. 2017;601–602:1192–1207. doi: 10.1016/j.scitotenv.2017.05.278. [DOI] [PubMed] [Google Scholar]
- 186.Franklin Associates. Cradle-to-resin life cycle analysis of polyethylene terephthalate resin. Franklin Associateshttps://napcor.com/wp-content/uploads/2020/05/Final-Revised-Virgin-PET-Resin-LCA.pdf (2020).
- 187.Watkins, E. et al. EPR in the EU Plastics Strategy and the Circular Economy: A focus on plastic packaging (Institute for European Environmental Policy, 2017).
- 188.Taylor, M. Can Norway help us solve the plastic crisis, one bottle at a time? (The Guardian, 2018).
- 189.The Japan Containers and Packaging Recycling Association (JCPRA). The containers and packaging recycling system in Japan. JCPRAhttps://www.jcpra.or.jp/Portals/0/resource/eng/JCPRAdocuments202012.pdf (2020).
- 190.Singh, S. India’s plastics sector looks at packaging EPR. Plastics Newshttps://www.plasticsnews.com/article/20180824/NEWS/180829927/india-s-plastics-sector-looks-at-packaging-epr (2018).
- 191.Kauertz, B. & Detzel, A. Verwendung und Recycling von PET in Deutschland. Institut für Energie- und Umweltforschunghttps://www.nabu.de/imperia/md/content/nabude/veranstaltungen/171025-nabu-01b_studie_verwendung-und-recycling-pet-deutschland.pdf (2017).
- 192.National Association for PET Container Resources (NAPCOR). Postconsumer PET recycling activity in 2018. NAPCORhttps://napcor.com/reports-resources/ (2019).
- 193.Welle F. Twenty years of PET bottle to bottle recycling - an overview. Resour. Conserv. Recycl. 2011;55:865–875. doi: 10.1016/j.resconrec.2011.04.009. [DOI] [Google Scholar]
- 194.Shen L, Worrell E, Patel MK. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recycl. 2010;55:34–52. doi: 10.1016/j.resconrec.2010.06.014. [DOI] [Google Scholar]
- 195.Park SH, Kim SH. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014;1:1. doi: 10.1186/s40691-014-0001-x. [DOI] [Google Scholar]
- 196.Demarteau J, Olazabal I, Jehanno C, Sardon H. Aminolytic upcycling of poly(ethylene terephthalate) wastes using a thermally-stable organocatalyst. Polym. Chem. 2020;11:4875–4882. doi: 10.1039/D0PY00067A. [DOI] [Google Scholar]
- 197.Hong M, Chen EYX. Towards truly sustainable polymers: a metal-free recyclable polyester from biorenewable non-strained γ-butyrolactone. Angew. Chem. Int. Ed. 2016;55:4188–4193. doi: 10.1002/anie.201601092. [DOI] [PubMed] [Google Scholar]
- 198.Alberti C, Enthaler S. Depolymerization of end-of-life poly(lactide) to lactide via zinc-catalysis. ChemistrySelect. 2020;5:14759–14763. doi: 10.1002/slct.202003979. [DOI] [Google Scholar]
- 199.Zhao D, Wang X, Miller JB, Huber GW. The chemistry and kinetics of polyethylene pyrolysis: a process to produce fuels and chemicals. ChemSusChem. 2020;13:1764–1774. doi: 10.1002/cssc.201903434. [DOI] [PubMed] [Google Scholar]
- 200.Williams PT, Williams EA. Interaction of plastics in mixed-plastics pyrolysis. Energy Fuels. 1999;13:188–196. doi: 10.1021/ef980163x. [DOI] [Google Scholar]
- 201.Yoshida S, et al. A bacterium that degrades and assimilates poly(ethylene terephthalate) Science. 2016;351:1196–1199. doi: 10.1126/science.aad6359. [DOI] [PubMed] [Google Scholar]
- 202.Austin HP, et al. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl Acad. Sci. USA. 2018;115:E4350–E4357. doi: 10.1073/pnas.1718804115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sivan A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011;22:422–426. doi: 10.1016/j.copbio.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 204.Wiesinger, H. et al. The Identity of Oxo-Degradable Plastics and their Use in Switzerland (Federal Office for the Environment (FOEN), 2020).
- 205.Lambert S, Wagner M. Environmental performance of bio-based and biodegradable plastics: the road ahead. Chem. Soc. Rev. 2017;46:6855–6871. doi: 10.1039/C7CS00149E. [DOI] [PubMed] [Google Scholar]
- 206.Deutsche Umwelthilfe. Bioplastik in der Kompostierung. Ergebnisbericht–Umfrage [German]. Deutsche Umwelthilfehttps://www.duh.de/fileadmin/user_upload/download/Projektinformation/Kreislaufwirtschaft/Verpackungen/180920_DUH_Ergebnisbericht_Kompostierungsumfrage.pdf (2018).
- 207.Hann, S. et al. in Report for European Commission, DG Environment (European Union, 2020).
- 208.Jarerat A, Tokiwa Y, Tanaka H. Production of poly(L-lactide)-degrading enzyme by Amycolatopsis orientalis for biological recycling of poly(L-lactide) Appl. Microbiol. Biotechnol. 2006;72:726–731. doi: 10.1007/s00253-006-0343-4. [DOI] [PubMed] [Google Scholar]
- 209.Lee A, ShanLiew M. Tertiary recycling of plastics waste: an analysis of feedstock, chemical and biological degradation methods. J. Mater. Cycles Waste Manag. 2021;23:32–43. doi: 10.1007/s10163-020-01106-2. [DOI] [Google Scholar]
- 210.Tournier V, et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020;580:216–219. doi: 10.1038/s41586-020-2149-4. [DOI] [PubMed] [Google Scholar]
- 211.Wei R, et al. Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv. Sci. 2019;6:1900491. doi: 10.1002/advs.201900491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pellis A, et al. Enzymatic hydrolysis of poly(ethylene furanoate) J. Biotechnol. 2016;235:47–53. doi: 10.1016/j.jbiotec.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 213.Weinberger S, et al. Enzymatic degradation of poly(ethylene 2,5-furanoate) powders and amorphous films. Catalysts. 2017;7:318. doi: 10.3390/catal7110318. [DOI] [Google Scholar]
- 214.Russell JR, et al. Biodegradation of polyester polyurethane by endophytic fungi. Appl. Environ. Microbiol. 2011;77:6076–6084. doi: 10.1128/AEM.00521-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Espinosa MJC, et al. Toward biorecycling: isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Front. Microbiol. 2020;11:404. doi: 10.3389/fmicb.2020.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen C, Dai L, Ma L, Guo R. Enzymatic degradation of plant biomass and synthetic polymers. Nat. Rev. Chem. 2020;4:114–126. doi: 10.1038/s41570-020-0163-6. [DOI] [PubMed] [Google Scholar]
- 217.Gewert B, Plassmann MM, Macleod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts. 2015;17:1513–1521. doi: 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- 218.Yang J, Yang Y, Wu W, Zhao J, Jiang L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014;48:13776–13784. doi: 10.1021/es504038a. [DOI] [PubMed] [Google Scholar]
- 219.Jaiswal S, Sharma B, Shukla P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 2020;17:100567. doi: 10.1016/j.eti.2019.100567. [DOI] [Google Scholar]
- 220.Gajendiran A, Krishnamoorthy S, Abraham J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech. 2016;6:52. doi: 10.1007/s13205-016-0394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Santo M, Weitsman R, Sivan A. The role of the copper-binding enzyme — laccase — in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeterior. Biodegrad. 2013;84:204–210. doi: 10.1016/j.ibiod.2012.03.001. [DOI] [Google Scholar]
- 222.Matos M, et al. A new generation of furanic copolyesters with enhanced degradability: poly(ethylene 2,5-furandicarboxylate)-co-poly(lactic acid) copolyesters. Macromol. Chem. Phys. 2014;215:2175–2184. doi: 10.1002/macp.201400175. [DOI] [Google Scholar]
- 223.Terzopoulou Z, et al. Biobased poly(ethylene furanoate-co-ethylene succinate) copolyesters: solid state structure, melting point depression and biodegradability. RSC Adv. 2016;6:84003–84015. doi: 10.1039/C6RA15994J. [DOI] [Google Scholar]
- 224.Liu X, Gao C, Sangwan P, Yu L, Tong Z. Accelerating the degradation of polyolefins through additives and blending. J. Appl. Polym. Sci. 2014;131:9001–9015. doi: 10.1002/app.40750. [DOI] [Google Scholar]
- 225.Harding KG, Dennis JS, von Blottnitz H, Harrison STL. Environmental analysis of plastic production processes: comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007;130:57–66. doi: 10.1016/j.jbiotec.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 226.United States Environmental Protection Agency (EPA). Inventory of U.S. Greenhouse Gas Emissions and Sinks 1990–2019 (EPA, 2021).
- 227.Themelis NJ, Ulloa PA. Methane generation in landfills. Renew. Energy. 2007;32:1243–1257. doi: 10.1016/j.renene.2006.04.020. [DOI] [Google Scholar]
- 228.Anenberg SC, et al. Global air quality and health co-benefits of mitigating near-term climate change through methane and black carbon emission controls. Environ. Health Perspect. 2012;120:831–839. doi: 10.1289/ehp.1104301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Stagner J. Methane generation from anaerobic digestion of biodegradable plastics–a review. Int. J. Environ. Stud. 2016;73:462–468. doi: 10.1080/00207233.2015.1108607. [DOI] [Google Scholar]
- 230.Hobbs SR, Parameswaran P, Astmann B, Devkota JP, Landis AE. Anaerobic codigestion of food waste and polylactic acid: effect of pretreatment on methane yield and solid reduction. Adv. Mater. Sci. Eng. 2019;2019:4715904. doi: 10.1155/2019/4715904. [DOI] [Google Scholar]
- 231.Aeschelmann F, Carus M. Biobased building blocks and polymers in the world: capacities, production, and applications–status quo and trends towards 2020. Ind. Biotechnol. 2015;11:154–159. doi: 10.1089/ind.2015.28999.fae. [DOI] [Google Scholar]
- 232.Technavio. Global Bioplastic Packaging Market 2019–2023. Market Report (Technavio, 2019).
- 233.bioplastics Magazine. Braskem to invest US$61 million to increase biopolymer production. bioplastics Magazinehttps://www.bioplasticsmagazine.com/en/news/meldungen/20210225-Braskem-invests-US-61-million-to-increase-biopolymer-production.php (2021).
- 234.Business Wire. NatureWorks Announces Key Milestones for Global Manufacturing Expansion with New 75kTa Facility for Producing Ingeo Biopolymer in Thailand (Business Wire, 2021).
- 235.Meereboer KW, Misra M, Mohanty AK. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020;22:5519–5558. doi: 10.1039/D0GC01647K. [DOI] [Google Scholar]
- 236.Choi, A. The business case for investing in sustainable plastics. World Economic Forumhttps://www.weforum.org/agenda/2020/01/the-business-case-for-investing-in-sustainable-plastics/ (2020).
- 237.Tullo, A. H. The Final Hour for Biobased Plastics Maker Metabolix (Chemical & Engineering News, 2015).
- 238.Holcomb, G. Bioplastics Manufacturing in the US. IBISWorld Industry Report OD4512 (IBISWorld, 2019).
- 239.Harper, J. Oil prices climb back to pre-pandemic levels. BBChttps://www.bbc.com/news/business-55975700 (2021).
- 240.The European Parliament and the Council of the European Union. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the reduction of the impact of certain plastic products on the environment in Official Journal of the European Union (The European Parliament and the Council of the European Union, 2019).
- 241.European Commission. Commission Delegated Regulation (EU) 2020/2174 in Official Journal of the European Union 1–9 (European Commission, 2020).
- 242.European Council. EUCO 10/20 Conclusions of the Meeting of the European Council (17, 18, 19, 20 and 21 July 2020) (2020).
- 243.European Commission. The European Green Deal (European Commission, 2019).
- 244.The Times Editorial Board. Editorial: China is putting the U.S. to shame in the fight against plastic trash (Los Angeles Times, 2020).
- 245.Yamada, S. & Fukumoto, Y. China aims to go as big in bioplastics as it did in solar panels. Nikkei Asiahttps://asia.nikkei.com/Spotlight/Environment/China-aims-to-go-as-big-in-bioplastics-as-it-did-in-solar-panels (2021).
- 246.Moshood TD. Expanding policy for biodegradable plastic products and market dynamics of bio-based plastics: challenges and opportunities. Sustainability. 2021;13:6170. doi: 10.3390/su13116170. [DOI] [Google Scholar]
- 247.Biden Administration. Executive Order on Tackling the Climate Crisis at Home and Abroad (Biden Administration, 2021).
- 248.Biden Harris. The Biden Plan for a Clean Energy Revolution and Environmental Justice. Biden Harrishttps://joebiden.com/climate-plan/ (2020).
- 249.Lowenthal, A. S. Break Free From Plastic Pollution Act. 116th US Congress 1–127 (US Congress, 2020).
- 250.Sicotte DM. From cheap ethane to a plastic planet: regulating an industrial global production network. Energy Res. Soc. Sci. 2020;66:101479. doi: 10.1016/j.erss.2020.101479. [DOI] [Google Scholar]
- 251.CIEL. How fracked gas, cheap oil, and unburnable coal are driving the plastics boom. Fueling Plastics - Center for International Environmental Law 1–9 (CIEL, 2017).
- 252.Coleman, C. & Dietz, E. Fact Sheet: Fossil Fuel Subsidies: A Closer Look at Tax Breaks and Societal Costs (EESI, 2019).
- 253.Coady, D., Parry, I., Le, N.-P. & Shang, B. Global Fossil Fuel Subsidies Remain Large: An Update Based on Country-Level Estimates. IMF Working Papers (IMF, 2019).
- 254.Beier, W. Biologisch abbaubare Kunststoffe. Umweltbundesamt (German Federal Office of Environment, 2009).
- 255.França DC, et al. Tailoring PBAT/PLA/Babassu films for suitability of agriculture mulch application. J. Nat. Fibers. 2019;16:933–943. doi: 10.1080/15440478.2018.1441092. [DOI] [Google Scholar]
- 256.Kunioka M, Ninomiya F, Funabashi M. Biodegradation of poly(butylene succinate) powder in a controlled compost at 58°C evaluated by naturally-occurring carbon 14 amounts in evolved CO2 based on the ISO 14855-2 method. Int. J. Mol. Sci. 2009;10:4267–4283. doi: 10.3390/ijms10104267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Eerhart AJJE, Faaij APC, Patel MK. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012;5:6407–6422. doi: 10.1039/c2ee02480b. [DOI] [Google Scholar]
- 258.Koch D, Mihalyi B. Assessing the change in environmental impact categories when replacing conventional plastic with bioplastic in chosen application fields. Chem. Eng. Trans. 2018;70:853–858. [Google Scholar]
- 259.Nakajima H, Dijkstra P, Loos K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers. 2017;9:523. doi: 10.3390/polym9100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kaminsky, W. in Ullmann’s Encyclopedia of Industrial Chemistry 1–16 (Wiley, 2005).
- 261.Albertsson A-C, Hakkarainen M. Designed to degrade. Science. 2017;358:872–874. doi: 10.1126/science.aap8115. [DOI] [PubMed] [Google Scholar]
- 262.Hocevar, J. Circular claims fall flat. Greenpeace reports. Greenpeacehttp://greenpeace.org/usa/plastic_recycling (2020).
- 263.United States Environmental Protection Agency (EPA). Advancing Sustainable Materials Management: 2017 Fact Sheet (EPA, 2019).
- 264.Tabuchi, H. & Choi-Schagrin, W. California Aims to Ban Recycling Symbols on Things That Aren’t Recyclable (New York Times, 2021).
- 265.TÜV Austria Belgium. OK compost INDUSTRIAL & Similar Standards. TÜV Austria Belgiumhttps://www.tuv-at.be/green-marks/doc-center/ (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.