Key Points
Question
Is N-terminal pro–brain natriuretic peptide (NT-proBNP) concentration in early pregnancy associated with development of hypertensive disorders of pregnancy and future hypertension?
Findings
In this cohort study including 4103 women, higher NT-proBNP concentrations in early pregnancy were associated with a significantly lower risk of hypertensive disorders of pregnancy and lower risk of incident hypertension 2 to 7 years after delivery.
Meaning
These findings suggest that normal early-pregnancy cardiovascular physiology, as assessed by NT-proBNP concentration, may be an important determinant of both pregnancy outcome and cardiovascular risk.
This cohort study investigates whether higher concentrations of N-terminal pro–brain natriuretic peptide (NT-proBNP) in early pregnancy are associated with hypertensive disorders of pregnancy and hypertension 2 to 7 years post partum.
Abstract
Importance
Hypertensive disorders of pregnancy are associated with future cardiovascular disease, perhaps because of subclinical cardiac dysfunction before pregnancy leading to impaired adaptation to pregnancy. Natriuretic peptides are promising biomarkers for detecting subclinical cardiac dysfunction outside of pregnancy.
Objective
To investigate whether higher concentrations of N-terminal pro–brain natriuretic peptide (NT-proBNP) in early pregnancy would be associated with hypertensive disorders of pregnancy and hypertension 2 to 7 years post partum.
Design, Setting, and Participants
This cohort study used data from the The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be Heart Health Study, a prospective multicenter observational study. A total of 4103 nulliparous women with complete data and no prepregnancy hypertension or diabetes who were treated at 8 clinical sites were included. Women were followed up with for 2 to 7 years after pregnancy. Data were collected from October 2010 to October 2017, and data were analyzed from August 2020 to November 2021.
Exposures
NT-proBNP concentration, measured using an electrochemiluminescence immunoassay from a first-trimester blood sample.
Main Outcomes and Measures
Hypertensive disorders of pregnancy and incident hypertension (systolic blood pressure of 130 mm Hg or diastolic blood pressure of 80 mm Hg or use of antihypertensive agents) at follow-up visit.
Results
A total of 4103 women met inclusion criteria; the mean (SD) age was 27.0 (5.6) years. Among these women, 909 (22.2%) had an adverse pregnancy outcome, and 817 (19.9%) had hypertension at the follow-up visit. Higher NT-proBNP concentrations were associated with a lower risk of hypertensive disorders of pregnancy (adjusted odds ratio per doubling, 0.81; 95% CI, 0.73-0.91), which persisted after adjustment for age, self-reported race and ethnicity, early-pregnancy body mass index, smoking, and aspirin use. Similarly, higher NT-proBNP concentration in early pregnancy was also associated with a lower risk of incident hypertension 2 to 7 years after delivery (adjusted odds ratio per doubling, 0.84; 95% CI, 0.77-0.93), an association that persisted after controlling for confounders, including hypertensive disorders of pregnancy.
Conclusions and Relevance
In this cohort study, higher NT-proBNP concentrations in early pregnancy were associated with a lower risk of hypertensive disorders of pregnancy and hypertension 2 to 7 years post partum. These findings suggest that normal early-pregnancy cardiovascular physiology, as assessed by NT-proBNP concentration, may provide biologic insights into both pregnancy outcome and cardiovascular disease risk.
Introduction
Adverse pregnancy outcomes (APOs), including hypertensive disorders of pregnancy (HDP), preterm birth, and small for gestational age (SGA), are associated with future maternal cardiovascular disease (CVD).1,2,3 In recently published findings from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b) Heart Health Study (HHS),4 31.5% of women with an APO had incident hypertension at 2 to 7 years post partum by the American College of Cardiology/American Heart Association criteria compared with 17.4% of women without an APO.5,6 The mechanisms linking APOs with future hypertension and CVD are unclear. One hypothesized mechanism is the presence of underlying subclinical cardiac dysfunction and impaired adaptation to pregnancy.7 In normal pregnancy, early hemodynamic adaptation involves a reduction in peripheral vascular resistance, an increase in plasma volume, and a concomitant rise in cardiac output. Impairments in these adaptations have been linked to both future CVD and APOs.8,9,10
The natriuretic peptides, including brain natriuretic peptide (BNP) and N-terminal pro–brain natriuretic peptide (NT-proBNP), are promising biomarkers for detecting subclinical cardiac dysfunction. BNP and NT-proBNP have established roles in the diagnosis, prognosis, and risk stratification of heart failure outside of pregnancy.11 Secreted predominantly by left ventricular cardiac myocytes in response to increased wall tension, BNP increases diuresis and decreases vascular tone. The byproduct of BNP production is the biologically inactive NT-proBNP, which has a longer half-life and increased sensitivity to detect left ventricular dysfunction.12 Sex steroid hormones may play a relevant physiological role in the regulation of production and secretion of cardiac natriuretic peptides, and female steroid hormones, in particular estrogens, have a stimulating effect on the cardiac natriuretic hormone system.13 Prior studies in pregnancy have focused mainly on assessment of NT-proBNP concentration at the time of diagnosis of HDP, immediate postpartum recovery, and association with echocardiography findings.14,15 NT-proBNP concentrations are elevated at the time of diagnosis in women with preeclampsia compared with those without the condition.14 These levels remain elevated 3 to 6 months post partum and are highly correlated with diastolic dysfunction on echocardiography.15 However, NT-proBNP has not been well studied early in gestation to predict APOs or future incident hypertension.
We sought to assess the association of NT-pro-BNP with pregnancy outcome and future cardiovascular risk through 2 objectives. We compared NT-proBNP concentrations in low-risk nulliparous women in early pregnancy based on (1) whether their pregnancies were uncomplicated or whether they developed HDP and other APOs and (2) whether women did or did not develop chronic hypertension years after pregnancy. We hypothesized that NT-proBNP concentrations in early pregnancy would be higher among women who developed HDP and other APOs and also among women who developed chronic hypertension at follow-up.
Methods
The nuMoM2b15 and subsequent nuMoM2b HHS16 are prospective cohort studies that enrolled nulliparous women with singleton pregnancies from 8 clinical centers in the US (Case Western Reserve University, Cleveland, Ohio; Columbia University, New York, New York; Indiana University, Indianapolis; University of Pittsburgh, Pittsburgh, Pennsylvania; Northwestern University, Chicago, Illinois; University of California at Irvine; University of Pennsylvania, Philadelphia; and University of Utah, Salt Lake City). Women were eligible if they had a viable singleton gestation, had no previous pregnancy that lasted more than 20 weeks of gestation, and were between 6 and less than 14 weeks of gestation at enrollment (first study visit). Participants provided a nonfasting blood sample in the first trimester (less than 14 weeks’ gestation), attended study visits at 3 time points prior to delivery, provided medical records access, and agreed to postpartum follow-up. All local institutional review boards approved the study protocol, and participants provided written informed consent prior to enrollment. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Following delivery, interviews were performed via telephone or email at 6-month intervals beginning at least 6 months after delivery to update health status and to schedule an in-person cardiovascular assessment visit at least 2 years after delivery. Women were eligible to participate in an in-person cardiovascular assessment if they participated in the nuMoM2b study, had obstetrical delivery information from the index pregnancy, agreed to be contacted for future studies, did not subsequently withdraw consent during an interval contact, were 18 years or older, were at least 2 years from the delivery of their index pregnancy, and were not currently pregnant.16,17 Race and ethnicity data were collected by self-report and included Asian, non-Hispanic Black, non-Hispanic White, Hispanic, and other (American Indian or Alaskan Native, Native Hawaiian, multiracial, and other). A total of 4508 women attended the nuMoM2b HHS follow-up visit during an interval of 2 to 7 years after their delivery that occurred during the nuMoM2b study. For the current analysis, women who experienced pregnancy loss before 20 weeks or pregnancy termination (n = 24) were abstraction-validated as having a prepregnancy diagnosis of chronic hypertension (n = 131) or prepregnancy diabetes (n = 43), or had missing data for early-pregnancy NT-proBNP concentration (n = 114) or early-pregnancy blood pressure (n = 93) were excluded.
Early-pregnancy blood specimens were taken via standard venipuncture. Serum, plasma, and whole blood specimens were stored at −80 °C in Fisher BioServices, Rockville, Maryland. NT-proBNP serum assays were completed during later analyses at the nuMoM2b HHS core laboratory (Lundquist Institute, Torrance, California) using the Roche Elecsys proBNP II electrochemiluminescence immunoassay (Roche Diagnostics). APOs during the index pregnancy were noted at delivery and verified by medical record abstraction and adjudication. An APO was defined as any of the following: HDP (gestational hypertension, preeclampsia, or eclampsia), spontaneous preterm birth, and SGA. Definitions for preeclampsia in the nuMoM2b study have been previously published.18 HDP included gestational hypertension, preeclampsia with and without severe features, and eclampsia. Spontaneous preterm birth was defined as a delivery between 20 weeks 0 days and 36 weeks 6 days secondary to preterm labor or preterm premature rupture of membranes. SGA was calculated using the Alexander growth curves from birth weight and gestational age at birth and defined as less than the fifth percentile. Blood pressure during early pregnancy was measured using manual sphygmomanometer, with a second measurement taken if the first measure indicated systolic blood pressure (SBP) of 140 mm Hg or higher or a diastolic blood pressure (DBP) of 90 mm Hg or higher; the second measurement was used for analysis when available, and otherwise, the first measurement was used. Hypertension status at the follow-up visit 2 to 7 years post partum was ascertained in triplicate via direct measurement of SBP and DBP following a standardized research protocol using the same device at all sites. Women were categorized as having hypertension at the follow-up visit according to the 2017 American College of Cardiology/American Heart Association blood pressure guidelines if they had an SBP of 130 mm Hg or higher or DBP of 80 mm Hg or higher or if they self-reported antihypertensive medication use. We also evaluated outcomes of elevated blood pressure (SBP of 120 to 129 mm Hg and DBP less than 80 mm Hg) and stage 1 hypertension (SBP of 130 to 139 mm Hg or DBP of 80 to 89 mm Hg) and stage 2 hypertension (SBP of 140 mm Hg or higher or DBP of 90 mm Hg or higher) in secondary analyses.19
Statistical analysis was conducted using SAS version 9.4 (SAS Institute). Continuous variables were summarized using means and SDs or medians and IQRs, as appropriate. Categorical variables were summarized using counts and percentages. NT-proBNP concentrations had a nonnormal distribution; therefore, they were included in statistical models after a logarithm transformation (base, 2). Odds ratios (ORs) for log-transformed quantities are interpreted on the multiplicative scale. We evaluated the association of early-pregnancy NT-proBNP concentration and the presence of HDP or any APO (in our first objective) and hypertension (in our second objective) using logistic regression with adjustment for covariates. Covariates were chosen a priori based on prior studies and included age, self-reported race and ethnicity, early-pregnancy body mass index (BMI), early-pregnancy blood pressure, smoking, and aspirin use in pregnancy. We performed several additional sensitivity analyses. We evaluated the association of NT-proBNP concentration in early pregnancy with severity of HDP. Given the association of NT-proBNP with race and ethnicity and with BMI in the nonpregnant population, we also evaluated whether our findings varied by maternal BMI or self-reported race or ethnicity.20 We repeated our analysis comparing the upper vs lower quartile of NT-proBNP concentration. Finally, we evaluated the association of elevated NT-proBNP concentration with APO and hypertension. There are limited normative data in pregnancy to establish a threshold for elevated NT-proBNP; thus, we used normative values for nonpregnant adults.21 Elevated NT-proBNP concentration was defined as a concentration greater than 125 pg/mL (nonpregnant adult threshold; to convert to nanograms per liter, multiply by 1) and greater than 200 pg/mL in sensitivity analyses (recently published pregnancy reference interval).22 Results are presented as adjusted ORs with corresponding 95% CIs. We used t tests for normally distributed continuous variables, Wilcoxon tests for medians, and χ2 tests for categorical variables. All P values were 2-sided, and P values less than .05 were considered statistically significant.
Results
Association of NT-proBNP Concentration With Pregnancy Outcome
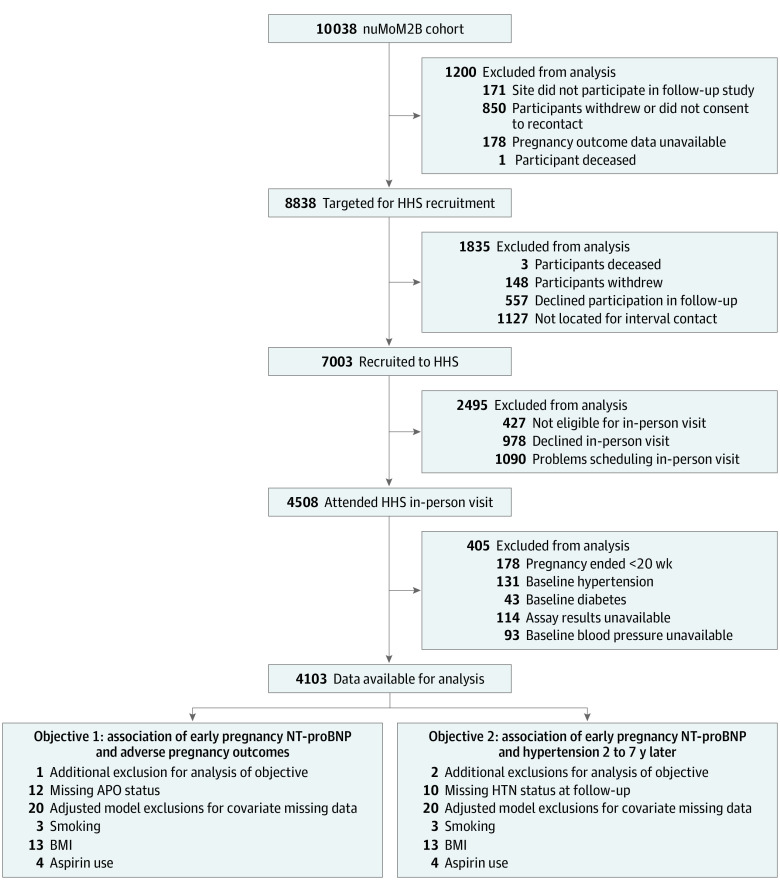
A total of 4103 women (mean [SD] age was 27.0 [5.6] years) had NT-proBNP results from blood specimens taken in early pregnancy, attended a nuMoM2b HHS follow-up visit, met our inclusion criteria for this study, and had no history of prepregnancy hypertension or diabetes per medical record abstraction (Figure). The median (IQR) NT-proBNP level was 62.0 (40.0-94.0) pg/mL. Among the women included in this analysis, 909 of 4103 (22.2%) had an APO during their index pregnancy. Women who experienced an APO were more likely to self-report being non-Hispanic Black and to have higher early-pregnancy BMIs compared with women without an APO. Additionally, they had higher blood pressures in early pregnancy, were more likely to develop gestational diabetes, had earlier gestational ages at delivery, and had lower infant birth weights (Table 1).
Figure. Study Enrollment.
APO indicates adverse pregnancy outcomes; BMI, body mass index; HHS, Heart Health Study; HTN, hypertension; NT-proBNP, N-terminal pro–brain natriuretic peptide; nuMoM2B, Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be.
Table 1. Demographic and Index Pregnancy Characteristics by Presence of Adverse Pregnancy Outcome (APO).
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| No APO (n = 3182) | Any APO (n = 909) | ||
| Age, mean (SD), y | 27.0 (5.5) | 26.8 (5.8) | .34 |
| Self-reported race and ethnicity | |||
| Asian | 104 (3.3) | 19 (2.1) | <.001 |
| Black | 368 (11.6) | 150 (16.5) | |
| White | 2053 (64.5) | 560 (61.6) | |
| Hispanic | 519 (16.3) | 129 (14.2) | |
| Othera | 138 (4.3) | 51 (5.6) | |
| Education level | |||
| <High school graduate | 201 (6.3) | 87 (9.6) | <.001 |
| High school graduate or GED completed | 337 (10.6) | 118 (13.0) | |
| Some college credit, no degree | 604 (19.0) | 187 (20.6) | |
| Associate’s/technical degree | 343 (10.8) | 116 (12.8) | |
| Bachelor’s degree | 952 (29.9) | 216 (23.8) | |
| Degree beyond Bachelor’s | 745 (23.4) | 185 (20.4) | |
| Type of health insurance | |||
| Commercial | 2268 (71.5) | 613 (68.0) | .09 |
| Government/military | 794 (25.0) | 258 (28.6) | |
| Self-pay/other | 108 (3.4) | 30 (3.3) | |
| Early pregnancy | |||
| BMI, median (IQR)b | 24.2 (21.8-28.2) | 26.3 (22.8-31.7) | <.001 |
| Waist circumference, mean (SD), cm | 93.9 (13.4) | 98.6 (15.5) | <.001 |
| Family history of hypertension, No./total No. (%) | 1726/3038 (56.8) | 557/874 (63.7) | <.001 |
| Blood pressure, mean (SD), mm Hg | |||
| Systolic | 108.2 (10.2) | 111.7 (11.1) | <.001 |
| Diastolic | 66.6 (7.9) | 69.0 (8.7) | <.001 |
| Gestational diabetes, No./total No. (%) | 111/3182 (3.5) | 56/909 (6.2) | <.001 |
| Current smoker, No./total No. (%) | 151/3165 (4.8) | 69/905 (7.6) | <.001 |
| Early-pregnancy aspirin use, No./total No. (%) | 51/3178 (1.6) | 20/909 (2.2) | .23 |
| Gestational age at delivery, median (IQR), wk | 39.9 (39.0-40.6) | 38.3 (36.1-39.7) | <.001 |
| Birth weight, mean (SD), g | 3401.2 (405.0) | 2844.9 (756.3) | <.001 |
Abbreviations: BMI, body mass index; GED, general educational development.
Other race included American Indian or Alaskan Native, Native Hawaiian, multiracial, and other.
Calculated as weight in kilograms divided by height in meters squared.
Women who went on to have an APO had lower NT-proBNP concentrations in early pregnancy than those who did not develop an APO (median [IQR], 56.0 [35.0-89.0] pg/mL vs 63.0 [41.0-95.0] pg/mL; P < .001). Higher NT-proBNP concentrations were associated with a lower risk of HDP (adjusted OR per doubling, 0.81; 95% CI, 0.73-0.91) after adjustment for age, self-reported race and ethnicity, early-pregnancy BMI, smoking, and aspirin use. Additionally, this association persisted after controlling for early-pregnancy blood pressure (SBP, DBP, and mean arterial pressure, each in separate models) (Table 2). We then sought to evaluate whether there was an association with the severity of HDP. The presence of a more severe phenotype of HDP (preeclampsia with severe features, preterm preeclampsia) was associated with lower NT-proBNP concentrations, with women with preterm preeclampsia having the lowest concentration of NT-proBNP. Higher NT-proBNP concentration was associated with a lower risk of preterm preeclampsia (adjusted OR per doubling, 0.75; 95% CI, 0.57-0.99), after controlling for confounders and early-pregnancy SBP (Table 3). There was no association of NT-proBNP concentration with risk of spontaneous preterm birth or SGA. These results were similar when comparing the upper vs lower quartile of NT-proBNP (eTable 7 in Supplement 1).
Table 2. Adjusted Association of Baseline N-Terminal Pro–Brain Natriuretic Peptide (NT-proBNP) and Blood Pressure With Adverse Pregnancy Outcome (APO)a,b.
| Measure | Adjusted OR (95% CI) | ||||
|---|---|---|---|---|---|
| No APO (n = 3185) | Any APO (n = 909) | HDP (n = 544) | Spontaneous preterm birth (n = 199) | SGA (n = 170) | |
| NT-proBNP, median (IQR), pg/mL | 63.0 (41.0-95.0) | 56.0 (35.0-89.0) | 52.0 (34.0-83.0) | 61.0 (33.0-104.0) | 61.0 (39.0-95.0) |
| NT-proBNP, per doubling in value | 1 [Reference] | 0.90 (0.82-0.98)c | 0.81 (0.73-0.91)c | 1.00 (0.85-1.18) | 1.04 (0.87-1.25) |
| NT-proBNP, per doubling in value, controlling for systolic blood pressure | 1 [Reference] | 0.91 (0.84-1.00)c | 0.84 (0.75-0.94)c | 1.00 (0.84-1.18) | 1.05 (0.88-1.26) |
Abbreviations: HDP, hypertensive disorders of pregnancy; OR, odds ratio; SGA, small for gestational age.
Adjusted for age, race and ethnicity, body mass index, smoking, and aspirin use.
This characteristic is included in statistical models after a logarithm transformation (base, 2). ORs for log-transformed quantities are interpreted on the multiplicative scale.
P < .05.
Table 3. Adjusted Association of Baseline N-Terminal Pro–Brain Natriuretic Peptide (NT-proBNP) and Blood Pressure With Severity of Hypertensive Disorders of Pregnancya,b.
| Measure | Adjusted OR (95% CI) | ||||
|---|---|---|---|---|---|
| No APO (n = 3182) | GH (n = 292) | Preeclampsia | |||
| Term (≥37 wk; n = 182) | Preterm (<37 wk; n = 70) | With severe features (n = 136) | |||
| NT-proBNP, median (IQR), pg/mL | 63.0 (41.0-95.0) | 54.0 (36.0-89.0) | 50.0 (33.0-76.0) | 42.0 (29.0-72.0) | 49.0 (31.5-74.0) |
| NT-proBNP, per doubling in value | 1 [Reference] | 0.86 (0.74-0.99)c | 0.78 (0.66-0.93)c | 0.71 (0.54-0.93)c | 0.77 (0.63-0.93)c |
| NT-proBNP, per doubling in value, controlling for systolic blood pressure | 1 [Reference] | 0.89 (0.77-1.03) | 0.80 (0.67-0.95)c | 0.75 (0.57-0.99)c | 0.80 (0.65-0.98)c |
Abbreviations: APO, adverse pregnancy outcome; GH, antepartum gestational hypertension; OR, odds ratio.
Adjusted for age, race and ethnicity, body mass index, smoking, and aspirin use.
This characteristic is included in statistical models after a logarithm transformation (base, 2). ORs for log-transformed quantities are interpreted on the multiplicative scale.
P < .05.
Association of NT-proBNP With Hypertension
Among the 4103 women included in our analysis, 817 (19.9%) met criteria for stage 1 hypertension or greater, and 49 (1.2%) self-reported the use of antihypertensive medication at the nuMoM2b HHS follow-up visit 2 to 7 years post partum. Women who had hypertension at follow-up were older at baseline, more likely to self-report being non-Hispanic Black, and had higher early-pregnancy BMIs compared with women who did not have hypertension at the follow-up visit (eTable 1 in Supplement 1). Women with incident hypertension 2 to 7 years after delivery had lower NT-proBNP concentrations in early pregnancy compared with women without hypertension (median [IQR], 54.0 [35.0-83.0] pg/mL vs 64.0 [41.0-96.0] pg/mL; P < .001). Higher NT-proBNP concentration in early pregnancy was associated with lower odds of incident hypertension 2 to 7 years after delivery (adjusted OR per doubling, 0.84; 95% CI, 0.77-0.93) after controlling for age, self-reported race and ethnicity, BMI, smoking, aspirin use, and early-pregnancy SBP (Table 4). Our findings were unchanged when controlling for the presence of an APO in the index pregnancy (adjusted OR per doubling, 0.85; 95% CI, 0.78-0.94) after adjustment for the presence of HDP. These results were similar when comparing the upper vs lower quartile of NT-proBNP (eTable 8 in Supplement 1). In additional sensitivity analyses, there was no evidence that the association between early-pregnancy NT-proBNP concentration and later hypertension varied according to BMI or maternal self-reported race and ethnicity (eTables 3 and 4 in Supplement 1).
Table 4. Adjusted Association of Baseline N-Terminal Pro–Brain Natriuretic Peptide (NT-proBNP) and Blood Pressure With Development of Hypertension at 2 to 7 Years After Deliverya,b.
| Measure | Hypertension, adjusted OR (95% CI) | |
|---|---|---|
| No (n = 3276) | Yes (n = 817) | |
| NT-proBNP, median (IQR), pg/mL | 64.0 (41.0-96.0) | 54.0 (35.0-83.0) |
| NT-proBNP, per doubling in value | 1 [Reference] | 0.82 (0.74-0.89)c |
| NT-proBNP, per doubling in value, controlling for systolic blood pressure | 1 [Reference] | 0.84 (0.77-0.93)c |
Abbreviation: OR, odds ratio.
Adjusted for age, race and ethnicity, body mass index, smoking, and aspirin use.
This characteristic is included in statistical models after a logarithm transformation (base, 2). ORs for log-transformed quantities are interpreted on the multiplicative scale.
P < .05.
In secondary analyses, we evaluated whether early-pregnancy NT-proBNP concentrations differed by hypertension stage at follow-up. Women with stage 2 hypertension had the lowest concentrations of NT-proBNP in early pregnancy, women with stage 1 hypertension had intermediate concentrations, and women without hypertension at follow-up had the highest concentrations of NT-proBNP in early pregnancy (median [IQR] concentration: stage 2, 52.0 [34.0-88.0] pg/mL; stage 1, 55.0 [35.0-82.0] pg/mL; no hypertension, 64.0 [41.0-96.0] pg/mL; Jonckheere-Terpstra P < .001).
Finally, we specifically investigated the association of elevated NT-proBNP concentration (greater than 125 pg/mL) with APOs and future hypertension. There were 479 women (11.7%) with an elevated NT-proBNP concentration. In early pregnancy in this group, the mean (SD) SBP was 107 (9) mm Hg, and the mean (SD) DBP was 66 (8) mm Hg. The overall frequency of APO occurrence within this subset of women was similar compared with women with a normal NT-proBNP concentration (96 of 479 [20.1%] vs 813 of 3624 [22.5%]; P = .23); however, the incidence of hypertension at follow-up was lower (69 of 479 [14.4%] vs 748 of 3624 [20.7%]; P = .001) (eTables 2, 5, and 6 in Supplement 1). Recently published work has identified trimester-specific reference intervals for NT-proBNP concentrations, with a cutoff greater than 200 pg/mL identified as abnormal in the first trimester. Thus, in a sensitivity analysis, we repeated our analysis using a cutoff of 200 pg/mL, and our findings were unchanged.
Discussion
In this cohort study, contrary to our hypothesis, we demonstrated that higher NT-proBNP concentrations in early pregnancy were associated with a lower risk of HDP. This association was present after controlling for early-pregnancy blood pressure and traditional risk factors. We found no association between NT-proBNP concentrations and risk of spontaneous preterm birth or SGA. Second, we demonstrated that women with higher NT-proBNP concentrations in early pregnancy had a lower risk of hypertension at 2 to 7 years post partum, even after controlling for APOs and confounders. Notably, an NT-proBNP concentration higher than the nonpregnant normal threshold (greater than 125 pg/mL) was associated with a significantly lower risk of future hypertension. We initially hypothesized that subclinical cardiac dysfunction as assessed by higher concentrations of NT-proBNP early in pregnancy would be linked to HDP and later hypertension. In contrast, our results suggest that higher levels of NT-proBNP early in pregnancy may represent a physiologically appropriate cardiovascular response to the first trimester volume expansion that is essential for healthy pregnancy. Marked maternal cardiovascular changes are a key component to the complex adaptive response to normal pregnancy to ensure adequate uterine blood flow to the developing fetoplacental unit, including reduced total vascular resistance to accommodate the greater intravascular volume.23 These results suggest the hypothesis that lower concentrations of NT-proBNP early in gestation may reflect impaired adaptation to pregnancy or impaired prepregnancy cardiovascular function, representing more vascular stiffness and a less robust volume expansion, which might portend risk of HDP and future hypertension. This is in contrast to the elevation in NT-proBNP concentration seen at the time of diagnosis of an HDP, which likely reflects the pathophysiology of the disease state.14
The association of early pregnancy hemodynamic adaptation and risk of APOs has been demonstrated in prior studies. Bioreactance cardiography to assess maternal hemodynamics longitudinally across pregnancy shows that women with impaired cardiovascular adaptation to pregnancy, specifically the failure to decrease vascular resistance and increase compliance, are more likely to develop APOs, particularly HDP and SGA.9,10 These studies note that women who later develop these APOs have decreased cardiac output and consistently increased peripheral vascular resistance in early pregnancy, suggesting a primary, persistent impairment of maternal cardiovascular adaptation to pregnancy. Likewise, other studies have noted increased arterial stiffness prior to pregnancy in women who subsequently develop preeclampsia.24 Pathologic evidence of this impaired adaptive response to pregnancy can be seen in the placentas of women with APOs in the form of maternal vascular malperfusion lesions. Women with maternal vascular malperfusion lesions have an abnormal blunting in early-pregnancy blood pressure, also suggesting an impaired early vascular response to pregnancy.25
The association of early-pregnancy cardiovascular physiologic adaptation and future CVD risk has not been as well characterized. One recent study demonstrated that higher early-pregnancy blood pressure is related to more adverse cardiovascular outcomes 6 years post partum, including pulse wave velocity, left ventricular mass, development of chronic hypertension, reduced coronary flow reserve, and myocardial scarring.8,26,27,28 Similarly, women with maternal vascular lesions of the placenta, potentially representing a maladaptive response to pregnancy, are at increased risk of hypertension 8 to 10 years post partum, independent of pregnancy outcome.29,30
Prior studies on NT-proBNP concentration in pregnancy have focused mainly on assessment at the time of diagnosis of HDP and immediate postpartum recovery.14,15 NT-proBNP concentrations are elevated at the time of diagnosis of preeclampsia,14 remain elevated 3 to 6 months post partum, and are highly correlated with diastolic dysfunction on echocardiography.15 Prior work has noted that pregnancy itself is associated with an increase in NT-proBNP concentration in the first trimester compared with a nonpregnant state (median [IQR] concentration, 56 [33-95] pg/mL vs 38 [22-62]; P < .001).31 Few prior studies have investigated the role of NT-proBNP as a biomarker early in gestation to suggest which women may develop APOs. Similar to our findings, a recent study from Pihl et al32 found a decreased concentration of NT-proBNP among women who developed term preeclampsia within a smaller cohort. The longer-term cardiovascular risk among women with relatively low concentrations of NT-proBNP in early pregnancy has not been well characterized. Our findings suggest the hypothesis that early-pregnancy NT-proBNP concentration is a novel biomarker that might inform future work exploring mechanisms underlying development of APOs and future hypertension. Longer-term studies are needed to evaluate the risk of CVD events in women with low NT-proBNP concentrations in early pregnancy. Further, our study used only early-pregnancy blood samples, and future studies would be strengthened with the inclusion of prepregnancy samples to allow for evaluation of longitudinal changes across early pregnancy.
It is increasingly recognized that there may be deficiency states of both atrial natriuretic peptide (ANP) and BNP, which may be driven to some extent by obesity or race.33,34 Other potential explanations for our findings may be related to alterations in natriuretic signaling pathways. BNP signals through the ANP receptor to elevate cyclic guanosine monophosphate and relax smooth muscle by decreasing peripheral vascular resistance.11,12 We did not assess concentrations of other enzymes or components of this pathway, which may be helpful in understanding the underlying mechanisms driving our findings, and future studies in pregnancy should assess BNP, ANP, and cyclic guanosine monophosphate. For example, neprilysin degrades natriuretic peptides, and a relative overexpression of this in early pregnancy could lead to decreased concentrations of NT-proBNP.35 Perhaps our findings of lower NT-proBNP concentrations in early pregnancy may lead to long-term epigenetic changes post partum, resulting in a higher risk of hypertension long term. In animal models, a lack of BNP leads to a relative cardiac fibrosis.36 In human studies, single-nucleotide variants have been identified that are associated with higher plasma concentrations of ANP, lower blood pressures, and reduced risk of hypertension.37 One specific BNP gene variant, the minor C allele of the BNP genetic variant rs198389, is associated with higher levels of BNP and a lower risk of hypertension and major adverse cardiovascular events in individuals at risk of heart failure. In those without this protective genetic variant, circulating BNP levels are lower and the risk of major cardiovascular events is higher.38 These epigenetic changes may be at play in early pregnancy, such that a BNP deficiency state could be the primary driver of an increased risk of HDP and future hypertension, which should be a focus of future work.
Strengths and Limitations
Our analysis includes a large socioeconomically and geographically diverse cohort from multiple institutions. The prospective design with enrollment beginning in the first trimester of the first pregnancy is a clear strength. This study is also strengthened by the rigorous data collection with adjudication of pregnancy and cardiovascular health outcomes.
Our study has limitations. Our findings are subject to the limitations of observational cohort studies. Additionally, our cohort only included nulliparous women. Prior studies have demonstrated a favorable association of pregnancy with vascular remodeling, thus it is possible that our findings may not be replicated in multiparous women.39,40 Future studies should include both nulliparous and multiparous women, including women with a history of APOs. It is possible that the women who did not attend an in-person follow-up cardiovascular screening visit differed from the women who returned for follow-up. Based on similar demographic characteristics among women who did and did not have visits, this is unlikely.5 It is possible that NT-proBNP concentration is affected by other unmeasurable factors within our study that confound our results. For example, while we adjust for BMI, this measure may be of limited utility in pregnancy. Whether alterations in NT-proBNP concentration are seen prepregnancy or persist at the time of diagnosis of APOs or at 2 to 7 years post partum is unclear, and we plan to explore this question further in future studies. Additionally, while the differences in NT-proBNP concentration that we see between groups are statistically significant, the absolute values are small and of questionable clinical significance. Additionally, the findings in this study have not been externally validated. We feel that our findings may provide important mechanistic insight for future work with a more targeted clinical focus.
Conclusions
Overall, findings from this cohort study support the importance of early-pregnancy cardiovascular adaptation not only for healthy pregnancy outcomes but also as a marker of future cardiovascular health. Taken together, these findings suggest that early-pregnancy cardiovascular physiology, as assessed with NT-proBNP concentration, may be an important determinant of both pregnancy outcome as well as future CVD.
eTable 1. Demographic characteristics by presence of hypertension at follow-up.
eTable 2. Demographic characteristics by presence of elevated NT-proBNP (>125 pg/mL).
eTable 3. Association of NT-proBNP with development of hypertension at 2 to 7 years after delivery stratified by body mass index during index pregnancy.
eTable 4. Association of NT-proBNP with development of hypertension at 2 to 7 years after delivery stratified by race and ethnicity.
eTable 5. Adjusted association of baseline NT-proBNP and blood pressure with adverse pregnancy outcome, including hypertensive disorders of pregnancy, spontaneous preterm birth, and small for gestational age among women with elevated NT-proBNP (>125 pg/mL).
eTable 6. Adjusted association of baseline NT-proBNP and blood pressure with development of hypertension at 2 to 7 years after delivery among women with elevated NT-proBNP (>125 pg/mL).
eTable 7. Adjusted association of baseline NT-proBNP (upper vs lower quartile) and blood pressure with adverse pregnancy outcomes, including hypertensive disorders of pregnancy, spontaneous preterm birth, and small for gestational age.
eTable 8. Adjusted association of baseline NT-proBNP (upper vs lower quartile) and blood pressure with development of hypertension at 2 to 7 years after delivery.
NICHD nuMoM2b and NHLBI nuMoM2b Heart Health Study Networks.
References
- 1.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003497. doi: 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 2.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918-930. doi: 10.1016/j.ahj.2008.06.042 [DOI] [PubMed] [Google Scholar]
- 3.Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol. 2018;41(2):239-246. doi: 10.1002/clc.22887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.nuMoM2b Heart Health Study (nuMoM2b-HHS). ClinicalTrials.gov identifier: NCT02231398. Updated July 23, 2020. Accessed July 26, 2021. https://clinicaltrials.gov/ct2/show/NCT02231398
- 5.Haas DM, Parker CB, Marsh DJ, et al. ; NHLBI nuMoM2b Heart Health Study . Association of adverse pregnancy outcomes with hypertension 2 to 7 years postpartum. J Am Heart Assoc. 2019;8(19):e013092. doi: 10.1161/JAHA.119.013092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 7.Thilaganathan B. Maternal cardiac dysfunction precedes development of preeclampsia. Hypertension. 2020;76(2):321-322. doi: 10.1161/HYPERTENSIONAHA.120.15281 [DOI] [PubMed] [Google Scholar]
- 8.Bergen NE, Schalekamp-Timmermans S, Roos-Hesselink J, Roeters van Lennep JE, Jaddoe VVW, Steegers EAP. Hypertensive disorders of pregnancy and subsequent maternal cardiovascular health. Eur J Epidemiol. 2018;33(8):763-771. doi: 10.1007/s10654-018-0400-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stott D, Papastefanou I, Paraschiv D, Clark K, Kametas NA. Longitudinal maternal hemodynamics in pregnancies affected by fetal growth restriction. Ultrasound Obstet Gynecol. 2017;49(6):761-768. doi: 10.1002/uog.17340 [DOI] [PubMed] [Google Scholar]
- 10.Stott D, Nzelu O, Nicolaides KH, Kametas NA. Maternal hemodynamics in normal pregnancy and in pregnancy affected by pre-eclampsia. Ultrasound Obstet Gynecol. 2018;52(3):359-364. doi: 10.1002/uog.18835 [DOI] [PubMed] [Google Scholar]
- 11.Oremus M, McKelvie R, Don-Wauchope A, et al. A systematic review of BNP and NT-proBNP in the management of heart failure: overview and methods. Heart Fail Rev. 2014;19(4):413-419. doi: 10.1007/s10741-014-9440-0 [DOI] [PubMed] [Google Scholar]
- 12.Tsai S-H, Lin Y-Y, Chu S-J, Hsu C-W, Cheng S-M. Interpretation and use of natriuretic peptides in non-congestive heart failure settings. Yonsei Med J. 2010;51(2):151-163. doi: 10.3349/ymj.2010.51.2.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerico A, Masotti S, Musetti V, Passino C. Pathophysiological mechanisms determining sex differences in circulating levels of cardiac natriuretic peptides and cardiac troponins. J Lab Precis Med. 2019;4:8. doi: 10.21037/jlpm.2019.01.03 [DOI] [Google Scholar]
- 14.Conti-Ramsden F, Gill C, Seed PT, Bramham K, Chappell LC, McCarthy FP. Markers of maternal cardiac dysfunction in pre-eclampsia and superimposed pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2019;237:151-156. doi: 10.1016/j.ejogrb.2019.04.034 [DOI] [PubMed] [Google Scholar]
- 15.Rafik Hamad R, Larsson A, Pernow J, Bremme K, Eriksson MJ. Assessment of left ventricular structure and function in preeclampsia by echocardiography and cardiovascular biomarkers. J Hypertens. 2009;27(11):2257-2264. doi: 10.1097/HJH.0b013e3283300541 [DOI] [PubMed] [Google Scholar]
- 16.Haas DM, Parker CB, Wing DA, et al. ; NuMoM2b study . A description of the methods of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-To-Be (nuMoM2b). Am J Obstet Gynecol. 2015;212(4):539.e1-539.e24. doi: 10.1016/j.ajog.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas DM, Ehrenthal DB, Koch MA, et al. ; National Heart, Lung, and Blood Institute nuMoM2b Heart Health Study Network . Pregnancy as a window to future cardiovascular health: design and implementation of the nuMoM2b heart health study. Am J Epidemiol. 2016;183(6):519-530. doi: 10.1093/aje/kwv309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31-41. doi: 10.1097/AOG.0000000000001805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 20.Patton KK, Heckbert SR, Alonso A, et al. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99(24):1832-1836. doi: 10.1136/heartjnl-2013-304724 [DOI] [PubMed] [Google Scholar]
- 21.Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330-337. doi: 10.1093/eurheartj/ehi631 [DOI] [PubMed] [Google Scholar]
- 22.Dockree S, Brook J, Shine B, James T, Vatish M. Pregnancy-specific reference intervals for BNP and NT-pro BNP—changes in natriuretic peptides related to pregnancy. J Endocr Soc. 2021;5(7):bvab091. doi: 10.1210/JENDSO/BVAB091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor RN, Roberts JM, Cunningham FG, Lindheimer MD, eds. Chesley’s Hypertensive Disorders in Pregnancy. 4th ed. Elsevier; 2015. [Google Scholar]
- 24.Hale SA, Badger GJ, McBride C, Magness R, Bernstein IM. Prepregnancy vascular dysfunction in women who subsequently develop hypertension during pregnancy. Pregnancy Hypertens. 2013;3(2):140-145. doi: 10.1016/j.preghy.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atlass J, Menke M, Parks WT, Catov JM. Pre-conception blood pressure and evidence of placental malperfusion. BMC Pregnancy Childbirth. 2020;20(1):25. doi: 10.1186/s12884-019-2699-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catov JM, McNeil RB, Marsh DJ, et al. ; NHLBI nuMoM2b Heart Health Study . Early pregnancy atherogenic profile in a first pregnancy and hypertension risk 2 to 7 years after delivery. J Am Heart Assoc. 2021;10(5):e017216. doi: 10.1161/JAHA.120.017216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quesada O, Park K, Wei J, et al. Left ventricular mass and myocardial scarring in women with hypertensive disorders of pregnancy. Open Heart. 2020;7(2):1273. doi: 10.1136/openhrt-2020-001273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park K, Quesada O, Cook-Wiens G, et al. Adverse pregnancy outcomes are associated with reduced coronary flow reserve in women with signs and symptoms of ischemia without obstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation–Coronary Vascular Dysfunction Study. J Womens Health (Larchmt). 2020;29(4):487-492. doi: 10.1089/jwh.2019.7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catov JM, Assibey-Mensah V, Muldoon MF, Sun B, Parks WT. Maternal vascular lesions in the placenta may identify women susceptible to masked hypertension a decade after pregnancy. Poster presented at: 2019 Scientific Sessions of the American Heart Association Epidemiology and Prevention and Lifestyle and Cardiometabolic Health; March 5-8, 2019; Houston, TX. [Google Scholar]
- 30.Holzman CB, Senagore P, Xu J, et al. Maternal risk of hypertension 7-15 years after pregnancy: clues from the placenta. BJOG. 2021;128(5):827-836. doi: 10.1111/1471-0528.16498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franz MB, Andreas M, Schiessl B, et al. NT-proBNP is increased in healthy pregnancies compared to non-pregnant controls. Acta Obstet Gynecol Scand. 2009;88(2):234-237. doi: 10.1080/00016340802596025 [DOI] [PubMed] [Google Scholar]
- 32.Pihl K, Sørensen S, Stener Jørgensen F. Prediction of preeclampsia in nulliparous women according to first trimester maternal factors and serum markers. Fetal Diagn Ther. 2020;47(4):277-283. doi: 10.1159/000503229 [DOI] [PubMed] [Google Scholar]
- 33.Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial differences in natriuretic peptide levels: the Dallas Heart study. JACC Heart Fail. 2015;3(7):513-519. doi: 10.1016/j.jchf.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109(5):594-600. doi: 10.1161/01.CIR.0000112582.16683.EA [DOI] [PubMed] [Google Scholar]
- 35.Jaffe AS. Unwinding the interaction of natriuretic peptides and neprilysin. J Am Coll Cardiol. 2015;65(7):666-667. doi: 10.1016/j.jacc.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 36.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97(8):4239-4244. doi: 10.1073/pnas.070371497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41(3):348-353. doi: 10.1038/ng.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannone V, Ledwidge M, Watson C, McKie PM, Burnett JC Jr, McDonald K. STOP-HF trial: higher endogenous BNP and cardiovascular protection in subjects at risk for heart failure. JACC Basic Transl Sci. 2021;6(6):497-504. doi: 10.1016/j.jacbts.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clapp JF III, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80(11):1469-1473. doi: 10.1016/S0002-9149(97)00738-8 [DOI] [PubMed] [Google Scholar]
- 40.Morris EA, Hale SA, Badger GJ, Magness RR, Bernstein IM. Pregnancy induces persistent changes in vascular compliance in primiparous women. Am J Obstet Gynecol. 2015;212(5):633.e1-633.e6. doi: 10.1016/j.ajog.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Demographic characteristics by presence of hypertension at follow-up.
eTable 2. Demographic characteristics by presence of elevated NT-proBNP (>125 pg/mL).
eTable 3. Association of NT-proBNP with development of hypertension at 2 to 7 years after delivery stratified by body mass index during index pregnancy.
eTable 4. Association of NT-proBNP with development of hypertension at 2 to 7 years after delivery stratified by race and ethnicity.
eTable 5. Adjusted association of baseline NT-proBNP and blood pressure with adverse pregnancy outcome, including hypertensive disorders of pregnancy, spontaneous preterm birth, and small for gestational age among women with elevated NT-proBNP (>125 pg/mL).
eTable 6. Adjusted association of baseline NT-proBNP and blood pressure with development of hypertension at 2 to 7 years after delivery among women with elevated NT-proBNP (>125 pg/mL).
eTable 7. Adjusted association of baseline NT-proBNP (upper vs lower quartile) and blood pressure with adverse pregnancy outcomes, including hypertensive disorders of pregnancy, spontaneous preterm birth, and small for gestational age.
eTable 8. Adjusted association of baseline NT-proBNP (upper vs lower quartile) and blood pressure with development of hypertension at 2 to 7 years after delivery.
NICHD nuMoM2b and NHLBI nuMoM2b Heart Health Study Networks.