Key Points
Question
Is there an association between invasive dental procedures (IDP) and late prosthetic joint infections (LPJI) in patients who did not receive antibiotic prophylaxis prior to IDP?
Findings
This cohort study of 9427 LPJI hospital admissions for which dental records were available from 15 months preceding admission found no evidence of a temporal association between IDP and LPJI.
Meaning
These findings refute recommendations to give antibiotic prophylaxis to patients with prosthetic joints prior to IDP, given the cost, adverse drug reaction risk, and potential for promoting antibiotic resistance associated with antibiotic prophylaxis.
This cohort assesses whether there is a temporal association between invasive dental procedures and subsequent later prosthetic joint infection among patients in England.
Abstract
Importance
Dentists in the United States are under pressure from orthopedic surgeons and their patients with prosthetic joints to provide antibiotic prophylaxis before invasive dental procedures (IDP) to reduce the risk of late prosthetic joint infection (LPJI). This has been a common practice for decades, despite a lack of evidence for an association between IDP and LPJI, a lack of evidence of antibiotic prophylaxis efficacy, cost of providing antibiotic prophylaxis, and risk of both adverse drug reactions and the potential for promoting antibiotic resistance.
Objective
To quantify any temporal association between IDP and subsequent LPJI.
Design, Setting, and Participants
This cohort study used a case-crossover and time trend design to examine any potential association between IDP and LPJI. The population of England (55 million) was chosen because antibiotic prophylaxis has never been recommended to prevent LPJI in England, and any association between IDP and LPJI would therefore be fully exposed. All patients admitted to hospitals in England for LPJI from December 25, 2011, through March 31, 2017, and for whom dental records were available were included. Analyses were performed between May 2018 and June 2021.
Exposures
Exposure to IDP.
Main Outcomes and Measures
The main outcome was the incidence of IDP in the 3 months before LPJI hospital admission (case period) compared with the incidence in the 12 months before that (control period).
Results
A total of 9427 LPJI hospital admissions with dental records (mean [SD] patient age, 67.8 [13.1] years) were identified, including 4897 (52.0%) men and 4529 (48.0%) women. Of these, 2385 (25.3%) had hip prosthetic joints, 3168 (33.6%) had knee prosthetic joints, 259 (2.8%) had other prosthetic joints, and 3615 (38.4%) had unknown prosthetic joint types. There was no significant temporal association between IDP and subsequent LPJI. Indeed, there was a lower incidence of IDP in the 3 months prior to LPJI (incidence rate ratio, 0.89; 95% CI, 0.82-0.96; P = .002).
Conclusions and Relevance
These findings suggest that there is no rationale to administer antibiotic prophylaxis before IDP in patients with prosthetic joints.
Introduction
Replacing arthritic joints with prosthetic joints is one of the great advances of modern medicine, with 2.9 million joints replaced annually worldwide.1,2 Periprosthetic joint infection (PJI) is a leading cause of arthroplasty failure. Early infections, within 3 months of joint replacement, are considered the result of wound contamination at the time of the surgical procedure. Early infection rates in the 1950s were approximately 12%, but antibiotic prophylaxis before joint replacement and lamina airflow operating rooms have reduced this to 1% to 2%,3 and refocused attention on late PJIs (LPJIs), which occur 3 months or longer after joint replacement operations.
LPJI may result in prosthesis removal, or more rarely, in loss of limb or life.4 The cost of treating LPJI is 4- to 6-fold that of the original arthroplasty5,6,7,8 and is projected to have cost $1.62 billion in the US in 2020,9 without taking account of the personal and societal costs of long-term disability or the impact on patients’ quality of life.10 Therefore, LPJI is of significant concern for the 28 000 US orthopedic surgeons and more than 7 million individuals with prosthetic joints.4,11 Furthermore, the number of individuals with prosthetic joints is increasing rapidly, with approximately 4 million new hip and knee arthroplasties projected annually in the US by 2030.12 Although LPJI incidence is comparatively low, it remains the most common mode of failure of knee replacement and the second most common of hip replacement, and the incidence is projected to increase with the increasing number of arthroplasties being performed.4,13,14
LPJI is most often attributed to hematogenous seeding of bacteria from another anatomical site,15,16 and this led US orthopedic surgeons to recommend patients with prosthetic joints be given antibiotic prophylaxis prior to invasive dental procedures (IDP),17,18,19 a practice that is supported by more than 90% of US orthopedic surgeons.20,21 Nonetheless, there are little data to support a causal link between IDP and LPJI, and there has never been a randomized clinical trial of antibiotic prophylaxis efficacy in preventing LPJI, to our knowledge. Furthermore, microbiological studies suggest that oral streptococci are an uncommon cause of LPJI, accounting for less than 10% of infections.4,22,23,24,25,26,27,28 These reasons may explain why antibiotic prophylaxis use for patients with prosthetic joints is not advocated in many countries, including the United Kingdom.28 The annual cost of providing antibiotic prophylaxis in the US has been calculated at approximately $59 640 000,11 but this does not take into account the cost of adverse drug reactions caused by antibiotic prophylaxis28,29,30 or the risk that antibiotic prophylaxis could contribute to selection of antibiotic resistant bacteria.28,31,32
For antibiotic prophylaxis to be effective, there must be a positive temporal association between IDP and LPJI, but data on this are lacking.25 In the absence of this evidence, the demand on US dentists to provide antibiotic prophylaxis for IDP remains controversial. The aim of this study was to determine whether there is a positive association between LPJI and IDP in the population of England where, because antibiotic prophylaxis is not recommended, the association should be fully exposed.
Methods
This cohort study used national data and necessitated the transfer of individually identifying National Health Service (NHS) information between NHS Digital and the NHS Business Services Authority (NHSBSA); therefore, we obtained national research ethics approval and Confidentiality Advisory Group approval to process patient identifiable information without consent from the NHS Health Research Authority. We also obtained approval from NHS Digital’s Data Access Request Service and the Independent Group Advising on the Release of Data. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Source
All hospital admissions in England are recorded in the Hospital Episode Statistics (HES) database of NHS Digital. This database was searched to identify all patients admitted between December 25, 2011, and March 31, 2017, with an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10)33 code for infection and inflammatory reaction due to internal joint prosthesis (T84.5) in any discharge diagnosis code field. From this list, NHS Digital created two data sets. The first data set was a full set of identifying patient details (NHS number, surname, forenames, date of birth, gender, full address, postcode). These identifiers, along with a unique study ID for each patient (encrypted HES ID), but no clinical information, were transferred to NHSBSA. The second data set was a full set of clinical, diagnostic, and procedural data for the period of January 1, 2000, through March 31, 2017, for all patients identified with ICD-10 code T84.5, as well as data relating to the PJI admission. This allowed us to identify the time between joint replacement and PJI, and the type of joint replaced (eg, hip, knee). Data set 2 also included the same encrypted HES ID field included in data set 1, but no other patient identifying information.
NHSBSA maintains dental records for all patients receiving NHS dental treatment in England. Using the personal identifiers received from NHS Digital in data set 1, NHSBSA created data set 3, including dental records for each individual from October 1, 2010, through March 31, 2017. All patient identifiers (except common encrypted HES ID) were removed from this data set before transfer to the study team. Thus, we were able to link medical (data set 2) and dental (data set 3) records of each patient using the common encrypted HES ID field.
IDP
Since April 2008, dentists working in the English NHS have been required to record if a patient had a dental extraction, scaling, or endodontic procedure as part of dental treatment. There is wide acceptance that these can result in bacteremia and were considered IDP for this study.34,35 As a control group, we identified courses of treatment restricted to a simple dental examination, with or without radiographs, that did not involve an IDP. Other courses of treatment were considered intermediate.
Previous studies have shown that more than 90% of distant site infections associated with IDP occur within 3 months, and this period is used widely to define distant site infections associated with IDP.4,36,37,38,39,40,41,42 Hence, we chose a 3-month window of risk to assess for a potential association between IDP and LPJI.
NHSBSA data provided start and end dates for each course of dental treatment. Rarely, treatment courses were not completed, such as owing to death or change of residence. Although such situations were rare, when they occurred, the clinical record was not always complete; therefore, for our main analysis, we used end date to define a course of treatment, thereby excluding any incomplete treatment.
LPJI Hospital Admissions
A cohort of individuals who had an LPJI between December 25, 2011, and March 31, 2017, were identified using ICD-10 code T84.5. By reviewing each patient’s HES record back to 2000, we identified the date and joint replaced using OPCS Classification of Interventions and Procedures version 4 (OPCS-4) joint replacement codes (eTable 1 in the Supplement). This allowed us to subanalyze data by joint replaced. Joint replacements were divided into all, hip (codes W37-W39, W46-W48), knee (codes W40-W42), other (codes W43-W45, W49-W51), and unknown. Unknown included joint replacements performed before 2000, for which no replacement code data were available to us, or when more than 1 type of joint was replaced (eg, hip and knee joints) and we did not know which had been infected. To ensure only patients with LPJI were analyzed, this information was also used to exclude patients admitted for PJI within 3 months of joint replacement. We also excluded any admission for PJI that occurred within 12 months of an earlier PJI admission as representing a reoccurrence of the same infection. In addition to the T84.5 code, coders can record supplementary codes to indicate the nature of the causal organism, and these were noted when recorded (eTable 2 in the Supplement).
Case-Crossover Design
Maclure43 proposed the case-crossover method for studying the association of transient events with subsequent outcomes while eliminating control selection bias and confounding by constant within-participant characteristics. In case-crossover studies, each individual serves as their own control.
This study examined individuals with LPJI as the outcome and evaluated exposure to IDP. We compared the incidence of IDP in a predefined 3-month case period immediately before LPJI hospital admission, with incidence of IDP in the preceding 12-month control period (months 4-15).43,44,45 To confirm the timing of events, the monthly incidence of dental procedures over the 15-month period before LPJI hospital admission was plotted. We also performed a post hoc sensitivity analysis in which we compared the effect of using a 3-, 4- or 5-month case period (eFigure 1 in the Supplement). Some case-crossover studies have compared case periods with 1 or several control periods of the same duration. However, Mittleman et al46 have shown that sampling the control exposure frequency over a full year was twice as efficient as sampling control-periods equal in duration to the case-period, even when many such control periods were sampled.
Statistical Analysis
The incidence of IDP was analyzed using a longitudinal negative binomial regression model in which the number of IDP was the dependent variable, the covariates were time period and admission number, and the duration of each period was the offset term. The primary contrast was the number of IDP in the 3 months prior to LPJI compared with the incidence of IDP over the previous 12 months. The type of IDP (ie, scaling, extraction, or endodontic), and the incidence of intermediate and noninvasive procedures were assessed using analogous models. Additional analyses were undertaken defined by the site of prosthesis (ie, hip, knee, other, or unknown).
Power calculations for self-controlled case series are given by Musonda.47,48 With a sample size of 9427 patients with LPJI with linked dental records, and assuming no association between IDP and LPJI, there was >90% power to detect a relative incidence of 1.09, ie, a 9% higher incidence of IDP, in the 3-month risk period compared with the matched control period.
All analyses were conducted using Stata statistical software version 16.1 (StataCorp). P values were 2-sided, and statistical significance was set at P < .05. Data were analyzed from May 2018 to June 2021.
Results
Population Characteristics
Of 23 133 patients admitted to hospitals with LPJI between December 25, 2011, and March 31, 2017, 9427 patients (40.8%) had dental records available and were included in analyses. The mean (SD) age of patients with dental records was 67.8 (13.1) years, 4897 (52.0%) were men and 4529 (48.0%) were women. The joints involved were hip (2385 patients [25.3%]), knee (3168 patients [33.6%]), other (259 patients [2.8%]), and unknown (3615 patients [38.4%]). The demographic characteristics of patients with LPJI with linked dental data were similar to all patients with LPJI overall (Table 1). In-hospital mortality associated with LPJI admission was 847 patients (3.7%) among all patients with LPJI and 211 patients (2.2%) among those with dental records. Causal organism data were recorded for 4338 patients (46.0%) for whom dental data were available. Within this group, 2314 LPJIs (53.3%) were recorded as caused by staphylococci, 408 LPJIs (9.4%) by oral streptococci, 213 LPJIs (4.9%) by other streptococci, 863 LPJIs (19.9%) by other organisms, and 540 LPJIs (12.5%) were recorded as mixed infection.
Table 1. Characteristics of the Study Population.
| Characteristics | LPJI hospital admissions, No. (%) | |
|---|---|---|
| With linked dental data (n = 9427) | All (N = 23 133) | |
| Age, y | ||
| Mean (SD) | 67.8 (13.1) | 69.4 (13.7) |
| Median (range) | 69 (3-101) | 71 (0-103) |
| Gender | ||
| Men | 4897 (52.0) | 11 697 (50.6) |
| Women | 4529 (48.0) | 11 429 (49.4) |
| Prosthetic joint type | ||
| Any | 9427 | 23 133 |
| Hip | 2385 (25.3) | 5861 (25.3) |
| Knee | 3168 (33.6) | 7349 (31.8) |
| Other | 259 (2.8) | 524 (2.3) |
| Unknowna | 3615 (38.4) | 9399 (40.6) |
| Causal organism recorded | ||
| No | 5089 (54.0) | 12 022 (52.0) |
| Yes | 4338 (46.0) | 11 111 (48.0) |
| Oral streptococcib | 408 (9.4) | 986 (8.9) |
| Other streptococcib | 213 (4.9) | 560 (5.0) |
| Staphylococcib | 2314 (53.3) | 5658 (50.9) |
| Other causal organismsb | 863 (19.9) | 2355 (21.2) |
| Mixedb | 540 (12.5) | 1552 (14.0) |
| Hospital admission details | ||
| All admissions | 9427 (100.0) | 23 133 (100.0) |
| Electivec | 5676 (60.2) | 12 973 (56.1) |
| Emergency | 3.751 (39.8) | 10 160 (43.9) |
| Length of stay, d | ||
| Mean (SD) | 17.3 (25.1) | 20.1 (28.3) |
| Median (range) | 9 (0-389) | 11 (0-646) |
| Discharged alive | ||
| No | 211 (2.2) | 847 (3.7) |
| Yes | 9191 (97.5) | 22 164 (95.8) |
| Dental treatment details | ||
| Course duration, d | ||
| Mean (SD) | 11.0 (31.0) | 10.6 (31.0) |
| Median (range) | 0 (0-854) | 0 (0-1486) |
| All procedures | 19 390 (100) | 255 437 (100) |
| Invasive procedures | 8930 (46.1) | 113 058 (44.3) |
| Extractionsd | 1850 (20.7) | 23 864 (21.1) |
| Scalingd | 7313 (81.9) | 92 102 (81.5) |
| Endodontic proceduresd | 308 (3.5) | 3891 (3.4) |
| Intermediate procedures | 2102 (10.8) | 28 370 (11.1) |
| Non-invasive procedures | 8358 (43.1) | 114 009 (44.6) |
Abbreviation: LPJI, late prosthetic joint infection.
Unknown includes joints inserted before 2000, when records started, and patients with multiple joints of different types for whom information on which joint was infected was not available.
Percentages shown are of LPJIs for which a causal organism was recorded.
Elective admissions include waiting list, booked, planned nonemergency transfers from another hospital, and admission method undefined.
Percentages shown are of invasive dental procedures. Numbers of dental procedures are the number of courses of dental treatment containing at least 1 of that type of procedure (the actual number of procedures could be more).
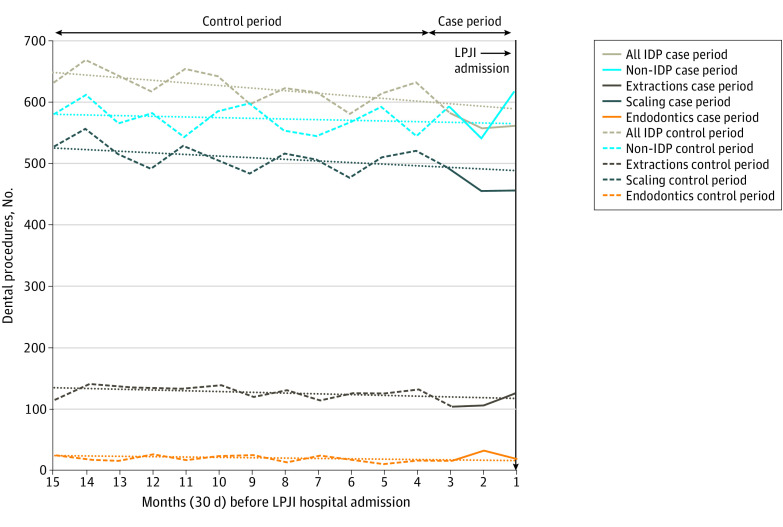
Incidence of Different Dental Procedures During 15 Months Before LPJI Admission
Among 9427 patients admitted to hospitals with LPJI with dental records, the monthly incidence of IDP and noninvasive dental procedures in the 15 months before LPJI hospital admission were similar (Figure 1). In both groups, there was no significant increase in IDP in the case period (months 1-3 before LPJI admission) compared with the control period. Indeed, there was a significant decrease in IDP in the 3 months before LPJI admission (incidence rate ratio [IRR], 0.89; 95% CI, 0.82-0.96; P = .002, Table 2), although the significance of this decrease was lost when individual types of IDP were examined (scaling: IRR, 0.88; 95% CI, 0.79-1.01; P = .07; extractions: IRR, 0.84; 95% CI, 0.69-1.04; P = .11; endodontics: IRR, 1.15; 95% CI, 0.86-1.52; P = .34) and pairwise differences between different IDP types were not statistically significant. Post hoc sensitivity analyses using 4- or 5-month case periods also found no significant increase (or decrease) in IDP compared with control periods (eFigure 1 in the Supplement).
Figure 1. Monthly Incidence of Different Types of Dental Procedure During 15 Months Before Late Prosthetic Joint Infection (LPJI) Hospital Admission.
Dotted lines show the linear trend for the case-crossover study control period (months 4-15 before LPJI admission, dashed lines of the same color). The control period trend lines have been extended through the case period (months 1-3) to allow comparison between the control period trend and any changes in the incidence of dental procedures in the case period. IDP indicates invasive dental procedure.
Table 2. Case-Crossover Analysis of LPJI Admissions With Linked Dental Data Comparing the Incidence of Dental Procedures in the 3-Month Case Period and the Preceding 12-Month Control Period.
| Procedure | LPJI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (N = 9427) | Hip (n = 2385) | Knee (n = 3168) | Other/unknown (n = 3874) | |||||||||||||
| Procedure, period | IRR (95% CI) | P value | Procedure, period | IRR (95% CI) | P value | Procedure, period | IRR (95% CI) | P value | Procedure, period | IRR (95% CI) | P value | |||||
| Case | Control | In Case | Control | Case | Control | Case | Control | |||||||||
| Type of dental procedure | ||||||||||||||||
| Invasive | 568 | 627 | 0.89 (0.82-0.96) | .002 | 153 | 156 | 0.91 (0.80-1.04) | .18 | 197 | 214 | 0.90 (0.80-1.01) | .07 | 219 | 258 | 0.81 (0.69-0.96) | .01 |
| Intermediate | 129 | 147 | 0.74 (0.59-0.96) | .01 | 29 | 38 | 0.82 (0.64-1.04) | .11 | 43 | 49 | 0.90 (0.73-1.11) | .33 | 56 | 60 | 0.96 (0.80-1.14) | .62 |
| Noninvasive | 585 | 572 | 1.06 (0.98-1.14) | .14 | 147 | 144 | 1.07 (0.92-1.24) | .40 | 198 | 195 | 1.13 (1.01-1.26) | .03 | 240 | 234 | 0.99 (0.85-1.14) | .86 |
| Type of invasive dental procedure | ||||||||||||||||
| Scaling | 468 | 512 | 0.88 (0.79-1.01) | .07 | 132 | 129 | 1.01 (0.91-1.12) | .82 | 160 | 169 | 0.97 (0.89-1.06) | .52 | 177 | 214 | 0.85 (0.78-0.92) | <.001 |
| Extractions | 115 | 131 | 0.84 (0.69-1.04) | .11 | 29 | 31 | 0.93 (0.72-1.20) | .60 | 42 | 50 | 0.86 (0.71-1.05) | .15 | 44 | 50 | 0.91 (0.75-1.10) | .32 |
| Endodontic | 23 | 21 | 1.15 (0.86-1.52) | .34 | 4 | 4 | 1.11 (0.56-2.17) | .77 | 8 | 8 | 0.95 (0.59-1.53) | .83 | 11 | 9 | 1.35 (0.90-2.05) | .15 |
Abbreviations: IRR, incidence rate ratio; LPJI, late prosthetic joint infection.
Site of Joint Replacement and LPJI
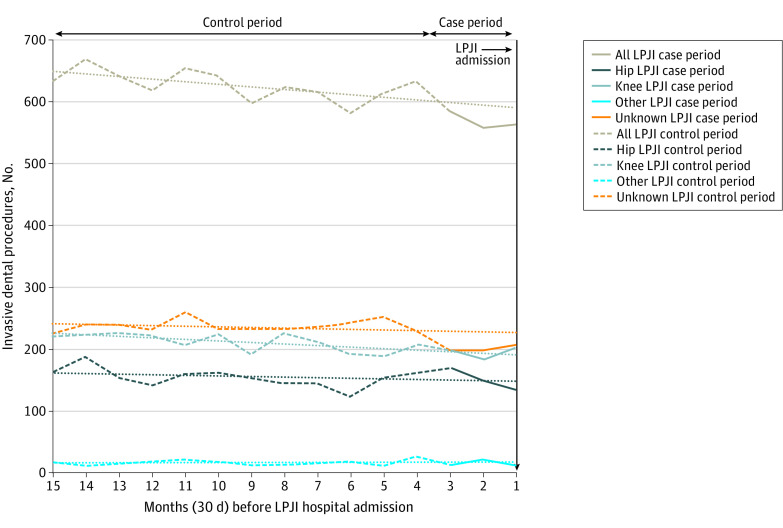
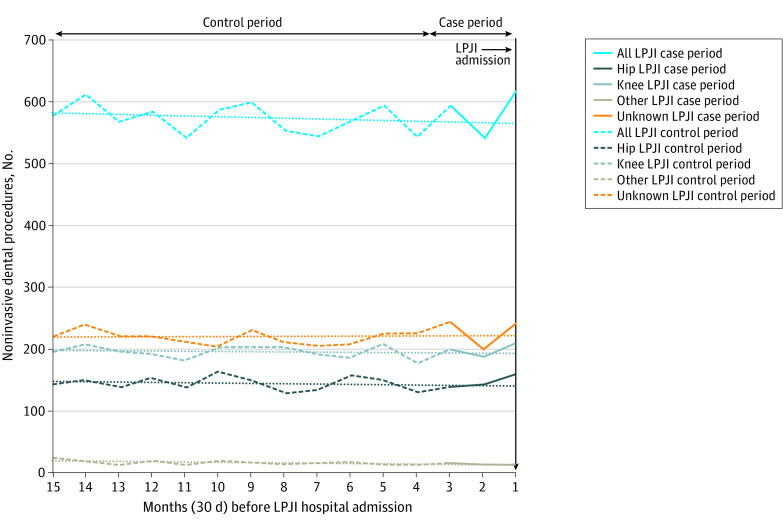
Data were available on the site of the LPJI for 5812 patients (61.6%) with dental records (Table 1). Most LPJIs were in knee replacements (3168 patients [33.6%]), followed by hip replacements (2385 patients [25.3%]) and other joint replacements (259 patients [2.8%]). The site of joint replacement was not associated with IDP and LPJI, with no significant increase in IDP in the 3 months before LPJI admission, and pairwise differences between sites were not statistically significant (Table 2, Figure 2, and Figure 3; eFigures 2-5 in the Supplement).
Figure 2. Monthly Incidence of Invasive Dental Procedures During 15 Months Before Admission to Hospital With Late Prosthetic Joint Infection (LPJI) .
Dotted lines show the linear trend for the case-crossover study control period (months 4-15 before LPJI admission, dashed lines of the same color). The control period trend lines have been extended through the case period (months 1-3, solid lines of the same color) to allow comparison between the control period trend and any changes in the incidence of dental procedures in the case period.
Figure 3. Monthly Incidence of Noninvasive Dental Procedures During 15 Months Before Admission to Hospital With Late Prosthetic Joint Infection (LPJI).
Dotted lines show the linear trend for the case-crossover study control period (months 4-15 before LPJI admission, dashed lines of the same color). The control period trend lines have been extended through the case-period (months 1-3, solid lines of the same color) to allow comparison between the control period trend and any changes in the incidence of dental procedures in the case period.
Discussion
In this cohort study of 9427 patients with dental records who developed LPJI, time trend and case-crossover analyses found no evidence of a temporal association between IDP and LPJI. In the 1970s and 1980s, the use of antibiotic prophylaxis to prevent infective endocarditis in individuals at high risk of LPJI undergoing IDP became well established and led US orthopedic surgeons to call for dentists to give antibiotic prophylaxis to patients with prosthetic joints.17,18,19,49 In 1988, the American Dental Association (ADA) sponsored a workshop to address this. Although there was limited evidence to support the use of antibiotic prophylaxis, they recommended its use until further evidence became available,50,51 and antibiotic prophylaxis was widely adopted by dentists.52 In 199753 and 200354 the ADA and American Academy of Orthopaedic Surgeons (AAOS) jointly published advisory statements recommending antibiotic prophylaxis for just 2 years after joint replacement but lifelong for patients with medical conditions that might put them at increased LPJI risk. However, in 2009, the AAOS unilaterally recommended that clinicians consider antibiotic prophylaxis for all patients with joint replacements prior to any invasive procedure that may cause bacteremia.55 This caused confusion among dentists and their patients.11 Several subsequent attempts were made by AAOS and ADA, either together or alone, to produce guidance,56,57,58 but these efforts only increased uncertainty about whether to provide antibiotic prophylaxis or not.59,60,61 As a result, in 2014, the ADA’s Council on Scientific Affairs assembled an expert panel to conduct a systematic review.61 They recommended: “In general, for patients with prosthetic joint implants, prophylactic antibiotics are not recommended prior to dental procedures.” Unfortunately, this advice lacked AAOS support. Consequently, there is still confusion among dentists and their patients, as well as ongoing pressure from orthopedic surgeons for their patients to receive antibiotic prophylaxis when undergoing IDP, and many dentists continue to give antibiotic prophylaxis for fear of being considered negligent.
There is little microbiological data to support a causal link between IDP and LPJI, and there has never been a randomized clinical trial of antibiotic prophylaxis to determine its safety and effectiveness in this context, to our knowledge. Unlike infective endocarditis, in which approximately 45% of infections are caused by oral streptococci, previous estimates suggest that oral streptococci are involved in less than 10% of LPJIs.4,22,23,24,25,26,27,28 Our study identified oral streptococci as a possible cause in approximately 9% of LPJIs. Nonetheless, we identified no increase in IDP prior to LPJI; if anything, there was a decrease. This suggests that those few LPJIs caused by oral streptococci were more likely a result of daily oral activities, such as toothbrushing, flossing, and mastication, particularly in patients with poor oral hygiene, rather than from IDP.62
For antibiotic prophylaxis to be effective, a positive causal link must exist between IDP and LPJI, and data on this are lacking.25 Only 5 studies have evaluated a potential association. In 1977, Waldman et al63 performed a retrospective case review of 62 patients who experienced late periprosthetic knee joint infection and identified 7 patients (11%) whose infections were temporally associated with IDP. In a related study, LaPorte et al24 temporally associated 3 of 52 (6%) late periprosthetic hip joint infections with IDP. However, neither study included a control group, making it impossible to draw any conclusions about the association between IDP and LPJI. In contrast, a case-control study by Kaandorp et al37 found that among 37 patients with LPJI, none had undergone an IDP in the previous 3 months, but 10% of control patients had. In a similar study of 42 Medicare patients with LPJI by Skaar et al,40 only 4 patients (9.5%) had undergone an IDP in the previous 3 months, compared to 15.9% of control patients. However, differences were not statistically significant in either study. In the largest study, by Berbari et al,64 48% of 303 patients with PJI had undergone an IDP in the previous 2 years compared with 34% of 318 control patients, but a high proportion had received antibiotic prophylaxis. A subanalysis of patients who had not had antibiotic prophylaxis found 33 patients with PJI (11%) had an IDP in the previous 2 years, compared with 49 control patients (14%). None of the differences were statistically significant, and each study was hindered by small sample sizes and lack of statistical power. The case-control studies also suffered from selection bias and risk factor confounding between cases and controls. Furthermore, there was confounding due to the widespread use of antibiotic prophylaxis in the populations studied, and recall bias for dental procedure data was a problem in some studies.
This study is 30-fold larger than any previous study, to our knowledge, involving 9427 LPJI episodes for which dental records were available, and power calculations show the study had 90% power to detect a 9% difference in dental procedure incidence between case and control periods, which would be more than sufficient to identify any clinically significant association between IDP and LPJI. Furthermore, the confounding caused by antibiotic prophylaxis use in previously investigated populations was avoided by using the English population, where the use of antibiotic prophylaxis to prevent LPJI has never been advocated.28 Recall bias was eliminated by using NHS records of all events and their timing, and a major advantage of the case-crossover design is the avoidance of selection bias. This study design also implicitly accounts for many potential confounders (eg, differences in oral hygiene, comorbidities), since each individual serves as their own control.43,44
Limitations
This study has several limitations. The T84.5 ICD-10 code identifies PJI but does not identify the joint infected or distinguish between early and late PJI. To determine this, we searched each patient’s record for earlier joint replacement admissions to exclude early PJI (within 3 months of joint replacement). OPCS-4 joint replacement codes allowed us to identify the type of joint replaced, and this was used to subdivide episodes. However, because we could only access records from January 2000, if joint replacement occurred before that, we did not know the joint type replaced and had to record it as unknown.
We used supplementary ICD-10 diagnosis codes to identify the LPJI causal organism. However, there are no ICD-10 codes for oral viridans group streptococci, and we could only estimate their involvement by excluding other streptococcal species for which codes exist and assuming that any other or unspecified streptococci were oral. Therefore, it is likely that the 9% of LPJI assigned an oral streptococcal cause is an overestimate.
Conclusions
The findings of this cohort study suggest that, in the absence of any increase in IDP prior to LPJI, there is no evidence to support a positive association between IDP and LPJI or the practice of administering antibiotic prophylaxis to patients with prosthetic joints undergoing IDP. The continuing use of antibiotic prophylaxis represents a large and unnecessary financial burden on individuals and the health care system as well as an unnecessary risk to patients, from adverse drug reactions, and society, owing to the potential development of antibiotic resistant bacteria, and should cease. However, our data suggest that maintenance of good oral hygiene may be important in preventing the small number of LPJIs in which oral bacteria are implicated. In addition, our findings should provide reassurance to orthopedic surgeons, dentists, and their patients in countries where antibiotic prophylaxis use in patients with prosthetic joints is not currently recommended.
eTable 1. OPCS-4 Procedure Codes for Joint Replacements
eTable 2. Supplementary ICD-10 Codes to Identify Whether a Causal Organism Was Recorded and the Nature of That Causal Organism
eFigure 1. Sensitivity Analysis Using 3-Month, 4-Month and 5-Month Exposure Windows to IDP Before LPJI Admission (Case-Periods) and Different Control Periods in the Case-Crossover Analyses
eFigure 2. Monthly Incidence of Different Dental Procedures Before Hip LPJI Admissions
eFigure 3. Monthly Incidence of Different Dental Procedures Before Knee LPJI Admissions
eFigure 4. Monthly Incidence of Different Dental Procedures Before Other LPJI Admissions
eFigure 5. Monthly Incidence of Different Dental Procedures Before Unknown LPJI Admissions
References
- 1.Colonna PC. An arthroplastic operation for congenital dislocation of the hip. Surg Gynecol Obstet. 1936;63:777-781. [Google Scholar]
- 2.Orthoworld . Orthopaedic Industry Annual Report—Focus on Joint Replacement. Orthoworld; 2012. [Google Scholar]
- 3.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-1654. doi: 10.1056/NEJMra040181 [DOI] [PubMed] [Google Scholar]
- 4.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302-345. doi: 10.1128/CMR.00111-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengtson S. Prosthetic osteomyelitis with special reference to the knee: risks, treatment and costs. Ann Med. 1993;25(6):523-529. doi: 10.1080/07853890.1993.12088578 [DOI] [PubMed] [Google Scholar]
- 6.Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res. 2010;96(2):124-132. doi: 10.1016/j.otsr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Peel TN, Cheng AC, Lorenzo YP, Kong DC, Buising KL, Choong PF. Factors influencing the cost of prosthetic joint infection treatment. J Hosp Infect. 2013;85(3):213-219. doi: 10.1016/j.jhin.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 8.Sculco TP. The economic impact of infected joint arthroplasty. Orthopedics. 1995;18(9):871-873. [PubMed] [Google Scholar]
- 9.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi: 10.2106/00004623-200704000-00012 [DOI] [PubMed] [Google Scholar]
- 10.Cahill JL, Shadbolt B, Scarvell JM, Smith PN. Quality of life after infection in total joint replacement. J Orthop Surg (Hong Kong). 2008;16(1):58-65. doi: 10.1177/230949900801600115 [DOI] [PubMed] [Google Scholar]
- 11.Little JW, Jacobson JJ, Lockhart PB; American Academy of Oral Medicine . The dental treatment of patients with joint replacements: a position paper from the American Academy of Oral Medicine. J Am Dent Assoc. 2010;141(6):667-671. doi: 10.14219/jada.archive.2010.0255 [DOI] [PubMed] [Google Scholar]
- 12.Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8)(suppl):61-5.e1. doi: 10.1016/j.arth.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 14.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24(6)(suppl):105-109. doi: 10.1016/j.arth.2009.04.027 [DOI] [PubMed] [Google Scholar]
- 15.Lew DP, Pittet D, Waldvogel FA. Infections that complicate the insertion of prosthetic devices. In: Mayhall CG, ed. Hospital Epidemiology and Infection Control. Third ed. Lippincott Williams & Wilkins; 2004:1181-1205. [Google Scholar]
- 16.Uçkay I, Pittet D, Bernard L, Lew D, Perrier A, Peter R. Antibiotic prophylaxis before invasive dental procedures in patients with arthroplasties of the hip and knee. J Bone Joint Surg Br. 2008;90(7):833-838. doi: 10.1302/0301-620X.90B7.20359 [DOI] [PubMed] [Google Scholar]
- 17.Ainscow DA, Denham RA. The risk of haematogenous infection in total joint replacements. J Bone Joint Surg Br. 1984;66(4):580-582. doi: 10.1302/0301-620X.66B4.6430907 [DOI] [PubMed] [Google Scholar]
- 18.Lattimer GL, Keblish PA, Dickson TB Jr, Vernick CG, Finnegan WJ. Hematogenous infection in total joint replacement. Recommendations for prophylactic antibiotics. JAMA. 1979;242(20):2213-2214. doi: 10.1001/jama.1979.03300200043023 [DOI] [PubMed] [Google Scholar]
- 19.Norden CW. Prevention of bone and joint infections. Am J Med. 1985;78(6B):229-232. doi: 10.1016/0002-9343(85)90390-0 [DOI] [PubMed] [Google Scholar]
- 20.Howell RM, Green JG. Prophylactic antibiotic coverage in dentistry: a survey of need for prosthetic joints. Gen Dent. 1985;33(4):320-323. [PubMed] [Google Scholar]
- 21.Jaspers MT, Little JW. Prophylactic antibiotic coverage in patients with total arthroplasty: current practice. J Am Dent Assoc. 1985;111(6):943-948. doi: 10.14219/jada.archive.1985.0224 [DOI] [PubMed] [Google Scholar]
- 22.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721-5732. doi: 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahrani-Mougeot FK, Paster BJ, Coleman S, Ashar J, Barbuto S, Lockhart PB. Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol. 2008;46(6):2129-2132. doi: 10.1128/JCM.02004-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaPorte DM, Waldman BJ, Mont MA, Hungerford DS. Infections associated with dental procedures in total hip arthroplasty. J Bone Joint Surg Br. 1999;81(1):56-59. doi: 10.1302/0301-620X.81B1.0810056 [DOI] [PubMed] [Google Scholar]
- 25.Lockhart PB, Loven B, Brennan MT, Fox PC. The evidence base for the efficacy of antibiotic prophylaxis in dental practice. J Am Dent Assoc. 2007;138(4):458-474. doi: 10.14219/jada.archive.2007.0198 [DOI] [PubMed] [Google Scholar]
- 26.Napeñas JJ, Kujan O, Arduino PG, et al. World Workshop on Oral Medicine VI: controversies regarding dental management of medically complex patients: assessment of current recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(2):207-226. doi: 10.1016/j.oooo.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 27.Trampuz A, Zimmerli W. Antimicrobial agents in orthopaedic surgery: prophylaxis and treatment. Drugs. 2006;66(8):1089-1105. doi: 10.2165/00003495-200666080-00005 [DOI] [PubMed] [Google Scholar]
- 28.Wahl MJ. Myths of dental-induced prosthetic joint infections. Clin Infect Dis. 1995;20(5):1420-1425. doi: 10.1093/clinids/20.5.1420 [DOI] [PubMed] [Google Scholar]
- 29.Thornhill MH, Dayer MJ, Durkin MJ, Lockhart PB, Baddour LM. Risk of adverse reactions to oral antibiotics prescribed by dentists. J Dent Res. 2019;98(10):1081-1087. doi: 10.1177/0022034519863645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother. 2015;70(8):2382-2388. doi: 10.1093/jac/dkv115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Dental Association Council on Scientific Affairs . Combating antibiotic resistance. J Am Dent Assoc. 2004;135(4):484-487. doi: 10.14219/jada.archive.2004.0214 [DOI] [PubMed] [Google Scholar]
- 32.Sweeney LC, Dave J, Chambers PA, Heritage J. Antibiotic resistance in general dental practice—a cause for concern? J Antimicrob Chemother. 2004;53(4):567-576. doi: 10.1093/jac/dkh137 [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization . International Statistical Classification of Diseases, Tenth Revision (ICD-10). World Health Organization; 1992. [Google Scholar]
- 34.Habib G, Lancellotti P, Antunes MJ, et al. ; ESC Scientific Document Group . 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)—endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi: 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 35.Wilson W, Taubert KA, Gewitz M, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group . Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754. doi: 10.1161/CIRCULATIONAHA.106.183095 [DOI] [PubMed] [Google Scholar]
- 36.Chen PC, Tung YC, Wu PW, et al. Dental procedures and the risk of infective endocarditis. Medicine (Baltimore). 2015;94(43):e1826. doi: 10.1097/MD.0000000000001826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaandorp CJ, Van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease: a prospective study. Arthritis Rheum. 1995;38(12):1819-1825. doi: 10.1002/art.1780381215 [DOI] [PubMed] [Google Scholar]
- 38.Lacassin F, Hoen B, Leport C, et al. Procedures associated with infective endocarditis in adults: a case control study. Eur Heart J. 1995;16(12):1968-1974. doi: 10.1093/oxfordjournals.eurheartj.a060855 [DOI] [PubMed] [Google Scholar]
- 39.Porat Ben-Amy D, Littner M, Siegman-Igra Y. Are dental procedures an important risk factor for infective endocarditis: a case-crossover study. Eur J Clin Microbiol Infect Dis. 2009;28(3):269-273. doi: 10.1007/s10096-008-0622-3 [DOI] [PubMed] [Google Scholar]
- 40.Skaar DD, O’Connor H, Hodges JS, Michalowicz BS. Dental procedures and subsequent prosthetic joint infections: findings from the Medicare Current Beneficiary Survey. J Am Dent Assoc. 2011;142(12):1343-1351. doi: 10.14219/jada.archive.2011.0134 [DOI] [PubMed] [Google Scholar]
- 41.Starkebaum M, Durack D, Beeson P. The “incubation period” of subacute bacterial endocarditis. Yale J Biol Med. 1977;50(1):49-58. [PMC free article] [PubMed] [Google Scholar]
- 42.Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis: a population-based, case-control study. Ann Intern Med. 1998;129(10):761-769. doi: 10.7326/0003-4819-129-10-199811150-00002 [DOI] [PubMed] [Google Scholar]
- 43.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144-153. doi: 10.1093/oxfordjournals.aje.a115853 [DOI] [PubMed] [Google Scholar]
- 44.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193-221. doi: 10.1146/annurev.publhealth.21.1.193 [DOI] [PubMed] [Google Scholar]
- 45.Smeeth L, Donnan PT, Cook DG. The use of primary care databases: case-control and case-only designs. Fam Pract. 2006;23(5):597-604. doi: 10.1093/fampra/cml025 [DOI] [PubMed] [Google Scholar]
- 46.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. Am J Epidemiol. 1995;142(1):91-98. doi: 10.1093/oxfordjournals.aje.a117550 [DOI] [PubMed] [Google Scholar]
- 47.Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case series studies. Stat Med. 2006;25(15):2618-2631. doi: 10.1002/sim.2477 [DOI] [PubMed] [Google Scholar]
- 48.HyLown Consulting . Test relative incidence in self controlled case series studies: SCCS, alt-2. Accessed December 6, 2021. http://powerandsamplesize.com/Calculators/Test-Relative-Incidence-in-Self-Controlled-Case-Series-Studies/SCCS-Alt-2
- 49.Pollard JP, Hughes SP, Scott JE, Evans MJ, Benson MK. Antibiotic prophylaxis in total hip replacement. Br Med J. 1979;1(6165):707-709. doi: 10.1136/bmj.1.6165.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Council on Dental Therapeutics . Management of dental patients with prosthetic joints. J Am Dent Assoc. 1990;121(4):537-538. doi: 10.14219/jada.archive.1990.0185 [DOI] [PubMed] [Google Scholar]
- 51.Nelson JP, Fitzgerald RH Jr, Jaspers MT, Little JW. Prophylactic antimicrobial coverage in arthroplasty patients. J Bone Joint Surg Am. 1990;72(1):1. doi: 10.2106/00004623-199072010-00001 [DOI] [PubMed] [Google Scholar]
- 52.Shrout MK, Scarbrough F, Powell BJ. Dental care and the prosthetic joint patient: a survey of orthopedic surgeons and general dentists. J Am Dent Assoc. 1994;125(4):429-436. doi: 10.14219/jada.archive.1994.0047 [DOI] [PubMed] [Google Scholar]
- 53.Whall CW Jr. Advisory statement: antibiotic prophylaxis for dental patients with total joint replacements:. American Dental Association; American Academy of Orthopaedic Surgeons. J Am Dent Assoc. 1997;128(7):1004-1008. doi: 10.14219/jada.archive.1997.0307 [DOI] [PubMed] [Google Scholar]
- 54.American Dental Association; American Academy of Orthopedic Surgeons . Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003;134(7):895-899. doi: 10.14219/jada.archive.2003.0289 [DOI] [PubMed] [Google Scholar]
- 55.American Academy of Orthopaedic Surgeons . Information statement: antibiotic prophylaxis for bacteremia in patients with joint replacements. Accessed April 28, 2021. http://pacosm.com/wp/wp-content/uploads/2015/08/Antibiotic-Prophylaxis-for-TJA-pts.-AAOS-March-2009.pdf [PubMed]
- 56.Watters W III, Rethman MP, Hanson NB, et al. ; American Academy of Orthopedic Surgeons; American Dental Association . Prevention of orthopaedic implant infection in patients undergoing dental procedures. J Am Acad Orthop Surg. 2013;21(3):180-189. doi: 10.5435/JAAOS-21-03-180 [DOI] [PubMed] [Google Scholar]
- 57.Rethman MP, Watters W III, Abt E, et al. ; American Academy of Orthopaedic Surgeons; American Dental Association . The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747. doi: 10.2106/00004623-201304170-00011 [DOI] [PubMed] [Google Scholar]
- 58.Watters W III, Rethman MP, Hanson NB, et al. ; American Academy of Orthopedic Surgeons; American Dental Association . Prevention of orthopaedic implant infection in patients undergoing dental procedures. J Am Acad Orthop Surg. 2013;21(3):180-189. doi: 10.5435/JAAOS-21-03-180 [DOI] [PubMed] [Google Scholar]
- 59.Lockhart PB. Antibiotic prophylaxis guidelines for prosthetic joints: much ado about nothing? Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):1-3. doi: 10.1016/j.oooo.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 60.Lockhart PB, Garvin KL, Osmon DR, et al. The antibiotic prophylaxis guideline for prosthetic joints: trying to do the right thing. J Am Acad Orthop Surg. 2013;21(3):193-194. [DOI] [PubMed] [Google Scholar]
- 61.Sollecito TP, Abt E, Lockhart PB, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: evidence-based clinical practice guideline for dental practitioners—a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2015;146(1):11-16.e8. doi: 10.1016/j.adaj.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 62.Lockhart PB, Brennan MT, Thornhill M, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140(10):1238-1244. doi: 10.14219/jada.archive.2009.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waldman BJ, Mont MA, Hungerford DS. Total knee arthroplasty infections associated with dental procedures. Clin Orthop Relat Res. 1997;(343):164-172. doi: 10.1097/00003086-199710000-00027 [DOI] [PubMed] [Google Scholar]
- 64.Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis. 2010;50(1):8-16. doi: 10.1086/648676 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. OPCS-4 Procedure Codes for Joint Replacements
eTable 2. Supplementary ICD-10 Codes to Identify Whether a Causal Organism Was Recorded and the Nature of That Causal Organism
eFigure 1. Sensitivity Analysis Using 3-Month, 4-Month and 5-Month Exposure Windows to IDP Before LPJI Admission (Case-Periods) and Different Control Periods in the Case-Crossover Analyses
eFigure 2. Monthly Incidence of Different Dental Procedures Before Hip LPJI Admissions
eFigure 3. Monthly Incidence of Different Dental Procedures Before Knee LPJI Admissions
eFigure 4. Monthly Incidence of Different Dental Procedures Before Other LPJI Admissions
eFigure 5. Monthly Incidence of Different Dental Procedures Before Unknown LPJI Admissions