Abstract
Background:
FOLFIRI [irinotecan, folinic acid (CF), and fluorouracil] is considered a standard second-line chemotherapy regimen for patients with metastatic colorectal cancer (mCRC) who failed first-line XELOX/FOLFOX regimens. However, it remains unknown whether fluorouracil is still necessary in this case. This trial was designed to test the superiority of FOLFIRI over single-agent irinotecan as a second-line treatment for patients with mCRC.
Methods:
This randomized clinical trial was conducted in five hospitals in China. From 4 November 2016 to 17 January 2020, patients aged 18 years or older with histologically confirmed unresectable mCRC and who had failed first-line XELOX/FOLFOX regimens were screened and enrolled. Patients were randomized to receive either FOLFIRI or irinotecan. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate (ORR), and toxicity. Data were analyzed on an intention-to-treat basis.
Results:
A total of 172 patients with mCRC were randomly treated with FOLFIRI (n = 88) or irinotecan (n = 84). The median PFS was 104 and 112 days (3.5 and 3.7 months) in the FOLFIRI and irinotecan groups, respectively [hazard ratio (HR) = 1.084, 95% confidence interval (CI) = 0.7911–1.485; p = 0.6094], and there was also no significant difference in OS and ORR between the two groups. The incidence of the following adverse events (AEs) was significantly higher in the FOLFIRI group than in the irinotecan group: any grade AEs including leucopenia (73.9% versus 55.4%), neutropenia (72.7% versus 56.6%), thrombocytopenia (31.8% versus 18.1%), jaundice (18.2% versus 7.2%), mucositis (40.9% versus 14.5%), vomiting (37.5% versus 21.7%), and fever (19.3% versus 7.2%) and grade 3–4 neutropenia (47.7% versus 21.7%).
Conclusion:
This is the first head-to-head trial showing that single-agent irinotecan yielded PFS, OS, and ORR similar to FOLFIRI, with a more favorable toxicity profile; therefore, it might be a more favorable standard chemotherapy regimen for mCRC patients who failed first-line XELOX/FOLFOX regimens.
Trial registration:
This study is registered with ClinicalTrials.gov, number NCT02935764, registered 17 October 2016, https://clinicaltrials.gov/ct2/show/NCT02935764.
Keywords: chemotherapy, FOLFIRI, irinotecan, metastatic colorectal cancer
Background
Colorectal cancer (CRC) is one of the most common malignant tumors.1,2 According to recent data, CRC is the third most common cancer worldwide and the second leading cause of cancer death. In China, CRC is the second most common cancer (12.2%).3–5 Nearly one third of patients have distant metastasis at first diagnosis, and the most common treatment for metastatic colorectal cancer (mCRC) is systemic chemotherapy and molecular targeted drugs. 6
Fluorouracil (5-FU) and folinic acid (CF) were the most essential therapeutic regimens for patients with mCRC in the first-line setting before 2000. In the 21st century, combination chemotherapies, such as FOLFOX (CF, 5-FU, and oxaliplatin) and FOLFIRI (CF, 5-FU, and irinotecan), began to be utilized, and patients who received all three agents including 5-FU, oxaliplatin, and irinotecan survived longer.7,8 Currently, the utility of molecular targeted drugs has further improved the efficacy of chemotherapy for mCRC.9–12
The V308 study showed that FOLFOX and FOLFIRI regimens could be the first or second line of each other in the treatment of mCRC. Patients with mCRC were randomly assigned to two groups: group A received FOLFIRI as first-line therapy and FOLFOX as second-line treatment; group B received FOLFOX as first-line treatment and FOLFIRI as second-line treatment. The median overall survival (mOS) of groups A and B was 21.5 months versus 20.6 months (p = 0.99); the median progression-free survival (mPFS) of first-line chemotherapy was 8.5 months versus 8.0 months (p = 0.9), and the objective response rate (ORR) was 56% versus 54% (p = 0.26); the mPFS of second-line chemotherapy was 4.2 months versus 2.5 months (p = 0.003), and ORR was 15% versus 4% (p = 0.05) in groups A and B. The results indicated that the overall survival (OS) of patients with mCRC who received FOLFOX-FOLFIRI or FOLFIRI-FOLFOX was similar, and FOLFOX or FOLFIRI as the first-line treatment showed similar efficacy. However, the mPFS and ORR of FOLFOX were significantly higher than those of FOLFIRI in the second-line treatment. 13
Based on the results of similar OS of the two groups in the V308 trial, FOLFOX as first-line treatment followed by FOLFIRI as second-line treatment, or in reverse order, has been considered the standard chemotherapy regimen for unresectable mCRC. In this study, 5-FU was used in both the first- and second-line treatments. However, cancer cells may have developed resistance to 5-FU after first-line 5-FU-containing regimen failure. Therefore, we considered whether it is still necessary to continue to administer 5-FU as a second-line treatment.
In a randomized study, mCRC patients who had failed first-line treatment with irinotecan and intravenous injection of 5-FU/CF (IFL) regimen were randomly divided into three groups: 5-FU/CF, single-agent oxaliplatin, and FOLFOX groups. The results showed that ORR of three groups was 0%, 1.3%, and 9.9%, and mPFS was 2.7, 1.6, and 4.6 months (p < 0.001), respectively. 14 This suggested that after the first-line treatment failure with IFL, the efficacy of oxaliplatin in the second-line treatment of mCRC was very limited, and the combination of 5-FU significantly improved the efficacy. Therefore, oxaliplatin needs to be combined with 5-FU as a second-line treatment.
In light of the V308 study, FOLFIRI has been widely recognized as the standard second-line treatment for mCRC after first-line treatment with FOLFOX. Meanwhile, irinotecan monodrug is also a second-line choice recommended by the NCCN guideline after two large phase 3 trials compared irinotecan with either best supportive care (V301 Study) or an infusional 5-FU/CF regimen (V302 Study) as second-line chemotherapy after prior treatment with 5-FU-based regimens and both obtained positive results.15,16 The FOLFOX and oxaliplatin monodrug comparative study led us to ponder whether FOLFIRI is better than irinotecan monotherapy as a second-line chemotherapy; however, there is no head-to-head comparative study between FOLFIRI and irinotecan. Therefore, we performed this randomized clinical study to investigate whether FOLFIRI is superior to irinotecan as a second-line treatment for patients with mCRC who failed 5-FU-based regimens.
Methods
Study design and participants
This study is a randomized, open-label, multicenter, phase 3 clinical trial performed at five hospitals in China designed to demonstrate the superiority of FOLFIRI over single-agent irinotecan as a second-line treatment of mCRC patients. Eligible patients were screened and enrolled between 4 November 2016 and 17 January 2020. Eligible patients included aged 18 years or older with histologically confirmed and unresectable colorectal adenocarcinoma and who had withdrawn from the first-line oxaliplatin combined with 5-FU or its derivative chemotherapy (FOLFOX or XELOX) with or without molecular targeted drugs because of disease progression (during chemotherapy or within 3 months after the final dose of chemotherapy), intolerable toxicity, or relapse less than 6 months after the final dose of adjuvant FOLFOX or XELOX chemotherapy. To be eligible, patients also had to have a life expectancy of at least 90 days, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, at least one measurable disease lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) criteria, and adequate bone marrow, liver, and renal function, and patients voluntarily agreed to participate in this clinical trial. As the molecular targeted drugs bevacizumab and cetuximab are expensive and not included in the Chinese medical insurance in the past, we informed the patients and only enrolled patients whose conditions were not suitable for bevacizumab (contraindications include hemorrhage, obstruction, thrombus within 6 months, uncontrolled hypertension, proteinuria, and unhealed wound) or cetuximab (K-RAS/N-RAS or B-RAF mutation) or were allergic to monoclonal antibodies or unable to afford them economically. Patients were excluded if they had received any anticancer treatment within 14 days before randomization or if irinotecan was used as first-line chemotherapy. Other reasons for exclusion included brain metastases, known deficiency of dihydropyrimidine dehydrogenase, previous chronic inflammatory bowel disease, chronic diarrhea or recurrent bowel obstruction, active severe infection, important organ failure, or other serious diseases.
The protocol was approved by Fudan University Shanghai Cancer Center ethics review board (approval ID: 1608162-17-1612A), and the study was conducted according to the principles of Good Clinical Practice and the Declaration of Helsinki. All patients signed randomization and treatment informed consent form.
Randomization and masking
Eligible patients were randomly assigned (1:1) to either the FOLFIRI or irinotecan treatment group via an interactive web-response system, stratified by ECOG score (0–1 versus 2) and number of metastatic sites (1–2 versus > 2). As this was an open-label study, the patients, investigators, and study team were not masked to the allocated treatment.
Procedures
Patients in the FOLFIRI group received irinotecan 180 mg/m2, CF 400 mg/m2, and fluorouracil 400 mg/m2 intravenously on day 1, followed by a 46-h continuous infusion of fluorouracil 2400 mg/m2, repeated every 14 days. Patients in the irinotecan group received irinotecan 180mg/m2 every 14 days. We did not define the minimum or maximum number of treatment cycles. Treatment continued until disease progression, intolerable toxicity, or patient refusal. Concurrent anticancer treatments were prohibited. In total, 71 (80.7%) patients in FOLFIRI group and 72 (85.7%) patients in IRI group were tested for UGT1A1 polymorphisms (UGT1A1*28 and UGT1A1*6) at baseline. 17
We performed computed tomography or magnetic resonance imaging (for only two patients who were allergic to the iodine contrast medium) scans at baseline and every 6 weeks thereafter. Tumor response or progression was evaluated using the RECIST 1.1 criteria. In addition, investigators graded AE (adverse events) using the National Cancer Institute Common Terminology Criteria (NCI-CTCAE version 4.0) and also recorded laboratory parameters of peripheral blood counts and blood biochemistry on the first day of each cycle (or the day before) during the protocol treatment period. Toxicities can be managed by dose reduction or discontinuation of irinotecan or fluorouracil.
Outcomes
The primary endpoint was PFS, defined as the time from the date of randomization to the date of confirmed progression or death from any cause, whichever occurred first. The secondary endpoints included the following: OS (time from the date of randomization to the date of death from any cause), ORR (the proportion of eligible patients with measurable lesions at baseline with the best overall response of complete response or partial response), toxicity and safety, and biomarker analysis. Safety analyses included all patients who received at least one dose of the study therapy. Biomarker analysis (the correlation between UGT1A1 polymorphisms with effects and toxicity) will be reported elsewhere.
Statistical analysis
This study was designed to assess the superiority of FOLFIRI compared with irinotecan monotherapy in terms of PFS. Considering that the mPFS was 2.6 months for patients treated with irinotecan after failure of oxaliplatin combined with 5-FU in the EPIC study, 18 and the study design was similar to our trial, we selected it to be our assumption for the IRI group mPFS. The mPFS was 2.5–4.7 months for patients treated with FOLFIRI in different trials,13,19,20 and in reference to the results of the mPFS of patients who received FOLFOX which was 4.6 months, which was superior to that of patients who received single-agent oxaliplatin at 1.6 months in the second-line treatment study, 14 the mPFS was assumed to be 2.5 months for patients treated with irinotecan single agent and 4.0 months for patients treated with FOLFIRI, which had a clinically valuable absolute benefit of 1.5 months longer, with a corresponding hazard ratio (HR) of 0.625. Under these assumptions, a two-sided significance level of 0.05 with 80% power shows the superiority of FOLFIRI versus irinotecan, an estimated 148 progression events are needed to show superiority and an enrollment of at least 164 patients (82 in each group) is required, accounting for a 10% dropout rate.
Assessment of treatment efficacy was performed on an intent-to-treat basis. Survival curves were generated using the Kaplan–Meier method, and log-rank tests were used to compare PFS and OS. We used Cox proportional hazards models to calculate HRs with 95% CIs and Pinteraction values for predefined subgroup analyses. The predefined subgroups included age, sex, ECOG performance status, pathological type, primary tumor location, primary tumor resection, number of metastatic sites, liver metastases, liver-limited metastases, stage at diagnosis (synchronous versus metachronous metastasis), RAS status, B-RAF status, and UGT1A1 polymorphism. We performed multivariable analysis using the Cox proportional hazard model, which included the treatment regimen and the above 13 factors. In addition, we assessed between-group differences in the overall response and AEs using χ² tests.
All statistical analyses were performed using SPSS (version 26), R project (version 3.6), and Stata (version 16). This study was registered with ClinicalTrials.gov (NCT02935764).
Results
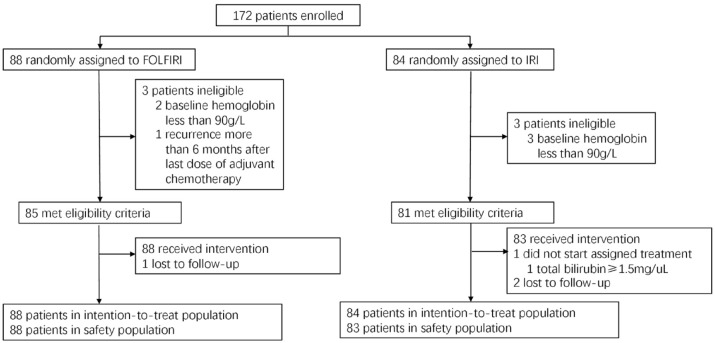
A total of 172 patients were enrolled and randomly assigned to receive either FOLFIRI (n = 88) or irinotecan (IRI, n = 84) as second-line therapy for mCRC. One patient in the irinotecan group did not receive any treatment (Figure 1). The baseline characteristics were well balanced between the two groups (Table 1).
Figure 1.
Patient flow diagram.
Table 1.
Baseline characteristics.
| No.(%) | ||
|---|---|---|
| FOLFIRI (n = 88) | IRI (n = 84) | |
| Age (years) | ||
| <65 | 59 (67) | 52 (62) |
| ⩾65 | 29 (33) | 32 (38) |
| Median (IQR) | 59 (50–66) | 61 (53–68) |
| Sex | ||
| Men | 52 (59) | 54 (64) |
| Women | 36 (41) | 30 (36) |
| ECOG performance status a | ||
| 0–1 | 86 (98) | 83 (99) |
| 2 | 2 (2) | 1 (1) |
| Stage at diagnosis | ||
| Synchronous metastasis | 56 (64) | 54 (64) |
| Metachronous metastasis | 32 (36) | 30 (36) |
| Primary tumor location | ||
| Right side | 27 (31) | 21 (25) |
| Left side | 61 (69) | 63 (75) |
| Primary tumor resection | ||
| Yes | 64 (73) | 54 (64) |
| No | 24 (27) | 30 (36) |
| Pathological type | ||
| Adenocarcinoma | 73 (83) | 73 (87) |
| Mucinous adenocarcinoma/signet ring | 15 (17) | 11 (13) |
| Number of metastatic sites | ||
| 1–2 | 50 (57) | 46 (55) |
| >2 | 38 (43) | 38 (45) |
| Liver metastases | ||
| Yes | 57 (65) | 56 (67) |
| No | 31 (35) | 28 (33) |
| Liver-limited metastases | ||
| Yes | 21 (24) | 20 (24) |
| No | 67 (76) | 64 (76) |
| UGT1A1 polymorphism | ||
| Wild | 37 (42) | 36 (43) |
| Single heterozygote | 26 (30) | 31 (37) |
| Double heterozygotes or homozygotes | 8 (9) | 5 (6) |
| Unknown | 17 (19) | 12 (14) |
| K-RAS and N-RAS status | ||
| Wild | 43 (49) | 32 (38) |
| Mutant | 36 (41) | 34 (41) |
| Unknown | 9 (10) | 18 (21) |
| BRAF status | ||
| Wild | 75 (85) | 63 (75) |
| Mutant | 4 (5) | 3 (4) |
| Unknown | 9 (10) | 18 (21) |
ECOG, Eastern Cooperative Oncology Group; FOLFIRI, folinic acid, fluorouracil, and irinotecan; IRI, irinotecan.
Data are n (%) or median (IQR).
Assessed according to ECOG guidelines.
The median treatment duration was 6 cycles (ranging 1–12 cycles) in the FOLFIRI group and 6 cycles (ranging 0–26 cycles) in the IRI group. Treatment was discontinued in 78 (88.6%) of 88 patients in the FOLFIRI group because of disease progression [58 (65.9%)], AE [9 (10.2%)], patient refusal [7 (8.0%)], and change to other therapy [4 (4.5%)] and 74 (89.2%) of 84 in the IRI group because of disease progression [50 (59.5%)], AE [4 (4.8%)], patient refusal [13 (15.9%)], change to other therapy [6 (7.1%)], and CR [1 (1.2%)]. In addition, the number of patients refused treatment after less than 3 times of treatment in two groups were actually similar (five in FOLFIRI group and seven in IRI group), and the exact reason of refusal was not clear. A delay in the treatment administration was necessary for 42 (47.7%) of 88 patients in the FOLFIRI group and 40 (47.6%) of 84 in the IRI group, and a dose reduction was needed for 17 (19.3%) patients in the FOLFIRI group and 14 (16.7%) in the IRI group, most of which (80%) occurred in patients with the UGT1A1 gene mutation. The relative dose intensity of irinotecan was 85.6% in the FOLFIRI group, and 88.7% in the IRI group.
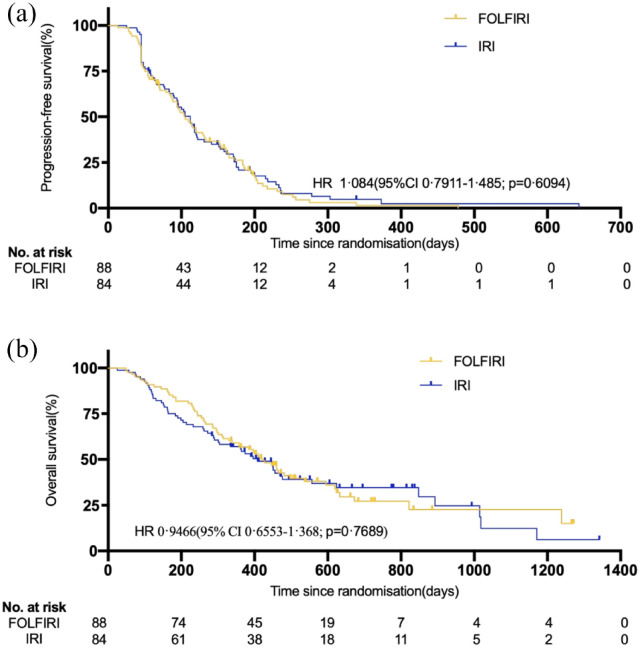
There was no significant difference in the mPFS between the two groups: 104 days (3.5 months; 95% CI = 85–123 days) in the FOLFIRI group and 112 days (3.7 months; 95% CI = 92–132 days) in the IRI group (HR = 1.084, 95% CI = 0.7911–1.485; p = 0.6094) (Figure 2(a)). At the cutoff date for the collection of survival data (14 January 2021), a total of 114 deaths were confirmed (59 in the FOLFIRI group and 55 in the IRI group). With a median follow-up of 388 days [12.9 months; interquartile range (IQR) = 221–521 days] for the entire study population, the mOS was 420 days (14 months; 95% CI = 349–491 days) in the FOLFIRI group and 408 days (13.6 months; 95% CI = 325–491 days) in the IRI group (HR = 0.9466, 95% CI = 0.6553–1.368; p = 0.7689) (Figure 2(b)). There were 83 progression or death events in the FOLFIRI group and 80 in the IRI group. Six of the 88 (6.8%, 6 partial remission (PR)) patients in the FOLFIRI group and 6 of 84 (7.1%, 1 complete remission (CR), and 5 PR) in the IRI group had an overall response to treatment (p = 0.933), which was not significantly different. Overall, all the patients in the FOLFIRI and IRI groups had at least one AE during the study (Table 2 listed AE with an incidence rate over 5%). Patients in the FOLFIRI group experienced significantly higher grade AEs, including leucopenia, neutropenia, thrombocytopenia, jaundice, mucositis, vomiting, and fever than those in the IRI group. Patients in the FOLFIRI group had a numerically higher incidence of grade 3–4 AEs than those in the IRI group [47 of 88 (53.4%) versus 24 of 83 (28.9%), p = 0.001]. This difference was mainly attributed to grade 3–4 neutropenia, which occurred more frequently in the FOLFIRI group than in the IRI group [42 of 88 (47.7%) versus 18 of 83 (21.7%), p < 0.001]. The severity of diarrhea was correlated with UGT1A1 (UGT1A1*6 and UGT1A1*28) polymorphisms in all patients (Spearman p = 0.016) (eFigure 1A). The severity of neutropenia was correlated with UGT1A1 polymorphisms in all patients (Spearman p = 0.073) (eFigure 1B), and the correlation was significant in the IRI group (Spearman p = 0.001) (eFigure 1 C), which may be due to the interference of neutropenia caused by 5-FU in the FOLFIRI group. One patient died 9 days after the last dose of treatment in the IRI group, which may be related to treatment; however, the cause of death was unknown. No other treatment-related deaths were observed.
Figure 2.
Kaplan–Meier survival estimates in the intention-to-treat population. (a) Progression-free survival and (b) overall survival in patients receiving FOLFIRI compared with patients receiving IRI.
Table 2.
Adverse events (safety population).
| Any grade | Grade ¾ | |||||
|---|---|---|---|---|---|---|
| IRI (N = 83) | FOLFIRI (N = 88) | p value | IRI (N = 83) | FOLFIRI (N = 88) | p value | |
| Hematological | ||||||
| Leucopenia | 46 (55) | 65 (74) | 0.012 | 14 (17) | 22 (25) | 0.192 |
| Neutropenia | 47 (57) | 64 (73) | 0.027 | 18 (22) | 42 (48) | 0.000 |
| Thrombocytopenia | 15 (18) | 28 (32) | 0.038 | 4 (5) | 2 (2) | 0.367 |
| Anemia | 26 (31) | 35 (40) | 0.249 | 0 (0) | 2 (2) | 0.173 |
| Non-hematological | ||||||
| ALP abnormality | 24 (29) | 33 (38) | 0.234 | 0 (0) | 0 (0) | |
| ALT abnormality | 7 (8) | 15 (17) | 0.093 | 0 (0) | 0 (0) | |
| AST abnormality | 8 (10) | 16 (18) | 0.108 | 0 (0) | 0 (0) | |
| Jaundice | 6 (7) | 16 (18) | 0.033 | 0 (0) | 0 (0) | |
| Diarrhea | 29 (35) | 39 (44) | 0.210 | 4 (5) | 5 (6) | 0.801 |
| Mucositis | 12 (14) | 36 (41) | 0.000 | 0 (0) | 0 (0) | |
| Anorexia | 31 (37) | 39 (44) | 0.354 | 1 (1) | 0 (0) | 0.303 |
| Nausea | 33 (40) | 42 (48) | 0.294 | 0 (0) | 0 (0) | |
| Vomiting | 18 (22) | 33 (38) | 0.024 | 1 (1) | 1 (1) | 0.967 |
| Alopecia | 46 (55) | 52 (59) | 0.628 | 0 (0) | 0 (0) | |
| Fatigue | 39 (47) | 49 (56) | 0.256 | 1 (1) | 0 (0) | 0.303 |
| Weight loss | 10 (12) | 12 (14) | 0.669 | 0 (0) | 0 (0) | |
| Peripheral sensory neuropathy | 8 (10) | 9 (10) | 0.898 | 0 (0) | 0 (0) | |
| Fever | 6 (7) | 17 (19) | 0.021 | 0 (0) | 0 (0) | |
| Febrile neutropenia | 3 (4) | 8 (9) | 0.145 | 3 (4) | 8 (9) | 0.145 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FOLFIRI, folinic acid, fluorouracil, and irinotecan; IRI, irinotecan.
Data are n (%).
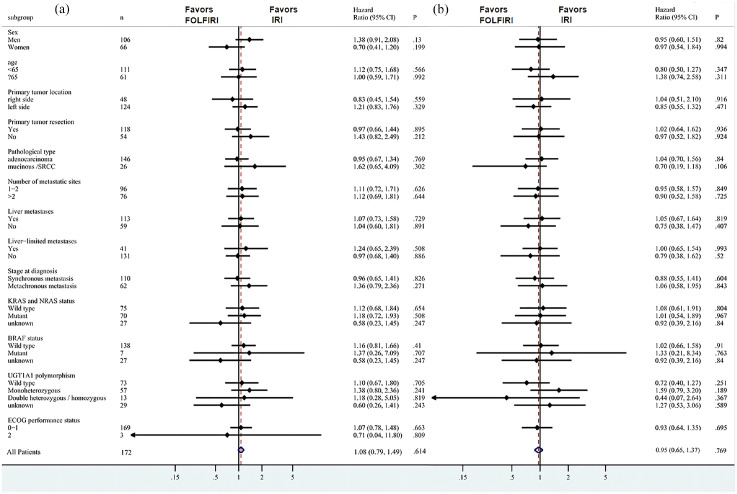
According to the exploratory subgroup analysis, FOLFIRI was not superior to IRI in all subgroups in terms of PFS (Figure 3(a)) and OS (Figure 3(b)). In addition, the results of the multivariable analysis using the Cox proportional hazard model, which included a second-line treatment regimen as a factor, revealed that with or without 5-FU, it was not a significant prognostic factor for PFS and OS (eTable1).
Figure 3.
Forest plots of exploratory subgroup analysis of (a) disease-free survival and (b) overall survival.
Discussion
The effect of the second-line FOLFOX was significantly better than that of single-agent oxaliplatin after the first-line 5-FU-containing regimen failure 14 ; however, there is no evidence that second-line FOLFIRI is superior to irinotecan alone. In the FOCUS study, which compared the effects of different strategies of sequential and combination chemotherapy for mCRC, patients were randomly assigned to three groups: group A received 5-FU single-agent until progression and then irinotecan single-agent as second-line treatment; group B received 5-FU single-agent until progression and was then divided into two subgroups that received different combined chemotherapy (irinotecan plus 5-FU or oxaliplatin plus 5-FU); group C received first-line combined chemotherapy. The results showed that in the second-line treatment, the ORR of single-agent irinotecan in group A and irinotecan combined with 5-FU in the subgroup of group B was 11.0% and 16.0%, respectively (p = 0.07), and the mPFS was 4.3 and 4.4 months, respectively (p = 0.75). Although the ORR of irinotecan combined with 5-FU was slightly higher than that of irinotecan alone, the PFS was similar. 21
However, there was no head-to-head trial to compare the effects of irinotecan combined with 5-FU regimen with single-agent irinotecan, and FOLFIRI with or without target drugs is considered the standard second-line treatment. Therefore, we conducted this study, assuming that the FOLFIRI regimen is superior to irinotecan monotherapy. However, the results showed that FOLFIRI was not superior to irinotecan in terms of PFS in all patients and every subgroup, and no significant differences were detected in OS and ORR between the two groups. Importantly, the PFS, OS, and ORR data between the two groups were very close, with HRs for PFS and OS close to 1. Furthermore, the multivariable analysis using the Cox proportional hazard model, which included the treatment regimen as a factor, showed that with or without 5-FU, it was not a significant independent factor associated with PFS or OS, which further demonstrated that adding 5-FU did not improve treatment efficacy and prognosis. Meanwhile, FOLFIRI resulted to more AEs. Patients in the FOLFIRI group had significantly higher grade AEs, including leucopenia, neutropenia, thrombocytopenia, jaundice, mucositis, and vomiting and had a numerically higher incidence of grade 3–4 AEs, especially neutropenia, compared with patients in the irinotecan group. Treatment discontinuation due to disease progression was similar in both groups; however, treatment discontinuations (10.2% versus 4.8%) were higher in the FOLFIRI group than in the irinotecan group. These results suggested that adding 5-FU to irinotecan after the first-line 5-FU-containing chemotherapy failure did not enhance the effects but rather increased the toxicity as the cancer cells already became resistant to 5-FU, and continuation of 5-FU in this case is no longer warranted. Most patients were enrolled because of progression after first-line treatment, only a few patients were enrolled because of intolerable toxicity in first-line treatment. Specifically, in FOLFIRI group, six patients were intolerable for oxaliplatin and two patients were intolerable for 5-FU; in IRI group, nine patients were intolerable for oxaliplatin and one patient was intolerable for 5-FU. Therefore, most patients have had the opportunity to develop resistance. The results could also answer the question why the effects of FOLFOX and FOLFIRI were similar in terms of ORR and PFS in the first-line treatment, but the effect of FOLFOX was better than that of FOLFIRI in the second-line treatment in the V308 study, as the application of 5-FU in the second-line treatment increased the effects of oxaliplatin but not irinotecan. The synergistic effect between oxaliplatin and 5-FU has been demonstrated in both in vitro and in vivo experiments 22 and clinical trials.23–25 Moreover, the addition of oxaliplatin could overcome resistance to 5-FU, 26 which could explain why the addition of 5-FU after development of drug resistance in the second-line oxaliplatin-based regimens could still improve the effects. However, the combination of irinotecan and 5-FU in previous studies was either antagonistic or synergistic,27–29 and irinotecan has not been reported to overcome resistance to 5-FU. Our study showed that the combination of 5-FU after development of drug resistance in the second-line irinotecan-based regimens did not improve the effects, which indicated that irinotecan might not overcome resistance to 5-FU.
To our knowledge, our study is the first head-to-head trial showing that FOLFIRI failed to improve PFS, OS, and ORR compared with irinotecan as second-line treatment for mCRC, and single-agent irinotecan demonstrated significantly less toxicity than FOLFIRI. Therefore, irinotecan as a single-agent might be a more favorable chemotherapy option and might be a choice for second-line treatment, as chemotherapy combined with target drugs is currently the standard of care for mCRC patients.
Oral 5-FU prodrug S-1 is widely used as a substitute for 5-FU continuous infusion. The FIRIS study demonstrated the noninferiority of irinotecan plus S-1 to FOLFIRI in terms of PFS and OS as second-line chemotherapy for mCRC,30,31 suggesting that the IRIS regimen could be an option as a second-line chemotherapy for mCRC. Subsequently, IRIS-based treatment (combined with target drugs) has been further investigated as a second-line treatment for mCRC.32,33 Another oral 5-FU prodrug capecitabine was also widely used. When combined with oxaliplatin as first-line treatment for mCRC, capecitabine can be used as a substitute for 5-FU continuous infusion, as the effects of oxaliplatin plus capecitabine (XELOX) were similar to FOLFOX.34,35 However, irinotecan combined with capecitabine (XELIRI) led to a significantly greater gastrointestinal toxicity and inferior PFS compared with FOLFIRI in the first-line setting in the BICC-C trial. 36 The AXEPT study showed that the modified XELIRI (mXELIRI) with or without bevacizumab was well tolerated and was noninferior to FOLFIRI with or without bevacizumab in terms of OS, suggesting that mXELIRI could be an alternative to FOLFIRI as a second-line treatment for mCRC, at least in Asian patients. 9 In FIRIS and AXEPT studies, the value of the continued use of 5-FU in the FOLFIRI regimen was not considered. The results of our study suggest that it is not necessary to continue 5-FU in the second-line irinotecan-based treatment after failure of the first-line 5-FU-based regimen, which only increased the toxicity but not the efficacy. S-1 or capecitabine might also be unnecessary in this setting, as they were transformed to 5-FU in vivo to exert anticancer effects, and the effects of IRIS or mXELIRI were similar to those of FOLFIRI. Therefore, single-agent irinotecan could be the best choice as a second-line chemotherapy for mCRC after failure of the first-line 5-FU-based treatment, as data showed its almost similar effects but with a significantly less toxicity compared with FOLFIRI in our study. Moreover, single-agent irinotecan could eliminate the inconvenience of a continuous infusion of 5-FU and implantation of an intravenous port system in the FOLFIRI regimen, as well as the side effects and expenses caused by S-1 or capecitabine in the IRIS or mXELIRI regimen.
Mutations in the drug metabolism enzyme UGT1A1 are associated with the severity of major AEs caused by irinotecan, including diarrhea and neutropenia, which indicates the value of UGT1A1 for toxicity prediction and is expected to guide clinical personalized medicine and dosage modification in the future.
Our study has some limitations. First, quality-of-life assessments are crucial to patients receiving palliative treatments, and relevant data might be useful in the treatment decision-making process regarding irinotecan monodrug or FOLFIRI regimens. Second, the trial was open-label, which might introduce a potential reporting bias. Third, the lack of standard monoclonals in first-line therapy may be a limitation to the external validity of this trial.
Conclusion
Our study showed that FOLFIRI failed to improve the effects compared with single-agent irinotecan as a second-line treatment for mCRC. However, single-agent irinotecan was significantly less toxic than FOLFIRI. The results suggested that single-agent irinotecan might be a more favorable second-line chemotherapy, with similar efficacy and less toxicity, and offers more convenience compared with FOLFIRI for patients with mCRC.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211068737 for FOLFIRI (folinic acid, fluorouracil, and irinotecan) increases not efficacy but toxicity compared with single-agent irinotecan as a second-line treatment in metastatic colorectal cancer patients: a randomized clinical trial by Xiaowei Zhang, Ran Duan, Yusheng Wang, Xin Liu, Wen Zhang, Xiaodong Zhu, Zhiyu Chen, Wei Shen, Yifu He, Hong Qiang Wang, Mingzhu Huang, Chenchen Wang, Zhe Zhang, Xiaoying Zhao, Lixin Qiu, Jianfeng Luo, Xuedan Sheng and Weijian Guo in Therapeutic Advances in Medical Oncology
Acknowledgments
We appreciate Dr. Xichun Hu for his advice on statistical analysis and manuscript revision.
Footnotes
Author contributions: Xiaowei Zhang: writing – review and editing; Ran Duan: data curation, formal analysis; visualization, writing – original draft, writing – review and editing; Yusheng Wang: methodology, project administration; Xin Liu: project administration; Wen Zhang: project administration; Xiaodong Zhu: project administration; Z Chen: project administration; Wei Shen: project administration; Yifu He: project administration; Hongqiang Wang: project administration; Huang Mingzhu: project administration; Chenchen Wang: project administration; Zhe Zhang: project administration; Xiaoying Zhao: project administration; Lixin Qiu: project administration; Jianfeng Luo: data curation, formal analysis, supervision; Xuedan Sheng: data curation, resources; Weijian Guo: conceptualization, methodology, project administration, resources, supervision.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: The protocol was approved by Fudan University Shanghai Cancer Center ethics review board (approval ID: 1608162-17-1612A), and the study conduct was conducted according to the principles of Good Clinical Practice and the Declaration of Helsinki. All patients had signed randomization and treatment informed consent form.
ORCID iD: Chenchen Wang  https://orcid.org/0000-0003-4930-3187
https://orcid.org/0000-0003-4930-3187
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xiaowei Zhang, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Ran Duan, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Yusheng Wang, Shanxi Tumor Hospital, Taiyuan, China; Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Xin Liu, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Wen Zhang, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Xiaodong Zhu, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Zhiyu Chen, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Wei Shen, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Yifu He, Department of Medical Oncology, The First Affiliated Hospital of USTC, Division of Life Science and Medicine, University of Science and Technology of China, Hefei, China.
Hong Qiang Wang, Department of Oncology, Zhejiang Province Zhoushan Hospital, Zhoushan, China.
Mingzhu Huang, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Chenchen Wang, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Zhe Zhang, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Xiaoying Zhao, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Lixin Qiu, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Jianfeng Luo, Department of Biostatistics, School of Public Health, Fudan University, Shanghai, China.
Xuedan Sheng, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
Weijian Guo, Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center and Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China.
References
- 1. Mo S, Cai X, Zhou Z, et al. Nomograms for predicting specific distant metastatic sites and overall survival of colorectal cancer patients: a large population-based real-world study. Clin Transl Med 2020; 10: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Yan S, Liu X, et al. Gender-related prognostic value and genomic pattern of intra-tumor heterogeneity in colorectal cancer. Carcinogenesis 2017; 38: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mo S, Zhou Z, Dai W, et al. Development and external validation of a predictive scoring system associated with metastasis of T1-2 colorectal tumors to lymph nodes. Clin Transl Med 2020; 10: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Latest global cancer data: cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020. International Agency for Research on Cancer, World Health Organization, https://www.iarc.who.int/wp-content/uploads/2020/12/pr292_E.pdf
- 5. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67: 177–193. [DOI] [PubMed] [Google Scholar]
- 6. Aparicio J, Esposito F, Serrano S, et al. Metastatic colorectal cancer. First line therapy for unresectable disease. J Clin Med 2020; 9: E3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer 2015; 14: 1–10. [DOI] [PubMed] [Google Scholar]
- 8. Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004; 22: 1209–1214. [DOI] [PubMed] [Google Scholar]
- 9. Xu RH, Muro K, Morita S, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol 2018; 19: 660–671. [DOI] [PubMed] [Google Scholar]
- 10. Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol 2018; 36: 350–358. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Qin S, Xu RH, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA 2018; 319: 2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015; 16: 619–629. [DOI] [PubMed] [Google Scholar]
- 13. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22: 229–237. [DOI] [PubMed] [Google Scholar]
- 14. Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol 2003; 21: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 15. Cunningham D, Glimelius B. A phase III study of irinotecan (CPT-11) versus best supportive care in patients with metastatic colorectal cancer who have failed 5-fluorouracil therapy. V301 Study Group. Semin Oncol 1999; 26(1, Suppl. 5): 6–12. [PubMed] [Google Scholar]
- 16. Van Cutsem E, Blijham GH. Irinotecan versus infusional 5-fluorouracil: a phase III study in metastatic colorectal cancer following failure on first-line 5-fluorouracil. V302 Study Group. Semin Oncol 1999; 26(1, Suppl. 5): 13–20. [PubMed] [Google Scholar]
- 17. Satoh T, Ura T, Yamada Y, et al. Genotype-directed, dose-finding study of irinotecan in cancer patients with UGT1A1*28 and/or UGT1A1*6 polymorphisms. Cancer Sci 2011; 102: 1868–1873. [DOI] [PubMed] [Google Scholar]
- 18. Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 2311–2319. [DOI] [PubMed] [Google Scholar]
- 19. Kim JH, Park SJ, Park MI, et al. FOLFIRI as second-line chemotherapy after failure of FOLFOX4 in advanced colorectal cancer: a Korean single-center experience. Korean J Gastroenterol 2014; 63: 18–24. [DOI] [PubMed] [Google Scholar]
- 20. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012; 30: 3499–3506. [DOI] [PubMed] [Google Scholar]
- 21. Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial [published correction appears in Lancet 2007; 370: 566]. Lancet 2007; 370: 143–152. [DOI] [PubMed] [Google Scholar]
- 22. Raymond E, Buquet-Fagot C, Djelloul S, et al. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs 1997; 8: 876–885. [DOI] [PubMed] [Google Scholar]
- 23. deBraud F, Munzone E, Nolè F, et al. Synergistic activity of oxaliplatin and 5-fluorouracil in patients with metastatic colorectal cancer with progressive disease while on or after 5-fluorouracil. Am J Clin Oncol 1998; 21: 279–283. [DOI] [PubMed] [Google Scholar]
- 24. de Gramont A, Figer M, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–2947. [DOI] [PubMed] [Google Scholar]
- 25. Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000; 18: 136–147. [DOI] [PubMed] [Google Scholar]
- 26. Garufi C, Brienza S, Pugliese P, et al. Overcoming resistance to chronomodulated 5-fluorouracil and folinic acid by the addition of chronomodulated oxaliplatin in advanced colorectal cancer patients. Anticancer Drugs 2000; 11: 495–501. [DOI] [PubMed] [Google Scholar]
- 27. Azrak RG, Cao S, Slocum HK, et al. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clin Cancer Res 2004; 10: 1121–1129. [DOI] [PubMed] [Google Scholar]
- 28. Yamada T, Furukawa K, Yokoi K, et al. Effects of irinotecan and 5-FU combination therapy in gastric cancer – is combination therapy synergic? Gan To Kagaku Ryoho 2010; 37: 2125–2129. [PubMed] [Google Scholar]
- 29. Goldberg RM, Erlichman C. Irinotecan plus 5-FU and leucovorin in advanced colorectal cancer: North American trials. Oncology 1998; 12(8, Suppl. 6): 59–63. [PubMed] [Google Scholar]
- 30. Muro K, Boku N, Shimada Y, et al. Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 2010; 11: 853–860. [DOI] [PubMed] [Google Scholar]
- 31. Yasui H, Muro K, Shimada Y, et al. A phase 3 non-inferiority study of 5-FU/l-leucovorin/irinotecan (FOLFIRI) versus irinotecan/S-1 (IRIS) as second-line chemotherapy for metastatic colorectal cancer: updated results of the FIRIS study. J Cancer Res Clin Oncol 2015; 141: 153–160. [DOI] [PubMed] [Google Scholar]
- 32. Miyamoto Y, Tsuji A, Tanioka H, et al. S-1 and irinotecan plus bevacizumab as second-line chemotherapy for patients with oxaliplatin-refractory metastatic colorectal cancer: a multicenter phase II study in Japan (KSCC1102) [published correction appears in Int J Clin Oncol 2017]. Int J Clin Oncol 2016; 21: 705–712. [DOI] [PubMed] [Google Scholar]
- 33. Takaoka T, Kimura T, Shimoyama R, et al. Panitumumab in combination with irinotecan plus S-1 (IRIS) as second-line therapy for metastatic colorectal cancer. Cancer Chemother Pharmacol 2016; 78: 397–403. [DOI] [PubMed] [Google Scholar]
- 34. Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008; 26: 2006–2012. [DOI] [PubMed] [Google Scholar]
- 35. Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study [published correction appears in J Clin Oncol 2008; 26: 3110] [published correction appears in J Clin Oncol 2009; 27: 653]. J Clin Oncol 2008; 26: 2013–2019. [DOI] [PubMed] [Google Scholar]
- 36. Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol 2007; 25: 4779–4786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211068737 for FOLFIRI (folinic acid, fluorouracil, and irinotecan) increases not efficacy but toxicity compared with single-agent irinotecan as a second-line treatment in metastatic colorectal cancer patients: a randomized clinical trial by Xiaowei Zhang, Ran Duan, Yusheng Wang, Xin Liu, Wen Zhang, Xiaodong Zhu, Zhiyu Chen, Wei Shen, Yifu He, Hong Qiang Wang, Mingzhu Huang, Chenchen Wang, Zhe Zhang, Xiaoying Zhao, Lixin Qiu, Jianfeng Luo, Xuedan Sheng and Weijian Guo in Therapeutic Advances in Medical Oncology