Abstract
Objective
Sensitivity for detection of precancers at colposcopy and reassurance provided by a negative colposcopy are in need of systematic study and improvement. We sought to evaluate whether selecting the appropriate women for multiple targeted cervical biopsies based on screening cytology, HPV testing, and colposcopic impression could improve accuracy and efficiency of cervical precancer detection.
Methods
690 women aged 18–67 referred to colposcopy subsequent to abnormal cervical cancer screening results were included in the study (ClinicalTrials.gov: NCT00339989). Up to four cervical biopsies were taken during colposcopy to evaluate the incremental benefit of multiple biopsies. Cervical cytology, HPV genotyping, and colposcopy impression were used to establish up to 24 different risk strata. Outcomes for the primary analysis were cervical precancers, which included p16-positive CIN2 and all CIN3 that were detected by colposcopy-guided biopsy during the colposcopy visit. Later outcomes in women without CIN2 or more at baseline were abstracted from electronic medical records.
Results
The risk of detecting precancer ranged from 2% to 82% across 24 strata based on colposcopy impression, cytology, and HPV genotyping. The risk of precancer in the lowest stratum increased only marginally with multiple biopsies. Women in the highest risk strata had risks of precancer consistent with immediate treatment. In other risk strata, multiple biopsies substantially improved detection of cervical precancer. Among 361 women with less than CIN2 at baseline, 195 (54%) had follow-up cytology or histology data with a median follow-up time of 508 days. Lack of detection of precancer at initial colposcopy that included multiple biopsies predicted low risk of precancer during follow-up.
Conclusion
Risk assessment at the colposcopy visit makes identification of cervical precancers more effective and efficient. Not finding precancer after a multiple-biopsy protocol provides high reassurance and allows releasing women back to regular screening.
Keywords: Biopsy, Cervical cancer screening, Colposcopy, Precancer, Risk
Condensation
Assessing the risk of cervical precancer at the colposcopy visit improves detection of precancers through multiple targeted biopsies and provides high reassurance of a negative colposcopy.
Introduction
Cervical cancer screening programs rely primarily on detection and removal of cervical precancers before they progress to invasive cancers. In most high-resource settings, referral to colposcopy with biopsy is prompted by positive primary screening (1, 2). The colposcopic evaluation with colposcopic biopsies is critical to decide between returning women to routine screening, more intensive surveillance, or treatment.
Taking a single biopsy from the most concerning area on the cervical transformation zone misses a substantial proportion of precancers (3–5). Taking multiple targeted biopsies during colposcopy improves detection of prevalent precancers (6), a practice that was recommended in recent colposcopy guidelines (7, 8). The routine use of 4-quadrant random biopsies for detection of precancer remains controversial (4, 9).
Apart from substantial variability of colposcopy practice, colposcopy populations differ in referral criteria and hence the underlying population risks of cervical precancer. Within a colposcopy population, women present with different absolute risks of cervical precancer based on their screening cytology and HPV test results. This information could be used to improve detection of cervical precancers, according to the principle of precision prevention (10). However, currently, results from cytology or HPV tests are not systematically used to modify colposcopy practice.
Because of limited sensitivity of colposcopy-biopsy procedures, the reassurance from a negative colposcopy result can be low. Women without precancer detected at colposcopy often undergo repeated colposcopy procedures for an extended period of time until they are released back to regular screening.
The Biopsy Study previously showed the incremental benefit of multiple lesion-directed colposcopic biopsies (6). Here, we evaluated whether selecting the appropriate women for multiple targeted cervical biopsies based on screening cytology, HPV testing, and colposcopic impression could improve accuracy and efficiency of cervical precancer detection. Furthermore, we studied outcomes following a negative colposcopy to evaluate the reassurance provided by negative colposcopy.
Materials and Methods
Population
The Biopsy Study included women 18 years and older with abnormal cervical cancer screening results referred to colposcopy at the University of Oklahoma Health Sciences Center (OUHSC) between February 2009 and August 2011, as previously described (6). Among 1,373 eligible women, 690 (50.3%) agreed to participate in the study. Written informed consent was obtained from all women enrolled and Institutional Review Board approval was provided by OUHSC and the US National Cancer Institute.
Colposcopy and biopsy protocol, histologic endpoints, cytology and HPV testing
Colposcopies were performed by experienced attendings and clinical fellows of the OUHSC colposcopy clinic. Six colposcopists performed between 60 and 179 colposcopic examinations each. The following grades of colposcopic impression were distinguished, based on the listed features: High grade colposcopy impression (rapidly appearing and slowly fading, thick acetowhitening; coarse mosaicism and coarse punctation; sharp border, inner border sign, ridge sign, peeling edges; flat contour); Low grade colposcopy impression (thin and rapidly fading acetowhitening; fine mosaicism and fine punctation; irregular border; raised contour); Acetowhitening (acetowhitening, but none of the features listed for high grade or low grade impression); Normal (no acetowhitening and none of the features listed for high grade or low grade impression). Up to four directed biopsies were taken from distinct areas of epithelium that turned white on the application of 5% acetic acid in the cervical transformation zone or large heterogeneous lesions extending over multiple quadrants (lesion-directed biopsies). If fewer than four lesion-directed biopsies were taken, a non-targeted biopsy was added at the transformation zone in a quadrant without acetowhite lesions. All biopsies were ranked by order of severity by the colposcopist and each biopsy specimen was evaluated separately in histology. An adjacent section was stained for p16 using the CINtec® p16 Histology assay (Roche mtm Laboratories, Mannheim, Germany) and evaluated as previously described (11). We adopted the Lower Anogenital Squamous Terminology (LAST) guidelines for this analysis (12): all CIN2 cases that stained diffusely positive for p16 were included as HSIL histology, along with all CIN3 regardless of p16 staining. Only 10% of 197 CIN2 were p16-negative, inclusion of which did not affect risk strata and disease yield in a meaningful way (6). In this population, endocervical sampling detected very little disease beyond cervical biopsies (13) and was not considered for histologic endpoints. Referral cytology was community-based and included conventional smears and liquid based cytology, using the Bethesda nomenclature with the categories “Atypical squamous cells of undetermined significance” (ASC-US), “Low grade squamous intraepithelial lesion” (LSIL), “Atypical squamous cells, HSIL cannot be excluded” (ASC-H), “High grade squamous intraepithelial lesion (HSIL)”, and “Atypical Glandular Cells (AGC)” (14). Women with “Normal for intraepithelial lesion or malignancy” (NILM) cytology results were not referred to colposcopy. During colposcopy, a cervical specimen was collected using a Wallach broom device and transferred to PreservCyt solution (Hologic, Marlborough, MA, USA) for HPV DNA analysis. HPV detection and genotyping was based on Linear Array (LA) HPV Genotyping Test (Roche Molecular Diagnostics, Branchburg, NJ), as previously described (15, 16).
Statistical analysis
The strata in this analysis were defined based on the risk measures that strongly predicted yield of cervical precancer in our previous analysis (6). The population was stratified into 24 subgroups based on colposcopy impression (High grade; Low grade; Acetowhitening; Normal), referral cytology (HSIL, including ASC-H and AGC; LSIL; ASC-US) and HPV16 status (positive; negative). We did not include age strata because age was not associated with yield of precancer in our population. A total of 647 women had information on all three risk measures and histologic endpoints. For each stratum, the absolute risk of HSIL histology was calculated by dividing the number of HSIL endpoints over all women in the stratum. Similar strata were formed for combinations of colposcopy impression and referral cytology alone (12 strata, n=650 women), as well as combinations of colposcopy impression and HPV16 status alone (8 strata, n=673 women). For subsequent analyses, we collapsed into 12 strata using two cytology categories (HSIL vs. <HSIL cytology), two HPV categories (HPV16-positive/HPV16-negative) and three colposcopy impression categories (High-grade impression, low-grade impression, acetowhitening/normal impression). Three strata with 12 or fewer women (i.e., acetowhitening or normal impression/ <HSIL/ HPV16-positive with 11 women; acetowhitening or normal impression/ HSIL/ HPV16-negative with 12 women; and acetowhitening or normal impression/ HSIL/ HPV16-positive with 6 women) were excluded from these analyses, leaving a total of nine combined strata including 619 (95.5%) of 648 women. We evaluated the incremental yield of taking multiple biopsies between risk strata by comparing the differences in risk of precancer for disease detected with the first biopsy vs. all biopsies. We compared the yield of histologic HSIL within risk strata to clinical management thresholds (17, 18), differentiating return to regular screening, increased surveillance, referral to colposcopy, and immediate treatment (19). Importantly, while all women included in this study were referred to colposcopy based on their cytology result, additional, more refined risk stratification may group individual women in other risk categories. A 10% risk was used to differentiate surveillance from immediate colposcopy referral, while a 61% risk (corresponding to HSIL cytology in this study) was used as a risk level that allows for immediate treatment per the American Society of Colposcopy and Cervical Pathology (ASCCP) management guidelines (20).
Follow-up of women without CIN2 or worse histopathology diagnosed at baseline was conducted through August 31, 2015 by searching medical records at the OUHSC and through contacting women who were not seen at OUHSC subsequently. One hundred and ninety-five of 361 (52%) women had follow-up information for additional outcomes after the initial visit. These outcomes were ascertained through standard community clinical practice, which may have differed from the study procedures at OUHSC. Median follow-up was 508 days, ranging up to 1913 days. We evaluated the risk of subsequent high grade histology (CIN2+) or high grade cytology (HSIL+) in women without HSIL histology in the cross-sectional study within risk strata (21).
Results
Cervical precancers in combined risk strata at colposcopy
We divided the colposcopy population into 24 strata based on referral cytology (HSIL+, LSIL, ASC-US), HPV16 status (HPV16+, other high-risk type or negative), and colposcopy impression (High grade, low grade, acetowhitening, normal). Table 1 shows the strata with number of women and the number of HSIL+ outcomes ranked by risk of HSIL+. The number of women in each stratum varied considerably, from 0 (normal colposcopy, HSIL+ cytology and HPV16-positive) up to 115 (low grade colposcopy, LSIL cytology, and HPV16-negative). Generally, there was a correlation between the different high risk criteria; e.g., women with high grade colposcopy impression were also more likely to have HSIL cytology and/or HPV16. Conversely, only four women with HPV16 and only two women with HSIL cytology had a completely normal colposcopy impression. The risk of histological HSIL+ ranged from 0% in the lowest categories up to 82% for the highest-risk stratum (High grade colposcopy, LSIL, HPV16+). Strata with at least two high risk measures (either HSIL cytology, HPV16 positivity, or high-grade colposcopy impression) had at least a 57% risk of precancer. Strata with one high risk measure had at least a 27% risk of precancer.
Table 1.
Cervical precancer risk in strata based on colposcopic impression, cytology and HPV16 status
| Colposcopy | Cytology | HPV16 | Total N | HSIL Histology |
Risk |
|---|---|---|---|---|---|
| High grade | LSIL | + | 17 | 14 | 0.82 |
| High grade | HSIL | + | 57 | 44 | 0.77 |
| Low grade | HSIL | + | 27 | 19 | 0.70 |
| High grade | HSIL | − | 50 | 32 | 0.64 |
| Low grade | LSIL | + | 41 | 25 | 0.61 |
| High grade | ASC−US | + | 7 | 4 | 0.57 |
| Low grade | HSIL | − | 53 | 22 | 0.42 |
| High grade | LSIL | − | 49 | 18 | 0.37 |
| High grade | ASC−US | − | 26 | 9 | 0.35 |
| Acetowhite | HSIL | + | 6 | 2 | 0.33 |
| Low grade | ASC−US | + | 17 | 5 | 0.29 |
| Acetowhite | HSIL | − | 11 | 3 | 0.27 |
| Acetowhite | LSIL | + | 4 | 1 | 0.25 |
| Low grade | LSIL | − | 115 | 22 | 0.19 |
| Low grade | ASC−US | − | 73 | 13 | 0.18 |
| Normal | LSIL | − | 28 | 2 | 0.07 |
| Acetowhite | LSIL | − | 29 | 2 | 0.07 |
| Normal | ASC−US | − | 8 | 0 | 0.00 |
| Normal | ASC−US | + | 2 | 0 | 0.00 |
| Normal | LSIL | + | 2 | 0 | 0.00 |
| Normal | HSIL | − | 2 | 0 | 0.00 |
| Acetowhite | ASC−US | − | 19 | 0 | 0.00 |
| Acetowhite | ASC−US | + | 4 | 0 | 0.00 |
| Normal | HSIL | + | None |
ASC-US: Atypical squamous cells of undetermined significance; LSIL: Low grade squamous intraepithelial lesion; HSIL: High grade squamous intraepithelial lesion; high-risk categories are shown in bold
To address clinical scenarios that do not have HPV genotyping available, we repeated these analyses for combinations of cytology and colposcopy impression. The risk of histological HSIL+ ranged from 0% in the lowest categories up to 71% for women with high grade colposcopy impression and HSIL cytology (Table 2). Similarly, in combinations of HPV genotyping and colposcopy impression the risk of histological HSIL+ ranged from 0%, to 77% for women with high grade colposcopy impression and HPV16 positivity (Table 3).
Table 2.
Cervical precancer risk in strata based on colposcopic impression and cytology
| Colposcopy | Cytology | Total N | HSIL Histology |
Risk |
|---|---|---|---|---|
| High grade | HSIL | 108 | 77 | 0.71 |
| Low grade | HSIL | 80 | 41 | 0.51 |
| High grade | LSIL | 66 | 32 | 0.48 |
| High grade | ASC-US | 33 | 13 | 0.39 |
| Low grade | LSIL | 156 | 47 | 0.30 |
| Acetowhite | HSIL | 17 | 5 | 0.29 |
| Low grade | ASC-US | 92 | 18 | 0.20 |
| Acetowhite | LSIL | 33 | 3 | 0.09 |
| Normal | LSIL | 30 | 2 | 0.07 |
| Normal | ASC-US | 10 | 0 | 0.00 |
| Normal | HSIL | 2 | 0 | 0.00 |
| Acetowhite | ASC-US | 23 | 0 | 0.00 |
ASC-US: Atypical squamous cells of undetermined significance; LSIL: Low grade squamous intraepithelial lesion; HSIL: High grade squamous intraepithelial lesion; high-risk categories are shown in bold
Table 3.
Cervical precancer risk in strata based on colposcopic impression and HPV16 status
| Colposcopy | HPV16 | Total N | HSIL Histology |
Risk |
|---|---|---|---|---|
| High grade | + | 83 | 64 | 0.77 |
| Low grade | + | 85 | 49 | 0.58 |
| High grade | − | 131 | 63 | 0.48 |
| Low grade | − | 249 | 60 | 0.24 |
| Acetowhite | + | 15 | 3 | 0.20 |
| Acetowhite | − | 62 | 5 | 0.08 |
| Normal | − | 43 | 2 | 0.05 |
| Normal | + | 5 | 0 | 0.00 |
HSIL: High grade squamous intraepithelial lesion; high-risk categories are shown in bold
Multiple biopsies in combined risk strata and risk thresholds
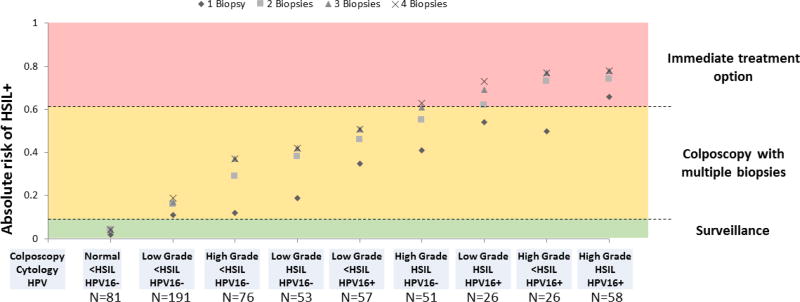
We evaluated the incremental benefit of taking multiple biopsies for detecting cervical precancers in nine risk strata representing 619 (95.5%) of 648 women (Table 4, Figure 1). The absolute increase in detection of cervical precancer from 1 to 4 biopsies varied widely across the groups. In most strata, three biopsies were sufficient to detect HSIL endpoints (Figure 1). The lowest increases in yield were found at the low end and at the high end of the risk spectrum. In the lowest risk group (Normal/acetowhitening colposcopy impression, HPV16-negative, <HSIL), taking multiple biopsies increased the HSIL yield only marginally from 2% to 4%, indicating that when disease risk is low, multiple biopsies are not very useful. In women with high grade colposcopy impression, HSIL cytology and HPV16, the yield of precancer increased from 66% to 78% from one to four biopsies, indicating that in the highest risk group, lesions are more easily detected with fewer biopsies. In the other risk categories, taking multiple biopsies led to increases of absolute yield up to 27% (in women with high grade colposcopy impression, <HSIL cytology, and HPV16-positivity), showing the importance of multiple biopsies for disease ascertainment.
Table 4.
Incremental yield of multiple biopsies in risk strata
| Stratum (Colposcopy impression/ cytology/ HPV16) |
Women in stratum |
Disease yield first biopsy |
Disease yield all biopsies |
Incremental yield |
Incremental sensitivity |
|---|---|---|---|---|---|
| High grade/ HSIL/ HPV16+ | 58 | 66% | 78% | 12% | 15% |
| High grade/ <HSIL/ HPV16+ | 26 | 50% | 77% | 27% | 35% |
| Low grade/ HSIL/ HPV16+ | 26 | 54% | 73% | 19% | 26% |
| High grade/ HSIL/ HPV16− | 51 | 41% | 63% | 22% | 35% |
| Low grade/ <HSIL/ HPV16+ | 57 | 35% | 51% | 16% | 31% |
| Low grade/ HSIL/ HPV16− | 53 | 19% | 42% | 23% | 55% |
| High grade/ <HSIL/ HPV16− | 76 | 12% | 37% | 25% | 68% |
| Low grade/ <HSIL/ HPV16− | 191 | 11% | 19% | 8% | 42% |
| Normal or acetowhitening/ <HSIL/ HPV16− | 81 | 2% | 4% | 2% | 50% |
HSIL: High grade squamous intraepithelial lesion; high-risk categories are shown in bold; disease indicates histological HSIL; incremental yield indicates the absolute difference in yield from all biopsies and the first biopsy; incremental sensitivity indicates the relative increase in detection from the first biopsy to all biopsies
Figure 1. Absolute risk of HSIL and greater in strata of colposcopy impression, cytology, and HPV result.
Absolute risk of cervical precancer (HSIL+) is shown for nine strata of colposcopy impression, cytology result and HPV result in the colposcopy population based on increasing number of biopsies (from 1–4). The green area of the graph shows the risk level at which surveillance is recommended. The yellow area shows the risk level at which colposcopy with biopsies is recommended. The red area shows the risk level at which immediate treatment is an option.
We compared the absolute risk of disease in risk strata to established clinical action thresholds (Figure 1). The risk in the lowest stratum (Normal colposcopy impression, <HSIL, HPV16−) including 81 women was 4%, a risk level at which current guidelines recommend surveillance rather than colposcopy referral (20). Four risk strata including 161 women who had at least two high risk categories had a risk above the threshold for which immediate treatment is acceptable per current management guidelines (20). Three of these strata, all HPV16-positive, had a risk considerably higher than the immediate treatment threshold (ranging from 73%–78% compared to 61% for HSIL cytology alone).
Follow-up of women without CIN2+ detected at colposcopy visit
To evaluate the safety of a multiple biopsy protocol, follow-up was conducted in women without precancer detected at the study colposcopy visit. Of the 619 women in the nine strata, 361 women had no CIN2 or greater detected at baseline (Table 5). Among these women, follow-up histology or cytology information was available for 195 (54%) women. The percentage of women with follow-up data ranged from 43%–75% within strata, with higher percentages in the higher risk strata. Among 195 women with follow-up, 8 CIN2+ histology or HSIL cytology results were observed, translating to a NPV of 96% for the multiple biopsy protocol in the overall population and a risk of any CIN2+ or cytologic HSIL in follow-up of 4%. There was one CIN3 observed during follow-up, with an NPV for CIN3+ of 99.5%. The other outcomes were 6 CIN2 histology results and 1 HSIL cytology result. All other women had <HSIL cytology results or <CIN2 histology results. In women from the lowest risk stratum (Normal or acetowhite colposcopy impression, <HSIL, HPV16−), no high grade histologic or cytologic abnormalities were found during follow-up. In other strata with at least 10 women who had follow up, the risk ranged from 0%–6%, translating into a high NPV independent of risk stratum for a multiple biopsy protocol to reassure against the presence of cervical precancer.
Table 5.
Disease detected in follow-up by risk strata
| Stratum (Colposcopy impression/ cytology/ HPV16) |
Women in stratum |
Women without CIN2+ at baseline |
Women with follow up |
N HSIL+ cytology or CIN2+ histology during follow-up |
Risk in women without CIN2+ at baseline |
NPV |
|---|---|---|---|---|---|---|
| High grade/ HSIL/ HPV16+ | 58 | 11 | 7 (64%) | 1 | 14% | 86% |
| High grade/ <HSIL/ HPV16+ | 26 | 6 | 4 (67%) | 2 | 50% | 50% |
| Low grade/ HSIL/ HPV16+ | 26 | 7 | 3 (43%) | 0 | 0% | 100% |
| High grade/ HSIL/ HPV16− | 51 | 16 | 12 (75%) | 0 | 0% | 100% |
| Low grade/ <HSIL/ HPV16+ | 57 | 25 | 17 (68%) | 1 | 6% | 94% |
| Low grade/ HSIL/ HPV16− | 53 | 29 | 20 (69%) | 0 | 0% | 100% |
| High grade/ <HSIL/ HPV16− | 76 | 45 | 20 (44%) | 1 | 5% | 95% |
| Low grade/ <HSIL/ HPV16− | 191 | 146 | 76 (52%) | 3 | 4% | 96% |
| Normal or acetowhitening/ <HSIL/ HPV16− | 81 | 76 | 36 (47%) | 0 | 0% | 100% |
| Total | 619 | 361 | 195 (54%) | 8 | 4% | 96% |
HSIL: High grade squamous intraepithelial lesion; high-risk categories are shown in bold; CIN2+: cervical intraepithelial neoplasia grade 2 or higher; NPV:negative predictive value
Comment
Efficient detection of cervical precancers for treatment is central to successful prevention of cervical cancer. Screening and triage tests, applied to large populations, identify the smaller subset of women at high enough risk of precancer that warrants referral to colposcopy for diagnostic evaluation. Current colposcopy approaches often lack sensitivity for detection of precancers and frequently result in recommendation of multiple repeat colposcopy exams when no precancer is found, to account for potentially false-negative findings (22). These repeated diagnostic evaluations are a considerable burden for affected women and increase the cost of cervical cancer screening programs.
We show that improved identification of cervical precancers at colposcopy can be achieved using risk assessment based on referral cytology and HPV tests as well as colposcopic visual examination, which allows tailoring colposcopy-biopsy procedures better to each individual woman, according to the principle of precision prevention (10). In the lowest risk group, taking multiple biopsies does not increase yield of disease meaningfully and biopsies can be restricted to visible abnormalities, including acetowhitening, metaplasia, or higher abnormalities. At the high end of the risk spectrum, immediate treatment in women >25 years is an alternative to multiple biopsy sampling. Particularly in women with at least two high risk features, the risk of precancer is substantially higher than the risk of HSIL cytology alone, for which immediate treatment is already an option according to current management guidelines (20). In other risk groups, taking multiple targeted biopsies from areas on the cervix with acetowhitening or more severe changes is important, because of the high absolute increase in yield of precancers.
For the first time, we report follow-up data for women evaluated with colposcopy using a multiple-biopsy protocol. Among 195 women who did not have precancer detected with up to 4 biopsies, only 8 cases of either HSIL cytology or CIN2+ were detected in follow-up, suggesting that a targeted multi-biopsy protocol has a very high sensitivity and negative predictive value for cervical precancer. We previously demonstrated that taking untargeted biopsies from areas on the cervix without acetowhitening yielded very little disease (6). Our follow-up data further supports that taking untargeted biopsies does not lead to meaningful disease detection. Another important observation from our study was that in women with high risk results (like HSIL cytology and HPV16), colposcopic abnormalities and biopsy targets are almost always found. Importantly, the negative predictive value of the multiple biopsy protocol was comparable across the different risk strata, indicating that this approach provides good reassurance for colposcopynegative women for the whole colposcopy population.
Taken together, our findings show that colposcopy can be optimized using a risk-based multiple-biopsy approach so that detection of prevalent precancers is highly sensitive, while at the same time providing great reassurance to women in whom precancer is not found. This strategy can accelerate detection and treatment of precancers, while allowing women without precancer to safely return to regular screening procedures more quickly and thereby reducing the number of colposcopies.
Of note, women age 40 years and older were underrepresented in our population. While age did not modify the findings in our study, it is possible that some results may be different in older women. In our population, endocervical curettage detected very little disease (Liu in press) and had no impact on the findings, but this could be different in older women. Our study was conducted in a population predominantly screened and managed by cytology. As primary screening increasingly moves to HPV-cytology co-testing or primary HPV screening alone, the composition of the colposcopy population will change. Sending all HPV-positive women to colposcopy is not practicable, since most infections are not associated with precancer and others do not produce lesions detectable at colposcopy initially. In current co-testing and HPV screening protocols, women who are negative for cytology, but who test positive for HPV16/18 once or for other high-risk types at two consecutive time points are referred to colposcopy. This group of women is not represented in our population, since all women had at least ASC-US cytology for colposcopy referral. Many of these women may not have precancers, and if they do, the lesions may be very small and harder to detect than lesions in cytology-positive women. Nevertheless, all risk strata described in our population are also present in populations screened with HPV, so the strata-specific findings are relevant for these populations. Future studies should particularly focus on risk strata and colposcopy-biopsy performance of HPV-positive, cytology-negative women.
When tailoring management to risk levels, it is important to consider the robustness of individual risk markers. Molecular risk markers such as HPV testing and genotyping or other biomarkers are objective and are highly reproducible. While morphological and visual markers are more limited, at high thresholds (HSIL cytology; high grade colposcopy impression), reproducibility and accuracy are sufficient for risk stratification. While it is possible to predict individual absolute risk with fine details, as evidenced by the risk distribution in the 24 strata, only few risk differences matter clinically. Therefore, it is practicable to condense risk strata using few strata that result in different clinical management.
Another important consideration for development of a risk-based colposcopy protocol is that results from primary screening need to be available for concurrent evaluation at the colposcopy visit. Integration of these results together with outcomes from previous screening rounds in electronic medical records will greatly facilitate the implementation of risk-based management. Of note, the findings from the Biopsy Study (6) have already influenced recently published ASCCP colposcopy recommendations (7, 8). The prospective findings presented here will further impact management recommendations, particularly with respect to management intervals after normal colposcopy.
In summary, we show how risk assessment at the colposcopy visit improves sensitivity for cervical precancers, while limiting multiple biopsies to those who need them. We demonstrate how biopsy protocols can be adapted to underlying risk, determined by measures readily available at the colposcopy visit. Importantly, we demonstrate that our approach provides great reassurance for women with a negative colposcopy result that they are at very low risk of precancer.
Implications and Contributions.
A. Why was this study conducted?
The study was conducted to evaluate how risk assessment can guide colposcopy and biopsy practice, and to evaluate what reassurance a negative colposcopy provides.
B. What are the key findings?
Taking multiple targeted biopsies is important for disease ascertainment for most women attending a colposcopy clinic. Exceptions are the lowest risk groups where multiple biopsies do not provide much benefit, and the highest risk groups, where immediate treatment is an option.
C. What does this study add to what is already known?
This is the first study to evaluate colposcopy performance in risk strata and to provide long-term follow up risk estimates in women with a negative colposcopy and biopsy.
Acknowledgments
Funding: Intramural Research Program of the National Cancer Institute (1ZIACP010124); the funding source had no role in collection, analysis or interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no conflict of interest.
References
- 1.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–7. doi: 10.1097/AOG.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gage JC, Hanson VW, Abbey K, Dippery S, Gardner S, Kubota J, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108(2):264–72. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 4.Pretorius RG, Belinson JL, Burchette RJ, Hu S, Zhang X, Qiao YL. Regardless of skill, performing more biopsies increases the sensitivity of colposcopy. J Low Genit Tract Dis. 2011;15(3):180–8. doi: 10.1097/LGT.0b013e3181fb4547. [DOI] [PubMed] [Google Scholar]
- 5.van der Marel J, van Baars R, Rodriguez A, Quint WG, van de Sandt MM, Berkhof J, et al. The increased detection of cervical intraepithelial neoplasia when using a second biopsy at colposcopy. Gynecol Oncol. 2014;135(2):201–7. doi: 10.1016/j.ygyno.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Wentzensen N, Walker JL, Gold MA, Smith KM, Zuna RE, Mathews C, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33(1):83–9. doi: 10.1200/JCO.2014.55.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wentzensen N, Massad LS, Mayeaux EJ, Khan MJ, Waxman AG, Einstein MH, et al. Evidence-based consensus recommendations for colposcopy practice for cervical cancer prevention in the United States. J Low Genit Tract Dis. 2017 doi: 10.1097/LGT.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 8.Wentzensen N, Schiffman M, Silver M, Khan MJ, Perkins RB, Smith KM, et al. The American Society for Colposcopy and Cervical Pathology Colposcopy Standards: Risk-Based Colposcopy Practice. J Low Genit Tract Dis. 2017 doi: 10.1097/LGT.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 9.Huh WK, Sideri M, Stoler M, Zhang G, Feldman R, Behrens CM. Relevance of random biopsy at the transformation zone when colposcopy is negative. Obstet Gynecol. 2014;124(4):670–8. doi: 10.1097/AOG.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 10.Vineis P, Wild CP. The science of precision prevention of cancer. Lancet Oncol. 2017;18(8):997–8. doi: 10.1016/S1470-2045(17)30331-5. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron C, Ordi J, Schmidt D, Trunk MJ, Keller T, Ridder R, et al. Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol. 2010;133(3):395–406. doi: 10.1309/AJCPXSVCDZ3D5MZM. [DOI] [PubMed] [Google Scholar]
- 12.Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16(3):205–42. doi: 10.1097/LGT.0b013e31825c31dd. [DOI] [PubMed] [Google Scholar]
- 13.Liu AH, Walker J, Gage JC, Gold MA, Zuna R, Dunn ST, et al. Diagnosis of Cervical Precancers by Endocervical Curettage at Colposcopy of Women With Abnormal Cervical Cytology. Obstet Gynecol. 2017;130(6):1218–25. doi: 10.1097/AOG.0000000000002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 15.Wentzensen N, Schiffman M, Dunn ST, Zuna RE, Walker J, Allen RA, et al. Grading the severity of cervical neoplasia based on combined histopathology, cytopathology, and HPV genotype distribution among 1,700 women referred to colposcopy in Oklahoma. Int J Cancer. 2009;124(4):964–9. doi: 10.1002/ijc.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wentzensen N, Schiffman M, Dunn T, Zuna RE, Gold MA, Allen RA, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125(9):2151–8. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbyn M, Xu L, Verdoodt F, Cuzick J, Szarewski A, Belinson JL, et al. Genotyping for Human Papillomavirus Types 16 and 18 in Women With Minor Cervical Lesions: A Systematic Review and Meta-analysis. Ann Intern Med. 2017;166(2):118–27. doi: 10.7326/M15-2735. [DOI] [PubMed] [Google Scholar]
- 18.Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S28–35. doi: 10.1097/LGT.0b013e318285423c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffman M, Wentzensen N, Khan MJ, Castle PE, Chelmow D, Huh WK, et al. Preparing for the Next Round of ASCCP-Sponsored Cervical Screening and Management Guidelines. J Low Genit Tract Dis. 2017;21(2):87–90. doi: 10.1097/LGT.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 21.Wentzensen N, Wacholder S. From differences in means between cases and controls to risk stratification: a business plan for biomarker development. Cancer Discov. 2013;3(2):148–57. doi: 10.1158/2159-8290.CD-12-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silver MI, Schiffman M, Fetterman B, Poitras NE, Gage JC, Wentzensen N, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: Reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682–6. doi: 10.1002/cncr.30277. [DOI] [PMC free article] [PubMed] [Google Scholar]