Abstract
As measles control and elimination campaigns progress, laboratory confirmation of clinically diagnosed measles cases becomes increasingly important. However, in many tropical countries collection and storage of clinical specimens for this purpose are logistically complicated. In this study it is shown that blood samples spotted on filter paper are suitable for the laboratory diagnosis of measles using a combination of reverse transcriptase PCR (RT-PCR) analysis and immunoglobulin M (IgM) detection. First, it was shown that in vitro measles virus (MV)-infected cells diluted in human blood and spotted on filter paper can be detected by RT-PCR. Small amounts of infected cells remained detectable after 25 weeks of storage of the filter paper at room temperature, 4 weeks at 37°C, or 2 weeks at 45°C. Subsequently, this RT-PCR was applied to filter paper blood samples collected from 117 clinically diagnosed measles patients in Sudan in 1997 and 1998. Prior laboratory diagnosis had confirmed 90 cases as acute MV infections, while 27 proved to be nonmeasles rash disease cases. Positive RT-PCR signals were detected in filter paper blood samples of 43 of the 90 confirmed cases (48%) but in none of the 27 nonmeasles cases. In addition, MV-specific IgM levels measured in reconstituted filter paper samples correlated well with those measured in plasma samples. Measles diagnosis based on the combination of filter paper RT-PCR and IgM detection had a sensitivity and specificity of 99 and 96%, respectively. An advantage of this diagnostic approach is that sequencing of RT-PCR products allows phylogenetic analysis of the MV strain involved.
Measles continues to be a major childhood disease, resulting in an estimated 1 million fatal cases each year (2). Live attenuated measles virus (MV) vaccines have successfully been used to control measles morbidity and mortality in the industrialized world, but vaccination has been less successful in developing countries. This is thought to be the result of a combination of insufficient vaccination coverage, logistical problems related to cold chain maintenance, civil wars, and safety issues related to the current AIDS pandemic (4, 16).
The diagnosis of measles in developing countries is based almost exclusively on the evaluation of clinical symptoms. The World Health Organization (WHO) defines a clinical measles case as one in which the patient has a generalized maculopapular rash, a fever of 38°C or more, and at least one of the symptoms cough, coryza, or conjunctivitis (8). However, similar symptoms may also be caused by infection with other infectious agents. In a cohort study of almost 200 clinically diagnosed Sudanese measles patients, we recently found that in approximately 25% of these cases the clinical symptoms were not related to acute MV infection (6). Other studies reported between 12% and more than 50% falsely diagnosed measles cases (5, 7, 11, 13, 14), with an apparent inverse relationship with vaccination coverage.
As measles control and elimination campaigns progress, the laboratory confirmation of clinically diagnosed measles cases becomes increasingly important. The “gold standard” for laboratory diagnosis of measles is the demonstration of specific serum immunoglobulin M (IgM) antibodies (9). However, these may be low or absent in patients sampled in an early stage of the infection or in immunocompromised patients. We have recently shown the usefulness of reverse transcriptase PCR (RT-PCR) analysis as an additional tool to help in the diagnosis of these patients (6).
In many tropical countries collection and storage of samples for laboratory diagnosis are logistically complicated due to a limited infrastructure. While the usefulness of filter paper blood samples for the measurement of MV-specific serum antibodies had been demonstrated before (3, 12), recent publications have suggested that filter paper blood samples may also be suitable for RT-PCR analyses to diagnose certain virus infections (1, 15). In the present study we show that blood samples spotted on filter paper are indeed suitable for use in MV-specific RT-PCR analysis. In combination with IgM detection carried out on the same filter paper samples, this provides an adequate method for the retrospective laboratory diagnosis of measles in tropical countries.
MATERIALS AND METHODS
Patients and samples.
Whole blood samples spotted on filter paper (no. 3; Whatman Inc., Clifton, N.J.) and plasma samples were collected in Khartoum, Sudan, in 1997 and 1998 from infants who met with the WHO clinical case definition of measles (6), after having obtained informed consent from their parents. The collection of these specimens was an integral part of a prospective measles study in Khartoum, which was approved by the medical ethical committee of the University of Khartoum. The clinical symptoms were always present at the time of sampling, and the time since the onset of rash varied from 1 to 6 days. Paired plasma and filter paper samples were available for 117 patients. Laboratory diagnosis of measles was carried out as previously described, based on MV-specific IgM and IgG antibody levels in plasma and, in doubtful cases, MV-specific RT-PCR on throat swab samples (6). Of the 117 patients, 90 were confirmed as true measles patients, while in 27 patients the clinical symptoms proved to have different causes. Filter paper samples had been stored frozen at −70°C in Khartoum for 1 to 2 years and subsequently at room temperature in The Netherlands for a period of 5 months.
Sample reconstitution for serology.
Using blood samples of healthy volunteers it was determined that a drop of 25 μl covered a circle with a diameter of approximately 1 cm of the filter paper. For serology, a circle of this size was cut out of the patient filter paper, making sure that both sides of the filter paper were saturated with blood. The filter paper fragment was incubated overnight at 4°C on a rotating device with 0.5 ml of phosphate-buffered saline supplemented with 2% fetal bovine serum. The next day the vial was centrifuged, and the supernatant was frozen at −20°C until analysis. Assuming that 25 μl of blood would contain approximately 10 μl of serum, the resulting sample was treated as a 1:50 dilution of the patient's serum.
Serology.
Levels of MV nucleoprotein (N)-specific IgM were measured in a capture enzyme-linked immunosorbent assay (ELISA) as previously described (6), using baculovirus-expressed MV N (a kind gift of T. F. Wild, Lyon, France) directly conjugated with peroxidase. The reconstituted filter paper samples were tested at an estimated final dilution of 1:100. Results are expressed as optical density at 450 nm.
Total IgM levels in the reconstituted filter paper samples were estimated in a sandwich ELISA, using a pool of eight human sera with a known total IgM concentration (0.7 mg/ml) as a standard. In this assay the same anti-human IgM-coated ELISA plates were used as in the MV N-specific capture ELISA. A calibration curve of twofold dilutions of the standard serum pool was prepared starting at 1:200; the reconstituted filter paper samples were tested at an estimated final dilution of 1:1,000. After incubation for 1 h at 37°C, the plates were washed and incubated with a peroxidase-labeled anti-human IgM conjugate. Following an additional 1 h of incubation at 37°C, the plates were washed again and subsequently stained using tetramethylbenzidine as a substrate. Sample IgM concentrations were estimated from the calibration curve.
In vitro MV infection.
A wild-type MV strain was isolated from one of the patients included in the Sudanese cohort (patient SM32) by cocultivation of phytohemagglutinin-stimulated peripheral blood mononuclear cells (PBMC) with a human B-lymphoblastic cell line (B-LCL) previously obtained from a healthy Dutch volunteer (6). A fourth passage of this virus on B-LCL was used as stock for in vitro infection experiments, in which the B-LCL was infected at a multiplicity of infection of 0.01. After 2 days the formation of syncytia was observed, and all cells expressed MV hemagglutinin at their surface as measured by immunofluorescence (not shown). The cells were washed five times (5 min, 300 × g) in RPMI 1640 medium supplemented with 5% fetal bovine serum, counted, and diluted in blood of a healthy human donor mixed with EDTA. Samples were spotted on 2992 filter paper (Schleicher & Schuell, Dassel, Germany).
Isolation of RNA from filter paper.
RNA was isolated from filter paper using a High Pure viral nucleic acid kit (Roche Diagnostics, Almere, The Netherlands), with minor modifications to the protocol. Briefly, a circle with a diameter of approximately 1 cm was cut out of the filter paper, inserted in an RNase-free vial, and incubated with 300 μl of kit binding buffer supplemented with poly(A) carrier RNA. After addition of 300 μl of distilled water and 60 μl of proteinase K (20 mg/ml), the sample was mixed using micropestles and vortexing and subsequently incubated at 72°C for 10 min. After this incubation, 150 μl of isopropanol was added and mixed using micropestles. Further steps were carried out according to the manufacturer's instructions.
RT-PCR.
RT-PCR was carried out as previously described (6), using forward primer 5′-TTAGGGCAAGAGATGGTAAGG-3′ (MV-N1, positions 1090 to 1110), reverse primer 5′-TTATAACAATGATGGAGGG-3′ (MV-N2, positions 1633 to 1615), and oligonucleotide probe 5′-GCCATGGCAGGAATCTCGGAA-3′ (MV-prN2, positions 1498 to 1518). A positive RT-PCR signal was defined as an amplified product of the right size (544 nucleotides) which hybridized with the specific oligonucleotide.
RESULTS
Sensitivity of filter paper RT-PCR.
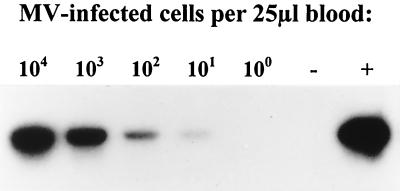
To evaluate the suitability of whole blood spotted on filter paper for use in MV-specific RT-PCR, in vitro MV-infected human B-LCL was diluted in human blood with EDTA and spotted on filter paper. RNA was isolated from the filter paper, and RT-PCR analysis was carried out. In a 10-fold dilution range of 104 to 10−1 infected cells per 25 μl of blood, all samples with 10 or more infected cells per drop of blood gave positive RT-PCR signals (Fig. 1).
FIG. 1.
RT-PCR detection of in vitro MV-infected cells diluted in human blood and spotted on filter paper. A human Epstein-Barr virus-transformed B-LCL was infected with a wild-type MV isolate from Khartoum, washed, counted, diluted in human blood with EDTA, and spotted on filter paper in 25-μl samples. After storage of the filter paper samples at room temperature for 6 weeks, RNA was isolated and RT-PCR was carried out with primers MV-N1 and MV-N2. The resulting amplicons were of the correct size as estimated on the gel using a 100-bp ladder as reference (not shown). The PCR products were blotted and hybridized with 32P-labeled oligonucleotide probe MV-prN2. The autoradiagram is shown, with numbers of MV-infected cells per 25 μl indicated above the respective lanes. The positive (MV Edmonston) and negative (untreated human blood with EDTA) controls are indicated by + and −, respectively.
Longevity of filter paper RT-PCR signals.
Freshly prepared filter paper samples spotted with 30 in vitro MV-infected cells per 25 μl of human blood were subsequently incubated at different temperatures, and RT-PCR analysis was carried out in duplicate after 1, 2, 4, 8, 12, and 25 weeks of dry storage at 20, 37, or 45°C. MV remained detectable by RT-PCR after storage for 25 weeks at 20°C, 4 weeks at 37°C, or 2 weeks at 45°C (Table 1). Positive RT-PCR signals were also detected in filter paper samples stored for 1 week in a humidified atmosphere at 37°C, but after 2 weeks the samples were no longer suitable for analysis due to fungal outgrowth.
TABLE 1.
RT-PCR detection of MV in filter paper samples after storage under different conditionsa
| Storage conditions | Detection after the following storage time (wk):
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 12 | 25 | |
| 20°C, dry | + | + | + | + | + | + |
| 37°C, dry | + | + | + | − | − | NTb |
| 45°C, dry | + | + | − | − | NT | NT |
| 37°C, humidified | + | − | NT | NT | NT | NT |
Each filter paper (four used) was spotted with 30 in vitro MV-infected B-LCL cells per 25 μl of human blood with EDTA (20 identical spots) and stored dry at 20, 37, or 45°C or in a humidified 37°C incubator. Two 25-μl spots were collected from each filter paper after 1, 2, 4, 8, 12, or 25 weeks of storage, RNA was isolated, and RT-PCR was carried out in duplicate. Results are shown as positive (+) or negative (−), results of duplicate measurements were in all cases concordant. Samples stored for more than 2 weeks at 37°C and humidified could not be processed due to fungal outgrowth.
NT, not tested.
IgM detection in Sudanese filter paper blood samples.
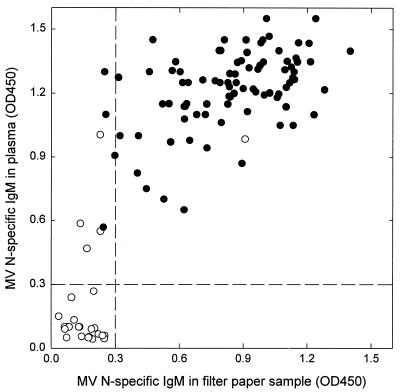
Subsequently, we tested filter paper blood samples collected from clinically diagnosed measles patients in Sudan in 1997 and 1998. First, MV-specific IgM levels measured in reconstituted filter paper samples were compared with those measured in plasma. In some cases the levels measured in filter paper samples were substantially lower than those measured in plasma, which prompted us to measure the total IgM level in the reconstituted filter paper samples using a sandwich ELISA. While IgM concentrations of approximately 1.75 and 0.5 μg/ml (reference serum pool dilutions of 1:400 and 1:1400) were required for 100% or 50 saturation of the capture ELISA plates, respectively, some of the reconstituted samples proved to contain less than that. Especially in samples with less than 0.2 μg of IgM per ml, the MV-specific signals measured were lower than those measured in plasma, so this level was defined as the critical total IgM value for a validated assay. As shown in Fig. 2, the final correlation between IgM levels measured in plasma and in filter paper after excluding the samples with less than 0.2 μg of IgM per ml (n = 3) was good (r2 = 0.65).
FIG. 2.
MV-specific IgM levels in paired filter paper and plasma samples collected from clinically diagnosed measles patients in Khartoum, Sudan. The samples were tested in an IgM capture ELISA, using peroxidase-labeled MV N as the conjugate. OD450, optical density at 450 nm. ●, laboratory-confirmed measles cases; ○, nonmeasles rash disease cases.
RT-PCR analysis of Sudanese filter paper blood samples.
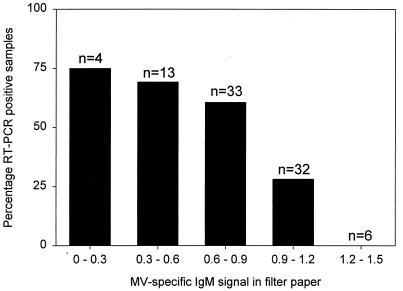
MV-specific RT-PCR signals were detected in filter paper samples of 43 out of 90 laboratory-confirmed acute measles cases (48%) and in none of 27 nonmeasles rash disease cases. Interestingly, the frequency of positive RT-PCR signals among confirmed measles cases was inversely correlated with the IgM level measured in filter paper samples (Fig. 3).
FIG. 3.
Frequency of RT-PCR-positive filter paper samples obtained from laboratory-confirmed measles patients in relation to the level of MV-specific IgM measured in the same filter paper samples. The cases correspond to the closed symbols in Fig. 2. The number of samples in each group is indicated above each bar.
Diagnostic value of combined RT-PCR analysis and IgM detection.
While specific IgM measurement in plasma samples had a high sensitivity but low specificity (100 and 78%, respectively), IgM measurement in filter paper blood samples had a slightly lower sensitivity but higher specificity (95 and 96%, respectively) (Table 2). A diagnosis based on the combination of RT-PCR analysis and IgM detection on filter paper samples, defining a measles case as MV-specific RT-PCR positive and/or IgM positive, had a sensitivity and specificity of 99 and 96%, respectively (Table 2).
TABLE 2.
Sensitivity, specificity, and positive predictive values of laboratory diagnosis of measles using filter paper blood samplesa
| Diagnostic method | Sensitivity (%) | Specificity (%) | Positive predictive value (%) |
|---|---|---|---|
| MV-specific IgM | 95 | 96 | 99 |
| MV-specific RT-PCR | 48 | 100 | 100 |
| Combination of IgM and RT-PCR | 99 | 96 | 99 |
As the gold standard, a previously described method for the laboratory diagnosis of measles was used, which is based on measurement of MV-specific IgM and IgG antibody levels in plasma and, in doubtful cases, MV-specific RT-PCR with throat swab samples (6).
DISCUSSION
This study shows that combined RT-PCR analysis and IgM detection on filter paper blood samples allows a highly accurate diagnosis of measles. Proof of principle of the suitability of filter paper samples for MV-specific RT-PCR was obtained from studies with in vitro MV-infected cells diluted in human blood. When applied to clinical materials from a cohort of clinically diagnosed Sudanese measles patients sampled in 1997 and 1998, we found the combination of RT-PCR and IgM detection to have a sensitivity, specificity, and positive predictive value of 99, 96, and 99%, respectively. An additional advantage of this approach is that sequence analysis of the PCR product allows phylogenetic analysis of the MV strain involved.
The incubation time of measles is 9 to 19 days, with the peak of MV replication preceding the appearance of the rash (10). Several of the clinical symptoms of measles, including rash and conjunctivitis, have an immunopathological basis: they coincide with the appearance of MV-specific serum antibodies and specific T lymphocytes. As a result of the MV-specific immune response, the viral load decreases rapidly after onset of disease. In a previous study, we could isolate MV from PBMC of 17 out of 23 laboratory-confirmed measles cases tested, and we found numbers of infected cells between 100.5 and 104 cells per 106 PBMC (6). Since these estimations are based on virus isolation, the frequency of in vivo MV-infected cells will probably be higher. With an estimated number of mononuclear leukocytes of between 104 and 105 per 25 μl of blood and a RT-PCR detection limit of three infected cells, about 0.03 to 0.003% of PBMC would need to be infected in vivo to give a positive RT-PCR signal in our assay. However, the rapidly decreasing virus load after the onset of rash (10) implies that measles diagnosis based on RT-PCR analysis alone may not be expected to be sensitive enough.
The IgM levels measured in filter paper samples were in most cases slightly lower than those measured in plasma samples, but in some cases the difference was more than threefold. Using a total IgM sandwich ELISA, we could demonstrate that these samples contained less than 0.2 μg of total IgM per ml. In a diagnostic setup it would be advisable to always test the MV-specific and total IgM levels at the same time and to consider negative data from samples with less than 0.2 μg of total IgM per ml to be a nonvalid.
As we have documented before, the diagnosis of measles based on serology alone has some shortcomings, especially when MV-specific IgM levels are low. This may be the case in patients sampled in an early stage of the infection, in patients with a secondary measles vaccine failure (who may mount a secondary immune response with low IgM and high IgG levels), and in immunocompromised patients. In these cases, the addition of RT-PCR analysis to IgM detection will reduce the number of false-negative diagnoses.
The Sudanese filter paper samples had first been stored frozen in Sudan, but after shipment to The Netherlands they were stored at 20°C for a period of 5 months before the assays were performed. However, our longevity data using in vitro MV-infected cells demonstrated that small amounts of infected cells spotted on filter paper and stored for 25 weeks at room temperature can still be detected by RT-PCR. Although we do not have sufficient data for an accurate estimation of the half-life of the RT-PCR signal at this temperature, it is at least several weeks.
The WHO is organizing a global laboratory network for the diagnosis of measles, as a first step in the preparation of a plan for the eventual eradication of measles. This laboratory network is organized as a tiered system, with global reference laboratories, and regional, national, and subnational laboratories. The collection of whole blood samples on filter paper would fit well within such an approach. A limited number of drops of blood could be collected from each patient, and filter paper samples could be sent to the respective laboratories to be analyzed as described here. Our longevity data suggest that even in countries with high ambient temperatures a transporation time of several weeks would result in only a limited loss of signal. In the laboratory the filter paper samples would probably best be stored frozen.
In conclusion, the combination of RT-PCR analysis and IgM detection on filter paper blood samples was shown to result in a highly sensitive and specific diagnostic method. The ease of sample collection and transport makes it especially attractive for use in tropical countries. In addition, the option for phylogenetic analysis of the MV strains involved, based on sequence analysis of the RT-PCR products, will be important for molecular epidemiological studies during the final stages of the envisaged measles eradication program.
ACKNOWLEDGMENTS
This work was supported by INCO-DC grant IC18CT96-0116 from the European Commission.
We thank K. H. Siebelink, O. M. Mustafa, M. M. Mukhtar, E. E. Zijlstra, H. W. Vos, and C. Copra for their contribution to these studies and R. S. van Binnendijk for critical comments on the manuscript.
REFERENCES
- 1.Abe K, Konomi N. Hepatitis C virus RNA in dried serum spotted onto filter paper is stable at room temperature. J Clin Microbiol. 1998;36:3070–3072. doi: 10.1128/jcm.36.10.3070-3072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. Measles: progress towards global control and regional elimination, 1990–1998. Wkly Epidemiol Rec. 1998;73:389–394. [PubMed] [Google Scholar]
- 3.Condorelli F, Scalia G, Stivala A, Gallo R, Marino A, Battaglini C M, Castro A. Detection of immunoglobulin G to measles virus, rubella virus, and mumps virus in serum samples and in microquantities of whole blood dried on filter paper. J Virol Methods. 1997;49:25–36. doi: 10.1016/0166-0934(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 4.Cutts F T, Henao-Restrepo A M, Olive J M. Measles elimination: progress and challenges. Vaccine. 1999;17:S47–S52. doi: 10.1016/s0264-410x(99)00309-6. [DOI] [PubMed] [Google Scholar]
- 5.Davidkin I, Valle M, Peltola H, Hovi T, Paunio M, Roivainen M, Linnavuori K, Jokinen S, Leinikki P. Etiology of measles- and rubella-like illnesses in measles, mumps, and rubella-vaccinated children. J Infect Dis. 1998;178:1567–1570. doi: 10.1086/314513. [DOI] [PubMed] [Google Scholar]
- 6.El Mubarak H S, Van de Bildt M W G, Mustafa O A, Vos H W, Mukhtar M M, Groen J, El Hassan A M, Niesters H G M, Ibrahim S A, Zijlstra E E, Wild T F, Osterhaus A D M E, De Swart R L. Serological and virological characterization of clinically diagnosed cases of measles in suburban Khartoum. J Clin Microbiol. 2000;38:987–991. doi: 10.1128/jcm.38.3.987-991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferson M J, Young L C, Robertson R W, Whybin L R. Difficulties in clinical diagnosis of measles: proposal for modified clinical case definition. Med J Aust. 1995;163:364–366. doi: 10.5694/j.1326-5377.1995.tb124630.x. [DOI] [PubMed] [Google Scholar]
- 8.Global Programme for Vaccines and Immunization/Expanded Programme on Immunization. Using surveillance data and outbreak investigations to strengthen measles immunization programmes (WHO/EPI/GEN/96.02) Geneva, Switzerland: World Health Organization; 1996. pp. 1–26. [Google Scholar]
- 9.Grandien M, Osterhaus A D M E, Rota P A, Smaron M F, Wild T F. Laboratory diagnosis of measles infection and monitoring of measles immunization: memorandum from a WHO meeting. Bull W H O. 1994;72:207–211. [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1267–1312. [Google Scholar]
- 11.Lambert S B, Kelly H A, Andrews R M, Catton M C, Lynch P A, Leydon J A, Gercovich D K, Hogg G G, Morgan M L, Lester R A. Enhanced measles surveillance during an interepidemic period in Victoria. Med J Aust. 2000;172:114–118. doi: 10.5694/j.1326-5377.2000.tb127934.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakano J H, Miller D L, Foster S O, Brink E W. Microtiter determination of measles hemagglutination inhibition antibody with filter papers. J Clin Microbiol. 1983;17:860–863. doi: 10.1128/jcm.17.5.860-863.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nur Y A, Groen J, Yusuf M A, Osterhaus A D M E. IgM antibodies in hospitalized children with febrile illness during an inter-epidemic period of measles, in Somalia. J Clin Virol. 1999;12:21–25. doi: 10.1016/s1386-6532(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 14.Oshitani H, Suzuki H, Mpabalwani M, Mizuta K, Kasolo F C, Luo N P, Numazaki Y. Laboratory diagnosis of acute measles infections in hospitalized children in Zambia. Trop Med Int Health. 1997;2:612–616. doi: 10.1046/j.1365-3156.1997.d01-346.x. [DOI] [PubMed] [Google Scholar]
- 15.Pitcovski J, Shmueli E, Krispel S, Levi N. Storage of viruses on filter paper for genetic analysis. J Virol Methods. 1999;83:21–26. doi: 10.1016/s0166-0934(99)00101-9. [DOI] [PubMed] [Google Scholar]
- 16.Wild T F. Measles vaccines, new developments and immunization strategies. Vaccine. 1999;17:1726–1729. doi: 10.1016/s0264-410x(98)00428-9. [DOI] [PubMed] [Google Scholar]